Abstract
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews on 'Feverfew for preventing migraine' (2004, Issue 1). Feverfew (Tanacetum parthenium L.) extract is a herbal remedy, which has been used for preventing attacks of migraine.
Objectives
To systematically review the evidence from double‐blind randomised controlled trials (RCTs) assessing the clinical efficacy and safety of feverfew monopreparations versus placebo for preventing migraine.
Search methods
For this updated version of the review we searched CENTRAL, MEDLINE, EMBASE and AMED to January 2015. We contacted manufacturers of feverfew and checked the bibliographies of identified articles for further trials.
Selection criteria
We included randomised, placebo‐controlled, double‐blind trials assessing the efficacy of feverfew monopreparations for preventing migraine in patients of any age. We included trials using clinical outcome measures, while we excluded trials focusing exclusively on physiological parameters. There were no restrictions regarding the language of publication.
Data collection and analysis
We systematically extracted data on patients, interventions, methods, outcome measures, results and adverse events. We assessed risk of bias using the Cochrane 'Risk of bias' tool and evaluated methodological quality using the Oxford Quality Scale developed by Jadad and colleagues. Two review authors (BW and MHP for this update, MHP and EE for the original version) independently selected studies, assessed methodological quality and extracted data. We resolved disagreements concerning evaluation of individual trials through discussion.
Main results
We identified one new study for this update, resulting in six trials (561 patients) meeting the inclusion criteria. Five of the six trials reported on the main outcome, migraine frequency. Although five of the trials were generally of good methodological quality, all studies were either of unclear or high risk of bias with regards to sample size. Pooled analysis of the results was not possible due to the lack of common outcome measures and heterogeneity between studies in terms of participants, interventions and designs.
The most recent trial added to this version of the review is rigorous and larger (n = 218), using a stable feverfew extract at a dose determined by a previous dose‐finding trial. It reports that feverfew reduced migraine frequency by 1.9 attacks from 4.8 to 2.9 and placebo by 1.3 from to 4.8 to 3.5 per month, resulting in a difference in effect between feverfew and placebo of 0.6 attacks per month. For the secondary outcome measures intensity and duration of migraine attacks, incidence and severity of nausea and vomiting, and global assessment no statistically significant differences were reported. Results of previous trials are not convincing: three trials reporting positive effects of feverfew are all of small sample size (17 to 60 participants), while two rigorous trials (n = 50, 147) did not find significant differences between feverfew and placebo. Only mild and transient adverse events, most commonly gastrointestinal complaints and mouth ulcers, were reported in the included trials.
Authors' conclusions
Since the last version of this review, one larger rigorous study has been included, reporting a difference in effect between feverfew and placebo of 0.6 attacks per month. This adds some positive evidence to the mixed and inconclusive findings of the previous review. However, this constitutes low quality evidence, which needs to be confirmed in larger rigorous trials with stable feverfew extracts and clearly defined migraine populations before firm conclusions can be drawn. It appears from the data reviewed that feverfew is not associated with any major safety concerns.
Plain language summary
Feverfew for preventing migraine
Migraine is a common, disabling headache disorder. Feverfew (Tanacetum parthenium L.) is a herbal remedy used for the prevention of migraine attacks. For this update of a previous Cochrane review, we reviewed the available evidence up to January 2015 for or against feverfew in the prevention of migraine and found six studies including 561 participants. Generally the studies were heterogeneous and their results were mixed. The previous version of this review showed no clear benefit of feverfew compared with placebo. We added a new study, which is larger and was carried out to high standards, to this review. It showed that feverfew reduced migraine frequency by a little more than half a migraine (0.6) per month compared to placebo. There was no difference in how severe the pain was, or how long it lasted. These results come from a single study of moderate size, therefore they must be viewed with caution until they are confirmed in other rigorous studies. No major adverse effects were associated with feverfew in the included studies.
Background
This is an update of a previously published review in the Cochrane Database of Systematic Reviews (2004, Issue 1) on feverfew for preventing migraine (Pittler 2004).
Description of the condition
Migraine is a common, incapacitating primary headache disorder. According to the International Headache Society it can be classified into two major sub‐types: migraine without aura and migraine with aura (previously called common and classical migraine) (IHS 2013). Migraine without aura manifests in attacks lasting 4 to 72 hours; the headaches are characterised by their unilateral location, pulsating quality, moderate or severe intensity, aggravation by routine physical activity and association with nausea and/or photophobia and phonophobia. Migraine with aura is primarily characterised by the focal neurological symptoms that usually precede the headache. Aura usually develops gradually over 5 to 20 minutes and last for less than 60 minutes. Headache with or without the features of migraine may accompany or follow the aura symptoms within 60 minutes (IHS 2013; World Health Organization 2012).
Apart from presenting a considerable personal burden with regards to pain, functional health and well being, the prevalence and socio‐economic impact of migraine are also significant. The Global Burden of Disease Study classified migraine as the third most common disorder to affect individuals globally in 2010, with a prevalence of 14.7% (Vos 2012). Migraine was also ranked seventh highest among specific causes of disability globally (Steiner 2013). A cross‐sectional survey of eight European Union (EU) countries representing 55% of the adult population estimated the average annual direct and indirect cost per person to be EUR 1222, and a total annual cost for the EU of EUR 111 billion for adults aged 18 to 65 years (Linde 2012). The economic burden of migraine in the US has been estimated at an average annual direct and indirect cost of USD 1757 for episodic migraine and USD 7750 for chronic migraine per person (Munakata 2009).
Description of the intervention
Feverfew (Tanacetum parthenium) is an herbaceous perennial native to Asia Minor and common in the Balkans, now also naturalised throughout most of Europe, the Americas and the rest of the world. It is a member of the Asteraceae family. The dried leaves or aerial parts are used for medicinal purposes. It has traditionally been used for fever, women's ailments, inflammatory conditions, psoriasis, toothache, insect bites, rheumatism, asthma and stomach‐ache. Today feverfew extract is predominantly used for preventing migraine attacks and alleviating accompanying symptoms (Ernst 2007; Natural Standard 2014; Pareek 2011). It is available in different forms and preparations such as fresh feverfew leaves, powdered dried feverfew leaves, alcoholic feverfew extracts and CO2‐feverfew extract. Concerning the safety of feverfew, toxicity studies have shown that chronic prophylactic use of feverfew does not affect the frequency of chromosomal aberration in lymphocytes or urine mutagenicity (Anderson 1988). Anecdotal reports describe contact dermatitis (e.g. Burry 1980; Hausen 1983).
How the intervention might work
The exact mechanisms of action of feverfew remain unknown. Its anti‐migraine action is probably related to its principally known bioactive ingredient, parthenolide, a sesquiterpene lactone (Knight 1995). Parthenolide comprises up to 85% of the total sesquiterpene content of feverfew and is found in the leaves but not the stems (Pareek 2011). Parthenolides probably inhibit prostaglandin production, interfere with both contractile and relaxant mechanisms in blood vessels and perhaps inhibit the secretion of serotonin (5‐HT) (ESCOP 2003; Heptinstall 1985; Heptinstall 1987; Makheja 1982; Pugh 1988; Taylor 2011). However, one study has suggested only a secondary role for serotonin in the aetiology of migraine (Goadsby 1997). Assuming whole‐leaf preparations are effective, this would direct attention more towards other components of the feverfew leaf (Awang 1998). This is also suggested by a Dutch study (de Weerdt 1996), which indicates that the essential oil constituent of feverfew, chrysanthenyl acetate, may be important. This component inhibits prostaglandin synthetase in vitro and seems to possess analgesic properties (Pugh 1988). Other investigators agree that parthenolide is not the only pharmacologically active constituent in feverfew (Brown 1997; Hendriks 1996). A link between the relatively high concentration of melatonin in different feverfew varieties (Murch 1997) and a decrease in melatonin excretion during migraine attacks has been suggested (Brun 1995). In conclusion, a definitive chemical link between the aetiology of migraine and parthenolide or any other of the feverfew constituents has still not been established (Kuritzky 1994).
As clinical trials of feverfew for the prevention of migraine attacks published in the 1980s and 1990s produced inconsistent results, wide variations in the strength of the parthenolides (Willigmann 1999) and differences in the stability of feverfew preparations (Pfaffenrath 2002) were suggested as explanations. One trial using an extract of feverfew with a standardised and constant concentration of parthenolide to treat migraine did not show any beneficial effect (de Weerdt 1996). This lack of efficacy might have been due to the absence of essential therapeutic components of the granulated feverfew leaves, which were either not sufficiently extracted, or perhaps degraded during the preparation (de Weerdt 1996). Subsequently, a new, more stable feverfew extract (MIG‐99) was developed, which was used in the trials by Pfaffenrath 2002 and Diener 2005 but trial results were not fully consistent.
Why it is important to do this review
Some of the existing standard prophylactic treatments, such as propranolol, metoprolol, flunarizine, valproic acid and topiramate, which reduce attack frequency in some patients, are associated with adverse effects (Dodick 2007). Furthermore, patients might find long‐term treatment of migraine with drugs unacceptable. An effective, safe and well‐tolerated prophylactic alternative would therefore be desirable. Herbal supplements are very popular and generally available over the counter. Although no specific prevalence data are available for feverfew, a 2013 systematic review of surveys of herbal medicine use in the UK reported a rate of 37.1% across a range of time periods. (Posadzki 2013). Given the high prevalence of use and the demand for non‐drug treatments, it is important to know about the safety and efficacy of herbal products including feverfew.
Objectives
To systematically review the evidence from double‐blind randomised controlled trials (RCTs) assessing the clinical efficacy and safety of feverfew monopreparations versus placebo for preventing migraine.
Methods
Criteria for considering studies for this review
Types of studies
We included placebo‐controlled trials that were both randomised (i.e. trials with a randomised sequence generation and concealed allocation of the sequence) and double‐blind (i.e. trials where neither patients nor care/treatment providers knew whether the patient had received feverfew or placebo).
Types of participants
We included studies with participants of all ages suffering from migraine. We did not impose any restrictions in terms of diagnostic criteria; information on the respective diagnostic criteria used in each study is provided in the Results section below, and in the Characteristics of included studies table.
Types of interventions
We included trials using oral preparations containing feverfew extract as the only component. We imposed no restrictions regarding dosage. We excluded trials assessing feverfew extract as one of several active components in a combination preparation or as a part of a combination treatment.
We considered both treatment studies and withdrawal studies (in which patients already on feverfew medication were randomised to continue on either placebo or feverfew) for inclusion.
Types of outcome measures
We included trials using clinical outcome measures, while we excluded trials focusing exclusively on physiological parameters. We collected and analysed trial data on migraine frequency, severity and duration, intensity and severity of nausea and vomiting, global assessment of efficacy, responders (patients with ≥ 50% reduction in headache frequency) and adverse events. We included data reported by participants and study assessors and applied no restrictions in terms of acceptable ways of measuring outcomes.
Primary outcomes
Frequency of migraine attacks
Secondary outcomes
Intensity and duration of migraine attacks
Incidence and severity of nausea/vomiting
Global assessment of efficacy
Adverse events
Search methods for identification of studies
We ran the search for the original review on 28 July 2003 and ran subsequent searches on 27 January 2009, 12 February 2014 and 27 January 2015. For this update, we contacted manufacturers in February 2014 and performed manual searches in February 2014 and January 2015.
Electronic searches
We searched for publications describing randomised, double‐blind trials of feverfew extract for migraine.
We performed searches in 2003 and then again in 2009 for past versions of the review in the following databases:
Trials Register of the Cochrane Pain, Palliative and Supportive Care Group (PaPaS) (last searched 27 January 2009);
Cochrane Central Register of Controlled Trials (CENTRAL);
PREMEDLINE/MEDLINE (from 1966 onwards);
EMBASE (from 1974 onwards);
Allied and Complementary Medicine Database (AMED) (from 1985 onwards).
For this version of the review, we updated the searches using the following electronic databases:
CENTRAL (2014, Issue 12);
MEDLINE (OVID) to 27 January 2015;
EMBASE (OVID) to 27 January 2015;
AMED (OVID) to 27 January 2015.
The strategies used to search the databases can be found in Appendix 1. We applied no filters for randomised controlled trials or controlled clinical trials.
We also searched for ongoing trials in the metaRegister of Controlled Trials (mRCT) (http://www.isrctn.com/), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/), all accessed on 4 February 2015.
Language
We imposed no restrictions regarding the language of publication (Egger 1997).
Searching other resources
Handsearching
We performed manual searches using the bibliographies of articles located through the electronic searches and through scanning our own files.
Personal contact
We contacted manufacturers of feverfew preparations in February and March 2014 and asked them to contribute relevant published and unpublished material. We received no responses from these manufacturers.
Data collection and analysis
Selection of studies
Two review authors independently screened the retrieved studies for inclusion in the review (BW and MHP for this update, MHP and EE for the previous version of this review).
Data extraction and management
Two review authors (BW and MHP for this update, MHP and EE for the previous version of this review) also systematically and independently extracted data from each trial report into a standardised data extraction form. Data extracted included patient characteristics, interventions, methods, outcome measures, efficacy results and adverse events. We resolved disagreements in all aspects of the data extraction and evaluation through discussion between all authors and reached consensus in all cases.
Assessment of risk of bias in included studies
We evaluated the methodological quality of the included studies using the Oxford Quality Scale developed by Jadad et al (Jadad 1996). This scale quantifies the likelihood of bias inherent in a trial based on the description of randomisation, blinding and withdrawals provided in the trial report. Two review authors (BW and MHP for this update, MHP and EE for the previous version of this review) independently performed the assessment. In addition, we completed Cochrane's 'Risk of bias' table for each study, using assessments of random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and bias due to small sample size. Two authors (BW and MHP) independently assessed all studies and resolved disagreements through discussion.
Measures of treatment effect
We used any clinical outcome measures in the efficacy assessment but not solely physiological outcomes. We planned to report standardised mean difference (SMD) for the primary outcome measure but had to abandon this plan due to the heterogeneity of studies. We also listed adverse events for all included studies.
Dealing with missing data
If data were missing or inadequate, we contacted the study authors to obtain further details.
Assessment of heterogeneity
We planned to assess heterogeneity of efficacy outcomes using the I² statistic (Higgins 2003), but the included studies were too heterogeneous in terms of participants, interventions, methods and outcome measures to be statistically combined. Differences in methods and clinical characteristics are described below in the Description of studies section.
Data synthesis
We considered meta‐analyses of data on the frequency, intensity and duration of migraine attacks, the incidence and severity of nausea/vomiting and the global assessment of efficacy, but abandoned the idea when the scarcity of common outcome measures and differences in methods became apparent. Quantitative data reported for the above outcomes are summarised in the Results section below.
Results
Description of studies
Results of the search
The literature search identified 11 double‐blind RCTs. Figure 1 presents the study flow diagram, which illustrates the study selection procedure.
1.
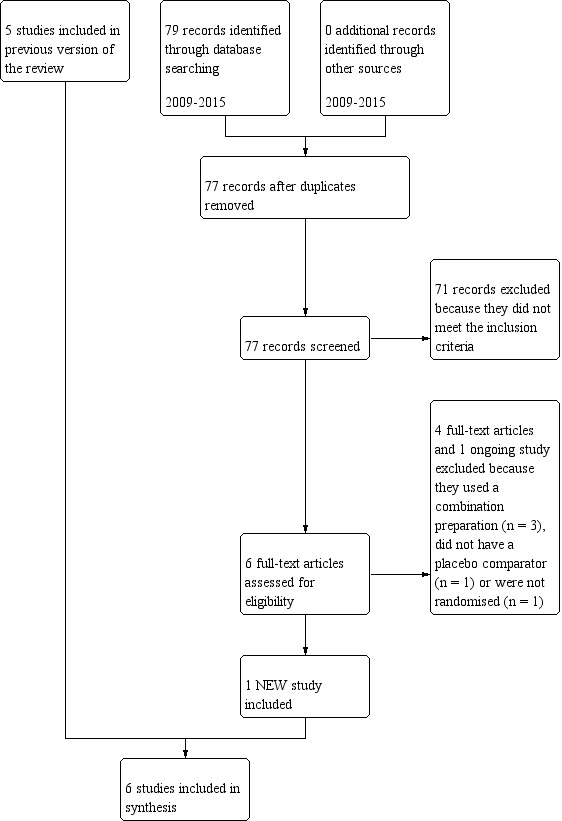
Study flow diagram.
See Characteristics of included studies and Characteristics of excluded studies.
Included studies
Six trials met the inclusion criteria and we reviewed them for this update. In this version of the review we included one new study (Diener 2005). We identified no relevant unpublished or ongoing trials.
In total, 561 patients participated in the included studies. Study sample sizes ranged from 17 to 218.
In three studies migraine was diagnosed according to the International Headache Society (IHS) criteria (de Weerdt 1996; Diener 2005; Pfaffenrath 2002), in one according to Blau 1984 (Murphy 1988), and in two the diagnostic criteria were not specified (Johnson 1985; Palevitch 1997).
Three trials were cross‐over trials (de Weerdt 1996; Murphy 1988; Palevitch 1997), and the other three used a parallel‐group design (Diener 2005; Johnson 1985; Pfaffenrath 2002). Four studies were treatment studies (de Weerdt 1996; Diener 2005; Murphy 1988; Pfaffenrath 2002), and two were withdrawal studies, i.e. participants already taking feverfew and responding positively to it were randomised to either continue receiving feverfew or to receive a placebo (Johnson 1985; Palevitch 1997).
In all studies feverfew was self‐administered orally one to three times daily in capsule form. Three trials administered dried, powdered feverfew extract (Johnson 1985; Murphy 1988; Palevitch 1997), one used an alcoholic feverfew extract (de Weerdt 1996), and two used a CO2‐extract (Diener 2005; Pfaffenrath 2002). Study duration ranged from two to eight months.
Excluded studies
For this update, we excluded two trials because they tested combination products (feverfew and ginger, Cady 2011; feverfew with riboflavin and magnesium, Maizels 2004), one because it did not report on clinical outcomes (Kuritzky 1994), and one because it did not use a placebo comparator (acupuncture versus feverfew versus acupuncture combined with feverfew, Ferro 2012).
We retrieved one ongoing trial through ICTRP. We excluded it because it used a combination product of lavender and feverfew (IRCT2014081218776N1 2015).
One trial is available as an abstract only (Karimooy 2011). The abstract does not contain sufficient details to ascertain that the trial meets the inclusion criteria. It is unclear whether the study is randomised and it appears to assess treatment rather than prevention of migraine. Despite contact with the author relevant information about methods and results could not be obtained and we excluded the study.
Risk of bias in included studies
We assessed the methodological quality of individual trials using the Oxford Quality Scale devised by Jadad and colleagues (Jadad 1996), allocating a total maximum of five points for the following areas:
Randomisation (R): Was the study described as randomised? (1 = yes; 0 = no). Was the method of randomisation well described and adequate? (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate).
Blinding (B): Was the study described as double‐blind? (1 = yes; 0 = no). Was the method of double‐blinding well described and adequate? (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate).
Withdrawals/drop‐outs (W/D): Was there a description of withdrawals and drop‐outs sufficient to determine the number of patients in each treatment group entering and completing the trial? (1 = yes; 0 = no).
Five out of the six included studies scored five (de Weerdt 1996; Diener 2005; Pfaffenrath 2002) or four (Johnson 1985; Murphy 1988) points on the Oxford scale used to assess methodological quality (Jadad 1996). One study yielded only two points (Palevitch 1997). The quality scores for each trial are reported in the Characteristics of included studies table.
We also assessed risk of bias using the 'Risk of bias' tool (Figure 2; Figure 3).
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Three studies were of low risk of bias for each risk of bias item (de Weerdt 1996; Diener 2005; Pfaffenrath 2002), and one was of unclear risk or high risk of bias (Palevitch 1997). The remaining studies were of mixed low and unclear risk (Johnson 1985; Murphy 1988). As per the inclusion criteria, all included studies were randomised and double‐blind.
Allocation
As per the inclusion criteria, all included studies were randomised. However, in two studies the random sequence generation was not described (Murphy 1988; Palevitch 1997), and three studies did not provide any details of allocation concealment (Johnson 1985; Murphy 1988; Palevitch 1997). This could be due to reporting flaws rather than actual methodological flaws.
Blinding
Two studies provided no further details of blinding such as who was blinded (Johnson 1985; Palevitch 1997).
Incomplete outcome data
In two studies the last observation was carried forward if data were missing for subsequent observation periods (Diener 2005; Pfaffenrath 2002). In the Diener 2005 study, 45 patients had been randomised without fulfilling the IHS criteria and were excluded from the intention‐to‐treat analysis. In two studies the analysis was restricted to participants who completed the study and for whom data were available (de Weerdt 1996; Murphy 1988). In one study all participants completed the study and were analysed (Johnson 1985). Only one study did not mention drop‐outs and it is unclear whether there were no drop‐outs at all or whether they were not reported (Palevitch 1997).
Selective reporting
Some studies appear at risk of selection bias with non‐reporting of outcomes for some of the clinically relevant and recommended outcome measures in migraine prevention trials (Tfelt‐Hansen 2012): frequency (Palevitch 1997), duration (de Weerdt 1996; Johnson 1985; Palevitch 1997), intensity (Johnson 1985), or nausea and vomiting (de Weerdt 1996; Diener 2005). Generally studies reported findings for their respective pre‐defined outcome measures.
Other potential sources of bias
All studies were either of unclear (Diener 2005; Murphy 1988; Palevitch 1997; Pfaffenrath 2002) or high (de Weerdt 1996; Johnson 1985) risk of bias with regards to sample size.
Effects of interventions
As stated in the 'Methods' section above, we did not conduct planned meta‐analyses of data on the frequency, intensity and duration of migraine attacks, the incidence and severity of nausea/vomiting and the global assessment of efficacy due to the lack of common outcome measures and heterogeneity between studies in terms of participants, interventions and designs. The methods and results of each of the six included studies are summarised below to provide an indication of the benefits and harms of feverfew for the prevention of migraine.
Efficacy
Johnson et al conducted a study including 17 patients who had taken raw feverfew leaves every day for the previous three to four years (Johnson 1985). Patients described as having a history of "common or classical migraine" (diagnostic criteria not specified by the authors) for a duration of at least two years, with eight or fewer attacks per month, were randomised to receive either two capsules of powdered, freeze‐dried feverfew leaves (50 mg total) daily or an identical placebo for 24 weeks. Diary cards were used to assess the frequency of migraine and the incidence of nausea and vomiting. The results showed a significant increase (P value < 0.02) in the number of attacks per month in the placebo group (mean 3.1; standard error (SE) 0.8) compared with baseline, while attack frequency remained constant in patients receiving feverfew (mean 1.7; SE 0.6). Forty‐two per cent and 79% of attacks were associated with nausea and vomiting in the feverfew and placebo groups, respectively (P value < 0.05). The incidence of bouts of nausea/vomiting was significantly lower in the feverfew group (P value < 0.05) than in the placebo group (39 and 116, respectively). The global assessment of efficacy by patients indicated a significant difference (P value < 0.01) in favour of feverfew: six of eight patients in the feverfew group rated the overall treatment effect as moderately good to excellent, while this result was reported by only three of nine patients in the placebo group. Due to the small sample size (n = 17), caution is advised with regards to the reliability and value of the data.
Murphy et al randomised 72 patients with common or classical migraine (as defined by Blau 1984) to receive either one capsule of dried feverfew leaves (mean weight, 82 mg) or placebo for four months after a one‐month placebo run‐in period (Murphy 1988). Patients were then transferred into the other group for the second four‐month period. There was no wash‐out period between the treatment periods. Outcomes were assessed using a diary of migraine symptoms provided every two months. The results suggested a significant difference (P value < 0.005) in the number of attacks per two‐month period during feverfew treatment (mean 3.6; SE 0.2) compared with placebo (mean 4.7; SE 0.3). Among patients with classical migraine (migraine with aura) (n = 17), the number of attacks per two‐month period was significantly lower (P value < 0.05) with feverfew (mean 2.9; SE 0.4) than with placebo (mean 4.3; SE 0.5); among patients with common migraine (n = 42), headache frequency was similar during the feverfew (mean 3.9; SE 0.3) and placebo (mean 4.9; SE 0.4) periods (P value = 0.06). In the study population as a whole, the total number of attacks rated as severe or very severe was 178/424 (42%) with feverfew and 258/559 (46%) with placebo. Nausea and vomiting accompanied the attacks in 207/424 (49%) and 313/559 (56%) cases treated with feverfew and placebo, respectively (P value < 0.02). The global assessment of efficacy, measured on a 100 mm visual analogue scale with 'worst ever' and 'best ever' as the two extremes, indicated a significant difference (P value < 0.0001) in favour of feverfew compared with placebo (mean 74; SE 2 versus mean 60; SE 3, respectively). Among patients with classical migraine, global assessment scores were significantly higher (P value < 0.01) during treatment with feverfew (mean 78; SE 4) than during treatment with placebo (mean 57; SE 5); among patients with common migraine, scores for the two treatment periods were similar (mean 72; SE 2 for feverfew and mean 61; SE 3 for placebo).
De Weerdt et al (de Weerdt 1996) assessed 50 patients diagnosed according to the criteria of the International Headache Society (IHS 1988). Patients suffering from migraine with or without aura received either one capsule of an alcoholic feverfew extract (143 mg) or placebo daily in a randomised cross‐over trial. A one‐month placebo run‐in phase was followed by two two‐month treatment periods. There was no wash‐out period between the treatment periods. The investigators reported that no significant effects on the number or intensity of headaches were observed. Again this study has a small sample size with only 44 patients being included in the final analysis and the results therefore need to be interpreted with caution.
The cross‐over trial conducted by Palevitch et al included 57 patients with migraine diagnosed by medical examination (diagnostic criteria not specified) (Palevitch 1997). Participants as young as nine years old (age range 9 to 65 years) were included despite migraine having different characteristics in young individuals. During the preliminary, open phase of the trial, each patient received 100 mg of powdered, dried feverfew leaves daily for two months. Thereafter, in the double‐blind, cross‐over phase, one group received placebo for 30 days, while the other continued taking feverfew. Patients in the active treatment group were then transferred to the placebo arm and vice versa. There was no wash‐out period between the treatment periods. The intensity of migraine attacks was measured by patients on a numerical scale of 0 ('no pain') to 10 ('most severe pain'), and the severity of nausea and vomiting was assessed using a numerical analogue scale and by questionnaire. The results of the preliminary, open phase showed a significant decrease by 4.27 points on a 10‐point scale in migraine intensity after treatment with feverfew compared with baseline (P value < 0.001). In the first cross‐over phase there was a further reduction of migraine intensity in the feverfew group (mean 1.5; SE 0.7), and an increase in intensity in the placebo group (mean 1.6; SE 0.9) (P value < 0.01). In the second phase of the cross‐over, these trends continued: migraine intensity decreased among patients taking feverfew (mean 4.0; SE 1.1) and increased among patients taking placebo (mean 1.4; SE 1.1). In addition, there was a significant difference (P value < 0.001) in the severity of nausea and vomiting in favour of feverfew.
Pfaffenrath et al conducted a double‐blind, placebo‐controlled, multicentre randomised controlled trial (RCT) (Pfaffenrath 2002). Three dosage regimens (2.08 mg versus 6.25 mg versus 18.75 mg, each administered three times daily for 12 weeks) of a novel CO2‐feverfew extract were compared with placebo. One hundred and forty‐seven patients with migraine with or without aura according to the IHS criteria (IHS 1988) were enrolled. The primary endpoint was pre‐defined as the total number of migraine attacks during the last 28 days of treatment compared to the four‐week baseline period. Secondary endpoints included total and average duration and intensity of migraine attacks, number of days with accompanying migraine symptoms and responder rates. There were no statistically significant effects for either primary or secondary outcomes measures. Accordingly, a dose‐response relationship could not be observed. Subgroup analysis including patients with at least four migraine attacks during baseline evaluations (n = 49) showed a significant effect when the 6.25 mg dose was compared with placebo (P value = 0.02).
Diener 2005 conducted a double‐blind, placebo‐controlled, multicentre RCT in patients with a diagnosis of migraine with or without aura according to the IHS criteria (IHS 2000). After a four‐week screening period without migraine prophylaxis, 218 participants were randomised to receive either 6.25 mg of a feverfew CO2‐extract (MIG‐99, n = 108) or placebo (n = 110) three times daily for 16 weeks. Patients documented each migraine attack in a diary. The primary outcome measure was the total number of migraine attacks within 28 days during the second and third 28‐day treatment period (mean of P2 and P3) compared with baseline (prophylaxis‐free period P0). Secondary efficacy parameters related to frequency, intensity and duration of migraine attacks, clinical global impression of efficacy, medication requirements, number of drop‐outs due to lack of effect of the study medication and responder rate (% of patients with a greater than 50% decrease of migraine attacks per 28 days). Data from 170 patients were available for efficacy analysis in the intention‐to‐treat (ITT) sample (feverfew n = 89, placebo n = 81). The migraine frequency decreased by 1.9 from 4.8 to 2.9 attacks per month in the MIG‐99 group and by 1.3 from 4.8 to 3.5 attacks per month in the placebo group in the weeks 5 to 12 (P value = 0.0456). For the secondary outcome measures, significant differences were reported for responder rates (30.3% in the MIG‐99 group versus 17.3% in the placebo group, P value = 0.047), number of migraine days per 28 days (P value = 0.0353), and global assessment of efficacy by patients (P value = 0.024) and investigators (P value = 0.035). No statistically significant differences were seen for any of the other outcome measures.
Adverse events/safety
Five out of the six studies reported on adverse events; only Palevitch did not assess these. The proportion of participants experiencing adverse events varied considerably between studies but there were no statistically significant differences in the occurrence of adverse events between the feverfew and placebo groups. Three studies reported a higher incidence of adverse events during treatment with placebo than with feverfew (Diener 2005; Johnson 1985; Murphy 1988). De Weerdt stated that none of the participants completing the study (one withdrawal) reported adverse events (de Weerdt 1996). In the Diener 2005 study, 8.4% of participants in the feverfew group and 10.2% in the placebo group experienced adverse events. The percentages in the Pfaffenrath 2002 study were between 12.8% and 30.6% in the high‐dose and low‐dose feverfew groups respectively and 28.6% in the placebo group. In the withdrawal study by Johnson 1985, 74% of participants in the feverfew group reported adverse events and 100% in the placebo group (see below). Murphy 1988 did not report numbers of patients experiencing adverse events but reported the incidence of adverse events, which were 28/72 in the feverfew group and 36/72 in the placebo group.
Adverse events were generally mild and reversible. Most common were gastrointestinal problems but a wide range of adverse events in the feverfew and placebo groups were reported. Johnson 1985 reported a high incidence of aches, pains and stiffness in joints and muscles, as well as central nervous system symptoms of nervousness, anxiety and poor sleep (in 13 out of 17 patients) during the placebo phase of their withdrawal study. The authors consider these effects occurring together with a rebound of migraine symptoms to constitute a genuine "post‐feverfew syndrome", which has been reported in long‐term users of feverfew.
No serious adverse events related to the study medication occurred in any of the included trials. In total, 13 withdrawals were necessitated by adverse events associated with feverfew, compared with eight withdrawals due to adverse events associated with placebo. Again, adverse events leading to withdrawals were mainly of a gastrointestinal nature. Feverfew did not appear to affect blood pressure, heart rate, body weight or haematological and biochemical safety parameters in any of the included studies.
Discussion
Summary of main results
Overall the results from the six included trials are mixed and provide low quality evidence. With the addition of the most recent and largest study, Diener 2005, to this updated Cochrane review, there is evidence from one medium‐sized, rigorous randomised controlled trial (RCT) that feverfew may reduce migraine attacks by 0.6 headaches per month compared to placebo. Results for the secondary outcome measures remain unconvincing.
In terms of the main outcome measure, frequency of migraine attacks, statistically significant reductions were reported in two studies (Diener 2005; Johnson 1985), as well as in subgroups described by the authors as suffering with classical migraine (with aura) but not common migraine (with aura) (Murphy 1988), and in the confirmatory subset but not the intention‐to‐treat (ITT) sample in the Pfaffenrath 2002 study. One study found no differences (de Weerdt 1996), and one did not report frequency (Palevitch 1997).
Of the five studies assessing intensity of migraine attacks (de Weerdt 1996; Diener 2005; Murphy 1988; Palevitch 1997; Pfaffenrath 2002), only Palevitch 1997 reported significant improvements. The three studies that assessed duration of migraine attacks did not report any significant differences between groups (Diener 2005; Murphy 1988; Pfaffenrath 2002). Global assessment was improved in the studies by Johnson 1985 and Diener 2005, as well as in the subgroup with classical migraine (with aura) in the study by Murphy 1988 (not assessed/reported in the other studies). The incidence of nausea and vomiting was reduced in the studies by Johnson 1985, Murphy 1988 and Palevitch 1997, while Pfaffenrath 2002 did not observe any differences.
Two of the three trials at low risk of bias did not report statistically significant improvements for any outcomes in their main analysis (de Weerdt 1996; Pfaffenrath 2002), while only one did (Diener 2005). The two studies reporting positive results for all pre‐defined outcome measures were both withdrawal studies (Johnson 1985; Palevitch 1997); the first is limited by a small number of participants (n = 17) (Tfelt‐Hansen 1987), and the latter was of unclear/high risk of bias and scored only two points on the Oxford Quality Scale. Generally the studies are of small sample size with four studies including fewer than 100 participants (de Weerdt 1996; Johnson 1985; Murphy 1988; Palevitch 1997).
In the included trials the adverse events reported were generally mild and reversible. Mouth ulceration and gastrointestinal symptoms were reported most frequently and were also experienced by long‐time feverfew users. Such problems can be avoided through the use of more carefully prepared formulations of feverfew. A post‐feverfew syndrome was reported by long‐time feverfew consumers after discontinuation of feverfew intake (Johnson 1985). Collectively these data imply that oral feverfew medication is relatively safe. However, more information is needed to detect serious or rare adverse events, and on long‐term clinical use.
Overall completeness and applicability of evidence
There is considerable variation in the six included trials of 561 participants in terms of designs, participants, interventions and outcome measures, which might explain the inconsistent results. This variation makes it difficult to apply these findings to the general population.
Participants
In terms of participants, the diagnostic criteria varied. While three studies used the International Headache Society (IHS) criteria (de Weerdt 1996; Diener 2005; Pfaffenrath 2002), one used Blau (Murphy 1988), and two did not specify the criteria used (Johnson 1985; Palevitch 1997). In some studies participants were feverfew‐naive (de Weerdt 1996; Palevitch 1997), while in the study by Johnson 1985 feverfew consumption for the last three to four years was an inclusion criterion. Palevitch 1997 included participants as young as nine years old (age range 9 to 65 years) despite migraine having different characteristics in young individuals.
Design
Most studies had small numbers of participants, particularly in the subgroups. The sample size of studies ranged between 17 and 218; four trials had 72 or fewer participants and one thereof (n = 17) had fewer than 10 patients per treatment group (Johnson 1985), which has an influence on effect sizes. Their statistical analyses therefore need to be interpreted with caution. Small sample size can expose studies to the hazards of random chance; at the same time studies with small sample sizes are more likely to overestimate results (Nüesch 2010).
The trials included treatment and withdrawal studies as well as parallel‐group and cross‐over studies. In order to reduce carry‐over effects the IHS recommends a wash‐out phase of at least one month. The two parallel‐group treatment studies by Pfaffenrath 2002 and Diener 2005 used a treatment‐free/placebo run‐in phase before the start of the trial.
Particularly in cross‐over trials carry‐over effects can present a significant problem. The effects of feverfew given in the first period might persist into the subsequent placebo period (or vice versa), thus interfering with the effects of the subsequent intervention. However, none of the three cross‐over studies used wash‐out phases between the two intervention phases. Two of them included a one‐month run‐in phase but no wash‐out before cross‐over (de Weerdt 1996; Murphy 1988), and one did not employ any wash‐out phase (Palevitch 1997). Murphy 1988 discussed the possibility of a carry‐over effect but concluded that their data did not support this. de Weerdt 1996 states that the average response to the two treatments was the same regardless of the order in which they were received. Similarly, no taper was used in the withdrawal studies to reduce the risk of a 'post‐feverfew syndrome', which has been described in long‐term users who abruptly discontinue use of feverfew (Pfaffenrath 2002). Post‐feverfew syndrome includes among others a rebound of migraine symptoms, which is consistent with the results in the two withdrawal studies by Palevitch 1997 and Johnson 1985. Treatment periods ranged from 60 days (Palevitch 1997) to nine months (de Weerdt 1996). The IHS recommends at least three months for phase II trials.
Intervention
Different types of medication were used in the studies: three trials administered dried, powdered feverfew extract (Johnson 1985; Murphy 1988; Palevitch 1997), one used an alcoholic feverfew extract (de Weerdt 1996), and two used a CO2‐extract (Diener 2005; Pfaffenrath 2002). In these two most recent trials care was given to using a stable extract of feverfew highly enriched with parthenolide. Furthermore the most recent trial by Diener 2005 used a dosage of 6.25 mg three times a day, which had shown the most promising results in the earlier dose‐finding study by the same author team (Pfaffenrath 2002),
Patients were asked to stop any other medication for migraine prevention but use of analgesics to treat acute attacks was allowed and reported in four studies (de Weerdt 1996; Diener 2005; Johnson 1985; Pfaffenrath 2002). Higher consumption of analgesics in the placebo groups was reported by two studies (de Weerdt 1996; Johnson 1985), one study found no differences between groups (Pfaffenrath 2002) and one did not report any results (Diener 2005). One study did not record concomitant medication (Palevitch 1997) and one stated that all migraine medication was stopped but did not specify whether analgesics were allowed as rescue medication (Murphy 1988). It is unlikely that the use of analgesics to treat acute attacks will have influenced the results for the incidence of migraine attacks.
Evaluation/outcome measures
Generally the trials used headache diaries to record a variety of outcomes. Although frequency, duration and intensity of migraine attacks, as well as incidence and severity of nausea and vomiting, were the most commonly used outcome measures, the individual studies varied in the outcome measures used and some did not report results for frequency (Palevitch 1997), duration (de Weerdt 1996; Johnson 1985; Palevitch 1997), intensity (Johnson 1985), or nausea and vomiting (de Weerdt 1996; Diener 2005). Migraine attacks were reported differently (e.g. as migraine days, migraine attacks, days with a certain attack intensity, use of analgesics, etc.) and over a range of time frames (e.g. last four weeks of the trial period, over total trial duration). They were also presented differently (values at a certain time interval with or without measures of variance, per cent change compared to baseline, etc.).
Quality of the evidence
Generally the quality of the evidence was good with five studies being of low risk of bias for most criteria and scoring four to five points on the Oxford scale. Only one study scored only two points on the Oxford scale and we considered it of unclear risk of bias for all criteria (Palevitch 1997). However, four out of the six studies had fewer than 100 participants; two studies are of high risk of bias due to small sample size (de Weerdt 1996; Johnson 1985), the others are of unclear risk of bias. Their results therefore need to be interpreted with caution. The two latest and largest trials, Diener 2005 and Pfaffenrath 2002, follow the IHS guidelines for controlled trials of drugs in migraine prevention (Tfelt‐Hansen 2012).
Potential biases in the review process
By carrying out a thorough search for relevant studies and following Cochrane review methods we aimed to minimise the risk of bias in the review process itself.
One trial is available as an abstract only (Karimooy 2011). It is a controlled clinical trial (n = 168) of feverfew for the treatment of migraine headache compared to dihydroergotamine and placebo. Relevant data about methods and results are missing in the abstract, which makes it impossible to assess the study. We contacted the authors who confirmed that the study has not been published in full but were unable to provide any further details of the trial. It therefore remains unclear whether the study assessed migraine prevention and was randomised. The use of dihydroergotamine suggests that treatment rather than prevention of migraine was investigated. We have therefore excluded the study and are confident that its results would not have changed the overall outcome of this review.
Agreements and disagreements with other studies or reviews
The findings from the randomised clinical trials have been reported in other reviews (Pareek 2011; Sun‐Edelstein 2009). The most recent systematic review of feverfew for migraine, published in 2009, also includes six trials (Saranitzky 2009). The authors do not include the Palevitch 1997 study, which is published in a journal not indexed by MEDLINE, but include a RCT of a feverfew combination product (Maizels 2004), which we excluded from this Cochrane review as it does not allow any conclusions about the role of feverfew alone. The review concludes that "currently available research examining feverfew for migraine is promising especially with the dried feverfew leaf formulations. However, more research ... is needed before it can be recommended to a general population." (Saranitzky 2009).
Authors' conclusions
Implications for practice.
Overall there is low quality evidence that feverfew is effective in migraine prevention. The most recent study, a medium‐sized, rigorously executed study, demonstrated a decrease in migraine frequency of 0.6 attacks per month with feverfew compared with placebo. Results from previous studies, which were smaller and used different designs and interventions, were mixed and not convincing. Use of feverfew appears to be well tolerated and no major safety issues have been reported.
Implications for research.
These cautiously positive results need to be confirmed in larger trials, which need to be rigorously executed and reported. Efforts should be undertaken to minimise heterogeneity between trials by using clearly defined outcome measures and methods to capture them as described in the International Headache Society (IHS) guidelines (Tfelt‐Hansen 2012). Future trials should use stable, standardised feverfew extracts and clearly defined migraine populations.
What's new
| Date | Event | Description |
|---|---|---|
| 11 March 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 20 April 2015 | Review declared as stable | This review will be assessed for further updating in 2020. |
| 26 June 2014 | New search has been performed | Searches updated on 27 January 2015. Review fully revised and updated. Formal 'Risk of bias' tables added. |
| 11 June 2014 | New citation required and conclusions have changed | Fully revised all sections and restructured them to use the recommended subheadings. One new study (n = 218) added. Conclusion changed. Recommend that previous authors re‐read update. |
| 10 November 2008 | Amended | New Contact Person and Author added (B Wider). |
| 28 August 2008 | Amended | Converted to new review format. |
| 18 November 2003 | New search has been performed | Search updated through 25 July 2003. One new included study added. |
| 18 November 2003 | New citation required but conclusions have not changed | One new included study added. No substantive change to conclusions. |
Notes
A restricted search in March 2020 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We thank Joanne Abbott, Trials Search Co‐ordinator for the Cochrane Pain, Palliative and Supportive Care Group, for conducting the searches for this update.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Search strategies
Search strategies used in the original version of this review (2003)
The search terms described in below (free text and controlled vocabulary) were applied to the databases listed (numbers in square brackets at the end of each line indicate the number of trial reports retrieved). All electronic searches were based on the subject only; no filters for randomised controlled trials or controlled clinical trials were applied.
1. CENTRAL
#1 FEVERFEW single term (MeSH) [1] #2 feverfew [17] #3 (tanacetum next parthenium) [10] #4 (chrysanthemum next parthenium) [2 ] #5 mutterkraut [2] #6 (#1 or #2 or #3 or #4 or #5) [18] #7 MIGRAINE explode all trees (MeSH) [897] #8 HEADACHE DISORDERS explode all trees (MeSH) [1005] #9 (migrain* or headache* or cephalgi*) [7329] #10 (#7 or #8 or #9) [7329] #11 (#6 and #10) [11]
2. PREMEDLINE/MEDLINE
1 FEVERFEW.mp. [mp=title, abstract, rw, subject headings] [112] 2 (tanacetum adj parthenium).mp [45] 3 (chrysanthemum adj parthenium).mp [7] 4 mutterkraut.mp [0] 5 or/1‐4 [122] 6 migraine.mp [13793] 7 headache disorders.mp [424] 8 (migrain$ or headache$ or cephalgi$).mp [40184] 9 or/6 ‐8 [40184] 10 5 and 9 [33]
3. EMBASE
1 FEVERFEW.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] [125] 2 (tanacetum adj parthenium).mp [61] 3 (chrysanthemum adj parthenium).mp [11] 4 mutterkraut.mp [2) 5 or/1‐4 [143] 6 migraine.mp [13170] 7 headache disorders.mp [181] 8 (migrain$ or headache$ or cephalgi$).mp [57094] 9 or/6‐8 [57094] 10 5 and 9 [54]
4. PaPaS Trials Register
The following terms were used to search the trials register of the Cochrane Pain, Palliative and Supportive Care group: ((feverfew OR "tanacetum parthenium" OR "chrysanthemum parthenium" OR mutterkraut) AND (migrain* OR headache* OR cephalgi*))
5. AMED
1 exp feverfew/ [8] 2 feverfew.mp. [mp=abstract, heading words, title] [38] 3 "tanacetum parthenium".mp [32] 4 "chrysanthemum parthenium".mp [4] 5 mutterkraut.mp [0] 6 or/1‐5 [47] 7 migraine/ [328] 8 exp Vascular headache/ [343] 9 exp Headache/ [861] 10 headache$ [924] 11 (migrain$ or cephalgi$).mp [408] 12 or/7‐11 [1023] 13 6 and 12 [21]
Search strategies used for the 27 January 2009 update
1. CENTRAL
#1 MeSH descriptor Tanacetum parthenium explode all trees #2 feverfew #3 tanacetum next parthenium #4 chrysanthemum next parthenium #5 mutterkraut #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Headache Disorders explode all trees #8 migrain* or headache* or cephalgi* or cephalalgi* #9 (#7 OR #8) #10 (#6 AND #9)
2. Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE and 3. EMBASE (same search strategy used for both databases)
1 TANACETUM PARTHENIUM.mp. 2 (tanacetum adj parthenium) or feverfew.mp. 3 (chrysanthemum adj parthenium).mp. 4 mutterkraut.mp. 5 or/1‐4 6 migraine.mp. 7 Exp HEADACHE DISORDERS.mp. 8 (migrain$ or headache$ or cephalgi$ or cephalalgi$).mp. 9 or/6‐8 10 5 and 9
4. PaPaS Trials Register
The following terms were used to search the trials register of the Cochrane Pain, Palliative and Supportive Care group: ((feverfew OR "tanacetum parthenium" OR "chrysanthemum parthenium" OR mutterkraut) AND (migrain* OR headache* OR cephalgi*))
5. AMED
1 TANACETUM PARTHENIUM/ 2 tanacetum parthenium or feverfew.mp. 3 chrysanthemum adj parthenium".mp. 4 mutterkraut.mp. 5 or/1‐4 6 exp Vascular headache/ 7 migrain$ or headache$ or cephalgi$ or cephalalgi$.mp. 8 or/6‐7 9 5 and 8
Search strategies used for the 12 February 2014 and 27 January 2015 update
CENTRAL
#1 MeSH descriptor: [Tanacetum parthenium] explode all trees
#2 FEVERFEW*:it,ab,kw (Word variations have been searched)
#3 (tanacetum next parthenium):it,ab,kw (Word variations have been searched)
#4 (chrysanthemum next parthenium):it,ab,kw (Word variations have been searched)
#5 mutterkraut:it,ab,kw (Word variations have been searched)
#6 #1 or #2 or #3 or #4 or #5
#7 MeSH descriptor: [Headache Disorders] explode all trees
#8 migrain* or headache* or cephalgi* or cephalalgi*:it,ab,kw (Word variations have been searched)
#9 #7 or #8
#10 #6 and #9 from 2009 to 2014
MEDLINE
1. Tanacetum parthenium/
2. FEVERFEW*.tw.
3. (tanacetum adj parthenium).tw.
4. (chrysanthemum adj parthenium).tw.
5. mutterkraut.tw.
6. or/1‐5
7. exp Headache Disorders/
8. exp Migraine Disorders/
9. (migrain$ or headache$ or cephalgi$ or cephalalgi$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
10. 7 or 8 or 9
11. 6 and 10
12. randomized controlled trial.pt.
13. controlled clinical trial.pt.
14. randomized.ab.
15. placebo.ab.
16. drug therapy.fs.
17. randomly.ab.
18. trial.ab.
19. or/12‐18
20. exp animals/ not humans.sh.
21. 19 not 20
22. 11 and 21
EMBASE
1. Tanacetum parthenium/
2. FEVERFEW*.tw.
3. (tanacetum adj parthenium).tw.
4. (chrysanthemum adj parthenium).tw.
5. mutterkraut.tw.
6. or/1‐5
7. exp Headache Disorders/
8. exp Migraine Disorders/
9. (migrain$ or headache$ or cephalgi$ or cephalalgi$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
10. 7 or 8 or 9
11. 6 and 10
12. random$.tw.
13. factorial$.tw.
14. crossover$.tw.
15. cross over$.tw.
16. cross‐over$.tw.
17. placebo$.tw.
18. (doubl$ adj blind$).tw.
19. (singl$ adj blind$).tw.
20. assign$.tw.
21. allocat$.tw.
22. volunteer$.tw.
23. Crossover Procedure/
24. double‐blind procedure.tw.
25. Randomized Controlled Trial/
26. Single Blind Procedure/
27. or/12‐26
28. (animal/ or nonhuman/) not human/
29. 27 not 28
30. 11 and 29
AMED
1. Tanacetum parthenium/
2. FEVERFEW*.tw.
3. (tanacetum adj parthenium).tw.
4. (chrysanthemum adj parthenium).tw.
5. mutterkraut.tw.
6. or/1‐5
7. (migrain$ or headache$ or cephalgi$ or cephalalgi$).mp. [mp=abstract, heading words, title]
8. Vascular headache/
9. 7 or 8
10. 6 and 9
Characteristics of studies
Characteristics of included studies [ordered by study ID]
de Weerdt 1996.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind trial. Cross‐over. Treatment study | |
| Participants | 50 patients aged 18 to 64 years. Data from 44 patients evaluated. Migraine with and without aura (IHS diagnostic criteria) | |
| Interventions | 1 capsule (143 mg) of dried alcoholic feverfew daily for 4 months; placebo | |
| Outcomes | Frequency and intensity of headache. Adverse events | |
| Notes | 1‐month placebo run‐in period. No wash‐out period. Oxford score: 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients received an identification number on study entry referring to numbered packet of trial medication; a lottery determined whether their pack contained placebo or phytotherapeutic drug for first treatment period |
| Allocation concealment (selection bias) | Low risk | Randomisation code was kept in sealed envelopes until all patients had completed the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neither patients nor the neurologist knew whether the placebo or the phytotherapeutic drug was being administered during the treatment periods. Placebo had the same colour as feverfew and was placed in the same type of gelatine capsules with the same weight |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs clearly reported |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures included; results reported for all pre‐defined outcome measures |
| Other bias | High risk | High risk of bias due to small sample size (n = 44 evaluated) |
Diener 2005.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, multicentre, parallel‐group study. Treatment study | |
| Participants | 218 patients aged 18 to 65 years, 170 analysed. Migraine with or without aura according to IHS criteria | |
| Interventions | 6.25 mg CO2‐extract (MIG‐99) or placebo 3 times per day for 16 weeks | |
| Outcomes | Primary: total number of migraine attacks within 28 days during the second and third 28‐day treatment period (mean of P2 and P3) compared with baseline Secondary: further outcomes related to frequency and intensity of migraine. Clinical global impression of efficacy. Vital signs and laboratory parameters. Drop‐outs due to inefficacy. Adverse events |
|
| Notes | 45 patients randomised without fulfilling IHS criteria, excluded from analysis. Design and conduct of trial as well as data analysis were supported by a grant from the manufacturers of MIG‐99. 4‐week treatment‐free run‐in phase Oxford score: 5/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of 4 in centre‐specific blocks on the basis of a randomisation code using a validated program |
| Allocation concealment (selection bias) | Low risk | Random numbers were allocated in consecutive order on enrolment into study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All persons active in the study, including the biometrician, remained blinded until the database was locked" "Study medications were identical in appearance, size, weight, taste and smell." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 170 of 218 randomised patients completed the study; 45 had been randomised without fulfilling all IHS criteria. This resulted in 170 patients available for efficacy analysis in the ITT sample. Violators of the inclusion criteria did not differ between groups |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures included; results reported for pre‐defined outcome measures |
| Other bias | Unclear risk | Unclear risk of bias due to sample size (n = 170 evaluated for efficacy) |
Johnson 1985.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind trial. 2 parallel groups. Withdrawal study | |
| Participants | 17 patients (age range not reported). Data from all 17 patients available and evaluated. Common and classical migraine (diagnostic criteria not specified) | |
| Interventions | 2 capsules (25 mg each) of powdered feverfew leaves daily for 6 months | |
| Outcomes | Frequency of headache. Incidence of nausea and vomiting. Global assessment of efficacy. Adverse events | |
| Notes | All patients had taken raw feverfew leaves for the previous 3 to 4 years. Small sample size. Oxford score: 4/5 (R = 1, B = 2, WD = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Successive patients were allocated randomly to receive either feverfew or identical placebo capsules in numbered packs |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study described as double‐blind and study medications described as identical but no further details provided on who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from all participants evaluated |
| Selective reporting (reporting bias) | Low risk | Results reported for all pre‐specified outcome measures (some outcome measures such as duration or intensity were not pre‐specified) |
| Other bias | High risk | High risk of bias due to small sample size (n = 17) |
Murphy 1988.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind trial. Cross‐over. Treatment study | |
| Participants | 72 patients aged 24 to 72 years. Data from 59 patients evaluated. Common and classical migraine (as defined by Blau 1984) | |
| Interventions | 1 capsule (mean weight 82 mg) of dried powdered feverfew or placebo daily for 4 months | |
| Outcomes | Frequency and intensity of headache. Incidence of nausea and vomiting. Global assessment of efficacy. Adverse events | |
| Notes | Heterogeneous patient sample. 1‐month placebo run‐in. No wash‐out period. Subgroup analysis in classical/common migraine patients. Oxford score: 4/5 (R = 2, B = 1, WD = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as random allocation but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Probably done. Described as double‐blind. Placebo capsules described as similarly prepared and not possessing "any anti‐secretory activity". Not specified who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear description of drop‐outs and reasons |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures included results reported for pre‐defined outcome measures |
| Other bias | Unclear risk | Unclear risk of bias due to sample size (n = 72) |
Palevitch 1997.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind trial. Cross‐over. Withdrawal study | |
| Participants | 57 patients aged 9 to 65 years. Migraine diagnosed by medical examination; probably migraine with and without aura, though this is unclear (diagnostic criteria not specified) | |
| Interventions | 2 capsules (50 mg) of powdered feverfew daily for 1 month or placebo (dry parsley leaves, Petroselinum crispum) after a 60‐day open‐label phase in which all participants received 100 mg feverfew | |
| Outcomes | Intensity of headache. Severity of nausea and vomiting | |
| Notes | Both groups were treated with feverfew in the preliminary open‐label period for 60 days. No wash‐out period. No mention of migraine history, inclusion criteria or drop‐outs/withdrawals Included participants as young as 9 years old Oxford score: 2/5 (R = 1, B = 1, WD = 0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind but does not mention who was blinded and how blinding was done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs not reported |
| Selective reporting (reporting bias) | High risk | Outcome measures not clearly stated. Important outcome measures like frequency of migraine attacks not included |
| Other bias | Unclear risk | Unclear risk of bias due to sample size (n = 57) |
Pfaffenrath 2002.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind trial. 4 parallel groups. Treatment study | |
| Participants | 147 patients aged (mean (SD)) 43 (11) years. Data from all 147 patients evaluated. Migraine with and without aura (IHS diagnostic criteria) | |
| Interventions | 3 different doses (2.08 mg, 6.25 mg, 18.75 mg) of CO2‐extract or placebo 3 times daily for 3 months | |
| Outcomes | Total number of migraine attacks during the last 28 days of treatment compared with the 4‐week baseline period. Duration and intensity of migraine attacks, number of days with accompanying migraine symptoms, inability to work, additionally taken medication. Adverse events | |
| Notes | ITT analysis and confirmatory subset of ITT patients who had at least 4 migraine attacks per month at baseline (per protocol) 4‐week treatment‐free run‐in phase Design and conduct of trial as well as data analysis were supported by a grant from the manufacturers of MIG‐99 Oxford score: 5/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in centre‐specific blocks on the basis of a randomisation code using a validated programme |
| Allocation concealment (selection bias) | Low risk | Random numbers were allocated in consecutive order on enrolment into study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators remained "blinded" through the whole study. All other persons active in the study including the biometrician remained blinded until the database was locked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All drop‐outs accounted for, reasons fully described |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures included; results reported for pre‐defined outcome measures |
| Other bias | Unclear risk | Unclear risk of bias due to sample size (n = 147) |
Abbreviation: B = blinding; IHS = International Headache Society; ITT = intention‐to‐treat; R = randomisation; SD = standard deviation; W = withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cady 2011 | Combination product of feverfew and ginger used |
| Ferro 2012 | No placebo comparator; acupuncture compared with feverfew or combined treatment |
| IRCT2014081218776N1 2015 | Combination product of feverfew and lavender |
| Karimooy 2011 | Available as abstract only; further details requested from author but not received. Unclear whether randomised; unclear whether prevention of migraine |
| Kuritzky 1994 | No clinical outcomes reported |
| Maizels 2004 | Combination product of riboflavin, magnesium and feverfew |
| Trader 2007 | Not randomised, n‐of‐1 trial |
Differences between protocol and review
None.
Contributions of authors
Conception and design: B Wider, MH Pittler, E Ernst Literature searches: B Wider, MH Pittler Analysis and interpretation of the data: B Wider, MH Pittler, E Ernst Drafting of the review: B Wider, MH Pittler, E Ernst Critical revision of the review for important intellectual content: B Wider, MH Pittler, E Ernst Full revision of the review for compliance with new Cochrane review format: B Wider Final approval of the review: B Wider, MH Pittler, E Ernst
Sources of support
Internal sources
No sources of support supplied
External sources
International Headache Society (for administrative costs associated with editorial review and peer review), Other
Declarations of interest
B Wider: None known.
MH Pittler: None known.
E Ernst: None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
de Weerdt 1996 {published data only}
- Weerdt CJ, Bootsma HPR, Hendriks H. Herbal medicines in migraine prevention: randomized double-blind placebo-controlled crossover trial of a feverfew preparation. Phytomedicine 1996;3:225-30. [DOI] [PubMed] [Google Scholar]
Diener 2005 {published data only}
- Diener HC, Pfaffenrath V, Schnitker J, Friede M, Henneicke-von-Zepelin H-H. Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention--a randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia 2005;25:1031-41. [DOI] [PubMed] [Google Scholar]
Johnson 1985 {published data only}
- Johnson ES, Kadam NP, Hylands DM, Hylands PJ. Efficacy of feverfew as prophylactic treatment of migraine. BMJ 1985;291:569-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 1988 {published data only}
- Murphy JJ, Heptinstall S, Mitchell JRA. Randomized double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet 1988;2:189-92. [DOI] [PubMed] [Google Scholar]
Palevitch 1997 {published data only}
- Palevitch D, Earon G, Carasso R. Feverfew (Tanacetum parthenium) as a prophylactic treatment for migraine: a double-blind placebo-controlled study. Phytotherapy Research 1997;11:508-11. [Google Scholar]
Pfaffenrath 2002 {published data only}
- Pfaffenrath V, Diener HC, Fischer M, Friede M, Henneicke-von Zeppelin HH. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis -- a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia 2002;22:523-32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cady 2011 {published data only}
- Cady RK, Goldstein J, Nett R, Mitchell R, Beach ME, Browning R. A double-blind placebo-controlled pilot study of sublingual feverfew and ginger (LipiGesic™ M) in the treatment of migraine. Headache 2011;51:1078-86. [DOI] [PubMed] [Google Scholar]
Ferro 2012 {published data only}
- Ferro EC, Biagini AP, da Silva ÍE, Silva ML, Silva JR. The combined effect of acupuncture and Tanacetum parthenium on quality of life in women with headache: randomised study. Acupuncture in Medicine 2012;30:252-7. [DOI] [PubMed] [Google Scholar]
IRCT2014081218776N1 2015 {published data only (unpublished sought but not used)}
- Rafi S. Investigation of efficacy of feverfew and lavander in migraine prophylaxis. International Clinical Trials Registry Platform. http://www.irct.ir/searchresult.php?id=18776&number=1 , accessed 30 January 2015.
Karimooy 2011 {published data only}
- Karimooy HN, Gholamnezhad Z, Talebi M. Effect of tanacetum parthenium (feverfew) in treatment of migraine headache comparing to dihydroergotamine and placebo. In: Pain Medicine. Vol. 12. 2011:487.
Kuritzky 1994 {published data only}
- Kuritzky A, Elhacham Y, Yerushalmi Z, Hering R. Feverfew in the treatment of migraine: its effect on serotonin uptake and platelet activity. Neurology 1994;44 Suppl 2:A201. [Google Scholar]
Maizels 2004 {published data only}
- Maizels M, Blumenfeld A, Burchetter R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache 2004;44:885-90. [DOI] [PubMed] [Google Scholar]
Trader 2007 {published data only}
- Trader J-M. Feverfew as preventive therapy for migraine: N-of-1-trial for verification of individual efficacy. Zeitschrift fuer Allgemeinmedizin 2007;83:238-41. [Google Scholar]
Additional references
Anderson 1988
- Anderson D, Jenkinson PC, Dewdney RS, Blowers SD, Johnson ES, Kadam NP. Chromosomal aberrations and sister chromatid exchanges in lymphocytes and urine mutagenicity of migraine patients: a comparison of chronic feverfew users and matched non-users. Human Toxicology 1988;7:145-52. [DOI] [PubMed] [Google Scholar]
Awang 1998
- Awang DVC. Prescribing therapeutic feverfew (Tanacetum parthenium (L.) Schultz-Bip., syn. Chrysanthemum parthenium (L.) Bernh.). Integrative Medicine 1998;1:11-3. [Google Scholar]
Blau 1984
- Blau JN. Towards a definition of migraine headache. Lancet 1984;1:444-5. [DOI] [PubMed] [Google Scholar]
Brown 1997
- Brown AMG, Edwards CM, Davey MR, Power B, Lowe KC. Pharmacological activity of feverfew (Tanacetum parthenium (L.) Schultz-Bip.): assessment by inhibition of human polymorphonuclear leucocyte chemiluminescence in-vitro. Journal of Pharmacy and Pharmacology 1997;49:558-61. [DOI] [PubMed] [Google Scholar]
Brun 1995
- Brun J, Claustrat B, Saddier P, Chazor G. Nocturnal melatonin excretion is decreased in patients with migraine without aura attacks associated with menses. Cephalalgia 1995;15:136-9. [DOI] [PubMed] [Google Scholar]
Burry 1980
- Burry JN. Compositae dermatitis in South Australia: contact dermatitis from Chrysanthemum parthenium. Contact Dermatitis 1980;6:445. [DOI] [PubMed] [Google Scholar]
Dodick 2007
- Dodick DW, Silberstein SD. Migraine prevention. Practical Neurology 2007;7:383-93. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Zellweger-Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350:326-9. [DOI] [PubMed] [Google Scholar]
Ernst 2007
- Ernst E, Pittler MH, Wider B, Boddy K. Complementary Therapies for Pain Management. Edinburgh: Elsevier, 2007. [DOI] [PubMed] [Google Scholar]
ESCOP 2003
- ESCOP European Scientific Cooperative on Phytotherapy. Tanaceti parthenii herba, feverfew. In: ESCOP Monographs. 2nd edition. Stuttgart: Thieme, 2003. [Google Scholar]
Goadsby 1997
- Goadsby PJ. How do the currently used prophylactic agents work in migraine? Cephalalgia 1997;17:85-92. [DOI] [PubMed] [Google Scholar]
Hausen 1983
- Hausen BM, Osmundsen PE. Contact allergy to parthenolide in Tanacetum parthenium (L.) Schulz-Bip. (feverfew, Asteraceae) and cross-reactions to related sesqiterpene lactone containing Compositae species. Acta Dermato-Venereologica 1983;63:308-14. [PubMed] [Google Scholar]
Hendriks 1996
- Hendriks H, Bos R, Woerdenbag HJ. The essential oil of Tanacetum parthenium (L.) Schultz-Bip. Flavour and Fragrance Journal 1996;11:367-71. [Google Scholar]
Heptinstall 1985
- Heptinstall S, White A, Williamson L, Mitchell JRA. Extracts of feverfew inhibit granule secretion in blood platelets and polymorphonuclear leucocytes. Lancet 1985;1(8437):1071-4. [DOI] [PubMed] [Google Scholar]
Heptinstall 1987
- Heptinstall S, Groenewegen WA, Spangenberg P, Loesche W. Extracts of feverfew may inhibit platelet behaviour via neutralization of sulphydryl groups. Journal of Pharmacy and Pharmacology 1987;39:459-65. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
IHS 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1-96. [PubMed] [Google Scholar]
IHS 2000
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine,second edition. Cephalalgia 2000;20:765–86. [DOI] [PubMed] [Google Scholar]
IHS 2013
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629-808. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carrol D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
Knight 1995
- Knight DW. Feverfew: Chemistry and biological activity. Natural Product Reports 1995;12:271-6. [DOI] [PubMed] [Google Scholar]
Linde 2012
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19:703-11. [DOI] [PubMed] [Google Scholar]
Makheja 1982
- Makheja AN, Bailey JM. A platelet phospholipase inhibitor from the medicinal herb feverfew (Tanacetum parthenium). Prostaglandins Leukotrienes and Medicine 1982;8:653-60. [PubMed] [Google Scholar]
Munakata 2009
- Munakata J, Hazard E, Serrano D, Klingman D, Rupnow MF, Tierce J, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2009;49:498-508. [DOI] [PubMed] [Google Scholar]
Murch 1997
- Murch SJ, Simmons CB, Saxena PK. Melatonin in feverfew and other medicinal plants. Lancet 1997;350:1598-9. [DOI] [PubMed] [Google Scholar]
Natural Standard 2014
- Natural Standard Research Collaboration. Feverfew. Natural Standard, available at www.naturalstandard.com (accessed 4 February 2014).
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;16:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pareek 2011
- Pareek A, Suthar M, Rathore GS, Bansal V. Feverfew (Tanacetum parthenium) L.): a systematic review. Pharmacognosy Reviews 2011;5:103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Posadzki 2013
- Posadzki P, Watson LK, Alotaibi A, Ernst E. Prevalence of herbal medicine use by UK patients/consumers: a systematic review of surveys. Focus on Alternative and Complementary Therapies 2013;18:19-26. [Google Scholar]
Pugh 1988
- Pugh WJ, Sambo K. Prostaglandin synthetase inhibitors in feverfew. Journal of Pharmacy and Pharmacology 1988;40:743-5. [DOI] [PubMed] [Google Scholar]
Saranitzky 2009
- Saranitzky E, White CM, Baker EL, Baker WL, Coleman CI. Feverfew for migraine prophylaxis: a systematic review. Journal of Dietary Supplements 2009;6:91-103. [DOI] [PubMed] [Google Scholar]
Steiner 2013
- Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. Cephalalgia 2013;33:289-90. [DOI] [PubMed] [Google Scholar]
Sun‐Edelstein 2009
- Sun-Edelstein C, Mauskop A. Foods and supplements in the management of migraine headaches. Clinical Journal of Pain 2009;25:446-52. [DOI] [PubMed] [Google Scholar]
Taylor 2011
- Taylor FR. Nutraceuticals and headache: the biological basis. Headache 2011;51:484-501. [DOI] [PubMed] [Google Scholar]
Tfelt‐Hansen 1987
- Tfelt-Hansen P, Nielsen SL. Patient numbers needed in prophylactic migraine trials. Neuroepidemiology 1987;6:214-9. [DOI] [PubMed] [Google Scholar]
Tfelt‐Hansen 2012
- Tfelt-Hansen P, Pascual J, Ramadan N, Dahlöf C, D'Amico D, Diener HC, et al, International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32:6-38. [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willigmann 1999
- Willigmann J, Freudenstein J. Production of a stable feverfew (Tanacetum parthenium) extract as an active substance for a pharmaceutical product. Internal document, Schaper Brummer GmbH and Co., 1999; Poster Symposium. Vienna: Society for Medicinal Plant Research; 1998.
World Health Organization 2012
- World Health Organization. Headache disorders. Fact sheet N°277. http://www.who.int/mediacentre/factsheets/fs277/en/ 2012 (accessed 29 January 2014).
References to other published versions of this review
Ernst 2000
- Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.): an update of a systematic review. Public Health Nutrition 2000;3:509-14. [DOI] [PubMed] [Google Scholar]
Pittler 2000
- Pittler MH, Vogler BK, Ernst E. Feverfew for preventing migraine. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002286] [DOI] [PubMed] [Google Scholar]
Pittler 2004
- Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002286.pub2] [DOI] [PubMed] [Google Scholar]
Vogler 1998
- Vogler BK, Pittler MH, Ernst E. Feverfew as a preventive treatment for migraine: a systematic review. Cephalalgia 1998;18:704-8. [DOI] [PubMed] [Google Scholar]


