Abstract
The Veterans Aging Cohort Study Index (VACS Index) is an index comprised of routine clinical laboratory tests that accurately and generalizably predicts all-cause mortality among those living with and without HIV infection. Increasing evidence supports its use as a measure of physiologic frailty among those aging with HIV because of its associations with frailty related outcomes including mortality, hospitalization, fragility fractures, serious falls, pneumonia, cognitive decline, delirium, and functional decline. In this review, we explore the evidence supporting the validity (construct, correlative, and predictive), responsiveness, and feasibility of the VACS Index as an early indicator of physiologic frailty. We also consider its limitations.
Keywords: HIV, epidemiology, antiretroviral therapy
Background
Frailty, a core concept in geriatrics, is defined by its prognostic implication; it is the decreased ability to recover from injury or a loss of reserve capacity indicating that a relatively minor stress may result in future, disproportionate, adverse health outcomes.1,2 Frailty-related outcomes include mortality, hospitalization, fragility fractures, serious falls, pneumonia, cognitive decline, delirium, and functional decline. Early frailty should trigger a full geriatric assessment to identify reversible causes.2 Advanced frailty suggests the need to avoid stressors, including aggressive medical treatments,2 which are likely to result in adverse, frailty-related health outcomes. Frailty increases with advancing chronologic age. However, frailty also differentiates risk of adverse health outcomes among those of similar age, that is, the difference between biologic and chronologic age.3 The etiology of frailty has not been definitively established, but is thought to result from the cumulative effects of molecular and cellular defects, chronic inflammation, immune exhaustion, sarcopenia, and cognitive decline.1,4–7 Corresponding to these hypothesized etiologies, specific frailty associated biomarkers have been proposed.8–11
For people aging with HIV (PAWH), major physiologic stresses occur at earlier ages. HIV infection and its treatment lead to microbial translocation, immune dysfunction, other viral coinfections, HIV-associated non-AIDS conditions, mitochondrial toxicity, and polypharmacy.12–15 In addition, PAWH are more likely to use harmful substances, including alcohol, tobacco, and other drugs of abuse.16 As a result, PAWH often develop early signs of frailty well before their seventh or eighth decade.8,17–25 PAWH with more advanced frailty may be susceptible to harm from aggressive medical management, including polypharmacy.
Multiple approaches to measuring frailty have been proposed. Fried describes a phenotype comprising any three of the following: weight loss, low physical activity, exhaustion, slowness (time to walk 15 feet), and weakness (by grip strength).26 Rockwood proposed an accumulation of deficits, measured as proportion present of 30 or more conditions.27 However, both the phenotype and accumulation of deficit approaches have limitations, in general and for PAWH.
In general, frailty measures lack standardization, have limited reproducibility, have limited agreement across measures, and have limited responsiveness to interventions.2,6,28 In addition, studies rarely consider the extent to which the frailty measure used captures differences between chronologic and biologic age by either controlling for or stratifying by age.29
Among PAWH, the Fried Frailty Phenotype and adaptations of it have been evaluated more than the Rockwood Accumulation of Deficits. When the Frailty Phenotype has been applied to PAWH, few patients demonstrate the full phenotype. Rather patients typically demonstrate only one or two of the criteria causing many studies to focus on “pre-frailty” rather than frailty.17,21,30,31
Because of the above limitations, many have proposed specific frailty associated biomarkers.2,9–11 But these biomarkers are not routinely available in clinical care. Investigators have also suggested that an index of biomarkers may provide an early indication of frailty (physiologic frailty) and might address limitations of previous measures—particularly if the biomarkers used are routinely measured. Of note, frailty is considered distinct from comorbidity and functional status, yet measures of comorbidity and/or functional status are included in over half the frailty metrics in common use, including the Frailty Phenotype which includes function.6 Consistent with this observation, some investigators have suggested that if the intended use is prognostic, it may be reasonable to include variables such as comorbidity or age known to improve accuracy of prediction for frailty associated outcomes.6
We have developed, and widely validated, using established measures of accuracy and generalizability (Appendix), the Veterans Aging Cohort Study Index (VACS Index), a physiologically-based index for predicting mortality. VACS Index is based on routine laboratory measures and age and has been shown to effectively discriminate risk of mortality among a wide variety of PAWH and several groups of uninfected individuals. Increasing evidence also supports its strong cross-sectional association with biomarkers and its predictive associations with other outcomes, considered indicative of frailty. As a result, many have suggested that the VACS Index may serve as a useful measure of frailty among PAWH.4,17,18,30,32–36 In this review, we explore the evidence supporting the validity (construct, correlative, and predictive), responsiveness, and feasibility of the VACS Index as an early indicator of physiologic frailty for people aging with and without HIV. We also consider its limitations.
Construct Validity
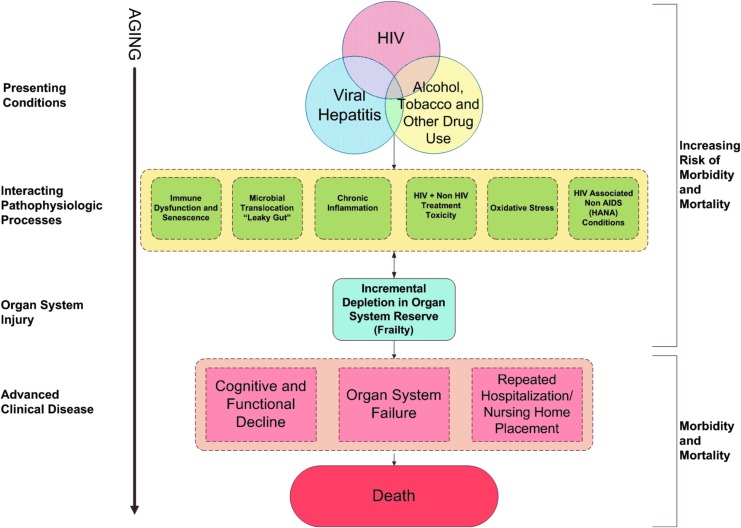
Our original conceptualization of the VACS Index was as an early summary indicator of multisystem injury reflecting reduced physiologic reserve across major organ systems (Fig. 1). Using an accumulation of deficit approach,27 and in accordance with a previous laboratory based frailty measure,9 we used a variety of clinical laboratory tests. A score is calculated based on preassigned points for routinely monitored, Clinical Laboratory Improvement Amendments (CLIA) certified (highly reproducible and standardized) laboratory tests (Table 1).37 Higher scores indicate increasing risk of mortality. Importantly, this approach is agnostic to specific disease diagnoses, which are susceptible to biases in ascertainment, and does not rely on self-report of health behaviors which can be susceptible to social desirability bias.38,39
FIG. 1.
Conceptual model for VACS Index. VACS, Veterans Aging Cohort Study.
Table 1.
Veterans Aging Cohort Study Index 1.0 Components and Weights
| Component | Level | Points |
|---|---|---|
| Age (years) | <50 | 0 |
| 50–64 | 12 | |
| ≥65 | 27 | |
| CD4 (cells/mm3) | ≥500 | 0 |
| 350–499 | 6 | |
| 200–349 | 6 | |
| 100–199 | 10 | |
| 50–99 | 28 | |
| <50 | 29 | |
| HIV-1 RNA (log copies/mL) | <500 | 0 |
| 500–99,999 | 7 | |
| ≥1 × 105 | 14 | |
| Hemoglobin (g/dL) | ≥14 | 0 |
| 12–13.9 | 10 | |
| 10–11.9 | 22 | |
| <10 | 38 | |
| FIB-4 | <1.45 | 0 |
| 1.45–3.25 | 6 | |
| >3.25 | 25 | |
| eGFR (mL/min) | >60 | 0 |
| 45–59.9 | 6 | |
| 30–44.9 | 8 | |
| <30 | 26 | |
| HCV coinfection | 5 |
eGFR, estimated glomerular filtration rate.
Nevertheless, in its original form, VACS Index (1.0) (Table 1) had important limitations. It categorized predictor variables, limiting its resolution and ability to respond to small changes within an individual. It was overly parsimonious; other routine laboratory tests were thought likely to increase its discrimination of mortality. We recently updated VACS Index 1.0–2.0 by adding albumin, white blood cell count, and body mass index (BMI) and treating all variables as continuous with somewhat complex functional forms (Table 2). While discrimination of the index improved with these modifications, our primary goal was to improve its sensitivity and resolution for detecting changes over time, thereby enhancing its utility for patient care and clinical research.
Table 2.
Veterans Aging Cohort Study Index 2.0 Cox Proportional Hazards Model, for 5-Year All-Cause Mortality, Estimated in Veterans Aging Cohort Study
| n | 28,390 | |||||
|---|---|---|---|---|---|---|
| Deaths | 7,293 | |||||
| Parameter | PE | SE | χ2 | p | HR | 95% CI |
| Age (years), censored at 30–75, centered at (age—50) | ||||||
| X | 0.056 | 0.012 | 22 | <.0001 | 1.06 | 0.00–0.00 |
| X2 | −0.004 | 0.004 | 2 | .22 | 1.00 | 0.00–0.00 |
| X3 | 0.005 | 0.001 | 29 | <.0001 | 1.01 | 0.00–0.00 |
| CD4 cell count (cells/mL), censored at 0–1,000, as ln (1,000-CD4) | ||||||
| X | −0.056 | 0.025 | 5 | .03 | 0.95 | 0.00–0.00 |
| X2 | −0.153 | 0.023 | 46 | <.0001 | 0.86 | 0.00–0.00 |
| X3 | 0.024 | 0.002 | 94 | <.0001 | 1.02 | 0.00–0.00 |
| HIV-1 RNA (log copies/mL), censored at 1.3–5.0, centered at (logVL—2) | ||||||
| X | 0.513 | 0.033 | 247 | <.0001 | 1.67 | 0.00–0.00 |
| X2 | −0.422 | 0.041 | 109 | <.0001 | 0.66 | 0.00–0.00 |
| X3 | 0.098 | 0.011 | 77 | <.0001 | 1.10 | 0.00–0.00 |
| Hemoglobin (g/dL), censored at 9–16, centered at (14—hemoglobin) | ||||||
| X | −0.134 | 0.011 | 141 | <.0001 | 0.88 | 0.00–0.00 |
| X2 | 0.026 | 0.006 | 16 | <.0001 | 1.03 | 0.00–0.00 |
| X3 | 0.005 | 0.001 | 10 | .002 | 1.01 | 0.00–0.00 |
| FIB-4, censored at 0.5–7.5 | ||||||
| X | 0.220 | 0.028 | 62 | <.0001 | 1.25 | 0.00–0.00 |
| X2 | −0.009 | 0.003 | 7 | .008 | 0.99 | 0.00–0.00 |
| eGFR (mL/min), censored at 0–180a | ||||||
| X1 | −0.031 | 0.028 | 1 | .28 | 0.97 | 0.00–0.00 |
| X2 | −0.077 | 0.045 | 3 | .0917 | 0.93 | 0.00–0.00 |
| X3 | 0.106 | 0.027 | 16 | <.0001 | 1.11 | 0.00–0.00 |
| X4 | 0.133 | 0.034 | 15 | .0001 | 1.14 | 0.00–0.00 |
| Hepatitis C coinfection | ||||||
| Yes | 0.342 | 0.028 | 147 | <.0001 | 1.41 | 0.00–0.00 |
| Albumin (g/dL), censored at 2–5, centered at (albumin—4) | ||||||
| X | −0.443 | 0.034 | 165 | <.0001 | 0.64 | 0.00–0.00 |
| X2 | 0.104 | 0.051 | 4 | .04 | 1.11 | 0.00–0.00 |
| X3 | 0.028 | 0.027 | 1 | .30 | 1.03 | 0.00–0.00 |
| White blood count (k/mL), censored at 2.5–11, centered at (WBC—5.5) | ||||||
| X | 0.126 | 0.011 | 130 | <.0001 | 1.13 | 0.00–0.00 |
| X2 | 0.020 | 0.004 | 30 | <.0001 | 1.02 | 0.00–0.00 |
| X3 | −0.004 | 0.001 | 23 | <.0001 | 1.00 | 0.00–0.00 |
| BMI, kg/m2, censored at 15–35, centered at (BMI—25) | ||||||
| X | −0.055 | 0.003 | 388 | <.0001 | 0.95 | 0.00–0.00 |
| X2 | 0.004 | 0.000 | 62 | <.0001 | 1.00 | 0.00–0.00 |
X1 = eGFR/10, X2 = (eGFR-35)/10, X3 = (eGFR-65)/10, X4 = (eGFR-115)/10.
BMI, body mass index; CI, confidence interval; HR, hazard ratio; PE, parameter estimate; SE, standard error; WBC, white blood cell.
In support of construct validity, several variables included in the VACS Index were previously included in the laboratory-based Frailty Index proposed by Blodgett et al.9 These include albumin, creatinine, hemoglobin, platelets, and liver function tests (we use aspartate and alanine transaminase [AST and ALT]; they used alkaline phosphatase, lactate dehydrogenase, and bilirubin). Consistent with the wasting construct in the Frailty Phenotype, VACS Index 2.0 adds BMI. Immune exhaustion an important manifestation of frailty, especially among PAWH,8,40,41 is represented by white blood cell count and CD4 cell count.42 Because hepatitis C virus (HCV), a common coinfection among PAWH, exacerbates inflammation, as well as directly causes injury to the liver and kidneys, we include an indicator for chronic HCV.43–45
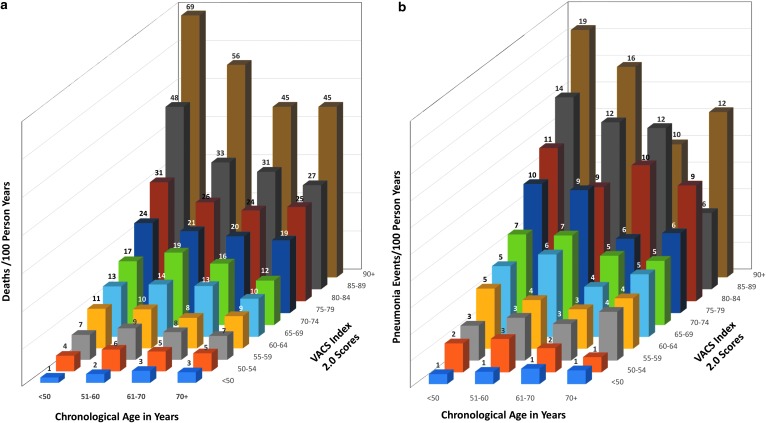
Finally, age independently predicts frailty associated biomarkers and outcomes and also modifies many other predictors. For example, age is incorporated in the estimation of liver fibrosis calculated with FIB-4, which includes AST, ALT, platelets, and age,46 and in the estimation of creatinine clearance using creatinine and age.47 To maximize discrimination and calibration, we retain age in the index and consider whether the VACS Index discriminates risk of frailty related outcomes among individuals of similar chronologic age (Fig. 2).
FIG. 2.
(a) Mortality by age and VACS Index 2.0. (b) Pneumonia events by age and VACS Index 2.0.
Correlative Validity
The VACS Index is strongly associated with a host of biomarkers and functional tests considered reflective of frailty.32,34,36,48 Among PAWH, the index is correlated with markers of chronic inflammation, microbial translocation, and hypercoagulability (cystatin C, tumor necrosis factor alpha, interleukin-6, soluble CD14, soluble CD163, and D-dimer) in resource rich49–52 and in resource limited settings.53 VACS Index is more strongly associated with biomarkers of hypercoagulability than the Framingham Index.51,53 The Index is associated with the Chronic Immune Activation and Senescence (CIADIS) score, composed of CD4 and CD8 activation, naive and terminally differentiated memory T cells, and CD57CD28 cells weighted by principal component analyses.54 The VACS Index is also associated with concurrent measures of neurocognitive test performance,55 functional performance,56,57 sarcopenia,58 and autonomic neuropathy.59
Predictive Validity
While mortality is not the only adverse health outcome associated with frailty, it is objective, important, and highly patient relevant. We first consider predictive validity of the VACS Index for mortality among PAWH and uninfected. We then address prognostic validity for other important frailty-related outcomes, including: hospitalization, falls, fractures, cognitive decline, delirium, and functional decline.
Mortality among PAWH
VACS Index 1.0 was developed in veteran patients,37 and its accuracy has been validated in other PAWH in North America and Europe.37,60 It discriminates risk of mortality more effectively than an index restricted to CD4 count, HIV-1 RNA, and age especially among those with undetectable HIV-1 RNA and those 50 or more years of age.37,60 The accuracy (discrimination and calibration) of the Index for predicting mortality among PAWH meets or exceeds that reported for indices currently used in clinical practice.61–63 VACS Index is consistently accurate for any length of time on antiretroviral treatment and is robust among important subgroups, including women, people of color, those with HCV coinfection, and those over 50 years of age.36,37,60 It also discriminates risk of mortality among young active duty military who are relatively free of comorbid disease64 and among highly frail individuals initiating salvage antiretroviral therapy (ART).65 It predicts cardiovascular mortality as accurately as all-cause mortality.66
Discrimination improved and resolution increased in VACS Index 2.0. Its generalizability was demonstrated in a European and North American cross cohort collaboration, the Antiretroviral Therapy Cohort Collaboration (ART-CC).42 Compared with VACS Index 1.0, improvements in discrimination were seen across all cohorts and in subgroups defined by age, gender, race/ethnicity, HIV RNA suppression, HCV status, and among low versus high risk patients. Of note, VACS Index 1.0 and 2.0 scores are 86% correlated, suggesting that work demonstrating the validity of 1.0 with respect to cross-sectional associations with biomarkers and predictive associations with hospitalizations, falls, cognitive function, and physical function likely applies to 2.0.
Mortality among PAWH of similar chronologic age
An important validity check when considering a frailty metric is whether it differentiates risk of frailty related, adverse health outcomes among individuals of similar chronologic age. We calculated observed mortality rates for PAWH stratified by age and VACS Index score (Fig. 2a), using data from the article originally reporting VACS Index 2.0,42 updated to 2018. For those 60–69 years of age overall observed mortality was 8.5 deaths/100 person-years. After stratifying by VACS Index score, observed mortality ranged from 3.3 deaths/100 PY to 45 deaths/100 PY or in other words from less than half to more than five times the overall rate in those aged 65–69. Discrimination of risk was even greater for younger individuals. VACS Index 2.0 successfully differentiates risk of mortality among PAWH of similar chronologic age over a wide range of age strata.
Mortality among uninfected individuals
If you assume that those without HIV infection have no HIV-1 RNA and a CD4 cell count above 500 cells/mm3 (i.e., a normal value), the VACS Index predicts mortality among those without HIV equally well as among PAWH. This has been shown for 30-day mortality after hospitalization that included medical intensive care67 and for long-term (median of 5 years) mortality68 within the Veterans Administration Healthcare System and for hospitalization and mortality among women with and without HIV infection in the Women's Interagency HIV Study.36,69 In the next section, we provide additional support for the accuracy of the VACS Index 2.0 in uninfected individuals compared with other risk indices.
Accuracy of predicted mortality exceeds other frailty and comorbidity metrics
Compared with an adapted version of the frailty related phenotype, the VACS Index more accurately predicted both all-cause mortality among PAWH and uninfected individuals.17,36 VACS Index 2.0 discriminates risk of all-cause mortality substantially better than the Care Assessment Need Score70 among veterans with HIV infection.71 More recent work in the Million Veteran Cohort, a general cohort of veterans in care volunteering for genetic research,72 demonstrated that VACS Index 2.0 discriminates risk of mortality more effectively than the Charlson Comorbidity Index (based on ICD coded diagnoses and age73). This was true overall, among those over 45 years of age, among those with and without HCV infection, and among all racial and ethnic groups considered. There was no subgroup evaluated in which Charlson discriminated better than VACS Index 2.0. Similarly, among HIV infected and uninfected patients with cancer, the VACS Index discriminates risk of mortality better than the Charlson Index.74 Given the long-standing use of Charlson Index in geriatric research, this finding is remarkable.
Other frailty-related outcomes
The VACS Index predicts other important frailty related adverse health outcomes, including hospitalization,17,69,75 medical intensive care unit admission,75 serious falls,76 fragility fractures,33,77 community acquired pneumonia,78 neurocognitive compromise,55,79–82 delirium, and functional decline. The index differentiates rates of pneumonia within age groups (Fig. 2b). When used in a time updated manner, the VACS Index predicts acute myocardial infarction better than time updated CD4 or HIV-1 RNA.83 The index also predicted length of stay and readmission after hospitalization for bacterial pneumonia among HIV infected and uninfected (age 50+ years) veterans.84 Finally, the VACS Index predicted delirium in the hospital setting among PAWH and uninfected individuals.85
Differentiation by exposures and response to change
VACS Index scores also differ by important exposures, including level of smoking, alcohol consumption, and hypertension.48,86,87 Values differ by number of non-antiretroviral (ARV) medications and physical function. Consistent with detecting reversible frailty, higher scores predict greater weight gain in the first 12 months after ART initiation.88
VACS Index scores also respond to important changes in health and health behaviors. Scores change in response to antiretroviral initiation89 and interruption64 and discriminate among levels of ART adherence.89 Changes in score correspond to changes in neurocognitive function.80 When levels of alcohol consumption change among HIV infected subjects, the score changes.90,91 Similarly, when HIV infected patients in treatment for substance abuse have positive urine toxicology screens, their scores are higher than when similar patients have negative toxicology screens (article in press). Because VACS Index scores rise during negative health behaviors (alcohol and stimulant use), it is likely that successful interventions in these domains would alter the VACS Index Score.
Feasibility
The VACS Index is in widespread use in research and clinical care. Online calculators can be found at the VACS website (http://vacs.med.yale.edu) and MDCalc (https://www.mdcalc.com/veterans-aging-cohort-study-vacs-index). These calculators have been accessed 88,000 times. In observational studies, the VACS Index has been used as a measure of frailty and as an adjustment for severity of illness.32,50,51,55,59,78,81,92–99 NIH funded alcohol intervention trials have included the VACS Index as an outcome100 and another is underway. The AIDS Clinical Trials Group has used the VACS Index in a randomized trial.101 The Public Health-Seattle & King County, HIV/STD Program and the Washington State Department of Health are using the VACS Index to monitor risk of mortality and burden of disease among PAWH.
Several health systems have incorporated the VACS Index as a decision support tool in their electronic health record, including: Fenway Healthcare System in Boston; the San Francisco General Hospital HIV clinic; and University of California, San Diego Owen Clinic. The latter has incorporated the index into their live HIV registry and reviews VACS Index scores on their patients during care team meetings. Providers caring for over 60,000 HIV infected patients (and nearly 600,000 uninfected patients) in 79 health care sites in the Observational Pharmaco-epidemiology Research & Analysis (OPERA) database directly access VACS Index scores for PAWH and uninfected individuals through a physician portal. Information provided includes VACS Index score trends over time, overall and for each component of the index. Some sites use these data to target care to their sickest patients, while others use it in provider meetings to discuss overall patient management. The VACS Index is calculated on every patient seen at the University of Modena Metabolic Clinic (a clinic of over 4,000 HIV patients in Modena, Italy) using an automated application.
Limitations
While the VACS Index has demonstrated strong construct, cross-sectional, and predictive validity as a measure of physiologic frailty, it is not a substitute for geriatric assessment. Rather, the VACS Index score might provide a useful indicator for when a full geriatric assessment is indicated. Furthermore, other measures, including medication count, physical and cognitive function, and selected diagnoses, might improve the ability of the index to identify those developing early signs of frailty or prefrailty. For example, while the Charlson Index demonstrated poorer discrimination than did the VACS Index, a model which included both Charlson and VACS Index components discriminated risk of mortality better than either alone.73 Although calibration of scores for VACS 1.0 is available, we are currently developing calibration curves for VACS Index 2.0 that will provide estimates of expected mortality over specified intervals of time from less than 1 year up to 10 years. Furthermore, we are still working on how to incorporate sustained viral response to HCV treatment and how to optimally handle missing data since healthier patients are less likely to have all required laboratory values for the VACS Index.
Conclusion
A “one size fits all” frailty index may not be a realistic goal. Rather, the frailty measure should be chosen based on its intended application. The VACS Index 2.0 may be a useful indicator of physiologic frailty indicating the need for more attentive management or a complete geriatric assessment. The VACS Index may also help alert providers and patients when life expectancy is short and end of life planning is indicated. The index reproducibly and generalizably discriminates risk of mortality in a wide variety of patient settings and it outperforms other general risk indices. It also predicts other frailty-associated outcomes, is cross-sectionally associated with frailty associated biomarkers, and differentiates risk of mortality and other frailty related outcomes among individuals of similar chronologic age. VACS Index 2.0 is based on readily available, standardized, clinical laboratory tests that are frequently obtained in routine care. Now that electronic health records make it easy to access these values and to automatically calculate scores using a validated algorithm, the VACS Index provides a practical and effective method to assess physiologic frailty.
Appendix
Accuracy and Generalizability
Accuracy has two components, discrimination and calibration.A1 Discrimination is the ability to accurately rank individuals according to risk. It is commonly measured using C statistics. A C statistic of 0.50 corresponds to no discrimination, and C statistic of 1.00 corresponds to perfect discriminationA2–A4; C statistics of 0.50–0.69 are considered fair, 0.70–0.79 are good, and >0.80 are excellent. Once a model has achieved good to excellent discrimination, it is difficult to improve the C statistic, since prognostic variables often covary. A change in c-statistic of 0.01 is usually clinically meaningful. Veterans Aging Cohort Study (VACS) Index 2.0 has demonstrated improved discrimination on the order of 0.02–0.03 over VACS Index 1.0, which previously demonstrated improved discrimination on the order of 0.05–0.10 over an index restricted to age, CD4 cell count, and HIV-1 RNA. Thus, the VACS Index offers substantial differentiation of risk controlling for age.
Calibration is the ability to consistently and accurately predict probability of an outcome over some specified interval of time. Calibration is often the more important component when using predictive indices in patient management. It can be evaluated using calibration curves or Kaplan–Meier Plots. VACS Index 1.0 has demonstrated consistent calibration, but this has yet to be determined for VACS Index 2.0.
Generalizability is the ability to be consistently accurate in a new, but plausibly related, sample.A1 VACS Index 1.0 predicts all-cause mortality in a wide range of HIV infected populations, including those first initiating antiretroviral therapy (ART),A5 after the first year of ART,A6,A7 among highly treatment experienced patientsA8 and among young military recruits.A9
Appendix References
- A1. Justice AC, Covinsky KE, Berlin JA: Assessing the generalizability of prognostic information. Ann Intern Med 1999;130:515–524 [DOI] [PubMed] [Google Scholar]
- A2. Harrell FE, Lee KL: A comparison of the discrimination of discriminant analysis and logistic regression under multivariate normality. In: Biostatistics: Statistics in Biomedical, Public Health and Environmental Sciences (Sen PK, ed.) Elsevier Science Publishers B.V., North-Holland, 1985, pp. 333–343
- A3. Harrell FE, Lee KL, Mark DB: Tutorial in biostatistics. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387 [DOI] [PubMed] [Google Scholar]
- A4. Hosmer DW, Lemeshow S: Applied Logistic Regression, 2nd ed. John Wiley & Sons, Inc., New York, 2000
- A5. Justice AC, McGinnis KA, Skanderson M, et al. : Towards a combined prognostic index for survival in HIV infection: The role of ‘non-HIV’ biomarkers. HIV Med 2009;11:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A6. Tate JP, Justice AC, Hughes MD, et al. : An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013;27:563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A7. Justice AC, Modur SP, Tate JP, et al. : Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: A North American cross cohort analysis. J Acquir Immune Defic Syndr 2013;62:149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A8. Brown ST, Tate JP, Kyriakides TC, et al. : The VACS index accurately predicts mortality and treatment response among multi-drug resistant HIV infected patients participating in the options in management with antiretrovirals (OPTIMA) study. PLoS One 2014;9:e92606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A9. Bebu I, Tate J, Rimland D, et al. : The VACS Index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2014;65:226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The following funding was received from the National Institute on Alcohol Abuse and Alcoholism (NIAAA): U10 AA013566, U24 AA020794, U01 AA020790, and U24 A022001.
References
- 1. Walston J, Hadley EC, Ferrucci L, et al. : Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006;54:991–1001 [DOI] [PubMed] [Google Scholar]
- 2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K: Frailty in elderly people. Lancet 2013;381:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitnitski A, Howlett SE, Rockwood K: Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci 2017;72:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hosaka KRJ, Greene M, Premeaux TA, et al. : Geriatric syndromes in older adults living with HIV and cognitive impairment. J Am Geriatr Soc 2019;67:1913–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faller JW, Pereira DDN, de Souza S, Nampo FK, Orlandi FS, Matumoto S: Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS One 2019;14:e0216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buta BJ, Walston JD, Godino JG, et al. : Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016;26:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson D, Jackson T, Sapey E, Lord JM: Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev 2017;36:1–10 [DOI] [PubMed] [Google Scholar]
- 8. Erlandson KM, Ng DK, Jacobson LP, et al. : Inflammation, immune activation, immunosenescence, and hormonal biomarkers in the frailty-related phenotype of men with or at risk for HIV infection. J Infect Dis 2017;215:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blodgett JM, Theou O, Howlett SE, Rockwood K: A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geroscience 2017;39:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calvani R, Marini F, Cesari M, et al. : Biomarkers for physical frailty and sarcopenia. Aging Clin Exp Res 2017;29:29–34 [DOI] [PubMed] [Google Scholar]
- 11. Mitnitski A, Collerton J, Martin-Ruiz C, et al. : Age-related frailty and its association with biological markers of ageing. BMC Med 2015;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. High KP, Brennan M, Clifford D, et al. : HIV and aging: State of knowledge and areas of critical need for research. A Report to the NIH office of AIDS research by the HIV and aging working group. J Acquir Immune Defic Syndr 2012;60 Suppl 1:S1–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Escota GV, O'Halloran JA, Powderly WG, Presti RM: Understanding mechanisms to promote successful aging in persons living with HIV. Int J Infect Dis 2018;66:56–64 [DOI] [PubMed] [Google Scholar]
- 14. Harris TG, Rabkin M, El-Sadr WM: Achieving the fourth 90: Healthy aging for people living with HIV. AIDS 2018;32:1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC: The next therapeutic challenge in HIV: Polypharmacy. Drugs Aging 2013;30:613–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park LS, Hernandez-Ramirez RU, Silverberg MJ, Crothers K, Dubrow R: Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: A meta-analysis. AIDS 2016;30:273–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akgun KM, Tate JP, Crothers K, et al. : An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr 2014;67:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akgun KM, Tate JP, Oursler KK, et al. : Association of chronic obstructive pulmonary disease with frailty measurements in HIV-infected and uninfected Veterans. AIDS 2016;30:2185–2193 [DOI] [PubMed] [Google Scholar]
- 19. Althoff KN, Jacobson LP, Cranston RD, et al. : Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014;69:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Desquilbet L, Jacobson LP, Fried LP, et al. : A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci 2011;66:1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erlandson KM, Wu K, Koletar SL, et al. : Association between frailty and components of the frailty phenotype with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017;215:933–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greene M, Justice AC, Covinsky KE: Assessment of geriatric syndromes and physical function in people living with HIV. Virulence 2017;8:586–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onen NF, Overton ET: A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Curr Aging Sci 2011;4:33–41 [PubMed] [Google Scholar]
- 24. Pathai S, Gilbert C, Weiss HA, et al. : Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr 2013;62:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piggott DA, Muzaale AD, Mehta SH, et al. : Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013;8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G: Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–263 [DOI] [PubMed] [Google Scholar]
- 27. Rockwood K, Bergman H: Frailty: A Report from the 3(rd) Joint Workshop of IAGG/WHO/SFGG, Athens, January 2012. Can Geriatr J 2012;15:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dent E, Kowal P, Hoogendijk EO: Frailty measurement in research and clinical practice: A review. Eur J Intern Med 2016;31:3–10 [DOI] [PubMed] [Google Scholar]
- 29. Stone ME, Lin J, Dannefer D, Kelley-Moore JA: The continued eclipse of heterogeneity in gerontological research. J Gerontol B Psychol Sci Soc Sci 2017;72:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blanco JR, Barrio I, Ramalle-Gomara E, et al. : Gender differences for frailty in HIV-infected patients on stable antiretroviral therapy and with an undetectable viral load. PLoS One 2019;14:e0215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Engel T, Raffenberg M, Marzolini C, et al. : HIV and aging—Perhaps not as dramatic as we feared? Gerontology 2018;64:446–456 [DOI] [PubMed] [Google Scholar]
- 32. Escota G, Patel P, Brooks JT, et al. : Short communication: The VACS Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses 2015;31:313–317 [DOI] [PubMed] [Google Scholar]
- 33. Womack JA, Goulet JL, Gibert C, et al. : Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis 2013;56:1498–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thurn M, Gustafson DR: Faces of frailty in aging with HIV infection. Curr HIV/AIDS Rep 2017;14:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shahani L, Breaux K, Lin M, Marcelli M, Rodriguez-Barradas M: Veterans aging cohort study index as a marker of bone disease in HIV-infected patients. AIDS Res Hum Retroviruses 2019 [Epub ahead of print]; DOI: 10.1089/AID.2019.0155 [DOI] [PubMed]
- 36. Gustafson DR, Shi Q, Holman S, et al. : Predicting death over 8 years in a prospective cohort of HIV-infected women: The Women's Interagency HIV Study. BMJ Open 2017;7:e013993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tate JP, Justice AC, Hughes MD, et al. : An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013;27:563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bajunirwe F, Haberer JE, Boum Y 2nd, et al. : Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in Southwestern Uganda. PLoS One 2014;9:e113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graham A, Goss C, Xu S, Magid DJ, DiGuiseppi C: Effect of using different modes to administer the AUDIT-C on identification of hazardous drinking and acquiescence to trial participation among injured patients. Alcohol Alcohol 2007;42:423–429 [DOI] [PubMed] [Google Scholar]
- 40. Appay V, Sauce D: Immune activation and inflammation in HIV-1 infection: Causes and consequences. J Pathol 2008;214:231–241 [DOI] [PubMed] [Google Scholar]
- 41. Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S: Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr 2010;54:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tate JP, Sterne JAC, Justice AC: Improved discrimination of mortality with Veterans Aging Cohort Study (VACS) Index 2.0 in HIV-positive individuals. AIDS 2019;33:903–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA: Projected cancer incidence rates and burden of incident cancer cases in HIV-infected adults in the United States Through 2030. Ann Intern Med 2018;168:866–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Byrne DD, Tate JP, Forde KA, et al. : Risk of acute liver injury after statin initiation by human immunodeficiency virus and chronic hepatitis C virus infection status. Clin Infect Dis 2017;65:1542–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gowda C, Newcomb CW, Liu Q, et al. : Risk of acute liver injury with antiretroviral therapy by viral hepatitis status. Open Forum Infect Dis 2017;4:ofx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sterling RK, Lissen E, Clumeck N, et al. : Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325 [DOI] [PubMed] [Google Scholar]
- 47. Barraclough K, Er L, Ng F, Harris M, Montaner J, Levin A: A comparison of the predictive performance of different methods of kidney function estimation in a well-characterized HIV-infected population. Nephron Clin Pract 2009;111:c39–c48 [DOI] [PubMed] [Google Scholar]
- 48. Justice AC, McGinnis KA, Tate JP, et al. : Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend 2016;161:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Justice AC, Freiberg MS, Tracy R, et al. : Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis 2012;54:984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Williams B, Livak B, Bahk M, Keating SM, Adeyemi OM: SCD14 and SCD163 levels are correlated with VACS Index scores: Initial data from the blunted immune recovery in CORE patients with HIV (BIRCH) cohort. AIDS Res Hum Retroviruses 2016;32:144–147 [DOI] [PubMed] [Google Scholar]
- 51. Mooney S, Tracy R, Osler T, Grace C: Elevated biomarkers of inflammation and coagulation in patients with HIV are associated with higher framingham and VACS Risk Index Scores. PLoS One 2015;10:e0144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williams B, Livak B, Bahk M, Keating SM, Adeyemi OM: Short communication: SCD14 and SCD163 levels are correlated with VACS Index Scores: Initial data from the blunted immune recovery in CORE patients with HIV (BIRCH) cohort. AIDS Res Hum Retroviruses 2016;32:144–147 [DOI] [PubMed] [Google Scholar]
- 53. Letizia A, Eller MA, Polyak C, et al. : Biomarkers of Inflammation Correlate With Clinical Scoring Indices in Human Immunodeficiency Virus-Infected Kenyans. J Infect Dis 2019;219:284–294 [DOI] [PubMed] [Google Scholar]
- 54. Duffau P, Wittkop L, Lazaro E, et al. : Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS 2015;29:2099–2108 [DOI] [PubMed] [Google Scholar]
- 55. Marquine MJ, Umlauf A, Rooney AS, et al. : The veterans aging cohort study index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr 2014;65:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Erlandson KM, Allshouse AA, Jankowski C, et al. : Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012;13:324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. John MD, Greene M, Hessol NA, et al. : Geriatric Assessments and Association With VACS Index Among HIV-Infected Older Adults in San Francisco. J Acquir Immune Defic Syndr 2016;72:534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oursler KK, Tate JP, Gill TM, et al. : Association of the veterans aging cohort study index with exercise capacity in HIV-infected adults. AIDS Res Hum Retroviruses 2013;29:1218–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Robinson-Papp J, Sharma SK: Autonomic neuropathy in HIV is unrecognized and associated with medical morbidity. AIDS Patient Care STDS 2013;27:539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Justice AC, Modur SP, Tate JP, et al. : Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: A North American cross cohort analysis. J Acquir Immune Defic Syndr 2013;62:149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vasan RS: Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 2006;113:2335–2362 [DOI] [PubMed] [Google Scholar]
- 62. D'Agostino RB, Sr., Grundy S, Sullivan LM, Wilson P: Validation of the Framingham coronary heart disease prediction scores: Results of a multiple ethnic groups investigation. JAMA 2001;286:180–187 [DOI] [PubMed] [Google Scholar]
- 63. Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK: Prognostic indices for older adults: A systematic review. JAMA 2012;307:182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bebu I, Tate J, Rimland D, et al. : The VACS Index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2014;65:226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brown ST, Tate JP, Kyriakides TC, et al. : The VACS index accurately predicts mortality and treatment response among multi-drug resistant HIV infected patients participating in the options in management with antiretrovirals (OPTIMA) study. PLoS One 2014;9:e92606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Justice AC, Tate JP, Freiberg MS, Rodriguez-Barradas MC, Tracy R: Reply to Chow et al.: Clin Infect Dis 2012;55:751–752
- 67. Akgun KM, Tate JP, Pisani M, et al. : Medical ICU admission diagnoses and outcomes in human immunodeficiency virus-infected and virus-uninfected veterans in the combination antiretroviral era. Crit Care Med 2013;41:1458–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tate JP, Brown ST, Rimland D, Rodriguez-Barradas M, Justice AC, Team VP: Comparison of VACS Index performance in HIV-infected and uninfected Veterans from 2000 to 2010. 18th International Workshop on HIV Observational Databases Sitges, IWHOD, March 27, 2014. Spain. Poster
- 69. Hotton AL, Weber KM, Hershow RC, et al. : Prevalence and predictors of hospitalizations among HIV-infected and at-risk HIV-uninfected women. J Acquir Immune Defic Syndr 2017;75:e27–e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang L, Porter B, Maynard C, et al. : Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care 2013;51:368–373 [DOI] [PubMed] [Google Scholar]
- 71. Quinton J, Krishnan S, Akgun K, et al.: Evaluation of mortality risk indexes for use in the Veteran's Healthcare Administration. 13th Annual Hopkins GIM Housestaff Research Awards National Competition, 2018. Baltimore, MD. Oral
- 72. Gaziano JM, Concato J, Brophy M, et al. : Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol 2016;70:214–223 [DOI] [PubMed] [Google Scholar]
- 73. Rentsch C, Tate J, Tarko L, et al.: Does an index composed of routine labs discriminate risk of mortality better than the Charlson Index?. MVP Science Meeting, 2019. Philadelphia, PA. Poster
- 74. Sigel K, Park LS, Stone K, et al.: VACS Index better predictor of mortality after cancer in HIV+ and HIV- than Charlson. 26th Conference on Retroviruses and Opportunistic Infections, 2019. Seattle, WA. Poster
- 75. Akgun KM, Gordon K, Pisani M, et al. : Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV infected Veterans. J Acquir Immune Defic Syndr 2013;62:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Womack JA, Murphy TE, Rentsch CT, et al.: Polypharmacy, hazardous alcohol and illicit substance use and serious falls among PLWH and uninfected comparators. J Acquir Immune Defic Syndr 2019 [Epub ahead of print]; DOI: 10.1097/QAI.0000000000002130 [DOI] [PMC free article] [PubMed]
- 77. Yin MT, Shiau S, Rimland D, et al. : Fracture prediction with modified-FRAX in older HIV-infected and uninfected men. J Acquir Immune Defic Syndr 2016;72:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Edelman EJ, Gordon KS, Crothers K, et al. : Association of prescribed opioids with increased risk of community-acquired pneumonia among patients with and without HIV. JAMA Intern Med 2019;179:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Franklin D, Heaton R, Woods S, et al.: Veterans Aging Cohort Study index score is associated with neurocognitive and functional impairment: A CHARTER Study. 20th Conference on Retroviruses and Opportunistic Infections (CROI), March 3, 2013. Atlanta, GA
- 80. Marquine MJ, Montoya JL, Umlauf A, et al. : The Veterans Aging Cohort Study (VACS) Index and neurocognitive change: A longitudinal study. Clin Infect Dis 2016;63:694–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marquine MJ, Sakamoto M, Dufour C, et al. : The impact of ethnicity/race on the association between the Veterans Aging Cohort Study (VACS) Index and neurocognitive function among HIV-infected persons. J Neurovirol 2016;22:442–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moore D: Higher Veterans Aging Cohort Study (VACS) Index scores are associated with concurrent risk of neurocognitive impairment. 3rd International Worshop on HIV & Aging, Baltimore, MD 2012
- 83. Salinas JL, Rentsch C, Marconi VC, et al. : Baseline, time-updated, and cumulative HIV care metrics for predicting acute myocardial infarction and all-cause mortality. Clin Infect Dis 2016;63:1423–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Barakat LA, Juthani-Mehta M, Allore H, et al. : Comparing clinical outcomes in HIV-infected and uninfected older men hospitalized with community-acquired pneumonia. HIV Med 2015;16:421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Akgun KM, Krishnan S, Tate JP, et al.: Potentially inappropriate medications increase delirium more in HIV+ than uninfected. Annual American Delirium Society (ADS) Conference, 2019. Boston, MA. Oral
- 86. Bryant K, McGinnis KA, Tate JP, Fiellin D, Justice AC: The VACS Index Score varies by alcohol level in those with HIV. Research Society on Alcoholism (RSA) Annual Conference, 2013. Orlando, FL
- 87. Tate J, Freiberg M, Justice AC: Do Risk Factors for cardiovascular disease improve VACS Index prediction of all cause mortality? 16th International Workshop on HIV Observational Databases (IWHOD), March 29, 2012. Athens, Greece
- 88. Yuh B, Tate J, Butt AA, et al. : Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015;60:1852–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tate JP, Hughes MD, Justice AC, for the VACS Project Team: Do risk factors for mortality change with time on antiretroviral therapy? 48th Annual Meeting of the Infectious Disease Society of America, 2010. Vancouver, British Columbia
- 90. Williams EC, McGinnis KA, Bobb JF, et al. : Changes in alcohol use associated with changes in HIV disease severity over time: A national longitudinal study in the Veterans Aging Cohort. Drug Alcohol Depend 2018;189:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Marshall BDL, Tate JP, McGinnis KA, et al. : Long-term alcohol use patterns and HIV disease severity. AIDS Behav 2017;31:1313–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Justice AC, Gordon KS, Skanderson M, et al. : Nonantiretroviral polypharmacy and adverse health outcomes among HIV-infected and uninfected individuals. AIDS 2018;32:739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weisberg DF, Gordon KS, Barry DT, et al. : Long-term prescription opioids and/or benzodiazepines and mortality among HIV-infected and uninfected patients. J Acquir Immune Defic Syndr 2015;69:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Adeyemi O, Livak B: Higher Veterans Aging Cohort Study (VACS) index scores in HIV-positive adults with CD4 counts <200 cells/mm3 despite viral suppression. J Acquir Immune Defic Syndr 2013;63:e78–e81 [DOI] [PubMed] [Google Scholar]
- 95. Furuya-Kanamori L, Kelly MD, McKenzie SJ: Co-morbidity, ageing and predicted mortality in antiretroviral treated Australian men: A quantitative analysis. PLoS One 2013;8:e78403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huggan PJ, Foo RM, Olszyna D, et al. : Presentation and outcome amongst older Singaporeans living with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): Does age alone drive excess mortality? Ann Acad Med Singapore 2012;41:581–586 [PubMed] [Google Scholar]
- 97. Cohen MH, Hotton AL, Hershow RC, et al. : Gender-related risk factors improve mortality predictive ability of VACS Index among HIV-infected women. J Acquir Immune Defic Syndr 2015;70:538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB: Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr 2013;63:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Erlandson KM, Allshouse AA, Jankowski CM, Mawhinney S, Kohrt WM, Campbell TB: Relationship of physical function and quality of life among persons aging with HIV infection. AIDS 2014;28:1939–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Edelman EJ, Maisto SA, Hansen NB, et al. : The starting treatment for ethanol in primary care trials (STEP Trials): Protocol for three parallel multi-site stepped care effectiveness studies for unhealthy alcohol use in HIV-positive patients. Contemp Clin Trials 2017;52:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tashima KT, Smeaton L, Klingman KL, et al.: Mortality among HIV+ participants randomized to omit NRTIs vs. add NRTIs in OPTIONS (ACTG A5241). 21st Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA 2014