Abstract
Background
The aim of the study was to observe the antimicrobial activity of Porphyromonas gingivalis and Treponema denticola as well as the effect on reducing volatile sulfur compounds (VSCs).
Materials and methods
After P. gingivalis and T. denticola were cultured with or without Streptococcus salivarius K12 and M18, VSCs were measured by Oral Chroma. In order to analyze the mechanism for malodor control, the antimicrobial activity of S. salivarius K12 and M18 against P. gingivalis and T. denticola was assessed. SPSS 21.0 was used for data analysis with the Kruskal–Wallis and Jonckheere–Terpstra tests. Mann–Whitney test was applied for post hoc analysis.
Results
P. gingivalis and T. denticola VSC levels were reduced by high concentrations of S. salivarius K12 and M18 during coculture. The concentrations were lower than those of single culture (p < .05). An antimicrobial effect was detected on P. gingivalis, and T. denticola by 50% S. salivarius K12 and M18. The spent culture medium and whole bacteria of S. salivarius K12 and M18 reduced the levels of VSCs below the amount in a single culture of P. gingivalis and T. denticola (p < .05).
Conclusion
S. salivarius K12 and M18 decreased the levels of VSCs originating from P. gingivalis and T. denticola.
Keywords: oral malodor, Streptococcus salivarius K12, Streptococcus salivarius M18
1. INTRODUCTION
Oral malodor is an unpleasant smell that occurs in the oral cavity and the nearby organs (Rosenberg et al., 1991). It is a foul odor that causes discomfort in others during expiration. In other words, oral malodor is defined not only as a foul odor that arises within the oral cavity, but also includes all unpleasant smells that pass through the oral cavity from other organs such as the stomach, the liver, and the lungs (Paik, Shin, Cho, Jang, & Lee, 2011).
Oral malodor has many potential causes, but 80–90% of cases occur because of factors within the oral cavity. It is usually the work of Gram negative anaerobic bacteria, especially Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Fusobacterium spp. (Fusobacterium nucleatum, Fusobacterium fusiform, Fusobacterium polymorphum), and Prevotella intermedia (Kim, 2008; Kishi et al., 2013). Of these, P. gingivalis and T. denticola are not only known to be related to periodontal diseases, but are also found on the tongue and are known to synthesize volatile sulfur compounds (VSCs) when attached to the dorsal side of the tongue (Kishi et al., 2013).
Probiotics are defined as “living bacteria that are beneficial to health when an appropriate amount is consumed” (Kopp‐Hoolihan, 2001). Nonpathogenic microorganisms such as yeast and lactobacilli exist in food and offer benefits to humans (Brown & Valiere, 2004). Probiotics can improve diseases such as diarrhea, enteritis, ulcerative colitis, deterioration of immunity, and hyperlipidemia. Recently, in dental medicine, there have been many studies on the effectiveness of using oral cavity probiotics against oral diseases including dental caries and halitosis (Burton et al., 2013). Comelli, Guggenheim, Stingele, and Neeser (2002) showed that Streptococcus thermophilus and Lactococcus lactis have preventive effects against dental caries due to their ability to weaken dental plaque. Burton, Chilcott, and Tagg (2005) reported that Streptococcus salivarius K12 reduces the synthesis of VSCs.
The purpose of this study is to research and analyze how probiotic bacteria such as S. salivarius K12 and M18 affect the synthesis of sulfur compounds and the growth of P. gingivalis and T. denticola, which cause oral malodor.
2. MATERIALS AND METHODS
2.1. Bacteria and bacterial culture
The major bacteria used in this experiment are the bacteria associated with oral malodor, P. gingivalis ATCC 33277 and T. denticola ATCC 35405, which were purchased from American Type Culture Collection (ATCC). P. gingivalis was cultured in brain heart infusion (BHI) liquid media, which included hemin (0.05 μg/mL; Sigma, St Louis, MO) and Vitamin K (1 μg/mL; Sigma). T. denticola was cultured in tryptone‐yeast extract‐gelatin‐volatile fatty acids‐serum (TYGVS) media in a 37°C anaerobic state (5% H2, 10% CO2, 85% N2) (Ohta, Makinen, & Loesche, 1986). S. salivarius K12 and M18 (Thera Breth; The California Clinics, Los Angeles, CA) were used as probiotics and cultured in BHI media at 37°C. For the coculture of oral cavity bacteria, P. gingivalis was cultured in BHI media containing hemin and vitamin K. T. denticola was cultured in a mixed media consisting of TYGVS liquid media and BHI liquid media in a one‐to‐one ratio at 37°C in an anaerobic condition.
2.2. Measurement of VSC synthesis in single culture or coculture of oral malodor causing bacteria with S. salivarius
After P. gingivalis, T. denticola alone or mixed incubation with S. salivarius, 1 ml of bacterial culture was transferred to a clean 50 ml conical tube. Afterwards, to explore the inhibitory mechanisms involved in the synthesis of VSCs, probiotics bacteria media was centrifuged at 7,000g for 10 min. The supernatant was sterilized by passing it through a polyvinylidene filter (Millipore Co., Belleica, MA) with the pore size of 0.22 μm. The precipitated S. salivarius was washed with a phosphate buffer. P. gingivalis or T. denticola media was mixed with 1 × 107, 2 × 107, or 3 × 107 of the filter sterilized S. salivarius and transferred to clean conical tubes. After vortexing for 30 min with a Vortex mixer (GENIE II; Scientific Industries, Bohemia, NY), 1 mL of the air above the media was sucked into a 10‐mL syringe. The syringe was pulled to the 10 mL line to dilute the air 10 times. Using Oral Chroma (ABILIT Corp., Tokyo, Japan), the amount of H2S, CH3SH, and (CH3)2S was measured.
2.3. The antimicrobial level of S. salivarius K12 and M18 against bacteria causing oral malodor
S. salivarius K12 and M18 were cultured and centrifuged at 7,000g for 10 min. The antimicrobial level was tested using the supernatant. The antimicrobial susceptibility test followed the protocols from the Clinical and Laboratory Standards Institute (CLSI) (Bosy, 1997). In each well of a 96‐well polystyrene culture plate, 180 μL of BHI containing hemin and Vitamin K was added. Media of the two probiotics bacteria (180 μL) was added to the first well and serially diluted, each time by half, using a multipipette. After incubating for 36 hr under anaerobic conditions at 37°C, absorbance at 650 nm was measured with a spectrophotometer. To evaluate the effect of S. salivarius colonies inside the oral cavity, the bacteria were cocultured using millicel inserts (Millipore Co., Belleica, MA).
The bacteria causing malodor were inoculated on the inside of the millicell insert, and S. salivarius was inoculated on the outside. They were then incubated for 36 hr under anaerobic conditions at 37°C. Contamination of the bacteria was verified using a phase‐contrast microscope (Nikon, Tokyo, Japan). The images of the bacteria were also obtained using a phase‐contrast microscope (Nikon).
2.4. Testing for statistical significance
Statistical significance between the case and control groups was tested with SPSS 21.0 (SPSS Inc., Chicago, IL), using Kruskal–Wallis and Jonckheere–Terpstra tests. Mann–Whitney test was used for post hoc analysis. We used the p value of .05 to test for significance.
3. RESULTS
3.1. The inhibitory effects of coculture of oral malodor causing bacteria and S. salivarius on the synthesis of VSCs
3.1.1. The inhibitory effects of coculture of P. gingivalis and S. salivarius on the synthesis of VSCs
After culturing S. salivarius by themselves or coculturing with oral malodor causing bacteria, Oral Chroma was used to measure the concentration of VSCs. When P. gingivalis and S. salivarius were cocultured, the level of VSCs that formed decreased significantly as the level of S. salivarius increased (Figure 1).
Figure 1.
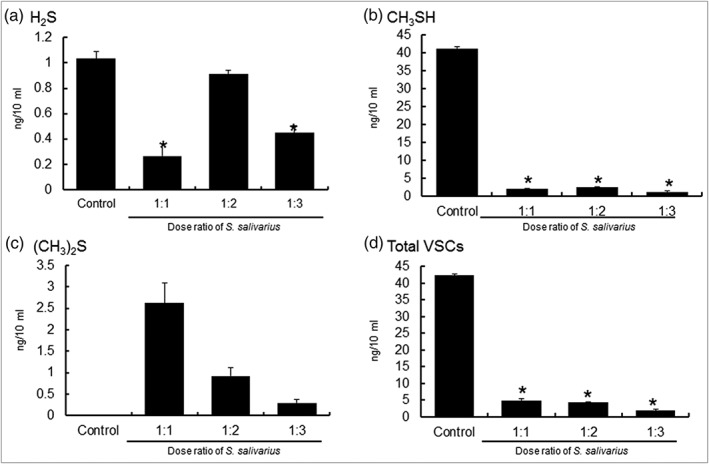
VSC of single‐cultured or cocultured Porphyromonas gingivalis with Streptococcus salivarius K12 and M18. VSC, volatile sulfur compound
Table 1 shows the concentration of VSCs from a single culture of P. gingivalis or a coculture of P. gingivalis and S. salivarius.
Table 1.
VSCs of single‐cultured or cocultured Porphyromonas gingivalis with Streptococcus salivarius K12 and M18
| Group | HS (n = 7) | MM (n = 7) | DMS (n = 7) | VSCs (n = 7) | ||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Control | 1.03 | 0.31 | 41.19** | 3.18 | 0.01 | 0.02 | 42.3** | 3.46 |
| 1:1 | 0.27* | 0.34 | 2.08* | 1.41 | 2.63 | 3.28 | 4.97* | 2.98 |
| 1:2 | 0.92 | 0.74 | 2.48* | 1.13 | 0.92 | 1.34 | 4.32* | 1.20 |
| 1:3 | 0.45* | 0.38 | 1.27* | 1.43 | 0.30 | 0.54 | 2.02* | 1.42 |
Note: Unit: ng/10 mL.
Abbreviations: DMS, dimethyl sulfide; HS, hydrogen sulfide; MM, methyl mercaptan; VSC, volatile sulfur compound.
*p < .05 by Kruskal–Wallis test; **p < .05 by Jonckheere–Terpstra test.
The total concentration of VSCs was highest in the single culture of P. gingivalis with 42.32 ng/10 mL. When 1 × 107 cells of S. salivarius were added, the total concentration dropped to 4.97 ng/10 mL. The concentration dropped to 4.32 and 2.02 ng/10 mL when 2 × 107 and 3 × 107 cells of S. salivarius were added, respectively. As the concentration of S. salivarius increased, the level of induction of VSCs for P. gingivalis decreased (p < .05).
3.1.2. The inhibitory effects of coculture of T. denticola and S. salivarius on the synthesis of VSCs
When T. denticola and S. salivarius were cocultured, the level of VSCs that formed decreased significantly as the level of S. salivarius increased (Figure 2).
Figure 2.

VSC of single‐cultured or cocultured Treponema denticola with Streptococcus salivarius K12 and M18. VSC, volatile sulfur compound
Table 2 shows the concentration of VSCs from a single culture of T. denticola or a coculture of T. denticola and S. salivarius.
Table 2.
VSC of single‐cultured or co‐cultured Treponema denticola with Streptococcus salivarius K12 and M18
| Group | HS (n = 7) | MM (n = 7) | DMS (n = 7) | VSCs (n = 7) | ||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Control | 10.82 | 7.81 | 37.32** | 16.46 | 10.17 | 26.90 | 58.30** | 8.39 |
| 1:1 | 13.08 | 8.93 | 27.27 | 19.16 | 19.01 | 31.01 | 59.36 | 7.06 |
| 1:2 | 9.31 | 8.78 | 17.63* | 16.69 | 12.69 | 16.23 | 39.64* | 11.74 |
| 1:3 | 2.16 | 1.75 | 10.67* | 6.38 | 0.43 | 0.34 | 13.25* | 8.06 |
Note: Unit: ng/10 mL.
Abbreviations: DMS, dimethyl sulfide; HS, hydrogen sulfide; MM, methyl mercaptan; VSC, volatile sulfur compound.
*p < .05 by Kruskal–Wallis test; **p < .05 by Jonckheere–Terpstra test.
The concentration of methyl mercaptan was highest at 37.32 ng/10 mL when T. denticola were cultured by themselves. When 1 × 107, 2 × 107, and 3 × 107 cells of S. salivarius were added and cocultured, the concentration dropped to 27.27, 17.63, and 10.67 ng/10 mL, respectively (p < .05). The total concentration of VSCs, when T. denticola were cultured by themselves, 58.30 ng/10 mL, was similar to the total concentration when T. denticola and 1 × 107 cells of S. salivarius were cocultured, 59.37 ng/10 mL. However, with 2 × 107 and 3 × 107 cells of S. salivarius, the total concentration dropped to 39.64 and 13.25 ng/10 mL, respectively (p < .05).
3.2. The antimicrobial activity of S. salivarius K12 and M18 on oral malodor causing bacteria
The synthesis of VSCs by oral malodor causing bacteria decreased in the presence of S. salivarius K12 and M18. To investigate the mechanism underlying S. salivarius' inhibition of the synthesis of VSCs by oral malodor causing bacteria, an antimicrobial activity test was performed. When the antimicrobial activity test recommended by CLSI was performed, S. salivarius K12 and M18 were found to have antimicrobial activity against P. gingivalis and T. denticola (Figure 3).
Figure 3.
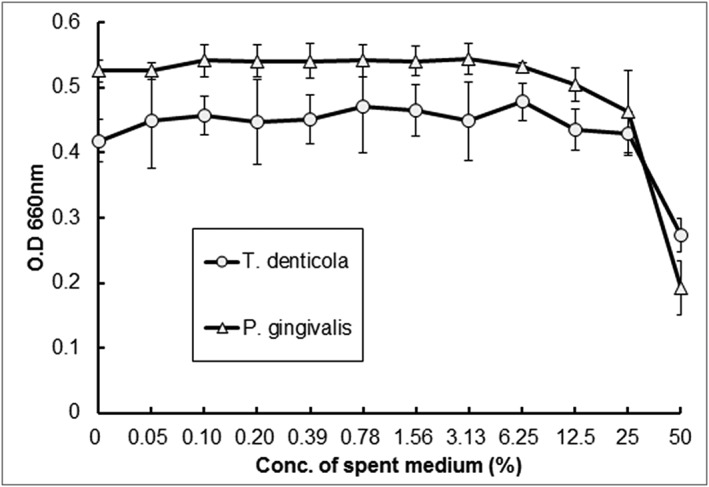
Antimicrobial activity of Streptococcus salivarius K12 and M18 against oral malodor generating bacteria
To study the effects of S. salivarius colonization in the oral cavity, we cocultured them with the malodor causing bacteria using a millicell insert and observed the cultures under a phase‐contrast microscope. Compared to a single culture of P. gingivalis, the coculture with S. salivarius saw a rapid decrease in the number of P. gingivalis (Figure 4).
Figure 4.
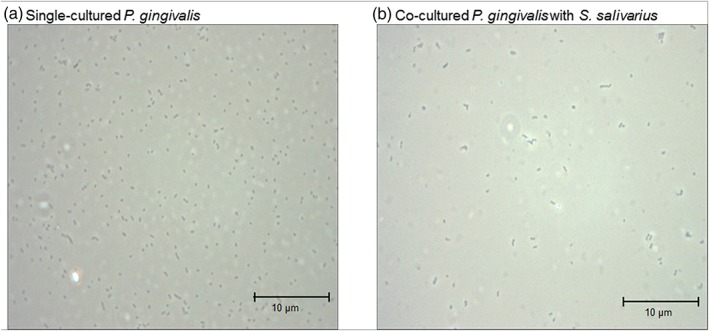
Phase‐contrast microscopic image of single‐cultured or cocultured Porphyromonas gingivalis with Streptococcus salivarius
Compared to a single culture of T. denticola, the coculture with S. salivarius saw a rapid decrease in the number of T. denticola (Figure 5).
Figure 5.
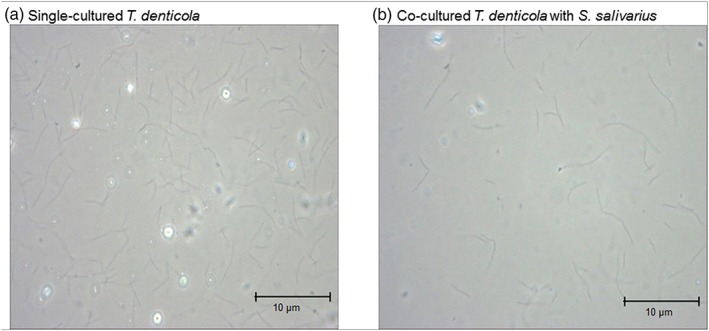
Phase‐contrast microscopic image of single‐cultured or cocultured Treponema denticola with Streptococcus salivarius
When oral malodor causing bacteria such as P. gingivalis and T. denticola were single cultured, the concentrations of VSCs were higher than when they were cocultured with S. salivarius (p < .05). As the concentrations of S. salivarius K12 and M18 increase, the occurrence of VSCs from oral malodor causing bacteria such as P. gingivalis and T. denticola decreases (p < .05) (Figures 6 and 7).
Figure 6.
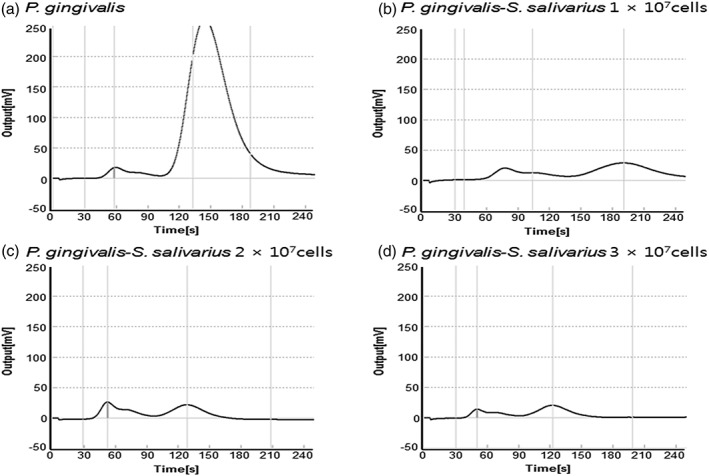
Oral Chroma graphs of single cultures of Porphyromonas gingivalis or cocultures with Streptococcus salivarius
Figure 7.
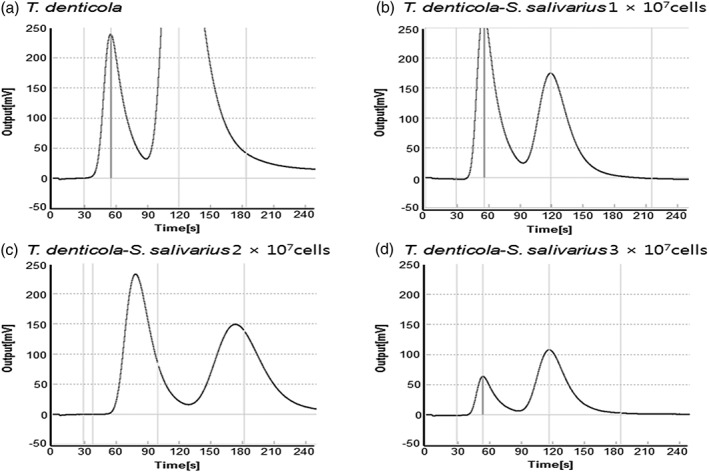
Oral Chroma graphs of single cultures of Treponema denticola or cocultures with Streptococcus salivarius
4. DISCUSSION
Oral malodor is an unpleasant smell that occurs in the oral cavity and in nearby organs. The prevalence has increased in modern society, which attaches an importance to social relationships. Bosy (1997) reported that the prevalence of oral malodor among adult's ranges from 25 to 50%, and 25% of afflicted individuals indicated that severe malodor affected their social lives. The American Dental Association reported that 50% of American adults complain of having oral malodor and about 25% have chronic oral malodor (ADA Council on Scientific Affairs, 2003). Kim and Cho (2011) reported that 45% of Korean people and 54% of teenager's desire treatment for oral malodor.
The causes of oral malodor arising from within the oral cavity include biofilms such as dental plaque and tongue coating as well as periodontal diseases. Especially, oral malodor comes from VSCs produced by anaerobic Gram negative bacteria inside biofilms (Amou, Hinode, Yoshioka, & Grenier, 2013; Kato, Yoshida, Awano, Ansai, & Takehara, 2005). Mitsuo et al. (2012) reported finding P. gingivalis, T. forsythia. and P. intermedia in the tongue coating of an individual with a healthy periodontium and they showed a correlation with the concentration of VSCs. On the other hand, T. denticola exists in dental plaque and their colonization is related to the concentration of VSCs (Paik et al., 2011). Tanaka et al. (2004) reported that P. gingivalis, T. forsythia, T. denticola, P. intermedia, and P. nigrescens living in the abdominal area of the tongue have a large effect on the synthesis of VSCs, and that patients with oral malodor showed a higher level of T. forsythia than healthy people. They also showed that the ratio of P. intermedia and P. nigrescens is related to hydrogen sulfide, and that P. gingivalis and P. nigrescens are associated with methyl mercaptan (Tanaka et al., 2004).
The prevalence of oral malodor shows an increasing trend but treatment methods have been limited to the improvement of oral hygiene involving the physical removal of biofilms by brushing the teeth and the tongue or chemical methods involving mouth wash (Broek, Feenstra, & Baat, 2007). Recently, however, many studies have reported the oral malodor decreasing effects of probiotics. Burton et al. (2005) described the possibility of decreasing oral malodor by using S. salivarius. As a result, a new treatment for controlling oral malodor causing bacteria through the use of the probiotic bacteria was proposed. S. salivarius can be used as a targeting system to remove harmful bacteria while adhering to the dorsal side of the tongue. They can also secrete a large amount of bacteriocins through saliva. In addition, S. salivarius can inhibit the synthesis of VSCs by blocking the colonization of bacteria responsible for synthesizing VSCs. However, the bacteria for which colonization was blocked to decrease oral malodor were unknown (Burton et al., 2005). Park, Auh, Chun, and Hong (2009) reported that S. salivarius showed inhibitory effects on P. intermedia, which cause periodontal diseases and oral malodor. Lee and Baek (2014) showed that both L. casei and L. rhamnosus showed inhibitory effects against VSCs synthesized by bacteria that cause periodontal diseases, such as P. gingivalis and F. nucleatum.
In this study, when S. salivarius was cocultured with oral malodor causing bacteria that produce VSCs such as P. gingivalis, T. denticola, T. forsythia, and F. nucleatum, there was a statistically significant decrease in oral malodor. In addition, as the concentration of probiotics increased, the decrease in oral malodor became more prominent.
S. salivarius K12 and M18 inhibit the synthesis of VSCs from oral malodor causing bacteria such as P. gingivalis and T. denticola.
Figures 6 and 7 show that when oral malodor causing bacteria such as P. gingivalis, T. denticola, T. forsythia, and F. nucleatum were single cultured, the concentrations of VSCs were higher than when they were cocultured with S. salivarius (p < .05). As the concentrations of S. salivarius K12 and M18 increase, the occurrence of VSCs from oral malodor causing bacteria such as P. gingivalis and T. denticola decreases (p < .05).
To investigate the mechanism behind the decrease of oral malodor, we used media containing S. salivarius to test for the minimum inhibitory concentration (50%). The results showed antimicrobial activity above a certain level of concentration. From these results, it can be inferred that the oral malodor decreasing effects of S. salivarius come from inhibiting the growth of oral malodor causing bacteria.
In the coculture of P. gingivalis, T. forsythia, and F. nucleatum with S. salivarius, there was a statistically significant decrease. However, the single cultures of oral malodor causing bacteria showed statistically significant decreases within a smaller range.
From these results, it can be inferred that S. salivarius shows a superior effect of reducing oral malodor through antimicrobial activity against oral malodor causing bacteria and the neutralization of sulfur compounds through the colonization of probiotic bacteria.
When S. salivarius K12 and M18 live in the oral cavity at concentrations above a certain level, they show antimicrobial activity against oral malodor causing bacteria, reducing the synthesis of VSCs. Using S. salivarius K12 and M18 as a treatment to clinically decrease oral malodor is a suitable application.
5. CLINICAL RELEVANCE
5.1. Scientific rationale for the study
This study provides information that S. salivarius inhibits halothane by inhibiting compound synthesis of halitosis‐inducing bacteria.
5.2. Principal findings
S. salivarius was incubated with the bad breath inducing strain, and the bad breath suppression was prominent with increasing concentration of probiotics.
5.3. Practical implication
Clinically, S. salivarius K12 and M18 can be used as therapeutic agents for halitosis suppression.
Yoo H‐J, Jwa S‐K, Kim D‐H, Ji Y‐J. Inhibitory effect of Streptococcus salivarius K12 and M18 on halitosis in vitro. Clin Exp Dent Res. 2020;6:207–214. 10.1002/cre2.269
REFERENCES
- ADA Council on Scientific Affairs (2003). Oral malodor. The Journal of the American Dental Association, 134(2), 209–214. 10.14219/jada.archive.2003.0135 [DOI] [PubMed] [Google Scholar]
- Amou, T. , Hinode, D. , Yoshioka, M. , & Grenier, D. (2013). Relationship between halitosis and periodontal disease—Associated oral bacteria in tongue coatings. International Journal of Dental Hygiene, 12(2), 145–151. 10.1111/idh.12046 [DOI] [PubMed] [Google Scholar]
- Bosy, A. (1997). Oral malodor: Philosophical and practical aspects. Journal of the Canadian Dental Association, 63, 196–201. [PubMed] [Google Scholar]
- Broek, A. V. D. , Feenstra, L. , & Baat, C. D. (2007). A review of the current literature on management of halitosis. Oral Diseases, 14(1), 30–39. 10.1111/j.1601-0825.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- Brown, A. C. , & Valiere, A. (2004). Probiotics and medical nutrition therapy. Nutrition in Clinical Care, 7(2), 56–68. [PMC free article] [PubMed] [Google Scholar]
- Burton, J. , Chilcott, C. , & Tagg, J. (2005). The rationale and potential for the reduction of oral malodour using Streptococcus salivarius probiotics. Oral Diseases, 11(s1), 29–31. 10.1111/j.1601-0825.2005.01084.x [DOI] [PubMed] [Google Scholar]
- Burton, J. P. , Drummond, B. K. , Chilcott, C. N. , Tagg, J. R. , Thomson, W. M. , Hale, J. D. F. , & Wescombe, P. A. (2013). Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: A randomized double‐blind, placebo‐controlled trial. Journal of Medical Microbiology, 62(Pt_6), 875–884. 10.1099/jmm.0.056663-0 [DOI] [PubMed] [Google Scholar]
- Comelli, E. M. , Guggenheim, B. , Stingele, F. , & Neeser, J.‐R. (2002). Selection of dairy bacterial strains as probiotics for oral health. European Journal of Oral Sciences, 110(3), 218–224. 10.1034/j.1600-0447.2002.21216.x [DOI] [PubMed] [Google Scholar]
- Kato, H. , Yoshida, A. , Awano, S. , Ansai, T. , & Takehara, T. (2005). Quantitative detection of volatile sulfur compound‐producing microorganisms in oral specimens using real‐time PCR. Oral Diseases, 11(s1), 67–71. 10.1111/j.1601-0825.2005.01096.x [DOI] [PubMed] [Google Scholar]
- Kim, Y. K. (2008). Oral malodor (pp. 17–18). Seoul, Republic of Korea: Shinhung International. [Google Scholar]
- Kim, Y. S. , & Cho, J. W. (2011). Volatile sulfar compound level in Korean measured by use of B&B checker. Int J Clin Prev Dent, 7(4), 167–177. [Google Scholar]
- Kishi, M. , Ohara‐Nemoto, Y. , Takahashi, M. , Kishi, K. , Kimura, S. , Aizawa, F. , & Yonemitsu, M. (2013). Prediction of periodontopathic bacteria in dental plaque of periodontal healthy subjects by measurement of volatile sulfur compounds in mouth air. Archives of Oral Biology, 58(3), 324–330. 10.1016/j.archoralbio.2012.09.019 [DOI] [PubMed] [Google Scholar]
- Kopp‐Hoolihan, L. (2001). Prophylactic and therapeutic uses of probiotics. Journal of the American Dietetic Association, 101(2), 229–241. 10.1016/s0002-8223(01)00060-8 [DOI] [PubMed] [Google Scholar]
- Lee, K. H. , & Baek, D. H. (2014). Beneficial effects of Lactobacillus casei ATCC 334 on halitosis induced by periodontopathogens. Int J Oral Biol, 39(1), 35–40. [Google Scholar]
- Ohta, K. , Makinen, K. K. , & Loesche, W. J. (1986). Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infection and Immunity, 53(1), 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik, D. I. , Shin, S. C. , Cho, J. W. , Jang, Y. S. , & Lee, M. G. (2011). Oral malodor control (pp. 9–13). Seoul, Republic of Korea: Pacific Books. [Google Scholar]
- Park, J. B. , Auh, Q. S. , Chun, Y. H. , & Hong, J. P. (2009). Effect of maintained microorganisms against to the phytoncide on Pr. Intermedia . Korean J Oral Medicine, 34(2), 153–167. [Google Scholar]
- Rosenberg, M. , Septon, I. , Eli, I. , Bar‐Ness, R. , Gelernter, I. , Brenner, S. , & Gabbay, J. (1991). Halitosis measurement by an industrial sulphide monitor. Journal of Periodontology, 62(8), 487–489. 10.1902/jop.1991.62.8.487 [DOI] [PubMed] [Google Scholar]
- Tanaka, M. , Yamamoto, Y. , Kuboniwa, M. , Nonaka, A. , Nishida, N. , Maeda, K. , … Shizukuishi, S. (2004). Contribution of periodontal pathogens on tongue dorsa analyzed with real‐time PCR to oral malodor. Microbes and Infection, 6(12), 1078–1083. 10.1016/j.micinf.2004.05.021 [DOI] [PubMed] [Google Scholar]


