Abstract
Background
Myasthenia gravis is an autoimmune disease in which autoantibodies interfere with neuromuscular transmission. As with other autoimmune diseases, people with myasthenia gravis would be expected to benefit from intravenous immunoglobulin (IVIg). This is an update of a review first published in 2003 and last updated in 2007.
Objectives
To examine the efficacy of IVIg for treating exacerbations of myasthenia gravis or for chronic myasthenia gravis.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (11 October 2011), CENTRAL (2011, Issue 3), MEDLINE (January 1966 to September 2011) and EMBASE (January 1980 to September 2011) using 'myasthenia gravis' and 'intravenous immunoglobulin' as the search terms.
Selection criteria
All randomised controlled trials (RCTs) or quasi‐RCTs in which IVIg was compared with no treatment, placebo or plasma exchange, in people with myasthenia gravis.
Data collection and analysis
One review author extracted the data and two others checked these data. For methodological reasons, no formal meta‐analysis was performed.
Main results
We identified seven RCTs. These trials differ in inclusion criteria, comparison with alternative treatment and outcomes. In a trial comparing IVIg with placebo, including 51 participants with myasthenia gravis worsening, the mean difference (MD) in quantitative myasthenia gravis score (QMGS) (MD 95% CI) after 14 days was: ‐1.60 (95% CI ‐ 3.23 to 0.03) this result being borderline statistically significant in favour of IVIg. In an unblinded study of 87 participants with exacerbation comparing IVIg and plasma exchange there was no difference in myasthenic muscle score (MMS) after 15 days (MD ‐1.00; 95% CI ‐7.72 to 5.72). In a study of 84 participants with worsening myasthenia gravis there was no difference in change in QMGS 14 days after IVIg or plasma exchange (MD ‐1.50; 95% CI ‐3.43 to 0.43). In a study of 12 participants with moderate or severe myasthenia gravis, which was at high risk of bias from skewed allocation, the mean fall in QMGS both for IVIg and plasma exchange after four weeks was significant (P < 0.05). A study with 15 participants with mild or moderate myasthenia gravis found no difference in change in QMGS 42 days after IVIg or placebo (MD 1.60; 95% CI ‐1.92 to 5.12). A study included 33 participants with moderate exacerbations of myasthenia gravis and showed no difference in change in QMGS 14 days after IVIg or methylprednisolone (MD ‐0.42; 95% CI ‐1.20 to 0.36). All these three smaller studies were underpowered. The last trial, including 168 people with exacerbations, showed no evidence of superiority of IVIg 2 g/kg over IVIg 1 g/kg on the change of MMS after 15 days (MD 3.84; 95% CI ‐0.98 to 8.66). Adverse events due to IVIg were moderate (fever, nausea, headache), self‐limiting and subjectively less severe than with plasma exchange (although, given the available data, no statistical comparison was possible). Other than where specific limitations are mentioned the trials were generally at low risk of bias.
Authors' conclusions
In exacerbation of myasthenia gravis, one RCT of IVIg versus placebo showed some evidence of the efficacy of IVIg and two did not show a significant difference between IVIg and plasma exchange. Another showed no significant difference in efficacy between 1 g/kg and 2 g/kg of IVIg. A further, but underpowered, trial showed no significant difference between IVIg and oral methylprednisolone. In chronic myasthenia gravis, there is insufficient evidence from RCTs to determine whether IVIg is efficacious.
Keywords: Humans; Chronic Disease; Disease Progression; Immunoglobulins, Intravenous; Immunoglobulins, Intravenous/therapeutic use; Methylprednisolone; Methylprednisolone/therapeutic use; Myasthenia Gravis; Myasthenia Gravis/therapy; Neuroprotective Agents; Neuroprotective Agents/therapeutic use; Plasma Exchange; Randomized Controlled Trials as Topic
Plain language summary
Intravenous immunoglobulin for myasthenia gravis
Myasthenia gravis is characterised by fluctuating muscle weakness and muscles that tire easily. An acute increase in symptoms can be life‐threatening because of swallowing difficulties or respiratory failure. Myasthenia gravis is an autoimmune disorder in which the body's own antibodies block the transmission of nerve impulses to muscles and damage the neuromuscular junction (where the nerve meets the muscle). With optimal treatment, including thymectomy, corticosteroids, immunosuppressive drugs and plasma exchange, most people with myasthenia gravis go into remission or improve but these treatments can cause many adverse events. Intravenous immunoglobulin (IVIg) (antibodies purified from human blood), is effective in other autoimmune diseases. The objective of this review was to examine the efficacy of IVIg for treating acute exacerbations or for chronic long‐term, persistent myasthenia. We identified seven randomised controlled trials (RCTs), all of which investigated short‐term benefit. Other than where study limitations are mentioned the risk of bias was generally low. Adverse events due to IVIg were observed in all trials. They were moderate (fever, nausea, headache), self‐limiting and are subjectively less severe than those with plasma exchange (although no statistical comparison was possible).
Five of the RCTs evaluated the efficacy of IVIg for the treatment of exacerbations or worsening (the former being usually more severe than the latter). One RCT of IVIg versus placebo, which included 51 participants, showed some evidence of the efficacy of IVIg for treating myasthenia gravis with worsening weakness. Two trials, the first of which included the first 87 and the second 84 participants, showed no significant difference between IVIg and plasma exchange. In the first of these trials there was a high risk of bias because the assigned treatments were not hidden. A trial including 33 participants showed no difference in efficacy between IVIg and a corticosteroid (methylprednisolone) but did not recruit enough participants to detect an effect, so there is insufficient evidence to favour IVIg over corticosteroids in moderate exacerbations. Another trial, which included 168 participants, showed no evidence of superiority of IVIg 2 g/kg over IVIg 1 g/kg on the change of myasthenic muscle score (MMS) after 15 days (MD 3.84; 95% CI ‐0.98 to 8.66).
Two RCTs evaluated the efficacy of IVIg for the treatment of moderate or severe myasthenia gravis. One compared, in 12 participants, IVIg and plasma exchange. The second, with 15 participants included, compared IVIg and a placebo. Both are underpowered and the first at some risk of bias, so no conclusion could be drawn from these two trials. There is no evidence from RCTs nor from other trials to determine whether IVIg improves function or reduces the need for steroids.
Background
Myasthenia gravis (MG) is an autoimmune disease. In up to 90% of generalised cases and in about half of ocular cases, IgG autoantibodies to the nicotinic acetylcholine receptor (AChR) are detectable, which mediate the neuromuscular transmission disorder. Antibodies directed against a muscle‐specific tyrosine kinase (MuSK) is detected among approximately 35% of MG patients without anti‐AChR antibody (Wolfe 2008). Some 'seronegative' patients may have antibodies to or near the acetylcholine binding site at the AChR which are not detected by the bungarotoxin‐bound AChR assay. These antibodies may have low avidity (Besinger 1983; Vincent 2003) or other autoantibodies may also be operative (McConville 2004). The disease is characterised by weakness and fatigability of voluntary muscle changing over shorter or longer periods. Acute exacerbations are life‐threatening because of swallowing difficulties or respiratory failure. Numerous observational studies and one randomised controlled trial (RCT) suggest that with optimal treatment, including thymectomy, corticosteroids and immunosuppressive drugs and plasma exchange, most people go into remission or improve (Hohlfeld 1996).
Plasma exchange was introduced in 1976 as a short‐term therapy for acute exacerbations (Dau 1977; Pinching 1976). A consensus conference concluded that plasma exchange induces short‐term improvement (NIH Consensus 1986). The use of repeated plasma exchange over a long period for refractory MG has also been reported (Kornfeld 1981; Rodnitzky 1984). Two controlled trials, including a small number of participants, failed to demonstrate a cumulative long‐term effect of plasma exchange (Gajdos 1983; Newsom‐Davis 1979). A Cochrane review on plasma exchange has been published (Gajdos 2002).
The efficacy of intravenous immunoglobulin (IVIg) was first shown for idiopathic thrombocytopenic purpura (Imbach 1981) at a daily dose of 0.4 g/kg for five days. Since then IVIg has been widely used in autoimmune diseases (Dwyer 1992). IVIg was used as early as 1984 for treatment of MG (Fateh‐Moghadam 1984; Gajdos 1984). In several open studies IVIg appeared promising especially in the acute phase of the disease (Arsura 1986; Cosi 1991; Gajdos 1987).
The improvement rate achieved by IVIg in MG calculated in two non‐systematic reviews from previously published uncontrolled studies was 73% and 76% (Arsura 1989; Van der Meché 1997). A 1999 review concluded that "the use of IVIg may be justified in lieu of plasmapheresis (1) for acutely worsening disease (2) to prepare a weak patient for thymectomy (3) as adjuvant to immunosuppressive therapy ... to minimize the long term side effects" (Dalakas 1999). Others recommend IVIg only as a second‐line regimen once plasma exchange has failed to improve the condition (Hohlfeld 1996). The optimal regimen of IVIg in terms of clinical benefits and costs has not been determined. After the dose of 0.4 g/kg/day for five days initially proposed for idiopathic thrombocytopenic purpura, other schedules have been proposed including a single daily dose of 0.8 g/kg or a daily dose of 0.5 g/kg or of 1.0 g/kg for two days (Blanchette 1994; Dalakas 1993; Godeau 1993).
The use of IVIg is claimed to be easier than plasma exchange and administration of IVIg is usually associated with less than a five per cent rate of mild and self‐limited adverse events (NIH Consensus 1990). More severe adverse effects have been reported, such as renal failure and aseptic meningitis, but in a large trial in Guillain‐Barré syndrome, the frequency and severity of adverse reactions were not significantly different after treatment with IVIg and after treatment with plasma exchange (PSGBS Group 1997).
Recently, the immunological mechanism of the action of IVIg has been widened by the direct observation of a neutralizing effect of commercial polyclonal IgG (IVIg) on the blocking effects of myasthenic IgG antibodies to nicotinic acetylcholine receptors (Buchwald and Toyka, unpublished observations 2005). Similar studies have been observed in the presynaptic counterpart of MG, the Lambert‐Eaton myasthenic syndrome and in Guillain‐Barré syndrome (that is neutralizing effects of IVIg in patch clamp studies at the mouse neuromuscular junction) (Buchwald 2002; Buchwald 2005).
This review has taken into account variables which could affect the efficacy of IVIg, including its use for exacerbation or the chronic phase and the concomitant use of corticosteroids or immunosuppressive drugs.
Objectives
To examine the efficacy of IVIg compared to plasma exchange, other treatments or placebo for treating of exacerbations of MG or the chronic phase.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all RCTs or quasi‐RCTs of IVIg for MG.
Types of participants
We included children and adults with generalised MG.
Diagnosis of MG was based on the following three criteria:
acquired weakness of voluntary muscles including those innervated by cranial nerves;
fluctuation of fatigability;
presence of anti‐AChR antibody or anti‐MuSK antibody or decremental electromyographic response (at least a 10% decrease in the amplitude of the muscle action potential when stimulated at three to five Hz), or positive single fiber electromyography (mean jitter more than 20 µs), or an objective improvement with anticholinesterase drugs (for example positive edrophonium test).
We considered two categories of participants.
Participants with exacerbations or worsening of generalised MG. As there is no precise definition of exacerbation or worsening (the former being usually more severe than the latter) we considered exacerbation or worsening as defined by the authors of the trials included in the review.
Participants with chronic generalised myasthenia (severe but stable) treated for reasons other than exacerbation (that is for pre‐operative management, chronic use of plasma exchange, MG refractory to corticosteroids or immunosuppressive drugs).
We did not include participants with pure ocular myasthenia.
Types of interventions
We included trials in which IVIg was compared with plasma exchange, other treatments, or placebo.
Types of outcome measures
Primary outcomes
In participants treated for exacerbation, the primary end point was a change in a specific score between the day before and days seven to fifteen after start of treatment (or day of randomisation). This score was a unified strength/fatigue measurement obtained from the various scores reported. If a unified score could not be obtained, the specific score reported in each individual study was used.
In participants treated for chronic (severe but stable) MG, the primary end point was an improvement by at least one grade in a functional scale (as described below) between the day before and at least six months after start of treatment (or day of randomisation).
Secondary outcomes
-
In participants treated for exacerbation:
improvement by at least one grade in a functional scale including five to six grades (from complete remission to very severe disease requiring admission to hospital) between the day before and seven to fifteen days after start of treatment;
weaning from ventilation before day 15 after start of treatment;
absolute mean reduction in circulating concentrations of anti‐AChR antibodies after treatment.
-
In participants treated for chronic (severe but stable) MG:
remission by the end of one year after starting treatment. Remission was defined as the absence of symptoms or symptoms that were infrequent or sufficiently mild that they did not interfere with normal activities;
delay of the first relapse.
Adverse events related to treatment. The following were included: haemorrhage requiring blood transfusion or a surgical procedure, hypotension requiring vascular expansion, fever (temperature greater than 38°C), acute renal failure, and aseptic meningitis.
Treatment discontinuation due to adverse events.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Disease Group Specialized Register for RCTs using 'myasthenia gravis' and 'intravenous immunoglobulin' as the search terms. We adapted this strategy to search CENTRAL (2011, Issue 3), MEDLINE (January 1966 to September 2011) and EMBASE (January 1980 to September 2011). For the detailed search strategies see Appendix 1 (MEDLINE), Appendix 2 (EMBASE) and Appendix 3 (CENTRAL).
Searching other resources
We checked the bibliographies in reports of the RCTs and contacted their authors (Ronager, Wolfe, Schuchardt) to identify additional published or unpublished data.
Data collection and analysis
Selection of studies
Two review authors (PG and SC) checked the titles and abstracts identified from the register and MEDLINE. All three review authors independently assessed the full texts of all potentially relevant studies. The review authors decided which trials fitted the inclusion criteria and graded their methodological quality. The authors resolved disagreements about inclusion criteria by discussion.
Data extraction and management
A single author (PG) performed data extraction and a second author (SC) checked it , and a third author (KVT) checked the results. We obtained missing data from the trial authors whenever possible.
Assessment of risk of bias in included studies
We assessed risk of bias using the following criteria: sequence generation, allocation concealment, binding, incomplete outcome data, selective outcome reporting and other sources of bias, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We judged the risk of bias for each entry "High risk of bias", "Low risk of bias" or "Unclear risk of bias". One author (PG) assessed the risk of bias and a second author (KVT) checked the risk of bias. We included a 'Risk of bias' summary figure (Figure 1).
1.
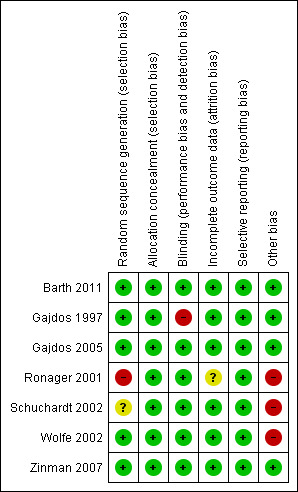
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Data synthesis
If the opportunity for meta‐analysis had arisen, we would have calculated a treatment effect (using a fixed‐effect method) across trials using the Cochrane statistical package Review Manager (RevMan). We would have expressed results as risk ratios (RRs) with 95% confidence intervals (CIs) and risk differences (RDs) with 95% CIs for dichotomous outcomes and mean differences (MDs) and 95% CIs for continuous outcomes.
We would have tested for heterogeneity in the results and undertaken sensitivity analyses on the basis of methodological quality when appropriate. We would also have analysed subgroups of interest. We chose the following predefined subgroups because they represent specific indications for IVIg:
patients who are being treated because of worsening during the initiation of steroids;
patients who are being treated before thymectomy;
patients who are being treated with IVIg or PE alone or patients who are being treated simultaneously with IVIg or PE and with steroids and/or immunosuppressive drugs.
Results
Description of studies
The number of papers found by the new, current strategies are Cochrane Neuromuscular Disease Group Specialized Register, 17 (4 new papers); CENTRAL,10; MEDLINE, 146 (102 new papers), EMBASE 74 (42 new papers).
For the first edition of this review we identified four RCTs (Gajdos 1997; Ronager 2001; Schuchardt 2002; Wolfe 2002). Since the initial publication of the review, one trial comparing two doses of IVIg (Gajdos 2005), one comparing IVIg and placebo (Zinman 2007) and one comparing IVIg and plasma exchange (Barth 2011) have been completed (see Characteristics of included studies). In this update we excluded one study (Liu 2009) because few data were available and we had no response to a request for further information. See Characteristics of excluded studies.
IVIg for treatment of MG worsening or exacerbation
Five trials evaluated IVIg for treatment of MG worsening or exacerbation (Barth 2011; Gajdos 1997; Gajdos 2005; Schuchardt 2002; Zinman 2007).
In the first included RCT (Gajdos 1997), the aim was to compare the efficacy and tolerability of IVIg and of plasma exchange in MG exacerbation and to compare two doses of IVIg. Patients were eligible if:
the diagnosis of MG was based on the following three criteria: (a) acquired weakness of voluntary muscles including those innervated by cranial nerves; (b) fluctuation of muscle fatigability; (c) concentration of anti‐AChR antibodies greater than 1 nM or a decremental electromyographic response (at least 10%) associated with a positive response to anticholinesterase drugs;
the participant had an exacerbation defined as the appearance of at least one of the following symptoms within the last month: difficulty in swallowing, acute respiratory failure or major functional disability responsible for the discontinuation of physical activity.
In the plasma exchange group, participants received three plasma exchanges of 1.5 plasma volume once every two days. The IVIg group had two treatment arms: in one participants received IVIg 0.4 g/kg for three days (total 1.2 g/kg) and in the other treatment arm participants received 0.4 g/kg for five days (total 2 g/kg).
Immunosuppressive treatment with corticosteroids or other drugs was continued without any change in dosage if participants were on these treatments before randomisation and was not allowed if participants had not received these treatments before randomisation.
The primary outcome was the variation of a myasthenic muscle score (MMS) between randomisation and day 15. The MMS is the sum of nine independent observations of trunk, limbs, neck and cranial muscles which when added yield an overall numerical rating between 0 for a maximum deficit and 100 for normal strength (Gajdos 1983) (see Table 1). Secondary outcomes were:
1. Myasthenic muscle score (Gajdos 1983).
| Item | Value | |
| (1) Maintain upper limbs horizontally outstretched | 1 point per 10 seconds | 0 to 15 |
| (2) Maintain lower limbs above bed plane | 1 point per 5 seconds | 0 to 15 |
| (3) Raise head above the plane, while lying on back | ||
| Against resistance | 10 | |
| Without resistance | 5 | |
| Impossible | 0 | |
| (4) Sit up from lying position | ||
| Without help of hands | 10 | |
| Impossible | 0 | |
| (5) Extrinsic ocular musculature | ||
| Normal | 10 | |
| Ptosis | 5 | |
| Double vision | 0 | |
| (6) Eyelid occlusion | ||
| Complete | 10 | |
| Incomplete with corneal covering | 5 | |
| Incomplete without corneal covering | 0 | |
| (7) Chewing | ||
| Normal | 10 | |
| Weak | 5 | |
| Impossible | 0 | |
| (8) Swallowing | ||
| Normal | 10 | |
| Impaired without aspiration | 5 | |
| Impaired with aspiration | 0 | |
| (9) Speech | ||
| Normal | 10 | |
| Nasal | 5 | |
| Slurred | 0 |
the time to the occurrence of a treatment response within the first two weeks, defined as an increase in MMS of at least 20 points compared with the initial value;
the relative variation of anti‐AChR antibody titre between day 0 and day 15;
adverse events.
A sample size of 86 participants was calculated to be sufficient to detect a 50% difference in the change in the mean MMS between the plasma exchange and the IVIg group with 85% power and P = 0.05.
Eighty‐seven participants were included: 41 in the plasma exchange group and 46 in the IVIg group (23 in the three‐day group and 23 in the five‐day group). Participants' characteristics at the time of randomisation were well balanced for age, disease duration, number of thymectomised participants, treatments with prednisone or azathioprine or presence of anti‐AChR antibodies. Mean (SD) MMS was 50.5 (15.7) in the plasma exchange group and 52.6 (15.4) in the IVIg group. Five participants in the plasma exchange group and four in the IVIg group were on mechanical ventilation.
An as yet unpublished RCT (Schuchardt 2002, manuscript available for personal review) compared IVIg to oral methylprednisolone in people with moderate exacerbations of MG. Criteria for inclusion were (1) an increase of at least one point of the Oosterhuis classification; and (2) a sum of the two most pathological criteria of at least three in the quantitative myasthenia gravis score (QMGS) (Besinger 1983; Toyka 2007) (see Table 2). Participants were randomised to receive either IVIg 30 g daily for five consecutive days and placebo tablets or methylprednisolone 1 mg/kg daily increased to 1.5 mg/kg daily on day seven and infusion of one per cent human albumin.
2. Quantitative MG score (Besinger 1983, modified Toyka 2007).
| Test Items | None (0) | Mild (1) | Moderate (2) | Severe (3) |
| 1. Arm outstretched (dominant arm, 90°) (sec) | > 180 | > 60 to180 | 10 to 60 | < 10 |
| 2. Leg outstretched (dominant leg, 45°, supine) (sec) | > 45 | > 30 to 45 | 5 to 30 | < 5 |
| 3. Head, lifted (45°, supine) (sec) | > 90 | > 30 to 90 | 5 to 30 | < 5 |
| 4. Vital capacity (L) | ||||
| Male | >4 | > 2.5 to 4 | 1.5 to 2.5 | < 1.5 |
| Female | > 3 | > 2 to 3 | 1.2 to 2 | < 1.2 |
| Bulbar muscles | ||||
| 5. Facial muscles | Normal | Mild weakness on lid closure, snarl | Incomplete lid closure | Non mimic expressions |
| 6. Chewing | Normal | Fatigue on chewing solid foods | Only soft foods | Gastric tube |
| 7. Swallowing | Normal | Fatigue on normal foods | Incomplete palatal closure, nasal speech | Gastric tube |
The primary outcome was the change in the two most affected criteria of the QMGS from day 0 to day 14. Secondary outcomes were the time needed to improve in strength by one point, the time to reach maximal improvement, the extent of clinical improvement on day 28 and the number of participants who improved by at least one point on day 28 irrespective of treatment after day 14. A sample size of 100 participants had a power of 90% to detect improvement in participants on IVIg compared to participants receiving methylprednisolone at the five per cent level of significance. Thirty‐three participants were included, 15 in the IVIg group and 18 in the methylprednisolone group. Participant groups were balanced except for an older mean age in the methylprednisolone group. The mean (SD) sum of the two most pathological items of the QMGS at day 0 was 3.9 (1.1) for the IVIg group and 4.2 (0.7) for the methylprednisolone group.
In the trial comparing two doses of IVIg (Gajdos 2005) patients were eligible if:
the diagnosis of MG was based on the following three criteria: (a) acquired weakness of voluntary muscles including those innervated by cranial nerves; (b) fluctuation of muscle fatigability; (c) serum concentration of anti‐AChR antibodies greater than 1 nM or a decremental electromyographic response (at least 10%) associated with a positive response to anticholinesterase drugs;
the participant had an exacerbation defined as the appearance of at least one of the following symptoms within the last month: difficulty in swallowing, acute respiratory failure or major functional disability responsible for the discontinuation of physical activity.
Participants were allocated to receive either IVIg 1 g/kg on day one and placebo on day two or IVIg 1 g/kg on days one and two.
The primary outcome was the change of MMS between randomisation and day 15. Secondary outcomes were:
the time to the occurrence of a treatment response within the first two weeks, defined as an increase in MMS of at least 20 points compared with the initial value;
the change of anti‐AChR antibody titres between day 0 and day 15;
adverse events.
A sample size of 170 participants was calculated to be sufficient to detect a 50% difference in the change in the mean MMS between the 1 g/kg IVIg group and the 2 g/kg IVIg group with 90% power and P = 0.05. Altogether 173 participants were randomised and 168 were available for the intention‐to‐treat efficacy analysis: 81 in the 1 g/kg IVIg group and 87 in the 2 g/kg IVIg group. For the safety analysis 172 were available. Participants' characteristics at the time of randomisation were well balanced for age, disease duration, number of thymectomised participants, treatments with prednisone or azathioprine or presence of anti‐AChR antibodies. At baseline, the mean (SD) MMS was 50.47 (15.62) in the 1 g/kg IVIg group and 49.56 (16.56) in the 2 g/kg IVIg group.
Zinman 2007 compared IVIg and placebo in the treatment of people with MG and worsening weakness. People aged 18 or older with a diagnosis of MG and worsening weakness were enrolled. Worsening weakness was defined as increasing symptoms or signs severe enough as judged by both patient and physician to warrant a change in therapy. People were excluded if they had respiratory distress requiring intensive care, a vital capacity less than 1 L, severe swallowing difficulties, a change in corticosteroid dosage in the two weeks prior to screening or other disorders causing weakness.
Participants were allocated to receive either IVIg 2g/kg or the equivalent volume of dextrose 5% over two days. The primary outcome was the change in QMGS modified by Tindall (Tindall 1987, see Table 3) from baseline (day 0) to day 14. Secondary outcomes were:
3. Quantified myasthenia gravis score (modified Tindall 1987).
| Test item | None | Mild | Moderate | Severe | Score |
| Grade | 0 | 1 | 2 | 3 | |
| Double vision on lateral gaze, seconds | 61 | 11 to 60 | 1 to 10 | Spontaneous | |
| Ptosis (upward gaze) seconds | 61 | 11 to 60 | 1 to 10 | Spontaneous | |
| Facial muscle | Normal lid closure | Complete, weak, some resistance | Complete without resistance | Incomplete | |
| Swallowing 4 oz water | Normal | Minimal coughing or throat clearing | Severe coughing/choking or nasal regurgitation | Cannot swallow | |
| Speech after counting aloud from 1 to 50 (onset of dysarthria) | None at 50 | Dysarthria at 30 to 49 | Dysarthria at 10 to 29 | Dysarthria at 9 | |
| Right arm outstretched (90° sitting) seconds | 240 | 90 to 239 | 10 to 89 | 0 to 9 | |
| Left arm outstretched (90° sitting) seconds | 240 | 90 to 239 | 10 to 89 | 0 to 9 | |
| Vital capacity,per cent predicted | >80 | 65 to 79 | 50 to 64 | <50 | |
| Right hand grip, kgW Men Women | > 45 > 30 | 15 to 44 10 to 29 | 5 to 14 5 to 9 | 0 to 4 0 to 4 | |
| Left hand grip, kgW Men Women | > 35 > 25 | 15 to 34 10 to 24 | 5 to 14 5 to 9 | 0 to 4 0 to 4 | |
| Head lifted (45° supine) seconds | 120 | 30 to 119 | 1 to 29 | 0 | |
| Right leg outstretched (45° supine) seconds | 100 | 31 to 99 | 1 to 30 | 0 | |
| Left leg outstretched (45° supine) seconds | 100 | 31 to 99 | 1 to 30 | 0 | |
| Total QMGS range 0 to 39 |
QMGS: quantitative myasthenia gravis score
the change in QMGS from day 0 to day 28 and from day 14 to day 28;
the change in single fibre electromyogram (SFEMG) and repetitive nerve stimulation (RNS) from day 0 to day 14;
post‐intervention status on day 14 and 28.
An analysis of the IVIg treatment effect was performed stratifying participants by baseline severity: mild MG (QMGS <10.5) and moderate to severe MG (QMGS > 10.5). A sample of 22 participants per treatment arm was calculated to be sufficient to detect a difference in mean change between the groups of 3.5 units in the QMGS at a significance level of 0.05 with 80% power.
Fifty‐one participants were included. The baseline characteristics were balanced between the IVIg and placebo groups without any significant difference in age, disease duration, thymoma, treatment with corticosteroids or immunosuppressant drugs, thymectomy, AChRAb level or EMG abnormalities. Mean (SD) QMGS at baseline was 12.3 (4.89) in the IVIg group and 12.5 (5.49) in the placebo group. Seventeen participants were classified as ocular MG, 23 as mild MG and 28 as moderate to severe MG.
Barth 2011 compared IVIg and plasma exchange in the treatment of people with moderate to severe MG, defined as a QMGS > 10.5 and worsening weakness requiring a change in treatment modality as judged by a neuromuscular expert.
Participants were allocated to receive either IVIg 2 g/kg over two days or five plasma exchanges of 1.0 plasma volume. The primary outcome was the change in QMGS (Table 3) from baseline (day 0) to day 14. Secondary outcomes were:
the change in QMGS from baseline to day 21 and 28;
the change in single fibre electromyogram (SFEMG) and repetitive nerve stimulation (RNS) from day 0 to days 14, 21 and 28;
post‐intervention status at days 14, 21 and 28;
change in AChRab titers from baseline to days 28 and 60;
the need for intensive care unit (ICU) admission, mechanical ventilation and additional therapy for MG.
Responders were defined as those who had a decrease in QMGS of ≥ 3.5 units.
A sample of 29 participants per arm was sufficient to detect a true difference in QMGS of 3.5 with 90% power and P = 0.05. Eighty‐four participants were randomised. The treatment groups were balanced for age, duration of disease, current medications, AChRab or anti‐MuSKab and EMG abnormalities. Mean (SD) QMGS at baseline was 14.3 (4.0) in the IVIg group and 14.35 (3.8) in the plasma exchange group.
IVIg for treatment of chronic (or severe but stable) MG
Two trials evaluated IVIg for the treatment of chronic (or severe but stable) MG (Ronager 2001; Wolfe 2002).
In Ronager 2001, the purpose was to compare the efficacy of IVIg versus plasma exchange in people with moderate to severe MG in a stable phase. It was a controlled cross‐over study. Participants were included if (1) they were in Osserman class III to V and if they were restricted in daily activities or completely dependent on skilled care for support; (2) if they were treated with prednisone or azathioprine; (3) they had anti‐AChR antibodies and a significant decrement (15 %) on EMG. Participants were randomly assigned to receive either IVIg 0.4 g/kg on five subsequent days and 16 weeks later five plasma exchanges every other day, or firstly five plasma exchanges and 16 weeks later IVIg. The primary outcome was the clinical improvement measured before and seven days after each treatment using the QMGS (see Table 3).
Secondary outcomes were decrease in anti‐AChR antibodies titre, change in decrement and the clinical effect assessed 4, 8 and 16 weeks after each treatment. A sample size of 20 participants was calculated as sufficient to identify a difference in QMGS of 0.3 or 20% in response with a power of 80% and P = 0.05. Twelve participants were included.
In Wolfe 2002, IVIg was compared to five per cent albumin as placebo. Criteria for inclusion were (1) mild or moderate generalised MG for participants who have never received corticosteroids or immunosuppressive therapy; (2) persistent symptoms and signs of generalised MG despite taking prednisone at a dose over 20 mg on alternate days. Participants were randomised to receive either IVIg 1 g/kg or five per cent albumin placebo on days one and two. A 1 g/kg infusion of IVIg or placebo was repeated on day 22. The primary outcome was the change in the QMGS (see Table 3) from baseline (day 0) to day 42. Secondary outcomes were the change from day 0 to day 42 in the decrement after repetitive nerve stimulation, of the mean jitter on single fiber electromyography and in the MG‐activities of daily living profile (MG‐ADL). At day 42 participants were invited into a six‐week open label study that followed an identical protocol to the randomised trial.
A sample size of 88 participants had a power of 80% to detect a difference of 3.5 units on the QMGS at the five per cent level of significance. Fifteen participants were included, six in the IVIg arm and nine in the placebo arm.
Risk of bias in included studies
In the Gajdos 1997 RCT, the method of randomisation was clearly described, stratified by centre and according to the previous use of corticosteroids or other immunosuppressive drugs. The number of participants needed was calculated. No participants were lost to follow‐up. Neither participants nor observers were blinded.
In the Ronager 2001 RCT, the method of randomisation was done by means of sealed envelopes. However, the allocation was skewed: eight participants were randomised to IVIg followed by plasma exchange and four to the opposite regimen. The number of participants required was calculated but not obtained. Observers, but not participants, were blinded.
In the Wolfe 2002 RCT, a computerised randomisation performed to stratify participants across 12 dichotomous variables and with a block size of four was used. Observers and participants were blinded. The study was underpowered (maximum power: 0.56).
In the Schuchardt 2002 RCT, allocation concealment was adequate. The number of participants required was calculated but not obtained. Observers and participants were blinded. The results were based on an intention‐to‐treat analysis. The participant groups were unbalanced only for age.
In the Gajdos 2005 RCT comparing two doses of IVIg, randomisation and concealment were adequate. Observers and participants were blinded. The number of participants required was calculated and obtained.
In the Zinman 2007 RCT comparing IVIg and a placebo, randomisation and concealment were adequate. Observers and participants were blinded. The number of participants required was calculated and obtained.
In the Barth 2011 RCT comparing IVIg and plasma exchange, randomisation and concealment were adequate. Observers and participants were blinded. The number of participants required was calculated and obtained.
See Characteristics of included studies for 'Risk of bias' tables and Figure 1 for a 'Risk of bias' summary figure.
Effects of interventions
IVIg for treatment of MG worsening or exacerbation
Primary outcome measure
Five RCTs reported the change in a specific score. In the Gajdos 1997 trial comparing IVIg and plasma exchange, the mean change in MMS between day 0 and day 15 was not significantly different between the two treatment groups: 15.60 in the IVIg group versus 16.60 in the PE group, MD ‐1.00 in favour of IVIg (P = 0.77) (Analysis 1.1). In the IVIg group, the mean change was 18.9 in the three‐day IVIg group and 12.4 in the five‐day IVIg group (P = 0.14 Wilcoxon test). Of the 87 participants included, 48 treatment responses were observed, 26 in the plasma exchange group and 22 in the IVIg group (14 in the three‐day and 8 in the five‐day group). A subgroup analysis was not available for 24 participants without anti‐AChR antibodies. In the second trial (Schuchardt 2002) comparing IVIg and methylprednisolone, the mean (SD) change between day 0 and day 14 in the two most abnormal items of the QMGS was 0.93 (1.10) in the IVIg group and 1.35 (1.17) in the methylprednisolone group, MD ‐0.42 (95% CI ‐1.20 to 0.36) (P = 0.29), slightly but not significantly in favour of IVIg (Analysis 2.1). The change in the total QMGS was 1.87 (2.82) in the IVIg and 2.53 (2.79) in the methylprednisolone group (P = 0.51).
1.1. Analysis.
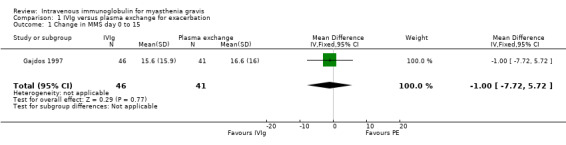
Comparison 1 IVIg versus plasma exchange for exacerbation, Outcome 1 Change in MMS day 0 to 15.
2.1. Analysis.
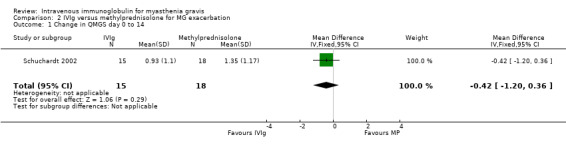
Comparison 2 IVIg versus methylprednisolone for MG exacerbation, Outcome 1 Change in QMGS day 0 to 14.
In the only RCT comparing IVIg with a placebo (Zinman 2007), the mean (SD) change on day 14 in QMGS was ‐2.5 (3.4) in the IVIg group and ‐0.9 (2.4) in the placebo group MD ‐1.60 (95% CI ‐3.23 to 0.03) (P = 0.05) this result being borderline statistically significant in favour of IVIg (Analysis 3.1).There was evidence of an interaction between treatment and disease stage (mild versus moderate and severe) both at day 14 (P < 0.025) and at day 28 (P < 0.025). In subgroup analyses (as specified in the trial protocol) there was no evidence of an effect in the mild MG but the effect was significant in the moderate to severe MG. The MD on day 14 was ‐3.40 (95% CI ‐5.74 to ‐1.06) (P = 0.004) in favour of IVIg (Analysis 3.5). On day 28 the MD in the change in QMGS was ‐1.80 (95% CI ‐3.64 to 0.04) (P = 0.06) (Analysis 3.2). However, on day 28 the effect was significantly in favour of IVIg in the moderate to severe MG group (Analysis 3.6) but not in the mild group (Analysis 3.4).
3.1. Analysis.

Comparison 3 IVIg versus placebo, Outcome 1 Change in QMGS day 0 to 14.
3.5. Analysis.
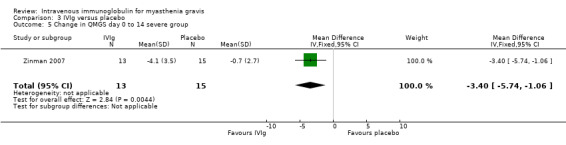
Comparison 3 IVIg versus placebo, Outcome 5 Change in QMGS day 0 to 14 severe group.
3.2. Analysis.

Comparison 3 IVIg versus placebo, Outcome 2 Change in QMGS day 0 to 28.
3.6. Analysis.
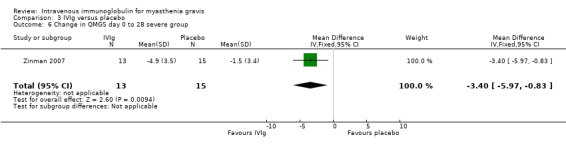
Comparison 3 IVIg versus placebo, Outcome 6 Change in QMGS day 0 to 28 severe group.
3.4. Analysis.
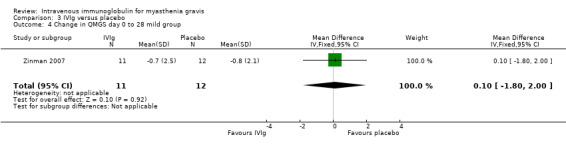
Comparison 3 IVIg versus placebo, Outcome 4 Change in QMGS day 0 to 28 mild group.
In the trial comparing two doses of IVIg (Gajdos 2005), the mean change in MMS between day 0 and day 15 was not significantly different between the two treatment groups, 15.49 points (95% CI 12.09 to 18.90) in the 1g/kg IVIg group and 19.33 points (95% CI 15.82 to 22.85) in the 2 g/kg IVIg group, MD 3.84 (95% CI ‐0.98 to 8.66) (P= 0.12) in favour of IVIg 2g/kg (Analysis 4.1). There was no evidence of a time‐by‐treatment interaction (P = 0.10). Similarly, no time‐by‐treatment interaction was found for the treatment response rate (P = 0.36). Similar numbers of participants responded at least once within the first two weeks (44 in the 1 g/kg IVIg group and 52 in the 2 g/kg IVIg group, respectively). In an analysis restricted to those who responded the median time to response was similar in the two groups (13.5 days and 12 days in the 2 groups respectively, P = 0.48). Participants' characteristics were well balanced between the two groups, nevertheless this result should be interpreted cautiously since the omission of non‐responders means that this is not a true randomised comparison.
4.1. Analysis.

Comparison 4 IVIg 1g/kg versus IVIg 2g/kg, Outcome 1 Change in MMS day 0 to 15.
In the trial comparing IVIg and plasma exchange for myasthenia worsening (Barth 2011), the mean change in QMGS was not significantly different between the two treatment groups. From baseline to day 14 the mean change in QMGS was 3.2 (95% CI 2 to 4.5) in the IVIg group and 4.7 (95% CI 3.2 to 6.9) in the plasma exchange group, MD ‐1.50 (95% CI ‐3.43 to 0.43) (P = 0.13) (Analysis 5.1). From baseline to day 21 and day 28 the MD in QMGS was respectively: ‐2.00 (95% CI ‐3.98 to ‐0.02) and ‐2.10 (95% CI ‐4.20 to ‐0.00) (Analysis 5.2; Analysis 5.3).
5.1. Analysis.

Comparison 5 IVIg versus plasma exchange for worsening MG, Outcome 1 Change in QMGS day 0 to day 14.
5.2. Analysis.
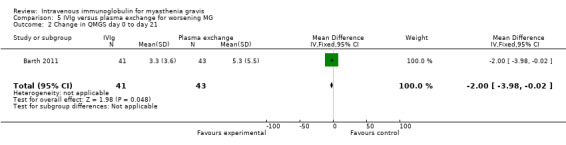
Comparison 5 IVIg versus plasma exchange for worsening MG, Outcome 2 Change in QMGS day 0 to day 21.
5.3. Analysis.
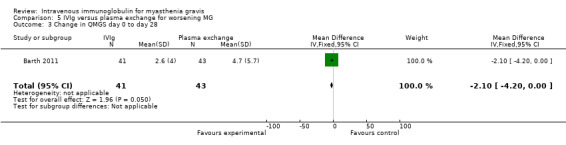
Comparison 5 IVIg versus plasma exchange for worsening MG, Outcome 3 Change in QMGS day 0 to day 28.
Secondary outcome measures
The change in functional scale and percentage of participants weaned from mechanical ventilation were not available from any of the trials. Among the 63 participants with detectable anti‐AChR antibodies in the Gajdos 1997 trial, 39 (62%) exhibited a decrease in concentration on day 15 compared with that measured at randomisation: 19 of 41 participants in the plasma exchange group and 20 of 46 participants in the IVIg group. The mean change in anti‐AChR antibody titres was not significantly different 14 days after IVIg when compared with plasma exchange (Gajdos 1997) or after 1 g/kg IVIg compared with 2 g/kg IVIg (Gajdos 2005). This information was not available in the trial comparing IVIg with a placebo or in the trial of IVIg versus methylprednisolone.
In the trial comparing IVIg and plasma exchange for myasthenia worsening (Barth 2011) responders were 51% of participants on IVIg and 57% of the participants on plasma exchange (Chi2 test P = 0.5). At day 14 the post‐intervention status improved in 69% of participants on IVIG and 65% of participants on plasma exchange, odds ratio 1.15 (95% CI 0.46 to 2.86). The mean change in anti‐AChR antibodies titre was not significantly different between the IVIg group and the plasma exchange group.
Since the comparisons in each of the five trials were different, a meta‐analysis was not considered appropriate.
IVIg for treatment of chronic (or severe but stable) MG
Primary outcome measure
Change in functional scale after treatment was not available in the RCT of IVIg versus plasma exchange (Ronager 2001). In the RCT comparing IVIg and placebo (Wolfe 2002), a functional scale (MG‐ADL) was used as a secondary outcome: the difference in the mean changes on this scale between the two groups was 2.30 (95% CI 0.06 to 4.54) in favour of the placebo group (P = 0.04) (Analysis 6.2). In fact in these two RCTs, the primary outcome measure was the change in QMGS after treatment. In the RCT of IVIg versus placebo (Wolfe 2002), mean (SD) change in the QMGS from day 0 to day 42 was 0.00 (3.8) in the IVIg group and ‐1.6 (2.7) in the placebo group, MD ‐1.60 (95% CI ‐1.92 to 5.12)(P = 0.37)(Analysis 6.1). No significant differences were observed in secondary outcome measurements. On conclusion of the open‐label segment the change in the QMGS for the participants who initially received placebo was ‐2.0 (2.1) from day 0 to day 42 (P = 0.03) and ‐3.6 (3.8) from day 0 to day 84 (P = 0.01) mean change in QMGS from baseline to day 42 was also not significantly different between the two treatment arms. In the Ronager 2001 trial comparing the clinical effect of plasma exchange or IVIg from baseline to one and four weeks, no significant difference could be detected (data not published). The authors report that the mean fall in QMGS was 0.23 (P < 0.05) after plasma exchange and 0.10 (NS) after IVIg from baseline to one week, although it should be recognised that such a comparison of P values is an inappropriate, and potentially misleading, way to report results from clinical trials. From baseline to four weeks, the mean fall in QMGS both after plasma exchange and after IVIg was significant (P < 0.05, mean values not published). The change from baseline to eight or 16 weeks was not significant for either plasma exchange or for IVIg.
6.2. Analysis.
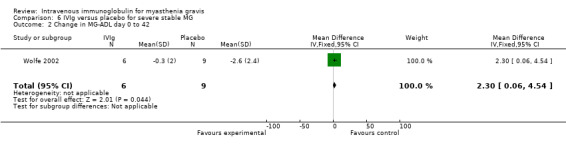
Comparison 6 IVIg versus placebo for severe stable MG, Outcome 2 Change in MG‐ADL day 0 to 42.
6.1. Analysis.
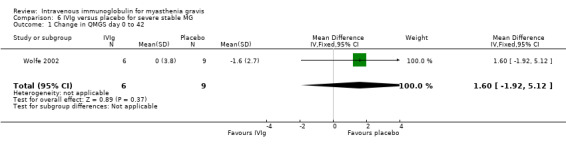
Comparison 6 IVIg versus placebo for severe stable MG, Outcome 1 Change in QMGS day 0 to 42.
Secondary outcome measures
The percentage of participants in remission by the end of one year and the delay from treatment to the first relapse were not reported in these RCTs.
Adverse events
Adverse events related to IVIg were observed in all the trials. One hundred and ninety adverse events were observed among 304 participants treated with IVIg in the six RCTs: fever or chills (13.8%), headaches (17.4%), nausea (6.9%), allergic reaction (1.3%), and others 11.5%. These adverse events would be considered subjectively as less severe than with plasma exchange where arterial bleeding, bleeding disorders, septicaemia and venous thrombosis were reported (Gajdos 1997; Ronager 2001) but, given the available data, no statistical comparison is possible. In the trial comparing two doses of IVIg (Gajdos 2005), serum creatinine elevation occurred in 20 of 172 participants (11.7%).
Discussion
Seven RCTs on IVIg were analysed, including one as yet unpublished study. The methodological quality of these trials was sometimes debatable.
IVIg for treatment of MG worsening or exacerbation
Five trials addressed the efficacy of IVIg for the treatment of MG worsening or exacerbation.
Overall these trials demonstrate the effectiveness of IVIg and a comparable efficacy of IVIg and plasma exchange for the treatment of MG worsening or exacerbation. The RCT comparing IVIg versus plasma exchange for exacerbation (Gajdos 1997) included the number of participants required but neither participants nor observers were blinded. However, the results were assessed by a score which has a good interobserver agreement (Sharshar 2000). Within these limitations, this RCT showed roughly the same efficacy of IVIg and plasma exchange for the treatment of MG exacerbation. The RCT comparing IVIg to methylprednisolone (Schuchardt 2002) did not recruit the number of participants required and so was underpowered in relation to the study objectives. The trial of IVIg versus placebo (Zinman 2007) was double blind, included the required number of participants and assessed the results with a score which is widely accepted for evaluation of clinical change. The very modest improvement observed with IVIg in this trial was probably due to the heterogeneity of the included population, that is to say the large number of people with ocular or mild MG. Nevertheless, this trial demonstrated the efficacy of IVIg in moderate or severe MG worsening. The same group comparing IVIg and plasma exchange in the treatment of patients with worsening myasthenia weakness (Barth 2011) found the same efficacy for the two treatments in a well designed trial. It is noteworthy that in the trial conducted by Zinman (Zinman 2007), people were excluded if they developed MG crisis or severe swallowing impairment and in the other RCTs dealing with exacerbation or worsening (Barth 2011; Gajdos 1997; Schuchardt 2002), the number of MG crises included was small or nil. So it is still not clear whether the conclusion concerning the efficacy of IVIg in the treatment of MG worsening or exacerbation is also valid if the patient is in MG crisis. The RCT comparing two doses of IVIg for the treatment of MG exacerbation (Gajdos 2005) had high methodological quality, large numbers and good compliance. It showed no significant superiority of IVIg 1 g/kg for two days (total 2 g/kg) as compared to IVIg 1 g/kg on a single day, although there was a trend toward a slight superiority of IVIg 2 g/kg. This result does not rule out a smaller benefit from the larger dose of IVIg, i.e. a smaller than 50% difference in MMS increase between 1 g/kg and 2 g/kg IVIg. The review authors felt that such a small difference would not be clinically relevant. Thus 1 g/kg IVIg on a single day may be a sufficient dose for the treatment of MG exacerbations.
IVIg for treatment of chronic (or severe but stable) MG
Two RCTs addressed the efficacy of IVIg for the treatment of moderate to severe but stable MG, the first versus plasma exchange and the second versus placebo. Neither showed a significant difference. However, because of an insufficient number of included participants, data from these trials could not support or refute a role for IVIg in chronic MG.
The changes in anti‐AChR antibody titre 15 days (Gajdos 1997), seven days (Ronager 2001) or 60 days (Barth 2011) after IVIg treatment were not significant. However, the use of change in antibodies titre as a secondary endpoint is debatable for theoretical reasons. We do not know the mechanisms of actions of IVIg, which might include down‐regulation of antibody production, idiotypic‐anti‐idiotypic interaction, modulation of T‐cell functions and neutralising effects (Buchwald 2002; Buchwald 2005; Dalakas 1999). On the other hand, if any change in anti AChR antibodies does occur, its kinetics are unknown and could be earlier than day 15.
No subgroup analysis is available concerning the efficacy of IVIg for anti‐Musk antibody positive or seronegative MG. A negative test for antibodies to AChR was an exclusion criterion in three trials. It is usually reported that anti‐MuSK antibody positive or seronegative MG, but otherwise typical patients, may respond to treatment in the same way as anti‐AChR antibody positive MG .
Adverse events due to IVIg were observed in all trials. They were moderate (fever, nausea, headache), self‐limiting and less severe than those seen with plasma exchange.
The treatment effect across trials was not calculated because it would have been inappropriate to calculate this effect with RCTs comparing IVIg to different alternatives (plasma exchange or corticosteroids or placebo). Neither were the participants identical in all these trials: participants with exacerbation in three trials, MG worsening in two and participants with moderate or severe but stable MG in two others. Furthermore, outcomes were not identical.
Several comparative, but non‐randomised, studies or case series reporting on the efficacy of IVIg for the treatment of MG have been published. All but one retrospective multicentre chart review (Qureshi 1999) support the efficacy of IVIg.
Several non‐randomised studies suggest the potential of IVIg for the treatment of MG in some situations which were not addressed by the published RCTs, namely the perioperative period and long‐term use of IVIg as a maintenance treatment.
Perez‐Nellar 2001 compared a prospective group of 33 people with MG treated with IVIg with a historical group of 38 people with MG treated with plasma exchange during the peri‐operative period of thymectomy. In the prospective group, participants received IVIg 2 g/kg (two‐thirds of the dose before thymectomy and one‐third after). In the retrospective group, participants were treated with three plasma exchanges on alternate days before thymectomy and two plasma exchanges on alternate days afterwards. The duration of mechanical ventilation was not different between the two treatment groups: 14.1 hours (95% CI 10.71 to 17.31) in the IVIg group and 17.24 hours (95% CI 12.54 to 21.94) in the plasma exchange group, MD ‐3.23 (95% CI ‐8.71 to 2.31). The time in the intensive care unit was shorter in the IVIg group: 3.36 days (95% CI 2.9 to 3.82) in the IVIg group compared with 4.34 days (95% CI 3.76 to 4.92) in the plasma exchange group, MD ‐ 0.98 ( 95% CI ‐1.72 to ‐0.24). More recently, Jensen published a retrospective study comparing IVIg used in nine patients and plasma exchange used in nine matched patients as prethymectomy treatment (Jensen 2008). The mean (SD) change in Osserman grade (Osserman 1971) from the pre‐ and postoperative period was 1.00 (0.71) (95% CI 0.53 to 1.47) for the plasma exchange group and 0.78 (0.83) (95% CI 0.23 to 1.33) (P= 0.55) for the IVIg group. There was no significant difference in the hospital stay between the two groups.
IVIg has been proposed as a maintenance therapy for MG. Achiron 2000 evaluated the efficacy of IVIg in an open study of 10 people with severe generalised MG and an acute deterioration that was unresponsive to conventional treatment with corticosteroids and immunosuppressive drugs. IVIg was administered at a loading dose of 2 g/kg over five days, and maintenance IVIg treatment at 0.4 g/kg once every six weeks. The mean (SD) severity of the disease decreased by 2.5 (0.8) grades on the Osserman scale after one year (P < 0.001) with a parallel reduction of prednisone and azathioprine doses. Hilkevich 2001 treated 11 people with generalised MG. All had severe bulbar involvement. IVIg was initiated at a dose of 2 g/kg over five days followed by 0.4 g/kg once monthly for a mean period of 20.3 (8.3) months. All improved. The Oosterhuis grade (Oosterhuis 1983) was 4.0 (0.7) before IVIg and 2.0 (0.8) at the end of follow‐up (P < 0.00005). At the same time prednisone dosage was reduced from 60 mg daily to 9.25 mg on alternate days (P < 0.0004) without any attempt to reduce the azathioprine dose. However, it was difficult to deduce from these uncontrolled studies whether there is indeed a drug sparing effect of IVIg.
In conclusion, three RCTs addressed the efficacy of IVIg compared with placebo (Zinman 2007) or with plasma exchange for the treatment of MG worsening or exacerbation (Barth 2011; Gajdos 1997) and demonstrated the efficacy of IVIg in this specific situation. This conclusion is in line with many case series. Improvement by IVIg in functional outcome of people with mild to severe but stable myasthenia gravis has not been demonstrated. There were methodological flaws in the two RCTs that addressed this problem. The efficacy of IVIg in the peri‐operative period and the sparing effect of IVIg on corticosteroids dosage are also suggested but not proved by two case series.
Therefore new and better designed RCTs should be planned. Issues of current concern are the confirmation of the efficacy of IVIg in the treatment of myasthenia gravis exacerbations or crisis, demonstrated only by one RCT, further determination of the dose of IVIg necessary for this indication evaluated only by one RCT, the long‐term effect of IVIg in moderate or severe MG and the sparing effect of IVIg on corticosteroid dosage.
It would be difficult to compare IVIg with a placebo for the treatment of MG exacerbations or crisis after the RCT which compared IVIg with plasma exchange. In other situations such as treatment of moderate or severe MG, the data in the literature are so inconclusive that comparison of IVIg with placebo would be justified. Study of the sparing effect of IVIg on corticosteroid dosage or the supplementary effect of IVIg on functional outcome when added to long‐term but ineffective immunosuppressive medication would be justified.
In future studies, inclusion criteria should be clearly defined and the severity of MG and concomitant treatment described. Outcome measures should be clinically and statistically significant and validated. The primary outcome measure for a RCT looking at the efficacy of IVIg for the treatment of MG exacerbations or crisis could be the change in a score of muscle strength 15 or 30 days after initiation of IVIg. In RCTs looking for an improvement in functional outcome in people with moderate or severe MG, the best outcome measure could be the change in functional class or the change in post‐intervention status as recently proposed by the Myasthenia Gravis Foundation of America (Jaretzki 2000). To demonstrate the sparing effect of IVIg on corticosteroid dosage, the outcome measure could be the cumulative dose of corticosteroid necessary to obtain and maintain a remission. The number of participants to be included in these trials should be carefully calculated so that the trial will have sufficient power to allow valid conclusions about the main comparison.
Authors' conclusions
Implications for practice.
In myasthenia gravis worsening, one RCT of IVIg versus placebo showed some evidence of the efficacy of IVIg and two did not show a significant difference between IVIg and plasma exchange in severe myasthenia gravis worsening or with exacerbation. Another showed no significant difference in efficacy between 1 g/kg and 2 g/kg of IVIg. A further, yet underpowered, trial showed no significant difference between IVIg and oral methylprednisolone.
In chronic (moderate or severe but stable) myasthenia gravis, there is insufficient evidence from RCTs to determine whether IVIg is efficacious.
Implications for research.
Further RCTs are needed to confirm the effectiveness of IVIg compared to plasma exchange for the treatment of MG crisis and to determine the indications for IVIg in moderate and severe but stable MG. More research is needed to determine whether IVIg improves MG in the perioperative period or reduces the need for corticosteroids as suggested by case series.
What's new
| Date | Event | Description |
|---|---|---|
| 8 December 2011 | New citation required but conclusions have not changed | Searches updated to 11 October 2011 |
| 8 December 2011 | New search has been performed | 'Risk of bias' tables added. One new trial of intravenous immunoglobulin included. Discussion modified. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 19 June 2008 | Amended | Converted to new review format. |
| 14 November 2007 | New citation required and conclusions have changed | We updated the search of the Cochrane Neuromuscular Disease Trials Register (April 2007) and MEDLINE (January 1966 to May 2007). We included one new randomised controlled trial comparing two doses of IVIg (Gajdos 2005). |
Acknowledgements
We thank Professor Djillali Annane for his technical assistance.
The editorial base of the Cochrane Neuromuscular Disease Group is supported by the MRC Centre for Neuromuscular Diseases and the Muscular Dystrophy Campaign.
Appendices
Appendix 1. MEDLINE (OvidSP) Search Strategy
1 randomized controlled trial.pt. (319110) 2 controlled clinical trial.pt. (83678) 3 randomized.ab. (224381) 4 placebo.ab. (129163) 5 drug therapy.fs. (1505532) 6 randomly.ab. (161540) 7 trial.ab. (232182) 8 groups.ab. (1070965) 9 or/1‐8 (2788051) 10 exp animals/ not humans.sh. (3698786) 11 9 not 10 (2365479) 12 myastheni$.tw. or exp Myasthenia Gravis/ (14782) 13 Immunoglobulins, Intravenous/ (8414) 14 (intravenous immunoglobulin$ or intra‐venous immunoglobulin$ or ivig).tw. (7664) 15 13 or 14 (11298) 16 11 and 12 and 15 (146)
Appendix 2. EMBASE (OvidSP) Search Strategy
1 crossover‐procedure/ (30907) 2 double‐blind procedure/ (100996) 3 randomized controlled trial/ (290224) 4 single‐blind procedure/ (14260) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1024327) 6 or/1‐5 (1094729) 7 exp animals/ (1655604) 8 exp humans/ (12628304) 9 7 not (7 and 8) (1260032) 10 6 not 9 (1058983) 11 limit 10 to embase (858133) 12 myasthenia gravis/ or myastheni$.tw. (17548) 13 exp immunoglobulin/iv [Intravenous Drug Administration] (17861) 14 (intravenous immunoglobulin$ or intra‐venous immunoglobulin$ or ivig).mp. (10754) 15 13 or 14 (23879) 16 11 and 12 and 15 (74)
Appendix 3. CENTRAL search strategy
#1(myasthenia gravis):ti,ab,kw #2MeSH descriptor Immunoglobulins, Intravenous, this term only #3immunoglobubin NEXT intravenous or immunoglobulins NEXT intravenous #4(#2 OR #3) #5(#1 AND #4)
Data and analyses
Comparison 1. IVIg versus plasma exchange for exacerbation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in MMS day 0 to 15 | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐7.72, 5.72] |
Comparison 2. IVIg versus methylprednisolone for MG exacerbation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in QMGS day 0 to 14 | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐1.20, 0.36] |
Comparison 3. IVIg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in QMGS day 0 to 14 | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐3.23, 0.03] |
| 2 Change in QMGS day 0 to 28 | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.8 [‐3.64, 0.04] |
| 3 Change in QMGS day 0 to 14 mild group | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.33, 2.13] |
| 4 Change in QMGS day 0 to 28 mild group | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.80, 2.00] |
| 5 Change in QMGS day 0 to 14 severe group | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.74, ‐1.06] |
| 6 Change in QMGS day 0 to 28 severe group | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.97, ‐0.83] |
3.3. Analysis.
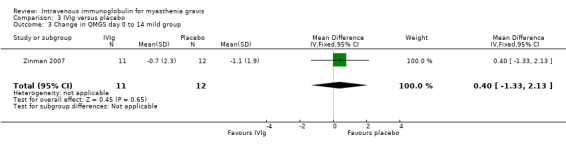
Comparison 3 IVIg versus placebo, Outcome 3 Change in QMGS day 0 to 14 mild group.
Comparison 4. IVIg 1g/kg versus IVIg 2g/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in MMS day 0 to 15 | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 3.84 [‐0.98, 8.66] |
Comparison 5. IVIg versus plasma exchange for worsening MG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in QMGS day 0 to day 14 | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐3.43, 0.43] |
| 2 Change in QMGS day 0 to day 21 | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.98, ‐0.02] |
| 3 Change in QMGS day 0 to day 28 | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐2.1 [‐4.20, ‐0.00] |
Comparison 6. IVIg versus placebo for severe stable MG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in QMGS day 0 to 42 | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [‐1.92, 5.12] |
| 2 Change in MG‐ADL day 0 to 42 | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [0.06, 4.54] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barth 2011.
| Methods | RCT: 2 parallel groups | |
| Participants | 84 participants with moderate to severe myasthenia gravis and worsening weakness | |
| Interventions | Group 1: IVIg 2g/kg Group 2: 5 PEs |
|
| Outcomes | Primary outcome: change in QMGS (Table 3) from baseline to day 14 Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation. Block of four |
| Allocation concealment (selection bias) | Low risk | Block of four with different order of treatment kept by Dr Barth who performed the treatment and not available to observer |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only observer were blinded (single blind study) but the non‐blinding of participants is unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The report include all expected outcomes |
| Other bias | Low risk | No other bias suspected |
Gajdos 1997.
| Methods | RCT: 2 parallel groups | |
| Participants | 87 participants with MG exacerbation | |
| Interventions | Group 1: 3 PEs Group 2: 2a: IVIg 2 g/kg; 2b: IVIg 1.2 g/kg | |
| Outcomes | Primary outcome: variation of a myasthenic muscle score (MMS) between randomisation and day 15 Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation through a centralised telephone, stratified by centre and previous treatment |
| Allocation concealment (selection bias) | Low risk | Central allocation and pharmacy controlled |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | The published report include all outcomes prespecified in the protocol |
| Other bias | Low risk | No other bias suspected |
Gajdos 2005.
| Methods | RCT: 2 parallel groups | |
| Participants | 173 participants with MG exacerbation | |
| Interventions | Group 1: IVIg 1g/kg Group 2: IVIg 2 g/kg | |
| Outcomes | Primary: change of MMS between randomisation and day 15 Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation through a centralised telephone, stratified by centre and previous treatment |
| Allocation concealment (selection bias) | Low risk | Drug container of identical appearance prepared by pharmacy in each centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias suspected |
Ronager 2001.
| Methods | RCT: cross‐over study | |
| Participants | 12 participants with stable moderate or severe MG | |
| Interventions | Group 1: IVIg 0.4 g/kg for 5 days and 16 weeks later 5 PE Group 2: opposite schedule | |
| Outcomes | Primary outcome: clinical improvement measured before and seven days after each treatment using the QMGS Secondary outcomes: decrease in anti‐AChR antibodies titre, change in decrement and the clinical effect assessed four, eight and 16 weeks after each treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Eight participants were randomised to IVIg followed by plasma exchange and four to the opposite regimen |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only observers were blinded (single blind study) but the non‐blinding of participants is unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this issue |
| Selective reporting (reporting bias) | Low risk | Protocol not available but the report includes all expected outcomes |
| Other bias | High risk | The number of participants required was calculated but not obtained |
Schuchardt 2002.
| Methods | RCT: 2 parallel groups | |
| Participants | 33 participants with MG exacerbation | |
| Interventions | Group 1: MP 1 to 1.5 mg/kg for 14 days Group 2: IVIg 30 g/d for 5 days | |
| Outcomes | Primary outcome: change in the two most affected criteria of the QMGS from day 0 to day 14 Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug container of identical aspect prepared in a central pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | High risk | 100 participants required, 33 included. Never published |
Wolfe 2002.
| Methods | RCT: 2 parallel groups | |
| Participants | 15 participants with mild or moderate MG | |
| Interventions | Group 1: IVIg 1 g/kg for 2 days
Group 2: 5% albumin for 2 days 1 g/kg infusion of IVIg or placebo repeated on day 22 |
|
| Outcomes | Primary outcome: change in the QMGS from baseline to day 42 Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation. Block of four |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug container of identical aspect |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The published report include all outcomes pre‐specified in the protocol |
| Other bias | High risk | 88 participants required/15 included |
Zinman 2007.
| Methods | RCT: 2 parallel groups | |
| Participants | 51 participants with worsening weakness | |
| Interventions | Group1: IVIg 1 g/kg for 2 days Group 2: 5% dextrose for 2 days | |
| Outcomes | Primary outcome: change in QMGS from baseline to day 14 .Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation. Block of four |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug container of identical aspect |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol not available but the report include all expected outcomes |
| Other bias | Low risk | No other bias suspected |
ICU: intensive care unit IVIg: intravenous immunoglobulin MG: myasthenia gravis MMS : myasthenic muscular score MP: methylprednisolone PE: plasma exchange QMGS: quantified myasthenia gravis score RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Liu 2009 | Study comparing double filtration plasmapheresis, immunoabsorption and IVIg for late onset myasthenia gravis, but with insufficient information for inclusion |
Differences between protocol and review
For this update, we changed an element of the third criterion for diagnosis of MG from "Concentration of anti‐AChR antibody greater than 1 nm" to "Presence of anti‐AChR antibody".
We decided to adopt the definition of exacerbation or worsening used by the authors of the included trials, which varies across the trials. We included information on the definition used for each trial.
We assessed study quality using the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
Philippe Gajdos extracted the data and wrote the first draft of the review and updates. Sylvie Chevret and Klaus Toyka checked the data and the drafts.
Declarations of interest
One review author (PG) conducted two trials on IVIg supported by the Laboratoire Francais de Biotechnologie (LFB). This support consisted of the provision of IVIg but no other financial support. One review author (KVT) participated in the trial by Schuchardt 2002 (unpublished) as the chairman of the Advisory Board (receiving compensation of the equivalent of 500 Euros) and as a senior co‐author of the manuscript originally submitted but not published (no payments received). He participated in a conference of IgG treatments in chronic inflammatory neruopathy (receiving a payment of 3000 US$) and performed a research project on the neutralising role of IgG in animal models of inflammatory neuropathies and of Lambert‐Eaton myasthenic syndrome (public funding, no payments).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barth 2011 {published data only}
- Barth D, Nabavi Nouri M, Ng E, Nwe P, Bril V. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011;76(23):2017‐23. [PUBMED: 21562253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gajdos 1997 {published data only}
- Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Clinical trial of plasma exchange and high‐dose intravenous immunoglobulin in myasthenia gravis. Myasthenia Gravis Clinical Study Group. Annals of Neurology 1997;41(6):789‐96. [PUBMED: 9189040] [DOI] [PubMed] [Google Scholar]
Gajdos 2005 {published data only}
Ronager 2001 {published data only}
- Ronager J, Ravnborg M, Hermansen I, Vorstrup S. Immunoglobulin treatment versus plasma exchange in patients with chronic moderate to severe myasthenia gravis. Artificial Organs 2001;25(12):967‐73. [PUBMED: 11843764] [DOI] [PubMed] [Google Scholar]
Schuchardt 2002 {unpublished data only}
- Schuchardt V, Kohler W, Hund E, Scheglmann K, Fleisher E, Jung K, et al. A randomized controlled trial of high dose intravenous immunoglobulin versus methylprednisolone in myasthenia gravis. An interim analysis. 2002. Data on file.
Wolfe 2002 {published data only}
- Wolfe GI, Barohn RJ, Foster BM, Jackson CE, Kissel JT, Day JW, et al. Randomized, controlled trial of intravenous immunoglobulin in myasthenia gravis. Muscle and Nerve 2002;26(4):549‐52. [PUBMED: 12362423] [DOI] [PubMed] [Google Scholar]
Zinman 2007 {published data only}
- Zinman L, Ng E, Bril V. IV immunoglobulin in patients with myasthenia gravis. A randomized controlled trial. Neurology 2007;68(11):837‐41. [PUBMED: 17353471] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Liu 2009 {published data only}
- Liu JF, Wang WX, Xue J, Zhao CB, You HZ, Lu JH, et al. Comparing the autoantibody levels and clinical efficacy of double filtration plasmapheresis, immunoadsorption, and intravenous immunoglobulin for the treatment of late‐onset myasthenia gravis. Therapeutic Apheresis and Dialysis 2009;14:153‐60. [PUBMED: 20438536] [DOI] [PubMed] [Google Scholar]
Additional references
Achiron 2000
- Achiron A, Barak Y, Miron S, Sarova‐Pinas I. Immunoglobulin treatment in refractory myasthenia gravis. Muscle & Nerve 2000;23(4):551‐5. [DOI] [PubMed] [Google Scholar]
Arsura 1986
- Arsura EL, Bick A, Brunner NG, Namba T, Grob D. High dose intravenous immunoglobulin in the management of myasthenia gravis. Archives of Internal Medicine 1986;146(7):1365‐8. [PubMed] [Google Scholar]
Arsura 1989
- Arsura EL. Experience with intravenous immunoglobulin in myasthenia gravis. Clinical Immunology and Immunopathology 1989;53(2 Pt 2):S170‐9. [DOI] [PubMed] [Google Scholar]
Besinger 1983
- Besinger UA, Toyka KV, Homberg M, Heininger K, Hohlfeld R, Fateh‐Moghadam A. Myasthenia gravis: long‐term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology 1983;33(10):1316‐21. [DOI] [PubMed] [Google Scholar]
Blanchette 1994
- Blanchette V, Imbach P, Andrew M, Adams M, McMillan J, Wang E, et al. Randomised trial of intravenous immunoglobulin G, intravenous anti‐D and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet 1994;344(8924):703‐7. [DOI] [PubMed] [Google Scholar]
Buchwald 2002
- Buchwald B, Ahangari R, Weishaupt A, Toyka KV. Intravenous immunoglobulins neutralize blocking antibodies in Guillain‐Barre syndrome. Annals of Neurology 2002;51(6):673‐80. [DOI] [PubMed] [Google Scholar]
Buchwald 2005
- Buchwald B, Ahangari R, Weishaupt A, Toyka KV. Presynaptic effects of immunoglobulin G from patients with Lambert‐Eaton myasthenic syndrome: their neutralization by intravenous immunoglobulins. Muscle and Nerve 2005;31(4):487‐94. [DOI] [PubMed] [Google Scholar]
Cosi 1991
- Cosi V, Lombardi M, Piccolo G, Erbetta A. Treatment of myasthenia gravis with high‐dose intravenous immunoglobulin. Acta Neurologica Scandinavica 1991;84(2):81‐4. [DOI] [PubMed] [Google Scholar]
Dalakas 1993
- Dalakas MC, Illa I, Dambrosia JM, Souiedan SA, Stein DP, Otero C, et al. A controlled trial of high dose intravenous immune globulin infusions as treatment for dermatomyositis. New England Journal of Medicine 1993;329(27):1993‐2000. [DOI] [PubMed] [Google Scholar]
Dalakas 1999
- Dalakas MC. Intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: present status and practical therapeutic guidelines. Muscle and Nerve 1999;22(11):1479‐97. [DOI] [PubMed] [Google Scholar]
Dau 1977
- Dau PC, Lindstrom JM, Cassel CK, Denys EH, Shev EE, Spitler LE. Plasmapheresis and immunosuppressive drug therapy in myasthenia gravis. New England Journal of Medicine 1977;297(21):1134‐40. [DOI] [PubMed] [Google Scholar]
Dwyer 1992
- Dwyer JM. Manipulating the immune system with immune globulin. New England Journal of Medicine 1992;326(2):107‐16. [DOI] [PubMed] [Google Scholar]
Fateh‐Moghadam 1984
- Fateh‐Moghadam A, Wick M, Besinger U, Geursen RG. High dose intravenous gammaglobulin for myasthenia gravis. Lancet 1984;1(8381):848‐9. [DOI] [PubMed] [Google Scholar]
Gajdos 1983
- Gajdos P, Simon N, Rohan‐Chabot P, Raphael JC, Goulon M. Long term effects of plasma exchange in myasthenia gravis. Results from a randomised study [Effet à long terme des échanges plasmatiques au cours de la myasthénie. Résultats d'une étude randomisée]. Presse Médicale 1983;12(15):939‐42. [PubMed] [Google Scholar]
Gajdos 1984
- Gajdos P, Outin HD, Elkharrat D, Brunel D, Rohan‐Chabot P, Raphaël JC, et al. High dose intravenous gammaglobulin for myasthenia gravis. Lancet 1984;1(8373):406‐7. [DOI] [PubMed] [Google Scholar]
Gajdos 1987
- Gajdos P, Outin HD, Morel E, Raphaël JC, Goulon M. High dose intravenous gamma globulin for myasthenia gravis: an alternative to plasma exchange. Annals of the New York Academy of Sciences 1987;505:842‐44. [IDS ‐ R6891, ISSN ‐ 0077 8923, Accession ‐ 1988146240] [Google Scholar]
Gajdos 2002
- Gajdos P, Chevret S, Toyka KV. Plasma exchange for generalised myasthenia gravis. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD002275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Godeau 1993
- Godeau B, Lesage S, Divine M, Wirquin V, Farcet JP, Bierling P. Treatment of adult chronic autoimmune thrombocytopenic purpura with repeated high dose intravenous immunoglobulin. Blood 1993;82(5):1415‐21. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hilkevich 2001
- Hilkevich O, Drory VE, Chapman J, Korczyn AD. The use of intravenous immunoglobulin as maintenance therapy in myasthenia gravis. Clinical Neurophamacology 2001;24(3):173‐6. [DOI] [PubMed] [Google Scholar]
Hohlfeld 1996
- Hohlfeld R, Melms A, Toyka KV, Drachman DB. [Therapy for myasthenia gravis]. Neurological disorders: course and treatment. San Diego, CA: Academic Press, 1996:947‐64.
Imbach 1981
- Imbach P, Barandun S, d'Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1981;1(8232):1228‐31. [DOI] [PubMed] [Google Scholar]
Jaretzki 2000
- Jaretzki A 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, et al. Myasthenia gravis: recommendations for clinical research standards. Neurology 2000;55(1):16‐23. [DOI] [PubMed] [Google Scholar]
Jensen 2008
- Jensen P, Bril V. A comparision of the effectiveness of intravenous immunoglobulin and plasma exchange as preoperative therapy of myasthenia gravis. Journal of Clinical Neuromuscular Disease 2008;9(3):352‐5. [DOI] [PubMed] [Google Scholar]
Kornfeld 1981
- Kornfeld P, Ambinder EP, Mittag T, Bender AN, Papatestas AE, Goldberg J, et al. Plasmapheresis in refractory generalized myasthenia gravis. Archives of Neurology 1981;38(8):478‐81. [DOI] [PubMed] [Google Scholar]
McConville 2004
- McConville J, Farrugia ME, Beeson D, Kishore U, Metcalfe R, Newsom‐Davis J, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Annals of Neurology 2004;55(4):580‐4. [DOI] [PubMed] [Google Scholar]
Newsom‐Davis 1979
- Newsom‐Davis J, Wilson SG, Vincent A, Ward CD. Long term effects of repeated plasma exchange in myasthenia gravis. Lancet 1979;1(8114):464‐8. [DOI] [PubMed] [Google Scholar]
NIH Consensus 1986
- NIH Consensus Conference. The utility of therapeutic plasmapheresis for neurological disorders. The Journal of the American Medical Association 1986;256(10):1333‐7. [PubMed] [Google Scholar]
NIH Consensus 1990
- NIH Consensus Conference. Intravenous immunoglobulin: prevention and treatment of disease. The Journal of the American Medical Association 1990;264(24):3189‐93. [PubMed] [Google Scholar]
Oosterhuis 1983
- Oosterhuis HJGH, Limburg PC, Hummel‐Tappel E, The TH. Anti‐acetylcholine receptor antibodies in myasthenia gravis. Part 2. Clinical and serological follow‐up of individual patients. Journal of the Neurological Sciences 1983;58(3):371‐85. [DOI] [PubMed] [Google Scholar]
Osserman 1971
- Osserman KE, Genkins G. Studies in myasthenia gravis: review of a twenty‐year experience in over 1200 patients. Mount Sinai Journal of Medicine 1971;38(6):497‐537. [PubMed] [Google Scholar]
Perez‐Nellar 2001
- Perez‐Nellar J, Dominguez AM, Llorens‐Figueroa JA, Ferra‐Betancourt A, Pardo A, Quiala M, et al. A comparative study of intravenous immunoglobulin and plasmapheresis preoperatively in myasthenia [Estudio comparativo entre immunoglobulina intravenosa y plasmaferesis en el perioperatorio de la miastenia gravis]. Revista de Neurologia 2001;33(5):413‐6. [IDS ‐ 487WH, ISSN ‐ 0210‐0010] [PubMed] [Google Scholar]
Pinching 1976
- Pinching AJ, Peters DK. Remission of myasthenia gravis following plasma exchange. Lancet 1976;2(8000):1373‐6. [DOI] [PubMed] [Google Scholar]
PSGBS Group 1997
- Plasma exchange/Sandoglobulin Guillain‐Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain‐Barré syndrome. Lancet 1997;349(9047):225‐30. [PubMed] [Google Scholar]
Qureshi 1999
- Qureshi AI, Choudhry MA, Akbar MS, Mohammad Y, Chua HC, Yahia AM, et al. Plasma exchange versus intravenous immunoglobulin treatment in myasthenic crisis. Neurology 1999;52(3):629‐32. [DOI] [PubMed] [Google Scholar]
Rodnitzky 1984
- Rodnitzky RL, Bosch EP. Chronic long interval plasma exchange in myasthenia gravis. Archives of Neurology 1984;41(7):715‐7. [DOI] [PubMed] [Google Scholar]
Sharshar 2000
- Sharshar T, Chevret S, Mazighi M, Chillet P, Huberfeld G, Berreotta C, et al. Validity and reliability of two muscle strength scores commonly used as endpoints in assessing treatment of myasthenia gravis. Journal of Neurology 2000;247(4):286‐90. [DOI] [PubMed] [Google Scholar]
Tindall 1987
- Tindall RSA, Rollins JT, Phillips, Greenlee RG, Wells L, Belendiuk G, et al. Preliminary results of a double blind, randomized, placebo controlled trial of cyclosporine in myasthenia gravis. New England Journal of Medicine 1987;316(12):719‐724. [DOI] [PubMed] [Google Scholar]
Toyka 2007
- Toyka KV, Gold R. Treatment of myasthenia gravis. Schweizer Archiv Fur Neurologie Und Psychiatrie 2007;158:309‐21. [Google Scholar]
Van der Meché 1997
- Meché FG, Doorn PA. The current place of high‐dose immunoglobulins in the treatment of neuromuscular disorders. Muscle & Nerve 1997;20(2):136‐47. [DOI] [PubMed] [Google Scholar]
Vincent 2003
- Vincent A, Bowen J, Newsom‐Davis J, McConville J. Seronegative generalised myasthenia gravis: clinical features, antibodies, and their targets. Lancet Neurology 2003;2(2):99‐106. [DOI] [PubMed] [Google Scholar]
Wolfe 2008
- Wolfe GI, Oh SJ. Clinical phenotype of muscle‐specific tyrosine kinase antibody positive myasthenia gravis. Annals of the New York Academy of Sciences 2008;1132:71‐5. [PUBMED: 18567855] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gajdos 2008
- Gajdos P, Chevret S, Toyka KV. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD002277.pub3] [DOI] [PubMed] [Google Scholar]


