Aging has a pronounced impact on the brain, changing its macroscopic structure as well as its cellular and molecular constituents. Aging also affects many other aspects of health, including co-morbidities such as chronic pain. Given that chronic pain is associated with brain alterations [2,3,6,8,9,14,16,20–22,24,25,27,31,36,38,39,41–43,45–47,50–52], the interplay of chronic pain, brain structure and aging is of considerable interest to scientists, clinicians and patients alike. In this issue of PAIN, Sörös and Bantel [44] tested whether chronic pain is associated with an older-appearing brain in chronic non-cancer pain patients (n=59) compared to healthy control subjects (n=60). The authors implemented a biomarker of brain aging, so-called ‘brain age’. Brain age condenses brain-wide information from structural neuroimaging into a single number, using a supervised machine learning model of healthy aging, defined in an independent training dataset [12]. A key advantage of the brain-age paradigm is that by using machine learning, the model can learn from a wide range of different brain structures involved in healthy aging. Having an older-appearing brain has been previously associated with neurological and psychiatric diseases [10,11], cognitive performance and poorer health outcomes [12,23,49]. Our own work in community-dwelling older adults found that chronic pain was associated with poorer brain aging cross-sectionally [13]. In contrast, this latest study by Sörös and Bantel did not find any significant cross-sectional differences in brain aging between pain patients and matched-controls. At face value, these results appear contradictory, however, we can offer some alternative explanations that may serve as potential future avenues of inquiry.
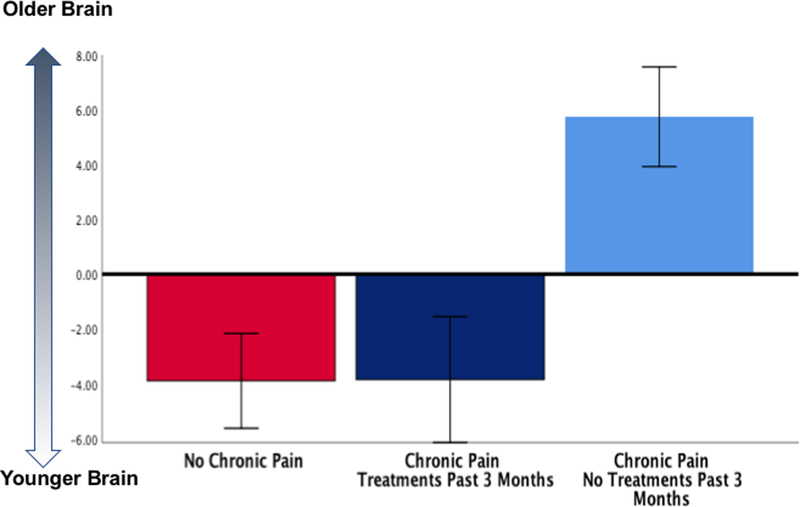
First, Sörös and Bantel included only patients seeking treatment for pain, while we included older adults from the community. Further, we asked our generally healthy older-adult participants “if they had received any treatments or tried any self-remedies (i.e., something they may have done at home) to relieve their worst pain during the past 3 months”. In those individuals with chronic pain (n=33), approximately half (n=19) reported having received pain treatments/ remedies during the past 3 months. Crucially, these individuals had significantly “younger” brains compared to those who did not report seeking pain treatments/ remedies (n=14)[13]. Older individuals not seeking treatments/ remedies during the past 3 months had brain ages that were significantly older (5.75±1.8 years) than those individuals reporting seeking treatments/remedies (−3.85±2.2 years, Bonferroni-corrected p=0.007) and no-pain controls (−3.90±1.7 years, Bonferroni-corrected p=0.001, Figure 1). In other words, our results are consistent with Sörös and Bantel’s null findings, where all individuals with chronic non-cancer pain were recruited from specialized pain clinics seeking and receiving treatment for their pain. Further examination of our data shows that most individuals who experienced chronic pain and reported using treatments/ remedies in the past 3 months (18/19, ~94%) reported receiving complementary and/or performing self-care strategies to treat their pain (Table 1). These converging findings pose a key question: what may explain the lack of a brain-aging effect in persons reporting seeking treatments, when our other chronic pain participants do show older-appearing brains?
Figure 1.
Brain-PAD in pain participants who reported having any treatments or trying any self-remedies (i.e., something they may have done at home) to relieve their worst pain during the past 3 months (n=19) compared with those who did not (n=14) and no chronic pain controls (n=14).
Table 1.
Qualitative data as reported by our subset of older adults reporting treatments/self-remedies in the past 3 months (n=19).
| Self-reported Pain Treatments/Remedies in the Past 3 Months (n=19) |
|---|
| Pain cream (n=1) |
| Weight lifting (n=1) |
| Exercise (n=1) |
| Exercise in water (n=1) |
| Heat (n=1) |
| Heating pad (n=3) |
| Icing and exercise (n=1) |
| Icing (n=1) |
| Exercise and knee massage (n=1) |
| Medication, icing and acupuncture (n=1) |
| Medication (n=1) |
| Salt bath and massaging (n=1) |
| Sitting and laying down (n=1) |
| Stretching and knee exercises (n=1) |
| Stretching and yoga (n=1) |
| Turmeric, ginger tea and diet change (n=1) |
| Walking, heating pad and TENS (n=1) |
Potentially, the brain-age biomarker used by us [13] and by Sörös and Bantel, could be partially driven by volumetric changes in brain circuits related to complex behaviors like treatment seeking; a circuitry that can also be impacted by aging. For example, the mesolimbic reward network underlies reward-seeking and hedonic responses to positive stimuli, and has been implicated in pain chronification [5,27,32,48]. Indeed, a protective role of medication use against pain chronification has been shown in patients with low back pain when treatment was started early with findings suggesting that treatment outcome was contingent on medial prefrontal (mPFC)–nucleus accumbens (NAc) functional connectivity [5,48]. However, while NAc age-related changes are not well-understood; aging is generally associated with volumetric decreases in mPFC regions [18,19,26,40]. Other important players in the reward circuitry include the hippocampus and amygdala. Emerging evidence in animals and humans suggests decreases in hippocampal volume in chronic pain states [7,15,21,32,33,37,54] as well as in normal and pathological aging processes [4,53] where stressors such as debilitating chronic pain may play a significant role [29,30,53]. Similar although subtle age-related changes also occur in the amygdala [1,28] and emerging evidence implicates the amygdala in pain modulation [17,34,35]. Thus, the brain aging biomarker may be picking up abnormal brain aging signatures associated with treatment-seeking behaviors.
Alternatively, it is also possible that the association between brain age and seeking treatment per se may in fact be due to those patients successfully obtaining actual pain relief. This is consistent with our previous findings of a correlation between brain age and self-reported pain relief levels. As Sörös and Bantel did not have information about recent use of non-pharmacological treatments, nor degree of pain relief, this possibility still requires further replication. Further, other factors known to have a positive influence on brain aging like exercise and practicing meditation[10] will also need to be considered in future work and unfortunately were not available in the patients studied by Sörös and Bantel.
More work is needed to understand how the brain-age paradigm can be beneficial for the understanding of chronic pain and its treatments. These findings may suggest that the brain aging biomarker is reflective of: a) reward neural circuitry; b) pain relief itself; c) pain relief specific to non-pharmacological treatments/self-remedies; or d) other non-specific, non-pain related brain circuitry, including findings due to random chance. Larger samples and wider range of study settings are needed to more rigorously test these different hypotheses. Nevertheless, we anticipate brain-age to play a role in the early identification of those at greater risk of functional decline and the evaluation of whether these trajectories can be improved by chronic pain treatment and relief.
Acknowledgements:
We are grateful to our volunteers for their participation and the NEPAL study team (Paige Lysne, Lorraine Hoyos, Darlin Ramirez, Brandon Apagueno and Rachna Sannegowda). This work was supported by the National Institutes of Health (NIA K01AG048259), the Center for Cognitive Aging & Memory, McKnight Brain Foundation, the University of Florida Claude D. Pepper Older Americans Independence Center (P30AG028740). James Cole was supported by the UK Research and Innovation Fellowship (reference # MR/R024790/1). All authors report no conflict of interest.
References
- [1].Aghamohammadi-Sereshki A, Hrybouski S, Travis S, Huang Y, Olsen F, Carter R, Camicioli R, Malykhin N V. Amygdala subnuclei and healthy cognitive aging. Hum Brain Mapp 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol 2009;87:81–97. doi: 10.1016/J.PNEUROBIO.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic Back Pain Is Associated with Decreased Prefrontal and Thalamic Gray Matter Density. J Neurosci 2004;24. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou YY, Toga AW, Thompson PM. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer disease. Alzheimer Dis Assoc Disord 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berger SE, Vachon-Presseau É, Abdullah TB, Baria AT, Schnitzer TJ, Apkarian AV. Hippocampal morphology mediates biased memories of chronic pain. Neuroimage 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. NeuroImage Clin 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen JYW, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res 2011. [DOI] [PubMed] [Google Scholar]
- [10].Cole JH, Franke K. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci 2017;40:681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- [11].Cole JH, Marioni RE, Harris SE, Deary IJ. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry 2018:1–16. doi: 10.1038/s41380-018-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cole JH, Ritchie SJ, Bastin ME, Valdés Hernández MC, Muñoz Maniega S, Royle N, Corley J, Pattie A, Harris SE, Zhang Q, Wray NR, Redmond P, Marioni RE, Starr JM, Cox SR, Wardlaw JM, Sharp DJ, Deary IJ. Brain age predicts mortality. Mol Psychiatry 2018;23:1385–1392. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cruz-Almeida Y, Fillingim RB, Riley JL, Woods AJ, Porges E, Cohen R, Cole JH, Riley III J, Adam W, Porges E, Cohen R, Cole JH. Chronic Pain is Associated with a Brain Aging Biomarker in Community-Dwelling Older Adults. Pain 2019;160:1119–1130. doi: 10.1097/j.pain.0000000000001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ellingson BM, Mayer E, Harris RJ, Ashe-Mcnally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ezzati A, Zimmerman ME, Katz MJ, Sundermann EE, Smith JL, Lipton ML, Lipton RB. Hippocampal subfields differentially correlate with chronic pain in older adults. Brain Res 2014;1573:54–62. doi: 10.1016/j.brainres.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Farmer MA, Huang L, Martucci K, Yang CC, Maravilla KR, Harris RE, Clauw DJ, MacKey S, Ellingson BM, Mayer EA, Schaeffer AJ, Apkarian AV. Brain white matter abnormalities in female interstitial cystitis/bladder pain syndrome: A MAPP network neuroimaging study. J Urol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fernando ABP, Murray JE, Milton AL. The amygdala: Securing pleasure and avoiding pain. Front Behav Neurosci 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High Consistency of Regional Cortical Thinning in Aging across Multiple Samples. Cereb Cortex 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gerstner G, Ichesco E, Quintero A, Schmidt-Wilcke T. Changes in regional gray and white matter volume in patients with myofascial-type temporomandibular disorders: a voxel-based morphometry study. J Orofac Pain 2011. [PubMed] [Google Scholar]
- [21].Khan SA, Keaser ML, Meiller TF, Seminowicz DA. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 2014. [DOI] [PubMed] [Google Scholar]
- [22].Lieberman G, Shpaner M, Watts R, Andrews T, Filippi CG, Davis M, Naylor MR. White matter involvement in chronic musculoskeletal pain. 2014;15:1110–1119. doi: 10.1016/j.jpain.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, Lampe L, Rahim M, Abraham A, Craddock RC, Riedel-Heller S, Luck T, Loeffler M, Schroeter ML, Witte AV, Villringer A, Margulies DS. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage 2017. [DOI] [PubMed] [Google Scholar]
- [24].Luchtmann M, Steinecke Y, Baecke S, Lützkendorf R, Bernarding J, Kohl J, Jöllenbeck B, Tempelmann C, Ragert P, Firsching R. Structural brain alterations in patients with lumbar disc herniation: A preliminary study. PLoS One 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maeda Y, Kettner N, Sheehan J, Kim J, Cina S, Malatesta C, Gerber J, McManus C, Mezzacappa P, Morse LR, Audette J, Napadow V. Altered brain morphometry in carpal tunnel syndrome is associated with median nerve pathology. NeuroImage Clin 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci 2013;16:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Vania Apkarian A. Brain white matter structural properties predict transition to chronic pain. Pain 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mather M. The Affective Neuroscience of Aging. Annu Rev Psychol 2016;67:213–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McEwen BS. Stress and the aging hippocampus. Front Neuroendocrinol 1999. [DOI] [PubMed] [Google Scholar]
- [30].Miller DB, O’Callaghan JP. Aging, stress and the hippocampus. Ageing Res Rev 2005. [DOI] [PubMed] [Google Scholar]
- [31].Moayedi M, Weissman-Fogel I, Salomons T V, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Abnormal gray matter aging in chronic pain patients. Brain Res 2012;1456:82–93. Available: http://linkinghub.elsevier.com/retrieve/pii/S0006899312005513. Accessed 20 Nov 2018. [DOI] [PubMed] [Google Scholar]
- [32].Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol 2014;111:1065–1076. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno M V, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci 2012;32:5747–56. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 2004;10:221–234. [DOI] [PubMed] [Google Scholar]
- [36].Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 2013. [DOI] [PubMed] [Google Scholar]
- [37].Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Riederer F, Marti M, Luechinger R, Lanzenberger R, Von Meyenburg J, Gantenbein AR, Pirrotta R, Gaul C, Kollias S, Sándor PS. Grey matter changes associated with medication-overuse headache: Correlations with disease related disability and anxiety. World J Biol Psychiatry 2012. [DOI] [PubMed] [Google Scholar]
- [39].Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain Gray Matter Decrease in Chronic Pain Is the Consequence and Not the Cause of Pain. J Neurosci 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- [41].Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology 2005. [DOI] [PubMed] [Google Scholar]
- [42].Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional Gray Matter Density Changes in Brains of Patients With Irritable Bowel Syndrome. Gastroenterology 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci 2011;31:7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sörös P, Bantel C. Chronic non-cancer pain is not associated with accelerated brain aging as assessed by structural MRI in patients treated in specialized outpatient clinics. Pain 2019. [DOI] [PubMed] [Google Scholar]
- [45].Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu Y Te, Yeh TC, Lirng JF, Hsieh JC. Brain morphological changes associated with cyclic menstrual pain. Pain 2010. [DOI] [PubMed] [Google Scholar]
- [46].Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cereb Cortex 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Unrath A, Juengling FD, Schork M, Kassubek J. Cortical grey matter alterations in idiopathic restless legs syndrome: An optimized voxel-based morphometry study. Mov Disord 2007. [DOI] [PubMed] [Google Scholar]
- [48].Vania Apkarian A, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang J, Knol MJ, Tiulpin A, Dubost F, de Bruijne M, Vernooij MW, Adams HHH, Ikram MA, Niessen WJ, Roshchupkin G V. Gray Matter Age Prediction as a Biomarker for Risk of Dementia. Proc Natl Acad Sci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wood PB, Glabus MF, Simpson R, Patterson JC. Changes in Gray Matter Density in Fibromyalgia: Correlation With Dopamine Metabolism. J Pain 2009. [DOI] [PubMed] [Google Scholar]
- [51].Woodworth D, Mayer E, Leu K, Ashe-McNalley C, Naliboff BD, Labus JS, Tillisch K, Kutch JJ, Farmer MA, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Landis JR, Deutsch G, Harris RE, Clauw DJ, Mullins C, Ellingson BM. Unique microstructural changes in the brain associated with urological chronic pelvic pain syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: A MAPP network neuroimaging study. PLoS One 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yang FC, Chou KH, Fuh JL, Huang CC, Lirng JF, Lin YY, Lin CP, Wang SJ. Altered gray matter volume in the frontal pain modulation network in patients with cluster headache. Pain 2013. [DOI] [PubMed] [Google Scholar]
- [53].Zannas AS, McQuoid DR, Payne ME, Steffens DC, MacFall JR, Ashley-Koch A, Taylor WD. Negative life stress and longitudinal hippocampal volume changes in older adults with and without depression. J Psychiatr Res 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: A pilot study. Neurology 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]