Abstract
Background
Azoospermia, the absence of sperm in ejaculated semen, is the most severe form of male‐factor infertility and is present in approximately 5% of all investigated infertile couples. The advent of intra‐cytoplasmic sperm injection (ICSI) has transformed treatment of this type of severe male‐factor infertility. Sperm can be retrieved for ICSI from either the epididymis or the testis, depending on the type of azoospermia.
Objectives
To evaluate the efficacy of the various surgical retrieval techniques for men with obstructive or non‐obstructive azoospermia prior to ICSI.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Trials Register (November 2007), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 4), MEDLINE (1966 to November 2007), EMBASE (1980 to November 2007), Biological Abstracts (1980 to November 2007), and reference lists of identified articles.
Selection criteria
Randomised controlled trials (RCTs) comparing the effectiveness of different sperm‐retrieval techniques in men with azoospermia prior to ICSI. Due to the lack of RCTs, non‐randomised trials that used the participants as their own control were also considered in the review but their results were not included in the meta‐analysis.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. Study authors were contacted for additional information.
Main results
The search was revised and re‐run in November 2007. No new trials were located therefore the results of the updated review remain unchanged from those published in 2006.
Two trials involving 98 men were included. The first small RCT had 59 participants and compared two epididymal techniques. The trial gave limited evidence that microsurgical epididymal sperm aspiration (MESA) achieved a significantly lower pregnancy rate (one pregnancy in 29 procedures compared with seven pregnancies in 30 procedures; OR 0.19, 95% CI 0.04 to 0.83) and fertilisation rate (OR 0.16, 95% CI 0.05 to 0.48) than the micropuncture with perivascular nerve stimulation technique. The other RCT comparing two testicular aspiration techniques (TSA) in 39 participants gave no statistically significant evidence for the superiority of the ultrasound‐guided technique compared to the aspiration technique without ultrasound. TSA with ultrasound resulted in pregnancy in three out of 16 participants compared with four out of 23 participants (OR 1.10, 95% CI 0.21 to 5.74).
Authors' conclusions
There is insufficient evidence to recommend any specific sperm retrieval technique for azoospermic men undergoing ICSI. In the absence of evidence to support more invasive or more technically difficult methods, the review authors recommend the least invasive and simplest technique available. Further randomised trials are warranted, preferably multi‐centred trials. The classification of azoospermia as obstructive and non‐obstructive appears to be relevant to a successful clinical outcome and a distinction according to the cause of azoospermia is important for future clinical trials.
Plain language summary
Techniques for surgical retrieval of sperm prior to intra‐cytoplasmic sperm injection (ICSI) because of absence of sperm in the semen (azoospermia).
It is not certain whether any particular surgical technique used to remove sperm for ICSI (sperm injection in vitro fertilisation or IVF) is better than another for the men involved or for leading to more pregnancies.
Some men are infertile because they produce sperm but a blockage in the testicle stops the sperm getting into the semen. In vitro fertilisation (IVF) is the only option for helping these men conceive with their own sperm.
The sperm are surgically removed from the testis gland or epididymis (tube leading from the testis towards the penis) and several micro‐surgical and suction techniques through hollow needles can be used for this. Sperm are then injected into an egg, an IVF procedure called ICSI. However, the review found there were too few trials to show which sperm removal technique might be better. Complications associated with surgical sperm‐retrieval techniques are haematoma and fibrosis, identified by ultrasound.
Background
Azoospermia, the absence of sperm in ejaculated semen, is the most severe form of male factor infertility and is present in approximately 5% of all investigated infertile couples (Irvine 1998). The condition is currently classified as 'obstructive' or 'non‐obstructive', although it is important to also consider the specific aetiology of each individual case (Sharif 2000). Obstructive azoospermia is the result of obstruction in either the upper or lower male reproductive tract (epididymis, vas deferens, seminal vesicles, or ejaculatory ducts). Sperm production may be normal (which may be verified through testicular biopsy) but the obstruction prevents the sperm from being ejaculated. Causes of obstructive azoospermia include vasectomy, congenital absence of vas deferens, scarring from past infections, and inguinal hernia or hydrocoele operations. Non‐obstructive azoospermia is the result of testicular failure where sperm production is either severely impaired or non‐existent, although in many cases sperm may be found and surgically extracted directly from the testicles. Causes of non‐obstructive azoospermia include genetic and hormonal disorders, testicular maldescent and torsion, systemic disease (including cancer), drugs, radiation, and toxins (Jansen 1997).
Some cases of obstructive azoospermia are treatable using microsurgical reconstruction of the seminal tract (for example vasectomy reversal). Pregnancy rates following reconstruction vary from 27 to 56% of cases and results are determined by a number of factors such as the site and duration of the obstruction (Belker 1991; Jarow 1997). Unreconstructable obstructive azoospermia and non‐obstructive azoospermia have historically been relatively untreatable conditions that required the use of donor spermatozoa for fertilisation. The advent of intra‐cytoplasmic sperm injection (ICSI), however, has transformed treatment of this type of severe male factor infertility. ICSI involves injecting a single sperm into an oocyte, making it an ideal treatment for male‐factor infertility that would otherwise be untreatable using conventional IVF techniques, which require large numbers of sperm (Palermo 1992). Even when aspirates with very good sperm concentration and motility are obtained from the testes or epididymis, low fertilisation and pregnancy rates are generally achieved with conventional IVF (Silber 1994).
Sperm can be retrieved for ICSI in a variety of ways, depending on the type of azoospermia. In non‐obstructive azoospermia sperm need to be directly obtained from the testis. Testicular sperm extraction (TESE), testicular biopsy, and testicular sperm aspiration (TESA) are techniques used on men with non‐obstructive azoospermia. The procedures can require multiple biopsies, sometimes in both testes. Testicular fine needle aspiration (TEFNA) is a relevantly new technique but seems to be a straightforward procedure that is well tolerated by men (Lewin 1999).
In many cases of obstructive azoospermia, sperm can be retrieved from either the epididymis or the testis (Jaroudi 1999a). Men with obstructive azoospermia can undergo microsurgical epididymal sperm aspiration (MESA), percutaneous epididymal sperm aspiration (PESA), or any of the testicular sperm extraction techniques. Of the two epididymal techniques, PESA is less invasive and does not require microsurgical skills or equipment (Sheynkin 1998).
Complications associated with surgical sperm‐retrieval techniques are haematoma and fibrosis, identified at ultrasound in the months immediately following the procedure (Amer 2000). Testicular atrophy and devascularization are considered to be long‐term physiological complications (Schlegel 1997).
In this review we evaluated the effectiveness of the techniques for surgical sperm retrieval for fertilisation, pregnancy and live birth outcomes, and investigated any adverse effects.
Objectives
To evaluate the efficacy of various surgical sperm‐retrieval techniques in retrieving a sufficient quantity and quality of sperm and the effect on subsequent fertilisation, pregnancy, and live birth rates in couples with non‐obstructive and obstructive azoospermia undergoing intra‐cytoplasmic sperm injection (ICSI).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that compared surgical sperm‐retrieval techniques. Due to the lack of RCTs in this topic area, non‐randomised comparative studies (where participants were their own controls) were also included in the review. These trials were commented on in the results and discussion sections but were not included in the meta‐analysis.
Types of participants
Inclusion criteria Participants in the trials had to meet all these inclusion criteria for the trial to be included in the review:
men with obstructive or non‐obstructive azoospermia;
men undergoing surgical retrieval of sperm for ICSI.
Exclusion criteria If participants in the trial met any of the exclusion criteria the trial was not included in the review:
men undergoing surgical retrieval were without azoospermia.
Types of interventions
RCTs involving any surgical sperm‐extraction technique prior to ICSI as treatment for azoospermia versus any other surgical sperm‐extraction technique or against variations of the same technique.
The specific comparisons considered were: (1) different epididymal sperm‐retrieval techniques versus each other (for obstructive azoospermia only); (2) different testicular sperm‐retrieval techniques versus each other (for either obstructive or non‐obstructive azoospermia); (3) epididymal sperm‐retrieval techniques versus testicular sperm‐retrieval techniques (for obstructive azoospermia only).
Surgical sperm‐extraction techniques include, but are not limited to, the following techniques:
microsurgical epididymal sperm aspiration (MESA);
percutaneous epididymal sperm aspiration (PESA);
testicular sperm extraction (TESE) or testicular biopsy;
testicular sperm aspiration (TESA) or testicular fine needle aspiration (TEFNA).
Different techniques of sperm extraction were considered as subcategories of the three comparisons mentioned above. Trials of surgically‐extracted sperm versus ejaculated sperm or of diagnostic biopsies with no sperm parameter information were not considered.
Types of outcome measures
Each of the following outcomes was recorded, where available.
Primary outcomes
Birth rate ‐ live birth per couple
Pregnancy rate per couple ‐ number of couples achieving a clinical pregnancy (which should be confirmed by ultrasound) divided by the number of couples
Adverse effects associated with sperm‐retrieval technique (e.g. haematoma, infection, severe bruising, pain)
Secondary outcomes
Pregnancy rate per cycle ‐ number of clinical pregnancies divided by the number of treatment cycles (data per couple is preferable, however pregnancy rate per completed cycle is the outcome measure used most often in infertility research and, therefore, remains an important outcome to express the efficacy of treatment despite its statistical inappropriateness)
Fertilisation rate ‐ number of oocytes fertilised divided by number sperm injected (preferably fertilisation should be determined by the presence of two pronuclei and the exclusion of a second polar body 16 to 18 hours after microinjection)
Implantation rate ‐ number of implanted embryos divided by the number transferred (should be determined by a gestational sac visible on transvaginal ultrasound)
Sperm parameters of tissue obtained from the surgical retrieval procedure; including fluid volume, sperm motility, sperm morphology, sperm density (however measured by each trial)
Multiple pregnancy rate (should be confirmed by ultrasound or delivery and, if possible, twins, triplets, and more distinguished)
Miscarriage rate (per intra‐uterine pregnancy and/or per woman)
Fetal abnormalities (any reported either in utero or after birth)
Search methods for identification of studies
All reports which described (or might describe) randomised controlled trials of surgical sperm extraction prior to ICSI as treatment for azoospermia were obtained using the following search strategy.
We searched the following.
(1) The Menstrual Disorders and Subfertility Group Trials Register was searched for any trials with azoospermia, ICSI, or intra‐cytoplasmic sperm injection in the title, abstract, or keywords sections (searched November 2007) (see the Review Group Module for more details on the register) and Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 4). (2) Electronic databases were searched using OVID software: MEDLINE (1966 to November 2007), EMBASE (1980 to December 2007), Biological Abstracts (1980 to November 2007). (3) CINAHL (Cumulative Index to Nursing & Allied Health Literature) (1982 to May 2007).
We searched the databases using the following subject headings and keywords.
MEDLINE and Biological Abstracts 1 Azoospermia/ (70) 2 Azoospermia.tw. (2958) 3 or/1‐2 (2984) 4 (sperm$ adj5 extract$).tw. (1553) 5 (sperm$ adj5 aspirat$).tw. (451) 6 (epididym$ adj5 sperm$).tw. (4437) 7 (testi$ adj5 sperm$).tw. (5785) 8 (sperm$ adj5 retrieval).tw. (359) 9 or/4‐8 (10208) 10 3 and 9 (835) 11 randomized controlled trial.pt. (234274) 12 controlled clinical trial.pt. (74820) 13 Randomized Controlled Trials/ (48327) 14 Random allocation/ (57750) 15 Double‐blind method/ (91028) 16 Single‐blind method/ (10880) 17 or/11‐16 (397294) 18 clinical trial.pt. (435392) 19 exp clinical trials/ (190560) 20 (clin$ adj25 trial$).ti,ab,sh. (129372) 21 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh. (90362) 22 Placebos/ (26128) 23 placebo$.ti,ab,sh. (114490) 24 random$.ti,ab,sh. (490003) 25 Research design/ (47276) 26 or/18‐25 (866440) 27 animal/ not (human/ and animal/) (3095759) 28 17 or 26 (873731) 29 28 not 27 (800552) 30 10 and 29 (69) 31 (2006$ or 2007$).ed. (885162) 32 30 and 31 (5) 33 from 32 keep 1‐5
Cochrane Central Register of Controlled Trials <2nd Quarter 2007>
1 Azoospermia/ (1) 2 Azoospermia.tw. (100) 3 or/1‐2 (101) 4 (sperm$ adj5 extract$).tw. (19) 5 (sperm$ adj5 aspirat$).tw. (16) 6 (epididym$ adj5 sperm$).tw. (16) 7 (testi$ adj5 sperm$).tw. (98) 8 (sperm$ adj5 retrieval).tw. (21) 9 or/4‐8 (135) 10 3 and 9 (30) 11 from 10 keep 1‐30
CINAHL ‐ Cumulative Index to Nursing & Allied Health Literature <1982 to May Week 1 2007>
1 Azoospermia/ (0) 2 Azoospermia.tw. (22) 3 or/1‐2 (22) 4 (sperm$ adj5 extract$).tw. (6) 5 (sperm$ adj5 aspirat$).tw. (6) 6 (epididym$ adj5 sperm$).tw. (8) 7 (testi$ adj5 sperm$).tw. (17) 8 (sperm$ adj5 retrieval).tw. (16) 9 or/4‐8 (39) 10 3 and 9 (4) 11 exp clinical trials/ (43840) 12 Clinical trial.pt. (20772) 13 (clinic$ adj trial$1).tw. (10271) 14 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$3 or mask$3)).tw. (6120) 15 Randomi?ed control$ trial$.tw. (8968) 16 Random assignment/ (15190) 17 Random$ allocat$.tw. (1026) 18 Placebo$.tw. (8576) 19 Placebos/ (3492) 20 Quantitative studies/ (3206) 21 Allocat$ random$.tw. (60) 22 or/11‐21 (61489) 23 10 and 22 (1) 24 from 23 keep 1
EMBASE <1980 to 2007 Week 18>
1 Azoospermia/ (3792) 2 Azoospermia.tw. (2635) 3 or/1‐2 (4311) 4 (sperm$ adj5 extract$).tw. (1305) 5 (sperm$ adj5 aspirat$).tw. (423) 6 (epididym$ adj5 sperm$).tw. (3369) 7 (testi$ adj5 sperm$).tw. (4696) 8 (sperm$ adj5 retrieval).tw. (366) 9 or/4‐8 (8049) 10 3 and 9 (1088) 11 Controlled study/ or randomized controlled trial/ (2405316) 12 double blind procedure/ (63789) 13 single blind procedure/ (6559) 14 crossover procedure/ (18585) 15 drug comparison/ (81250) 16 placebo/ (97915) 17 random$.ti,ab,hw,tn,mf. (367123) 18 latin square.ti,ab,hw,tn,mf. (1064) 19 crossover.ti,ab,hw,tn,mf. (32554) 20 cross‐over.ti,ab,hw,tn,mf. (11275) 21 placebo$.ti,ab,hw,tn,mf. (146355) 22 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf. (106285) 23 (comparative adj5 trial$).ti,ab,hw,tn,mf. (5769) 24 (clinical adj5 trial$).ti,ab,hw,tn,mf. (483066) 25 or/11‐24 (2886258) 26 nonhuman/ (2878264) 27 animal/ not (human/ and animal/) (12847) 28 or/26‐27 (2881866) 29 25 not 28 (1695407) 30 10 and 29
(4) The National Research Register (NRR), a register of ongoing and recently completed research projects funded by or of interest to the United Kingdom's National Health Service as well as entries from the Medical Research Council's Clinical Trials Register and containing details on reviews in progress collected by the NHS Centre for Reviews and Dissemination, was searched for any trials with ICSI, intra‐cytoplasmic sperm injection, or azoospermia as keywords. We also searched the Clinical Trials Register, a registry of federally and privately funded US clinical trials, using the same keywords.
5) The citation lists of relevant publications, review articles, abstracts of scientific meetings, and included studies were also searched.
Data collection and analysis
Two review authors (MW and AMvP) independently selected trials for inclusion in the review. Where differences of opinion arose which could not be resolved by discussion, as occurred in the decision of whether to include the De Croo 2000 trial, this was resolved in discussion with a third author (NJ).
We analysed included trials for the following quality criteria and methodological details. This information, if available, was presented in the table 'Characteristics of included studies' and the 'Description of studies' and 'Methodological quality of included studies' sections of the review. The information extracted from the trials provided a context for discussing the reliability of results.
Trial characteristics (1) Method of randomisation (2) Presence or absence of blinding to treatment allocation (3) Number of participants and oocytes randomised, excluded, or lost to follow up (4) Whether an intention‐to‐treat' analysis was done (5) The presence of a power calculation (6) Duration, timing, and location of the study (7) Sources of any funding
Characteristics of the study participants (1) Definition and duration of pre‐existing infertility in both male and female (2) Method of assessment of azoospermia (3) Previous administered treatment(s) (4) Information on sperm concentration, motility and morphology, and spermatogenesis (5) Age of participants, both male and female (6) Follicle stimulating hormone (FSH) levels of males
Interventions used (1) Types of techniques used (2 Methodology of techniques used (3) Site of sperm retrieval (e.g. epididymis, testis) (4) Number of interventions (5) Number of cycles (6) Methods of ovarian stimulation and oocyte retrieval (7) Methods used in performing ICSI (8) Sperm preparation methods used (9) Number of embryos implanted (10) Use of spermatids or spermatozoa (11) Presence or absence of blood or debris, or both, in sample
Outcomes (1) Definition of clinical pregnancy used (2) Methods used to assess all outcomes
Two review authors (MW and AMvP) independently assessed the quality of trials and performed data extraction using forms designed according to Cochrane guidelines. A third author (NJ) was available to resolve any discrepancies, although none occurred. We sought additional information on trial methodology or original trial data, or both, from the authors of the three RCTs identified by the search strategy and from comparative trials when it was unclear if they were randomised. No reply has been received from authors of Yamamoto 1996, Amer 1999b, Atassi 2000, or Belenky 2001. De Croo and Dhont kindly supplied additional data (multiple pregnancy rates, miscarriage rates, and some sperm parameters) and methodological information regarding the De Croo 2000 trial.
Allocation concealment was scored according to the categories used by The Cochrane Collaboration; allocation concealment was assessed as: adequate (A), unclear (B), inadequate (C), or that it was not used (D). Other aspects that were reported in the Methodological quality section of the review are: use of blinding, use of intention‐to‐treat analysis, power calculations, numbers lost to follow up, and the criteria for including participants and assessing outcomes.
Each type of retrieval technique was considered as a separate comparison. It was intended to perform subgroup analysis according to the type of azoospermia (obstructive and non‐obstructive) however this was not necessary due to the small number of RCTs identified. We performed statistical analysis in accordance with the guidelines for statistical analysis developed by the Cochrane Menstrual Disorders and Subfertility Group.
A priori, it was planned to perform sensitivity analyses on results to look at the possible contributions of: (1) differences in methodological quality of the trials (trials rated A or B versus trials rated with a C), (2) differences in number of treated cycles used (trials analysing one treated cycle versus those with more than one). However, these analyses were not possible as an adequate number of trials were not found for inclusion.
The majority of outcome measures for this review used binary data (for example, pregnancy rate per woman is the number of women becoming pregnant over the number of women treated). Results for each study were expressed as odds ratio with 95% confidence intervals and combined for meta‐analysis with RevMan software using the Peto‐modified Mantel‐Haenszel method with a fixed‐effect model.
The outcome measures of sperm parameters were the only outcomes to use continuous data. For these outcomes, results for each study were expressed as weighted mean differences (WMD) with 95% confidence intervals (CI) and combined for meta‐analysis using RevMan software and a fixed‐effect model.
Differences in clinical parameters are considerable in subfertility trials (clinical heterogeneity). These differences need to be taken into account when analysing and interpreting the results. Clinical heterogeneity in subfertility cannot be avoided because most centres use their own 'materials and methods', which vary for a number of parameters. When trials meet the inclusion criteria, have performed the same intervention and are statistically homogenous we considered it appropriate to pool their results, although due to the lack of RCTs identified no pooling of results occurred.
Pregnancy outcomes are considered positive consequences of treatment; therefore, a higher proportion of women achieving pregnancy is considered a benefit. Adverse effects are a negative consequence and therefore higher numbers are considered to be detrimental. This needs to be taken into consideration when viewing the summary graphs.
It is the intention of the review authors that a new search for RCTs will be performed yearly and the review updated accordingly.
Results
Description of studies
The search was revised and re‐run in November 2007. No new trials were located therefore the results of the updated review remain unchanged from the review published in 2006.
Trials included in this review
The Japanese trial (Yamamoto 1996) compared epididymal micropuncture with perivascular nerve stimulation versus microsurgical epididymal sperm aspiration (MESA). Only one cycle of treatment with the sperm‐retrieval intervention and ICSI was performed. The micropuncture procedure involved the use of general anaesthesia. A scrotal incision was made and testis and epididymis were retrieved and stabilised in a testicular holder. The micro pipettes were placed in a micromanipulator that was connected to a syringe. Electrodes were placed around the spermatic cord for electrical nerve stimulation with direct current (intensity 136 V, frequency 20 Hz) for 30 seconds. This was repeated every minute until no further fluid was obtained.
The comparative procedure was MESA, which involved incision of an epididymal tubule and aspiration of the expressed fluid with a 22 gauge angiocatheter and a 1ml tuberculin syringe. Sperm was prepared in the same way regardless of the intervention, in a medium containing 10% polyvinylpyrrolidone. Micropuncture was performed repeatedly only if excessive blood contamination occurred (no information was presented on whether this was necessary in any of the participants). The ICSI procedure was performed within four hours after both methods of sperm retrieval.
Included participants were men with congenital bilateral absence of the vas deferens (CBAVD) or unreconstructable vasal obstruction (failed vasovasostomy, or epididymovasostomy). There were no specified exclusion criteria. The age of participants (male and female) in both groups was comparable as was the mean duration of fertility. The study's main outcomes were pregnancy rate per couple (confirmed by fetal heartbeat at ultrasound), fertilisation rate per couple and sperm parameters (fluid volume, sperm density, motility and morphology).
The RCT from Tel Aviv, Israel (Belenky 2001) compared percutaneous testicular aspiration with ultrasound guidance (USTSA) versus percutaneous testicular aspiration without ultrasound guidance (TSA).
Both procedures were done under general anaesthesia. This was preferred by the study authors as it allowed an open biopsy procedure to be performed immediately if no sperm was retrieved via aspiration.
In the USTSA group, the 21 gauge butterfly needle was directed into the testicular regions to be sampled under real‐time gray‐scale and power Doppler sonographic guidance, avoiding the echogenic mediastinum testis and the vascular plexus of the tunica albuginea as well as the prominent testicular parenchymal vessels.
The 'blind' TSA procedure included three passes, one each in the upper, middle and lower thirds of each testis, with a 21 gauge butterfly needle and tubing connected to a 20 ml syringe placed in a syringe holder designed to facilitate manual aspiration. The needle was inserted in the testis and, after negative pressure was created, was moved slowly back and forth four or five times in different directions within the sampled volume.
Before the procedures were terminated a laboratory technologist examined the aspirate microscopically to ensure that at least 10 motile sperm could be identified. If no motile sperm could be found, the same aspiration technique was performed in the contralateral testis. If insufficient motile sperm were found in the contralateral testis the patient underwent open biopsy. All aspiration procedures were performed one or two days before the man's female partner underwent oocyte retrieval for ICSI.
The included participants were men with azoospermia aged between 27 and 41 years (mean 33 years). There was no information about the cause of the azoospermia (obstructive or non‐obstructive). No exclusion criteria were specified.
The study's main outcomes were pregnancy rate and percentage of complications. Although not mentioned as a trial outcome, sufficient material for ICSI procedure was also documented.
Trials excluded from the meta‐analysis The Belgian trial (De Croo 2000) compared epididymal spermatozoa obtained by microsurgical epididymal sperm aspiration (MESA) with testicular spermatozoa obtained by testicular sperm extraction (TESE) for ICSI from men with obstructive azoospermia and normal spermatogenesis. Couples with at least eight mature oocytes were included. Half of the oocytes were randomised by day of the week to injection with epididymal spermatozoa and half to testicular spermatozoa. The subsequent selection of embryos to transfer from these two groups was based on the investigator's clinical decision, not randomisation. The trial was unblinded.
A study comparing testicular sperm extraction through multiple testicular biopsies with single biopsy in men with functional azoospermia was excluded from this review (Amer 1999b). This study was an abstract of the 15 th annual meeting of the ESHRE in Tours, France and there was insufficient information published to determine if the trial was a case series or a comparative trial. Randomisation and blinding were not mentioned in the abstract. We sought further information from the authors in order to determine trial design however no response was received. See the 'Characteristics of excluded studies' table for more information. A study comparing testicular sperm retrieval by the open biopsy method with testicular sperm retrieval by needle aspiration was also excluded from the review (Atassi 2000). One hundred and forty‐nine participants underwent a needle aspiration technique and 60 underwent the open biopsy technique. Randomisation and blinding were not mentioned in the trial report. Further information was sought from the authors in order to determine trial design however no response was received. See the table 'Characteristics of excluded studies' for more information. Non‐randomised comparative trials
We identified seven other non‐randomised trials using participants as their own control; both procedures were performed one after the other in all participants. The characteristics of these trials are summarised in Table 3 of the 'Additional tables' section. The trials are commented on in the Results and Discussion sections but were not included in the meta‐analysis.
1. Characteristics of comparative studies of sperm retrieval techniques.
| Study ID | Methods | Participants | Interventions | Outcomes |
| Collins 1996 | Not random Patients as their own control Single blinded 20 participants with an additional 5 as a control group of men with known fertility wanting a vasectomy. 4 participants were excluded (2 had solitary systems and 2 had unilateral exploration) | Inclusion criteria: Men admitted for reversal of vasectomy with previous proven fertility (obstructive azoospermia) Location: Manchester, UK | Percutaneous epididymal sperm aspiration with needle in situ (PESA1) or while withdrawing the needle (PESA2) versus Microscopic epididymal sperm aspiration with needle in situ (MESA1) or while withdrawing needle (MESA2), a 23G needle was used in all procedures. Procedures were performed on both testes and both procedures (PESA then MESA) were performed on all men | Success of sperm retrieval. Aspiration was considered successful when enough sperm (with sufficient quality) was retrieved to perform ICSI (however ICSI results are not presented) |
| Ezeh 1998 | Not random Patients as their own control 35 participants | Inclusion criteria: Men with azoospermia due to defective spermatogenesis‐testicular atrophy/raised plasma FSH/exposure to gonadotoxins/testicular torsion etc. Exclusion criteria: azoospermia due to retrograde ejaculation, obstruction of the genital tract and endocrine disorders Location: Sheffield, UK. | Multiple fine needle biopsies versus open window (simple) testicular biopsy Multiple needle biopsies performed on all men, then open biopsy performed | Success of sperm retrieval Adverse effects |
| Friedler 1997 | Not random Patients as their own control 37 participants | Inclusion criteria: Men with non‐obstructive azoospermia/testicular failure. Exclusion criteria: pseudo azoospermia. Age mean 32.7 (range 24‐47) years Location: Zerifin, Israël | Testicular fine needle aspiration versus Testicular sperm extraction (open biopsy) TEFNA performed with a 21G needle in 6 different entries, then TESE performed in same men with up to 3 biopsies per testicle | Success of sperm retrieval (comparison between TEFNA and TESE) Pregnancy and fertilisation with TESE |
| Rosenlund 1998 | Not random. Patients as their own control 22 participants, 10 with a 19 G needle, 12 with a 21 G needle | Inclusion criteria: Men with non‐obstructive azoospermia, testicular volume 8‐25 ml. Location: Göteborg, Sweden | Testicular biopsies with 19 G or 21 G butterfly needles versus open biopsy Percutaneous needle testicular biopsy performed first in all men (12 men with a 21G needle, 17 procedures; 10 men with a 19G needle, 16 procedures), then an open biopsy performed immediately over the same site | Testicular spermatozoa in sample (focus is on histological outcome) |
| Segal 1995 | Not random Patients as their own control 7 participants + 2 as control who underwent needle biopsy only | Inclusion criteria: Men who had undergone MESA and had no spermatozoa in their aspirate. Location: Barzilai, Israël | Testicular sperm aspiration with ACECUT (automatic biopsy system) versus open biopsy. ACECUT performed first in all men then open biopsy over the puncture site | Tubular length obtained and spermatozoa/5 mm tubule |
| Sheynkin 1998 | Not random Patients as their own control. | Inclusion criteria: Men with obstructive azoospermia due to bilateral absence of vas, unilateral epididymal obstruction, or previous failed attempt of vasoepididymostomy. Location: New York, USA | Testicular fine needle aspiration (TFNA) versus percutaneous testicular needle biopsy versus Microsurgical epididymal sperm aspiration (MESA). At a planned MESA, sperm retrieval was also attempted on the same site with TFNA and percutaneous testicular needle biopsy. | Success of sperm retrieval. (sperm count/motility) |
| Amer 2000 | Not random Patients as their own control. | Inclusion criteria:Men with non‐obstructive azoospermia. Exclusion criteria: Men with bilateral non‐identical testicular histopathology. | Conventional and microsurgical testicular sperm extraction. Procedures performed at different sites. | Sperm recovery rate (SSR%). Histological outcomes. Adverse effects. |
Four trials included men with non‐obstructive azoospermia (Amer 2000; Ezeh 1998; Friedler 1997; Rosenlund 1998). Two trials included men with obstructive azoospermia due to vasectomy, with previous proven fertility (Collins 1996) or with congenital bilateral absence of vas deferens (Sheynkin 1998). One trial included men with azoospermia who had undergone MESA with no sperm in the retrieved aspirate (Segal 1995).
Four trials compared a minimally invasive technique, such as testicular fine needle aspiration or multiple needle biopsies, with an open‐window biopsy. Procedures were performed at the same site in the testis in men with non‐obstructive azoospermia (Ezeh 1998; Friedler 1997Rosenlund 1998; Segal 1995). The Amer 2000 trial performed each procedure in a different testis. Participants underwent the procedures under local or general anaesthetic. Twenty‐one or 19 gauge butterfly needles attached to a syringe under negative pressure were used to aspirate the fluid containing the spermatozoa. An incision was made at the same site and a small sample of testis tissue was excised.
One trial compared two different types of percutaneous sperm aspiration with two different types of microsurgical sperm aspiration (Collins 1996). Microsurgical epididymal sperm aspiration (MESA) was performed with the needle in situ and while withdrawing the needle and compared with percutaneous epididymal sperm aspiration (PESA) with the needle in situ and while withdrawing the needle. Sheynkin 1998 compared testicular fine needle aspiration (for which the abbreviation TFNA was used) and percutaneous testicular needle biopsy with microsurgical sperm aspiration (MESA) in participants with obstructive azoospermia. At a planned MESA, sperm retrieval was also attempted on the same testis with TFNA and percutaneous testis needle biopsy. The procedures were done under local or general anaesthesia based on patient preference.
The main outcome used in most the trials was successful retrieval of spermatozoa.
Risk of bias in included studies
The Japanese RCT (Yamamoto 1996) was given a B as its allocation score due to unclear allocation concealment. The trial (Yamamoto 1996) mentioned that couples were randomly assigned to one of the two treatments but gave no information on methodology, allocation concealment, or blinding. Additional information regarding methodology has been sought from the author. Twenty‐nine couples underwent microsurgical sperm aspiration and 30 couples underwent micropuncture with perivascular nerve stimulation. None of the randomised couples withdrew from the trial. The treatment and control groups were comparable for duration of infertility. The mean age was younger in both the males and females in the MESA group. This has the potential for bias in favour of the younger group as certainly the age of the female is one of the most important factors in predicting the success of treatment (Silber 1995a). There is no other information on other possible female confounding factors, such as smoking or body mass index.
The RCT from Israel (Belenky 2001) was given a C as its allocation score. The azoospermic men were randomised by the final digit of their patient ID number, therefore, allocation concealment was inadequate. It remains unclear how blinding was achieved. Therefore the results of this trial could be prone to bias and should be dealt with cautiously. Sixteen men underwent ultrasound‐guided percutaneous testicular sperm aspiration and 23 men underwent percutaneous testicular sperm aspiration without ultrasound guidance. Primary outcomes were pregnancy rate and the percentage of complications. There was no information reported in the trial regarding the baseline comparability of the patients in each group, the mean age of the female partners, or other characteristics of the included patients (for example duration of infertility). All patients provided written informed consent before the procedure was undertaken.
Trials excluded from the meta‐analysis
The Belgian trial (De Croo 2000) was given an allocation score of C. Oocytes rather than couples were randomised by days of the week to receive either epididymal or testicular sperm. Seven embryo transfers were carried out with epididymal spermatozoa and 10 embryo transfers with testicular sperm. In 11 cases a mixed transfer was done, although these were not considered here. Only the fertilisation rates for epididymal or testicular sperm were considered by this review. The decision to transfer embryos fertilised with either testicular or epididymal sperm was based on the investigator's clinical judgement of the best embryos. Therefore, all outcomes post‐embryo transfer were unrelated to the original randomisation and were prone to bias. The trial was not blinded. Twenty‐two couples dropped out of the study because they had less than eight oocytes available after hyperstimulation. The mean age of participants was younger, for both males and females, in the TESE group compared to the MESA group, again a potential source of bias. There was no information about a power calculation or intention‐to‐treat analysis.
Non‐randomised comparative studies
In the seven trials which used participants as their own controls, both procedures were performed one after the other in all participants. These trials were commented on in the Results and Discussion sections but were not included in the meta‐analysis as they did not meet the inclusion criteria and were prone to bias. When one procedure is performed after another, often in the same site, it is difficult to separately evaluate the efficacy of the two interventions.
None of these trials were randomised. One was single blind (Collins 1996), the participants were unaware of the procedure performed by the surgeon, the rest failed to mention blinding.
One of the trials reported that four of the 20 participants were excluded (Collins 1996), an exclusion rate of 20%. Amer 2000 excluded patients with non‐identical testicular histopathology to make sure there was no advantage for either technique. The other trials made no mention of exclusions or withdrawals.
One of the trials was in abstract form only (Segal 1995), the rest were published articles.
Effects of interventions
Because of the lack of RCTs all study results were described.
Epididymal versus epididymal techniques
There was one trial comparing epididymal sperm aspiration versus micropuncture with perivascular nerve stimulation (Yamamoto 1996). All outcomes but one (enough sperm retrieved for ICSI) suggested micropuncture was the more favourable technique. (a) Live birth per couple No data were available.
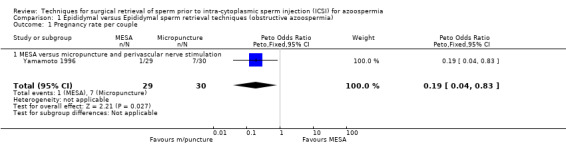
(b) Pregnancy rate per couple The MESA group had significantly lower pregnancy rates than the micropuncture with perivascular nerve stimulation group (OR 0.19, 95% CI 0.04 to 0.83). A pregnancy rate per couple of 3.4% was seen in the MESA group compared to 23.3% in the micropuncture group. The MESA group pregnancy rate was substantially lower than would have been expected in 1996 (Van Steirteghem 1998).
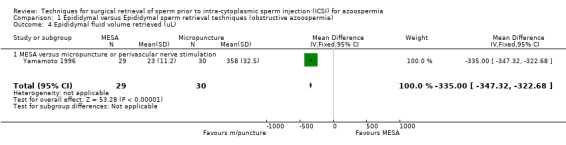
(c) Sperm parameters of tissue obtained from the surgical‐retrieval procedure With both techniques, enough sperm was retrieved for ICSI in all participants. The MESA group had a smaller volume of retrieved epididymal sperm (WMD ‐335.00, 95% CI ‐347.32 to ‐322.67), lower sperm density (x 106/mL) (WMD ‐12.70, 95% CI ‐14.78 to ‐10.61), and lower motility (WMD ‐21.10, 95% CI ‐24.78 to ‐17.42) than the micropuncture group. There was a non‐significant result for normal sperm morphology (WMD 1.00, 95% CI ‐0.46 to 2.46).Table 4
2. Results from comparative studies of sperm retrieval techniques.
| Study ID | Intervention | N | Sperm retrieved | Sperm Parameters |
| Collins 1996 | PESA MESA | 20 | Overall success: PESA ‐ 15/20 MESA ‐ 13/20 Bilateral success: PESA ‐ 8/20 MESA ‐ 5/20 | |
| Ezeh 1998 | Needle biopsy TESA Open TESE | 35 | Needle ‐ 22/35 Open ‐ 5/35 | |
| Friedler 1997 | TEFNA Open TESE | 37 | TEFNA ‐ 4/37 TESE ‐ 16/37 | |
| Roselund 1998 | 19G Needle biopsy TESA 21G Needle biopsy TESA Open TESE | 19G needle vs open ‐ 10 men 21G needle vs open ‐ 12 men | 19G needle ‐ 6/10 Open ‐ 8/10 21G needle ‐ 2/12 Open ‐ 8/10 | |
| Segal 1995 | TESA ‐ ACECUT Open TESE | 7 | Sperm was retrieved in all cases | |
| Sheynkin 1998 | TFNA, Percutaneous testicular needle biopsy, MESA | 9 | Sperm retrieved: TFNA 6/9, percutaneous testicular needle biopsy 9/9, MESA 9/9 | Sperm count/motility for TFNA and percutaneous testicular needle biopsy: poor. |
| Amer 2000 | Conventional testicular sperm extraction, Microsurgical testicular sperm extraction | 100 | Microsurgery: SSR=47%. Conventional=30% |
(d) Adverse effects associated with sperm‐retrieval technique The authors did not report any serious adverse effects. (e) Pregnancy rate per cycle No data were available.
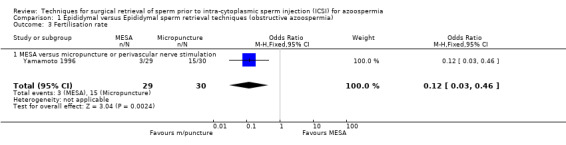
(f) Fertilisation rate The MESA group had a significantly lower fertilisation rate than the micropuncture group (OR 0.16, 95% CI 0.05 to 0.48).
(g) Implantation No data were available.
(h) Multiple pregnancy rate No data were available.
(i) Miscarriage rate No data were available.
(j) Fetal abnormalities No data were available.
Testicular versus testicular techniques
One RCT compared ultrasound‐guided testicular sperm aspiration (USTSA) with 'blind' testicular sperm aspiration (TSA).
(a) Live birth per couple No data were available.
(b) Pregnancy rate per couple There was no significant difference in pregnancy rates between the two groups: 3/16 (19%) in the USTSA group and 4/23 (17%) in the TSA group.
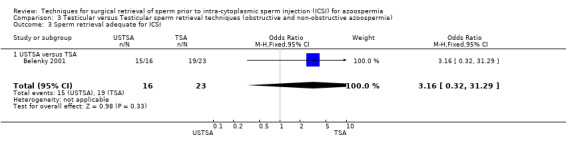
(c) Sperm parameters of tissue obtained from the surgical retrieval procedure Enough sperm was retrieved if at least 10 motile sperm were identified microscopically. In 15/16 patients (94%) in the USTSA group enough sperm was obtained compared to 19/23 (83%) in the TSA group. The results were not statistically significant.
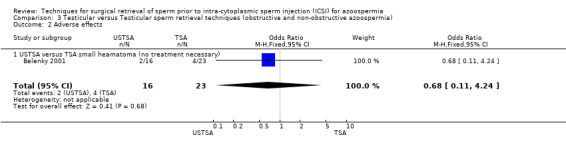
(d) Adverse effects associated with sperm‐retrieval technique No adverse effects needing medical intervention were reported. In the USTSA group 2/16 (13%) reported a small haematoma after one month compared with 4/23 (17%) in the TSA group. The result was not statistically significant.
(e) Pregnancy rate per cycle No data were available.
(f) Fertilisation rate No data were available.
(g) Implantation No data were available.
(h) Multiple pregnancy rate No data were available.
(i) Miscarriage rate No data were available.
(j) Fetal abnormalities No data were available.
Epididymal versus testicular techniques
One RCT compared the use of epididymal spermatozoa obtained with MESA with the use of testicular spermatozoa obtained with TESE (De Croo 2000). This RCT was excluded from the meta‐analysis due to poor randomisation however its results are reported here for extra information. The decision to transfer embryos fertilised with either testicular or epididymal sperm was based on the investigator's clinical judgement of the best embryos. Therefore, all outcomes post‐embryo transfer (for example implantation rate etc) were prone to bias. (a) Live birth per couple No data were available. (b) Pregnancy rate per couple There was no significant difference in pregnancy rates: MESA had a pregnancy rate per couple of 14.3% (4/28 couples) and TESE had a pregnancy rate per couple of 17.9 % (5/28 couples). (c) Sperm parameters of tissue obtained from the surgical retrieval procedure TESE had higher numbers of sperm retrieval adequate for ICSI: 50/50 couples versus 36/50 couples for MESE. The trial showed that 16% of the obtained epididymal sperm were motile compared to 14% of the testicular sperm; the mean concentration of epididymal spermatozoa was (106/ml) 13.7 ± 25.1; the mean number of testicular sperm was 1414 ± 1601, after extrapolation of the spermatozoa seen in one microscopic field (x 320).Table 4 (d) Adverse effects associated with sperm‐retrieval technique No data were available. (e) Pregnancy rate per cycle No data were available.
(f) Fertilisation rate There was no significant difference in fertilisation rates between the two groups: 173/208 (83%) fertilised oocytes with MESE versus 142/177 (80%) with TESE. (g) Implantation There was no significant difference in implantation rates: 5/14 implantations per embryo transferred occurred in the MESA group and 9/18 in the TESE group. (h) Multiple pregnancy rate There was no significant difference in multiple pregnancies: 0/4 pregnancies were multiples in the MESA group and 2/5 pregnancies in the TESE group. (i) Miscarriage rate There was no significant difference in miscarriage rates: 1/28 in the MESA group and 2/28 in the TESE group. (j) Fetal abnormalities No data were available.
Non‐randomised comparative trials
Although mentioned in this section of the review, the trials were not included in the meta‐analysis and served only as extra information in light of the lack of RCTs. In five trials comparing a minimally invasive technique with open‐window biopsy a small number of participants were used. Amer 2000 included 100 participants.
The main outcome for all these trials was sperm retrieval for ICSI. Four trials reported better results for sperm retrieval with the open biopsies. Friedler 1997 reported successful sperm retrieval in 4/37 participants for TEFNA and 16/37 for open biopsy. The Ezeh 1998 trial reported 5/35 for needle biopsies and 22/35 for open biopsy. The Rosenlund 1998 results showed 2/12 for the 21 gauge needle compared with 6/12 for open biopsy and 6/10 for the 19 gauge needle compared with 8/10 for open biopsy. Sheynkin 1998 reported on sperm count and motility per participant. All participants showed a better result for MESA.Table 4
The Amer 2000 trial compared microsurgical extraction with conventional (open) extraction and reported better results in the microsurgical group.
The Segal 1995 abstract did not report exact numbers on sperm retrieval but stated simply that ACECUT biopsy was an efficient device for obtaining a sufficient amount of tubules for the retrieval of spermatozoa.
Collins 1996 compared two types of PESA with two different types of MESA. The main outcome was the retrieval of sperm. The trial reported success in 15/20 participants for PESA and 13/20 participants for MESA. PESA while withdrawing the needle was successful in five more cases compared to PESA with the needle in situ.
Discussion
If sperm is present in the ejaculate, ICSI is clearly of benefit for male factor infertility (Van Rumste 2003). Unprecedented success has also been seen in previously hopeless cases of infertility because of azoospermia with surgically retrieved sperm for ICSI. It has been unclear which of the increasing number of methods for surgical retrieval of sperm might be superior. This review has drawn on the results of two RCTs examining surgical techniques for retrieval of sperm prior to ICSI. One RCT compared two techniques for the retrieval of epididymal sperm and the other compared techniques for harvesting testicular sperm. It is very difficult to draw conclusions about the efficacy of the retrieval techniques with only two trials.
The classification of azoospermia into obstructive and non‐obstructive appears to have become a relevant factor for a successful clinical outcome. In obstructive azoospermia, the prognosis for surgical retrieval of sperm and ICSI is generally good. In non‐obstructive azoospermia whether sperm can be surgically retrieved is by no means certain. Significantly lower rates of fertilisation and clinical pregnancy rates after ICSI have been found in men with non‐obstructive azoospermia compared to men with obstructive azoospermia (Mansour; Nicoupoullous 2004; Vernaeve 2003). One of the trials included in this review (Yamamoto 1996) included only participants with obstructive azoospermia, however the other included trial (Belenky 2001) failed to specify the etiology of the azoospermia. Therefore, this review is unable to comment on success rates in relation to classification of azoospermia. In future trials a clear differentiation in aetiology of azoospermia should be achieved. This might only be possible with histopathological evaluation of the testis.
The importance of assessing the appropriate outcome measures is illustrated by this review. Successful retrieval of sperm for ICSI (the outcome measure of many of the non‐randomised trials) is simply the first step towards the achievement of the more clinically relevant outcomes of fertilisation, embryo development, implantation, a clinical pregnancy, a live birth and finally, the most relevant outcome to every couple, a healthy baby without abnormality. The debate continues as to whether the non‐natural selection of a sperm for ICSI could subtly increase the likelihood of 'abnormal' offspring. This question is even more pressing for testicular surgically retrieved sperm. Although testicular retrieved sperm achieves comparable fertilization and implantation rates after ICSI compared to normal semen (Silber 1995b), it is still unclear if testicular sperm is as safe to use as epididymal or ejaculated sperm with respect to abnormality in the offspring. The evidence to date would suggest that the abnormality rate of offspring remains low in this context (Meuleman 1998). However, the limitations of using the successful retrieval of ICSI‐suitable sperm as the primary outcome is emphasised further by the Yamamoto 1996 trial. The results suggest that micropuncture with nerve stimulation is preferable compared to the MESA technique. Both procedures retrieved enough sperm for ICSI in all participants but significant differences were found for sperm parameters (except sperm morphology), pregnancy, and fertilisation rates.
The micropuncture technique was developed by the authors of the Yamamoto 1996 trial because contamination with blood cells was believed to adversely affect fertilisation rates. Unfortunately the trial does not report how many times excessive blood was found in the micropuncture samples or the number of repeated micro punctures that were performed, making it difficult to appraise success of the technique. Also, it is unclear if repeat procedures were performed in the MESA group. This is a possible source for bias. In essence the trial could be considered a randomised comparison of MESA versus a three‐intervention package consisting of micropuncture with nerve stimulation and repeated micro punctures until no blood contamination occurred. It is unclear as to which of these three interventions conferred the efficacy for the men in this group. Micropuncture with nerve stimulation is not a technique in widespread use. Additionally this technique carries many of the disadvantages of a formal MESA approach as it is invasive and requires general anaesthesia; it is a complex procedure requiring a high degree of operator skill. These features may explain why the micropuncture technique has not been widely used since this trial was published. Finally, the number of participants in each treatment group was small so no strong conclusions should be drawn about the efficacy or the safety of these techniques.
There may be differences in spermatogenesis between individuals with congenital bilateral absence of vas deferens (CBAVD) and individuals with failed vasovasostomy (Jarow 1985); some men with CBAVD may have a degree of abnormal spermatogenesis in addition to obstructive azoospermia. Although the Yamamoto 1996 trial was randomised, there is possible heterogeneity in the treatment group. However, since only four participants with CBAVD were included in this trial this is unlikely to have compromised the outcomes. It is also unclear how those four cases were distributed in the treatment groups.
The authors of Belenky 2001 compared sperm aspiration with or without ultrasound. It was hypothesised that using ultrasound during the aspiration procedure could prevent complications occurring, such as haematoma and lacerations in the testis. Allocation of a procedure using the final digits of the patient ID number is a randomisation technique that is prone to bias as there is little way of concealing potential treatment allocation. The small sample size of this trial (n = 39) meant that the small difference between treatments was not statistically significant. Therefore, no conclusion can be drawn about the usefulness of ultrasound without further trials. This trial also failed to report information on parameters such as age (both male and female), hormonal status, and duration of fertility. The De Croo 2000 trial, which was excluded from the meta‐analysis due to inadequate randomisation and the use of clinical judgement rather than randomisation in deciding embryo transfer, showed better overall fertilisation rates compared to the Yamamoto 1996 trial. It highlights an important problem with reviews of rapidly changing areas such as subfertility and further emphasises the importance of including only randomised trials in meta‐analyses. Techniques in subfertility treatment are constantly improving and being refined. Therefore, the use of historically‐controlled trials could introduce an even larger bias as the control group would represent not only a different intervention but also the efficacy of techniques earlier in time.
The De Croo 2000 trial had a small number of participants; only 28 couples (or 50 oocytes) were randomised. There was a high dropout rate (initially 50 couples had their oocytes randomised). The trial did not report on the various aetiologies of azoospermia in the participants. The only significant result showed TESE as more favourable to MESA for the retrieval of spermatozoa adequate for ICSI. Sperm parameters in the De Croo trial were not comparable because they were assessed after extrapolation from a microscopic field and also due to the lack of consistency in the way sperm parameters were reported. Should TESE then be the technique of first choice, given it is less invasive, may be performed under local anaesthesia, and is a simpler procedure than MESA? The trial reported a higher incidence of uni‐pronuclear (abnormal) fertilisation with testicular sperm obtained with the TESE procedure. This might support the theoretical concern that testicular sperm is less mature compared to epididymal sperm. Overall, no firm conclusions can be drawn from this small trial.
The non‐randomised comparative trials serve as extra information; owing to the lack of RCTs. Friedler 1997, Ezeh 1998, Rosenlund 1998, and Sheynkin 1998 all stated that open testicular biopsy appears to be more effective than less invasive needle biopsy for the retrieval of testicular spermatozoa in azoospermic men with defective spermatogenesis. One of the main reasons for this outcome seems to be histopathological. A general problem with the needle techniques is the difficulty in choosing the right site in the testis. There is evidence of focal areas of normal and abnormal tissue within the testis of participants with non‐obstructive azoospermia (Amer 1999b) so that different methods of biopsy require randomised evaluation. Better retrieval results are often reported with open biopsy simply because there is greater potential for more suitable tissue to be obtained. This problem with the needle techniques has been remedied to some extent since practitioners started using a microscope during retrieval procedures (Schlegel 1999). Thorough exploration with a microscope enables the surgeons to choose the largest tubules in the testis, which are associated with more spermatozoa. Evaluating this newer technique, Amer 2000 reports better retrieval results with microsurgical sperm extraction compared to conventional open testicular retrieval. The Collins 1996 trial performed MESA in a less invasive manner. It reported taking only one or two samples during the procedure compared to the usual procedure with multiple samples. Careful dissection of the testis and taking multiple samples has traditionally been considered to be a strength of the technique. A limitation of this trial is that it did not mention the length of time since the vasectomy was performed. Epididymal scarring and scarring of the vas deferens may compromise spermatogenesis of the testis and influence MESA recovery. The Segal 1995 abstract did not give enough information to draw any conclusions. The technique seems to be a variation of other automatic biopsy systems. Some critics believe that such a system is associated with increased morbidity should vascular injury occur.
The various aetiologies of obstructive and non‐obstructive azoospermia make it difficult to investigate this group of men as a whole. In fact any degree of hypospermatogenesis has the ability to compromise outcomes. Those conducting future research must be aware of this problem and clearly define the included population.
The poor reporting of data and the small number of included studies make it difficult to comment on any of the hypotheses outlined in the Objectives section of the review.
Authors' conclusions
Implications for practice.
There is insufficient data from randomised trials to recommend any particular surgical sperm‐retrieval techniques for either obstructive or non‐obstructive azoospermia. Non‐obstructive azoospermia is a difficult area to analyse as the physiology of the testis may be very different between individuals. Techniques are modified rapidly and there is much variation between different centres and surgeons. It is logical for the least invasive and simplest technique method for surgical retrieval of sperm to be used, which would be one of the needle retrieval techniques, usually under local anaesthetic, in the absence of evidence to support more invasive or more technically difficult methods. It seems that percutaneous aspiration techniques are now widely used for this reason. The more invasive methods should currently be reserved for situations where sperm cannot be retrieved by a less invasive technique (such as ultrasound‐guided needle aspiration of the epididymis or testis) or for evaluation in the context of a randomised trial.
Implications for research.
The onus remains with those in support of the more invasive techniques of surgical retrieval of sperm, which require greater surgical expertise, to demonstrate by performing suitably powered RCTs that these techniques can be justified. Such trials need to have a particular focus upon: 1) a clear definition of the population of men studied in terms of aetiology of azoospermia; 2) use of clinically relevant outcomes, not only clinical pregnancy and live birth rates but also the rate of birth of a normal healthy baby, and certainly not simply the success of retrieval of sperm suitable for ICSI; and 3) cost effectiveness (with inclusion of a cost‐benefit analysis). As the prevalence of azoospermia is low it remains unlikely that a single unit will attain numbers to confer sufficient power to such a trial. Large multi‐centre trials would increase the power and confer generalisability to the results.
What's new
| Date | Event | Description |
|---|---|---|
| 11 December 2008 | New search has been performed | Update complete |
| 6 November 2008 | Amended | Converted to new review format. |
| 11 December 2007 | Review declared as stable | This review is now stable |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 11 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
New conflict of interest declared.
The search was revised and re ran in November 2007 and no new trials were located therefore the results of the updated review as published in 2006 remain unch
Acknowledgements
M Dhont and I De Croo for supplying additional information. Special thanks are due to the marvellous support from the Cochrane office in Auckland; Anne Lethaby for all her advice, Review Group Coordinator Jane Clarke for her help with all the inevitable problems, Trials Search Coordinator Sue Furness for her assistance with identifying trials, and to Sue Hall, Secretary of the Review Group, for her secretarial help.
Data and analyses
Comparison 1. Epididymal versus Epididymal sperm retrieval techniques (obstructive azoospermia).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate per couple | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.04, 0.83] |
| 1.1 MESA versus micropuncture and perivascular nerve stimulation | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.04, 0.83] |
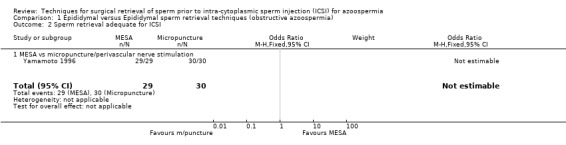
| 2 Sperm retrieval adequate for ICSI | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 MESA vs micropuncture/perivascular nerve stimulation | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Fertilisation rate | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.46] |
| 3.1 MESA versus micropuncture or perivascular nerve stimulation | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.46] |
| 4 Epididymal fluid volume retrieved (uL) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐335.0 [‐347.32, ‐322.68] |
| 4.1 MESA versus micropuncture or perivascular nerve stimulation | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐335.0 [‐347.32, ‐322.68] |
| 5 Sperm density (x 10 6/mL) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐12.70 [‐14.79, ‐10.61] |
| 5.1 MESA versus micropuncture or perivascular nerve stimulation | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐12.70 [‐14.79, ‐10.61] |
| 6 Sperm morphology (% of normal sperm) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.46, 2.46] |
| 6.1 MESA versus micropuncture or perivascular nerve stimulation | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.46, 2.46] |
| 7 Motility of sperm (%) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐21.10 [‐24.78, ‐17.42] |
| 7.1 MESA versus micropuncture or perivascular nerve stimulation | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐21.10 [‐24.78, ‐17.42] |
1.1. Analysis.
Comparison 1 Epididymal versus Epididymal sperm retrieval techniques (obstructive azoospermia), Outcome 1 Pregnancy rate per couple.
1.2. Analysis.
Comparison 1 Epididymal versus Epididymal sperm retrieval techniques (obstructive azoospermia), Outcome 2 Sperm retrieval adequate for ICSI.
1.3. Analysis.
Comparison 1 Epididymal versus Epididymal sperm retrieval techniques (obstructive azoospermia), Outcome 3 Fertilisation rate.
1.4. Analysis.
Comparison 1 Epididymal versus Epididymal sperm retrieval techniques (obstructive azoospermia), Outcome 4 Epididymal fluid volume retrieved (uL).
1.5. Analysis.
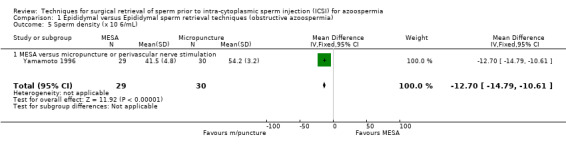
Comparison 1 Epididymal versus Epididymal sperm retrieval techniques (obstructive azoospermia), Outcome 5 Sperm density (x 10 6/mL).
1.6. Analysis.
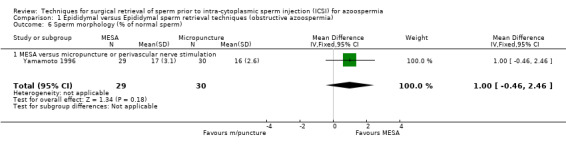
Comparison 1 Epididymal versus Epididymal sperm retrieval techniques (obstructive azoospermia), Outcome 6 Sperm morphology (% of normal sperm).
1.7. Analysis.
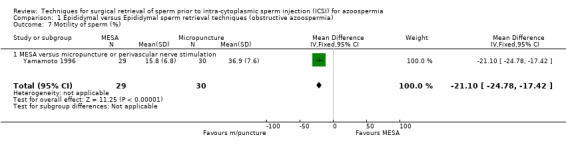
Comparison 1 Epididymal versus Epididymal sperm retrieval techniques (obstructive azoospermia), Outcome 7 Motility of sperm (%).
Comparison 3. Testicular versus Testicular sperm retrieval techniques (obstructive and non‐obstructive azoospermia).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate per couple | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.21, 5.74] |
| 1.1 Percutaneous testicular aspirtion with ultrasound guidance versus blind percutaneous testicular aspiration | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.21, 5.74] |
| 2 Adverse effects | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.11, 4.24] |
| 2.1 USTSA versus TSA small heamatoma (no treatment necessary) | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.11, 4.24] |
| 3 Sperm retrieval adequate for ICSI | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.32, 31.29] |
| 3.1 USTSA versus TSA | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.32, 31.29] |
3.1. Analysis.
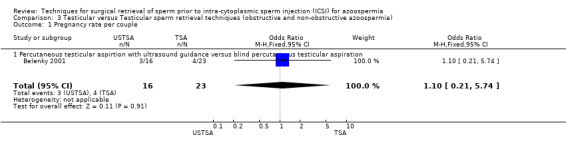
Comparison 3 Testicular versus Testicular sperm retrieval techniques (obstructive and non‐obstructive azoospermia), Outcome 1 Pregnancy rate per couple.
3.2. Analysis.
Comparison 3 Testicular versus Testicular sperm retrieval techniques (obstructive and non‐obstructive azoospermia), Outcome 2 Adverse effects.
3.3. Analysis.
Comparison 3 Testicular versus Testicular sperm retrieval techniques (obstructive and non‐obstructive azoospermia), Outcome 3 Sperm retrieval adequate for ICSI.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belenky 2001.
| Methods | Randomisation by final digit of patient ID number Blinding: unclear 16 ultrasound guided percutaneous testicular sperm aspirations, 23 percutaneous testicular aspirations without ultrasound guidance | |
| Participants | Inclusion: azoospermic men Exclusion not specified Age: 27‐41 (mean 33) years Location: Tel Aviv, Israel | |
| Interventions | Group (1) Percutaneous testicular aspiration with ultrasound guidance (USTSA) Group 2) Percutaneous testicular aspiration without ultrasound guidance (TSA) | |
| Outcomes | Pregnancy rates Percentage of complications Sufficient material (for ICSI) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Yamamoto 1996.
| Methods | Random, method unclear (couples randomised) Blinding unclear Parallel trial Group 1: 29 couples Group 2: 30 couples No withdrawals or dropouts | |
| Participants | Inclusion: Men with congenital bilateral absence of the vas deferens (CBAVD) or unreconstructable vasal obstruction (failed vasovasostomy, failed epididymovasostomy) Exclusion: not specified Age: Group 1 men 34±2.3, women 28±2.1; Group 2 men 36±1.8, women 31±2.5 Location: Nagoya, Japan | |
| Interventions | Group (1) Microsurgical epididymal sperm aspiration (MESA) Group 2) Epididymal micropuncture with perivascular nerve stimulation followed by aspiration of fluid Duration: 1 cycle | |
| Outcomes | Fertilisation rate per couple Pregnancy rate per couple (ultrasound) Sperm parameters (density, motility, morphology) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amer 1999b | It remains unclear if this was a comparative trial. No randomisation or blinding was mentioned. The numbers of participants in both group suggest failure to randomise. Contact with the author was sought, but unfortunately no reply followed. |
| Atassi 2000 | Randomisation or blinding was not mentioned in the trial. Further information was sought from the authors in order to determine trial design however no response was received. |
| De Croo 2000 | The study randomised oocytes not women |
Contributions of authors
Arno‐Maarten van Peperstraten: took the lead in writing the review and the updates, he was involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included and non randomised trials, and was responsible for statistical analysis and interpretation of data.
Michelle Proctor: took the lead in writing the protocol, performed initial searches, involved in selecting trials for inclusion, performed independent data extraction and quality assessment from the included and non‐randomised trials.
Greg Philipson: involved in selecting trials for inclusion, commented on drafts of protocol and review, and added clinical expertise to the discussion.
Neil Johnson: initiated and conceptualised the review, commented on drafts of the protocol, review, updates and added clinical expertise to the discussion.
Sources of support
Internal sources
University of Auckland, School of Medicine, Auckland, New Zealand.
External sources
Princess of Wales Memorial Trust Fund administered by the Mercia Barnes Fund, New Zealand.
Declarations of interest
NJ works as a gynaecologist at Auckland City Hospital (a public hospital) in the National Women's Minimal Access Surgery and Endometriosis Service. NJ is also a private gynaecologist with groups called Endometriosis Auckland and IVF Auckland. Within the last three years NJ has received financial support to attend conferences or to arrange research meetings from the following companies: Organon, Serono, Schering and Device Technologies. NJ is an author of the Auckland LUNA Trial and of the Cochrane systematic review on neuroablation and LUNA.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Belenky 2001 {published data only}
- Belenky A, Avrech O, Nissim Bachar G, Zuckerman Z, Ben Rafael Z, Fisch B, et al. Ultrasound‐guided testicular sperm aspiration in azoospermic patients: A new sperm retrieval method in intracytoplasmic sperm injection. Journal of Clinical Ultrasound 2001;29(6):339‐43. [DOI] [PubMed] [Google Scholar]
Yamamoto 1996 {published data only}
- Yamamoto M, Hibi H, Miyake K, Asada Y, Suganuma N, Tomoda Y. Microsurgical epididymal sperm aspiration versus epididymal micropuncture with perivascular nerve stimulation for intracytoplasmic sperm injection to treat unreconstructable obstructive azoospermia. Archives of Andrology 1996;36:217‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amer 1999b {published data only}
- Amer M, Haggar S, Moustafa T, Abd El‐Naser T, Elhelw B. Testicular sperm extraction through multiple testicular biopsies versus single biopsy in functional azoospermia and the predictive value of May‐Grunwald‐Giemsa stain for semen. Abstract of the 15th Annual Meeting of the ESHRE. 1999:148.
Atassi 2000 {published data only}
- Atassi M, Takkla M, Sambasivarao K, Yassa D, Idriss W. Comparative study of testicular sperm retrieval between the open biopsy method and the needle aspiration technique in azoospermic patients. Middle East Fertility Society Journal 2000;5(2):147‐53. [Google Scholar]
De Croo 2000 {published and unpublished data}
- Croo I, Elst J, Everaert K, Sutter P, Dhont M. The use of epididymal versus testicular spermatozoa for ICSI on sibling oocytes: a prospective, randomized study in azoospermic patients with normal spermatogenesis. Human Reproduction. Abstracts of the 16th Annual Meeting of ESHRE, Bologna, Italy. June, 2000:98.
Additional references
Amer 2000
- Amer A, Ateyah A, Hany R, Zohdy W. Prospective comparative study between microsurgical and conventional testicular sperm extraction in non‐obstructive azoospermia: follow‐up by serial ultrasound examinations. Human Reproduction 2000;15(3):653‐6. [DOI] [PubMed] [Google Scholar]
Belker 1991
- Belker AM, Thomas AJ, Fuchs EF, Konnak JW, Sharlip ID. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. Journal of Urology 1991;145(3):505‐11. [DOI] [PubMed] [Google Scholar]
Collins 1996
- Collins G, Critchlow J, Lau M, Payne S. Open versus closed epididymal sperm retrieval in men with secondarily obstructed vasal systems ‐ a preliminary report. British Journal of Urology 1996;78:437‐9. [DOI] [PubMed] [Google Scholar]
Ezeh 1998
- Ezeh U, Moore H, Cooke I. A prospective study of multiple needle bopsies versus a single open biopsy for testicular sperm extraction in men with non‐obstructive azoospermia. Human Reproduction 1998;13(11):3075‐80. [DOI] [PubMed] [Google Scholar]
Friedler 1997
- Friedler S, Raziel A, Strassburger D, Soffer Y, Komarovsky D, Ron‐El R. Testicular sperm retrieval by percutaneous fine needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non‐obstructive azoospermia. Human Reproduction 1997;12(7):1488‐93. [DOI] [PubMed] [Google Scholar]
Irvine 1998
- Irvine DS. Epidemiology and aetiology of male infertility. Human Reproduction 1998;13(Suppl 1):33‐44. [DOI] [PubMed] [Google Scholar]
Jansen 1997
- Jansen R. Overcoming infertility. New York: WH Freeman, 1997. [Google Scholar]
Jaroudi 1999a
- Jaroudi K, Coskun S, Hollanders J, Al‐Hassan S, Al‐Sufayan H, Atared A, et al. Advanced surgical sperm recovery is a viable option for intracytoplasmic sperm injection in patients with obstructive or nonobstructive azoospermia. Fertility and Sterility 1999;72(3):479‐83. [DOI] [PubMed] [Google Scholar]
Jarow 1985
- Jarow J P, Budin R E, Dym M, Zirkin BR, Noren S, Marshall FF. Quantitative pathologic changes in the human testis after vasectomy. A controlled study. New England Journal of Medicine 1985;313:1252‐6. [DOI] [PubMed] [Google Scholar]
Jarow 1997
- Jarow JP, Oates RD, Buch JP, Shaban SF, Sigman M. Effect of level of anastomosis and quality of intraepididymal sperm on the outcome of end‐to‐side epididymovasostomy. Urology 1997;49(4):590‐5. [DOI] [PubMed] [Google Scholar]
Lewin 1999
- Lewin A, Reubinoff B, Porat‐Katz A, Weiss D, Eisenberg V, Arbel R, et al. Testicular fine needle aspiration: the alternative method for sperm retrieval in non‐obstructive azoospermia. Human Reproduction 1999;14(7):1785‐90. [DOI] [PubMed] [Google Scholar]
Mansour
- Mansour R. Intracytoplasmic sperm injection in obstructive and non‐obstructive azoospermia. Human Reproduction 12;9:1974‐9. [DOI] [PubMed] [Google Scholar]
Meuleman 1998
- Meuleman E, Moorselaar R. Ovum fertilisation using surgically obtained sperm: additional risk to offspring unlikely with meticulous procedure [Eicelbevruchting met chirurgisch verkregen zaad: extra risico's voor het nageslacht onwaarschijnlijk bij zorgvuldige toepassing]. Nederlands Tijdschrift voor Geneeskunde 1998;142(3):108‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Nicoupoullous 2004
- Nicopoullos JD, Gilling‐Smith C, Almeida PA, Norman‐Taylor J, Grace I, Ramsay JW. Use of surgical sperm retrieval in azoospermic men: a meta‐analysis. Fertility and Sterility 2004;82(3):691‐701. [DOI] [PubMed] [Google Scholar]
Palermo 1992
- Palermo G, Joris H, Devroey P. Pregnancies after intracytoplasmic sperm injection of single spermatozoon into an oocyte. Lancet 1992;340:17‐8. [DOI] [PubMed] [Google Scholar]
Rosenlund 1998
- Rosenlund B, Kvist U, Plöen L, Lundh Rozell B, Sjöblom P, Hillensjö T. A comparison between open and percutaneous needle biopsies in men with azoospermia. Human Reproduction 1998;13(5):1266‐71. [DOI] [PubMed] [Google Scholar]
Schlegel 1997
- Schlegel P, Su L. Physiological consequences of testicular sperm extraction. Human Reproduction 1997;12(8):1688‐92. [DOI] [PubMed] [Google Scholar]
Schlegel 1999
- Schlegel P. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Human Reproduction 1999;14(1):131‐5. [DOI] [PubMed] [Google Scholar]
Segal 1995
- Segal S, Zohav E, Katz N, Kinchin B, Popescu M, Gemer O, et al. The use of an automatic biopsy system for the retrieval of testicular spermatozoa for intracytoplasmic sperm injection. Human Reproduction. Abstracts of the 11th Annual meeting of ESHRE, Book 2 1995;10:101. [Google Scholar]
Sharif 2000
- Sharif K. Reclassification of azoospermia: the time has come?. Human Reproduction 2000;15(2):237‐8. [DOI] [PubMed] [Google Scholar]
Sheynkin 1998
- Sheynkin Y, Zhen Y, Menendez S, Liotta D, Veeck L, Schlegel P. Controlled comparison of percutaneous and microsurgical sperm retrieval in men with obstructive azoospermia. Human Reproduction 1998;13(11):3086‐9. [DOI] [PubMed] [Google Scholar]
Silber 1994
- Silber SJ, Nagy ZP, Liu J. Conventional in‐vitro fertilization vs ICSI for patients requiring microsurgical sperm aspiration. Human Reproduction 1994;9:1705‐9. [DOI] [PubMed] [Google Scholar]
Silber 1995a
- Silber SJ, Nagy Z, Liu J, Tournaye H, Lissens W, Ferec C, et al. The use of epididymal and testicular spermatozoa for intracytoplasmic sperm injection: the genetic implications for male infertility. Human Reproduction 1995;10(8):2031‐43. [DOI] [PubMed] [Google Scholar]
Silber 1995b
- Silber S, Steirteghem A, Liu J, Nagy Z, Tournaye H, Devroey P. High fertilization and pregnancy rate after intracytoplasmic sperm injection with spermatozoa from testicle biopsy. Human Reproduction 1995;10:148‐52. [DOI] [PubMed] [Google Scholar]
Van Rumste 2003
- Rumste MME, Evers JLH, Farquhar CM. Intra‐cytoplasmic sperm injection versus conventional techniques for oocyte insemination during in vitro fertilisation in patients with non‐male subfertility. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Van Steirteghem 1998
- Steirteghem A, Nagy P, Joris H, Janssenswillen C, Staessen C, Verheyen G, et al. Results of intracytoplasmic sperm injection with ejaculated, fresh and frozen‐thawed epididymal and testicular spermatozoa. Human Reproduction 1998;13:134‐42. [DOI] [PubMed] [Google Scholar]
Vernaeve 2003
- Vernaeve V, Tournaye H, Osmanagaoglu K, Verheyen G, Steirteghem A, Devroey P. Intracytoplastic sperm injection with testicular spermatozoa is less successful in men with nonobstructive azoospermia than in men with obstructive azoospermia. Fertility and Sterility 2003;79(3):529‐33. [DOI] [PubMed] [Google Scholar]


