Abstract
Background
Drug resistance often occurs in the treatment of gastric cancer, which is the main cause of poor prognosis of chemotherapy. c-Met is overexpressed in a variety of tumors including gastric cancer, often leads to poor prognosis of gastric cancer, therefore regarded as a key target for the treatment of gastric cancer. This study aims to determine whether exosomes with si-c-Met could inhibit the resistance to cisplatin in gastric cancer (GC).
Methods
The protein expression levels of c-Met in tumor tissues and normal tissues of patients were evaluated by Western blot (WB) and immunohistochemistry (IHC), HEK293T cells were transfected with si-c-Met, exosomes were isolated, then co-cultured with gastric cancer cell lines and confirmed that it was incorporated into the cells by transmitted electron microscopy. Functional experiments were performed to examine the inhibitory effect of exo-si-c-Met on gastric cancer cell resistance in vitro, and xenograft models were used to reveal that exo-si-c-Met can enhance the sensitivity of tumors to cisplatin in vivo.
Results
High expression of c-Met is associated with poor prognosis of GC patients. si-c-Met significantly inhibited migration, invasion and promoted apoptosis in vitro, which indicated that si-c-Met sensitizes the response of gastric cancer cells to cisplatin. Exo-si-c-Met sharply reduced c-Met expression in gastric cancer cells and reverse the resistance to cisplatin in vitro and in vivo.
Conclusion
Our results indicate that exo-si-c-Met can inhibit the invasion and migration of gastric cancer cells and promote apoptosis in vitro and inhibit tumor growth in vivo, reversing the resistance to cisplatin in gastric cancer.
Keywords: exosomes, c-Met, siRNA, chemoresistance, gastric cancer
Introduction
Gastric cancer is the fifth most commonly diagnosed cancer in the world with mortality ranking third, with an estimated more than 1000,000 new cases diagnosed and 783,000 deaths in 2018.1 Systemic chemotherapy based on platinum is the most commonly used first-line solution for advanced gastric cancer, merely with a median overall survival time of approximately 1 year.2 One of the main causes of poor prognosis is that cisplatin could induce drug resistance after treatment.3 Cisplatin is one of the most commonly used chemotherapeutic drugs in the treatment of different types of tumors, including gastric cancer, ovarian cancer, head and neck cancer, lung cancer and breast cancer.4–6 It inhibits tumors by damaging DNA, inhibiting DNA synthesis and mitosis then inducing apoptosis to kill tumor cells.7 However, long-term use of platinum usually causes acquired drug resistance, which reduces therapeutic effects.8
Cisplatin (diamminedichloroplatinum II) is employed for the treatment of a variety of solid malignancies, including ovarian, colorectal, gastric, head and neck, bladder and lung cancers.7 Cisplatin treatment often leads to therapeutic failure without a consistent rate of initial responses. The mechanisms of which including reduced drug uptake or enhanced efflux, changes in molecular targets of anticancer drugs and enhancement of DNA damage repair.9 As in some clinical settings cisplatin constitutes the major therapeutic option, the development of chemosensitization strategies constitutes a goal with important clinical implications.
c-Met is a tyrosine kinase receptor of hepatocyte growth factor (HGF), Met promotes tissue remodeling by integrating growth, survival and migration in response to environmental stimuli or cellular autonomic perturbations, as the basis for morphogenesis, wound repair, organ homeostasis and cancer metastasis, which plays an important role in various morphological events.10 When activated by some abnormal factors, HGF c-Met pathway is involved in the development of different types of malignancies and plays a key role in tumor proliferation, invasion and metastasis, acts as a “driver” for carcinogenesis and promotes tumorigenesis.11
Crizotinib and Cabozantinib are selective inhibitors of c-Met. In vitro studies have shown that crizotinib can effectively inhibit the phosphorylation of Met kinase and inhibit the proliferation, migration and invasion of Met-dependent tumor cells.12 Carbotinib and crizotinib were approved by FDA for the treatment of thyroid malignant thyroid cancer and NSCLC, respectively. However, c-MET amplification is observed in only 2–10% of GC cases,13 which is relatively low and c-Met inhibitors can only respond to a small percentage of patients. It is urgent to find another way to inhibit c-Met. We find c-Met is highly expressed in epithelium.14 Met is detected in a variety of carcinomas including gastric cancer, and the overexpression or abnormal expression of Met usually associates with poor prognosis.15 Studies have shown that c-Met overexpression is associated with poor prognosis in 22–82% of patients with gastric cancer16,17 and the overall survival (OS) of c-Met negative is significantly longer than that of c-Met-positive patients in advanced gastric cancer.18
Small interfering RNAs (siRNA) are double-stranded, non-coding RNAs of about 20 base pairs in length and knockdown target genes by attaching to complementary target mRNAs, leading to the inhibited expression of specific genes.19–21
As an emerging approach, siRNA has great potential for the treatment of diseases caused by pathogenic genes.22 Studies have shown that the combination of cisplatin and CS nanoparticles loaded with Mad2 siRNA was demonstrated to be an effective method for the treatment of cisplatin-resistant tumors.23 Similarly, small interfering RNA (siRNA) downregulated P-glycoprotein, which mediates multidrug resistance (MDR), was sent into tumor cells to overcome MDR.24 Therefore, knocking down the expression level of c-Met by small interfering RNA could be an approach to enhance the sensitivity of chemotherapy in cancer cells.
Exosomes are vesicles secreted by a variety of mammalian cell types with a diameter of approximately 30–100 nm.25 Exosome consists of lipid bilayer membrane with no organelles inside. It can carry various molecular components including proteins and nucleic acid.26 Because of its advantages of being non-toxic, low-immunogenic and higher biocompatibility than liposomes, exosome recently emerged as a new carrier for delivering a variety of biologically active substances for treatment.27 Exosomes carry EGFR to regulate liver microenvironment and promote liver metastasis in gastric cancer.28 siRNA against RAD51 delivered by exosomes could cause massive of Hela and HT1080 cell death.29 Exosome-mediated siRNAs downregulate CDK4 specifically and induce Cell Cycle Arrest.30 Plasma exosomes can deliver siRNA into lymphocytes and monocytes to silence mitogen-activated protein kinase 1 selectively.31 Studies have shown that exosomes have a negative impact on chemotherapy treatment by delivering chemotherapeutic agents to target cancer cells.
The current study aimed to reverse the resistance by decreasing the expression of c-Met. This process was achieved by exosome-delivered siRNA, exosomes were then used to carry si-c-Met and act in vitro and in vivo. It was found to reverse the drug resistance of gastric cancer cells in vitro, and significantly inhibited the growth of tumors in mouse xenograft models. It has been shown to increase the sensitivity of gastric cancer cells to cisplatin. The above results indicate that patients with DDP refractory gastric cancer may have treatment options in the future.
Materials and Methods
Tumor Models
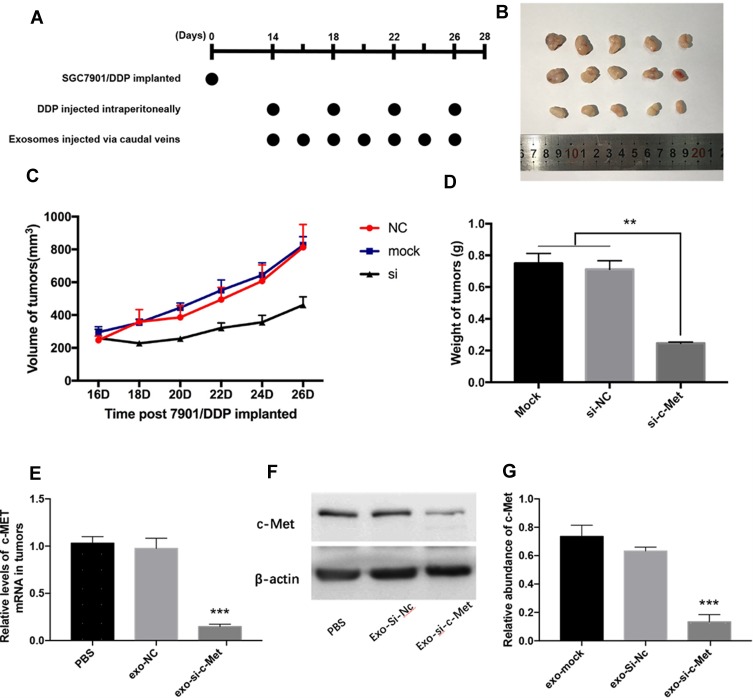
All experimental procedures were approved by the Institutional Animal Care and Research Advisory Committee of Tianjin Medical University. Female nude mice (BALB/c-nu, 4 weeks) were purchased from the Model Animal Center of Nanjing University and barrier facilities on a high-efficiency particulate air (HEPA) filter rack. Feeding in the middle. Since si-c-Met could increase the sensitivity of the two cell lines to cisplatin and the resistant effect is more significant in SGC7901/DDP cells, so 3×106 SGC7901/DDP cells were injected into the subcutaneous region of the left groin area of each mouse, 5 mice in each group. On the 14th day, a tumor model was established subcutaneously in nude mice (n = 15), when the tumor grows to a diameter of about 8 mm, 20μg of different exosomes (suspended in 40μL PBS) or PBS are injected through the tail vein every 2 days, and 5 mg/kg DDP is injected intraperitoneally every 4 days (Figure 1A). The long and short diameters of tumors were recorded every other day and then calculated tumor volume.
Figure 1.
Exo-si-c-Met increases the sensitivity of gastric cancer tissues to cisplatin in vivo. (A) A flow diagram depicting the in vivo experimental design. (B) the morphology of xenografted tumor tissues (n = 5). (C, D) Quantitative analysis of tumor volume (C) and weight (D). (E, F) Expression levels of c-Met in implanted tumors by qRT-PCR (E) and Western blot analysis (F). (G) Gray analysis of (F). **p < 0.01; ***p < 0.001. All error bars stand for SE.
Cell Culture and Human Sample Collection
The human gastric adenocarcinoma cell line SGC7901 and human embryonic kidney epithelial cell line HEK293T were obtained from the Chinese Academy of Sciences Cell Bank in Shanghai, China. The human DDP-resistant gastric adenocarcinoma cell line SGC7901/DDP was purchased from Gefan Biotechnology (Shanghai, China). According to the instructions, the maximum tolerated dose of DDP was reported to be 800ng/mL, mycoplasma contamination was excluded from these cell lines. Cells were incubated in a humidified, 95% air and 5% CO2 incubator at 37°C in DMEM (GIBCO) containing 10% fetal bovine serum (GIBCO) and 1% penicillin/streptomycin (Solarbio, China). Five pairs of gastric cancer tissues and paired adjacent normal tissues were collected from patients underwent surgery at the Tumor Research Institute and Hospital of Tianjin Medical University (Tianjin, China). Specimens and patient information of this study were approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital, and the written consent of the patients was provided.
Isolation of Exosomes from Cell Culture Media
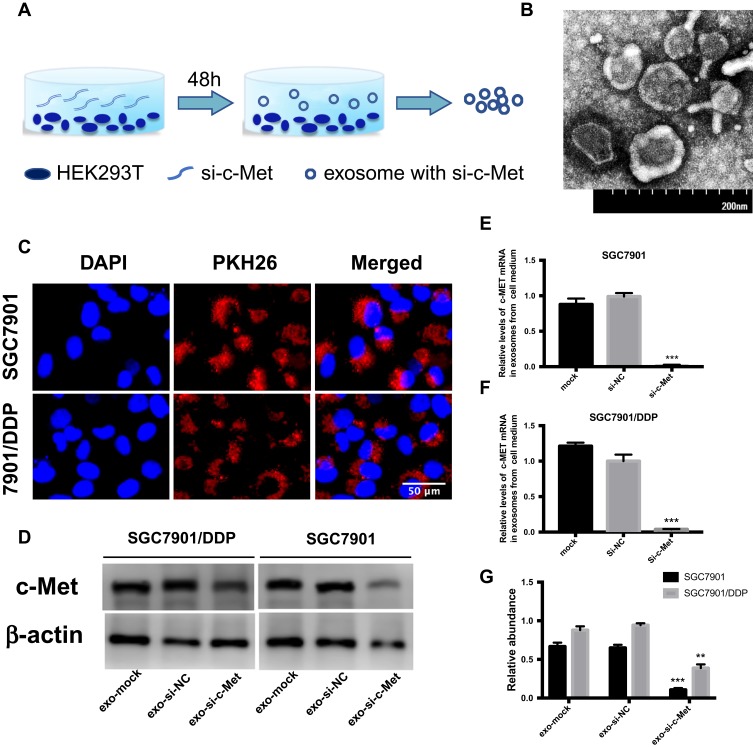
After transfection of HEK293T cells for 48h, the medium was collected and centrifuged at 3,000g for 20 mins to remove cells and other debris, the supernatant was then centrifuged at 10,000g for 20 mins to remove larger sized vesicles. Finally, the supernatant was centrifuged at 110,000 g for 80 mins, then the supernatant was discarded and 100μL of PBS was added to the sediment, gently pipetting several times and filtered by a 0.2-mm filter to obtain an exosomal suspension. All steps were carried out at 4°C.
Transmission Electron Microscopy
For transmission electron microscopy, exosomes were infiltrated in a droplet of 2.5% glutaraldehyde and fixed for more than 12hrs at 4°C. Samples were washed thrice in PBS and fixed in 1% osmium tetroxide for 1 hr (RT), embedded in 10% gelatin and fixed in glutaraldehyde at 4°C, then cut into blocks (<1 mm3). The specimens were dehydrated with alcohol of increasing concentrations (30%, 50%, 70%, 90%, 95%, and 100%; ×3), pure alcohol was replaced with propylene oxide and the samples were then infiltrated with increasing concentrations (25%, 50%, 75%, and 100%) of Quetol-812 epoxy resin mixed with propylene oxide. Then, all specimens were embedded in pure Quetol-812 epoxy resin. Ultrathin sections (100 nm) were cut with a Leica UC6 ultramicrotome, followed by dying with uranyl acetate (10 mins) and lead citrate (5 min) at RT before observation via FEI Tecnai T20 transmission electron microscope (120 kV).
PKH26 Staining
PKH26 Red Fluorescent Cell Linker Kits (Sigma) for labeling lipid bilayer. Exosomes were first resuspended in 100μL of diluent C. Adding 0.4 μL PKH26 ethanolic dye solution into 100μL of diluent C to make the dye solution (4×10−6M). Then, the 100μL exosome suspension was mixed and the 100ul dye solution by pipetting. The cell/dye suspension was incubated for 1–5 mins and periodically mixed, then the staining was terminated by adding 200μL of serum and then incubated for 1 min. The stained exosomes were finally washed twice with 1×PBS, then resuspended them in a fresh sterile tapered polypropylene tube. Eventually, moderate amounts of stained exosomes were added to the cell culture medium and incubated for 4hrs before pictured.
Cell Transfection
SGC7901 and SGC7901/DDP were seeded in 6-well plates, HEK293T was seeded in 10-cm dishes, transfected with Lipofectamine 2000 (Invitrogen) and Opti-MEM (GIBCO) according to the manufacturer’s instructions. siRNA of c-Met and its nonsense NC (Santa Cruz) were used to downregulate c-Met mRNA. The medium was replaced with complete medium 4–6hrs after transfection.
Immunohistochemistry Assay
Paraffin-embedded tissue samples of gastric cancer tissues and paired adjacent noncancerous tissues (8 pairs, total of 16 samples) were sectioned and stained with a 1:50 dilution of anti-c-Met monoclonal antibody (Santa Cruz Biotechnology). Using the DAB system (Zhongshan Jinqiao, China) to identify positive staining. Five regions were randomly selected for each specimen.
Protein Extraction and Western Blotting
The expression of c-Met was evaluated by Western blotting and normalized to β-actin. Cells and tissues were lysed in RIPA buffer with a newly added Protease inhibitor cocktail. The pyrolysate was separated on SDS-PAGE Gel and then transferred onto polyvinylidene fluoride membrane (Millipore). The immunoblots were blocked with 2% BSA for 0.5 hrs at room temperature, then incubated at 4 °C with anti-c-Met (1:150, Santa Cruz Biotechnology) and anti-β-actin (1:3000, Abcam) antibodies for more than 12hrs. After incubation with the corresponding secondary antibody, membranes were visualized by enhanced chemiluminescence system Kit (Millipore, USA).
RNA Isolation and qRT-PCR
Total RNA of the cultured cells and tissues was extracted with TRIzol reagent (Invitrogen). cDNA was obtained via avian myelobastosis virus (AMV) reverse transcriptase (TaKaRa), the reaction procedure as follows: 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. Then, real-time qPCR was initiated by at 95°C for 5min; then the cDNA was denatured at 95°C for 15s followed by extension at 60°C for 1 min, which was performed for 40 cycles. All assayed in triplicate. After the reactions were complete, the cycle threshold (Ct) data were calculated according to the formula that ΔCt = Ct gene _ Ct control. The relative levels of genes were normalized with the equation 2−ΔCt. The c-Met and GAPDH primers were designed as follows:
5ʹ-TGTCCCGAGAATGGTCATAA-3ʹ (c-Met, sense),
5ʹ-AGGGAAGGAGTGGTACAACA-3ʹ (c-Met, anti-sense),
5ʹ-AGAAGGCTGGGGCTCATTTG-3ʹ (GAPDH, sense),
5ʹ-AGGGGCCATCCACAGTCTTC-3ʹ (GAPDH, anti-sense).
CCK-8 Cell Viability Assay
Cell viability was tested with CCK-8 kit (MY Biotech, China). SGC7901 or SGC7901/DDP cells were seeded into 96-well plates and transfected or co-incubated with exosomes, different doses of DDP (Jiangsu, China) were added after 24hrs. Forty-eight hours later, 10μL of CCK-8 reagent was added to each well and incubated at 37°C for 1–3 hrs. The optical density (OD) values were detected at a wavelength of 460nm by a microplate reader (Thermo). The assay was performed at least in triplicate. Cell viability was calculated using the following formula:
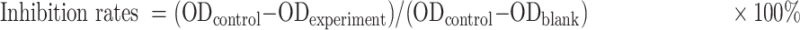 |
Edu Cell Proliferation Assay
The proliferation ability of SGC7901 and SGC7901/DDP cells was determined by the EdU proliferation assay (RiboBio). After co-incubation with different exosomes for 24 hrs, 50M EdU was added into cells and incubated for 5hrs, then fixed, filtered, and stained according to the instructions.
Transwell Cell Migration Assay
Cell migration ability was assessed using a Boyden chamber covered with 8.0 um pores (Costar) polycarbonate membrane. After the pretreatment described above, a cell suspension of about 105 SGC7901/DDP cells in 200μL serum-free medium was transferred to the upper chamber, and 500μL of complete medium was added to the lower chamber. After 8–10hrs, the non-migrated cells on the upper surface of the membrane were gently wiped with a cotton swab, and the cells that migrated to the lower surface of the membrane were fixed and subjected to three-step staining (Thermo Fisher). The number of migrated cells was counted under an optical microscope.
Flow Cytometry
SGC7901 or SGC7901/DDP was pretreated as described previously, added 3 mg/mL DDP and incubated for 48 hrs. Subsequently, adherent cells and floating cells were harvested and analyzed using Annexin V-FITC (fluorescein isothiocyanate)/PI (propidium iodide) staining kit (BD Biosciences). Cells were washed with PBS, suspended in binding buffer, stained with annexin V-FITC for 10 mins and PI for 5 mins in the dark. Apoptotic cells were quantified by fluorescence-activated cell sorting (FACS) flow cytometry (BD Biosciences).
Statistical Analyses
All experiments were repeated at least three times in parallel and the data were expressed as mean ± SE. The p value < 0.05 was considered statistically significant by using Student’s t test: * p < 0.05; ** p < 0.01; and *** p < 0.001.
Results
High Expression of c-Met Is Associated with Poor Prognosis of GC Patients
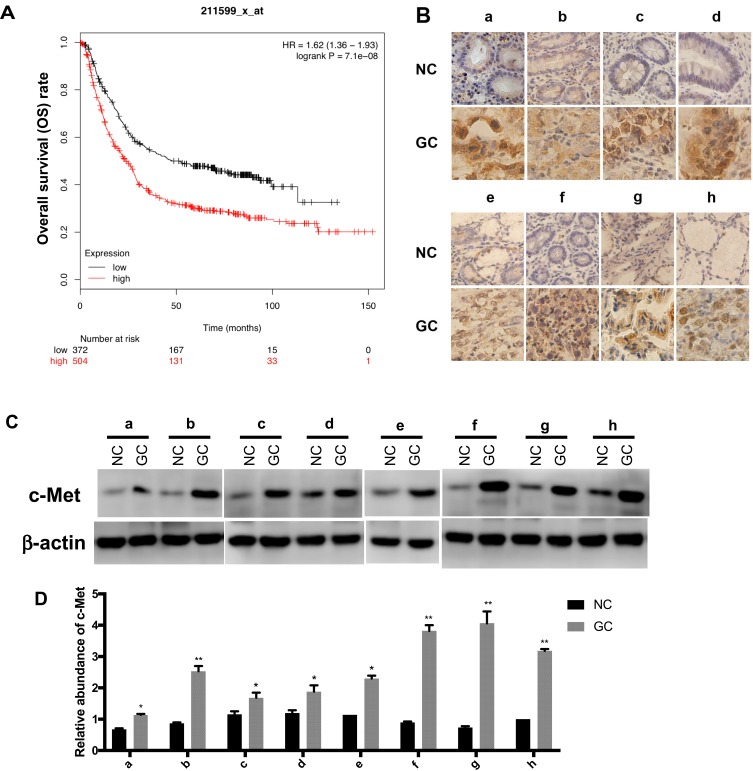
c-Met plays an important role in a variety of malignancies,32 we analyzed the effect of c-Met on the prognosis of GC by Kaplan–Meier plotter (http://kmplot.com/analysis/index.php? p=service&cancer=gastric), the survival curve of GC patients with c-Met expression is shown in Figure 2A. Briefly, during the follow-up, the survival rate of the high c-Met expression group was consistently higher than that of the group with low expression. It can be seen from the graph that the overall survival rate of patients with c-Met high expression group was lower than that with low expression group during the follow-up period.
Figure 2.
The expression level of c-Met in GC. (A) The survival curve of GC patients associated with c-Met expression. (p < 0.001). (B) Immunohistochemistry of the paraffin-embedded human gastric cancer tissues and paracancerous normal tissues (n=8, total of 16 samples). (C) Western blotting analysis of c-Met expression in GC tissues and the corresponding adjacent normal tissues (n=8, total of 16 samples). (D) Gray analysis of (C). *p < 0.05, **p < 0.01. All error bars stand for SE.
c-Met Protein Is Upregulated in GC Tissues Compared to Normal Adjacent Tissues
We selected eight pairs of cancer tissues and adjacent normal tissues from GC patients. Level of c-Met was detected by immunohistochemistry, and it was found that the expression of c-Met was significantly upregulated in cancer tissues compared with that in noncancerous tissues (Figure 2B), followed by Western blot and got consistent results (Figure 2C and D).
si-c-Met Sensitizes the Response of Gastric Cancer Cells to Cisplatin
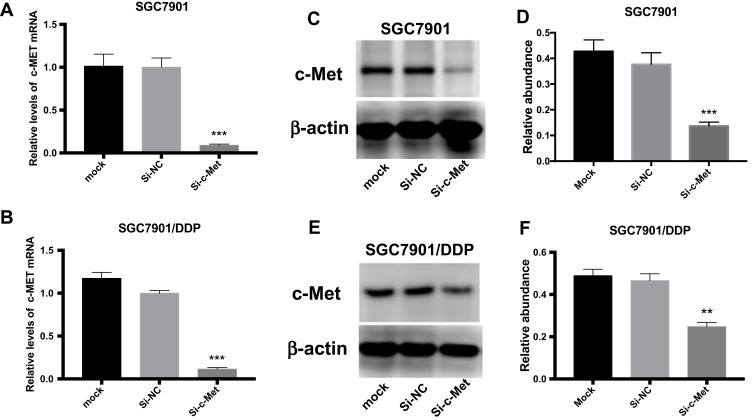
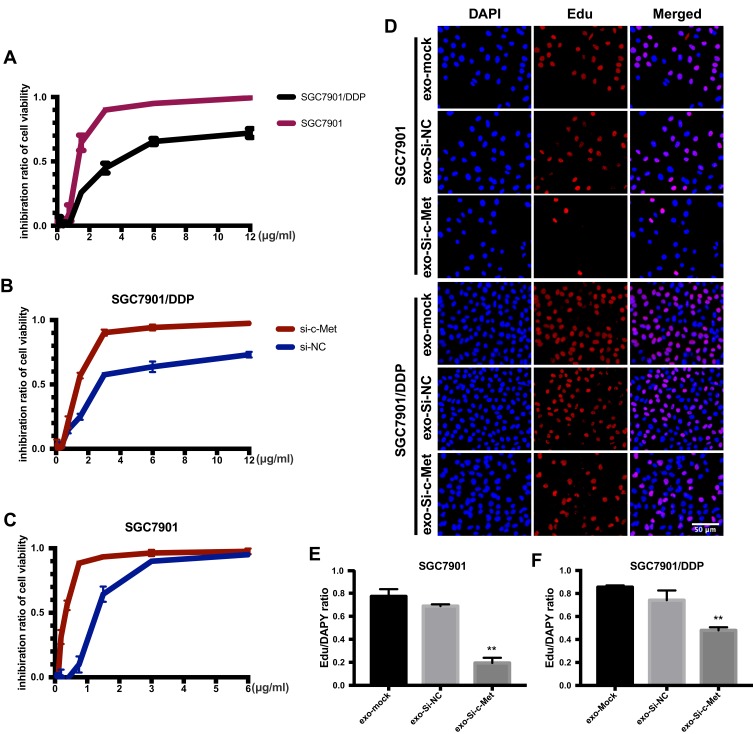
To explore the inhibitory effect of small interfering RNA on c-Met, si-c-Met and si-NC were transfected into SGC7901 and SGC7901/DDP cells, the expression level of c-Met was detected by qPCR (Figure 3A and B), and c-Met protein was verified by Western blotting (Figure 3C–F) results indicate that si-c-Met could significantly reduce the expression level of c-Met. We added gradient concentrations of cisplatin to SGC7901 and SGC7901/DDP cells, based on preliminary results, the concentration of cisplatin in SGC7901 was 0.09375–6μg/mL, while ranged from 0.1875–12μg/mL in SGC7901/DDP. We found that it has the most significant difference of inhibition rate at the concentration of 3μg/mL (Figure 4A), so 3μg/mL is the most suitable concentration for subsequent experiments. Afterwards, we transfected si-c-Met and si-NC into SGC7901 and SGC7901/DDP cells in 96-well plates. After 24h, gradient concentrations of cisplatin were added to each well of two cell lines. After 48h of dosing, we tested cell viability by CCK-8 reagent and then calculate the inhibition rate. Si-c-Met could increase the sensitivity of two cell lines to cisplatin and significantly reversed the resistance of SGC7901/DDP cells to cisplatin (Figure 4B and C).
Figure 3.
The role of si-c-Met. (A, B) qRT-PCR analysis was done to exam the inhibition to the expression level of c-Met mRNA. (C, D) Western blotting analysis was done to confirm the inhibiting effect of si-c-Met in gastric cancer cells. (E, F) Gray analysis of (C, D). **p < 0.01, and ***p < 0.001. All error bars stand for SE.
Figure 4.
Exo-si-c-met fuses into cells and downregulates the expression c-met. (A) The inhibition rate of two cell lines was observed by CCK-8. (B, C) Cell viability of different drug concentrations in SGC7901 and SGC7901/DDP was tested by CCK-8, and the inhibition ratio was calculated. (D) EdU was stained to confirm the number of newly proliferating cells. (E, F) Quantitative analysis of (D) **, p<0.01; All error bars stand for SE.
Exosome-Delivered c-Met siRNA Sharply Reduced c-Met Expression in Gastric Cancer Cells
Exosomes were obtained from HEK293-T medium (Figure 5A) then photographed by transmission electron microscopy (Figure 5B). After stained with PKH26 dye, exosomes were co-cultured with SGC7901 and SGC7901/DDP cells, small clusters were observed to fuse into the cytoplasm surrounding the nucleus or beside them (Figure 5C). To deliver small interfering RNA into gastric cancer cells, HEK293-T cells were transfected with si-c-Met and si-NC and incubated for 48h, then the exosomes were extracted and co-cultured with gastric cancer cells. It was confirmed by qRT-PCR that exosomes loaded si-c-Met could significantly downregulate the expression of c-Met at the gene level compared with exosomes carrying si-NC (Figure 5E and F) and obtained consistent results by Western blot (Figure 5D and G) that exo-si-c-Met could downregulate the expression of c-Met consistent with transfecting si-c-Met directly.
Figure 5.
Exo-si-c-met fuses into cells and downregulates the expression c-met. (A) Extraction of exosomes from HEK293-T cells. (B) Electron microscope scanning of exosome from HEK293T cells. (C) Exosomes were stained with PKH26 and then fused into gastric cancer cells. (D–F) Expression levels of c-Met in exosomes from medium of SGC7901 and SGC7901/DDP were detected by Western blotting (D) and qRT-PCR (E, F) assays. (G) Gray analysis of (D). **p < 0.01, and ***p < 0.001. All error bars stand for SE.
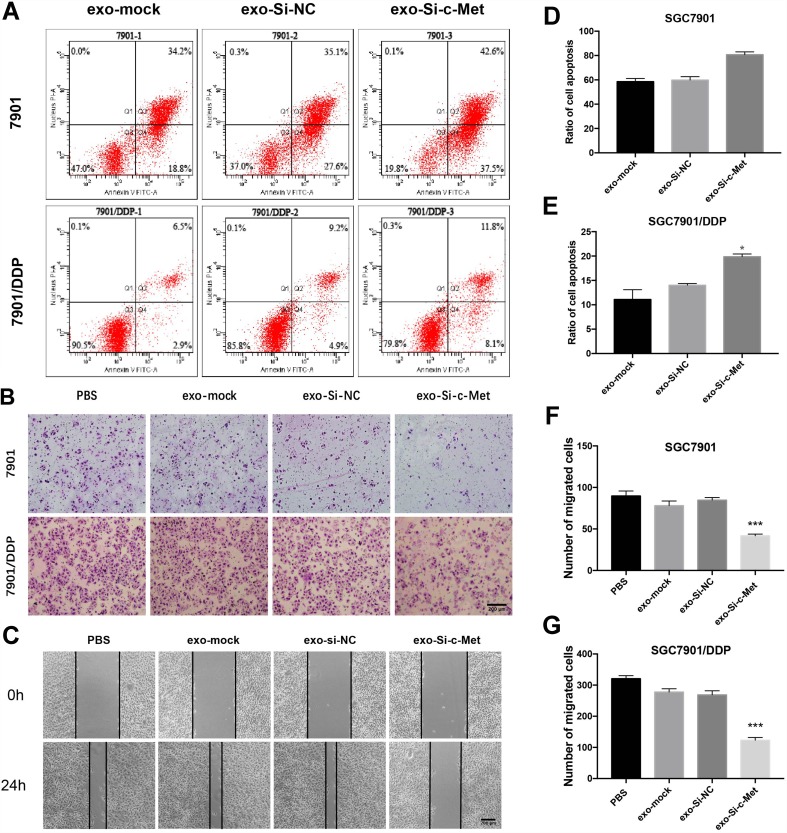
Exo-si-c-Met Reverses the Resistance to Cisplatin in Gastric Cancer Cells
The ratio of apoptosis was detected by flow cytometry. The apoptosis rate was high in SGC7901 and there was no significant difference among groups. Compared with the other two control groups, the number of early apoptotic cells was significantly reduced and the late apoptotic cells were increased in the exo-si-c-Met group. In SGC7901/DDP cells, exo-si-c-Met reversed the resistance to cisplatin and improve the proportion of apoptosis (Figure 6A, D and E). Cell migration ability was analyzed by transwell assay, 3μg/mL of cisplatin was added to all cells, in SGC7901 cell line, the ability of migration was significantly inhibited in all groups. For the drug-resistant cell line SGC7901/DDP, after adding cisplatin at a concentration of 3 μg/mL, the migration ability was maintained in groups of, exo-si-NC and exo-si-Mock, while significantly reduced in exo-si-c-Met group (Figure 6B, F and G) consistent results were also obtained in wound-healing assay (Figure 6C) which indicated that exo-si-c-Met could increase the effect of cisplatin on cell migration ability and reversed the resistance to cisplatin. The effect of exo-si-c-Met on cell proliferation was examined by EDU assay. It was found that in the concentration of 3 μg/mL, cell proliferation ability was inhibited apparently in group of exo-si-c-Met both in SGC7901 and in SGC7901/DDP (Figure 4D–F).
Figure 6.
Exo-si-c-met increases the sensitivity of gastric cancer cells to cisplatin (A) Apoptotic rate was tested by flow cytometry. (B) Transwell assays were completed to examine the cell migration ability. (C) Wound-healing assay demonstrated that exo-si-c-Met increased SGC7901/DDP migration. (D, E) Quantitative analysis of (A). (F, G) Quantitative analysis of (B). ***p < 0.001. All error bars stand for SE.
In vivo Role of Exo-si-c-Met in Reversing Chemotherapy Resistance
Since the drug resistance reversal effect in vitro was more pronounced in SGC7901/DDP than in SGC7901 cells, SGC7901/DDP was selected to set up tumor models in nude mice subcutaneously (n = 15). When tumors grew to about 8mm in diameter, 20 μg of different exosomes (suspended in 40μL PBS) or PBS were injected through the caudal vein every other day, and intraperitoneal injection of 5 mg/kg DDP every other 3 days (Figure 1A). The length and short diameter of tumors were recorded every 2 days and calculate the volume (Figure 1C). Compared with two control groups, the tumor volume did not increase significantly in the treatment group (Figure 1B). After 2 weeks, tumors were removed and weighed (Figure 1D). Extracted RNA from each tumor and qRT-PCR was performed to detect the level of mRNA of c-Met in each group of tumors. The results showed that the level of c-Met mRNA in the Exo-si-c-Met group was significantly lower than that in control groups (Figure 1E), then verified by Western blot, the expression level of c-Met was also significantly decreased in Exo-si-c-Met group (Figure 1F and G). The above results demonstrate that Exo-si-c-Met could reverse the resistance of cisplatin in vivo.
Discussion
Chemotherapy is the main treatment strategy for patients with advanced gastric cancer. However, the median survival time (mOS) of patients with advanced gastric cancer who received standard chemotherapy is only 10–12 months, and the 5-year survival rate is less than 10%.33,34 One of the important reasons for the low efficiency of chemotherapy in gastric cancer is drug resistance. There are several molecular mechanisms of cisplatin resistance, among which the over expression of c-Met is an important factor that cannot be ignored. We found that the expression level of c-Met in gastric cancer-resistant cell lines was higher than that in sensitive cell lines and that it has a certain mechanism for cisplatin resistance. Therefore, we tried to use si-c-Met to inhibit the expression of c-Met and enhance sensitivity to chemotherapy. It was found that transfected si-c-Met into gastric cancer cells directly could increase the sensitivity to cisplatin. Then, a series of cell function experiments showed that Exo-Si-c-Met significantly increases the inhibition rate of cisplatin, reduces the ability of cell proliferation and migration, and promotes cell apoptosis. Exo-si-c-Met combined with intraperitoneal injection of cisplatin in mice could inhibit tumor growth and reverse the resistance of gastric cancer to cisplatin analogously.
As a receptor tyrosine kinase for HGF, c-Met plays an important role in drug resistance of a variety of tumors. CAF-derived HGF plays vital roles in drug resistance of ovarian cancer by inhibiting c-Met/PI3K/Akt and GRP78 signal pathways.35 c-Met/β1 complex could drive invasive oncologic processes.36 The resistance to inhibitor of EGFR (AC0010) could be overcome by targeting c-Met.37 Exosomes are low-immunogenic and low-toxic vesicles secreted by various cell types and could carry proteins, nucleic acids and lipids, which involves in mediating a variety of biological processes.38,39 Exosomes with miR-128-3p increase chemosensitivity of oxaliplatin in drug-resistant colorectal cancer.40 Exosome-delivered nucleic acid and proteins partly facilitate the resistance of breast cancer.41 Exosomes derived from HEK293 could deliver siRNA that target PKL-1 and reduce expression of PLK-1 in bladder cancer cells.42 In this study, extracted exosomes from HEK293T cell culture media, then injected intravenously to downregulate the expression of c-Met in tumors of mice. In this way, the expression of c-Met in tumors was decreased. The sensitivity of refractory gastric cancer to DDP was improved and suppressed the growth of tumor at the same time.
Given this, exo-si-c-Met could be delivered to reverse drug resistance of cisplatin in gastric cancer, suppress proliferation and accelerate apoptosis in vitro, inhibit tumor growth in vivo. It can be a new approach to treat DDP-resistant gastric cancer.
Conclusions
In summary, we provided evidence that Exo-si-c-Met can inhibit the invasion and migration of gastric cancer cells and promote apoptosis in vitro and inhibit tumor growth in vivo, reversing the resistance to cisplatin in gastric cancer. Our results demonstrate a method that Exo-si-c-Met may be a potential choice for cisplatin-resistant treatment of refractory gastric cancer.
Acknowledgment
The authors thank the Tianjin Medical University Cancer Institute and Hospital for providing pathological specimens and diagnostic guidance.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81772629, 81602158, 81602156, 81702275, 81802363, 81702431, 81702437, 81772843) and the Demonstrative Research Platform of Clinical Evaluation Technology for New Anticancer Drugs (No. 2018ZX09201015). This work was also supported by the Tianjin Science Foundation (Nos. 18JCQNJC81900, 18JCYBJC92000, 18JCYBJC25400, 16PTSYJC00170) and the Science & Technology Development Fund of the Tianjin Education Commission for Higher Education (2018KJ046, 2017KJ227, 2017KJ204). The funders had no role in the study design, the data collection and analysis, the interpretation of the data, the writing of the report, and the decision to submit this article for publication.
Abbreviations
c-Met: cellular-mesenchymal to epithelial transition factor; HGF: hepatocyte growth factor; DDP: cis-diamine dichloro platinum; OS: the overall survival.
Ethics Approval and Consent to Participate
All animal studies were performed by skilled experimenters under the approval of the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital and the informed consent was obtained from all patients. All experimental procedures were under an approved protocol in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903–2909. doi: 10.1200/JCO.2005.05.0245 [DOI] [PubMed] [Google Scholar]
- 3.Xu S, Zhu X, Huang W, Zhou Y, Yan D. Supramolecular cisplatin-vorinostat nanodrug for overcoming drug resistance in cancer synergistic therapy. J Control Release. 2017;266:36–46. doi: 10.1016/j.jconrel.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, Shin SW, Kim BS, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85(2):295–301. doi: 10.1002/(ISSN)1097-0142 [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH. Current standards of care in small-cell and non-small-cell lung cancer. Oncology. 2001;61(Suppl 1):3–13. doi: 10.1159/000055386 [DOI] [PubMed] [Google Scholar]
- 6.Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34(10):1522–1534. doi: 10.1016/S0959-8049(98)00224-X [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi: 10.1038/onc.2011.384 [DOI] [PubMed] [Google Scholar]
- 8.McKeage MJ, Mistry P, Ward J, et al. A Phase I and pharmacology study of an oral platinum complex, JM216: dose-dependent pharmacokinetics with single-dose administration. Cancer Chemother Pharmacol. 1995;36(6):451–458. doi: 10.1007/BF00685793 [DOI] [PubMed] [Google Scholar]
- 9.Marin JJ, Al-Abdulla R, Lozano E, et al. Mechanisms of resistance to chemotherapy in gastric cancer. Anticancer Agents Med Chem. 2016;16(3):318–334. doi: 10.2174/1871520615666150803125121 [DOI] [PubMed] [Google Scholar]
- 10.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11(12):834–848. doi: 10.1038/nrm3012 [DOI] [PubMed] [Google Scholar]
- 11.Yao JF, Li XJ, Yan LK. et al. Role of HGF/c-Met in the treatment of colorectal cancer with liver metastasis. J Biochem Mol Toxicol.2019;e22316. doi: 10.1002/jbt.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui JJ, Tran-Dube M, Shen H, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem. 2011;54(18):6342–6363. doi: 10.1021/jm2007613 [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Chon HJ, Kim H, et al. MET in gastric cancer with liver metastasis: the relationship between MET amplification and Met overexpression in primary stomach tumors and liver metastasis. J Surg Oncol. 2018;117(8):1679–1686. doi: 10.1002/jso.25097 [DOI] [PubMed] [Google Scholar]
- 14.Otte JM, Schmitz F, Kiehne K, et al. Functional expression of HGF and its receptor in human colorectal cancer. Digestion. 2000;61(4):237–246. doi: 10.1159/000007764 [DOI] [PubMed] [Google Scholar]
- 15.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–925. doi: 10.1038/nrm1261 [DOI] [PubMed] [Google Scholar]
- 16.Yonemura Y, Kaji M, Hirono Y, et al. Correlation between overexpression of c-met gene and the progression of gastric cancer. Int J Oncol. 1996;8(3):555–560. [PubMed] [Google Scholar]
- 17.Taniguchi K, Yonemura Y, Nojima N, et al. The relation between the growth patterns of gastric carcinoma and the expression of hepatocyte growth factor receptor (c-met), autocrine motility factor receptor, and urokinase-type plasminogen activator receptor. Cancer. 1998;82(11):2112–2122. doi: 10.1002/(ISSN)1097-0142 [DOI] [PubMed] [Google Scholar]
- 18.Fuse N, Kuboki Y, Kuwata T, et al. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer. 2016;19(1):183–191. doi: 10.1007/s10120-015-0471-6 [DOI] [PubMed] [Google Scholar]
- 19.Burnett JC, Rossi JJ, Tiemann K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J. 2011;6(9):1130–1146. doi: 10.1002/biot.201100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung RK, Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107(2):222–239. doi: 10.1016/j.pharmthera.2005.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittrup A, Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat Rev Genet. 2015;16(9):543–552. doi: 10.1038/nrg3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Zhang W, Qi R, Mao ZW, Shen H. Engineering functional inorganic-organic hybrid systems: advances in siRNA therapeutics. Chem Soc Rev. 2018;47(6):1969–1995. doi: 10.1039/C7CS00479F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nascimento AV, Singh A, Bousbaa H, Ferreira D, Sarmento B, Amiji MM. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater. 2017;47:71–80. doi: 10.1016/j.actbio.2016.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yhee JY, Song S, Lee SJ, et al. Cancer-targeted MDR-1 siRNA delivery using self-cross-linked glycol chitosan nanoparticles to overcome drug resistance. J Control Release. 2015;198:1–9. doi: 10.1016/j.jconrel.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 25.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32(3–4):623–642. doi: 10.1007/s10555-013-9441-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107(9):1047–1057. doi: 10.1161/CIRCRESAHA.110.226456 [DOI] [PubMed] [Google Scholar]
- 27.Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083 [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Deng T, Liu R, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z, Xie J, Zhu J, et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release. 2016;243:160–171. doi: 10.1016/j.jconrel.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 31.Wahlgren J, De LKT, Brisslert M, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):e130. doi: 10.1093/nar/gks463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89–103. doi: 10.1038/nrc3205 [DOI] [PubMed] [Google Scholar]
- 33.Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol. 2016;22(8):2403–2414. doi: 10.3748/wjg.v22.i8.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–1649. doi: 10.3748/wjg.v20.i7.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deying W, Feng G, Shumei L, Hui Z, Ming L, Hongqing W. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci Rep. 2017;37:2. doi: 10.1042/BSR20160470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahangiri A, Nguyen A, Chandra A, et al. Cross-activating c-Met/beta1 integrin complex drives metastasis and invasive resistance in cancer. Proc Natl Acad Sci U S A. 2017;114(41):E8685–e8694. doi: 10.1073/pnas.1701821114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, Tang W, Li T, Zhang X, Sun Y. Overcoming resistance to AC0010, a third generation of EGFR inhibitor, by targeting c-MET and BCL-2. Neoplasia (New York, NY). 2019;21(1):41–51. doi: 10.1016/j.neo.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14(10):1036–1045. doi: 10.1038/ncb2574 [DOI] [PubMed] [Google Scholar]
- 39.Rani S, Ritter T. The exosome - A naturally secreted nanoparticle and its application to wound healing. Adv Mater. 2016;28(27):5542–5552. doi: 10.1002/adma.v28.27 [DOI] [PubMed] [Google Scholar]
- 40.Liu T, Zhang X, Du L, et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol Cancer. 2019;18(1):43. doi: 10.1186/s12943-019-0981-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Yu DD, Wu Y, Shen HY, et al. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci. 2015;106(8):959–964. doi: 10.1111/cas.12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greco KA, Franzen CA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN. PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology. 2016;91:241.e241–247. doi: 10.1016/j.urology.2016.01.028 [DOI] [PubMed] [Google Scholar]