Abstract
Background
Rotavirus is a common neonatal nosocomial viral infection and epidemics with the newer P(6)G9 strains have been reported. Local mucosal immunity in the intestine to rotavirus is important in the resolution of infection and protection against subsequent infections. Oral administration of anti‐rotaviral immunoglobulin preparations might be a useful strategy in preventing rotaviral infections, especially in low birth weight babies.
Objectives
To determine the effectiveness and safety of oral immunoglobulin preparations for the prevention of rotavirus infection in hospitalized low birthweight infants (birthweight < 2500 g)
Search methods
The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), MEDLINE, EMBASE, CINAHL, biological Abstracts (BIOSIS), Science Citation Index for articles citing Barnes 1982 and the proceedings of the Pediatric Academic Societies from 1991 onwards were searched in July 2011. Ongoing trials were also searched at clinicaltrials.gov and controlled‐trials.com
Selection criteria
The criteria used to select studies for inclusion were: 1) design: randomized or quasi‐randomized controlled trials; 2) participants: hospitalized low birthweight infants; 3) intervention: oral immunoglobulin preparations for prevention of rotavirus infection compared to placebo OR no intervention; 4) at least one of the following outcomes were reported: all cause mortality during hospital stay, mortality due to rotavirus infection during hospital stay, rotavirus infection , duration of diarrhea, need for rehydration, duration of viral excretion, duration of infection control measures, length of hospital stay in days, recurrent diarrhea or chronic diarrhea.
Data collection and analysis
The two review authors independently abstracted data from the included trials.
Main results
One published study (Barnes 1982) was eligible for inclusion in this review. Barnes 1982 found no significant difference in the rates of rotavirus infection after oral gammaglobulin versus placebo in hospitalized low birthweight babies [RR 1.27 (95% CI 0.65 to 2.37)]. In the subset of infants who became infected with rotavirus after receiving gammaglobulin or placebo for prevention of rotavirus infection, there was no significant difference in the duration of rotavirus excretion between the group who had gammaglobulin (mean 2 days, range 1 to 4 days) and the group who had placebo (mean 3 days, range 1 to 6 days). Barnes 1982 reported no adverse effects after administration of oral immunoglobulin preparations.
Authors' conclusions
Current evidence does not support the use of oral immunoglobulin preparations to prevent rotavirus infection in low birthweight infants. Researchers are encouraged to conduct well‐designed neonatal trials using the newer preparations of anti‐rotaviral immunoglobulins (colostrum, egg yolk immunoglobulins) and include cost effectiveness evaluations.
Plain language summary
Oral immunoglobulin for the prevention of rotavirus infection in low birth weight infants
Rotavirus infection can cause significant problems including diarrhea in the newborn. This is particularly true in babies weighing less than 2500 g (low birthweight infants). Rotavirus infection is becoming more common in newborn babies and can spread from one baby to another in the neonatal unit. Administration of antibodies against rotavirus to babies may prevent this infection and its spread in the neonatal unit. In this review, only one small trial was identified. Currently, there is not enough evidence to recommend the use of antibodies against rotavirus to babies exposed to rotavirus infection. More research is needed.
Background
Description of the condition
Group A rotavirus infection is a major cause of diarrheal morbidity in children. Globally, it is estimated that in children < 5 years of age, rotavirus causes 111 million episodes of gastro‐enteritis requiring only home care, 25 million clinic visits, two million hospitalizations and 440,000 deaths. 82% of deaths occurred in the poorest countries (Parashar 2003). It has also been recognized as the most common neonatal nosocomial viral infection (Strodtbeck 1986). Several outbreaks of rotaviral infection in neonatal nurseries in different countries have been reported (Bryden 1982; Shif 1983; Akinci 1991; Omoigberale 1995). Rotavirus infection epidemics appear to be seasonal, being more common in the colder winter months. Infection rates range from 13% to 78% of neonates in the neonatal unit during epidemics (Tufvesson 1986; Cicirello 1994; Kilgore 1996; Widdowson 2000). The main reservoir of rotavirus infection in neonatal nurseries seems to be the infected neonate (Grillner 1985) and most infection occurs in the first few days of life (Cicirello 1994; Kilgore 1996). Therefore, preventive strategies might be useful if administered during this time period. Premature and low birth‐weight infants and infants staying in the neonatal unit longer have a greater risk of acquiring the infection (Dearlove 1983; Walther 1984; Dennehy 1985). Rotavirus, especially the newer strains, can cause severe diarrhea and dehydration in already sick neonates. Rotavirus infection has been shown to be associated with necrotizing enterocolitis (NEC) in premature infants (Mogilner 1983). In another study, 29% of neonates with NEC were stool rotavirus positive. Although these infants had lower Bell's Staging of NEC, the outcome of these infants regarding mortality or complication rates did not differ from rotavirus negative infants who had NEC (Sharma 2004). A significantly higher incidence of bradycardia‐apnoea episodes (BAE) has been noticed two days before and two days after the diagnosis of rotavirus infection in infants. These episodes were followed by cyanosis and required intervention more often than did BAE episodes in rotavirus negative infants (Riedel 1996). Rotavirus infected neonates stay in the hospital longer than non‐infected neonates, causing increased stress to the family and increased cost to the neonatal unit (Strodtbeck 1986). Strict infection control measures have been advocated including hand protection, hand disinfection, individual nursing sets and cohorting infected babies (Grehn 1990). Closure of neonatal units (Valmari 1984) has been recommended to control and eradicate nosocomial outbreaks of rotavirus infections, placing considerable stress on busy neonatal units.
Newer strains of rotavirus P(6)G9 genotypes which previously have not been known to cause outbreaks of diarrhea have been identified to cause epidemics in the UK (Cubitt 2000), US (Ramachandran 1998), Bangladesh (Unicomb 1999) and Europe (Widdowson 2000; Widdowson 2002). Unlike previous strains, the new P(6)G9 strains can cause serious outbreaks of diarrhea in neonatal units and cause severe symptoms in most infected neonates. Most mothers have not been exposed to these new strains and thus a high proportion of neonates lack protective antibodies which could explain high attack rates in the neonatal unit and the severity of symptoms. Predominance of neonatal cases compared to few cases in older children may indicate that neonates have an increased risk of infection by P(6)G9 strains.
Description of the intervention
Determinants of protective immunity against rotavirus are unclear. It has been suggested (Molyneaux 1995) that local mucosal immunity in the intestine may protect against rotavirus illness. While local antibodies may be important in the resolution of infection and protection from subsequent infections, there is no specific antibody that could be used reliably as a marker of protection (Ward 1996). Breast‐fed infants are less susceptible to rotavirus infection and this is probably due to the presence of anti‐rotaviral secretory IgA and trypsin inhibitors in the breast milk (McLean 1981; Jayashree 1988). The protective efficacy of breast milk correlates positively with the concentrations of anti‐rotaviral secretory IgA in the breast milk (Jayashree 1988). Breast‐fed infants tend to excrete fewer viruses than bottle‐fed infants after infection with rotavirus (Chrystie 1978).
Oral administration of immunoglobulin containing preparations of bovine colostrum from immunized cows, egg yolk immunoglobulin from immunized hens (Mine 2002) or pooled plasma derived immunoglobulins can provide passive immunity. The highest titers of neutralizing anti‐rotaviral antibodies are present in bovine colostrum, then in egg yolk immunoglobulin followed by human pooled plasma derived immunoglobulin (Bogstedt 1996). These preparations may inhibit intestinal viral adherence or viral replication and therefore may have a role in the prevention or treatment of rotavirus infections. Oral immunoglobulin preparations are resistant to proteolytic digestion and retain significant neutralizing activity in the stools of treated infants. The newborn infant's immaturity of proteolytic enzymes or rapid gastrointestinal transit time permits intact or nearly intact Ig to pass throughout the gastrointestinal system (Blum 1981; Hilpert 1987). In a prospective randomized placebo controlled study of oral human plasma immunoglobulin in children (but not neonates) with acute rotaviral gastroenteritis, there was a reduction in total duration of viral diarrhea and viral excretion, and a faster clinical improvement compared to controls (Guarino 1994). Bovine colostrum from hyperimmunized pregnant cows has been shown to reduce viral excretion, stool output and the need for rehydration when used in the treatment of acute rotaviral gastroenteritis in children other than neonates (Hilpert 1987; Mitra 1995; Sarker 2001). It has also been shown to prevent diarrhea from rotavirus infection when used as prophylaxis (Turner 1993; Ebina 1996). Antibodies derived from the yolk of rotavirus immunized hens have been tested in acute rotaviral gastroenteritis in children beyond the neonatal period in a randomized placebo controlled trial, and found to cause earlier clearance of virus from the stools and an improvement of diarrhea (Sarker 1998).
How the intervention might work
Advantages of oral immunoglobulin containing preparations in neonates, especially low birthweight infants, would be a reduction in morbidity, reduction in the need for rehydration, earlier clearance of the virus thereby reducing the duration of infection control measures, and a reduction in hospital stay. Infection control measures are expensive and sometimes involve closure of infected units; oral immunoglobulins could be a cheaper and an easier alternative.
Why it is important to do this review
Rotavirus infection in low birthweight infants is a major problem and a systematic review on the efficacy and safety of oral immunoglobulin therapy in low birthweight infants for prevention of rotavirus infection is appropriate.
Objectives
To determine the effectiveness and safety of oral immunoglobulin preparations for the prevention of rotavirus infection in hospitalised low birthweight (birthweight < 2500 g) infants .
Methods
Criteria for considering studies for this review
Types of studies
Studies in which hospitalized low birthweight infants are randomized or quasi randomized to receive oral immunoglobulin preparations or either a placebo or no intervention for prevention of rotavirus infection.
Types of participants
Hospitalized low birthweight infants (birth weight < 2500 grams) not known to have rotavirus infection at study entry.
Types of interventions
Oral immunoglobulin preparations, specifically a) pooled plasma, b) colostrum from rotavirus immunized cows or c) egg yolk immunoglobulin from rotavirus immunized hens, used for prevention of rotavirus infection at any dose or duration.
Types of outcome measures
Primary outcomes
All cause mortality during hospital stay.
Mortality due to rotavirus infection during hospital stay.
Rotavirus infection.
Duration of diarrhea.
Need for rehydration.
Secondary outcomes
Duration of viral excretion.
Duration of infection control measures.
Length of hospital stay in days.
Recurrent diarrhea.
Chronic diarrhea.
Definitions
Rotavirus infection ‐ detection of rotavirus or antigen in the stools.
Rotavirus infection rates ‐ percentage of infants acquiring rotavirus infection.
diarrhea ‐ loose watery stools.
Recurrent diarrhea ‐ recurrence of diarrhea after 48 hrs of normal stools.
Chronic diarrhea ‐ persistence of diarrhea beyond 14 days.
Rehydration for rotavirus diarrhea ‐ fluid needed beyond maintenance requirements to maintain normal hydration by any route.
Duration of diarrhea ‐ time till the last loose watery stools from the onset of diarrhea measured in days.
Duration of viral excretion ‐ time till two rotavirus negative stools from the time of positive diagnosis measured in days.
Mortality due to rotavirus infection during hospital stay ‐ deaths directly attributable to rotavirus infection.
Duration of infection control measures ‐ days per infant of extra infection control measures as a result of rotavirus infection above what is normally practiced in that hospital for that infant infected with rotavirus.
Search methods for identification of studies
See: Collaborative Review Group search strategy The search strategy used to identify studies was devised according to the guidelines of the Cochrane Neonatal Review Group. The search was updated in July 2011. Relevant trials in any language were identified through 1. The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2011). 2. Electronic journal reference databases‐ MEDLINE (1966 to present) and PREMEDLINE EMBASE (1980 to present) CINAHL (1982 to present) Biological Abstracts (BIOSIS) (1980 to present)
3. Science citation index search for all articles, which quoted Barnes 1982 was performed. 4. Abstracts of conferences ‐proceedings of the Pediatric Academic Societies (American Pediatric Society, Society for Pediatric Research) and the European Society for Paediatric Research. The reference list of identified trials and abstracts published in Pediatric Research (1991 to present) and Abstracts online (2000 to 2011) were searched in MEDLINE and EMBASE for full published articles.
5. Ongoing trials were searched at clinicaltrials.gov and controlled‐trials.com 6. Communication was made with published authors for more information and other prominent authors in the field for possible unpublished studies, which may or may not have been presented as abstracts. 7. Additional searches were made in reference lists of identified clinical trials and in the reviewers' personal files.
MEDLINE, PREMEDLINE, EMBASE, CINAHL, Biological Abstracts search strategy #1 Search Rotavirus #2 Search Infant, newborn #3 Search Infant, newborn, diseases #4 Search neonat* #5 Search Infant, low birth weight #6 Search Infant, Very Low birth weight #7 #2 OR #3 OR #4 OR #5 OR #6 #8 Search Immunoglobulin AND Oral #9 Search Antibodies AND Oral #10 Search Gammaglobulin AND Oral #11 #8 OR #9 OR #10 #12 #1 AND #7 AND #11 #13 Limit #12 to (TG = Human) and (PG = Clinical trial)
No language restriction was applied. The reviewers erred on the side of over inclusion and later the articles, which did not meet the eligibility criteria were excluded
Data collection and analysis
The standard methods of the Cochrane Neonatal Review were employed in creating this update.
Selection of studies
The titles and the abstracts of studies identified by the search strategy were assessed by the two reviewers independently. Differences were resolved by mutual discussion. Full text versions of any eligible studies were obtained for quality assessment.
All randomized and quasi‐randomized controlled trials fulfilling the selection criteria described in the previous section were included. Both authors reviewed the results of the search and separately select the studies for inclusion. The review authors resolved any disagreement by discussion.
Data extraction and management
Data extraction was done independently by the authors using paper proforma and compared for any differences, which were then resolved by discussion. Any article for which either person felt that the reference list should be searched was retrieved. Information regarding all infants randomized in the trial by Barnes 1982 was requested and limited information was obtained from the authors. Details of the study by Tam JS (unpublished) including particular details of the participants, interventions and outcomes were requested and obtained from the principal investigator and the sponsor (Numico Research, previously Northfield Laboratories).
Assessment of risk of bias in included studies
The standard methods of the Cochrane Neonatal Review Group were employed. We assessed the methodological quality of the studies using the following key criteria: allocation concealment (blinding of randomization), blinding of intervention, completeness of follow‐up, and blinding of outcome measurement/assessment. We included this information in the Characteristics of Included Studies Table.
For the update in 2011, we evaluated the following issues to address the Risk of Bias (Higgins 2011):
1) Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated? For each included study, we categorized the method used to generate the allocation sequence as:
‐ low risk (any truly random process e.g. random number table; computer random number generator);
‐ high risk (any non random process e.g. odd or even date of birth; hospital or clinic record number);
‐ unclear risk.
(2) Allocation concealment (checking for possible selection bias). Was allocation adequately concealed? For each included study, we categorized the method used to conceal the allocation sequence as:
‐ low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
‐ high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
‐ unclear risk.
(3) Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment? For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
‐ low risk, high risk, or unclear risk for participants;
‐ low risk, high risk, or unclear risk for personnel;
‐ low risk, high risk, or unclear risk for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
‐ low risk (< 20% missing data);
‐ high risk (≥ 20% missing data):
‐ unclear risk.
(5) Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting? For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
‐ low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
‐ high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
‐ unclear risk.
(6) Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias? For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
‐ low risk, high risk, or unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
The standard methods of the Neonatal Review Group were used. Statistical analyses were performed using Review Manager software. Categorical data were analyzed using relative risk (RR), risk difference (RD) and the number needed to treat (NNT). Continuous data were analyzed using weighted mean difference (WMD). The 95% Confidence interval (CI) was reported on all estimates.
Assessment of heterogeneity
Heterogeneity was not assessed as only one trial is included in this review. If multiple trial were identified, we planned to estimate the treatment effects of individual trials and examine heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I‐2 statistic. If we detected statistical heterogeneity, we planned to explore the possible causes (for example, differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc subgroup analyses.
Data synthesis
The meta‐analysis was performed using Review Manager software (RevMan 5), supplied by the Cochrane Collaboration. For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. All meta‐analyses were done using the fixed effect model.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were prespecified in the protocol but were not possible: 1) birthweight: birthweight < 1500 grams; birthweight from 1500 to 2500 grams.
2) type of preparation: oral immunoglobulin derived from pooled plasma; oral immunoglobulin from the colostrum of rotavirus immunized cows; oral immunoglobulins from egg yolk of rotavirus immunized hens.
3) strains of rotavirus: newer G(9) strains; non G(9) strains.
Results
Description of studies
Details of the included studies are provided in the table "Characteristics of Included Studies". The following studies for possible inclusion in this review were identified by our search strategy: Barnes 1982; Tam JS (unpublished); Ventura 1993.
Barnes 1982 was included in this review. Seventy‐five infants whose birthweight ranged from 2000 to 2500 g and who were admitted to a special care baby unit where rotavirus infection was endemic were randomized to oral gammaglobulin or placebo within 12 hours of birth. The intervention (gammaglobulin or placebo) was given in a dose of 4 ml, four times a day for seven days. The following outcomes were reported: i) timing of excretion of rotavirus, ii) quantity or grading of rotavirus excreted in the stools, iii) duration of excretion of rotavirus and iv) the incidence of clinically important diarrhea. The report of this trial analyzed only those 25 infants who were subsequently found to be rotavirus positive; infants who did not excrete rotavirus in the first two weeks of life were excluded from the analysis. As diarrhea or rotavirus infection were not criteria for entry into the study, we considered this to be a study of prevention of rotavirus infection in low birthweight infants admitted to a neonatal unit, where rotavirus was known to be endemic. In order to conduct analyses based on all infants randomized, we requested and obtained limited information from the authors regarding the outcomes of the infants, who were randomized but not infected with rotavirus.
Studies excluded
Tam JS (unpublished) Two hundred and thirty four infants with birthweight > 1500 g in Hong Kong and Vellore (India) were randomized into three groups to receive: i) colostrum (powder) from rotavirus vaccinated cows, ii) colostrum (powder) from unvaccinated cows and iii) standard infant milk powder (Lactogen) respectively at a dose of 550 mg three times a day during hospital stay and seven days after discharge. Physicians were blinded to the intervention. Outcomes reported were i) rotavirus infection from stools ii) Ig A or IgM seroconversion to rotavirus (defined as a four fold increase in titre in paired samples) and iii) adverse effects. Only 61% (76/234) infants completed the study. The mean age of infants recruited was 16 months and highly unlikely to have any low birthweight infants recruited in the trial. The investigators cannot separately identify the number of low birthweight infants randomized in this trial. There are unresolved discrepancies regarding rotavirus infection status of the participants in the data received from the principal investigator and the sponsor (Numico, previously Northfield Laboratories) and hence the study is excluded. Ventura 1993 As a part of the prevention arm of a trial that also assessed the efficacy of treatment of acute diarrhea with oral gammaglobulin or placebo, 16 infants hospitalised during the rotavirus epidemic were randomized to gammaglobulin or no intervention for prevention of rotavirus infection and diarrhea. Data regarding hospitalized low birthweight infants without known rotavirus infection at study entry who were randomized in the trial are unavailable and hence this study was excluded.
Risk of bias in included studies
Barnes 1982 This is a randomized, placebo controlled study. Randomization was performed in another institution and concealment of allocation (or blinding of randomisation) was noted. Physicians were blinded to the intervention. Assessment of outcomes was also blinded. Fifty enrolled infants who were not diagnosed with rotavirus infection were excluded from analysis in the report of this trial, but have been included in this review in analyzing effect on incidence of rotavirus infection.
Effects of interventions
i) Rotavirus infection In the included study (Barnes 1982), there was no significant difference in rotavirus infection rates in infants who received the gammaglobulin compared to infants who received placebo [RR 1.24 (95% CI 0.65 to 2.37) RD 0.07 (95% CI ‐0.14 to 0.28)].
ii) Duration of viral excretion In the subset of infants in Barnes 1982 who developed rotavirus infection after randomization to oral gammaglobulin or placebo, there was no significant difference in the duration of excretion of rotavirus between the gammaglobulin group (mean two days and range 1 to 4 days) and the placebo group (mean three days and range 1 to 6 days).
iii) Adverse effects No adverse effects were reported in the included study (Barnes 1982).
The following outcomes could not be evaluated: all cause mortality during hospital stay, mortality due to rotavirus infection during hospital stay, duration of diarrhea, need for rehydration, duration of infection control measures, length of hospital stay in days, recurrent diarrhea and chronic diarrhea.
Discussion
The single study included in this review (Barnes 1982) is too small to reliably answer the objectives of this review. This study was analyzed in the report as a trial of treatment of rotavirus infection with oral gammaglobulin. Infants were randomized within 12 hours of birth and neither rotavirus infection nor diarrhea was a criterion for entry into the trial. Twenty‐five of the 75 infants randomized were diagnosed to have rotavirus infection. It was assumed that 50 infants who were rotavirus negative were not exposed to rotavirus infection and excluded from analysis. However, it is difficult to assume so in a neonatal unit where rotavirus infection is known to be endemic and hence this study is included as a trial for prevention of rotavirus infection in low birthweight infants. There was no significant difference in the infection rates between the two randomized groups nor was there any significant difference in the duration of rotavirus excretion in the subset of infants who were infected with rotavirus.
Newer immunoglobulin preparations, namely bovine colostrum from rotavirus immunized cows and egg yolk of rotavirus immunized hens (Mine 2002), have been shown to be safe and effective in preventing rotavirus diarrhea and reducing morbidity when used to treat rotavirus infections in children other than neonates (Hilpert 1987; Turner 1993; Mitra 1995; Ebina 1996; Sarker 1998; Sarker 2001). These preparations have not been tested in neonatal trials for prevention or treatment of rotavirus infection. The obvious advantages are ease of administration, lack of adverse effects and potentially important clinical benefits.
Future investigators should be encouraged to plan large multicenter trials using the newer preparations of anti‐rotaviral immunoglobulin for the prevention of rotavirus infection during epidemics of rotavirus infections or in neonatal units where rotavirus is endemic. They would be well advised to use sensitive rotavirus detection methods and include all low birth weight infants, especially the smaller ones who are at a much greater risk of rotavirus infection. These large trials should be planned during the months of the year when rotavirus infections occur. Large multicenter trials would be able to recruit units with different degrees of endemicity for rotavirus infections. In theory, enhancing local mucosal immunity has the potential to reduce rates of rotavirus infections and attenuate symptoms of infection.
Active immunization with rotavirus vaccines can be an effective strategy to prevent rotavirus infections. The initial vaccine licensed was a tetravalent rhesus‐human reassortment vaccine (Rotashield). Vaccination with this vaccine was discontinued because of safety concerns, as there was an association with intussuception. Currently available vaccines RotaTeq and RIX4414 (Rotarix) have not been shown to be associated with intussuception and are 70% effective against any rotavirus disease and 90 to 100% effective in preventing severe rotavirus disease (Vesikari 2006). The American Academy of Pediatrics currently recommends routine rotavirus vaccination with either bovine reassortment rotavirus vaccine (RV5) or human attenuated virus vaccine (RV1), to be given orally for three doses or two doses respectively (AAP 2009). However, current rotavirus vaccines do not include the newer P(6)G9 rotavirus strains and immunizations start from six weeks through 14 weeks of age. Therefore, vaccination would not be expected to prevent neonatal rotavirus infection in hospitalized low birthweight infants. Alternate methods of prevention of rotavirus infection therefore become important. Oral immunoglobulin therapy may have the potential to prevent rotavirus infection in hospitalized low birthweight neonates and reduce neonatal morbidity, mortality and health resource utilization.
Authors' conclusions
Implications for practice.
Current evidence from one small randomized controlled trial does not support the routine use of oral immunoglobulin preparations in the prevention of rotavirus infections in hospitalized low birth weight infants who are exposed to rotavirus.
Implications for research.
The efficacy of the newer preparations of anti‐rotaviral immunoglobulins, namely colostrum from cows immunized against rotavirus and egg yolk immunoglobulins from hens immunized against rotavirus, have not been assessed for the prevention of rotavirus infections in the newborn. Well designed, large randomized controlled trials are needed to address the effectiveness and safety of these preparations in hospitalized neonates, in the prevention of rotavirus infections. Premature and or low birth weight infants are likely to have a higher mortality and morbidity after infection with the new strains of rotavirus and it will be prudent to test in this group of infants. Such randomized controlled trials should report effects on mortality, morbidity and health resource utilization. Key outcomes to be looked for would be efficacy in the prevention of rotavirus infection, reduction of morbidity and a reduction in the duration of viral excretion which will determine the duration of expensive infection control measures. The design of these randomized controlled trials should also include cost‐effectiveness evaluations.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2011 | New search has been performed | This updates the review 'Oral immunoglobulin for the prevention of rotavirus infection in low birth weight infants' published in the Cochrane Database of Systematic Reviews (Mohan 2002). |
| 7 July 2011 | New citation required but conclusions have not changed | Updated search in July 2011 did not identify any new trials for inclusion. No change to conclusions. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 7 December 2010 | Amended | Contact details updated. |
| 10 June 2008 | Amended | Converted to new review format. |
| 30 April 2007 | New search has been performed | This review is an update of the existing review "Oral immunoglobulin for the prevention of rotavirus infection in low birth weight infants", published in The Cochrane Library, Issue 3 2003 (Mohan 2002). There were no new trials identified with our updated search in April 2007. Two studies (Tam JS unpublished, Ventura 1993) were excluded due to nonavailability of relevant data. Five new references were added to the additional references section. The background and the discussion section have been updated with new references |
| 2 April 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the help of 1) Prof. L. Doyle, Dr. G.L. Barnes and R. Bishop for providing us with unpublished information regarding the trial by Barnes 1982. 2) Prof. Davidson for bringing to our notice the trial by Tam JS (unpublished). 3) Prof. Tam and Prof Tai Fok and Mr. Ivan Clarke of Numico Research (previously Northfield Laboratories) for providing us with details of the study Tam JS (unpublished). 4) Nicola Bexon, Information Services Manager, Institute of Health Sciences, Oxford, for developing search strategies of literature conducted in June 2002. 5) Elena Telaro from the Italian Cochrane Center for the translation of an article from Italian to English. 6) Maura Moggia from the Italian Cochrane center for providing us the contact details of A. Ventura, the principal author of the article Ventura 1993.
Data and analyses
Comparison 1. Oral immunoglobulin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rotavirus infection | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.37] |
1.1. Analysis.
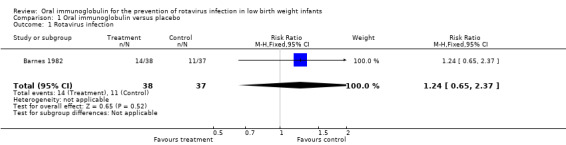
Comparison 1 Oral immunoglobulin versus placebo, Outcome 1 Rotavirus infection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barnes 1982.
| Methods | Single center, randomised placebo controlled trial Block randomised in blocks of 4 in another institution Blinding of randomization‐ yes Clinician blinded to the intervention Blinding of outcome measurement‐ yes Completeness of follow‐up ‐ 50/75 of enrolled infants excluded in the report of this trial, but included in this review. | |
| Participants | Low birth weight infants (2000‐2500 g) Admitted to special care unit, Royal Women's Hospital, Melbourne Period‐ September 1978 to April 1981 75 infants enrolled 25/75 infants subsequently diagnosed with rotavirus infection | |
| Interventions | Human gammaglobulin (Commonwealth Serum Laboratories) 16 g/dl preparation‐4 ml, 4 times a day for 7 days Placebo‐Methyl‐cellulose (2% w/v 20 ml); glycerol (20ml); Intravite injection (0.04 ml); water (1 dl) similar dosage to gammaglobulin 38/75 infants had gammaglobulin and 37/75 had placebo | |
| Outcomes | Outcomes were reported for 25 rotavirus positive infants. Outcomes for the 50 rotavirus negative infants were requested from the authors Reported outcomes i) timing of excretion of rotavirus ii) duration of excretion of rotavirus iii) quantification or grading of excretion of rotavirus iv) incidence of clinically important diarrhea (defined as diarrhea necessitating change of milk to low lactose feeds) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unsatisfactory ‐ 50/75 of enrolled infants excluded |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Tam JS (unpublished) | Reliable data regarding low birthweight infants and rotavirus infection in the participants are not available. |
| Ventura 1993 | Data regarding hospitalised low birthweight infants without known rotavirus infection at study entry who were randomised in the trial are not available. |
Contributions of authors
Mohan Pammi Literature search and identification of trails for inclusion. Contacting prominent authors in the field for more data and unpublished trials. Abstraction of data from eligible studies. Evaluation of methodological quality of included trials. Verifying and entering data in RevMan. Writing the text of the review.
Khalid Haque Abstraction of data from eligible studies. Evaluation of methodological quality of included trials. Writing the text of the review.
Sources of support
Internal sources
National Perinatal Epidemiology Unit, Oxford, UK.
Epsom & St.Helier NHS trust, UK.
External sources
No sources of support supplied
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barnes 1982 {published and unpublished data}
- Barnes GL, Doyle LW, Hewson PH, Knoches AML, McLellan JA, Kitchen WH, Bishop RF. A randomised trial of oral gammaglobulin in low‐birth‐weight infants infected with rotavirus. Lancet 1982;1:1371‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Tam JS (unpublished) {unpublished data only}
- Tam JS, Cheng AFB, Fok T, Leung D, Oppenheimer SJ. Passive prevention of rotavirus infection in neonates by oral administration of hyperimmune bovine colostrum containing antibodies to four human rotavirus serotypes.
Ventura 1993 {published data only}
- Ventura A, Nassimbeni G, Martelossi S, Bohm P, D'Agro PL. Experience with gamma globulins per os in the therapy and prevention of infectious diarrhea [Esperienze con le gammaglobuline per os nella terapia e prevenzione della diarrea infettiva]. La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics 1993;15:343‐6. [PubMed] [Google Scholar]
Additional references
AAP 2009
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of rotavirus disease: updated guidelines for use of rotavirus vaccine. Pediatrics 2009;123:1412‐20. [DOI] [PubMed] [Google Scholar]
Akinci 1991
- Akinci A, Tezic T, Gur I, Cetin H, Hatun S. Rotavirus diarrhoea in newborn infants. Turkisk Journal of Pediatrics 1991;33:153‐7. [PubMed] [Google Scholar]
Blum 1981
- Blum PM, Phelps DL, Ank BJ, Krantman HJ, Stiehm ER. Survival of oral human serum globulin in the gastrointestinal tract of low birth weight infants. Pediatric Research 1981;15:1256‐60. [DOI] [PubMed] [Google Scholar]
Bogstedt 1996
- Bogstedt AK, Johansen K, Hatta H, Kim M, Casswell T, Svensson L, Hammarstrom L. Passive immunity against diarrhoea. Acta Paediatrica 1996;85:125‐8. [DOI] [PubMed] [Google Scholar]
Bryden 1982
- Bryden AS, Thouless ME, Hall CJ, Flewett TH, Wharton BA, Mathew PM, Craig I. Rotavirus infections in a special‐care baby unit. Journal of Infection 1982;4:43‐8. [DOI] [PubMed] [Google Scholar]
Chrystie 1978
- Chrystie IL, Totterdell BM, Banatvala JE. Asymptomatic endemic rotavirus infections in the newborn. Lancet 1978;1:1176‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cicirello 1994
- Cicirello HG, Das BK, Gupta A, Bhan MK, Gentsch JR, Kumar R, Glass RI. High prevalence of rotavirus infection among neonates born at hospitals in Delhi, India: predisposition of newborns for infection with unusual rotavirus. Pediatric Infectious Disease Journal 1994;13:720‐4. [DOI] [PubMed] [Google Scholar]
Cubitt 2000
- Cubitt WD, Steele AD, Iturriza M. Characterisation of rotaviruses from children treated at a London hospital during 1996: emergence of strains G9P2A[6] and G3P2A[6]. Journal of Medical Virology 2000;61:150‐4. [DOI] [PubMed] [Google Scholar]
Dearlove 1983
- Dearlove J, Latham P, Dearlove B, Pearl K, Thomson A, Lewis IG. Clinical range of neonatal rotavirus gastroenteritis. British Medical Journal 1983;286:1473‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dennehy 1985
- Dennehy PH, Peter G. Risk factors associated with nosocomial rotavirus infection. American Journal of Diseases of Children 1985;139:935‐9. [DOI] [PubMed] [Google Scholar]
Ebina 1996
- Ebina T. Prophylaxis of rotavirus gastroenteritis using immunoglobulin. Archives of Virology Suppl 1996;12:217‐23. [DOI] [PubMed] [Google Scholar]
Grehn 1990
- Grehn M, Kunz J, Sigg P, Slongo R, Zbinden R. Nosocomial rotavirus infections in neonates: means of prevention and control. Journal of Perinatal Medicine 1990;18:369‐74. [DOI] [PubMed] [Google Scholar]
Grillner 1985
- Grillner L, Broberger U, Chrystie I, Ransjo U. Rotavirus infections in newborns: an epidemiological and clinical study. Scandinavian Journal of Infectious Diseases 1985;17:349‐55. [DOI] [PubMed] [Google Scholar]
Guarino 1994
- Guarino A, Canani RB, Russo S, Albano F, Canani MB, Ruggeri FM, Donelli G, Rubino A. Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics 1994;93:12‐6. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hilpert 1987
- Hilpert H, Brussow H, Mietens C, Sidoti J, Lerner L, Werchau H. Use of bovine milk concentrate containing antibody to rotavirus to treat rotavirus gastroenteritis in infants. Journal of Infectious Diseases 1987;156:158‐66. [DOI] [PubMed] [Google Scholar]
Jayashree 1988
- Jayashree S, Bhan MK, Kumar R, Bhandari N, Sazawal S. Protection against neonatal rotavirus infection by breast milk antibodies and trypsin inhibitors. Journal of Medical Virology 1988;26:333‐8. [DOI] [PubMed] [Google Scholar]
Kilgore 1996
- Kilgore PE, Unicomb LE, Gentsch JR, Albert MJ, McElroy CA, Glass RI. Neonatal rotavirus infection in Bangladesh: strain characterization and risk factors for nosocomial infection. Pediatric Infectious Disease Journal 1996;15:672‐7. [DOI] [PubMed] [Google Scholar]
McLean 1981
- McLean BS, Holmes IH. Effects of antibodies, trypsin, and trypsin inhibitors on susceptibility of neonates to rotavirus infection. Journal of Clinical Microbiology 1981;13:22‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mine 2002
- Mine Y, Kovacs‐Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: A review. Journal of Medicinal Food 2002;5:159‐69. [DOI] [PubMed] [Google Scholar]
Mitra 1995
- Mitra AK, Mahalanabis D, Ashraf H, Unicomb L, Eeckels R, Tzipori S. Hyperimmune cow colostrum reduces diarrhoea due to rotavirus: a double‐blind, controlled clinical trial. Acta Paediatrica 1995;84:996‐1001. [DOI] [PubMed] [Google Scholar]
Mogilner 1983
- Mogilner BM, Bar‐Yochai A, Miskin A, Shif I, Aboudi Y. Necrotizing enterocolitis associated with rotavirus infection. Israel Journal of Medical Science 1983;19:894‐6. [PubMed] [Google Scholar]
Molyneaux 1995
- Molyneaux PJ. Human immunity to rotavirus. Journal of Medical Microbiology 1995;43:397‐404. [DOI] [PubMed] [Google Scholar]
Omoigberale 1995
- Omoigberale AI, Abiodun PO. Nosocomial rotavirus infection in newborns. East African Medical Journal 1995;72:220‐1. [PubMed] [Google Scholar]
Parashar 2003
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerging Infectious Diseases 2003;9:565‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramachandran 1998
- Ramachandran M, Gentsch JR, Parashar UD, Jin S, Woods PA, Holmes JL, Kirkwood CD, Bishop RF, Greenberg HB, Urasawa S, Gerna G, Coulson BS, Taniguchi K, Bresee JS, Glass RI. Detection and characterisation of novel rotavirus strains in the United States. Journal of Clinical Microbiology 1998;36:3223‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riedel 1996
- Riedel F, Kroener T, Stein K, Nuesslein TG, Rieger CH. Rotavirus infection and bradycardia‐apnoea‐episodes in the neonate. European Journal of Pediatrics 1996;155:36‐40. [DOI] [PubMed] [Google Scholar]
Sarker 1998
- Sarker SA, Casswall TH, Mahalanabis D, Alam NH, Albert MJ, Brussow H, Fuchs GJ, Hammarstrom L. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. Pediatric Infectious Disease Journal 1998;17:1149‐54. [DOI] [PubMed] [Google Scholar]
Sarker 2001
- Sarker SA, Casswall TH, Juneja LR, Hoq E, Hossain I, Fuchs GJ, Hammarstrom L. Randomized placebo‐controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. Journal of Pediatric Gastroenterology and Nutrition 2001;32:19‐25. [DOI] [PubMed] [Google Scholar]
Sharma 2004
- Sharma R, Garrison RD, Tepas JJ 3rd, Mollitt DL, Pieper P, Hudak ML, Bradshaw JA, Stevens G, Premachandra BR. Rotavirus‐associated necrotizing enterocolitis: an insight into a potentially preventable disease. Journal of Pediatric Surgery 2004;39:453‐7. [DOI] [PubMed] [Google Scholar]
Shif 1983
- Shif I, Aboudy A, Mogilner B, Bar‐Yochai A, Miskin A. Rotavirus infection in a neonatal intensive care nursery. Israeal Journal of Medical Science 1983;19:860‐2. [PubMed] [Google Scholar]
Strodtbeck 1986
- Strodtbeck F. The epidemiology of nosocomial viral infections in infants who are long term residents of the neonatal intensive care unit. Indiana University School of Nursing. Doctoral thesis 1986.
Tufvesson 1986
- Tufvesson B, Polberger S, Svanberg L, Sveger T. A prospective study of rotavirus infections in neonatal and maternity wards. Acta Paediatrica Scandinavica 1986;75:211‐5. [DOI] [PubMed] [Google Scholar]
Turner 1993
- Turner RB, Kelsey DK. Passive immunization for prevention of rotavirus illness in healthy infants. Pediatric Infectious Disease Journal 1993;12:718‐22. [DOI] [PubMed] [Google Scholar]
Unicomb 1999
- Unicomb LE, Podder G, Gentsch JR, Woods PA, Hasan KZ, Faruque AS, Albert MJ, Glass RI. Evidence of high frequency reassortment of Group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. Journal of Clinical Microbiology 1999;37:1885‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valmari 1984
- Valmari P, Pontynen S, Sunila R. Rotavirus infection in a neonatal unit. Annals of Clinical Research 1984;16:167‐70. [PubMed] [Google Scholar]
Vesikari 2006
- Vesikari T, Giaquinto C, Huppertz HI. Clinical trials of rotavirus vaccines in Europe. Pediatric Infectious Disease Journal 2006;25(1 suppl):S42‐7. [DOI] [PubMed] [Google Scholar]
Walther 1984
- Walther FJ, Bruggeman C, Daniels‐Bosman MS. Rotavirus infections in high‐risk neonates. Journal of Hospital Infection 1984;5:438‐43. [DOI] [PubMed] [Google Scholar]
Ward 1996
- Ward RL. Mechanisms of protection against rotavirus in humans and mice. Journal of Infectious Diseases 1996;174:S51‐8. [DOI] [PubMed] [Google Scholar]
Widdowson 2000
- Widdowson MA, Doornum GJJ, Poel WH, Boer AS, Mahdi U, Koopmans M. Emerging group‐A rotavirus and a nosocomial outbreak of diarrhoea. Lancet 2000;356:1161‐2. [DOI] [PubMed] [Google Scholar]
Widdowson 2002
- Widdowson MA, Doornum GJ, Poel WH, Boer AS, HR, Mahdi U, Haanen P, Kool JL, Koopmans M. An outbreak of diarrhea in a neonatal medium care unit caused by a novel strain of rotavirus: investigation using both epidemiologic and microbiological methods. Infection Control and Hospital Epidemiology 2002;23:665‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mohan 2002
- Pammi M, Haque K. Oral immunoglobulin for the prevention of rotavirus infection in low birth weight infants. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003740] [DOI] [PubMed] [Google Scholar]


