Short abstract
Objective
Galla chinensis ointment can inhibit the proliferation of keloid fibroblasts and decrease keloid formation. We investigated whether Galla chinensis ointment inhibits keloid fibroblast proliferation through expression of microRNA-21, phosphorylated (p)-phosphatidylinositol 3-kinase (p-PI3K), chromosome 10 neutropenic protein phosphatase (PTEN), protein kinase B (p-Akt), and mammalian target of rapamycin (p-mTOR).
Methods
A keloid mouse model and human keloid-derived fibroblasts were developed and treated with Galla chinensis. Immunohistochemistry, western blot, and reverse transcription-PCR were used to detect miR-21, PI3K, PTEN, Akt, and mTOR in keloid tissues.
Results
p-Akt and p-mTOR were highly expressed in the control group, PTEN was highly expressed in the treatment group, and p-PI3K was highly expressed in keloid tissue in both groups. Galla chinensis reduced miR-21 expression and increased PTEN mRNA expression in keloid fibroblasts compared with the control group, resulting in increased PTEN protein and decreased p-Akt and p-mTOR protein. Galla chinensis had no effect on p-PI3K.
Conclusion
Galla chinensis might inhibit proliferation of keloid fibroblasts by upregulating PTEN, thus inhibiting expression of miR-21 and downregulating p-Akt and p-mTOR expression. These results confirm the effect of Galla chinensis ointment on fibroblasts and suggest that it could be used to manage keloids clinically.
Keywords: Galla chinensis ointment, keloid, fibroblast, miR-21, PTEN, mTOR
Introduction
Keloids are scars that grow into the surrounding normal skin; they occur when excessive collagen is deposited during wound healing. There is often a family history of these disfiguring scars.1 Many studies have been conducted on the pathogenesis of keloids, but the exact mechanism has not yet been fully elucidated. The most common causes of keloids are trauma and acne, mainly in the trunk.2 The occurrence of keloid has significant racial differences and is more common among blacks and Asians,3 with an incidence of 0.09% to 16% in the African population.4 The incidence rate is slightly higher in women than in men.5 Although keloids can occur at any age, most occur between 11 and 30 years,6 with a high incidence around puberty and pregnancy.7 Risk factors include pigmented skin; black, Hispanic, or Asian heritage; age ≤30 years; and elevated hormone levels.8
Traditional Chinese medicine has a long history of preventing and treating keloids. Galla chinensis scar paste (also called Galla chinensis ointment or Wubeizi ointment) is a compound preparation developed by Ouyang Heng, a famous Chinese medicine dermatologist, under guidance of the theory of traditional Chinese medicine. It is made of seven Chinese medicines: black vinegar, Galla chinensis, centipede, Salvia miltiorrhiza, veeringxian, ground bone skin, and alum, and it is processed using modern technology. Preliminary clinical studies showed that the external use of Galla chinensis scar paste is effective in the treatment of keloid, with a total effective rate of 96.6%. In addition, an aqueous solution of Galla chinensis scar paste can inhibit the proliferation of keloid fibroblasts9 and affect the collagen synthesis of scar fibroblasts at the level of gene transcription.10 At present, the specific mechanism of Galla chinensis scar paste in preventing keloid has not been fully elucidated, so it is necessary to conduct in-depth research to reveal the underlying mechanism and effective targets of this traditional Chinese medicine in preventing keloid.
The finding that microRNAs (miRNA) play a role in the development of keloids provides new insights into the prevention and treatment of keloids. Profiling studies show that miRNAs are differentially expressed in keloid tissues compared with normal skin tissues.11 In particular, expression of miR-21 is significantly higher in hypertrophic keloid tissues than in normal skin.12 miR-21 is involved in a number of complex signaling pathways that are still being revealed, but one important pathway is the PTEN/Akt/mTOR signaling pathway, which has been shown to be important in extracellular matrix degradation.13 This pathway involves phosphatidylinositol 3-kinase (PI3K), chromosome 10 neutropenic protein phosphatase (PTEN), protein kinase B (Akt), and mammalian target of rapamycin (mTOR), and it may also be important in fibroblast proliferation. The expression of PTEN, a direct target of miR-21, has been shown to be lower in keloid tissues than in adjacent normal skin tissues.12 Consistent with this observation, overexpression of miR-21 in normal skin fibroblasts increased cell proliferation with decreased PTEN levels, whereas a miR-21 inhibitor increased the expression of PTEN and decreased Akt levels.12 In addition, keratinocyte growth factor and activation of its downstream signaling, including the PI3K-Akt-mTORC1 pathway, have been indicated in keloid formation,14 whereas mTOR inhibitor has been shown to have an antiproliferative effect in keloid fibroblast cells.15 The above evidence suggests that miR-21, PTEN, PI3K, Akt, and mTOR are all involved in the pathogenic process of keloid formation.
The transplantation of human keloid tissue into nude mice to create keloid models began in the mid-1980s.16–18 Human keloid tissue is transplanted to the subcutaneous surface of the back of nude mice, where it survives and maintains the original pathological features and morphological characteristics.16–18 To verify the feasibility of a nude mouse keloid model in the study of pathological scar, pieces of keloid tissue were transplanted subcutaneously into nude mice. The histological characteristics, acid mucopolysaccharides, and collagen type I and III contents did not change following transplantation, and the biological characteristics remained stable.19 At present, this model of keloid in nude mice has been widely used in the study of keloid.20–22
In this study, our aim was to test whether Galla chinensis ointment could inhibit the proliferation of keloid fibroblasts by modulating the expression of miR-21, PTEN, PI3K, Akt, and mTOR.
Materials and methods
Human keloid tissue samples
Anterior chest keloid tissue was collected from 18 patients after dermatological resection, and normal skin tissues were excised from urological foreskin from another 18 patients treated in the Xuzhou Hospital of Traditional Chinese Medicine. The patients’ details are shown in Table 1. This study was ethically approved by the independent ethics committee of Xuzhou Hospital of Traditional Chinese Medicine affiliated with the Nanjing University of Traditional Chinese Medicine, in Xuzhou, China (date of approval: September 5, 2016). Written informed consent was obtained from all participants.
Table 1.
Patients’ sex, age, and area and duration of lesions (mean ± standard deviation).
| Patient group | Number of cases |
Sex |
Age (years) | Area | Duration of lesions (years) | |
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Keloid | 18 | 10 | 8 | 23.6 ± 2.4 | Xuzhou, China | 1.8 ± 0.5 |
| Control (foreskin tissue) | 18 | 18 | 21.3 ± 2.6 | Xuzhou, China | 21.3 ± 2.6 | |
Preparation of drugs
Galla chinensis ointment was made following the method for preparing Wubeizi ointment.23 The active component of Galla chinensis ointment was prepared by water extraction and alcohol precipitation. The ingredients were decocted in water and then evaporated in a rotary vacuum. Ethanol was slowly added, the mixture was agitated refrigerated for 24 to 48 hours, and then filtered by solid–liquid separation. The filtrate is the active component of the ointment. The mass/volume concentration was adjusted to 50 mg/mL for storage. The Galla chinensis ointment was prepared by adding 50 g of white vanillin, 20 g of hexadecyl alcohol, 20 g of octadecyl alcohol, 100 g of stearic acid, 75 g of monostearate glyceride, and 20 g of lanolin melted at 80°C, followed by the addition of 10 g of borneol and ethyl niperkin.
The pharmaceutical composition of the Galla chinensis ointment aqueous solution was the same as that of the Galla chinensis ointment. Its extraction method was the same as that used for Wubeizi ointment aqueous solution.9 It was diluted with Dulbecco’s modified Eagle medium to a concentration of 0.5 mg/mL [concentration screening was obtained in accordance with its concentration when the median inhibitory concentration (IC50) of cells in the experiment ranged from 1/3 to 2/3].
Animal model
Sixteen-week-old female nude mice (BALB/C-nunu strain), weighing 18 to 22 g, were purchased from the Experimental Animal Center of Xuzhou Medical University. According to the Kischer method,24 36 nude mice were subjected to adaptive feeding for 1 week, and their body weight was recorded. The mice were anesthetized with an intraperitoneal injection of pentobarbital sodium at 0.8 mg/10 g of body weight. When the mice were completely unconscious, the back skin was cut with a disposable scalpel with the skin fully exposed. The human keloid sample was cut into 5- × 5-× 5-mm pieces using a sterile blade. and the keloid pieces were fixed to the subcutaneous tissue with a No. 1 suture to prevent the keloid from shifting or falling off. Then, the keloid tissue was neatly sewn into the surrounding skin using a No. 4 suture. Erythromycin ointment was applied to the wound and fixed using a pressure dressing. The mice were resuscitated by thermostat anesthesia. After surgery, the keloid model was established at around 14 days.
The mice were divided into Galla chinensis ointment treatment and control groups (18 mice/group). Mice in the treatment group had 5% Galla chinensis ointment applied to the skin (0.5 g/10 mm2, 3 times a day, prepared by Xuzhou Traditional Chinese Medicine). Mice in the control group had the matrix (white vanillin, hexadecyl alcohol, octadecyl alcohol, stearic acid, monostearate glyceride, borneol, and ethyl niperkin) for making the Galla chinensis ointment applied. The treatment (ointment or matrix) was applied three times a day for 30 consecutive days.
At the end of the treatment, the mice were anesthetized by intraperitoneal injection of sodium pentobarbital and then killed by cervical dislocation. The keloid tissues were dissected and the size of the keloid tissues was measured using a Vernier caliper. The keloid tissues were removed and cut into 5- × 5- × 5-mm pieces for further analyses. The mouse model study was ethically approved by the independent ethics committee of Xuzhou Hospital of Traditional Chinese Medicine, and all procedures were undertaken to ensure the animals were treated humanely.
Separation and culture of fibroblast cells
Tissue specimens from keloids and foreskin (normal skin used as control25–28) were cut under aseptic conditions, and epithelial and subcutaneous tissues were removed carefully with ophthalmic scissors. The specimens were washed three times with D-Hanks’ solution and sheared into tissue pieces of 1 mm3. The specimens were placed in a sterile bottle, filtered after being digested into flocculence with 20% trypsin-EDTA solution, and centrifuged at 650 × g for 5 minutes. Then, 5 mL of 20% DMEM containing 10% fetal bovine serum (FBS) was added to deposit cells. The specimens were placed in a 25-mL culture bottle and cultivated in a 5% CO2 incubator at 37°C. The liquid was replaced by 20% DMEM containing 10% FBS every 24 hours, and growth was observed under a microscope. When the cell growth was close to confluence, the cells were passaged at a proportion of 1:3.
Experimental groups and intervention methods
Fifth-generation keloid fibroblasts were inoculated into 96-well culture plates and divided into two groups: Galla chinensis ointment group (Galla group) and the control group. Another normal skin group (normal group) was set up to inoculate normal skin tissue fibroblasts.
The fifth-generation fibroblast cells were diluted into a single cell suspension with DMEM containing 10% FBS, plated in 96-well culture plate at a density of 1.0 × l04 cells/mL with 200 µL per well, and placed into a CO2 incubator for routine culture. When the cells had adhered, the culture medium was replaced with 180 µL of DMEM containing no FBS in each well. Then, 20 µL of Galla chinensis ointment aqueous solution was added to cells in the Galla group, and 20 µL of DMEM containing 10% FBS was added to cells of the control and normal groups. Cells were harvested 24 and 48 hours after culture to extract total RNA and protein.
Immunohistochemistry
Protein expression of PI3K, PTEN, Akt, and mTOR in keloid tissues was assessed by using immunohistochemical staining. Tissues were fixed and stored in 4% paraformaldehyde at low temperatures for more than 72 hours. The samples were cut into 4-μm-thick paraffin sections, placed in a 62°C oven for 30 minutes, dewaxed with xylene, hydrated, and rinsed three times with phosphate-buffered saline (PBS). Endogenous peroxidase was blocked by 1% H2O2. Non-specific antigens were blocked with normal goat serum for 10 minutes. Then, sections were incubated with mouse anti-human primary antibodies against mTOR, Akt, PTEN, PI3K (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight, after which the sections were incubated with biotin-labeled goat anti-nude mouse/rabbit IgG at 37°C for 30 minutes. Horseradish peroxidase (HRP)-labeled streptavidin solution was added and incubated for 10 minutes, and the cells were placed into 3,3′-diaminobenzidine (DAB) stain solution from a DAB horseradish peroxidase Color Kit (Beijing Zhongshang Jinqiao Biotechnology Co. Ltd., Beijing, China) at room temperature. Negative control staining was done by incubating tissue sections with PBS instead of the primary antibody.
Under a standard optical microscope, positive staining of mTOR, Akt, and PI3K was observed as pale yellow, brown-yellow, or yellow-brown cytoplasm, and positive staining of PTEN was observed as pale yellow, brown-yellow, or yellow-brown nucleus. According to the Shimizu method,29 0, 1, 2, and 3 points were assigned according to staining differences of each section: no staining, light yellow, brown, or tan, respectively. In addition, 0, 1, 2, and 3 points were assigned according to the proportion of positive staining area/photo area under the light microscope: no staining, less than one-third, one-third to two-thirds, and more than two-thirds. A positive result was defined as the sum of the two scores ≥3.29 Hematoxylin staining was done to evaluate morphology.
Expression of miR-21 and PTEN mRNA in keloid fibroblast cells detected by reverse transcription PCR
A TRIzol reagent total RNA extraction kit (Invitrogen, Carlsbad, CA, USA) was used to extract RNA from cells harvested into an Eppendorf tube, and the procedures were in strict accordance with the instructions. Electrophoresis was used to determine the integrity of RNA extraction. The proportion of RNA light absorption at 260 and 280 nm (A260/A280) values of all the samples was >1.8, indicating that sample purity was high without obvious protein contamination. The total RNA concentration was regulated at 0.5 g/L and preserved at −70°C.
A real-time PCR instrument from Funglyn Biotech (Richmond Hill, ON, Canada) was used. A volume of 5 μL of RNA per sample was used for reverse transcription. The expression of miR-21 and PTEN was measured by reverse transcription (RT)-PCR with the following procedures: predenaturation for 4 minutes at 94°C, denaturation at 94°C for 20 seconds, annealing at 60°C for 30 seconds, 30 cycles in total, and extension at 72°C for 5 minutes. The relative expression level (RQ) of mRNA was expressed as the ratio of the intensity value of each target gene to the internal reference gene U6. The expression level of the gene was RQ = 2−ΔΔCt, where Ct is the cycle threshold. Specific primers were designed based on the sequences obtained from the GenBank database; the primer sequences are shown in Table 2. Each PCR experiment was repeated three times.
Table 2.
Primers used for the detection of miR-21.
| Gene | Primer sequence |
|---|---|
| miR-21 | Forward: 5′-CGGTAGCTTATCAGACTG-3′Reverse: 5′-GAGCAGGCTGGAGAA-3′ |
| PTEN | Forward: 5′-TTGAAGACCATAACCCACCA-3′Reverse: 5′-CACATAGCGCCTCTGACTGG-3′ |
| U6 | Forward: 5′-CTCGACGCTTCGGCAGCACATA-3′Reverse: 5′-CGCTTCACGAATTCGTTGCGT-3′ |
Expression of PTEN, PI3K, p-Akt, and p-mTOR protein in keloid fibroblast cells detected by western blot analysis
Cultured fibroblast cells were collected, washed twice in cooled PBS, lysed with a homogenizer, and then placed on ice for 10 to 20 minutes. The samples were then centrifuged at 8,500 × g for 10 minutes at 4°C, and the supernatant was removed. The Lowry method was used for supernatant protein quantification; 50 µg of protein was taken for 15% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with an SDS-PAGE Gel Preparation Kit (Shanghai Suo Lai Biotechnology Co. Ltd., Shanghai, China) and electrophoresis instrument (Bio-Rad Laboratories, Hercules, CA, USA). When bromophenol blue reached the bottom of the gel, the protein was transferred to a nitrocellulose filter, and the primary antibody was added for incubation overnight at 4°C. Corresponding HRP-labeled goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) was added, and the cells were incubated for 1 hour at room temperature. After the membrane was washed, an Odyssey infrared fluorescence imaging system (LI-COR Biosciences, Lincoln, NE, USA) was used to scan it and provide an image on a GDS8000 gel scanning system (UVP LLC, Upland, CA, USA), which was analyzed by using the Image-Pro Plus imaging analysis system (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
The experimental data were statistically analyzed by SPSS version 20.0 software (IBM Corp., Armonk, NY, USA). The measurement data were expressed as mean ± standard deviation. A multi-sample mean comparison was performed by the F test. The q-test was used for pairwise comparisons between multiple sample means. The count data were analyzed by the chi-square test. P < 0.05 was used to indicate statistical significance.
Results
Mouse model
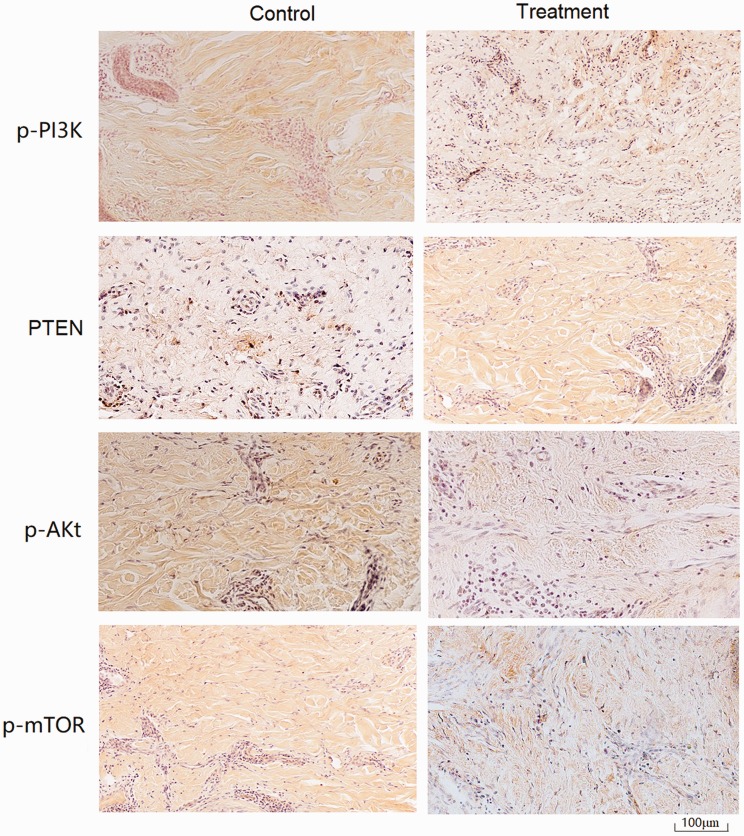
We detected an obvious effect of the ointment in all Galla chinensis–treated implants. The size of the transplanted keloid from Galla chinensis ointment–treated nude mice averaged 258.36 ±37.42 mm3 (n = 18), which was significantly smaller than that from control nude mice (764.56 ± 75.47 mm3, n = 18) (P < 0.05). In keloid tissues isolated from the mouse model, PTEN was mainly detected in the nucleus with weak signals in the cytoplasm, whereas PI3K, Akt, and mTOR were mainly found in the cytoplasm but not in the nucleus (Figure 1). The staining level suggested that PTEN was expressed at a higher level in keloid tissues of the treatment group than in the control group, whereas Akt and mTOR were expressed at lower levels in keloid tissues of the treatment group than in the control group. PI3K was highly expressed in both the control and treatment groups.
Figure 1.
Expression of PTEN, PI3K, Akt, and mTOR in keloid tissues. Keloid tissues were removed at the end of treatment and subjected to immunohistochemistry for the detection of PTEN, PI3K, Akt, and mTOR. As shown in the images, PTEN was mainly detected in the nucleus (tan stain), whereas PI3K, Akt, and mTOR were mainly found in the cytoplasm (tan stain). As shown in the images, PTEN was expressed at a higher level in keloid tissues of the treatment group than in the control group, whereas Akt and mTOR were expressed at lower levels in keloid tissues of the treatment group than in the control group. PI3K was highly expressed in both the control and treatment groups. PTEN, chromosome 10 neutropenic protein phosphatase; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin.
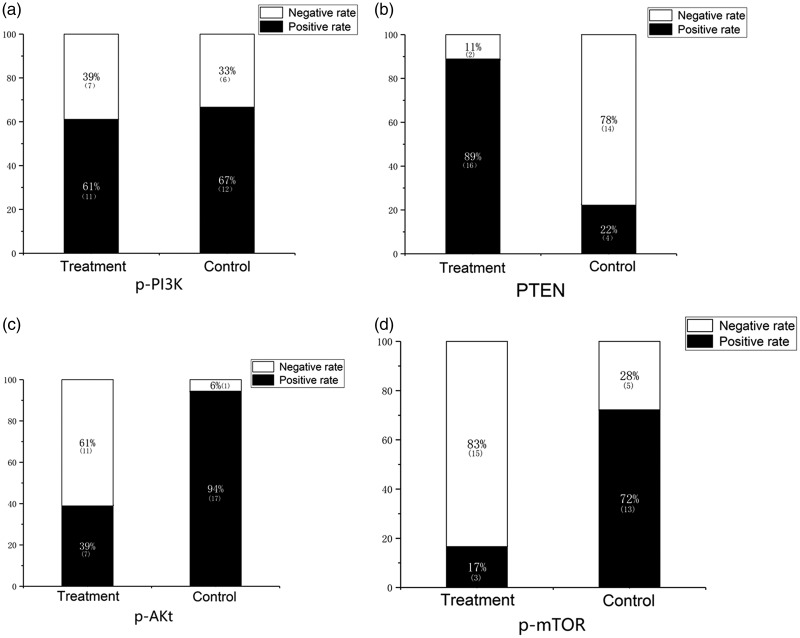
When expression was quantified, as shown in Figure 2, the positive expression rates of PTEN, Akt, and mTOR were 88.9% (16/18), 38.9% (7/18), and 16.7% (3/18) in the treatment group and 22.2% (4/18), 94.4% (17/18), and 72.2% (13/18) in the control group, respectively. The difference between the two groups was significant (P < 0.05). The positive expression rate of PI3K was 61.1% (11/18) in the treatment group and 66.7% (12/18) in the control group, with no significant difference between the groups.
Figure 2.
Quantification of PTEN, PI3K, Akt, and mTOR expression in keloid tissues. The immunohistochemistry staining was quantified using the Shimizu method.29 (a) Expression of phosphorylated (p)-PI3K in keloid tissue sections. There was no significant difference between the treatment and control groups. (b) p-Akt expression differed significantly in the experimental and control groups (P < 0.05). (c) p-mTOR expression differed significantly in the experimental and control groups (P < 0.05). (d) PTEN expression differed significantly in the experimental and control groups (P < 0.05). PTEN, chromosome 10 neutropenic protein phosphatase; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin.
RT‑PCR
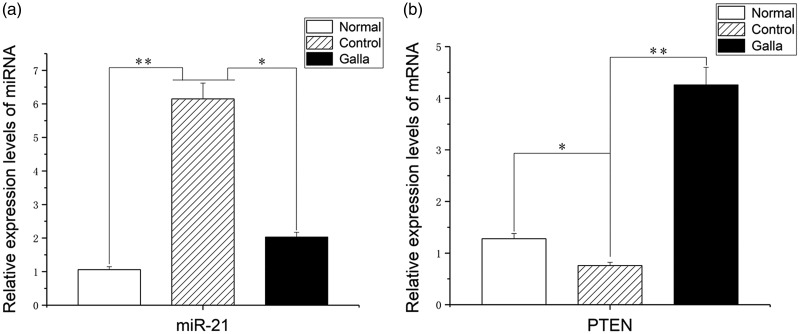
RT-PCR showed that the relative expression of miR-21 mRNA in the control and Galla group fibroblasts was higher than that of fibroblasts in the normal group (q = 13.23, 8.46; P < 0.05). The relative expression of miR-21 mRNA in fibroblasts of the Galla group was significantly lower than that of the control group (q = 11.34, P < 0.05). The relative expression level of PTEN mRNA was lower in the control group than in the Galla and the normal groups (q = 10.52, 9.78; P < 0.05). In contrast, the relative expression of PTEN was significantly higher in fibroblasts of the Galla group than in the normal group (q = 8.95; P < 0.05) (Figure 3).
Figure 3.
Expression levels of miR-21 and PTEN mRNA as calculated by reverse transcription-PCR. The relative mRNA expression levels in normal (untreated normal tissue), Galla (keloid tissue treated with Galla chinensis ointment), and control (untreated keloid tissue) samples were compared. (a) miR-21 expression levels; (b) PTEN mRNA expression levels. *Significant difference between the Galla and control groups (P < 0.05); **significant difference between normal and control groups (P < 0.05). PTEN, chromosome 10 neutropenic protein phosphatase.
Western blot analysis
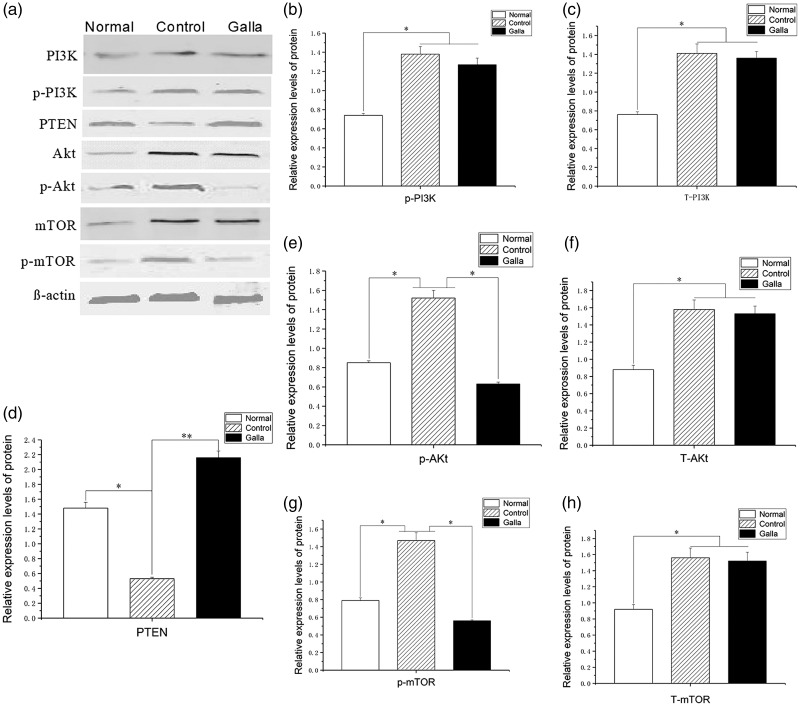
Western blot analysis showed no significant difference in PI3K protein expression levels between control and Galla group fibroblasts (q = 1.85, 2.04), but both groups had higher expression than the normal group (q = 10.74, 8.63; P < 0.05). The expression of PTEN protein was significantly lower in fibroblasts of the control group than in the normal group (q = 13.7; P < 0.05) but significantly increased in fibroblasts of the Galla group compared with the control group (q = 15.31; P < 0.05). Expression of phosphorylated (p)-Akt and p-mTOR protein was significantly higher in fibroblasts of the control group than in the normal group (q = 12.16, 10.36; P < 0.05) but significantly lower in fibroblasts of the Galla group compared with the control group (q = 16.24, 13.12; P < 0.05) (Figure 4).
Figure 4.
Quantification of PTEN, PI3K, Akt, and mTOR protein expression in normal (untreated normal fibroblasts), Galla (keloid fibroblasts treated with Galla chinensis ointment), and control (untreated keloid fibroblasts) samples were compared. (a) Bands from the western blot showing expression of PTEN, p-PTEN, PI3K, p-P13K, Akt, p-Akt, mTOR, and p-mTOR protein, together with the β-actin loading control. (b) Quantification of p-P13K expression. (c) Quantification of T-PI3K expression. (d) Quantification of PTEN expression. (e) Quantification of p-AKt expression.(f) Quantification of T-Akt expression. (g) Quantification of p-mTOR expression. (h) Quantification of T-mTOR expression. p-, phosphorylated protein; T-, total protein. *Significant difference compared with control group (P < 0.05). PTEN, chromosome 10 neutropenic protein phosphatase; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin.
Discussion
Our previous study showed that the use of Galla chinensis ointment to treat keloids in the clinic achieved promising results based on the Vancouver Scar Scale and evaluation of clinical efficacy. The overall effective rate was 96.6%, as measured by the percentage of patients in which the symptoms of keloid improved.23 In addition, experimental studies have shown that Galla chinensis ointment can inhibit proliferation of keloid fibroblasts.9
miRNAs are a class of endogenous small non-coding RNAs that regulate gene expression, thus affecting a variety of biological processes such as proliferation, differentiation, apoptosis, and metabolism.30 Differential expression of miRNAs has been demonstrated between pathological scar tissue and normal skin tissue using microarrays,11 in which miR-21 was significantly more highly expressed in hypertrophic scar tissues than in normal skin.12 This is consistent with results from the present study, which suggested that miR-21 is closely related to the occurrence of keloids (Figure 3).
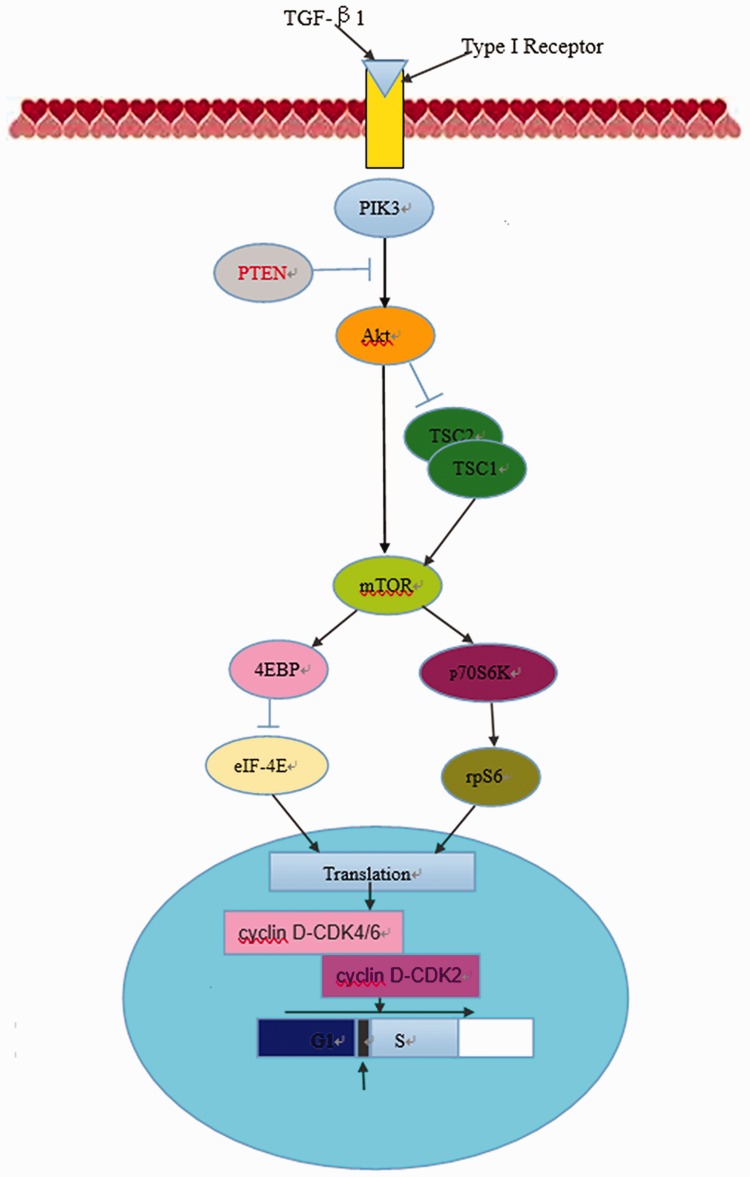
Keloids have tumor-like properties and, based on results of recent studies,12,14,15 we propose that miR-21, PTEN, PI3K, Akt, and mTOR may interact with each other, as has been shown in the PI3K-Akt-mTORC1 signaling pathway represented in Figure 5, and that this pathway may play an important role in the pathogenic process of keloid formation.
Figure 5.
Graphical representation of the PI3K-Akt-mTORC1 signaling pathway. TGF, transforming growth factor; PTEN, chromosome 10 neutropenic protein phosphatase; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin.
Thus, we tested whether Galla chinensis ointment exerted its therapeutic effect by regulating the expression of miR-21, PTEN, PI3K, Akt, and mTOR. To confirm this hypothesis, we used RT-PCR, western blot analysis, and immunohistochemistry to investigate the expression of miR-21 and key components in mTOR signaling pathways in keloids altered by Galla chinensis ointment. The RT-PCR results showed that expression of miR-21 in the normal and Galla groups was significantly lower than that of the control group, indicating that miR-21 is expressed at a low level in normal skin tissues but highly expressed in keloids and that Galla chinensis ointment can reverse the abnormal expression of miR-21 (Figure 3). The expression of PTEN was also significantly higher in the normal group and the Galla group than in the control group (Figure 3). The immunohistochemistry results showed that expression of Akt and mTOR was high in the control group but low in the Galla group (Figure 2). PI3K was highly expressed in both the control group and the Galla group, whereas PTEN showed low-level expression in the control group and high-level expression in the Galla group (Figure 2). The results of western blot analysis showed that the expression of PTEN protein was lower in keloid fibroblasts than in normal skin fibroblasts, whereas expression of PI3K, p-Akt, and p-mTOR protein was higher in keloid fibroblasts than in normal skin fibroblasts (Figure 4). The Galla chinensis ointment increased the expression of PTEN protein and decreased the expression of p-Akt and p-mTOR protein in keloid fibroblasts, but it had no significant effect on the expression of PI3K protein. Importantly, the observation that PTEN mRNA expression levels were similar between the normal and control groups but much higher in the Galla chinensis ointment group was unexpected. Because the ointment is a complex mixture of seven ingredients, different active compounds may activate a wide variety of pathways. Only one pathway was examined in the present study, and future studies should examine other pathways that could lead to increased PTEN expression by Galla chinensis ointment. PTEN is a tumor suppressor gene and because keloids share some characteristics with cancer,12,14,15 the increase in PTEN expression could be one mechanism by which Galla chinensis ointment controls the growth of keloids. This will have to be examined more closely in future studies.
Galla chinensis has a wide range of actions in modern medicine.31,32 Further study is needed to fully evaluate the specific actions of the main ingredients in treating keloid, but we can speculate on some of them. The composition of Galla chinensis ointment is gallnut, medlar, Salvia miltiorrhiza, clematis, Chinese wolfberry root bark, and black vinegar. The main active components of Galla chinensis are enamel and gallic acid. We suggest that enamel could combine with collagen in the extracellular matrix of keloid fibroblasts to form a complex that cannot be decomposed and that inhibits the proliferation of fibroblasts. Enamel can also promote contraction of small blood vessels, so blood supply to the keloid is reduced, causing keloid to soften and shrink. The main components of medlar are proteins and polysaccharides, which can interfere with mitosis of tumor cells, inhibit cell proliferation, and induce apoptosis. Tanshinone IIA contained in Salvia miltiorrhiza can promote the apoptosis of fibroblasts, reduce the content of collagen fibers in keloid tissue, inhibit the autocrine action of transforming growth factor-β1 in fibroblasts, and effectively inhibit keloid tissue proliferation.33 The main components of clematis are pulsatilla and saponins. The main function of pulsatilla is to inhibit the growth of bacteria.34 Clematis saponins can eliminate superoxide radicals and inhibit the formation of lipid peroxides.35 The main components of the Chinese wolfberry root bark are alkaloids and flavonoids, which antagonize histamine and inhibit the inflammatory reaction,36 thereby reducing the irritating effect of medlar and gallnut on the skin and reducing the incidence of drug dermatitis. Black vinegar can neutralize the alkaloids in herbs to form a salt substance, which can fully bind to the skin tissue and promote drug absorption.
Because we studied animal models of keloid and in vitro–cultured keloid fibroblasts, our experimental results may differ somewhat from treatment of keloid in the clinical setting. Therefore, our future research will include a clinical trial to determine the reliability of the experimental results. In addition, this study reveals only one aspect of the mechanism of Galla chinensis ointment in the treatment of keloid, providing a theoretical basis for its application in clinical practice. Nevertheless, the pathogenesis of keloid is complex and still mostly unknown, and its formation probably involves multiple genes and multiple signaling pathways.8,37 Therefore, whether there are other mechanisms and targets of Galla chinensis ointment remains to be studied.
This study has some limitations. The subjects in this study were animal keloid models, which are different from human keloids. In addition, there are other animal models of keloid,38,39 but we used only one in the present study. Future studies could use multiple models for confirmation. Furthermore, the environment in which the keloid fibroblasts were cultured in vitro is different from that of the human body, so its biological characteristics and signal transduction may be inconsistent with the actual situation in the body. Therefore, the experimental results do not fully reflect the clinical situation.
Taken together, our results indicate that miR-21, PTEN, Akt, and mTOR might be involved in the formation of keloids, and Galla chinensis ointment can affect the expression of these key molecules related to the mTOR signaling pathway to inhibit the proliferation of keloid fibroblasts. Our study sheds light on some mechanisms underlying the therapeutic effects of Galla chinensis ointment on keloid, which supports further translational research.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The work was supported by the following two projects: National Natural Science Foundation of China (81673976); Clinical Chinese Medicine Plateau Discipline Construction Project of Shanghai Pudong New Area Health Committee (PDZY-2018-0604).
ORCID iD
Xiaoxiang Zhai https://orcid.org/0000-0003-1527-1236
References
- 1.Tsai CH, Ogawa R. Keloid research: current status and future directions. Scars Burn Heal 2019; 5: 2059513119868659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belie O, Ugburo AO, Mofikoya BO. Demographic and clinical characteristics of keloids in an urban center in Sub-Sahara Africa. Niger J Clin Pract 2019; 22: 1049–1054. [DOI] [PubMed] [Google Scholar]
- 3.Allah KC, Yeo S, Kossoko H, et al. [Keloid scars on black skin: myth or reality]. Ann Chir Plast Esthet 2013; 58: 115–122. [DOI] [PubMed] [Google Scholar]
- 4.Alhady SM, Sivanantharajah K. Keloids in various races. A review of 175 cases. Plast Reconstr Surg 1969; 44: 564–566. [DOI] [PubMed] [Google Scholar]
- 5.Kelly AP. Medical and surgical therapies for keloids. Dermatol Ther 2004; 17: 212–218. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan KM, Thomas KP, Sundararajan CR. Study of 1,000 patients with keloids in South India. Plast Reconstr Surg 1974; 53: 276–280. [DOI] [PubMed] [Google Scholar]
- 7.Akoz T, Gideroglu K, Akan M. Combination of different techniques for the treatment of earlobe keloids. Aesthetic Plast Surg 2002; 26: 184–188. [DOI] [PubMed] [Google Scholar]
- 8.Arno AI, Gauglitz GG, Barret JP, et al. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns 2014; 40: 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding JC, Tang ZM, Zhai XX, et al. The effects of Wubeizi ointment on the proliferation of keloid-derived fibroblasts. Cell Biochem Biophys 2015; 71: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai XX, Ding JC, Tang ZM, et al. Effect of Wubeizi ointment aqueous solution on the expression of type I and III procollagen genes in keloid fibroblasts. Exp Ther Med 2017; 13: 503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Yang D, Xiao Z, et al. miRNA expression profiles in keloid tissue and corresponding normal skin tissue. Aesthetic Plast Surg 2012; 36: 193–201. [DOI] [PubMed] [Google Scholar]
- 12.Zhu HY, Li C, Bai WD, et al. MicroRNA-21 regulates hTERT via PTEN in hypertrophic scar fibroblasts. PLoS One 2014; 9: e97114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WJ, Yang W, Ouyang ZH, et al. MiR-21 promotes ECM degradation through inhibiting autophagy via the PTEN/akt/mTOR signaling pathway in human degenerated NP cells. Biomed Pharmacother 2018; 99: 725–734. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YM, Zhang ZQ, Liu YY, et al. Requirement of Galphai1/3-Gab1 signaling complex for keratinocyte growth factor-induced PI3K-AKT-mTORC1 activation. J Invest Dermatol 2015; 135: 181–191. [DOI] [PubMed] [Google Scholar]
- 15.Ong CT, Khoo YT, Mukhopadhyay A, et al. mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Exp Dermatol 2007; 16: 394–404. [DOI] [PubMed] [Google Scholar]
- 16.Shetlar MR, Shetlar CL, Hendricks L, et al. The use of athymic nude mice for the study of human keloids. Proc Soc Exp Biol Med 1985; 179: 549–552. [DOI] [PubMed] [Google Scholar]
- 17.Robb EC, Waymack JP, Warden GD, et al. A new model for studying the development of human hypertrophic burn scar formation. J Burn Care Rehabil 1987; 8: 371–375. [DOI] [PubMed] [Google Scholar]
- 18.Polo M, Kim YJ, Kucukcelebi A, et al. An in vivo model of human proliferative scar. J Surg Res 1998; 74: 187–195. [DOI] [PubMed] [Google Scholar]
- 19.Jin PS, Cen Y, Liu XX, et al. [Replication of pathological scar in nude mice]. Zhonghua Shao Shang Za Zhi 2007; 23: 126–129. [PubMed] [Google Scholar]
- 20.Waki EY, Crumley RL, Jakowatz JG. Effects of pharmacologic agents on human keloids implanted in athymic mice. A pilot study. Arch Otolaryngol Head Neck Surg 1991; 117: 1177–1181. [DOI] [PubMed] [Google Scholar]
- 21.Philandrianos C, Bertrand B, Andrac-Meyer L, et al. Treatment of keloid scars with a 1210-nm diode laser in an animal model. Lasers Surg Med 2015; 47: 798–806. [DOI] [PubMed] [Google Scholar]
- 22.Ishiko T, Naitoh M, Kubota H, et al. Chondroitinase injection improves keloid pathology by reorganizing the extracellular matrix with regenerated elastic fibers. J Dermatol 2013; 40: 380–383. [DOI] [PubMed] [Google Scholar]
- 23.Ding JC, Yan YH, Zhai XX. The efficacy of Wubeizi Ointment in treating keloid. Journal of Hebei Medical University 2007; 28: 356–359. [Google Scholar]
- 24.Kischer CW, Pindur J, Shetlar MR, et al. Implants of hypertrophic scars and keloids into the nude (athymic) mouse: viability and morphology. J Trauma 1989; 29: 672–677. [DOI] [PubMed] [Google Scholar]
- 25.Tu T, Huang J, Lin M, et al. CUDC907 reverses pathological phenotype of keloid fibroblasts in vitro and in vivo via dual inhibition of PI3K/Akt/mTOR signaling and HDAC2. Int J Mol Med 2019; 44: 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Nahas Z, Feng F, et al. Tissue engineering for in vitro analysis of matrix metalloproteinases in the pathogenesis of keloid lesions. JAMA Facial Plast Surg 2013; 15: 448–456. [DOI] [PubMed] [Google Scholar]
- 27.Fong CY, Biswas A, Subramanian A, et al. Human keloid cell characterization and inhibition of growth with human Wharton’s jelly stem cell extracts. J Cell Biochem 2014; 115: 826–838. [DOI] [PubMed] [Google Scholar]
- 28.Tian Y, Jin L, Zhang W, et al. AMF siRNA treatment of keloid through inhibition signaling pathway of RhoA/ROCK1. Genes Dis 2019; 6: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha-ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol 1990; 21: 607–612. [DOI] [PubMed] [Google Scholar]
- 30.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010; 11: 597–610. [DOI] [PubMed] [Google Scholar]
- 31.Djakpo O, Yao W. Rhus chinensis and Galla Chinensis–folklore to modern evidence: review. Phytother Res 2010; 24: 1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Li L, Kim SH, et al. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res 2009; 26: 2066–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, Jiang H, Xu X, et al. Tanshinone IIA mediates SMAD7-YAP interaction to inhibit liver cancer growth by inactivating the transforming growth factor beta signaling pathway. Aging (Albany NY) 2019; 11: 9719–9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HS, Beon MS, Kim MK. Selective growth inhibitor toward human intestinal bacteria derived from Pulsatilla cernua root. J Agric Food Chem 2001; 49: 4656–4661. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, Fang W, Han D, et al. Clematichinenoside attenuates myocardial infarction in ischemia/reperfusion injury both in vivo and in vitro. Planta Med 2013; 79: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 36.Wang S, Suh JH, Zheng X, et al. Identification and quantification of potential anti-inflammatory hydroxycinnamic acid amides from wolfberry. J Agric Food Chem 2017; 65: 364–372. [DOI] [PubMed] [Google Scholar]
- 37.Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 2011; 17: 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marttala J, Andrews JP, Rosenbloom J, et al. Keloids: animal models and pathologic equivalents to study tissue fibrosis. Matrix Biol 2016; 51: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Supp DM. Animal models for studies of keloid scarring. Adv Wound Care (New Rochelle) 2019; 8: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]