Abstract
Background
This retrospective study aimed to investigate the risk factors associated with the recurrence of L5–S1 disc herniation after percutaneous endoscopic transforaminal discectomy (PETD).
Material/Methods
There were 484 patients L5–S1 disc herniation who underwent PETD who were divided into the recurrence group (n=46) and the non-recurrence group (n=438). Transforaminal endoscopic approaches included modifications of the Yeung endoscopy spine system (YESS) (the intraforaminal intradiscal approach) and the transforaminal endoscopic spine system (TESSYS) (intraforaminal extradiscal approach). Demographic and clinical characteristics and imaging data were analyzed. The two study groups were compared to determine the factors associated with the recurrence of L5–S1 disc herniation. The patients underwent postoperative follow-up for between one and four years.
Results
At follow-up, 9.504% of patients (46/484) with the recurrence of L5–S1 disc herniation following PETD when compared with the non-recurrence group showed no significant difference for time to return to work, gender, history of diabetes mellitus, trauma, duration of symptoms, smoking and alcohol history, hypertension, location of disc herniation, transverse process length, intervertebral space height, and pelvic incidence angle (P>0.05). However, age, body mass index (BMI), the degree of disc degeneration, sagittal range of motion, lumbar lordosis angle, and sacral slope were significantly associated with the recurrence of L5–S1 disc herniation following PETD (P<0.05). Logistic regression analysis supported these main associations.
Conclusions
The recurrence of L5–S1 disc herniation following PETD was significantly associated with increased age and BMI, more severe disc degeneration, increased sagittal range of motion, increased lumbar lordosis, and sacral slope.
MeSH Keywords: Diskectomy, Percutaneous; Endoscopy; Intervertebral Disc
Background
Percutaneous endoscopic lumbar discectomy (PELD) is a minimally invasive procedure for lumbar disc space decompression and removal of the nucleus pulposus that uses a posterolateral approach and is a technique increasingly used by spinal surgeons [1,2]. The PELD procedure has similar characteristics and therapeutic effects to traditional surgical treatments, but also has the advantages of a small incision, less blood loss, soft tissue injury, and fewer complications, with more rapid recovery and reduced surgical costs [3–5]. However, there has been some recent concern regarding postoperative complications as a result of the widespread use of PELD, with the recurrence of disc herniation as the most common complication. Choi et al. [1] studied 10, 228 patients who underwent PELD with follow-up for12 years and found that 4.3% of the procedures failed, mainly because of the recurrence of disc herniation.
Currently, there have been few studies on the risk factors associated with the recurrence of lumbar disc herniation after PELD, and there has been little in-depth analysis. In particular, the L5–S1 segment has special anatomical associations with the iliac crest, the oblique endplate, and the long transverse process [6,7]. Unlike other segments, these anatomical structures may affect the adjacent L5–S1 intervertebral disc. The two types of PELD currently used include percutaneous endoscopic transforaminal discectomy (PETD) and percutaneous endoscopic interlaminar discectomy (PEID). There is an ongoing debate regarding the most suitable PELD method for patients with L5–S1 disc herniation [8–11]. Currently, the choice of surgery is largely based on the experience of the surgeon.
Therefore, this retrospective study was conducted at a single center and aimed to investigate the risk factors associated with the recurrence of L5–S1 disc herniation after PETD. In this study, transforaminal endoscopic approaches for the surgical management of lumbar disc herniation included modifications of the Yeung endoscopy spine system (YESS) (the intraforaminal intradiscal approach) and the transforaminal endoscopic spine system (TESSYS) (intraforaminal extradiscal approach) techniques.
Material and Methods
Patients
This retrospective study included patients who were treated with percutaneous endoscopic transforaminal discectomy (PETD) for L5–S1 disc herniation from October 2014 to June 2018. The surgeons who participated in the study were assessed as having sufficient experience and competency in PETD. The patients underwent postoperative follow-up for between one and four years to identify patients with recurrent disc herniation. All procedures are carried out in compliance with the resolution of Helsinki. This study was approved by the Local Ethics Committee, and all patients provided written informed consent to participate in the study.
Study groups and clinical data
The study included 484 patients with L5–S1 disc herniation who underwent PETD. The patients were divided into the recurrence group (n=46) and the non-recurrence group (n=438). Clinical and demographic data were recorded and the two groups were compared. The clinical data included age, gender, body mass index (BMI), duration of symptoms, duration of follow-up, current tobacco and alcohol consumption, a history of hypertension, diabetes mellitus, trauma, the surgical procedure time, and time to return to work. Imaging parameters recorded included the location of disc herniation, intervertebral foramen, disc degeneration, ilium wing height, transverse process length, intervertebral space height, sagittal range of motion, lumbar lordosis angle, pelvic incidence angle, and sacral slope.
Study inclusion and exclusion criteria
The study inclusion criteria were disc herniation at L5–S1 level that was confirmed by magnetic resonance imaging (MRI) or computed tomography (CT), nerve root pain with a distribution that that conformed with imaging findings, pain reduction after surgery with no short-term recurrence (<3 month), and no strenuous exercise for three months after surgery. The exclusion criteria included lumbar instability and spondylolisthesis on X-ray of the lumbar spine during hyperextension and hyperflexion, the slip distance between the L5–S1 of >3 mm, or a sagittal rotation angle of >20° [12,13]. Patients were also excluded from the study if they had lumbar spondylolysis, lumbar scoliosis or kyphosis, who did not participate in follow-up, or who did not comply with the guidance of doctors postoperatively. Recurrent disc herniation was defined as the recurrence of the same pain as at the initial presentation, or MRI or CT imaging findings that confirmed recurrent disc herniation at the same segment.
Surgery using percutaneous endoscopic transforaminal discectomy (PETD) techniques
In this study, transforaminal endoscopic approaches for the surgical management of lumbar disc herniation included modifications of the Yeung endoscopy spine system (YESS) (the intraforaminal intradiscal approach) and the transforaminal endoscopic spine system (TESSYS) (intraforaminal extradiscal approach) techniques.
In the transforaminal endoscopic spine system (TESSYS) (intraforaminal extradiscal approach), a working tunnel was first created from the foraminal annular window, and the nucleus pulposus fragments were removed, and the internal disc was [14–16]. The modified Yeung endoscopy spine system (YESS) (the intraforaminal intradiscal approach) involved retrograde resection of the herniated disc within the spinal canal and was performed intradiscally through the annulus defect, and selectively included foraminal annular window fenestration [16–18].
The modified transforaminal endoscopic spine system (TESSYS) (intraforaminal extradiscal approach)
This surgical approach was chosen for patients with foraminal disc herniation and partial paramedian disc herniation, with or without spinal stenosis, for central disc herniation with intervertebral foramen stenosis, and for free central disc herniation. In this surgical method for PETD, the patient was in the lateral recumbent position with the symptomatic leg upward. Local anesthesia was used and included 5 ml of ropivacaine and 20 ml of lidocaine in 20 ml of normal saline. The mobile C-arm fluoroscopic X-ray system (Siemens, Munich, Germany) was used for guidance during surgery. The iliac crest was used as a marker to locate the L5–S1 intervertebral space, and a straight line was drawn from the highest point of the iliac crest, perpendicular to the midline of the spinous process. The puncture point was marked on the skin, about 12 cm from the midline of the spinous process, and the symptomatic side of the line. The angle between the guide puncture line and the anatomic level was between 25–30 degrees. Using the C-arm imaging window, the puncture needle reached the superior articular process of S1, and more local anesthesia was injected. The skin at the puncture point was incised (0.5–0.8 mm), and a cannula was sited while the puncture needle was removed. The superior articular process of S1 was reduced with a trephine.
A transforaminal endoscope (Maxmorespine, Unterföhring, Bavaria, Germany) was sited outside the intervertebral disc and through the superior articular process of S1 to the lateral recess. Foraminoplasty was performed after identification of the nerve root and the important tissues around it, followed by the removal of the nucleus pulposus that was pressing on the nerve root. Any surrounding adhesions and calcified tissue were removed. The annulotomy was performed selectively, and the clearance was assessed as successful when the color of the nerve roots changed from pale to pink under microscopy, the straight leg elevation test was negative, and the range of movement of nerve roots was 2–3 mm. The endoscope was removed, the drainage tube was sited, and the skin wound was sutured.
The modified Yeung endoscopy spine system (YESS) (the intraforaminal intradiscal approach)
This surgical approach was chosen for patients with containable, central, and partial paramedian disc herniation, with or without foramen stenosis. The modified YESS procedure for PETD was identical to the TESSYS method and used the mobile C-arm fluoroscopic X-ray system (Siemens, Munich, Germany) for imaging during surgery but used different puncture angles. The puncture needles were sited using the Kambin’s triangle subpedicular approach. Discography was performed to confirm the location of the disc. The cannula was gradually expanded and the working cannula was finally sited. The annulus fibrosus was surgically opened to remove the disc, radiofrequency hemostasis and nucleus pulposus ablation (annulotomy) were performed. Selective laminoplasty was performed.
Definitions of the study parameters
The BMI was defined as the patient weight (kg)/height2 (m2) [19]. The two main surgical procedures used for PETD included foraminoplasty and annulotomy. Annulotomy was used to remove the nucleus pulposus and destroy the annulus fibrosus. The location of the disc herniation was central, paramedian, and foraminal. The Lee grading approach was used as the basis for the classification of the foramen size [20]. Intervertebral foramen stenosis was divided into four degrees based on sagittal MRI: grade 0, none; grade 1, mild with perineural fat occlusion in the vertical or lateral directions; grade 2, moderate with fat occlusion in all four directions, but without morphological change; grade 3, severe intervertebral foramen stenosis with nerve root collapse or morphological changes. Intervertebral disc degeneration was classified according to the Pfirrmann MRI classification [21]. The ilium wing height was the distance from the top of the iliac crest to the horizontal plane of the upper edge of the S1 endplate measured on X-ray (Figure 1). The transverse process length was the distance from the outer edge of the transverse process to the outer edge of the vertebral body measured on X-ray (Figure 2). The intervertebral space height was the anterior height of the intervertebral space between the anterior upper edge of S1 and the anterior lower edge of L5. The posterior height of the intervertebral space was the distance from the posterior lower edge of S1 to the upper posterior edge of L5. The average height of the intervertebral space was calculated as: (the anterior height of the intervertebral space+the posterior height of intervertebral space)/2 (Figure 3). The sagittal range of motion was the angle (A) of the intervertebral space measured on the X-ray film in the lumbar hyperflexion position, and the angle (B) of the intervertebral space was measured on the X-ray film in the lumbar hyperextension position. The sagittal range of motion was the absolute value of angle A - angle B (Figures 4, 5). The lumbar lordosis angle was the angle between the vertical line of the upper edge of L1 and the vertical line of the upper endplate of S1 (Figure 6) [22]. The pelvic incidence angle was the vertical line of the upper sacral endplate through the mid-point of the upper sacral endplate, with the straight line passing through the mid-point of the upper sacral endplate and the center line of the bilateral femoral head. The pelvic incidence is the angle between the two lines (Figure 7) [22]. The sacral slope was the angle between the plane of the S1 endplate and the horizontal plane (Figure 8) [22]
Figure 1.
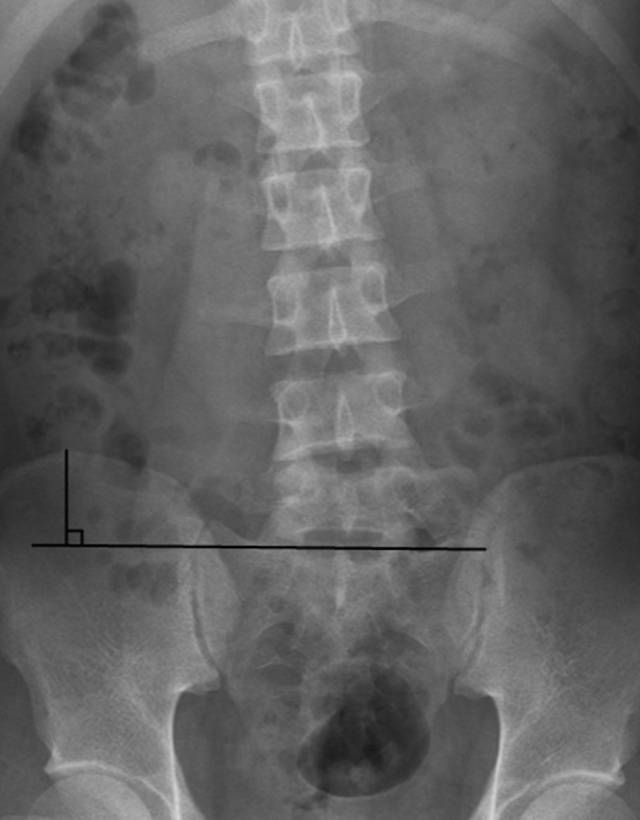
The ilium wing height. The ilium wing height is the distance from the top of the iliac crest to the horizontal plane of the upper edge of the S1 endplate measured on X-ray.
Figure 2.
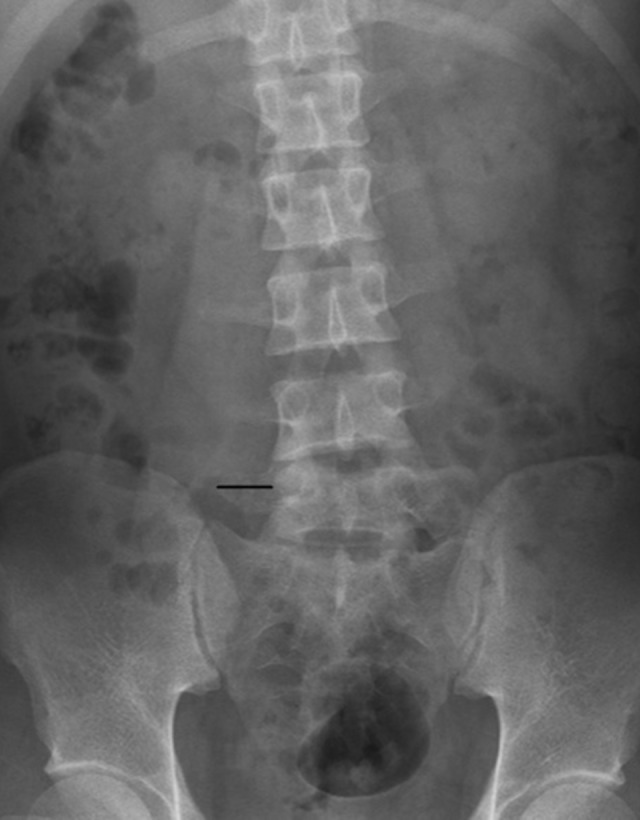
The transverse process length. The transverse process length is the distance from the outer edge of the transverse process to the outer edge of the vertebral body measured on X-ray.
Figure 3.
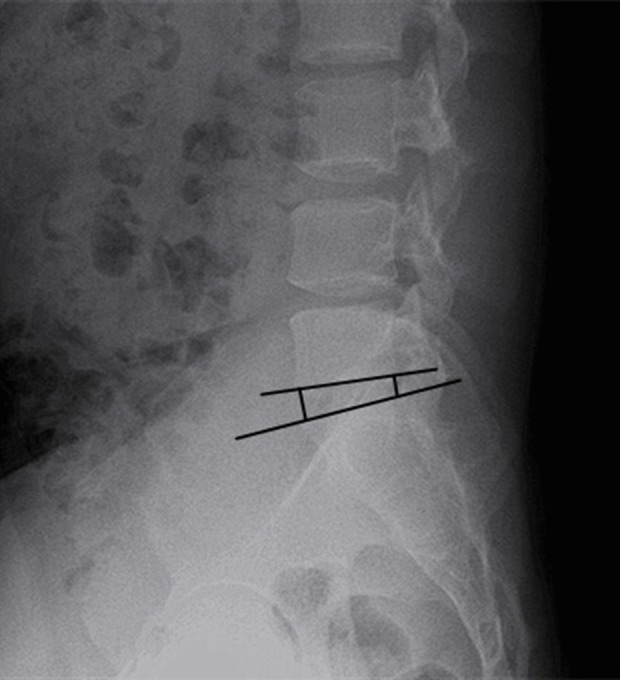
Intervertebral space height. The intervertebral space height is the anterior height of the intervertebral space between the anterior upper edge of S1 and the anterior lower edge of L5. The average height of the intervertebral space is calculated as: (the anterior height of the intervertebral space+the posterior height of intervertebral space)/2.
Figure 4.
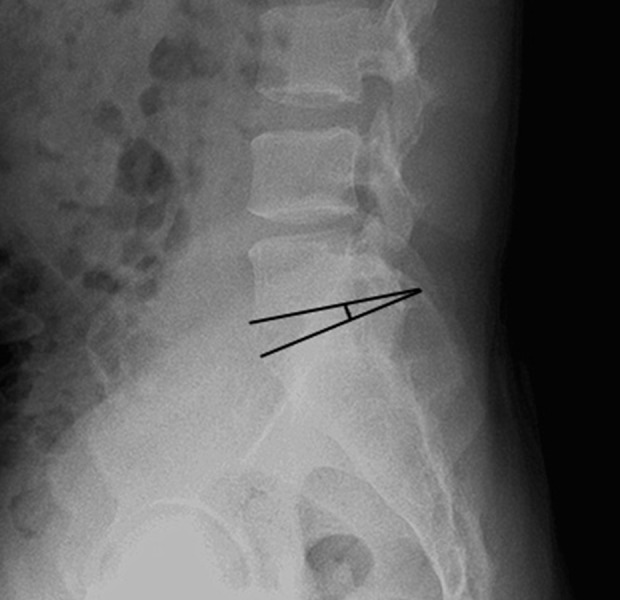
The sagittal range of motion. The sagittal range of motion is the angle (A) of the intervertebral space measured on the X-ray film in the lumbar hyperflexion position. The angle (B) of the intervertebral space is measured on the X-ray film in the lumbar hyperextension position. The sagittal range of motion is the absolute value of angle A–angle B.
Figure 5.
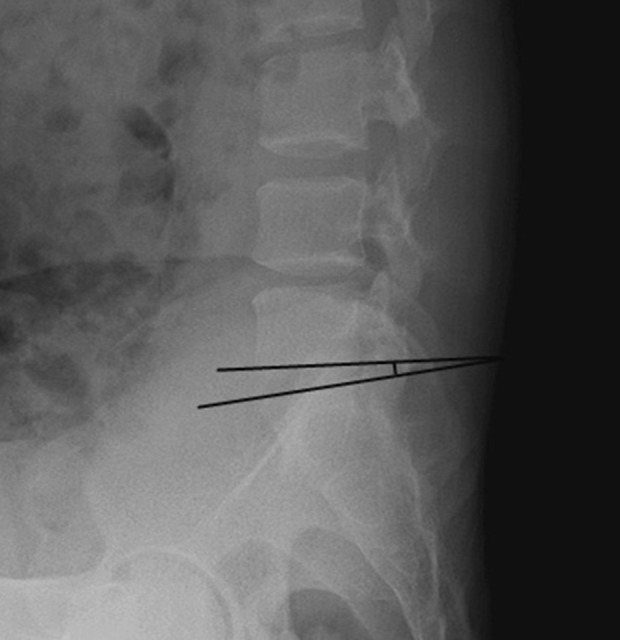
The sagittal range of motion. The sagittal range of motion is the angle (A) of the intervertebral space measured on the X-ray film in the lumbar hyperflexion position. The angle (B) of the intervertebral space is measured on the X-ray film in the lumbar hyperextension position. The sagittal range of motion is the absolute value of angle A–angle B.
Figure 6.
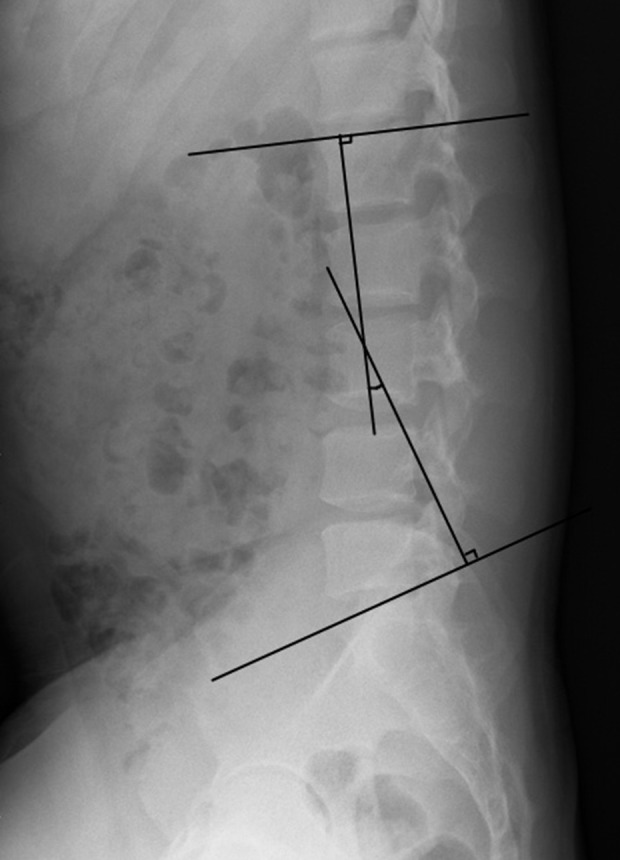
The lumbar lordosis angle. The lumbar lordosis angle is the angle between the vertical line of the upper edge of L1 and the vertical line of the upper endplate of S1.
Figure 7.
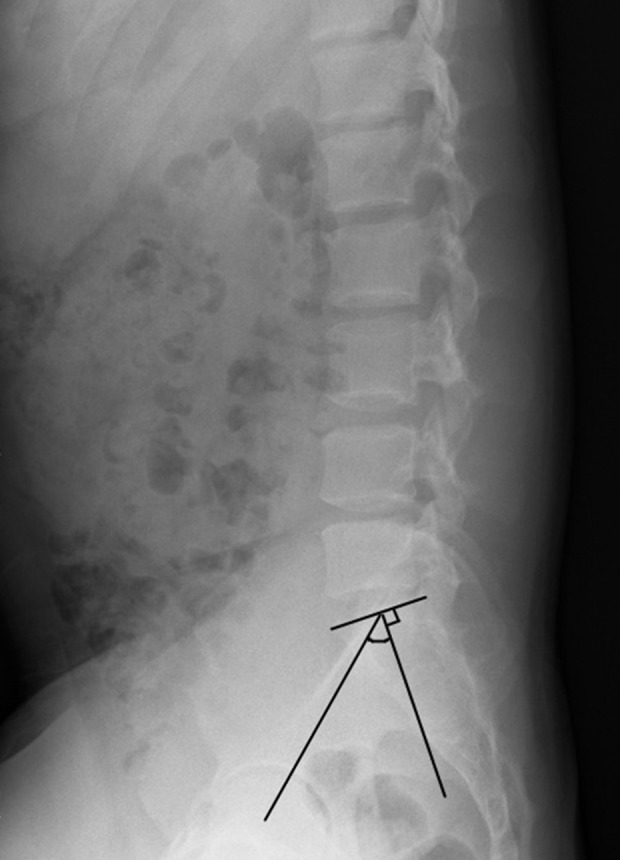
The pelvic incidence angle. The pelvic incidence angle is the vertical line of the upper sacral endplate through the mid-point of the upper sacral endplate, with the straight line passing through the mid-point of the upper sacral endplate and the center line of the bilateral femoral head. The pelvic incidence is the angle between the two lines.
Figure 8.
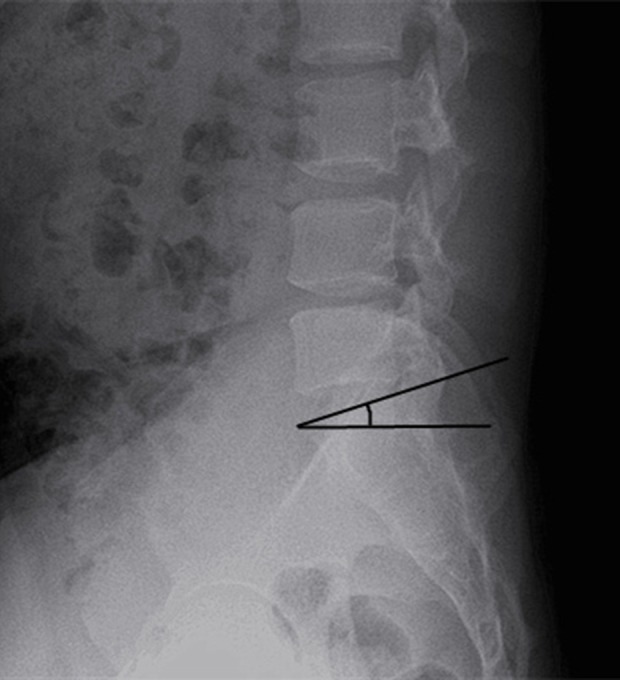
The sacral slope. The sacral slope is the angle between the plane of the S1 endplate and the horizontal plane.
Statistical analysis
The data were analyzed using SPSS version 17.0 software (IBM, Chicago, IL, USA). Measurement data were expressed as the mean±standard standard deviation (SD). Comparison between two groups was performed using the chi-squared (χ2) test and Student’s t-test. The determination of the independent risk factors of recurrent disc herniation was analyzed using the binary logistic regression analysis. P<0.05 was considered to indicate statistical significance.
Results
Patient data and surgical procedures
In this study, patients were treated with percutaneous endoscopic transforaminal discectomy (PETD) for L5–S1 disc herniation. The transforaminal endoscopic approaches for the surgical management of lumbar disc herniation included modifications of the Yeung endoscopy spine system (YESS) (the intraforaminal intradiscal approach) and the transforaminal endoscopic spine system (TESSYS) (intraforaminal extradiscal approach) techniques. A total of 506 patients with L5–S1 disc herniation underwent PETD.
There were initially 484 patients who were enrolled in the study but 22 patients were not followed-up, 212 patients had no intervertebral foramen stenosis, and 272 patients had mild to severe intervertebral foramen stenosis (grade 1, 207; grade 2, 59; and grade 3, 6). There were 319 patients who underwent the TESSYS with an intraforaminal extradiscal approach, and 165 patients underwent the YESS with an intraforaminal intradiscal approach. The surgical procedures included 171 cases of foraminoplasty, 109 cases of annulotomy, and 204 cases of foraminoplasty and annulotomy. The symptoms of lumbar and leg pain were significantly reduced after surgery, and there was no increase in symptoms in the short term.
Comparison between the nonrecurrence group and the recurrence group
The nonrecurrence group (n=438) and recurrence group (n=46, 9.504%) were compared. There was no significant difference between the two groups in the symptom duration, follow-up time, time to return to work, location of disc herniation, and the intervertebral foramen (P>0.05). Table 1 shows the patient data. The age of the patients ranged from 20–81 years (mean, 48.242±9.677 years). The body mass index (BMI) ranged from 15.54–32.28 kg/m2 (mean, 23.775±3.159 kg/m2), and the follow-up time ranged from 1–4 years (mean, 1.847±0.673 years).
Table 1.
The demographic and clinical characteristics of the patients in the non-recurrence group and recurrence group for L5–S1 disc herniation after percutaneous endoscopic transforaminal discectomy (PETD).
| Non-recurrence group n=438 | Recurrence group n=46 | |
|---|---|---|
| General data | ||
| Age (years) | 47.744±9.793 | 52.978±6.962* |
| Gender | ||
| Male/Female | 229/209 | 25/21 |
| Body mass index (BMI) (kg/m2) | 23.585±3.120 | 25.576±2.987* |
| Symptom duration (months) | 5.452±1.761 | 5.348±1.958 |
| Follow-up (months) | 1.858±0.675 | 1.739±0.648 |
| Current smoking | ||
| Yes/No | 383/55 | 38/8 |
| Alcohol | ||
| Yes/No | 282/156 | 32/14 |
| Hypertension | ||
| Yes/No | 413/35 | 41/5 |
| Diabetes mellitus | ||
| Yes/No | 410/28 | 42/4 |
| Trauma history | ||
| Yes/No | 357/81 | 35/11 |
| Surgical procedure | ||
| Foraminoplasty | 156 | 15 |
| Annulotomy | 101 | 8 |
| Foraminoplasty and annulotomy | 181 | 23 |
| Time to return to work (months) | 4.171±1.166 | 4.130±1.067 |
| Imaging data | ||
| Location of disc herniation | ||
| Foraminal | 101 | 6 |
| Paramedian | 237 | 27 |
| Central | 100 | 13 |
| Intervertebral foramen (grading) | ||
| 0/1/2/3 | 192/187/55/4 | 20/20/4/2 |
| Disc degeneration | ||
| I/II/III/IV | 118/182/120/18 | 4/21/16/5* |
| Ilium wing height (cm) | 3.544±0.605 | 3.647±0.531 |
| Transverse process length (cm) | 2.428±0.576 | 2.518±0.475 |
| Intervertebral space height (cm) | 1.156±0.433 | 1.062±0.452 |
| Sagittal range of motion (°) | 8.066±3.011 | 11.096±2.356* |
| Lumbar lordosis angle (°) | 39.304±12.889 | 43.389±10.981* |
| Pelvic incidence angle (°) | 46.671±10.963 | 47.440±11.325 |
| Sacral slope (°) | 29.083±7.320 | 34.087±7.187* |
P<0.05.
The 46 patients with recurrent symptoms of L5–S1 disc herniation after PETD had low back pain or increased radiation pain, which were consistent with the finding from magnetic resonance imaging (MRI) or computed tomography (CT). The time to recurrence ranged from 1–4 years. The mean patient age was 52.978±6.962 years, and the mean BMI was 25.576±2.987 kg/m2. There were 26 patients who received conservative treatment, 12 patients underwent repeat surgery, and eight patients underwent further treatment. From the patients who underwent repeat surgery, nine patients underwent traditional open surgery, two patients received percutaneous endoscopic interlaminar discectomy (PEID), and one patient received PETD. There were no recurrences during the follow-up of patients after the second operation (Table 1).
Univariate analysis of the parameters of age, gender, body mass index (BMI), smoking and alcohol history, a history of hypertension, diabetes mellitus, trauma, surgery, disc degeneration, ilium wing height, transverse process length, intervertebral space height, sagittal range of motion, lumbar lordosis angle, pelvic incidence angle, and sacral slope showed that there was no significant difference between the two groups (P<0.05). Age, BMI, sagittal range of motion, lumbar lordosis angle, and sacral slope of the recurrence group was significantly greater than the non-recurrence group. The grade of disc degeneration of the recurrence group was significantly greater than that of the non-recurrence group (Table 1)
Age, BMI, disc degeneration, sagittal range of motion, lumbar lordosis angle, and sacral slope were assigned values before logistic regression analysis, as shown in Table 2. The results of logistic regression analysis indicated that age, BMI, disc degeneration, sagittal range of motion, lumbar lordosis angle, and sacral slope were correlated with the recurrence of disc herniation, as shown in Table 3. Age, BMI, disc degeneration, sagittal range of motion, lumbar lordosis angle, and sacral slope are all risk factors for recurrence of disc herniation.
Table 2.
Assigned values of the parameters of age, body mass index (BMI), disc degeneration, sagittal range of motion, lumbar lordosis angle, and sacral slope before logistic regression analysis.
| Factor | Variable | Assignment description | |
|---|---|---|---|
| Age | X1 | <50.361=1; ≥50.361=2 | Average (47.744+52.978)/2=50.361 |
| Body mass index (BMI) | X2 | <24.581=1; ≥24.581=2 | Average (23.585+25.576)/2=24.581 |
| Disc degeneration | X3 | I=1; II=2; III=3; IV=4 | |
| Sagittal range of motion | X4 | <9.581=1; ≥9.581=2 | Average (8.066+11.096)/2=9.581 |
| Lumbar lordosis angle | X5 | <41.347=1; ≥41.347=2 | Average (39.304+43.389)/2=41.347 |
| Sacral slope | X6 | <31.585=1; ≥31.585=2 | Average (29.083+34.087)/2=31.585 |
| Recurrence | Y | Yes=1; No=2 | |
Table 3.
Logistic regression analysis results of age, body mass index (BMI), disc degeneration, sagittal range of motion, lumbar lordosis angle, and sacral slope were correlated with the recurrence of disc herniation.
| β | SE | Wald | P-value | OR | 95% confidence interval (CI) | ||
|---|---|---|---|---|---|---|---|
| Low value | High value | ||||||
| Age | 0.908 | 0.355 | 6.535 | 0.011* | 2.480 | 1.236 | 4.975 |
| BMI | 1.103 | 0.358 | 9.500 | 0.002* | 3.013 | 1.494 | 6.075 |
| Disc degeneration | 0.584 | 0.209 | 7.779 | 0.005* | 1.793 | 1.189 | 2.702 |
| Sagittal range of motion | 1.901 | 0.371 | 26.245 | 0.000* | 6.694 | 3.234 | 13.855 |
| Lumbar lordosis angle | 0.876 | 0.348 | 6.326 | 0.012* | 2.402 | 1.213 | 4.754 |
| Sacral slope | 0.955 | 0.349 | 7.489 | 0.006* | 2.598 | 1.311 | 5.146 |
| Constant | −12.196 | 1.540 | 62.720 | 0.000* | 0.000 | ||
P<0.05.
OR – odds ratio; BMI – body mass index.
Discussion
The aim of this retrospective study, conducted at a single center, was to investigate the risk factors associated with the recurrence of L5–S1 disc herniation after percutaneous endoscopic transforaminal discectomy (PETD). In this study, transforaminal endoscopic approaches for the surgical management of lumbar disc herniation included modifications of the Yeung endoscopy spine system (YESS) (the intraforaminal intradiscal approach) and the transforaminal endoscopic spine system (TESSYS) (intraforaminal extradiscal approach) techniques.
Disc herniation is the most common cause of low back pain and can seriously affect the quality of life for patients. Surgical treatment for disc herniation includes several approaches, including open lumbar microdiscectomy and micro-endoscopic discectomy [23–26]. Percutaneous endoscopic lumbar discectomy (PELD) is a minimally invasive procedure that has recently become popular, and the procedure was originally described in 1993 by Kambin [27]. In 2000, Yeung developed the intraforaminal intradiscal approach, now termed the Yeung endoscopy spine system (YESS) [23–26].
Surgical discectomy may be associated with the recurrence of disc herniation. Despite its numerous advantages, the recurrence of disc herniation after PETD is common. The recurrence rate of PELD in different studies conducted at different spinal levels is between 0–7.4% [1,8,10,30–32]. The L5–S1 vertebral segment includes two structures, the lumbar spine and the sacral vertebra. The endplate of L5–S1 has a greater angle of inclination relative to the horizontal plane, and there are nearby associated structures that include the pelvis and inferior transverse processes. However, there have been few previous studies on lumbar disc herniation after discectomy at the L5–S1 level. In the present study, the recurrence rate of the intervertebral disc herniation was 9.504%.
Kim et al. [32] conducted a follow-up of between 24–108 months on patients following microendoscopic discectomy, and 39 (8.351%) patients had recurrence of disc herniation. These findings support those of the present study. However, there may be different risk factors for recurrence following different types of surgical procedure, but the effects of PETD on recurrence of L5–S1 disc herniation has not been previously studied. The findings from the present study showed that lumbar spondylolisthesis often occurs at the L5–S1 level. This finding may indicate that the junction of the lumbar and sacral vertebrae undergoes greater shear forces due to the inherently unique structures of the L5–S1 segment. This effect is likely to have an impact on disc herniation and recurrence. The instability of the section increases after removal of the intervertebral disc, and this increases the imbalance of shear forces between the lumbar and sacral vertebrae. As a consequence, the risk of recurrence of disc herniation increases at the L5–S1 level. In this retrospective clinical study of 484 patients, the parameters that were found to be significantly associated with the recurrence of L5–S1 disc herniation following PETD included increased age, body mass index (BMI), disc degeneration, sagittal range of motion, lumbar lordosis angle, and sacral slope.
The recurrence of disc herniation is more likely to recur with age, although this remains controversial [33]. It may be assumed that the intervertebral disc slowly degenerates with age and has the tendency to deform more easily, which is a potential risk for the recurrence of disc herniation. Intervertebral disc degeneration involves the alteration of chemical and physical properties, and in the absence of trauma, the recurrence of disc herniation is significantly associated with disc degeneration. Also, healing in elderly patients after surgery can be impaired, and increased age may result in delayed healing of the annulus fibrosus. In the long-term, an incomplete fibrous annulus results in recurrence of disc herniation, as shown by previous studies [30,34–36]. In 2018, Wu et al. [37] showed that the age of more than 40 years was a predisposing factor for recurrence. In 1998, Cinotti et al. [38] found that male patients with significantly degenerated discs were at increased risk of recurrence of disc herniation after microdiscectomy. In 2017, Yao et al. [34] showed that elderly patients had several factors that increased the incidence of recurrence. Further studies are needed to evaluate the factors associated with age and intervertebral disc degeneration.
In 2018, Suk et al. [39] studied the associations between percutaneous endoscopic lumbar reoperation for recurrent symptoms of sciatica and showed that both age and male gender were risk factors for the recurrence of disc herniation. Cinotti et al. [38] found that male patients with significant degenerative disc disease were more likely to have recurrent disc herniation after isolated injuries or unexpected events. Gender differences might be associated with the effects of differences in estrogen and androgen levels, but these effects may be small.
In 2005, Kara et al. [40] showed that there was no significant association between the recurrence of disc herniation and BMI. However, some studies have suggested that weight loss may reduce the burden on the disc [30,34]. BMI may have an effect on the biomechanical properties and morphology of the intervertebral disc. The findings from the present study showed that the higher the BMI, the greater the risk of recurrence of disc herniation. An increased BMI may have a more significant effect when there is degenerative disc disease and also on postoperative pressure that increases the deformation of the disc.
In the present study, the follow-up time in the two study groups was the same. Consistent with the findings from previous studies, the surgical learning curve had an impact on the therapeutic effect of PETD [34,41,42]. Surgical experience is required to be able to identify and remove the nucleus pulposus, and other aspects of the surgery for PETD to reduce the rate of recurrence. The location of disc herniation affects the choice of surgical approach, and surgery affects the size of the postoperative foramen. In the present study, there was no significant difference between the two study groups in the position of disc herniation and the intervertebral foramen.
In 2013, Huang et al. [43] reported meta-analysis data on the risk factors associated with recurrent lumbar disc herniation and showed that more attention should be paid to the prevention of recurrence after surgery in patients who were smokers and those with diabetes mellitus. In 2015, Wang et al. [44] showed that patients with diabetes had an increased risk of PELD failure, particularly in the early stages of surgery. The present study investigated the association between recurrence and smoking and alcohol use, hypertension, and diabetes, but did not identify these as significant risk factors. Following surgery, some patients make lifestyle changes that include weight loss, reduced smoking and reduced alcohol intake. However, long-term studies are needed to determine the effects of lifestyle, hypertension, and diabetes on recurrence following disc surgery.
Trauma is the cause of disc herniation in some patients. The annulus fibrosus surrounds the nucleus pulposus that protects the integrity of the intervertebral disc. Trauma to the disc may result in rupture of the annulus fibrosus and protrusion of the nucleus pulposus. However, a previous history of trauma was not identified as a risk factor for recurrence following surgery in the present study. Although the annulus fibrosus is damaged in trauma, the nucleus pulposus can be surrounded by the growth of scar tissue after surgery. The growth of scar tissue contributes to the stability of the intervertebral space and can protect the nucleus pulposus.
This study included patients who underwent two types of PETD surgical approaches, the TESSYS intraforaminal extradiscal approach, and the YESS intraforaminal intradiscal approach. These surgical procedures were not separately evaluated in the risk assessment, as they included several procedures, such as foraminoplasty and annulotomy. According to the operative characteristics of the annulus fibrosus and facet joints, they were divided into categories of surgical procedure, as shown in Table 1. The annulus fibrosus is destroyed by surgery and then reconstructed. In 1994, Ethier et al. [45] found that annulus fibrosus defects resulted in reduced strength, which could increase the risk of recurrence. Ahlgren et al. [46] showed that direct annulus fibrous incision repair had no positive effect on vertebral segment stability. In 2018, Qian et al. [47] showed that removing one-quarter of the superior articular had a partial effect on the mechanical properties of the lumbar spine and in affecting its stability and that the removal of one-half or more would significantly destroy the stability of the lumbar spine. However, in 2013, Karakaşlı et al. [48] reported that there were no biomechanical or clinical disadvantages associated with PETD. The findings from the present study showed that after annulotomy and laminoplasty, some tissues were damaged during surgery. These changes are gradual and do not significantly affect the pressure on the intervertebral disc. The L5–S1 segment is surrounded by ligaments and muscles that are adequate to withstand the increased pressure on the intervertebral disc caused by annulotomy and laminoplasty.
The L5–S1 segment has unique anatomical structures that include a large transverse process, sacrum, ilium, and strong ligaments. These anatomical structures may have a local biomechanical effect on the intervertebral disc. In 2015, Kim et al. [32] studied the biomechanical risk factors for the recurrence of L5–S1 disc herniation following micodiscectomy and found that the sagittal range of movement was associated with the recurrence of disc herniation. These findings were supported by those of the present study that showed that one of the risk factors for the recurrence of disc herniation, a large sagittal range of motion, was impaired. Ilium wing height, transverse process length, and intervertebral space height had no significant effect on recurrence, which might mean that they had little effect on the distribution of disc stress. Increased sagittal range of motion indicates insufficient intervertebral space stability rather than increased disc pressure. Following surgery, if the patient has increased lumbar activity, the balance of disc pressures may be disrupted. This pressure imbalance may be reduced by wearing a protective waist belt after surgery to restrict the initial movement of intervertebral space and prevent the recurrence of disc herniation in patients with an increased sagittal range of motion.
In addition to the anatomical structures, the morphology of the spine and pelvis may have an overall biomechanical impact on the recurrence of L5–S1 disc herniation. Previous studies have shown that the abnormalities in the spine and pelvis sagittal anatomy limit not only balance and posture, but are also closely linked to spinal disease [49,50]. Labelle et al. [51] showed that a large lumbar lordosis angle, pelvic incidence angle, or sacral slope resulted in L5–S1 vertebral spondylolisthesis. Roussouly et al. [52] studied the cause of spondylolisthesis and showed that it might result in increased stress at the L5–S1 level from a large pelvic incidence angle and sacral slope. In 2011, Chaléat-Valayer et al. [50] found that patients with chronic low back pain had a significantly reduced lumbar lordosis angle, pelvic incidence angle, and sacral slope. Barrey et al. [53] showed that a small lumbar lordosis angle, pelvic incidence angle, and sacral slope were associated with disc degeneration. In 2017, Fei et al. [54] showed that the pelvic incidence angle was not significantly correlated with disc herniation in young patients.
In the present study, the lumbar lordosis angle, the pelvic incidence angle, and the sacral slope were among the several spinal and pelvic parameters studied. These three criteria were analyzed, and the recurrence of disc herniation was significantly associated with a large lumbar lordosis angle or high sacral slope. For sagittal spine balance, normal spine-pelvic sagittal morphology may be essential, especially for the regulation of stress balances in the L5–S1 disc segment. Although it may be anticipated that long-term disc herniation results in straightening of the physiological curvature of the lumbar spine, an increased lumbar lordosis angle may unidirectionally increase the forward spinal pressure. Also, a wide lumbar lordosis angle creates increased mechanical stress on the posterior joint, especially on the posterior plane of the joint, which can exacerbate joint degeneration. An increased sacral slope suggests a more bent endplate and a change in the top-down stress from gravity and shear force. These factors contribute to the abnormally increased shear force on the L5–S1 segment, which disrupts the equilibrium of local stress and weakens intervertebral space stability. Although the underlying ligaments are sufficiently strong to support the strength of the lower lumbar spine, the L5–S1 segment is the most susceptible segment to spondylolysis, which also indicates that it bears large shear forces. Although Roussouly et al. [22] previously showed that the lumbar lordosis angle, pelvic incidence angle, and sacral slope were significantly correlated with spinal and pelvic parameters, the findings from the present study showed no effect of pelvic incidence angle on the recurrence of disc herniation.
This study had several limitations. This study included a small number of patients from a single surgical center, and the demographic and clinical data obtained relied on the accuracy and availability of clinical records. Also, because this was a retrospective study, it was difficult to conduct further research on some study parameters. Associations between some of the parameters, particularly the imaging, may have been missed due to the complex nature of the imaging data, which requires further study. Also, in this study, the postoperative follow-up time was relatively short, and the long-term recurrence rate remains to be studied further. Therefore, because this was a retrospective clinical study, conducted at a single center, and had a small study sample size, further large-scale prospective studies are required to compare the long-term outcome following different PETD procedures.
Conclusions
This study aimed to investigate the risk factors associated with the recurrence of L5–S1 disc herniation after percutaneous endoscopic transforaminal discectomy (PETD). The recurrence of L5–S1 disc herniation following PETD was significantly associated with increased age and body mass index (BMI), more severe disc degeneration, increased sagittal range of motion, increased lumbar lordosis, and sacral slope.
Acknowledgments
The authors thank Guangxi Gushang Hospital for supporting this research.
Footnotes
Conflict of interest
None.
Source of support: This study was funded by the Regional Science Foundation Project, National Natural Science Foundation of China (No. 81560359)
References
- 1.Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: A single-center experience of 10,228 cases. Neurosurgery. 2015;76:372–80. doi: 10.1227/NEU.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 2.Eun SS, Lee SH. Long-term follow-up results of percutaneous endoscopic lumbar discectomy. Pain Physician. 2016;19:E1161–66. [PubMed] [Google Scholar]
- 3.Lee DY, Shim CS, Ahn Y, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for recurrent disc herniation. J Korean Neurosurg Soc. 2009;46:515–21. doi: 10.3340/jkns.2009.46.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruan W, Feng F, Liu Z, et al. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: A meta-analysis. Int J Surg. 2016;31:86–92. doi: 10.1016/j.ijsu.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Ruetten S, Komp M, Merk H. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: A prospective, randomized, controlled study. Spine. 2008;33:931–39. doi: 10.1097/BRS.0b013e31816c8af7. [DOI] [PubMed] [Google Scholar]
- 6.Reulen HJ, Müller A. Microsurgical anatomy of the lateral approach to extraforaminal lumbar disc herniations. Neurosurgery. 1996;39:345–50. doi: 10.1097/00006123-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Osman SG. Endoscopic transiliac approach to L5–S1 disc and foramen. A cadaver study. Spine. 1997;22:1259–63. doi: 10.1097/00007632-199706010-00020. [DOI] [PubMed] [Google Scholar]
- 8.Choi KC, Kim JS, Ryu KS, et al. Percutaneous endoscopic lumbar discectomy for L5–S1 disc herniation: transforaminal versus interlaminar approach. Pain Physician. 2013;16:547–56. [PubMed] [Google Scholar]
- 9.Du J, Tang X, Jing X, et al. Outcomes of percutaneous endoscopic lumbar discectomy via a translaminar approach, especially for soft, highly down-migrated lumbar disc herniation. Int Orthop. 2016;40:1247–52. doi: 10.1007/s00264-016-3177-4. [DOI] [PubMed] [Google Scholar]
- 10.Ruetten S, Komp M, Merk H. Recurrent lumbar disc herniation after conventional discectomy: A prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech. 2009;22:122–29. doi: 10.1097/BSD.0b013e318175ddb4. [DOI] [PubMed] [Google Scholar]
- 11.Choi G, Lee SH, Raiturker PP, et al. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery. 2006;58:ONS59–68. doi: 10.1227/01.neu.0000192713.95921.4a. [DOI] [PubMed] [Google Scholar]
- 12.Boden SD, Wiesel SW. Lumbosacral segmental motion in normal individuals. Have we been measuring instability properly? Spine. 1990;15:571–76. doi: 10.1097/00007632-199006000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Nizard RS, Wybier M. Radiologic assessment of lumbar intervertebral instability and degenerative spondylolisthesis. Radiol Clin North Am. 2001;39:55–71. doi: 10.1016/s0033-8389(05)70263-3. [DOI] [PubMed] [Google Scholar]
- 14.Hoogland T, Schubert M, Miklitz B. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: A prospective randomized study in 280 consecutive cases. Spine. 2006;31:E890–97. doi: 10.1097/01.brs.0000245955.22358.3a. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Adsul N, Kapoor A, et al. A mobile outside-in technique of transforaminal lumbar endoscopy for lumbar disc herniations. J Vis Exp. 2018;7(138) doi: 10.3791/57999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorenza V. Percutaneous endoscopic transforaminal outside-in outside technique for foraminal and extraforaminal lumbar disc herniations-operative technique. World Neurosurg. 2019;130:244–53. doi: 10.1016/j.wneu.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Yeung AT. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine. 2002;27:722–31. doi: 10.1097/00007632-200204010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SB, Lin GX, Jabri H, et al. Radiographic and clinical outcomes of huge lumbar disc herniations treated by transforaminal endoscopic discectomy. Clin Neurol Neurosurg. 2019;185:105485. doi: 10.1016/j.clineuro.2019.105485. [DOI] [PubMed] [Google Scholar]
- 19.Kapetanakis S, Gkantsinikoudis N, Chaniotakis C, et al. Percutaneous transforaminal endoscopic discectomy for the treatment of lumbar disc herniation in obese patients: Health-related quality of life assessment in a 2-year follow-up. World Neurosurg. 2018;113:e638–49. doi: 10.1016/j.wneu.2018.02.112. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Lee JW, Yeom JS, et al. A practical MRI grading system for lumbar foraminal stenosis. Am J Roentgenol. 2010;194(4):1095–98. doi: 10.2214/AJR.09.2772. [DOI] [PubMed] [Google Scholar]
- 21.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–78. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 22.Roussouly P, Gollogly S, Berthonnaud E. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine. 2005;30:346–53. doi: 10.1097/01.brs.0000152379.54463.65. [DOI] [PubMed] [Google Scholar]
- 23.Lee DY, Shim CS, Ahn Y, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for recurrent disc herniation. J Korean Neurosurg Soc. 2009;46:515–21. doi: 10.3340/jkns.2009.46.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin R, Liu B, Hao J, et al. Percutaneous endoscopic lumbar discectomy versus posterior open lumbar microdiscectomy for the treatment of symptomatic lumbar disc herniation: A systemic review and meta-analysis. World Neurosurg. 2018;120:352–62. doi: 10.1016/j.wneu.2018.08.236. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Yuan S, Tian Y, et al. Comparison of percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, and microdiscectomy for symptomatic lumbar disc herniation: Minimum 2-year follow-up results. J Neurosurg Spine. 2018;28:317–25. doi: 10.3171/2017.6.SPINE172. [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Guo D, Sun T. A comparative study on short-term therapeutic effects of percutaneous transforaminal endoscopic discectomy and microendoscopic discectomy on lumbar disc herniation. Pak J Med Sci. 2019;35:426–31. doi: 10.12669/pjms.35.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kambin P. Percutaneous endoscopic discectomy. J Neurosurg. 1993;79:968–69. [PubMed] [Google Scholar]
- 28.Yeung AT. The evolution of percutaneous spinal endoscopy and discectomy: State of the art. Mt Sinai J Med. 2000;67:327–32. [PubMed] [Google Scholar]
- 29.Hoogland T, Schubert M, Miklitz B. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: A prospective randomized study in 280 consecutive cases. Spine. 2006;31:E890–97. doi: 10.1097/01.brs.0000245955.22358.3a. [DOI] [PubMed] [Google Scholar]
- 30.Kim JM, Lee SH, Ahn Y, et al. Recurrence after successful percutaneous endoscopic lumbar discectomy. Minim Invasive Neurosurg. 2007;50:82–85. doi: 10.1055/s-2007-982504. [DOI] [PubMed] [Google Scholar]
- 31.Eun SS, Lee SH. Long-term follow-up results of percutaneous endoscopic lumbar discectomy. Pain Physician. 2016;19:E1161–66. [PubMed] [Google Scholar]
- 32.Kim KT, Lee DH, Cho DC, et al. Preoperative risk factors for recurrent lumbar disk herniation in L5–S1. J Spinal Disord Tech. 2015;28:E571–77. doi: 10.1097/BSD.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 33.Swartz KR. Recurrent lumbar disc herniation. Neurosurg Focus. 2003;15:E10. doi: 10.3171/foc.2003.15.3.10. [DOI] [PubMed] [Google Scholar]
- 34.Yao Y, Liu H, Zhang H, et al. Risk factors for recurrent herniation after percutaneous endoscopic lumbar discectomy. World Neurosurg. 2017;100:1–6. doi: 10.1016/j.wneu.2016.12.089. [DOI] [PubMed] [Google Scholar]
- 35.Yin S, Du H, Yang W, et al. Prevalence of recurrent herniation following percutaneous endoscopic lumbar discectomy: A meta-analysis. Pain Physician. 2018;21:337–50. [PubMed] [Google Scholar]
- 36.Wang H, Zhou Y, Li C, et al. Risk factors for failure of single-level percutaneous endoscopic lumbar discectomy. J Neurosurg Spine. 2015;23:320–25. doi: 10.3171/2014.10.SPINE1442. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Zhang C, Lu K, et al. Percutaneous endoscopic lumbar reoperation for recurrent sciatica symptoms: A retrospective analysis of outcomes and prognostic factors in 94 patients. World Neurosurg. 2018;109:e761–69. doi: 10.1016/j.wneu.2017.10.077. [DOI] [PubMed] [Google Scholar]
- 38.Cinotti G, Roysam GS, Eisenstein SM. Ipsilateral recurrent lumbar disc herniation. A prospective, controlled study. J Bone Joint Surg Br. 1998;80:825–32. doi: 10.1302/0301-620x.80b5.8540. [DOI] [PubMed] [Google Scholar]
- 39.Suk KS, Lee HM, Moon SH. Recurrent lumbar disc herniation: Results of operative management. Spine. 2001;26:672–76. doi: 10.1097/00007632-200103150-00024. [DOI] [PubMed] [Google Scholar]
- 40.Kara B, Tulum Z. Functional results and the risk factors of reoperations after lumbar disc surgery. Eur Spine J. 2005;14:43–48. doi: 10.1007/s00586-004-0695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DY. Learning curve for percutaneous endoscopic lumbar discectomy. Neurol Med Chir. 2008;48:383–88. doi: 10.2176/nmc.48.383. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Huang B, Li C, et al. Learning curve for percutaneous endoscopic lumbar discectomy depending on the surgeon’s training level of minimally invasive spine surgery. Clin Neurol Neurosurg. 2013;115:1987–91. doi: 10.1016/j.clineuro.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Huang W, Han Z, Liu J, et al. Risk factors for recurrent lumbar disc herniation: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e2378. doi: 10.1097/MD.0000000000002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Zhou Y, Li C, et al. Risk factors for failure of single-level percutaneous endoscopic lumbar discectomy. J Neurosurg Spine. 2015;23:320–25. doi: 10.3171/2014.10.SPINE1442. [DOI] [PubMed] [Google Scholar]
- 45.Ethier DB, Cain JE, Yaszemski MJ, et al. The influence of anulotomy selection on disc competence. A radiographic, biomechanical, and histologic analysis. Spine. 1994;19:2071–76. doi: 10.1097/00007632-199409150-00012. [DOI] [PubMed] [Google Scholar]
- 46.Ahlgren BD, Vasavada A, Brower RS, et al. Annular incision technique on the strength and multidirectional flexibility of the healing intervertebral disc. Spine. 1994;19(8):948–54. doi: 10.1097/00007632-199404150-00014. [DOI] [PubMed] [Google Scholar]
- 47.Qian J, Yu SS, Liu JJ, et al. [Biomechanics changes of lumbar spine caused by foraminotomy via percutaneous transforaminal endoscopic lumbar discectomy]. Zhonghua Yi Xue Za Zhi. 2018;98:1013–18. doi: 10.3760/cma.j.issn.0376-2491.2018.13.012. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 48.Karakaşlı A, Yildiz DV, Kumtepe E, et al. Biomechanical comparison of intact lumbar lamb spine and endoscopic discectomized lamb spine. Eklem Hastalik Cerrahisi. 2013;24:33–38. doi: 10.5606/ehc.2013.08. [DOI] [PubMed] [Google Scholar]
- 49.Mac-Thiong JM, Labelle H, Charlebois M, et al. Sagittal plane analysis of the spine and pelvis in adolescent idiopathic scoliosis according to the coronal curve type. Spine. 2003;28:1404–9. doi: 10.1097/01.BRS.0000067118.60199.D1. [DOI] [PubMed] [Google Scholar]
- 50.Chaléat-Valayer E, Mac-Thiong JM, Paquet J, et al. Sagittal spino-pelvic alignment in chronic low back pain. Eur Spine J. 2011;20(Suppl 5):634–40. doi: 10.1007/s00586-011-1931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labelle H, Mac-Thiong JM. Spino-pelvic sagittal balance of spondylolisthesis: A review and classification. Eur Spine J. 2011;20(Suppl 5):641–46. doi: 10.1007/s00586-011-1932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roussouly P, Gollogly S, Berthonnaud E, et al. Sagittal alignment of the spine and pelvis in the presence of L5–S1 isthmic lysis and low-grade spondylolisthesis. Spine. 2006;31:2484–90. doi: 10.1097/01.brs.0000239155.37261.69. [DOI] [PubMed] [Google Scholar]
- 53.Barrey C, Jund J, Noseda O. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16:1459–67. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fei H, Li WS, Sun ZR, et al. Analysis of spino-pelvic sagittal alignment in young Chinese patients with lumbar disc herniation. Orthop Surg. 2017;9:271–76. doi: 10.1111/os.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]


