Abstract
Achieving minimal residual disease (MRD) negativity in the bone marrow is one of the strongest prognostic factors in multiple myeloma. Consequently, MRD testing is routinely performed in clinical trials and moving towards standard of care. This review focuses on the role of next generation sequencing (NGS) of tumor-specific immunoglobulin V(D)J sequences for MRD tracking. The immunoglobulin variable regions are ideal targets for tracking, because every tumor cell shares an identical gene sequence, which is stable over time and generally distinct from the immunoglobulin sequences of normal B-cells. Several excellent assays for NGS-based MRD testing are available, both commercial and community-based, including one that is FDA-approved. These assays can achieve the gold standard analytical sensitivity of one tumor cell per million (10−6), requiring a minimum input of 3 million bone marrow cells. On-going clinical trials will outline how MRD testing should be used to inform dynamic risk-adopted therapy.
Keywords: Multiple Myeloma, minimal residual disease, next generation sequencing
Introduction
Minimal residual disease (MRD) assessment refers to the dynamic estimation of the number of tumor cells remaining after treatment and during follow-up. With novel active treatment regimens for multiple myeloma (MM) yielding deep and sustained responses, MRD has become an increasingly important tool in clinical trials as well as routine patient care (1, 2). Achieving MRD negativity has been established as a strong prognostic biomarker in MM (3, 4). Indeed, becoming MRD negative more strongly affects progression free survival (PFS) than the therapeutic regimens used to get there, as evidenced by recent randomized clinical trials in the newly diagnosed setting (5, 6) and relapse setting (7–9). Furthermore, in these studies, MRD status had a stronger PFS impact than conventional biomarkers of high-risk disease such as the International Staging System, t(4;14) and del(17p), raising the exciting possibility that MRD status may be used prospectively to guide treatment (8, 10).
Although randomized clinical trials of MRD-directed therapy are still ongoing, clinicians are increasingly adopting MRD testing to inform challenging clinical decisions. It is likely that in the near future, in a routine setting, MRD testing will help clinicians adopt treatment intensity to patients’ individual risk (1). Importantly, MRD testing may guide the use of high-dose melphalan with autologous stem cell transplant (HDM-ASCT), and the type and duration of maintenance therapy (11). Until recently, the use of MRD testing in MM was confined to clinical trials and a few dedicated academic centers (12). This landscape is rapidly changing, with emerging data supporting the clinical utility and increasing availability of high-quality MRD assays (1, 13, 14). To ensure validity and reproducibility of MRD testing, on-going efforts are directed to optimize and standardize MRD testing procedures (15–17).
The topic of this review is MRD testing by next generation sequencing (NGS) of the bone marrow, while a companion review (Roshal et al) will address MRD testing by next generation flow cytometry. We will begin by discussing the underlying assumptions of NGS-based MRD testing, before providing an overview of the testing strategies employed in current state-of-the-art assays. Next, we will discuss the sensitivity limitations and appropriate timing of bone marrow based MRD testing. Finally, we point to important areas of ongoing and future development.
Tumor-specific immunoglobulin V(D)J rearrangements
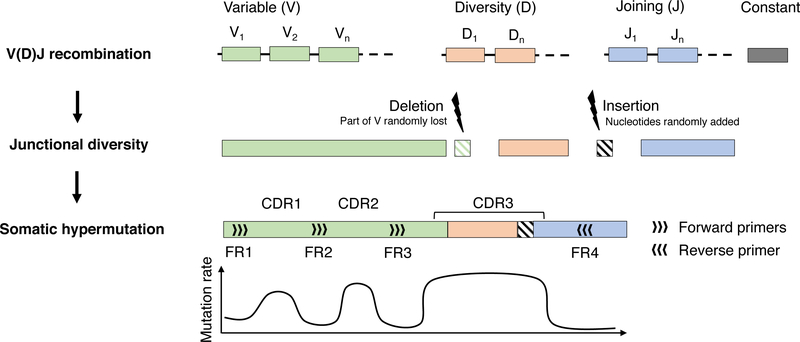
Tracking of genomic alterations in the tumor cells is an attractive strategy for MRD testing. However, somatic alterations must fulfill three criteria before they are suitable biomarkers for tracking: 1) presence in all tumor cells; 2) stability over time throughout tumor evolution; and 3) absence in normal cells. In MM, examples of poor biomarkers are gene mutations, as they are usually present in a subset of tumor cells and are subject to clonal evolution. On the other hand, the immunoglobulin heavy chain (IGH) variable region is an ideal target (Figure 1). Research and MRD assay development has focused mainly on the third complementarity determining region (CDR3), a sequence of 30–70 nucleotides, at the intersection of variable (V), diversity (D) and joining (J) immunoglobulin gene segments (18). During formation of the mature immunoglobulin gene, vast diversity is introduced through V(D)J recombination, junctional insertions/deletions and somatic hypermutation (Figure 1) (18). The probability of two identical IGH variable regions arising in independent B-cell clones is very low, and in practice, these sequences are considered fully tumor-specific (14, 19, 20). A growing body of evidence has also demonstrated that IGH CDR3 sequences are shared across all cells within a tumor and remain stable during long-term follow-up (19, 21, 22).
Figure 1: Development of the mature immunoglobulin heavy chain gene.
Schematic representation of IGH gene development from the germline configuration (top) through V(D)J recombination with junctional insertions/deletions (middle), followed by somatic hypermutation (bottom) in the germinal center when the B-cell has encountered its antigen. Insertions and deletions may involve any or all of the segment junctions. Light chain gene development follows an analogous pattern, except for the absence of a D-segment, resulting in one less junction in the CDR3 and considerably lower diversity.
Use of immunoglobulin kappa (IGK) and lambda (IGL) light chain sequences for tracking is less straight-forward. The IGK and IGL variable regions lack a D-segment, resulting in lower diversity and a higher probability that tumor and normal B-cells will share an identical CDR3 sequence (18–20). For this reason, IGH-tracking remains the backbone of NGS-based MRD tracking. Some assays also include the possibility for light chain tracking, which is particularly beneficial in cases where no clonal IGH sequence can be identified (23, 24). Furthermore, the theoretical repertoire of paired heavy and light chain sequences in a given individual has been estimated in the range of 10−16-10−18(20). Tracking more than one sequence may therefore increase the sensitivity and specificity of MRD assays, but to our knowledge, this is not yet supported by published data. If MRD tracking is to be based solely on a light chain sequence, it will be necessary to determine which sequences are sufficiently unique for tracking. We recently showed that the degree of junctional diversity and somatic hypermutation of the light chain CDR3 is highly correlated with uniqueness (19). This is logical, as more complex sequences are less likely to appear by chance.
Each tumor clone can have up to six trackable immunoglobulin sequences. This follows from the order in which immunoglobulin genes are rearranged during B-cell development: First IGH, then IGK and finally IGL (each gene has two copies)(18, 25). The cell continues to rearrange one allele at a time until it has one productive heavy and light chain sequence, leaving the remaining alleles in the germline configuration. For tracking purposes, the immunoglobulin alleles have to be rearranged, making them as unique as possible (i.e. tumor-specific); but they do not have to be productive. For example, a patient with kappa-restricted multiple myeloma will have productive IGH and IGK rearrangements and may also have an unproductive IGH and/or IGK rearrangement, but both the IGL alleles will be in the germline configuration (24, 26). Patients with lambda-restricted multiple myeloma will be in the same situation with regards to IGH and IGL but will also have two unproductive IGK rearrangements that can potentially be used for tracking (24).
Assays for NGS-based MRD
All NGS-based MRD assays that are currently in clinical use employ a similar workflow (14). One or more immunoglobulin variable regions are amplified using multiplex PCR, followed by NGS of the PCR product and computational processing of the sequencing data. This procedure is first performed on a baseline sample with high tumor cell infiltration, to define the tumor-specific sequences for tracking by ultra-deep sequencing of subsequent samples.
The current market-leader in NGS-based MRD is Adaptive Biotechnologies, providing the ClonoSeq assay as a service (10, 27–30). Although the details of their assay are not public, their main practical selling-point is to identify and track tumor-specific rearrangements of all three immunoglobulin genes in a single tube. Their assay is also, to our knowledge, the only one that is currently FDA approved for multiple myeloma. The main commercial contender, Invivoscribe, Inc., follows a different model with their LymphoTrack assays, marketing them as kits for pathologists to set up and use in their own laboratories (31). LymphoTrack has four assays for the IGH locus, with primers targeting different framework regions (FR1, FR2, FR3 and the upstream Leader region (see Figure 1), a separate assay for IGK, and another for IGL currently under development (24). We have implemented LymphoTrack as standard of care in the pathology laboratory at Memorial Sloan Kettering Cancer Center and have excellent experiences using both assays (24, 28, 29, 31). As an non-commercial alternative, a new set of NGS-based MRD assays were recently published by the EuroClonality/BIOMED-2 consortium (32, 33). Although the research efforts of the BIOMED-2 consortium is primarily acute lymphoblastic leukemia, their assays have an excellent track-record, and the previous version has been applied successfully to multiple myeloma (34).
One of the main challenges with NGS-based MRD has been failure to identify a trackable clone at baseline (24, 29). This is a particular challenge with multiple myeloma, because all tumors have undergone antibody affinity maturation through somatic hypermutation of the immunoglobulin variable regions (18, 25). Although somatic hypermutation primarily affects the CDRs, the framework regions where PCR primers bind may also be affected (Figure 1). This may result in poor PCR efficiency and failure to amplify the tumor sequence, making it indistinguishable from the normal polyclonal background (19, 24). Modern assays have included additional primers, essentially to get more shots on the goal, as somatic hypermutation is largely random and impossible to avoid completely. Another important factor is sample quality. Peripheral blood hemodilution and sampling bias from a patchy bone marrow may both result in failure to identify the tumor clone (24, 35). An early-pull bone marrow aspirate should be used, to avoid hemodilution, ideally followed by enrichment of CD138+ cells. Baseline clonality detection rates of at least 95% can be achieved by either LymphoTrack or ClonoSeq, as long as the sample quality is adequate (24, 29, 31).
Sensitivity and the rule of three
Current IMWG guidelines define MRD negativity as fewer than one tumor cell in 100000 bone marrow cells (10−5) in a patient who fulfills the criteria for complete response (CR) (15). However, there has been considerable advances in the field since these criteria were developed in 2014. Recent studies using highly sensitive assays have demonstrated a dose-response relationship between the concentration of residual tumor cells and patient outcomes (10, 36). The strongest impact on progression free survival (PFS) was seen with a sensitivity of 10−6, which is the highest that can be achieved with current methods.
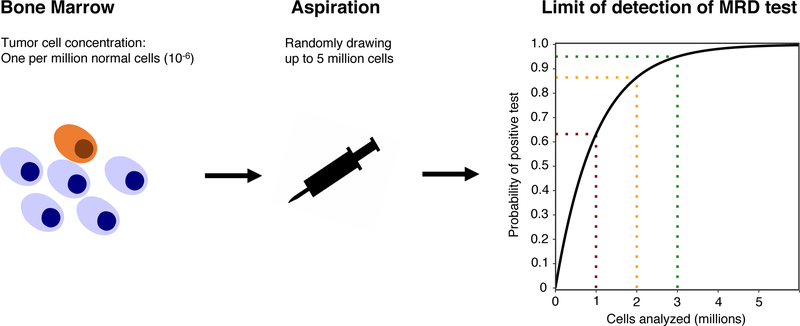
The first bar that any MRD test must pass is high sensitivity: at least 10−5, preferably 10−6 However; in practice, the limiting factor for sensitivity is often the sample available for analysis rather than the method itself. A sensitivity (or “limit of detection”) of 10−6 means that if the actual concentration of tumor cells in a sample is one per million, the test will be positive at least 95 % of the time (Figure 2) (37). To reach a given level of certainty that a sample is really negative at the desired sensitivity (e.g., 95 % confidence at 10−6), a minimum number of bone marrow cells must be analyzed. We can estimate this number using the binomial distribution, assuming that each cell drawn from the bone marrow is like a coin-toss, with a 10−6 probability of landing on its head, or being a tumor cell. As it turns out, the number of cells required is always approximately three times the desired sensitivity: in this case three million. This useful statistical rule of thumb is sometimes referred to as “the rule of three”(38).
Figure 2: Sensitivity of MRD testing depends on the number of cells analyzed.
Illustration of the sensitivity of MRD testing in a patient where the true bone marrow infiltration is one tumor cell per million (10−6). Aspiration of cells from the bone marrow can be modeled as a series of Bernoulli trials where the probability of “success” (i.e., drawing a tumor cell) is equal to the tumor burden (in this case 10−6). The probability of a positive MRD test given a number of cells analyzed is shown on the right, with dashed lines indicating the probability at 1, 2 and 3 million input cells. With 3 million input cells, the probability of a positive test is 95 %, which is usually considered as the required confidence level for a clinical assay.
The rule of three gives the lowest possible number of cells required to reach a given level of sensitivity, given a perfect assay. Consequently, no matter how good an MRD assay is, its sensitivity in practical application will never be higher than one tumor cell in the number of cells analyzed divided by three. This is why a sensitivity of 10−7 is more than a technical challenge – you would need to analyze at least 30 million bone marrow cells!
In reality, the number of cells required will be higher than the rule of three suggests. For example, cells may be lost during sample preparation, effectively reducing the input; or a single tumor cell may not be enough to confidently call the sample as positive. For these reasons, the number of cells required to reach 10−6 sensitivity with published assays ranges from three to 10 million (13, 14, 17). Because NGS-based MRD assays do not require live cells, and have extremely low error-rates, DNA from 3 million cells has been reported as sufficient input material (17).
Tumor dynamics and the timing of MRD assessment
The optimal time to test for MRD in the bone marrow during the course of treatment depends on the dynamics of response to a particular therapeutic strategy. Historically, myeloma therapies resulted in gradual reduction of tumor burden over the course of several months, mirroring the slow clearance of serum monoclonal immunoglobulins (the half-life of IgG is ~25 days) (2, 39). In this setting, it is logical to only apply invasive MRD testing when monoclonal immunoglobulins have become undetectable, which was the basis for current IMWG response criteria (15). This strategy of MRD assessment has been challenged by novel highly effective therapies (e.g. CAR-T cells and four-drug combinations) capable of eradicating all measurable tumor cells from the bone marrow long before monoclonal proteins have cleared from the serum (40, 41). Most of these patients go on to achieve a conventional CR up to several months later, indicating that early MRD negativity in the bone marrow is a biomarker for deep and durable responses irrespective of the serum protein levels at the time of assessment. A recent “MRD expert meeting”-report addressed this as one of the new important aspects going forward (17).
Future directions
MRD testing in multiple myeloma is here to stay. Anticipating data from well-designed clinical trials, many physicians and patients find that MRD testing is already a useful tool for clinical decision-making (1, 11). In parallel, efforts are ongoing to establish MRD negativity as a surrogate endpoint for PFS in clinical trials, aiming to accelerate drug development (42, 43).
The current state-of-the-art in bone marrow based MRD testing is any assay that can achieve 10–6 sensitivity. Our objective in this review has been to lay out why NGS is a highly suitable platform for MRD testing and how these assays work in practice. Similar analytical sensitivity can also be achieved with a well-designed flow cytometry assay in expert hands (44, 45). Both platforms require a minimum number of cells to guarantee the desired sensitivity, and usually NGS can get away with a lower input.
Based on the idea of MRD as a surrogate for cure, the MRD field is continuously searching for more sensitive methods. As we have discussed above, limitations on sample volume probably means that bone marrow-based testing will not become much more sensitive than the current optimum of 10−6. Furthermore, a single bone marrow aspirate will not fully capture the spatial heterogeneity of multiple myeloma. Thus, going forward, still smaller quantities of disease within the patient can only be detected by combining bone marrow-based MRD testing with other modalities, such as imaging and blood-based assays (46–51).
Summary
MRD negativity in the bone marrow by a highly sensitive assay has emerged as one of the strongest prognostic factors in multiple myeloma, surpassing conventional risk stratifications such as ISS and cytogenetics. Indeed, becoming MRD negativity appears to be more important than what therapy was used to get there. Initially established in the newly diagnosed setting, recent studies have shown compelling evidence of the impact of MRD in relapsed myeloma as well. One of the most attractive strategies for MRD testing is NGS of the bone marrow, taking advantage of the immunoglobulin V(D)J sequences that are shared by all tumor cells. Each tumor may have up to six trackable heavy and light chain sequences, yielding ample opportunities to define a tumor-specific signature that can separate the tumor from normal B-cells. Modern NGS-based MRD assays are applicable to >95 % of patients, as long as a tumor sample of adequate quality can be obtained for baseline characterization of clonal immunoglobulin sequences. The maximum sensitivity of any MRD test is determined by the number of cells available for analysis. To achieve the gold standard sensitivity of one tumor cell per million (10−6) with 95 % confidence, DNA from at least 3 million bone marrow cells must be analyzed. On-going clinical trials will outline how MRD testing should be used to inform dynamic risk-adopted therapy in multiple myeloma.
Practice points.
MRD status in the bone marrow is one of the strongest prognostic factors in multiple myeloma, with higher sensitivity providing more accurate information.
The current gold standard sensitivity for MRD is one tumor cell per million (10−6), which required at least 3 million bone marrow cells for analysis for NGS and sometimes up to 10 million for flow cytometry.
NGS-based MRD assays have >95 % applicability, provided a baseline sample with high tumor cell infiltration is available for calibration
There is currently no evidence that one MRD assay is technically superior to another, be it flow cytometry or NGS-based, as long as the analytical sensitivity is equivalent
Research agenda.
There is a great need for clinical trials of MRD-directed therapy.
The optimal timing and interval of MRD testing remains unknown.
Imaging and peripheral blood based MRD tests will most likely be necessary to overcome patchy bone marrow infiltration and identify residual extramedullary disease.
Acknowledgements
This work is supported by the Memorial Sloan Kettering Cancer Center NCI Core Grant (P30 CA 008748).
Footnotes
Conflicts of interest
No conflict of interests to declare.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Landgren O. MRD Testing in Multiple Myeloma: From a Surrogate Marker of Clinical Outcomes to an Every-Day Clinical Tool. Semin Hematol. 2018;55(1):1–3. [DOI] [PubMed] [Google Scholar]
- 2.Landgren O, Lu SX, Hultcrantz M. MRD Testing in Multiple Myeloma: The Main Future Driver for Modern Tailored Treatment. Semin Hematol. 2018;55(1):44–50. [DOI] [PubMed] [Google Scholar]
- 3.Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone marrow transplantation. 2016;51(12):1565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avet-Loiseau H, Lauwers-Cances V, Corre J, Moreau P, Attal M, Munshi N. Minimal Residual Disease in Multiple Myeloma: Final Analysis of the IFM2009 Trial. Blood. 2017;130(Suppl 1):435-. [Google Scholar]
- 5.Perrot A, Lauwers-Cances V, Tournay E, Hulin C, Chretien M-L, Royer B, et al. Development and Validation of a Cytogenetic Prognostic Index Predicting Survival in Multiple Myeloma. Journal of Clinical Oncology. 2019;37(19):1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;394(10192):29–38. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. The New England journal of medicine. 2016;375(8):754–66. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. The New England journal of medicine. 2016;375(14):1319–31. [DOI] [PubMed] [Google Scholar]
- 9.Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazandjian D, Landgren O. Delaying the use of high-dose melphalan with stem cell rescue in multiple myeloma is ready for prime time. Clinical advances in hematology & oncology : H&O. 2019;17(10):559–68. [PMC free article] [PubMed] [Google Scholar]
- 12.Salem D, Stetler-Stevenson M, Yuan C, Landgren O. Myeloma minimal residual disease testing in the United States: Evidence of improved standardization. Am J Hematol. 2016;91(12):E502–E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roshal M. Minimal Residual Disease Detection by Flow Cytometry in Multiple Myeloma: Why and How? Semin Hematol. 2018;55(1):4–12. [DOI] [PubMed] [Google Scholar]
- 14.Ho C, Arcila ME. Minimal residual disease detection of myeloma using sequencing of immunoglobulin heavy chain gene VDJ regions. Semin Hematol. 2018;55(1):13–8. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The lancet oncology. 2016;17(8):e328–e46. [DOI] [PubMed] [Google Scholar]
- 16.Rawstron AC, Paiva B, Stetler-Stevenson M. Assessment of minimal residual disease in myeloma and the need for a consensus approach. Cytometry Part B, Clinical cytometry. 2016;90(1):21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landgren O, Rustad EH. Meeting report: Advances in minimal residual disease testing in multiple myeloma 2018. ADVANCES IN CELL AND GENE THERAPY. 2019;2(1):e26. [Google Scholar]
- 18.Dunn-Walters D, Townsend C, Sinclair E, Stewart A. Immunoglobulin gene analysis as a tool for investigating human immune responses. Immunol Rev. 2018;284(1):132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rustad EH, Misund K, Bernard E, Coward E, Yellapantula VD, Hultcrantz M, et al. Stability and uniqueness of clonal immunoglobulin CDR3 sequences for MRD tracking in multiple myeloma. American Journal of Hematology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briney B, Inderbitzin A, Joyce C, Burton DR. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature. 2019;566(7744):393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puig N, Conde I, Jiménez C, Sarasquete ME, Balanzategui A, Alcoceba M, et al. The predominant myeloma clone at diagnosis, CDR3 defined, is constantly detectable across all stages of disease evolution. Leukemia. 2015;29:1435. [DOI] [PubMed] [Google Scholar]
- 22.Ralph QM, Brisco MJ, Joshua DE, Brown R, Gibson J, Morley AA. Advancement of multiple myeloma from diagnosis through plateau phase to progression does not involve a new B-cell clone: evidence from the Ig heavy chain gene. Blood. 1993;82(1):202–6. [PubMed] [Google Scholar]
- 23.Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. The New England journal of medicine. 2018;378(6):518–28. [DOI] [PubMed] [Google Scholar]
- 24.Rustad EH, Hultcrantz M, Yellapantula VD, Akhlaghi T, Ho C, Arcila ME, et al. Baseline identification of clonal V(D)J sequences for DNA-based minimal residual disease detection in multiple myeloma. PloS one. 2019;14(3):e0211600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez D, van der Burg M, Garcia-Sanz R, Fenton JA, Langerak AW, Gonzalez M, et al. Immunoglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood. 2007;110(9):3112–21. [DOI] [PubMed] [Google Scholar]
- 26.Perfetti V, Vignarelli MC, Palladini G, Navazza V, Giachino C, Merlini G. Insights into the regulation of immunoglobulin light chain gene rearrangements via analysis of the kappa light chain locus in lambda myeloma. Immunology. 2004;112(3):420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korde N, Roschewski M, Zingone A, Kwok M, Manasanch EE, Bhutani M, et al. Treatment With Carfilzomib-Lenalidomide-Dexamethasone With Lenalidomide Extension in Patients With Smoldering or Newly Diagnosed Multiple Myeloma. JAMA oncology. 2015;1(6):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hultcrantz M, Rustad EH, Yellapantula V, Akhlaghi T, Jacob A, Patel A, et al. Capture Rate of the Adaptive Next Generation Sequencing VDJ Assay in Multiple Myeloma. Blood. 2018;132(Suppl_1):3184-. [Google Scholar]
- 30.Martinez-Lopez J, Lahuerta JJ, Pepin F, Gonzalez M, Barrio S, Ayala R, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arcila ME, Yu W, Syed M, Kim H, Maciag L, Yao J, et al. Establishment of Immunoglobulin Heavy (IGH) Chain Clonality Testing by Next-Generation Sequencing for Routine Characterization of B-Cell and Plasma Cell Neoplasms. J Mol Diagn. 2019;21(2):330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruggemann M, Kotrova M, Knecht H, Bartram J, Boudjogrha M, Bystry V, et al. Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study. Leukemia. 2019;33(9):2241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheijen B, Meijers RWJ, Rijntjes J, van der Klift MY, Mobs M, Steinhilber J, et al. Next-generation sequencing of immunoglobulin gene rearrangements for clonality assessment: a technical feasibility study by EuroClonality-NGS. Leukemia. 2019;33(9):2227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Lopez J, Sanchez-Vega B, Barrio S, Cuenca I, Ruiz-Heredia Y, Alonso R, et al. Analytical and clinical validation of a novel in-house deep-sequencing method for minimal residual disease monitoring in a phase II trial for multiple myeloma. Leukemia. 2017;31(6):1446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vij R, Mazumder A, Klinger M, O’Dea D, Paasch J, Martin T, et al. Deep sequencing reveals myeloma cells in peripheral blood in majority of multiple myeloma patients. Clinical lymphoma, myeloma & leukemia. 2014;14(2):131–9.e1. [DOI] [PubMed] [Google Scholar]
- 36.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. The New England journal of medicine. 2017;376(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29 Suppl 1(Suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 38.Jovanovic BD, Levy PS. A Look at the Rule of Three. The American Statistician. 1997;51(2):137–9. [Google Scholar]
- 39.Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, et al. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. The Journal of laboratory and clinical medicine. 1988;112(5):634–40. [PubMed] [Google Scholar]
- 40.Landgren O, Hultcrantz M, Lesokhin AM, Mailankody S, Hassoun H, Smith EL, et al. , editors. Weekly Carfilzomib, Lenalidomide, Dexamethasone and Daratumumab (wKRd-D) Combination Therapy Provides Unprecedented MRD Negativity Rates in Newly Diagnosed Multiple Myeloma: A Clinical and Correlative Phase 2 Study. American Society of Hematology Annual Meeting; 2019; Abstract number: 653. [Google Scholar]
- 41.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. New England Journal of Medicine. 2019;380(18):1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gormley NJ, Farrell AT, Pazdur R. Minimal Residual Disease as a Potential Surrogate End Point-Lingering Questions. JAMA oncology. 2017;3(1):18–20. [DOI] [PubMed] [Google Scholar]
- 43.Avet-Loiseau H, Ludwig H, Landgren O, Paiva B, Morris C, Yang H, et al. Minimal Residual Disease Status as a Surrogate Endpoint for Progression-free Survival in Newly Diagnosed Multiple Myeloma Studies: A Meta-analysis. Clinical lymphoma, myeloma & leukemia. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roshal M, Flores-Montero JA, Gao Q, Koeber M, Wardrope J, Durie BGM, et al. MRD detection in multiple myeloma: comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood advances. 2017;1(12):728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, Garcia-Sanchez O, Bottcher S, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31(10):2094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thoren KL. Mass spectrometry methods for detecting monoclonal immunoglobulins in multiple myeloma minimal residual disease. Semin Hematol. 2018;55(1):41–3. [DOI] [PubMed] [Google Scholar]
- 47.Pugh TJ . Circulating Tumour DNA for Detecting Minimal Residual Disease in Multiple Myeloma. Semin Hematol. 2018;55(1):38–40. [DOI] [PubMed] [Google Scholar]
- 48.Rustad EH, Coward E, Skytoen ER, Misund K, Holien T, Standal T, et al. Monitoring multiple myeloma by quantification of recurrent mutations in serum. Haematologica. 2017;102(7):1266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hillengass J, Merz M, Delorme S. Minimal residual disease in multiple myeloma: use of magnetic resonance imaging. Semin Hematol. 2018;55(1):19–21. [DOI] [PubMed] [Google Scholar]
- 50.Pandit-Taskar N. Functional Imaging Methods for Assessment of Minimal Residual Disease in Multiple Myeloma: Current Status and Novel ImmunoPET Based Methods. Seminars in hematology. 2018;55(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waldschmidt JM, Anand P, Knoechel B, Lohr JG. Comprehensive characterization of circulating and bone marrow-derived multiple myeloma cells at minimal residual disease. Semin Hematol. 2018;55(1):33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]