Abstract
In this study of three-dimensional (3D) printed composite β-tricalcium phosphate (β-TCP)-/hydroxyapatite/poly(ɛ-caprolactone)-based constructs, the effects of vertical compositional ceramic gradients and architectural porosity gradients on the osteogenic differentiation of rabbit bone marrow-derived mesenchymal stem cells (MSCs) were investigated. Specifically, three different concentrations of β-TCP (0, 10, and 20 wt%) and three different porosities (33% ± 4%, 50% ± 4%, and 65% ± 3%) were examined to elucidate the contributions of chemical and physical gradients on the biochemical behavior of MSCs and the mineralized matrix production within a 3D culture system. By delaminating the constructs at the gradient transition point, the spatial separation of cellular phenotypes could be specifically evaluated for each construct section. Results indicated that increased concentrations of β-TCP resulted in upregulation of osteogenic markers, including alkaline phosphatase activity and mineralized matrix development. Furthermore, MSCs located within regions of higher porosity displayed a more mature osteogenic phenotype compared to MSCs in lower porosity regions. These results demonstrate that 3D printing can be leveraged to create multiphasic gradient constructs to precisely direct the development and function of MSCs, leading to a phenotypic gradient.
Impact Statement
In this study, three-dimensional (3D) printed ceramic/polymeric constructs containing discrete vertical gradients of both composition and porosity were fabricated to precisely control the osteogenic differentiation of mesenchymal stem cells. By making simple alterations in construct architecture and composition, constructs containing heterogenous populations of cells were generated, where gradients in scaffold design led to corresponding gradients in cellular phenotype. The study demonstrates that 3D printed multiphasic composite constructs can be leveraged to create complex heterogeneous tissues and interfaces.
Keywords: gradients, osteogenic differentiation, multiphasic scaffold, bone tissue engineering, construct
Introduction
In clinical practice, autogenous bone grafts are the gold standard for the treatment of bone defects, delayed unions, and nonunions.1 However, due to drawbacks associated with these grafts, such as donor site morbidity, surgical site pain, limited availability, and failure to adequately address the soft tissue-to-bone interface, research into future treatment methods increasingly focuses on the substantial advancement of constructs for tissue engineering.2–4
Native bone tissue has a complex heterogenous architecture that possesses regional differences in structure and composition.5 For example, cortical bone has a higher degree of mineralization and a 20–30% higher tissue compressive strength compared to trabecular bone.6–8 In addition, long bones have a radial gradient in porosity when transitioning from trabecular to cortical bone.9 Cortical bone has an average porosity of 10–20%, while for trabecular bone, the porosity ranges between 50% and 90%.10 Furthermore, defects of soft tissue-to-bone interfaces, such as the osteochondral unit, demonstrate an even more heterogenous structure because they are composed of relatively simple building blocks that combine to form exceedingly complex structures.11 With the advent of a diverse array of biofabrication techniques, one can now fabricate multiphasic constructs that recreate the complex architectural and compositional gradients present in tissues and interfaces.12
To better recapitulate the complex heterogenous structure of tissue interfaces, several different techniques have been investigated that aim to restore the native zonal architecture and cellular phenotypic transitions. One such approach takes inspiration from nature, where different biological processes, such as wound healing, nerve regeneration, immune response, angiogenesis, and tissue architecture, are mediated by bioactive molecule gradients.13,14 In this type of approach, the spatiotemporal patterning and dispersion of bioactive molecules such as growth factors are specifically controlled to create gradients in biochemical signaling, leading to the formation of heterogeneous tissues.15–18 As an example, the delivery of transforming growth factor-β1 and bone morphogenetic protein-2 (BMP-2) was leveraged in a graded manner to control the arrangement of regenerated cartilage and subchondral bone tissue.17
Another creative approach has leveraged modified viral vectors rather than growth factors to accomplish spatially stratified phenotypic changes in cultured cells.19–21 Such demonstrations display the utility of viral vectors for therapeutic benefit. For example, the zonal organization of the soft tissue-to-bone interface can be recapitulated by seeding fibroblasts onto polymeric constructs that contained a graded distribution of a retroviral vector for transcription factor Runx2.21 However, both of these approaches leverage exogenous biologics or viral vectors to ultimately control the zonal organization, whereas it may be desirable to exclude these added factors.
One can additionally leverage three-dimensional (3D) synthetic constructs for tissue engineering without relying on viral vectors or biologics and still impart a variety of mechanical and biochemical stimuli to affect cell behavior and phenotype. With respect to the physical attributes of the scaffold or environment, several studies have demonstrated that, in particular, porosity and pore size have significant implications on the osteogenic differentiation of mesenchymal stem cells (MSCs).22–26 In vitro, it was demonstrated that a construct's mean pore size affects the cellular activity of cultured preosteogenic cells, and that small changes in the mean pore size can dramatically change cell adhesion and distribution, thereby affecting the osteogenic differentiation by impacting the paracrine signaling distance.27,28
Many cytocompatible synthetic polymers exhibit a bioinert surface resulting in minimal tissue response or capability for direct cell interaction without the assistance of additives or adsorbed proteins.29,30 By incorporating additives within synthetic polymers, such as poly(ɛ-caprolactone) (PCL), one can improve the attachment, proliferation, and ultimately affect the osteogenesis of MSCs.31–38 Among other such additives, ceramic materials, such as hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP), are widely used as synthetic bone substitutes that have been shown to affect the osteogenic differentiation of MSCs.5,11,39–44 Recently, in the field of tissue engineering, 3D printing has emerged as an attractive technique for the development of biphasic, triphasic, and even multiphasic constructs that better mimic the complexities of tissues and interfaces.45–48 The improved understanding of the effects of architectural and compositional aspects of graded constructs on osteogenic differentiation of MSCs will further guide construct design and fabrication for interfacial tissue engineering.
In this work, 3D printed porous composite PCL-based constructs with vertical gradients in porosity and pore size, as well as ceramic content of β-TCP were fabricated and seeded with MSCs to direct spatially dependent osteogenic differentiation. The constructs were then evaluated using a variety of biochemical assays to elucidate the individual and combined effects of compositional and architectural gradients on the osteogenic differentiation and mineralized matrix development of MSCs in a 3D culture environment. Specifically, this study focused on the effects of (i) a vertical porosity gradient; (ii) a vertical ceramic gradient; and 3) a combined dual vertical porosity/ceramic gradient on the spatiotemporal osteogenic differentiation of cultured MSCs and mineralized matrix deposition.
Materials and Methods
Experimental design
To evaluate the specific objectives of the study as outlined above, seven different experimental groups (Table 1) were examined for directing the spatiotemporal osteogenic differentiation of MSCs. As presented in Figure 1, groups contained uniform architecture and composition, a vertical architectural gradient, a vertical compositional gradient, or a combined vertical architectural/compositional (dual) gradient. The bulk material used for control scaffolds PoreU and PoreG was 10 wt% HA in PCL. To observe baseline effects of uniformly increased ceramic content, the bulk material for control scaffolds TCP10PoreU and TCP10PoreG was 10 wt% HA and 10 wt% β-TCP in PCL. To elucidate the individual effects of a ceramic gradient, the TCPGPoreU and TCPGPoreG groups were composed of three different bulk materials: 0 wt% β-TCP/10 wt% HA in the top section, 10 wt% β-TCP/10 wt% HA in the middle section, and 20 wt% β-TCP/10 wt% HA in the bottom section.
Table 1.
β-Tricalcium Phosphate/Hydroxyapatite/Poly(ɛ-Caprolactone) Formulations for Three-Dimensional Printed Constructs
| Formulation | HA (wt%) | β-TCP (wt%) | Pore gradient |
|---|---|---|---|
| PoreU | 10 | 0 | Uniform (M) |
| PoreG | 10 | 0 | Gradient (S, M, L) |
| TCP10PoreU | 10 | 10 | Uniform (M) |
| TCP10PoreG | 10 | 10 | Gradient (S, M, L) |
| TCPGPoreU | 10 | Gradient (0, 10, 20) | Uniform (M) |
| TCPGPoreG | 10 | Gradient (0, 10, 20) | Gradient (S, M, L) |
| TCPGPoreGI | 10 | Gradient (0, 10, 20) | Gradient (L, M, S) |
Constructs were fabricated with three distinct fiber spacings: 0.2 mm (small, S), 0.6 mm (medium, M), and 1.0 mm (large, L). Porosity and gradient ceramic concentrations are listed in the orientation, from top to bottom, during cell culture.
β-TCP, β-tricalcium phosphate; HA, hydroxyapatite.
FIG. 1.
Cross-sectional view of architectural, compositional, and dual gradient constructs presented in the same orientation that was maintained over the duration of the study (the section that was resting on the bottom of the well plate corresponds to the section at the bottom of the figure). Each construct comprised three sections that had a fiber spacing of 0.2 mm (S), 0.6 mm (M), or 1.0 mm (L) and a concentration of β-TCP of 0, 10, or 20 wt% as described in Table 1. β-TCP, β-tricalcium phosphate.
The TCPGPoreU and TCPGPoreG groups were then compared to the PoreU/TCP10PoreU and PoreG/TCP10PoreG groups, respectively. To elucidate the individual effects of a porosity gradient, groups PoreU, TCP10PoreU, and TCPGPoreU were compared with groups PoreG, TCP10PoreG, and TCPGPoreG, respectively. Finally, the combined effects of a dual porosity/ceramic gradient were evaluated by comparing the first experimental group, TCPGPoreG, with the uniform porosity and ceramic negative controls, PoreU and TCP10PoreU. The second experimental group, TCPGPoreGI, was included to additionally observe the effect of an inverted pore gradient relative to the TCPGPoreG group.
Fabrication of the 3D printed β-TCP/HA/PCL composite constructs
The 3D printed composite scaffolds were fabricated by adapting previously established protocols in our laboratory.49,50 Briefly, HA (average particle size 208 nm; Sigma-Aldrich, St. Louis, MO), PCL (nominal molecular weight 50,000 Da, Mn = 41,000 ± 900 Da, Mw = 56,000 ± 1200 Da; Polysciences, Warrington, PA), and β-TCP (average particle size 237 nm; Sigma-Aldrich) were homogeneously mixed in a mortar and pestle at varying weight percentages, as outlined in Table 1. The molecular weight of PCL was determined using an ACQUITY Advanced Polymer Chromatography as previously described.49 Square models (8 × 8 × 4.32 mm) were designed in SketchUp (Trimble, Sunnyvale, CA) and sliced at a layer thickness of 0.36 mm in BioplotterRP Software for 3D printing (EnvisionTEC, Gladbeck, Germany).
Throughout the document, the word “fiber” is used to describe filaments printed to form each scaffold. Three distinct fiber spacing patterns—0.2 mm (small, S), 0.6 mm (medium, M), and 1.0 mm (large, L)—were created by altering the spacing between adjacent fibers. β-TCP/HA/PCL mixtures were printed using a 3D Bioplotter (EnvisionTEC) extrusion printer. Printing conditions (pressure, speed, preflow and postflow, and wait time) were set for each composition to maintain a fiber diameter of 400 ± 60 μm (15% quality control window) and are shown in Table 2. To manufacture constructs with porosity gradients, three separate square models corresponding to the selected fiber spacings were stacked within the Bioplotter RP Software and sliced with the same layer thickness as stated above (0.36 mm). Finally, scaffolds that had a compositional gradient were printed in a similar manner, but three separate high temperature cartridges were employed.
Table 2.
List of Scaffold Printing Conditions/Parameters
| Material | Temperature (°C) | Pressure (bar) | Speed (mm/s) | Preflow (s) | Postflow (s) |
|---|---|---|---|---|---|
| 0 wt% β-TCP | 160 | 5.2 | 1.1 | 0.85 | 0.55 |
| 10 wt% HA | |||||
| 10 wt% β-TCP | 160 | 6.0 | 1.3 | 0.7 | 0.45 |
| 10 wt% HA | |||||
| 20 wt% β-TCP | 160 | 6.2 | 0.7 | 0.8 | 0.7 |
| 10 wt% HA |
Structural architecture of 3D printed composites
Constructs were analyzed by microcomputed tomography to quantify the fiber diameters and porosity gradient.49,51 Briefly, 3D printed constructs (n = 3) were scanned using a Skyscan 1272 (Ver 1.1.11; Bruker, Kontich, Belgium) at an X-ray voltage and current of 40 kV and 250 μA, respectively. Scans were performed with a rotation step size of 0.6°, 16.0 μm/pixel, frame averaging of 4, and a random movement of 10. Raw images were reconstructed using NReconn (Ver 1.7.1.0; Bruker). To determine the fiber size and porosity present in each section of the constructs, a 7.5 × 7.5 × 1.2 mm region of interest was used in each subsection of the construct and analyzed in CTan (Ver 1.17.7.2+; Bruker). To determine the fiber diameter in each section, 10 longitudinal slices were randomly selected, and an average fiber diameter for 1 scaffold section was obtained using n = 10 fibers per slice. Group average fiber diameters were obtained using n = 3 sections per group.
Rabbit bone marrow mesenchymal stem cells harvest
All experimental and surgical protocols were reviewed and approved by the Rice University Institutional Animal Care and Use Committee and were conducted following the animal care and use guidelines set forth by the National Institutes of Health. MSCs were isolated from the bone marrow of 6-month-old New Zealand male white rabbits as previously described.52 After induction of anesthesia, bone marrow was aspirated into 10 mL syringes that contained 1000 U of heparin. The bone marrow aspirate was cultured in 225-cm2 tissue culture flasks for 3 weeks in an incubator at 37°C and 5% CO2, with medium changes every 3 days, in general medium (GM) containing low-glucose Dulbecco's modified Eagle's medium (DMEM-LG), 10% v/v fetal bovine serum (FBS), 1% v/v GlutaMAX, and 1% v/v penicillin/streptomycin/fungizone (PSF). After which, the adherent fraction of cells from the six rabbits was pooled to minimize interanimal variability and designated as “MSCs,” given the established osteogenic potential of these cells.1,53 MSCs were expanded in GM and seeded on the constructs at passage 4.
Seeding of 3D printed scaffolds
After fabrication, 3D printed constructs were sterilized in a 12-h ethylene oxide cycle (Anprolene AN74i; Anderson Sterilizers, Haw River, NC) and allowed to vent for 24 h before cell seeding. Constructs were immersed in a sterile gradient of ethanol and phosphate-buffered saline (PBS; Gibco, Waltham, MA) (100%, 75%, 50%, 25%, and 0%), washed three times with PBS, and incubated in 2 mL of complete medium overnight on a shaker table. On the following day, four constructs were placed into a 10 mL syringe with 3 mL of complete medium and a concentration of 5.0 × 105 cells/mL measured by a hemocytometer.
Syringe plungers were compressed to remove any air and capped with a sterile syringe filter. After which, syringes with the constructs were placed on a rotating wheel (5 RPM) in an incubator for 14 h to facilitate cell adhesion. Individual constructs were transferred to 24-well plates (ultralow attachment plates) with 1 mL of complete osteogenic medium (DMEM-LG, 10% v/v FBS, 10 mM β-glycerol-2-phosphate, 10 nM dexamethasone, 50 mg/mL ascorbic acid, 1% v/v GlutaMAX, and 1% v/v PSF) per well, which was exchanged every 2 days. Seeded constructs were cultured for 3, 14, or 28 days. To evaluate cell proliferation between days 1 and 3 of culture, gradient ceramic constructs (TCPGPoreU, TCPGPoreG, and TCPGPoreGI) were separately seeded and cultured for 1 or 3 days following the same methods described above.
Biochemical assays
After the respective culture periods, constructs were rinsed twice in PBS, cut in half longitudinally, delaminated, and placed into 400 μL of sterile-filtered Millipore water and frozen at −20°C for further analysis. Each construct underwent three freeze-thaw cycles followed by ultrasonication to lyse the cells to assay the delaminated constructs for cellularity and alkaline phosphatase (ALP) activity. The amount of DNA was quantified using a Quant-iT PicoGreen dsDNA Assay Kit (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Briefly, cell lysate, assay buffer, and dye solution were placed into the well of a black 96-well plate and incubated for 10 min at room temperature. Fluorescence was measured with an excitation wavelength of 490 nm and emission wavelength of 520 nm (BioTek FLX800, Winooski, VT).
Following previous established protocols, the amount of DNA present in the cell population used to seed the scaffolds was determined to be 4.4 ± 0.2 pg/cell, using n = 3 samples of 100,000 MSCs. Results are expressed as cells per section by normalizing the measured DNA content from experimental samples to this value.54 ALP enzymatic activity was determined according to an established colorimetric assay that relies on the conversion of para-nitrophenylphosphate to para-nitrophenol (Abcam, Cambridge, MA).55
To measure the calcium content, acetic acid was added to the samples (n = 4) with a final concentration of 0.5 M and incubated on a shaker table at room temperature overnight. Calcium arsenazo III (Sigma-Aldrich) reagent was then added and the absorbance at 650 nm was measured with a (BioTek Powerwave x340) plate reader. Values were normalized to cell number for each respective delaminated section. Unseeded constructs were incubated under identical conditions as the cultured constructs for 3, 14, and 28 days, at which point the calcium was measured (Supplementary Fig. S1) to correct for calcium deposited from the medium or present within the constructs to ensure that the calcium measured accurately reflected active mineralization by the cultured cells.
Real-time reverse transcription–polymerase chain reaction
To evaluate the change of osteogenic genes, constructs were rinsed twice in PBS, delaminated, and cut into small pieces. To reproducibly delaminate the constructs, cutting guides were designed using CAD software (Autodesk Inventor, San Rafael, CA) and 3D printed such that a low-profile, 0.26 mm thick microtome blade (AccuEdge® 4689) could be used to cut the constructs at the architectural or compositional transition points. The guide referenced the surface of the construct that was adjacent to the printer bed during fabrication to ensure sections were flat and parallel to the horizontal faces. Sections of compositions from similar constructs were combined to ensure the collection of a sufficient amount of RNA, as has been described previously, placed in an Eppendorf tube with 1 mL of TRIzol (Invitrogen, Carlsbad, CA), and stored at −80°C.24 RNA was isolated with a TRIzol Plus RNA Purification Kit (Invitrogen) following the manufacturer's instructions for isolation of RNA from animal cells. The concentration and the purity of RNA were evaluated with a Nanodrop 2000 (Thermo Fisher, Waltham, MA). Reverse transcription was performed using One-Step SYBR™ GreenER™ Kit, with premixed ROX (Invitrogen) and the primers listed in Table 3.55–57
Table 3.
Primer Sequences for Reverse Transcription–Polymerase Chain Reaction
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| GAPDH | TCACCATCTTCCAGGAGCGA | CACAATGCCGAAGTGGTCGT |
| Ocn | CAAAGCCCAGCGGTGCAGAGTCT | AGCTCCCTGCCCGTCGATCAGTT |
| Opn | CACCATGAGAATCGCCGT | CGTGACTTTGGGTTTCTACGC |
| Runx2 | CCTTCCACTCTCAGTAAGAAGA | TAAGTAAAGGTGGCTGGATAGT |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Ocn, osteocalcin; Opn, osteopontin.
Polymerase chain reaction (PCR) was performed on the CFX96Touch (Bio-Rad Laboratories, Hercules, CA) under the recommended conditions: cDNA was synthesized at 60°C for 10 min and denatured at 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 65°C for 60 s. The expression of target genes was normalized to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase in the samples (ΔCt) (n = 1). Results are expressed as fold induction in mRNA expression normalized to the gene expression of the MSC cell stock used to seed the scaffolds.
Histological processing
Constructs were longitudinally sectioned in half and sent to the MD Anderson Cancer Center Research Histology Core Laboratory (Houston, TX) for embedding and cryosectioning. Sections (5 μm) were taken from the center of the constructs and staining was performed with von Kossa using a 2% silver nitrate solution incubated under ultraviolet for 30 min.
Confocal microscopy
After 1 and 3 days in culture, transverse sections (1 mm thick) of constructs were rinsed twice in PBS, fixed in Richard-Allan Scientific Neutral Buffered Formalin (10%) (Thermo Fisher) for 30 min, and permeabilized with CytoVista permeabilization buffer (Invitrogen) for 30 min at room temperature. Samples were then washed three times in CytoVista wash buffer (Invitrogen) for 5 min at room temperature and submerged in Image-IT FX Signal Enhancer (Invitrogen). Samples were then stained for actin with Alexa Fluor 488 Phalloidin (Invitrogen) at a concentration of 6 μL/mL and the nuclei were stained with 4,6-diamidino-2-phenylindole (Abcam, Boston, MA) staining solution (2.5 μM in PBS) and washed three times in PBS. Samples were then visualized with a Nikon A1 confocal microscope (Minato, Tokyo, Japan).
Fluorescence microscopy
After 1 and 3 days, the TCPGPoreU group was assessed for cell aggregations within the pores by using fluorescence microscopy following previous protocols.58 Briefly, constructs were rinsed twice in PBS, fixed in 4% paraformaldehyde for 30 min, and permeabilized with CytoVista permeabilization buffer (Invitrogen) for 30 min at room temperature. Constructs were stained with Alexa Fluor 488 Phalloidin (Invitrogen) at a concentration of 6 μL/mL and three different sections (top, middle, and bottom) were visualized with a Nikon Ti2-U with a DS-FI3 microscope camera.
Statistical analysis
JMP Pro 14 (SAS, Cary, NC) was used to evaluate the data set using a one-way ANOVA (analysis of variance) test with post hoc analysis by Tukey's honestly significant difference. Results were considered significant at p < 0.05. Results are presented as mean ± standard deviation.
Results
Scaffold architecture
The average fiber diameters measured for each scaffold section and group are presented in Figure 2. A ±15% quality control window on the desired nominal fiber diameter of 400 μm was applied to all scaffolds. Scaffolds with average fiber diameters outside of this criterion were not included in the study, and replacement scaffolds were printed with the same conditions. As seen in Figure 3, for gradient scaffolds, sections of significantly different (p < 0.05) porosities were incorporated by varying the lateral fiber spacing between sections. Specifically, the porosities (Fig. 3) of individual zones were 33% ± 4%, 50% ± 4%, and 65% ± 3% for the small (0.2 mm), medium (0.6 mm), and large (1.0 mm) fiber spacings, respectively. Furthermore, among uniform and gradient scaffolds, the porosity of the uniform scaffolds (0.6 mm fiber spacing) was similar (p > 0.05) to the porosity of the 0.6 mm zone of gradient scaffolds.
FIG. 2.
Average fiber diameters within each section of the different formulations investigated (Fig. 1). Data are reported as the mean ± standard deviation. Top, middle, and bottom refer to the location of the section within the construct during cell culture. Dashed green lines represent 400 ± 60 μm (15% quality control window), while the dashed black line represents the 400 μm desired fiber diameter (n1 = 3 scaffolds/group, n2 = 3 sections/scaffold, and n3 = 10 fibers/section).
FIG. 3.
Porosity of the β-TCP/HA/PCL composites (Fig. 1) evaluated by μCT. Top, middle, and bottom refer to the location of the section within the construct during cell culture. Data are reported as the mean ± standard deviation. Groups that do not share the same letter are significantly different (n = 3, p < 0.05). μCT, microcomputed tomography; HA, hydroxyapatite; PCL, poly(ɛ-caprolactone).
Cellularity
The cellularity of the constructs was analyzed on days 3, 14, and 28 (Fig. 4). On day 3, there was no discernible difference in the cellularity between the individual sections of the constructs (p > 0.05). After 14 days, significant differences between sections could be observed. Specifically, sections that contained 20 wt% β-TCP (bottom section of TCPGPoreU, TCPGPoreG, and TCPGPoreGI) had lower cell content compared to sections containing 0 wt% β-TCP within the same construct (top section of TCPGPoreU, TCPGPoreG, and TCPGPoreGI, p < 0.05).
FIG. 4.
Cellularity of the β-TCP/HA/PCL constructs (Fig. 1) at 3 days (A), 14 days (B), and 28 days (C) of culture. Data are reported as the mean ± standard deviation. Top, middle, and bottom refer to the location of the section within the construct during cell culture. Within each time point, sections that do not share the same letter are significantly different (p < 0.05, n = 4).
In addition, equivalent cellularity was observed between sections of groups that contained a uniform ceramic concentration independent of the pore gradient (PoreU and PoreG, TCP10PoreU, and TCP10PoreG, p > 0.05). On day 28, the majority of sections had similar cellularity (p > 0.05). The only noticeable difference was between the top and bottom section of TCP10PoreG (p < 0.05). Finally, the cell distribution is presented in Supplementary Figure S2, and it was observed that dynamic seeding resulted in a homogenous attachment of cells.
The cellularity of gradient ceramic constructs (TCPGPoreU, TCPGPoreG, and TCPGPoreGI) was separately analyzed on days 1 and 3 (Supplementary Fig. S3). On both day 1 and 3, there were no discernable differences in the cellularity between individual construct sections containing either the same ceramic composition or the same pore architecture (p > 0.05). On day 3, the cellularity of all construct sections trended higher compared to day 1, with statistically significant increases observed in all construct sections containing 20 wt% β-TCP (bottom sections of TCPGPoreU, TCPGPoreG, and TCPGPoreGI, p < 0.05) as well as the middle section of the TCPGPoreG group and the top and middle sections of the TCPGPoreGI group (p < 0.05).
Confocal microscopy of gradient ceramic constructs (TCPGPoreU, TCPGPoreG, and TCPGPoreGI, Fig. 5) demonstrated uniform spreading of seeded cells across the constructs on day 1. In addition, fluorescence microscopy of the TCPGPoreU formulation (Supplementary Fig. S4) demonstrated the formation cell aggregates on top of construct fibers. Also, in both Figure 5 and Supplementary Figure S4, cellular material can be observed extending into the construct pores.
FIG. 5.
Confocal images of TCPGPoreU, TCPGPoreG, and TCPGPoreGI, on day 1. Samples were stained for actin with phalloidin (green) and nuclei with DAPI (blue). Yellow asterisks represent pore spaces within the scaffold. Scale bar is 100 μm. DAPI, 4,6-diamidino-2-phenylindole.
Alkaline phosphatase activity
To evaluate the early commitment to osteogenic differentiation, ALP activity was measured on days 3, 14, and 28 (Fig. 6). On day 14, equivalent ALP activity was observed between sections of scaffolds that contained 0 wt% β-TCP (PoreU vs. PoreG, p > 0.05) and 10 wt% β-TCP (TCP10PoreU vs. TCP10PoreG, p > 0.05), while a significantly higher ALP activity was observed in the sections containing 20 wt% β-TCP when compared to the 0 wt% β-TCP sections within the same construct for groups with a β-TCP gradient (TCPGPoreG and TCPGPoreGI, p < 0.05). A temporal increase in ALP activity was observed on day 14 in groups that contained a ceramic gradient independent of the presence of a porosity gradient compared to the same sections on 3 days (TCPGPoreU, TCPGPoreG, and TCPGPoreGI, p < 0.05). Finally, peak ALP activity was observed on day 14 in groups that contained a ceramic gradient (TCPGPoreU, TCPGPoreG, and TCPGPoreGI, p < 0.05) followed by a decrease in ALP activity on day 28.
FIG. 6.
ALP activity of the β-TCP/HA/PCL constructs (Fig. 1) at 3 days (A), 14 days (B), and 28 days (C) of culture. Data are reported as the mean ± standard deviation. Top, middle, and bottom refer to the location of the section within the construct during cell culture. Within each time point, sections that do not share the same letter are significantly different. ^, *, and + indicate significant differences for the same experimental group between 3 and 14, 14 and 28, and 3 and 28 days, respectively (p < 0.05, n = 4). ALP, alkaline phosphatase.
Osteogenic differentiation
To understand the effects of physical and biochemical gradients on early and late osteogenic differentiation, quantitative reverse transcription–polymerase chain reaction (RT-PCR) for specific osteogenic markers was performed (Figs. 7–9). Maximum Runx2 expression (Fig. 7) was observed in all groups on day 3 followed by a decline for the remainder of the study. The concentration of β-TCP appeared to affect the upregulation of Runx2 on day 3. Specifically, the expression level of Runx2 trended higher in sections that contained 20 wt% β-TCP compared to sections that contained 0 wt% β-TCP within the same construct (TCPGPoreU, TCPGPoreG, and TCPGPoreGI).
FIG. 7.
Runx2-fold induction of the β-TCP/HA/PCL constructs (Fig. 1) at 3 days (A), 14 days (B), and 28 days (C) of culture. Data are reported as single measurements from four combined sections of similar constructs (n = 1). Top, middle, and bottom refer to the location of the section within the construct during cell culture.
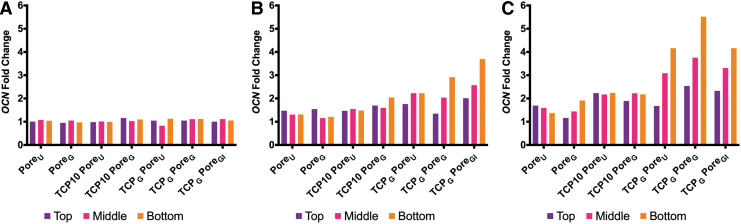
FIG. 9.
Ocn fold induction of the β-TCP/HA/PCL constructs (Fig. 1) at 3 days (A), 14 days (B), and 28 days (C) of culture. Data are reported as single measurements from four combined sections of similar constructs (n = 1). Top, middle, and bottom refer to the location of the section within the construct during cell culture. Ocn, osteocalcin.
Within the TCP10PoreG group on day 3, the highest porosity section appeared to demonstrate an upregulation of Runx2 compared to the lowest porosity section, although the effect of porosity on Runx2 expression was not observed for the PoreG group. On day 3, the expressions of late osteogenic differentiation (osteopontin [Opn; Fig. 8] and osteocalcin [Ocn; Fig. 9]) were similar. However, on day 14, scaffold sections containing 20 wt% β-TCP and the highest porosity (bottom section of TCPGPoreG) tended to have higher expression of Opn. While the apparent upregulation of Opn due to β-TCP concentration was observed after 14 days, the impact of porosity on trends in Opn expression was not observed until day 28. Specifically, at 28 days in both the PoreG and TCP10PoreG groups, sections of higher porosity tended to have upregulated Opn levels compared to sections of lower porosity.
FIG. 8.
Opn fold induction of the β-TCP/HA/PCL constructs (Fig. 1) at 3 days (A), 14 days (B), and 28 days (C) of culture. Data are reported as single measurements from four combined sections of similar constructs (n = 1). Top, middle, and bottom refer to the location of the section within the construct during cell culture. Opn, osteopontin.
Finally, a temporal increase in Opn expression was observed for sections that had at least 10 wt% β-TCP present. Regarding Ocn expression (Fig. 9), upregulation was observed in all groups after 14 days with maximum expression detected on day 28. Also, increasing concentration of β-TCP from 0 to 20 wt% resulted in an upward trend in Ocn expression after day 14.
Mineralized matrix
A calcium deposition assay (Figs. 10 and 11) was used to assess the amount of mineralized matrix, which is considered to be evidence of full osteogenic maturation.59 At all time points, no spatial differences in mineralization were observed between sections of groups with uniform porosity and composition (PoreU and TCP10PoreU, p > 0.05) (Fig. 10). At 14 days, MSCs that were seeded in sections that contained higher concentrations of β-TCP demonstrated higher levels of mineralization per cell. Specifically, layers that contained 20 wt% β-TCP had significantly higher mineralization when compared to layers that contained 0 wt% or 10 wt% β-TCP (TCPGPoreU and TCPGPoreGI, p < 0.05). In fact, a 2-fold increase of total ceramic content after the addition of β-TCP resulted in over a 10-fold increase in mineralization after 14 days (PoreU vs. TCP10PoreU).
FIG. 10.
Calcium deposition in the β-TCP/HA/PCL constructs (Fig. 1) on a per-section basis at 3 days (A), 14 days (B), and 28 days (C) of culture. Data are reported as the mean ± standard deviation. Top, middle, and bottom refer to the location of the section within the construct during cell culture. Within each time point, sections that do not share the same letter are significantly different. ^, *, and + indicate significant differences for the same experimental group between 3 and 14, 14 and 28, and 3 and 28 days, respectively (p < 0.05, n = 4).
FIG. 11.
Calcium deposition in the β-TCP/HA/PCL constructs (Fig. 1) on a per-cell basis at 3 days (A), 14 days (B), and 28 days (C) of culture. Top, middle, and bottom refer to the location of the section within the construct during cell culture. Data are reported as the mean ± standard deviation. Within each time point, sections that do not share the same letter are significantly different. ^, *, and + indicate statistical differences for the same experimental group between 3 and 14, 14 and 28, and 3 and 28 days, respectively (p < 0.05, n = 4).
Interestingly, at 14 days, there were no observable differences in mineralization between groups that only had a pore gradient compared to their respective uniform pore controls (PoreU and PoreG, and TCP10PoreU and TCP10PoreG, p > 0.05). After 28 days, the section that contained 20 wt% β-TCP and the highest porosity (bottom section of TCPGPoreG) had a higher level of mineralization (on a per cell basis) than any other section (p < 0.05) (Fig. 11). Finally, differences in mineralization were detected after 28 days between sections that contained the highest and lowest porosity in uniform ceramic and gradient porosity constructs (PoreG and TCP10PoreG, p < 0.05). Representative histological sections at 28 days are presented in Supplementary Figure S5 to visualize the amount of mineralization. It was observed that increasing concentrations of β-TCP resulted in enhanced mineralization, as noted by the enhanced black staining around the perimeter of the pores.
Discussion
In this study, porous 3D printed composite PCL-based constructs with vertical gradients in porosity and β-TCP concentration were fabricated, to create osteoblastic phenotypic gradients of MSCs for the generation of complex heterogenous constructs. Specifically, the study focused on the effects of (i) a vertical porosity gradient; (ii) a vertical ceramic gradient of β-TCP; and (iii) a combined dual porosity/ceramic gradient on the generation of phenotypic gradients of seeded MSCs by individually evaluating separate construct sections.
To this end, three different concentrations of β-TCP (0, 10, and 20 wt%) and three different porosities (33% ± 4%, 50% ± 4%, and 65% ± 3%) were investigated. The results of this study demonstrated distinct differences in the osteogenic differentiation of MSCs depending on their spatial location within graded constructs. In particular, regions with the highest concentrations of β-TCP (20 wt%) stimulated mineralization in comparison to sections with the lowest concentration of β-TCP (0 wt%) on days 14 and 28, independent of pore size. However, differences in mineralization were not observed until day 28 for regions that had differing porosities, but uniform β-TCP concentrations.
Early and late markers of osteogenic differentiation were assayed to understand the effects of β-TCP concentration and construct porosity on the osteogenic differentiation of MSCs. Specifically, ALP and Runx2 are early transient markers of osteogenic differentiation, while mineralization, Opn, and Ocn are indicative of late-stage osteogenic differentiation.60,61 The paradigmatic increase and subsequent decrease of ALP activity were observed in all groups with peak ALP activity observed on day 14. ALP activity between different construct sections was found to be directly related to the amount of β-TCP present within those sections, but independent of the porosity. This observation indicates that, during early osteogenic lineage development, β-TCP concentration more strongly influences MSC differentiation toward an osteoblastic phenotype compared to porosity.
In a previous study, Takahashi et al. demonstrated that β-TCP had a positive, dose-dependent effect on the osteogenic differentiation of MSCs that were cultured within gelatin sponges.62 Similarly, a recent study by Diaz-Gomez et al. demonstrated a dose-dependent effect of HA composition on calcium deposition when radially gradient PCL-based scaffolds were incubated in simulated body fluid. Specifically, outermost sections of 30 wt% HA/PCL demonstrated increased calcium deposition compared to middle sections of 15 wt% HA/PCL and inner sections of pure PCL.63 In this study, a dose-dependent effect of β-TCP on mineralization and ALP activity was shown. Furthermore, several studies have observed that β-TCP initiates osteogenic differentiation within 3 days.64–66 These observations corroborate with the results of this study, as significant dose-dependent upregulation of the osteogenic marker ALP was observed as early as 3 days.
Also, the further increase in osteogenic differentiation seen in sections with a higher concentration of β-TCP may be explained by the dissolution of calcium ions, which have been shown to play a critical role on the osteogenic differentiation of MSCs. Specifically, extracellular calcium activates Class C G protein-coupled calcium-sensing receptors, increasing the expression of type L voltage-gated calcium channels.67,68 This channel activation triggers a signaling cascade that leads to the upregulation of BMP-2 and thus enhanced osteogenic differentiation.69–71
No differences in ALP activity expression were observed between sections of uniform material, gradient porosity constructs (PoreG and TCP10PoreG). However, within the uniform material, gradient porosity constructs, sections with higher porosity displayed enhanced markers of late osteogenic differentiation compared to sections with lower porosity. These observations imply that porosity predominately influences late-stage osteogenic differentiation, which is characterized by the degree of mineralization, rather than early-stage osteogenic differentiation.
The mineralization of all groups monotonically increased over the course of the study. On a per-section basis, the sections with 20 wt% β-TCP (TCPGPoreU, TCPGPoreG, and TCPGPoreGI) resulted in the highest amounts of mineralized matrix again corroborating the influence of β-TCP on the osteogenic differentiation. However, normalizing to the cell number present in each section, one can see that the combination of 20 wt% β-TCP and the highest porosity (TCPGPoreG) resulted in the greatest amount of mineralized matrix per cell. This specific observation again demonstrates the influence of porosity on late-stage osteogenic differentiation. The mineralization observed in this study is similar to that of previous in vitro studies that investigated PCL-based constructs for bone tissue engineering.72–75
After 28 days in this study, regions with higher porosity within uniform composition constructs demonstrated enhanced osteogenic differentiation and mineralization, further supporting the hypothesis that porosity affects osteogenesis at later stages as has been shown previously.76–79 It has been hypothesized that the presence of larger pores facilitates the migration of cells and diffusion of bioactive molecules, leading to a more mature osteogenic phenotype.79 Matsiko et al. postulated that cell morphology can also drive the differences seen in the cellular response due to scaffold porosity since the process of differentiation is known to be dependent upon cell morphology.80,81
Another plausible explanation for the differences in the osteoblastic phenotype between sections with smaller and larger pores could be due to diffusion limitations within the smaller pores as a result of the cell aggregates present on day 1 and 3. It has been postulated that cells are more likely to remain in a pluripotent state when nutrients are limited due to diffusion.82
In gradient composition constructs (TCPGPoreU, TCPGPoreG, and TCPGPoreGI), porosity had a comparatively lower effect on markers of late-stage osteogenic differentiation than uniform composition constructs (PoreG and TCP10PoreG). This observation suggests that β-TCP concentration was the dominant factor in influencing the osteogenic differentiation of MSCs under the conditions in this study. One plausible explanation for a phenotypic gradient driven by a gradient in ceramic content, as observed in this study, is the selective adsorption of proteins and other soluble factors, whether added to the media or secreted by seeded cells, to scaffold sections of higher ceramic concentration. It has been widely demonstrated that protein adsorption to a scaffold surface is highly dependent on the physicochemical makeup of that surface.83
As applied to bone tissue formation and mineralization, proteins often demonstrate a selective affinity to surfaces with calcium phosphate moieties.84 A similar ceramic dose-dependent phenomenon was observed in the radial gradient study by Diaz-Gomez et al., where regions of higher HA composition led to increased calcium deposition, despite significantly lower porosity in the outer regions containing a high HA composition compared to inner regions with lower or no HA content.63
We have previously demonstrated how 3D printing can be leveraged to fabricate a wide range of complex multiphasic constructs.49–51 As such, the most notable result from this study is that, by altering the porosity and ceramic composition within a construct, one can direct the osteoblastic phenotype of MSCs within distinct regions of the construct to create cell phenotype gradients. Furthermore, it was observed that the spatial orientation of the sections influenced the osteogenic differentiation of adjacent layers. In fact, sections that contained 0 wt% β-TCP within compositional gradient constructs (TCPGPoreU, TCPGPoreG, and TCPGPoreGI) had a higher level of mineralization compared to 0 wt% β-TCP sections within uniform composition constructs (TCP10PoreU and TCP10PoreG).
We acknowledge that this study had several limitations. While an inverse pore gradient group (TCPGPoreGI) was included in the study, the 20 wt% β-TCP section was always located on the bottom of the construct. As a result, it is difficult to determine if the enhanced osteogenic differentiation observed in the 20 wt% β-TCP sections was due to the ceramic concentration independent of orientation. As such, it would be interesting to investigate the effects of an inverse compositional gradient to further understand this observation. We also acknowledge that the literature surrounding bone infill in tissue engineering has identified pore sizes >300 μm to be optimal for bone tissue engineering scaffolds.85 However, the scope of this study was to investigate topological changes observed during osteogenic differentiation caused by gradients in pore size and ceramic content. The results from the study demonstrate the physicochemical properties of each parameter and significant differences between different scaffold sections, highlighting the importance of gradients in tissue-engineered scaffolds.
In addition, the time points selected for this study were ideal for measuring end-stage mineralization, as previously outlined in the objectives. Therefore, since ALP and Runx2 are transient markers of osteogenic differentiation, it is possible that the time points selected did not capture global peak expression during the culture period, but rather a local maximum. However, in this study, the cellularity of the constructs was not dependent on the surface area of an individual section. This is likely due to the cell aggregates that formed within the pore spaces during the seeding of the scaffolds. While the constructs had exhibited a high rate of proliferation between days 1 and 3, after day 3, the cellularity did not change for the remainder of the study.
It is well established that cell proliferation and differentiation are inversely related processes.86 As an immature cell begins to differentiate into more a mature phenotype, especially in the presence of osteogenic cell culture supplements, there is a restriction in the cellular proliferative capacity. Ultimately, the tissue architecture and function are dependent upon the maintenance of cell cycle arrest in a terminally differentiated cell.87 Therefore, one plausible explanation for the observed plateau in cell proliferation, after 3 days, is that the cells began differentiating down an osteogenic lineage by day 3, which is supported by the increased expression observed in ALP.
Finally, as described in the Materials and Methods section, similar construct sections of the same formulation were combined for biochemical analysis by RT-PCR to ensure a sufficient amount of RNA was collected. As the reported values Runx2, Ocn, and Opn presented in this study represent the means of replicates, no inferences of statistical significance can be drawn; however, the results align with prior observations in the literature as well as the more definite indications of osteogenic differentiation (ALP activity, and particularly calcium deposition) observed in this study.
The results of this study demonstrated that both β-TCP and porosity can be leveraged to influence the osteogenic differentiation of MSCs that are seeded in a 3D system, leading to the development of heterogenous graded tissue with phenotypic gradients. Furthermore, this study underscores the importance additive manufacturing plays in further controlling and harnessing the interactions between cells and biomaterials for the generation of complex heterogenous tissues and interfaces.
Conclusion
This study demonstrates that the osteogenic differentiation of MSCs seeded on porous 3D polymeric constructs can be precisely controlled by modulating the local concentration of β-TCP and the construct porosity. Expression of osteogenic markers was stimulated by an increased concentration of β-TCP and a higher porosity for early- and late-stage osteogenesis, respectively. In particular, significantly higher ALP activity was observed in 20 wt% β-TCP sections compared to 0 wt% β-TCP sections within ceramic gradient constructs, and these constructs likewise demonstrated a characteristic temporal increase and subsequent decrease in ALP activity.
Similarly, on both day 14 and 28, sections that contained 20 wt% β-TCP had significantly higher mineralization than other sections of the same construct, while in uniform ceramic constructs, a 2-fold increase of total ceramic content resulted in over a 10-fold increase in mineralization on day 14. Finally, on day 28, significant differences in mineralization were observed between sections of highest and lowest porosity within pore gradient scaffolds. Also, the spatial arrangement of the layers, which was well controlled by a 3D printing fabrication strategy, was shown to affect the osteogenic differentiation.
The observations from this study suggest that inclusion of β-TCP in HA/PCL constructs enhances markers of both early and late osteogenic differentiation, while construct porosity additionally affects markers of late osteogenic differentiation. Furthermore, differences in construct composition and architecture yielded heterogeneous population of cells within the same construct. These results highlight the potential utility of multiphasic ceramic/polymeric composite constructs for the generation of complex heterogeneous tissues and interfaces.
Supplementary Material
Acknowledgments
We acknowledge the assistance of Gabriel Vazquez Velez for his assistance with RT-PCR.
Disclosure Statement
No competing financial interests exist.
Funding Information
Support was provided by the National Institutes of Health (P41 EB023833 and R01 AR068073) and the RegenMed Development Organization (2017-601-002) in the preparation of this work. S.M.B. and M.M.S. also acknowledge support from the National Science Foundation Graduate Research Fellowship Program. B.T.S., E.W., and E.R.M. received support from Ruth L. Kirschstein Fellowships from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (F30 AR071258), the National Institute of Dental and Craniofacial Research (F31 DE027586), and the National Cancer Institute (F31 CA213994), respectively. B.T.S., E.W., and E.R.M. acknowledge the Baylor College of Medicine Medical Scientist Training Program. L.D.-G. acknowledges Consellería de Cultura, Educación e Ordenación Universitaria for a Postdoctoral Fellowship (Xunta de Galicia, ED481B 2017/063). Finally, we acknowledge the MD Anderson Cancer Center Research Histology Core Laboratory (NCI CA16672).
Supplementary Material
References
- 1. Thibault R.A., Scott Baggett L., Mikos A.G., and Kasper F.K.. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A 16, 431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keating J.F., and McQueen M.M.. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Br 83, 3, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Habibovic P., and de Groot K.. Osteoinductive biomaterials—properties and relevance in bone repair. J Tissue Eng Regen Med 1, 25, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Lu H.H., Subramony S.D., Boushell M.K., and Zhang X.. Tissue engineering strategies for the regeneration of orthopedic interfaces. Ann Biomed Eng 38, 2142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bracaglia L.G., Smith B.T., Watson E., Arumugasaamy N., Mikos A.G., and Fisher J.P.. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater 56, 3, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bayraktar H.H., Morgan E.F., Niebur G.L., Morris G.E., Wong E.K., and Keaveny T.M.. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J Biomech 37, 27, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Oftadeh R., Perez-Viloria M., Villa-Camacho J.C., Vaziri A., and Nazarian A.. Biomechanics and mechanobiology of trabecular bone: a review. J Biomech Eng 137, 010802, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gong J.K., Arnold J.S., and Cohn S.H.. Composition of trabecular and cortical bone. Anat Rec 149, 325, 1964 [DOI] [PubMed] [Google Scholar]
- 9. Wegst U.G., Bai H., Saiz E., Tomsia A.P., and Ritchie R.O.. Bioinspired structural materials. Nat Mater 14, 23, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Sikavitsas V.I., Temenoff J.S., and Mikos A.G.. Biomaterials and bone mechanotransduction. Biomaterials 22, 2581, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Bittner S.M., Guo J.L., Melchiorri A., and Mikos A.G.. Three-dimensional printing of multilayered tissue engineering scaffolds. Mater Today 21, 861, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yousefi A.M., Hoque M.E., Prasad R.G., and Uth N.. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res A 103, 2460, 2015 [DOI] [PubMed] [Google Scholar]
- 13. Oh S.H., Kim T.H., and Lee J.H.. Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials 32, 8254, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Bittner S.M., Guo J.L., and Mikos A.G.. Spatiotemporal control of growth factors in three-dimensional printed scaffolds. Bioprinting 12, e00032, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ilkhanizadeh S., Teixeira A.I., and Hermanson O.. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 28, 3936, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Seidi A., Ramalingam M., Elloumi-Hannachi I., Ostrovidov S., and Khademhosseini A.. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater 7, 1441, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Han F., Zhou F., Yang X., Zhao J., Zhao Y., and Yuan X.. A pilot study of conically graded chitosan-gelatin hydrogel/plga scaffold with dual-delivery of TGF-beta1 and BMP-2 for regeneration of cartilage-bone interface. J Biomed Mater Res B Appl Biomater 103, 1344, 2015 [DOI] [PubMed] [Google Scholar]
- 18. Mikos A.G., Herring S.W., Ochareon P., et al. Engineering complex tissues. Tissue Eng 12, 3307, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lipner J., Shen H., Cavinatto L., et al. In vivo evaluation of adipose-derived stromal cells delivered with a nanofiber scaffold for tendon-to-bone repair. Tissue Eng Part A 21, 2766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kameda T., Koike C., Saitoh K., Kuroiwa A., and Iba H.. Developmental patterning in chondrocytic cultures by morphogenic gradients: BMP induces expression of Indian hedgehog and noggin. Genes Cells 4, 175, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Phillips J.E., Burns K.L., Le Doux J.M., Guldberg R.E., and Garcia A.J.. Engineering graded tissue interfaces. Proc Natl Acad Sci U S A 105, 12170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roy T.D., Simon J.L., Ricci J.L., Rekow E.D., Thompson V.P., and Parsons J.R.. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J Biomed Mater Res A 66, 283, 2003 [DOI] [PubMed] [Google Scholar]
- 23. O'Brien F.J., Harley B.A., Waller M.A., Yannas I.V., Gibson L.J., and Prendergast P.J.. The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol Health Care 15, 3, 2007 [PubMed] [Google Scholar]
- 24. Di Luca A., Ostrowska B., Lorenzo-Moldero I., et al. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci Rep 6, 22898, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Story B.J., Wagner W.R., Gaisser D.M., Cook S.D., and Rust-Dawicki A.M.. In vivo performance of a modified csti dental implant coating. Int J Oral Maxillofac Implants 13, 749, 1998 [PubMed] [Google Scholar]
- 26. Kruyt M.C., de Bruijn J.D., Wilson C.E., et al. Viable osteogenic cells are obligatory for tissue-engineered ectopic bone formation in goats. Tissue Eng 9, 327, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Kim K., Dean D., Mikos A.G., and Fisher J.P.. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules 10, 1810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy C.M., Haugh M.G., and O'Brien F.J.. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31, 461, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Kim S.S., Ahn K.M., Park M.S., Lee J.H., Choi C.Y., and Kim B.S.. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J Biomed Mater Res A 80, 206, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Mano J.F., Sousa R.A., Boesel L.F., Neves N.M., and Reis R.L.. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: state of the art and recent developments. Compos Sci Technol 64, 789, 2004 [Google Scholar]
- 31. Liao H.T., Lee M.Y., Tsai W.W., Wang H.C., and Lu W.C.. Osteogenesis of adipose-derived stem cells on polycaprolactone-beta-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J Tissue Eng Regen Med 10, E337, 2016 [DOI] [PubMed] [Google Scholar]
- 32. Park C.-H., Kim E.K., Tijing L.D., et al. Preparation and characterization of La/Pcl composite fibers containing beta tricalcium phosphate (B-TCP) particles. Ceram Int 40, 5049, 2014 [Google Scholar]
- 33. Yeo M., Lee H., and Kim G.. Three-dimensional hierarchical composite scaffolds consisting of polycaprolactone, beta-tricalcium phosphate, and collagen nanofibers: fabrication, physical properties, and in vitro cell activity for bone tissue regeneration. Biomacromolecules 12, 502, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Lohfeld S., Cahill S., Barron V., et al. Fabrication, mechanical and in vivo performance of polycaprolactone/tricalcium phosphate composite scaffolds. Acta Biomater 8, 3446, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Azevedo M.C., Reis R.L., Claase M.B., Grijpma D.W., and Feijen J.. Development and properties of polycaprolactone/hydroxyapatite composite biomaterials. J Mater Sci Mater Med 14, 103, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Shalumon K.T., Sowmya S., Sathish D., Chennazhi K.P., Nair S.V., and Jayakumar R.. Effect of incorporation of nanoscale bioactive glass and hydroxyapatite in PCL/Chitosan nanofibers for bone and periodontal tissue engineering. J Biomed Nanotechnol 9, 430, 2013 [DOI] [PubMed] [Google Scholar]
- 37. Guarino V., Causa F., Netti P.A., et al. The role of hydroxyapatite as solid signal on performance of PCL porous scaffolds for bone tissue regeneration. J Biomed Mater Res B Appl Biomater 86, 548, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Roohani-Esfahani S.I., Nouri-Khorasani S., Lu Z., Appleyard R., and Zreiqat H.. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites. Biomaterials 31, 5498, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Ohgushi H., Okumura M., Tamai S., Shors E.C., and Caplan A.I.. Marrow cell induced osteogenesis in porous hydroxyapatite and tricalcium phosphate: a comparative histomorphometric study of ectopic bone formation. J Biomed Mater Res 24, 1563, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Bergmann C., Lindner M., Zhang W., et al. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J Eur Ceram Soc 30, 2563, 2010 [Google Scholar]
- 41. Bergmann C.J., Odekerken J.C., Welting T.J., et al. Calcium phosphate based three-dimensional cold plotted bone scaffolds for critical size bone defects. Biomed Res Int 2014, 852610, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inzana J.A., Olvera D., Fuller S.M., et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 35, 4026, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin L., Chow K.L., and Leng Y.. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A 89, 326, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Samavedi S., Whittington A.R., and Goldstein A.S.. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater 9, 8037, 2013 [DOI] [PubMed] [Google Scholar]
- 45. Szpalski C., Wetterau M., Barr J., and Warren S.M.. Bone tissue engineering: current strategies and techniques—part I: scaffolds. Tissue Eng Part B Rev 18, 246, 2012 [DOI] [PubMed] [Google Scholar]
- 46. Spalazzi J.P., Dagher E., Doty S.B., Guo X.E., Rodeo S.A., and Lu H.H.. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A 86, 1, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Dorozhkin S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater 8, 963, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Catledge S.A., Clem W.C., Shrikishen N., et al. An electrospun triphasic nanofibrous scaffold for bone tissue engineering. Biomed Mater 2, 142, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Diaz-Gomez L., Smith B.T., Kontoyiannis P.D., Bittner S.M., Melchiorri A.J., and Mikos A.G.. Multimaterial segmented fiber printing for gradient tissue engineering. Tissue Eng Part C Methods 25, 12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bittner S.M., Smith B.T., Diaz-Gomez L., et al. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater 90, 37, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trachtenberg J.E., Placone J.K., Smith B.T., Fisher J.P., and Mikos A.G.. Extrusion-based 3D printing of poly(propylene fumarate) scaffolds with hydroxyapatite gradients. J Biomater Sci Polym Ed 28, 532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lam J., Lu S., Meretoja V.V., Tabata Y., Mikos A.G., and Kasper F.K.. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater 10, 1112, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 8, 315, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Bancroft G.N., Sikavitsas V.I., van den Dolder J., et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A 99, 12600, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lu S., Lee E.J., Lam J., Tabata Y., and Mikos A.G.. Evaluation of gelatin microparticles as adherent-substrates for mesenchymal stem cells in a hydrogel composite. Ann Biomed Eng 44, 1894, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Du Y., Liu H., Yang Q., et al. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 137, 37, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang W., Carlsen B., Wulur I., et al. BMP-2 exerts differential effects on differentiation of rabbit bone marrow stromal cells grown in two-dimensional and three-dimensional systems and is required for in vitro bone formation in a PLGA scaffold. Exp Cell Res 299, 325, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Smoak M.M., Han A., Watson E., et al. Fabrication and characterization of electrospun decellularized muscle-derived scaffolds. Tissue Eng Part C Methods 25, 276, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sikavitsas V.I., Bancroft G.N., Holtorf H.L., Jansen J.A., and Mikos A.G.. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A 100, 14683, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lian J.B., and Stein G.S.. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med 3, 269, 1992 [DOI] [PubMed] [Google Scholar]
- 61. Lian J.B., and Stein G.S.. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J 15, 118, 1995 [PMC free article] [PubMed] [Google Scholar]
- 62. Takahashi Y., Yamamoto M., and Tabata Y.. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and beta-tricalcium phosphate. Biomaterials 26, 3587, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Diaz-Gomez L., Kontoyiannis P.D., Melchiorri A.J., and Mikos A.G.. Three-dimensional printing of tissue engineering scaffolds with horizontal pore and composition gradients. Tissue Eng Part C Methods 25, 411, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smoak M., Hogan K., Kriegh L., et al. Modulation of mesenchymal stem cell behavior by nano-and micro-sized B-tricalcium phosphate particles in suspension and composite structures. J Nanopart Res 17, 182, 2015 [Google Scholar]
- 65. Marino G., Rosso F., Cafiero G., et al. Beta-tricalcium phosphate 3D scaffold promote alone osteogenic differentiation of human adipose stem cells: in vitro study. J Mater Sci Mater Med 21, 353, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Lu Z., and Zreiqat H.. Beta-tricalcium phosphate exerts osteoconductivity through alpha2beta1 integrin and down-stream MAPK/Erk signaling pathway. Biochem Biophys Res Commun 394, 323, 2010 [DOI] [PubMed] [Google Scholar]
- 67. Wen L., Wang Y., Wang H., et al. L-Type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem Biophys Res Commun 424, 439, 2012 [DOI] [PubMed] [Google Scholar]
- 68. Aquino-Martínez R., Artigas N., Gámez B., Rosa J.L., and Ventura F.. Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on Smad signalling. PLoS One 12, e0178158, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Surmenev R.A., Surmeneva M.A., and Ivanova A.A.. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—a review. Acta Biomater 10, 557, 2014 [DOI] [PubMed] [Google Scholar]
- 70. dos Santos E.A., Farina M., Soares G.A., and Anselme K.. Chemical and topographical influence of hydroxyapatite and β-tricalcium phosphate surfaces on human osteoblastic cell behavior. J Biomed Mater Res A 89A, 510, 2009 [DOI] [PubMed] [Google Scholar]
- 71. Barradas A.M.C., Fernandes H.A.M., Groen N., et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 33, 3205, 2012 [DOI] [PubMed] [Google Scholar]
- 72. Heo S.J., Kim S.E., Wei J., et al. Fabrication and characterization of novel nano-and micro-HA/PCL composite scaffolds using a modified rapid prototyping process. J Biomed Mater Res A 89, 108, 2009 [DOI] [PubMed] [Google Scholar]
- 73. Williams J.M., Adewunmi A., Schek R.M., et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 26, 4817, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Park S.A., Lee S.H., and Kim W.D.. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess Biosyst Eng 34, 505, 2011 [DOI] [PubMed] [Google Scholar]
- 75. Park J., Lee S.J., Jo H.H., et al. Fabrication and characterization of 3D-printed bone-like B-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J Ind Eng Chem 46, 175, 2017 [Google Scholar]
- 76. Kim K., Yeatts A., Dean D., and Fisher J.P.. Stereolithographic bone scaffold design parameters: osteogenic differentiation and signal expression. Tissue Eng Part B Rev 16, 523, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huri P.Y., Ozilgen B.A., Hutton D.L., and Grayson W.L.. Scaffold pore size modulates in vitro osteogenesis of human adipose-derived stem/stromal cells. Biomed Mater 9, 045003, 2014 [DOI] [PubMed] [Google Scholar]
- 78. Kasten P., Beyen I., Niemeyer P., Luginbühl R., Bohner M., and Richter W.. Porosity and pore size of B-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: an in vitro and in vivo study. Acta Biomater 4, 1904, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Kim K., Dean D., Wallace J., Breithaupt R., Mikos A.G., and Fisher J.P.. The influence of stereolithographic scaffold architecture and composition on osteogenic signal expression with rat bone marrow stromal cells. Biomaterials 32, 3750, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Matsiko A., Gleeson J.P., and O'Brien F.J.. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng Part A 21, 486, 2014 [DOI] [PubMed] [Google Scholar]
- 81. Harris G.M., Piroli M.E., and Jabbarzadeh E.. Deconstructing the effects of matrix elasticity and geometry in mesenchymal stem cell lineage commitment. Adv Funct Mater 24, 2396, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McMurtrey R.J. Roles of diffusion dynamics in stem cell signaling and three-dimensional tissue development. Stem Cells Dev 26, 1293, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rabe M., Verdes D., and Seeger S.. Understanding protein adsorption phenomena at solid surfaces. Adv Colloid Interface Sci 162, 87, 2011 [DOI] [PubMed] [Google Scholar]
- 84. Kretlow J.D., and Mikos A.G.. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng 13, 927, 2007 [DOI] [PubMed] [Google Scholar]
- 85. Karageorgiou V., and Kaplan D.. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26, 5474, 2005 [DOI] [PubMed] [Google Scholar]
- 86. Stein G.S., Lian J.B., and Owen T.A.. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J 4, 3111, 1990 [DOI] [PubMed] [Google Scholar]
- 87. Zhu L., and Skoultchi A.I.. Coordinating cell proliferation and differentiation. Curr Opin Genet Dev 11, 91, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.