Abstract
Background
The rate of operative deliveries (both caesarean sections, vacuum extractions and forceps), continues to rise throughout the world. These are associated with significant maternal and neonatal morbidity. The most common reasons for operative births in nulliparous women are labour dystocia (failure to progress), and non‐reassuring fetal status. Epidural analgesia has been shown to slow the progress of labour, as well as increase the rate of instrumental deliveries. However, it is unclear whether the use of oxytocin in women with epidural analgesia results in a reduction in operative deliveries, and thereby reduces both maternal and fetal morbidity.
Objectives
To determine whether augmentation of women using epidural analgesia with oxytocin will decrease the incidence of operative deliveries and thereby reduce fetal and maternal morbidity.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2013).
Selection criteria
All published and unpublished randomised and quasi‐randomised trials that compared augmentation with oxytocin of women in spontaneous labour with epidural analgesia versus intent to manage expectantly were included. Cluster‐randomised trials were eligible for inclusion but none were identified.
Cross‐over study designs were unlikely to be relevant for this intervention, and we planned to exclude them if any were identified. We did not include results that were only available in published abstracts.
Data collection and analysis
The two review authors independently assessed for inclusion the 16 studies identified as a result of the search strategy. Both review authors independently assessed the risk of bias for each included study. Both review authors independently extracted data. Data were checked for accuracy.
Main results
We included two studies, involving 319 women. There was no statistically significant difference between the two groups in either of the primary outcomes of caesarean section (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.42 to 2.12) or instrumental delivery (RR 0.88, 95% CI 0.72 to 1.08). Similarly, there were no statistically significant differences between the two groups in any of the secondary outcomes for which data were available. This included Apgar score less than seven at five minutes (RR 3.06, 0.13 to 73.33), admission to neonatal intensive care unit (RR 1.07, 95% CI 0.29 to 3.93), uterine hyperstimulation (RR 1.32, 95% CI 0.97 to 1.80) and postpartum haemorrhage (RR 0.96, 95% CI 0.58, 1.59).
Authors' conclusions
There was no statistically significant difference identified between women in spontaneous labour with epidural analgesia who were augmented with oxytocin, compared with those who received placebo. However, due to the limited number of women included in the studies, further research in the form of randomised controlled trials are required.
Plain language summary
Oxytocin for reducing operative births in women with epidurals in labour
The rate of operative births (caesarean sections, forceps and vacuum extraction) continues to rise throughout the world. All three types of delivery are associated with significant complications for both the mother and her baby such as traumatic birth injuries, increased blood loss and placental complications in future pregnancies. One of the most common reasons for a woman to require an operative birth is because the labour does not progress adequately. Increasingly, epidurals are used to manage the pain during labour, however, epidurals may also slow the progression of labour. Oxytocin is a hormone that stimulates uterine contractions in labour and is given to women who are slow to progress in labour. By giving oxytocin to all women with epidurals during labour, the rate of operative deliveries, and the associated complications, could be reduced.
Data were collected from two randomised studies (involving 319 women) which compared women with epidurals who were given either oxytocin, or a placebo. The rates of operative deliveries were not clearly different between the two groups There were also no significant differences between the other outcomes analysed, such as the Apgar scores of the newborn babies, admissions to the neonatal nursery, rates of post birth haemorrhage or rates of over stimulation of the uterus. Both studies appeared to have a low risk of bias.
Overall, there was no significant difference between the rates of operative deliveries in women with epidurals who were given oxytocin compared with those who received the placebo. However, as there were limited data available, in order to fully determine whether augmentation of women with epidurals reduces the rate of operative deliveries and therefore reduces the complications associated, further studies are required.
Background
The rate of operative deliveries, both caesarean sections and instrumental deliveries (vacuum extractions and forceps), continues to rise throughout the world (Hamilton 2009). In Australia, the rate of operative deliveries rose from 32% in 1997 to 42.5% in 2008 (Day 1999; Laws 2010), with currently more than 30% of deliveries being performed by caesarean section. A 2007 WHO global survey into mode of delivery amongst 24 countries worldwide showed that the rate of all operative deliveries varied between 2.3% and 47.4%, with an average caesarean section rate of 25.7% throughout the countries involved (Souza 2010). Similarly, Betran 2007 showed that the caesarean section rates in developed countries averaged 21.1%, compared with developing countries which had an average rate of just 2%.
Operative deliveries are associated with significant fetal and maternal morbidity, both short and long term, and impact upon subsequent pregnancies. All operative deliveries are associated with increased rates of maternal mortality, as well as increased rates of blood transfusions, hysterectomy and intensive care unit admissions when compared with spontaneous vaginal delivery (Souza 2010).
Instrumental deliveries are associated with an increased risk of third‐ and fourth‐degree perineal tears, increased pain and incontinence. They are also associated with fetal injuries such as facial and scalp injuries, and cephalohaematomas (O'Mahony 2010). Furthermore, a previous operative delivery is associated with an increased rate of operative deliveries in subsequent pregnancies (Melamed 2009).
Caesarean sections are also associated with significant maternal morbidity including increased abdominal pain and postpartum haemorrhage (Wang 2010). Pregnancies following caesarean sections are associated with obstetric complications such as placenta praevia, placenta accreta and uterine rupture (Al‐Zirqi 2010; Hemminki 1996).
Description of the condition
The most common reasons for operative deliveries in nulliparous women are labour dystocia (failure to progress), and non‐reassuring fetal status (Dencker 2009; Shields 2007). The most common reason in multiparous women is previous operative delivery (Laws 2010). The frequent incidence of labour dystocia is further enhanced by the increasingly common use of epidural analgesia, especially amongst nulliparous women (Dickinson 2002; Hamilton 2009; Laws 2010). Epidural analgesia has been shown to slow significantly the second stage of labour, as well as increase the rate of instrumental deliveries (Alexander 2002; Anim‐Somuah 2011; Liu 2004; Newton 1995). The effect on the first stage of labour is less clear. Anim‐Somuah 2011's review of epidural analgesia showed no significant increase in the first stage of labour, however, there was significant heterogeneity amongst the studies included. There is also some discrepancy in the literature as to whether or not epidural analgesia alone results in an increased rate of caesarean section (Liu 2004; Thorp 1993; Zimmer 2000). Anim‐Somuah 2011 showed no significant increase in the rate of caesarean section in their review which included 8895 women.
Description of the intervention
Labour dystocia is most commonly managed by augmentation with a synthetic form of the hormone oxytocin, which stimulates uterine contractions in labour. Adequate uterine contractions are essential for labour to progress.
How the intervention might work
Augmentation with oxytocin has been shown to significantly reduce the length of both the first and second stages of labour (Hinshaw 2008; Sadler 2000). It has also demonstrated an increase in the rate of spontaneous vaginal deliveries (Wei 2009); however, there is uncertainty as to whether oxytocin augmentation results in a statistically significant reduction in caesarean sections (Fraser 1998; Sadler 2000). Oxytocin augmentation has not been shown to affect the rate of maternal satisfaction, postpartum haemorrhage or uterine rupture in nulliparous women (Akoury 1991; Sadler 2000). Studies also show that it does not appear to negatively impact on fetal morbidity or mortality in both nulliparous (Akoury 1991; Sadler 2000; Wei 2009) and multiparous women (Ben‐Aroya 2001), although uterine rupture has been associated with oxytocin use in multiparous women (Ozdemir 2005).
Why it is important to do this review
Oxytocin augmentation is often used in women with epidural analgesia to improve uterine contractibility and reduce the effect of labour dystocia. However, it is unclear whether the use of oxytocin in women with epidural analgesia results in a significant reduction in operative deliveries, and thereby reduces both maternal and fetal morbidity.
Objectives
The objective of this study is to determine whether augmentation of women using epidural analgesia with oxytocin will decrease the incidence of operative deliveries and thereby reduce fetal and maternal morbidity.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised and quasi‐randomised trials that compared augmentation of women with epidural analgesia versus intent to manage expectantly. Cross‐over study designs were unlikely to be relevant for this intervention, and we planned to exclude them if any were identified. We did not include results that were only available in published abstracts.
Types of participants
All women in spontaneous labour with epidural analgesia who have not been augmented or induced with oxytocin prior to randomisation.
Types of interventions
Augmentation with oxytocin versus intent to manage expectantly.
Types of outcome measures
Primary outcomes
Caesarean section
Instrumental delivery (forceps and vacuum)
Secondary outcomes
Apgar scores less than seven at five minutes
Apgar scores less than four at five minutes
Admission to neonatal intensive care unit
Uterine hyperstimulation
Uterine rupture
Postpartum haemorrhage
Breastfeeding
Maternal satisfaction
Maternal anxiety
Episiotomy
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 June 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
The two review authors (Philippa Costley and Christine East) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, both review authors extracted the data using the agreed form. We planned to resolve any discrepancies through discussion. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
Assessment of risk of bias in included studies
The two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to resolve any disagreement by discussion.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We did not identify any studies for inclusion that reported continuous data. If we identify studies in the future that report continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
If we had identified cluster‐randomised trials, we planned to include them in the analyses along with individually randomised trials. If such trials are identified in the future, we will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
As a cross‐over trial design would be inappropriate in this clinical setting, we planned to exclude such trials.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If we had included 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots. If we identify additional trials in the future, we will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes, we will use the test proposed by Egger 1997, and for dichotomous outcomes, we will use the test proposed by Harbord 2006. If we detect asymmetry in either of these tests or by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, we presented the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses:
nulliparous and multiparous;
augmentation timing: cervical dilatation less than 5 cm, between 5 cm and 9 cm, second stage of labour (from 10 cm dilatation).
We planned to use the primary outcomes in subgroup analysis.
The data available in the included studies did not allow us to perform the prespecified subgroup analysis. There were no data on multiparous women, so we were unable to subdivide by parity.
The available data allowed for subgroup analysis of augmentation in women with incomplete (< 10 cm) and full (10 cm) cervical dilatation, rather than the prespecified breakdown as stated in the protocol. We therefore entered the data in the available format. If future studies present data in our prespecified format, we will revise our subgroup analyses using the prespecified categories.
For fixed‐effect inverse variance meta‐analyses, we planned to assess differences between subgroups by interaction tests. For random‐effects and fixed‐effect meta‐analyses using methods other than inverse variance, we planned to assess differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
We planned to carry out sensitivity analysis of the primary outcomes (caesarean section and instrumental delivery) to explore the effect of trial quality, including studies assessed as having adequate controls in place for the prevention of potential bias. However, due to the limited number and quality of studies, this was not required.
Results
Description of studies
Results of the search
In total, the search identified 16 studies for consideration. Two studies were included and 14 were excluded.
Included studies
The two studies identified for inclusion in the systematic review were Saunders 1989 and Shennan 1995. The two studies involved a total of 319 women; 154 women randomised to receive oxytocin infusion and 165 women randomised to a placebo treatment.
The two studies used similar augmentation protocols with oxytocin, and compared with a normal saline placebo infusion. Interestingly, both studies only included nulliparous women, however, importantly, the studies focused on different stages of labour. Saunders 1989 focused on women who had reached full dilatation, whereas Shennan 1995 included women who were ≤ 6 cm dilated.
For further details about the included studies' designs, seeCharacteristics of included studies.
Excluded studies
Fourteen studies were excluded. The most common reasons for exclusion were studies not being specific to women with epidural analgesia (eight studies), the analysis not being relevant to this study question (two studies) and being an unacceptable study design (two studies). One study (Pickrell 1989) fulfilled the inclusion criteria, however, was only published in abstract form. For this reason it was also excluded from the analysis.
For more details on reasons for exclusion, seeCharacteristics of excluded studies.
Risk of bias in included studies
The two included studies were both double blinded randomised controlled trials of adequate quality. Both studies specified the methods of randomisation and allocation concealment, however, Saunders 1989 did not specifically mention the method of random number generation. Neither of the studies had pre‐published protocols, so the risk of selective reporting bias was unclear. It was also unclear from both studies whether the outcome assessors were also blinded. Overall, both studies appear to have a low risk of bias.
SeeFigure 1 and Figure 2 for a 'Risk of bias' graph and summary table.
1.
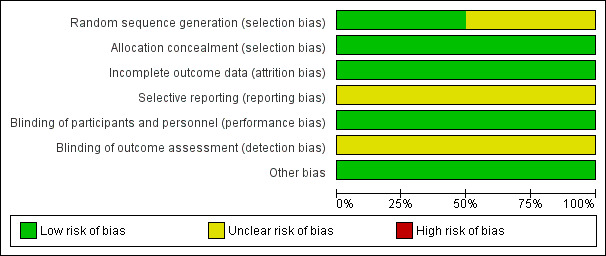
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
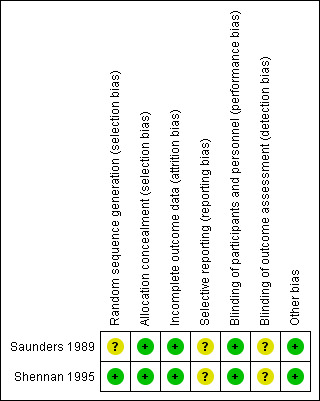
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
There was no statistically significant difference between the two groups in either of the primary outcomes of caesarean section (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.42 to 2.12; two studies, 319 women; Analysis 1.1) or instrumental delivery (RR 0.88, 95% CI 0.72 to 1.08; two studies, 319 women; Analysis 1.2). Furthermore, there was no statistically significant difference between the rate of combined operative deliveries between the oxytocin and placebo groups (average RR 1.01, 95% CI 0.68 to 1.50; two studies, 319 women; random effects, T² =0.06, I² = 77%; Analysis 1.3).
1.1. Analysis.
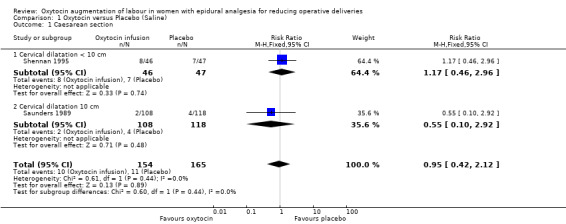
Comparison 1 Oxytocin versus Placebo (Saline), Outcome 1 Caesarean section.
1.2. Analysis.
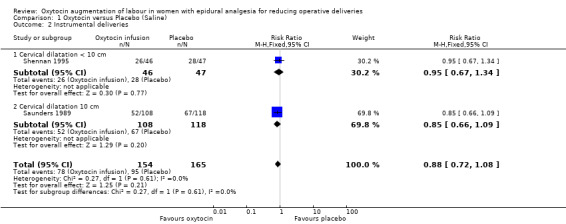
Comparison 1 Oxytocin versus Placebo (Saline), Outcome 2 Instrumental deliveries.
1.3. Analysis.
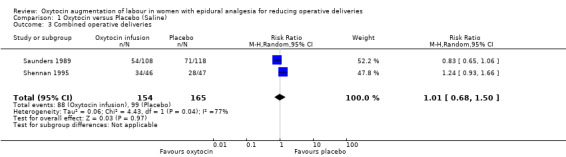
Comparison 1 Oxytocin versus Placebo (Saline), Outcome 3 Combined operative deliveries.
Similarly, there were no statistically significant differences between the two groups in any of the secondary outcomes for which there were data available. This included Apgar score less than seven at five minutes (RR 3.06, 95% CI 0.13 to 73.33; two studies, 319 women; Analysis 1.4), admission to neonatal intensive care unit (RR 1.07, 95% CI 0.29 to 3.93; two studies, 319 women; Analysis 1.6), uterine hyperstimulation (RR 1.32, 95% CI 0.97 to 1.80; one study, 226 women; Analysis 1.7) and postpartum haemorrhage (RR 0.96, 95% CI 0.58 to 1.59; two studies, 319 women; Analysis 1.8).
1.4. Analysis.
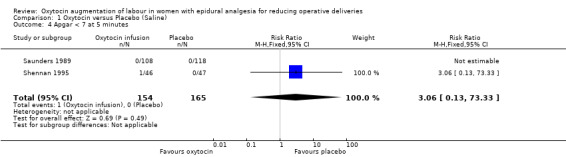
Comparison 1 Oxytocin versus Placebo (Saline), Outcome 4 Apgar < 7 at 5 minutes.
1.6. Analysis.
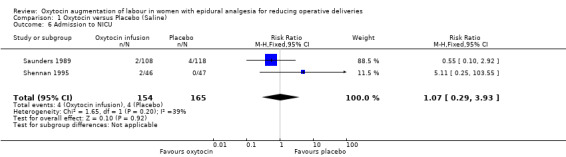
Comparison 1 Oxytocin versus Placebo (Saline), Outcome 6 Admission to NICU.
1.7. Analysis.
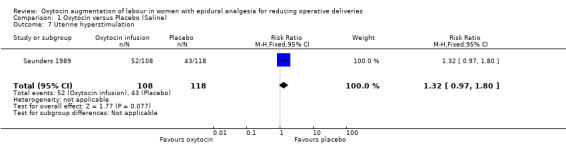
Comparison 1 Oxytocin versus Placebo (Saline), Outcome 7 Uterine hyperstimulation.
1.8. Analysis.
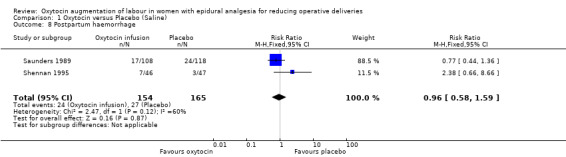
Comparison 1 Oxytocin versus Placebo (Saline), Outcome 8 Postpartum haemorrhage.
Discussion
The review identified two randomised controlled trials which compared oxytocin augmentation with placebo in women in spontaneous labour with epidural analgesia. The studies included a total of 319 women; 154 women randomised to receive oxytocin infusion and 165 women randomised to a placebo treatment with the primary outcome being the rate of operative deliveries, both caesarean section and instrumental deliveries.
There were no statistically significant differences identified in any of the outcomes measured. The groups had similar rates of caesarean section with 6.5% in the oxytocin group and 6.7% in the placebo group. The rates of instrumental deliveries were also very similar with 50.6% in the oxytocin group and 57.6% in the placebo group.
There is a marked contrast in the caesarean section rates in the included studies (2% to 17%) compared with the currently published rates in developed countries of 21.1% (Betran 2007). Furthermore, it is well known that the rate of operative deliveries had risen dramatically over the past 10 years. In Australia, the overall rate of operative deliveries rose from 32% in 1997 to 42.5% in 2008 (Day 1999; Laws 2010). This rate may be further increased in women with epidural analgesia (Anim‐Somuah 2011). This decade of change calls into question the generalisability of the findings from both of the included studies that were published more than ten years prior to this review. On this basis, it is reasonable to question whether the lack of a statistically significant difference observed can be enough evidence to impact clinical practice.
There were no significant difference in any of the secondary outcomes which were studied including Apgar score less than seven at five minutes, admission to neonatal intensive care unit, uterine hyperstimulation and postpartum haemorrhage. There were no data available to compare the other prespecified secondary outcomes including Apgar scores less than four at five minutes, uterine rupture, rates of breastfeeding, maternal satisfaction, maternal anxiety or episiotomy rates.
The limited number of relevant studies available for inclusion in this review may have also potentially impacted on the lack of a statistically significant difference between the two groups. Larger numbers are required to more accurately assess the treatment effect.
Neither of the included studies included multiparous women. It would be difficult to develop a randomised controlled trial including these women, as many clinicians may be concerned about potential complications, in particular, the risk of uterine rupture associated with a multiparous uterus. Despite widespread caution in the augmentation of a multiparous woman, limited evidence from the literature is available to support this perceived increase rate of rupture of an unscarred uterus. Two retrospective studies both show an increased rate of uterine rupture amongst multiparous women, however, the absolute risk is very low. (Ozdemir 2005; Lao 1987). Furthermore, in clinical practice, multigravid women have a reduced rate of operative deliveries overall, and reducing the rate of operative deliveries in nulliparous women would be associated with a larger clinical effect.
The studies enrolled women in whom epidurals had been sited at different times during labour. Saunders 1989 included women at full cervical dilatation, whereas Shennan 1995 included women who were ≤ 6 cm dilated when they were randomised. As we were unable to perform the subgroup analysis as prespecified in our protocol, we performed a subgroup analysis of women who were fully dilated (10 cm) at randomisation, and those who were less than 10 cm dilated. There were no statistically significant differences in the rates of operative deliveries between the patients who were augmented with oxytocin as opposed to those who received placebo in either of the subgroups. These subgroups are associated with different clinical situations. Women who are augmented at full dilatation would have a reduced rate of operative deliveries, in particular, caesarean section as compared with those augmented during the first stage of labour. If future trials are included in updates of this review, we will endeavour to undertake the subgroup analyses as planned.
Overall, the review did not identify any statistically significant difference between the rates of operative deliveries amongst women with epidural analgesia who received oxytocin augmentation as opposed to those who received placebo. To accurately determine whether oxytocin augmentation in women with epidural analgesia would reduce the rates of operative deliveries and thereby decreases maternal and neonatal morbidity, a large double blinded randomised controlled trial is required. Currently, there is not enough evidence to support or refute routine oxytocin augmentation of women with epidural analgesia.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to guide practice in regards to routine oxytocin augmentation of nulliparous women with epidural analgesia to reduce operative deliveries. There is no evidence from randomised trials to guide practice in multiparous women.
Implications for research.
Well conducted randomised controlled trials with sufficient numbers are required to accurately determine whether augmentation of women in spontaneous labour with epidural analgesia will reduce the rate of operative deliveries.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2013 | New citation required but conclusions have not changed | Review updated. |
| 30 June 2013 | New search has been performed | Search updated. No new trial reports identified. |
Acknowledgements
As part of the pre‐publication editorial process, the first version of this review (Costley 2012) was commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Oxytocin versus Placebo (Saline).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.42, 2.12] |
| 1.1 Cervical dilatation < 10 cm | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.46, 2.96] |
| 1.2 Cervical dilatation 10 cm | 1 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.10, 2.92] |
| 2 Instrumental deliveries | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 2.1 Cervical dilatation < 10 cm | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.34] |
| 2.2 Cervical dilatation 10 cm | 1 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.09] |
| 3 Combined operative deliveries | 2 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.68, 1.50] |
| 4 Apgar < 7 at 5 minutes | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.13, 73.33] |
| 5 Apgar < 4 at 5 minutes | 1 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Admission to NICU | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.29, 3.93] |
| 7 Uterine hyperstimulation | 1 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.97, 1.80] |
| 8 Postpartum haemorrhage | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.59] |
1.5. Analysis.
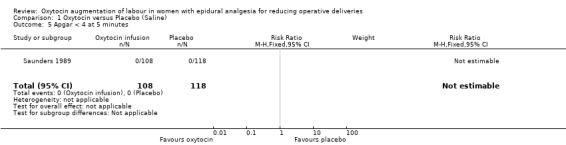
Comparison 1 Oxytocin versus Placebo (Saline), Outcome 5 Apgar < 4 at 5 minutes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Saunders 1989.
| Methods | Double blinded randomised controlled trial of oxytocin versus placebo. | |
| Participants | 226 Nulliparous women (108 intervention, 118 placebo) with epidural analgesia, 37‐42 weeks' gestation, singleton fetus in vertex presentation, fully dilated cervix, no oxytocin prior to randomisation. | |
| Interventions | Oxytocin (initial dose 2 mU/min increasing to maximum of 16 mU/min) versus placebo. | |
| Outcomes | Duration of second stage, mode of delivery, postpartum haemorrhage, fetal condition (Apgar scores, neonatal jaundice, nursery admission, cord pH). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as random number allocation although sequencing not specified. |
| Allocation concealment (selection bias) | Low risk | Sheffield: infusions of oxytocin added to saline or saline alone were prepared by the pharmacy. London: coded vials of oxytocin or saline alone were used which were added to the infusion by labour ward staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for. |
| Selective reporting (reporting bias) | Unclear risk | This cannot be assessed as there was no published protocol or trial registration. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Other bias | Low risk | None identified. |
Shennan 1995.
| Methods | Double blinded randomised controlled trial of oxytocin versus placebo. | |
| Participants | 93 Nulliparous women (46 intervention, 47 placebo) with epidural analgesia, > 35 weeks' gestation, singleton fetus in vertex presentation, 6 cm dilated or less. | |
| Interventions | Artificial rupture of membranes and oxytocin (initial dose 2 mU/min increasing to maximum of 32 mU/min) versus placebo. | |
| Outcomes | Duration of first and second stage, mode of delivery, postpartum haemorrhage, fetal condition (Apgar scores, intubation, nursery admission, cord pH). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Coded ampoules of oxytocin or saline were prepared by pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for. |
| Selective reporting (reporting bias) | Unclear risk | This cannot be assessed as there was no published protocol or trial registration. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded, unless slow progress noted and code was broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Other bias | Low risk | None identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bidgood 1987a | Randomised controlled trial of placebo versus low dose ocytocin versus high dose oxytocin to reduce caesarean section rates. The study was not specific to patients with epidural analgesia and therefore the study population was not relevant for this review. |
| Bidgood 1987b | Randomised controlled trial of placebo versus low dose ocytocin versus high dose oxytocin in regards to uterine activity. The study was not specific to patients with epidural analgesia and therefore the study population was not relevant for this review. |
| Cammu 1996 | Randomised controlled trial of early versus delayed amniotomy and oxytocin infusion. The study was not specific to patients with epidural analgesia and therefore the study population was not relevant for this review. |
| Cardozo 1990 | Randomised controlled trial of placebo versus oxytocin infusion in relation to cervical dilatation. The study was not specific to patients with epidural analgesia and therefore the study population was not relevant for this review. |
| Girard 2009 | Non‐randomised controlled trial of continuing versus discontinuing oxytocin in the active phase of labour on various obstetric outcomes. The intervention was not relevant to this review. |
| Goodfellow 1979 | A controlled trial of epidural analgesia versus no epidural analgesia in regards to forceps deliveries. As all participants received oxytocin prior to randomisation, this was not a relevant study population for this review. |
| Hinshaw 2008 | Randomised controlled trial of placebo versus oxytocin infusion in nulliparous women. The study was not specific to patients with epidural analgesia and therefore the study population was not relevant for this review. |
| Hogston 1993 | A non‐randomised observational study of active management of labour. This type of study design was not included in this review. |
| Ladfors 2005 | A randomised controlled trial of oxytocin versus placebo in nulliparous women. The study was not specific to patients with epidural analgesia and therefore the study population was not relevant for this review. Furthermore, rates of epidural analgesia usage in each group was an outcome studied, not a pre‐requisite. |
| Pickrell 1989 | A randomised controlled trial of delayed pushing and augmentation in the second stage of labour in regards to rates operative deliveries. This study was only published in abstract form and therefore excluded from this review. |
| Rogers 1997 | A randomised controlled trial of active management of labour. The study was not specific to patients with epidural analgesia and therefore the study population was not relevant for this review. A second publication retrospectively compared patients with epidural analgesia as compared to controls. This again was not relevant for this review. |
| Sadler 2001 | A randomised controlled trial of maternal satisfaction with active management of labour. The study was not specific to patients with epidural analgesia and therefore the study population was not relevant for this review. |
| Treisser 1981 | An observational study of oxytocin augmentation of labour. This type of study design was not included in this review. |
| Ustunyurt 2007 | A randomised controlled trial of continuing versus discontinuing oxytocin in the active phase of labour on various obstetric outcomes. The intervention was not relevant to this review. |
Differences between protocol and review
We were unable to perform the planned subgroup analysis as specified in the protocol. Due to the available data, we modified one of the planned subgroup analyses to augmentation timing of full dilatation (10 cm) or less than 10 cm dilated. We have also added a new secondary outcome for episiotomy.
Contributions of authors
Philippa Costley and Christine East contributed to both the protocol design and the review. Philippa Costley is guarantor for the review. All authors approved the 2013 update.
Sources of support
Internal sources
Royal Women's Hospital, Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Saunders 1989 {published data only}
- Saunders NJStG, Spiby H, Gilbert L, Fraser RB, Hall JM, Mutton PM, et al. Oxytocin infusion during second stage of labour in primiparous women using epidural analgesia: a randomized double blind placebo controlled trial. BMJ 1989;299:1423‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiby H. Report of a trial of low dose oxytocin infusion for primiparae with epidural analgesia. Research and the Midwife Conference. Manchester, UK, 1990:96‐102.
Shennan 1995 {published data only}
- Shennan AH, Smith R, Browne D, Edmonds DK, Morgan B. The elective use of oxytocin infusion during labour in nulliparous women using epidural analgesia. International Journal of Obstetric Anesthesia 1995;4:78‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bidgood 1987a {published data only}
- Bidgood KA, Steer PJ. A randomized control study of oxytocin augmentation of labour. 1. Obstetric outcome. British Journal of Obstetrics and Gynaecology 1987;94:512‐7. [DOI] [PubMed] [Google Scholar]
- Bidgood KA, Steer PJ. Oxytocin augmentation of labour. Proceedings of the 24th British Congress of Obstetrics and Gynaecology;1986 April 15‐18; Cardiff, UK. Cardiff UK, 1986:239.
Bidgood 1987b {published data only}
- Bidgood KA, Steer PJ. A randomized control study of oxytocin augmentation of labour. 2. Uterine activity. British Journal of Obstetrics and Gynaecology 1987;94:518‐22. [DOI] [PubMed] [Google Scholar]
Cammu 1996 {published data only}
- Cammu H, Eeckhout E. A randomised controlled trial of early vs delayed use of amniotomy and oxytocin infusion in nulliparous labor. British Journal of Obstetrics and Gynaecology 1996;103:313‐8. [DOI] [PubMed] [Google Scholar]
Cardozo 1990 {published data only}
- Cardozo L, Pearce JM. Oxytocin in active‐phase abnormalities of labor: a randomized study. Obstetrics & Gynecology 1990;75:152‐7. [PubMed] [Google Scholar]
- Cardozo LD. Dysfunctional labour. Proceedings of Silver Jubilee British Congress of Obstetrics and Gynaecology; 1989 July 4‐7; London, UK. London, UK, 1989:76.
Girard 2009 {published data only}
- Girard B, Vardon D, Creveuil C, Herlicoviez M, Dreyfus M. Discontinuation of oxytocin in the active phase of labor. Acta Obstetricia et Gynecologica Scandinavica 2009;88(2):172‐7. [DOI] [PubMed] [Google Scholar]
Goodfellow 1979 {published data only}
- Goodfellow CF, Studd C. The reduction of forceps in primigravidae with epidural analgesia ‐ a controlled trial. British Journal of Clinical Practice 1979;33(10):287‐8. [PubMed] [Google Scholar]
Hinshaw 2008 {published data only}
- Hinshaw K, Moustafa A, Wilson K, Boyd P, Fawzi H, Kumarendran K. Oxytocin augmentation versus conservative management for primary dysfunctional labour in nulliparous women: a randomised controlled trial. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:Abstract no: 207.
- Hinshaw K, Simpson S, Cummings S, Hildreth A, Thornton J. A randomised controlled trial of early versus delayed oxytocin augmentation to treat primary dysfunctional labour in nulliparous women. BJOG: an international journal of obstetrics and gynaecology 2008;115(10):1289‐95. [DOI] [PubMed] [Google Scholar]
Hogston 1993 {published data only}
- Hogston P, Noble W. Active management of labour ‐ the Portsmouth experience. Journal of Obstetrics and Gynaecology 1993;13:340‐2. [Google Scholar]
Ladfors 2005 {published data only}
- Ladfors L, Dencker A, Nyberg K, Thorsen LS, Bergqvist L, Lilja H. A randomized trial of labor augmentation by oxytocin vs delayed oxytocin treatment or no oxytocin in nulliparous women with spontaneous contractions [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S44. [Google Scholar]
Pickrell 1989 {published data only}
- Pickrell MD, Buxton EJ, Redman CWE, Obhrai M. Epidural analgesia: delayed pushing and augmentation in the second stage do not reduce operative deliveries. Proceedings of Silver Jubilee British Congress of Obstetrics and Gynaecology; 1989 July 4‐7; London, UK. 1989:128.
Rogers 1997 {published data only}
- Rogers R, Gilson G, Kammerer‐Doak D. Epidural analgesia and active management of labor: effects on length of labor and mode of delivery. Obstetrics & Gynecology 1999;93(6):995‐8. [DOI] [PubMed] [Google Scholar]
- Rogers R, Gilson GJ, Miller AC, Izquierdo LE, Curet LB, Qualls CR. Active management of labor: does it make a difference?. American Journal of Obstetrics and Gynecology 1997;177(3):599‐605. [DOI] [PubMed] [Google Scholar]
- Rogers RG, Gardner MO, Tool KJ, Ainsley J, Gilson G. Active management of labor: a cost analysis of a randomized controlled trial. Western Journal of Medicine 2000;172:240‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadler 2001 {published data only}
- Sadler LC, Davison T, McCowan LME. Maternal satisfaction with active management of labor: a randomized controlled trial. Birth 2001;28(4):225‐35. [DOI] [PubMed] [Google Scholar]
Treisser 1981 {published data only}
- Treisser A, Breart G, Blum F, Jouhet P, Pigne A, Barrat J. Dystocia at the onset of labour. An evaluation of the different treatments available. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1981;10:91‐8. [PubMed] [Google Scholar]
Ustunyurt 2007 {published data only}
- Ustunyurt E, Ugur M, Ustunyurt BO, Iskender TC, Ozkan O, Mollamahmutoglu L. Prospective randomized study of oxytocin discontinuation after the active stage of labor is established. Journal of Obstetrics and Gynaecology Research 2007;33(6):799‐803. [DOI] [PubMed] [Google Scholar]
Additional references
Akoury 1991
- Akoury HA, MacDonald FJ, Brodie G, Caddick R, Chaudhry NM, Frize M. Oxytocin augmentation of labor and perinatal outcome in nulliparas. Obstetrics & Gynecology 1991;78(2):227‐30. [PubMed] [Google Scholar]
Al‐Zirqi 2010
- Al‐Zirqi I, Stray‐Pedersen B, Forsen L, Vangan S. Uterine rupture after previous caesarean section. BJOG: an international journal of obstetrics and gynaecology 2010;117(7):809‐20. [DOI] [PubMed] [Google Scholar]
Alexander 2002
- Alexander JM, Sharma SK, McIntire DD, Leveno KJ. Epidural analgesia lengthens the Friedman active phase of labor. Obstetrics & Gynecology 2002;100:46‐50. [DOI] [PubMed] [Google Scholar]
Anim‐Somuah 2011
- Anim‐Somuah M, Smyth R, Jones L. Epidural versus non‐epidural or no analgesia in labour. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000331.pub3] [DOI] [PubMed] [Google Scholar]
Ben‐Aroya 2001
- Ben‐Aroya Z, Yochai D, Silberstein T, Friger M, Hallak M, Katz M, et al. Oxytocin use in grand‐multiparous patients: safety and complications. Journal of Maternal‐Fetal Medicine 2001;10(5):328‐31. [DOI] [PubMed] [Google Scholar]
Betran 2007
- Betrán AP, Merialdi M, Lauer JA, Bing‐Shun W, Thomas J, Look P, et al. Rates of caesarean section: analysis of global, regional and national estimates. Paediatric and Perinatal Epidemiology 2007;21(2):98‐113. [DOI] [PubMed] [Google Scholar]
Day 1999
- Day P, Sullivan EA, Ford J, Lancaster P. Australia's Mothers and Babies 1997. Perinatal Statistics Series No. 9, Cat. No. PER 12. Sydney: AlHW National Perinatal Statistics Unit, 1999. [Google Scholar]
Dencker 2009
- Dencker A, Berg M, Bergqvist L, Ladfors L, Thorsen LS, Lilja H. Early versus delayed oxytocin augmentation in nulliparous women with prolonged labour‐a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2009;116(4):530‐6. [DOI] [PubMed] [Google Scholar]
Dickinson 2002
- Dickinson JE, Paech MJ, McDonald SJ, Evans SF. The impact of intrapartum analgesia on labour and delivery outcomes in nulliparous women. Australian & New Zealand Journal Of Obstetrics & Gynaecology 2002;42(1):59‐66. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraser 1998
- Fraser W, Vendittelli F, Krauss I, Breart G. Effects of early augmentation of labour with amniotomy and oxytocin in nulliparous women: a meta‐analysis. British Journal of Obstetrics and Gynaecology 1998;105:189‐94. [DOI] [PubMed] [Google Scholar]
Hamilton 2009
- Hamilton BE, Martin JA, Ventura SJ. Division of vital statistics births: preliminary data 2007. National Vital Statistics Report 2009;57(12):1‐23. [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Hemminki 1996
- Hemminki E. Impact of caesarean section on future pregnancy: a review of cohort studies. Paediatric and Perinatal Epidemiology 1996;10(4):366‐79. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lao 1987
- Lao T, Leung B. Rupture of the gravid uterus. European Journal of Obstetrics & Gynecology and Reproductive Biology 1987;25(3):175‐80. [DOI] [PubMed] [Google Scholar]
Laws 2010
- Laws P, Li Z, Sullivan EA. Australia's Mothers and Babies 2008. Perinatal statistics series no.24. Cat. No. PER 50. Canberra: AIHW National Perinatal Statistics Unit, 2010. [Google Scholar]
Liu 2004
- Liu EH, Sia AT. Rates of caesarean section and instrumental vaginal delivery in nulliparous women after low concentration epidural infusions or opioid analgesia: systematic review. BMJ 2004;328:1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melamed 2009
- Melamed N, Ben‐Haroush A, Chen R, Pardo J, Hod M, Yogev Y. Pregnancy outcome and mode of delivery after a previous operative vaginal delivery. Obstetrics & Gynecology 2009;114(4):757‐63. [DOI] [PubMed] [Google Scholar]
Newton 1995
- Newton ER, Schroeder BC, Knape KG, Bennett BL. Epidural analgesia and uterine function. Obstetrics & Gynecology 1995;85:749‐55. [DOI] [PubMed] [Google Scholar]
O'Mahony 2010
- O'Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD005455.pub2] [DOI] [PubMed] [Google Scholar]
Ozdemir 2005
- Ozdemir I, Yucel N, Yucel O. Rupture of the pregnant uterus: a 9‐year review. Archives of Gynecology and Obstetrics 2005;272:229‐31. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sadler 2000
- Sadler LC, Davidson T, McCowan LM. A randomised controlled trial and meta‐analysis of active management of labour. BJOG: an international journal of obstetrics and gynaecology 2000;107(7):909‐15. [DOI] [PubMed] [Google Scholar]
Shields 2007
- Shields SG, Ratcliff SD, Fontaine P, Leeman L. Dystocia in nulliparous women. American Family Physician 2007;75(11):1671‐8. [PubMed] [Google Scholar]
Souza 2010
- Souza JP, Gülmezoglu AM, Lumbiganon P, Laopaiboon M, Carroli G, Fawole B, et al. Caesarean section without medical indications is associated with an increased risk of adverse short‐term maternal outcomes: the 2004‐2008 WHO Global Survey on Maternal and Perinatal Health. BMC Medicine 2010;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorp 1993
- Thorp JA, Hu DH, Albin RM, McNitt J, Meyer BA, Cohen GR, et al. The effect of intrapartum epidural analgesia on nulliparous labor: a randomized, controlled, prospective trial. American Journal of Obstetrics and Gynecology 2003;169(4):851‐8. [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang B, Zhou L, Coulter D, Liang H, Zhong Y, Guo, et al. Effects of caesarean section on maternal health in low risk nulliparous women: a prospective matched cohort study in Shanghai, China. BMC Pregnancy and Childbirth 2010;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2009
- Wei SQ, Luo ZC, Xu H, Fraser WD. The effect of early oxytocin augmentation in labor: a meta‐analysis. Obstetrics & Gynecology 2009;114(3):641‐9. [DOI] [PubMed] [Google Scholar]
Zimmer 2000
- Zimmer EZ, Jakobi P, Itskovitz‐Eldor J, Weizman B, Solt I, Glik A, et al. Adverse effects of epidural analgesia in labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 2000;89(2):153‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Costley 2012
- Costley PL, East CE. Oxytocin augmentation of labour in women with epidural analgesia for reducing operative deliveries. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009241.pub2] [DOI] [PubMed] [Google Scholar]


