Abstract
Background:
Exercise training (ET) with blood flow restriction (BFR) is becoming increasingly popular, but the majority of BFR ET studies have evaluated skeletal muscle strength and hypertrophy. The favorable effect of BFR ET on skeletal muscle and the vasculature appears to improve aerobic capacity (AC) although conflicting results have been observed.
Purpose: The purposes of this systematic review with meta- analysis were to examine the effects of aerobic ET with and without BFR on AC and to compare the effect of low-to-moderate aerobic ET with and without BFR to high-intensity aerobic ET with and without BFR on AC.
Study Design:
Systematic Review with Meta-analysis.
Methods:
A comprehensive search for studies examining the effects of aerobic ET with and without BFR on AC was performed. Inclusion criteria were: (a) the study was conducted in healthy individuals, (b) there was random allocation of study participants to training and control groups, (c) BFR was the sole intervention difference between the groups.
Results:
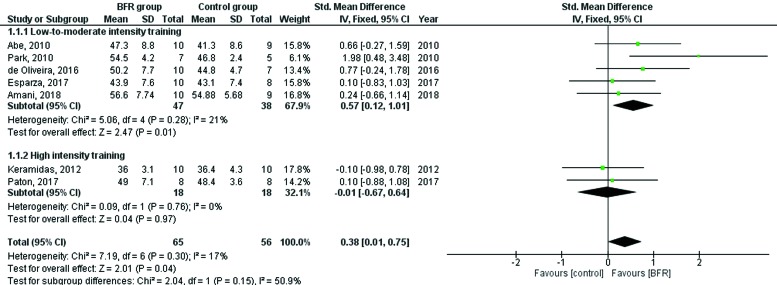
A total of seven studies (5 low-to-moderate ET and 2 high-intensity ET) were included in the meta-analysis providing data from 121 subjects. There was a significant standardized mean difference (SMD) of 0.38 (95% CI = 0.01, 0.75) in AC between the BFR and non-BFR groups of all seven studies (z = 2.01; p = 0.04). Separate analyses of the five low-to-moderate aerobic ET studies found similar results with aerobic ET with BFR eliciting a significantly greater AC (z = 2.47; p=0.01) than aerobic ET without BFR (SMD of 0.57; 95% CI = 0.12, 1.01). Separate analyses of the two high-intensity aerobic ET studies with and without BFR found no significant difference in AC between the groups (SMD of - 0.01; 95% CI = - 0.67, 0.64).
Conclusion:
Aerobic ET with BFR elicits a significantly greater AC than aerobic ET without BFR in healthy young adults. However, low-to-moderate intensity aerobic ET with BFR elicited a greater improvement in AC than aerobic ET without BFR while high-intensity aerobic ET with BFR did not elicit an improvement in AC over high-intensity aerobic ET without BFR.
Level of Evidence:
1a
Keywords: aerobic capacity, blood flow restriction, maximal oxygen consumption, meta-analysis, oxygen uptake, vascular occlusion training, VO2max.
INTRODUCTION
Exercise training (ET) with blood flow restriction (BFR) is becoming increasingly popular in rehabilitation, allowing skeletal muscle strengthening and hypertrophy to be accomplished using lower workloads, fewer repetitions, and shorter durations.1 These benefits have been seen across a variety of musculoskeletal conditions and age ranges. Furthermore, research on different cuff width, size, and pressure distribution has led to the development and implementation of more sophisticated cuffs for a safer and more precise reduction in blood flow to the exercising limb.2 While the majority of BFR ET studies have evaluated these effects on skeletal muscle strength and hypertrophy, the effects of BFR ET on aerobic capacity (AC) have also been studied, albeit on a smaller scale and with conflicting conclusions. Just as BFR with low-load resistance ET elicits a localized metabolic response similar to high-load resistance ET without BFR, it is reasonable to question whether BFR with aerobic ET may have superior cardiovascular effects compared to aerobic ET without BFR.
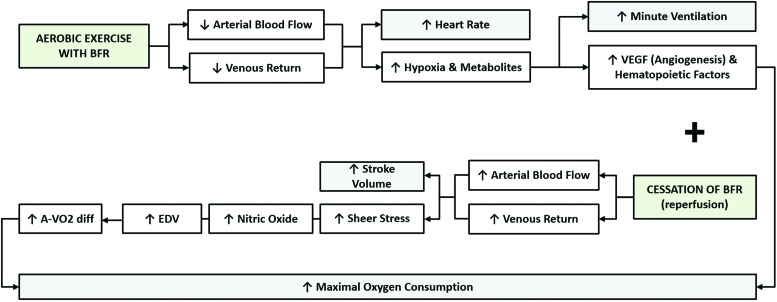
Aerobic ET with BFR has the potential to improve AC due to improvements in components of the Fick equation [VO2 = HR x SV x (a-vO2 difference)] as well as several other factors including the effects of hypoxia on vascular endothelial growth factor (VEGF) during BFR and the increase in endothelium-dependent vasodilation from increased shear stress and nitric oxide production during cuff release and reperfusion after BFR, as shown in Figure 1.3 Increased VEGF and endothelium-dependent vasodilation from BFR ET have the capacity to improve oxygen delivery and uptake, but may not have the same degree of improvement during high-intensity aerobic ET compared to low-to-moderate aerobic ET due to the possibility of a limited training duration and muscle damage associated with high-intensity aerobic ET with BFR.4,5
Figure 1.
Potential mechanisms of action during and immediately post-blood flow restricted exercise contributing to improvements in aerobic capacity.
However, a recent systematic review on the effects of BFR ET on AC and exercise performance suggested that aerobic ET with BFR improved AC irrespective of training intensity.6 In view of these findings, the purposes of this systematic review with meta-analysis were to examine the effects of aerobic ET with and without BFR on AC and to compare the effect of low-to-moderate aerobic ET with and without BFR to high-intensity aerobic ET with and without BFR on AC hypothesizing that (a) AC would be greater with aerobic ET and BFR compared to aerobic ET without BFR and (b) AC would be greater during low-to-moderate intensity aerobic ET with BFR compared to high-intensity aerobic ET with BFR.
METHODS
Search strategy and inclusion criteria
A comprehensive literature review was performed in PubMed and the Cochrane library through December 2018. The search strategy was conducted in English and included a mix of terms for the key concepts Blood Flow Restriction, Maximal Oxygen Consumption, Oxygen Consumption, Oxygen Uptake, Aerobic Capacity, Exercise Training, and Exercise and these were later combined with an advanced search strategy (Appendix 1) to identify randomized controlled trials for inclusion purposes. The reference list of eligible studies was also screened to identify other potentially relevant publications. To be included in the systematic review and meta-analysis, a study had to meet the following criteria: (a) the study was conducted in healthy individuals of all ages (i.e. free of overt acute or chronic diseases), (b) there was random allocation of study participants to training and control groups, (c) BFR was applied during aerobic ET, (d) BFR was the sole intervention difference between the groups, and (e) direct measurement rather than estimated maximal oxygen consumption was reported for each group. Any studies not meeting these criteria were excluded. Disagreement related to eligibility of studies was resolved through discussions among all authors.
To assist with the interpretation of results, all included studies were assessed for methodological quality using the PEDro scale, which is comprised of 11 items to evaluate the risk of bias and statistical reporting of randomized control trials (Table 1). The first item in the scale relates to external validity and items 2-11 assess the internal validity of a trial. Each item in the scale was scored yes (1 point) or no (0 points). Since the first item is not included in the total PEDro score of an article, a maximum of 10 points was possible for each study with scores ranging from 0 to 10. Higher scores indicate greater methodological quality.
Table 1.
Methodological quality of the included studies assessed with the PEDro scale.
| PEDro* | Abe, 2010 | Park, 2010 | Keramidas, 2012 | de Oliveira, 2016 | Paton, 2017 | Esparza, 2017 | Amani, 2018 |
|---|---|---|---|---|---|---|---|
| Eligibility criteria | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Randomized allocation | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Concealed allocation | No | No | No | No | No | No | No |
| Groups similar at baseline | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Blind subjects | No | No | No | No | No | No | No |
| Blind therapists | No | No | No | No | No | No | No |
| Blind assessors | No | No | No | No | No | No | No |
| Measure of one key outcome obtained from > 85% initial subjects | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Intention-to-treat | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Between-group comparisons | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Point measures and measures of variability | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| TOTAL | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
Eligibility criteria is not used to calculate the PEDro score.
Data extraction
Two authors independently read and coded each study for descriptive information including: (a) publication year (b) source of publication (i.e. journal article or published theses) (c) gender (1 = only males; 2 = only females; 3 = mixed) and (d) age of the participants in the studies. For both BFR and standard training protocols, the mode of ET and ET intensity were coded (1 = walking/treadmill protocol, 2 = bicycle protocol and 1 = low-to-moderate intensity if ET intensity was < 80% of maximal capacity, 2 = high-intensity if ET intensity was ≥ 80% of maximal capacity, respectively). Means and standard deviations of post-intervention maximal oxygen consumption were recorded as continuous variables in mL/kg/min. Means and standard deviations of post-intervention minute ventilation (VE) in L.min-1 and isometric knee extension strength in N/m were also recorded as continuous variables when available for supplementary pooled analyses carried out for discussion purposes. Inter-rater reliability of the coding by the two authors was calculated for all continuous and categorical variables. Cohen's Kappa determined that the raters were in complete agreement (k = 1). Pearson correlation analysis also demonstrated complete consistency among coders (r = 1).
Data analysis
Hedge's g was computed for each study using the metafor package with the statistical software R (3.0.2 version), providing an unbiased estimate of the population standardized mean difference. The overall effect was computed from effect sizes extracted from the individual studies, each of which was weighted by its inverse of the associated variance. Review Manager (RevMan, 5.3 version) was also used for data analyses to measure the standardized mean difference and I2. Heterogeneity of effect sizes was examined using the Q statistic, a standardized measure of the total amount of variation observed across studies. A nonsignificant Q statistic indicated that a fixed-effects model, rather than a random-effects model, was preferred for the analysis. Subgroup analyses were carried out based on ET intensity. Statistical significance was set at a p-value < 0.05.
Risk of publication bias could not be assessed because of the low number of included studies. As a rule of thumb, publication bias assessment can only be performed when there are at least 10 studies entered in the meta-analysis.
RESULTS
Selected studies
A total of seven trials were identified as eligible.7-13 A flow diagram of the studies retrieved for the meta-analysis is presented in Figure 2, as per PRISMA reporting guidelines. The studies were all randomized controlled trials with a BFR ET group and an ET control group with no vascular occlusion. The included studies evaluated a total of 121 subjects from both genders (79.3% men), with a mean sample size of 17.3 (SD = 2.7). The age across studies ranged from 20-25 years (combined mean ± SD age = 23.5 ± 4.1). The methodological quality of the studies using the PEDro scale was moderate with all studies scoring 6 of 10, as shown in Table 1. Four of the studies examined treadmill training with and without BFR and the other three studies examined cycling with and without BFR. Baseline characteristics of the study participants are shown in Table 2 with the majority of subjects being physically active except for the subjects in the study by Keramidas et al in which physically inactive subjects were enrolled. Table 3 provides information about the protocols and outcomes of each study.
Figure 2.
Flow diagram of study selection.
Table 2.
Overall characteristics of participants per study.
| Author, year | Sample Size (N) | Age (years) | Training Status | VO2max (mL/kg/min)* |
|---|---|---|---|---|
| Abe, 2010 | N = 19; 19 M 0 F | 23.0 ± 1.7 | Physically active | 42.8 ± 8.1 |
| Park, 2010 | N = 12; 12 M 0 F | 20.3 ± 1.23 | College basketball athletes | 48.2 ± 4.5 |
| Keramidas, 2012 | N = 20; 6 M 14 F | 22.9 ± 4.2 | Physically inactive | 37.4 ± 5.1 |
| de Oliveira, 2016 | N = 17; 12 M 5 F | 23.8 ± 4 | Physically active | 46.3 ± 6.5 |
| Paton, 2017 | N = 16; 10 M 6 F | 24.9 ± 6.9 | Physically active | 46.3 ± 0.2 |
| Esparza, 2017 | N = 18; 18 M 0 F | 23.8 ± 4.4 | Physically active | 41.1 ± 7.3 |
| Amani, 2018 | N = 19; 19 M 0 F | 23.89 ± 2.2 | Soccer players | 54.3 ± 6.1 |
M = males, F = females. Values expressed in mean ± standard deviation.
Baseline VO2max information for the entire sample per study.
Table 3.
Summary of protocols and outcomes from the included studies.
| Author, year | Exercise mode | Group comparisons | Exercise Intensity | Frequency of Training | BFR protocol | VO2max % change | |
|---|---|---|---|---|---|---|---|
| BFR group | Non-BFR group | ||||||
| Abe, 2010 | Cycling | Low intensity training with (n = 9) and without (n = 10) BFR | 40% of VO2max | 3 sessions per week, for 8 weeks | Cuff width: 50 mm; Occlusion pressure: 160-210 mmHg; Total time of occlusion: 18 minutes (preparation and exercise session; protocol had no rest periods) | 5.1 | 0.7 |
| Park, 2010 | Walking | Low intensity training with (n = 7) and without (n = 5) BFR | 40% of VO2max | 12 sessions per week, for 2 weeks | Cuff width: 110 mm; Occlusion pressure: 160-210 mmHg; Total time of occlusion: 19 minutes (preparation and exercise session, including rest periods) | 11.6 | -0.6 |
| Keramidas, 2012 | Cycling | High intensity training with (n = 10) and without (n = 10) BFR | 90% of VO2max | 3 sessions per week, for 6 weeks | Cuff width: NR; Occlusion pressure: 90 mmHg; Total time of occlusion: NR (exercise session until exhaustion; cuff deflated during rest periods) | -2.2 | -4.2 |
| de Oliveira, 2016 | Cycling | Low intensity interval training with (n = 10) and without (n = 7) BFR | 30% of VO2max | 3 sessions per week, for 4 weeks | Cuff width: 180 mm; Occlusion pressure: 140-220 mmHg; Total time of occlusion: 20-32 minutes (during exercise only; cuff deflated during rest periods) | 5.5 | 0.4 |
| Paton, 2017 | Running | High intensity training with (n = 8) and without (n = 8) BFR | 80% of peak running velocity | 2 sessions per week, for 4 weeks | Elastic wrap width: 75 mm; Occlusion pressure: NR; Total time of occlusion: 10-28 minutes (during exercise only; unwrapped during rest periods) | 6.3 | 4.0 |
| Esparza, 2017 | Running | Low intensity training with (n = 10) and without (n = 8) BFR | 30-45% VO2 reserve | 3 sessions per week, for 6 weeks | Cuff width: 50 mm; Occlusion pressure: 120-210 mmHg; Total time of occlusion: 30 minutes. (exercise session) | 7.3 | 4.1 |
| Amani, 2018 | Running | Moderate intensity interval training with (n = 10) and without (n = 9) BFR | 60% of maximum heart rate reserve | 4 sessions per week, for 2 weeks | Cuff width: NR; Occlusion pressure: 140-180 mmHg; Total time of occlusion: NR (exercise session until exhaustion) | 3.6 | 1.4 |
Abbreviations: BFR = Blood flow restriction; NR = not reported; VO2max = maximal oxygen uptake.
All included studies except for possibly the work by Amani et al, which was difficult to interpret, applied BFR bilaterally at the most proximal portion of the subject's thighs with all but the study by Paton et al using pressurized cuffs. Paton et al used elastic wraps instead (Get Strength Heavy Duty 75 mm, Waiuku, New Zealand) that were wrapped to a pressure that elicited a subject-perceived (self-reported) pressure of 7 out of 10 described as moderate, but pain free.11 The elastic wraps used in the Paton et al study were unwrapped between treadmill sets for a period of 150 seconds to provide a break from wearing the wraps and to provide time to re-apply the wraps. Two other studies deflated the pressure in the cuffs during rest periods.9,10 The size of the occlusion devices varied from 50 mm to 180 mm and the protocols to occlude the thighs also varied. The pressure used to occlude the thighs also varied with one study using perceived pressure,11 several other studies gradually increasing the occlusion pressure until a maximal level was achieved,7,8,10,12,13 and one study applying a cuff pressure of 90 mm Hg.9 (Table 3)
The exercise prescriptions used in each study also varied with the shortest duration of exercise training being two weeks in two studies,8,13 and the longest duration being eight weeks.7 The majority of studies performed exercise 3x/week,7,9,10,12 but one study performed exercise 2x/week,11 while another study performed exercise 12x/week.8 Amani et al did not report the frequency of exercise per week or the duration of exercise for each session.13 The duration of exercise for each session varied from 15 minutes to 30-33 minutes. The intensity of exercise was similar in four of the studies with three using an intensity of 40% of AC,7,8,10 and one using 30-45% of the oxygen reserve.12 One additional study grouped with the above low-intensity aerobic exercise studies used an intensity of 60-70% of the heart rate reserve.13 (Table 3)
The two high-intensity studies used an intensity of 90% of AC and 80% of peak running velocity. The two high-intensity studies performed exercise for shorter periods of time and with more frequent rest periods. Keramidas et al had subjects cycle for two minutes at 90% of AC followed by two minutes of cycling at 50% of AC (with thigh cuffs deflated) which was repeated for a total exercise duration of 30 minutes.9 Paton et al had subjects run for 30 seconds at 80% of peak running velocity followed by 30 seconds of rest while straddling the treadmill and repeating this five to eight times after which 150 seconds of rest was provided and the elastic wraps were unwrapped.11 One to two additional sets of the above procedures were performed for a total exercise duration of 12 minutes and 12 minutes of rest.11
The rate of perceived exertion (RPE) during BFR ET was compared to ET without BFR in four of the seven studies with two of the studies finding significantly greater RPE with BFR compared to ET without BFR.7,11 Park et al demonstrated the progressive increase in RPE during each of the five sets of walking with BFR, but did not statistically compare the RPE between sets or between BFR and non-BFR conditions.8 Keramidas et al found no significant difference in RPE between the BFR and non-BFR groups.9 No complications or adverse events were reported in any of the studies. (Table 3)
Maximal exercise testing methods
The methods used to determine maximal AC included bicycle exercise testing in four of the studies,7-10 and treadmill exercise testing in three of the studies.11-13 All studies performed a ramping protocol to exhaustion and utilized calibrated respiratory gas analysis systems, but only three studies utilized strict criteria (i.e. plateau in oxygen consumption, attainment of near maximal age predicted heart rate, respiratory exchange ratio > 1.10) to identify maximal oxygen consumption (VO2max).7-9 Because only three studies utilized strict VO2max criteria, the term maximal AC was used rather than VO2max. All studies identified maximal AC using the highest oxygen consumption during the final 15 to 30 seconds of exercise testing. Despite only three of the studies using strict VO2max criteria, the maximal AC of the seven studies was similar and reflective of the activity level of the subjects in each study. (Table 2)
Synthesized findings
A test of heterogeneity yielded a non-significant Q-statistic of 7.19 (df = 6, p = 0.30) indicating that no between-study variance in observed effects for AC existed. Under the fixed-effects model, the overall standardized mean difference of all seven studies was 0.38 (SE = 0.18), which was found to be statistically significant (z = 2.01, p = 0.04; 95% CI = 0.01, 0.75). Differences across subgroups classified according to ET intensity were also assessed using fixed-effects models, given that tests of heterogeneity performed for both the low-to-moderate ET intensity [Q (df = 4) = 5.06, p = 0.28] and the high-intensity ET [Q (df = 1) = 0.09, p = 0.76] groups indicated that effects were from the same population. A significant standardized mean difference of 0.57 (SE = 0.22) in AC was found between BFR and non-BFR groups in studies examining low-to-moderate ET intensity (z = 2.47, p = 0.01; 95% CI 0.12, 1.01) while no significant mean difference in AC was found between the groups when the high-intensity ET studies were analyzed together (z = 0.04, p = 0.97; 95% CI -0.67, 0.64). Forest plots for the overall and sub-analyses are shown in Figure 3.
Figure 3.
Forest plot of the overall and subgroup effects of blood flow restricted exercise on aerobic capacity.
Supplementary pooled analyses revealed no significant standardized mean difference in either VE [Q (df = 2) = 1.4, p = 0.49; fixed-effects model: z = 0.69, p = 0.48; 95% CI -0.36, 0.77)] or isometric knee extension strength [Q (df = 2) = 7.9, p = 0.01; random-effects model: z = 1.56, p = 0.11; 95% CI -0.23, 2.05)] post-intervention between the groups.
DISCUSSION
The overall findings from this systematic review with meta-analysis reveal that aerobic ET performed with BFR significantly improves AC more than aerobic exercise without BFR. A recent systematic review also concluded that aerobic ET with BFR increased AC,6 but provided no meta-analytic results making this the first pooled analysis of previous studies assessing the effects of aerobic ET with BFR on AC. Furthermore, although the finding that no significant improvement in AC was observed when high-intensity aerobic ET was combined with BFR is important, the results should be cautiously interpreted highlighting the need for further investigation of high-intensity ET with and without BFR.
Nonetheless, the effects of low-to-moderate intensity aerobic ET with BFR demonstrate significant and consistent improvements in AC compared to low-to-moderate intensity aerobic ET without BFR in healthy mostly active individuals. Furthermore, the RPE was significantly greater during BFR ET compared to non-BFR ET in two 7,10 of the four studies in which it was measured and importantly, no complications or adverse events were reported in any of the studies.
The reasons for the findings observed in this meta-analysis and the differences found between low-to-moderate intensity aerobic ET with BFR compared to high-intensity aerobic ET with BFR are likely due to the effects of BFR ET on components of the Fick equation, the physiological differences between low-to-moderate versus high-intensity aerobic ET, and possibly muscle damage and oxidative stress from high-intensity aerobic ET with BFR compared to low-to-moderate intensity aerobic ET with BFR.
The Impact of the Fick Equation on the Observed Results
The Fick equation [VO2 = HR x SV x (a-vO2 difference)] is understandably responsible for the changes observed in this meta-analysis and provides a framework to understand the effects of both low-to-moderate intensity and high-intensity aerobic ET with BFR. Figure 1 provides several possible explanations of how aerobic ET with BFR may improve AC, including the effects of hypoxia on VEGF during BFR as well as the increase in endothelium-dependent vasodilation from increased shear stress and nitric oxide production during cuff release and reperfusion after BFR. Increased VEGF and endothelium-dependent vasodilation from BFR ET have the capacity to improve oxygen delivery and uptake. In fact, Sundberg et al found an improvement in capillary density, oxidative metabolism, and AC after four weeks of one-legged cycle ET with BFR “at the highest tolerable workload that could be sustained” for 45 minutes.14 Furthermore, the study by de Oliveira et al included in this meta-analysis examined the effects of low-to-moderate intensity aerobic ET with and without BFR on the onset of blood lactate accumulation and found that the BFR group improved 16% compared to the 6% improvement in the non-BFR group reinforcing the above findings of Sundberg et al.10 Several of the studies included in this meta-analysis examined one or more components of the Fick equation besides VO2,7-12 which will provide insight into the physiologic mechanisms responsible for the observed findings.
Although the changes in resting 8,12 and peak heart rate7-9,12 were similar after aerobic ET with and without BFR, the heart rate during aerobic ET with BFR was significantly greater than aerobic ET without BFR in three studies,7,10,11 while one study observed similar heart rates during such ET.9 The increased training intensity observed during aerobic ET with BFR in the above three studies may be partly responsible for the greater increase in AC after aerobic ET with BFR compared to aerobic ET without BFR. A possible reason for the similar resting and peak heart rates after aerobic ET with and without BFR may be due to the relatively high activity level and fitness of the subjects in all of the studies except Keramidas et al,9 and attainment of maximal or near maximal heart rates during maximal exercise testing, respectively.
Park et al did not compare heart rate response between aerobic ET with and without BFR, but they did examine heart rate change during the first and last BFR ET session and found that heart rate was significantly lower at the mid-point and maximal point of BFR ET after performing BFR 2x/day, 6 days/week, for two weeks.8 Associated with the reduced heart rate in the Park et al study was a significant increase in stroke volume (approximately 22%) during the last aerobic ET with BFR session compared to the first aerobic ET with BFR session. The study by Esparza also examined the effects of aerobic ET with and without BFR on stroke volume and despite finding no significant difference between groups, the BFR group experienced a 5% increase in stroke volume while the stroke volume of the non-BFR group was unchanged.12 Therefore, an improvement in stroke volume from aerobic ET with BFR may be partly responsible for the significant increase in AC we observed in this meta-analysis, but further investigation of this is needed.
Another factor that may have contributed to the significant improvement in AC during aerobic ET with BFR is the effect that BFR ET appears to have on minute ventilation (VE). Three of the studies included in this meta-analysis examined change in VE after aerobic ET with and without BFR.8,9,11 Park et al found that VE increased significantly in the BFR group (10%), but was unchanged in the non-BFR group.8 The two high-intensity aerobic ET studies with and without BFR found similar results with both the BFR and non-BFR groups increasing VE.9,11 Although the BFR group in the Paton et al study had a 6.8% increase compared to the 0.8% increase in VE in the non-BFR group, the difference was not statistically significant. Keramidas et al found more similar increases in VE in the BFR and non-BFR groups (15.6% versus 13.7%, respectively). Thus, both low-to-moderate and high-intensity ET appear to elicit improvements in VE, but the results of an additional meta-analysis performed on these three studies demonstrated a non-significant effect of aerobic ET with BFR which was likely due to the different ET intensities and the small number of studies and subjects included in the studies. Therefore, due to the small number of studies that examined VE, further investigation of the effects of low-to-moderate and high-intensity aerobic ET with and without BFR is needed to determine the role VE may have in the improvement of AC.
An additional factor that may be responsible for the effects of aerobic ET with BFR on improving AC in this meta-analysis is an increase in hematopoietic factors such as erythropoietin, hemoglobin, and hematocrit concentrations during hypoxic conditions such as that during BFR.15,16 Only one of the studies in this meta-analysis examined hemoglobin and hematocrit and found no change in either measure after six weeks of cycling at 90% of AC for two minutes followed by two minutes of cycling at 50% of AC with BFR cuffs deflated which was repeated for 30 minutes, 3x/week.9 Despite Keramidas et al observing no change in hemoglobin or hematocrit concentration, near-infrared spectroscopy applied to the right vastus lateralis muscle during a submaximal exercise test found the change in total hemoglobin and oxyhemoglobin increased in both the BFR and non-BFR groups. Furthermore, after ET oxygen consumption was significantly lower in both groups during the submaximal exercise test at the same relative workload reflecting greater muscular efficiency.9 Additionally, Paton et al observed an improvement in running economy only in the BFR group despite AC improving similarly in both the BFR and non-BFR groups. Therefore, the Fick equation peripheral component (a-vO2 difference) and possibly the central components (HR and SV) contributed to greater muscular efficiency during submaximal exercise as a result of high-intensity ET.9,11 In view of these results, further investigation of aerobic ET with and without BFR on muscular efficiency and hematopoietic factors appears warranted.
Change in particular characteristics of skeletal muscle associated with aerobic ET and BFR may also be responsible for the improvements that were observed in AC. All included studies but the work by Amani et al examined some characteristic of skeletal muscle including strength (n=3), hypertrophy (n=1), power (n=3), and peak running velocity (n=1). Across the studies, isometric knee extension strength tended to be greater post-ET with BFR when compared to ET alone, even though no significant standardized mean difference between the groups was observed in our supplementary analysis. Improvements in hypertrophy and power also seemed to be greater in the aerobic ET with BFR groups when compared to the non-BFR groups, but the methodology that the above characteristics were measured and reported prevented these data from being subjected to an additional pooled analysis. Therefore, increased skeletal muscle strength, hypertrophy, and power may be partly responsible for the improvement in AC observed in this meta-analysis, but further investigation is warranted.
Minimal Clinically Important Difference in Aerobic Capacity
It is important to interpret the change in AC from aerobic ET with and without BFR presented in this meta-analysis with the minimal clinically important difference (MCID) in AC in healthy adults. Hays and Woolley suggest that the threshold for a MCID corresponds to a small effect size (0.20) while others suggest that a MCID reflects a difference or change of ½ of the baseline standard deviation (SD).17,18 The data presented in Figure 2 for the combined high-intensity and the low-to-moderate intensity ET studies shows that the effect size (std. mean difference) exceeds 0.20 (0.38) and that the low-to-moderate intensity ET studies with BFR far exceeds this threshold (0.57) while the high-intensity ET studies fall far below it (-0.01). Furthermore, half of the baseline SD data from both the low-to-moderate and high-intensity ET studies reveals that a threshold of 3.3 ml/kg/min in AC was needed to achieve a MCID which was exceeded by two of the five low-to-moderate intensity ET studies with BFR,7,8 and neither of the high-intensity ET studies with BFR exceeded this value.9,11 The above effect size (std. mean difference) results are also supported by a previous study which found that a 3.4% increase in AC was considered to be a MCID in healthy adults.19 In view of the results of this meta-analysis, all included studies but the work by Keramidas et al had an increase in AC in the BFR group that exceeded 3.4% and only two of the seven studies (i.e. Esparza and Paton et al) observed an improvement in AC in the non-BFR group that exceeded 3.4%. The change in AC of the non-BFR groups in the five other studies ranged from – 4.2% to + 0.7%.7-10,13 Finally, the one study in which the BFR group did not exceed a 3.4% improvement in AC (a high-intensity study) did observe a decrease in AC that was less than the non-BFR group (– 2.2% versus – 4.2%, respectively).9 Thus, BFR with aerobic ET appears to facilitate an improvement in AC with a MCID in AC in the majority of studies.
Low-to-Moderate Intensity Versus High-Intensity Exercise with and without Blood Flow Restriction
The results of this systematic review with meta-analysis suggest that low-to-moderate intensity aerobic ET with BFR physiologically elicits more AC compared to high-intensity ET with BFR which appears to elicit more anaerobic capacity.7-13 However, high-intensity aerobic ET without BFR also elicits improvements in AC as observed in two comprehensive meta-analyses.20,21 One meta-analysis found that ET intensity divided into tertiles based on intensity (60-70%, 80-92.5%, and over 100% of VO2max) had no statistically significant effect on the magnitude of improvement in AC in healthy adults with effect sizes of 0.77, 0.68, and 0.80, respectively.20 Thus, the highest ET intensity (over 100% of VO2max) produced the greatest effect on AC. The second meta-analysis found that both low-to-moderate aerobic ET and high-intensity ET elicit significant increases in AC in healthy adults with greater gains in AC following high-intensity ET compared to endurance training.21 However, the effect of high-intensity aerobic ET with BFR requires further investigation and discussion in view of the results observed in this systematic review and meta-analysis.
The two high-intensity ET studies included in this meta-analysis used an intensity of 90% of AC 9 and 80% of peak running velocity 11 and performed exercise for shorter periods of time and with more frequent rest periods than the low-to-moderate intensity ET studies. Also, during high-intensity aerobic ET with BFR, both studies eliminated BFR repeatedly during the rest periods. The results of high-intensity ET with BFR found an increase in AC in only one study (i.e. Paton et al) which increased more in the BFR group compared to the non-BFR group (2.9 versus 1.8 ml/kg/min, respectively), but it was not a statistically significant difference.11 In contrast, Keramidas et al found AC to decrease in both the BFR and non-BFR groups with less of a decrease in the BFR group (0.8 versus 1.6 ml/kg/min, respectively), which was also statistically insignificant. In view of the above, a limited ET duration and more frequent rest periods with deflated cuffs may be responsible for the lack of improvement in AC during high-intensity aerobic ET with BFR observed in this meta-analysis. This is particularly interesting given that only one of the low-to-moderate intensity studies provided reperfusion during one-minute passive rest periods.10 Thus, further investigation of low-to-moderate and high-intensity ET with BFR followed by reperfusion on AC is needed.
An important consideration given the above studies and the findings of high-intensity aerobic ET with BFR is the manner by which high-intensity aerobic ET with BFR may potentially damage skeletal muscle compared to low-to-moderate intensity aerobic ET with BFR. Studies of high-intensity resistance ET ( ≥ 70% 1RM) of a large muscle mass have consistently observed substantial increases in blood oxidative stress markers while low-intensity resistance training ( ≤ 30% 1RM) with BFR has not been found to increase oxidative stress.4 Similarly, high-intensity resistance ET has been observed to significantly increase creatine kinase values while low-intensity resistance and aerobic ET has not been found to increase creatine kinase or myoglobin content.4 Although the above research on skeletal muscle damage is limited, from the available literature it appears that low-to-moderate intensity ET with BFR is less likely to damage skeletal muscle compared to high-intensity ET with BFR. In fact, Loenneke et al concluded that low-intensity ET with BFR does not produce skeletal muscle damage in view of the absence of prolonged decrements in muscle function, no prolonged swelling, soreness ratings were similar to a submaximal low load control, and no elevation in blood biomarkers of muscle damage have been reported.5 Additionally, no complications or adverse events were reported in any of the studies included in this meta-analysis. In view of the above, further investigation of the effects of low-to-moderate and high-intensity aerobic ET with and without BFR on skeletal muscle damage is needed since little literature appears to exist.4,5
Limitations
The limitations of this meta-analysis include a variety of aerobic ET regimens and methods to employ BFR as well as the limited number of high-intensity aerobic ET studies comparing the effects of aerobic ET with and without BFR on AC. Other limitations include the absence of consistent and similar measures of skeletal muscle characteristics such as strength, hypertrophy, and power as well as the absence of data on the effects of varying intensities of aerobic ET with BFR on skeletal muscle damage. Further research in the above areas is needed to fully understand the effects of aerobic ET with BFR.
CONCLUSIONS
The results of this systematic review and meta-analysis reveal that aerobic ET with BFR elicits a greater improvement in AC than aerobic ET without BFR. Although high-intensity aerobic ET with BFR did not appear to elicit an improvement in AC over high-intensity aerobic ET without BFR only two studies were available to be included in this analysis for which reason these results should be interpreted cautiously. Further investigation of the effects of low-to-moderate and high-intensity aerobic ET with and with BFR on AC as well as the components of the Fick equation and VE are needed.
Appendix 1.
The search strategy was developed based on the following clinical question and its respective concept map: What is the effectiveness of exercise training (ET) with blood flow restriction (BFR) compared to ET without BFR on maximal aerobic capacity in healthy adults?
| P: Healthy adults | I: ET with BFR | C: ET without BFR | 0: Maximal aerobic capacity |
|---|---|---|---|
| Aerobic Exercise (MeSH) | Oxygen Consumption (MeSH) | ||
| Exercise Training (MeSH) | Aerobic Capacity (KW) | ||
| Blood Flow Restriction (KW) | Oxygen Uptake (KW) | ||
| Kaatsu Training (KW) | V02 (KW) |
Search: (((((aerobic exercise) OR exercise training) AND “blood flow restriction”) OR “kaatsu training”)) AND (((((“oxygen consumption”[MeSH Terms] OR “oxygen consumption” [All Fields])) OR “aerobic capacity”) OR “oxygen uptake”) OR “vo2”)
REFERENCES
- 1.Hughes L Paton B Rosenblatt B Gissane C Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51(13):1003-1011. [DOI] [PubMed] [Google Scholar]
- 2.Ipavec M Grapar Zargi T Jelenc J Kacin A. Efficiency of pneumatic tourniquet cuff with asymmetric pressure distribution at rest and during isometric muscle action. J Strength Cond Res. 2018;33(9):2570-2578. [DOI] [PubMed] [Google Scholar]
- 3.Wong ML Formiga MF Owens J Asken T Cahalin LP. Safety of blood flow restricted exercise in hypertension: a meta-analysis and systematic review with potential applications in orthopedic care. Tech Orthop. 2018;33(2):80-88. [Google Scholar]
- 4.Loenneke JP Wilson JM Wilson GJ Pujol TJ Bemben MG. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports. 2011;21(4):510-518. [DOI] [PubMed] [Google Scholar]
- 5.Loenneke JP Thiebaud RS Abe T. Does blood flow restriction result in skeletal muscle damage? A critical review of available evidence. Scand J Med Sci Sports. 2014;24(6):e415-422. [DOI] [PubMed] [Google Scholar]
- 6.Bennett H Slattery F. Effects of blood flow restriction training on aerobic capacity and performance: a systematic review. J Strength Cond Res. 2019;33(2):572-583. [DOI] [PubMed] [Google Scholar]
- 7.Abe T Fujita S Nakajima T et al. Effects of low-intensity cycle training with restricted leg blood flow on thigh muscle volume and VO2max in young men. J Sport Sci Med. 2010;9(3):452-458. [PMC free article] [PubMed] [Google Scholar]
- 8.Park S Kim JK Choi HM Kim HG Beekley MD Nho H. Increase in maximal oxygen uptake following 2-week walk training with blood flow occlusion in athletes. Eur J Appl Physiol. 2010;109(4):591-600. [DOI] [PubMed] [Google Scholar]
- 9.Keramidas ME Kounalakis SN Geladas ND. The effect of interval training combined with thigh cuffs pressure on maximal and submaximal exercise performance. Clin Physiol Funct Imaging. 2012;32(3):205-213. [DOI] [PubMed] [Google Scholar]
- 10.de Oliveira MF Caputo F Corvino RB Denadai BS. Short-term low-intensity blood flow restricted interval training improves both aerobic fitness and muscle strength. Scand J Med Sci Sports. 2016;26(9):1017-1025. [DOI] [PubMed] [Google Scholar]
- 11.Paton CD Addis SM Taylor LA. The effects of muscle blood flow restriction during running training on measures of aerobic capacity and run time to exhaustion. Eur J Appl Physiol. 2017;117(12):2579-2585. [DOI] [PubMed] [Google Scholar]
- 12.Esparza BN. The effects of a short-term endurance training program with blood flow restriction cuffs versus ACSM recommended endurance training on arterial compliance and muscular adaptations in recreationally active males. ProQuest Dissertations Publishing. Published 2017. Accessed 2018.
- 13.Amani A Sadeghi H Afsharnezhad T. Interval training with blood flow restriction on aerobic performance among young soccer players at transition phase. Monten J Sports Sci Med. 2018;7(2):5-10. [Google Scholar]
- 14.Sundberg CJ Eiken O Nygren A Kaijser L. Effects of ischaemic training on local aerobic muscle performance in man. Acta Physiol Scand. 1993;148(1):13-19. [DOI] [PubMed] [Google Scholar]
- 15.Dale EA Ben Mabrouk F Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology. 2014;29(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koistinen PO Rusko H Irjala K et al. EPO, red cells, and serum transferrin receptor in continuous and intermittent hypoxia. Med Sci Sports Exerc. 2000;32(4):800-804. [DOI] [PubMed] [Google Scholar]
- 17.Hays RD Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? PharmacoEconomics. 2000;18(5):419-423. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke R Singer J Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407-415. [DOI] [PubMed] [Google Scholar]
- 19.Clark NA Edwards AM Morton RH Butterly RJ. Season-to-season variations of physiological fitness within a squad of professional male soccer players. J Sport Sci Med. 2008;7(1):157-165. [PMC free article] [PubMed] [Google Scholar]
- 20.Scribbans TD Vecsey S Hankinson PB Foster WS Gurd BJ. The effect of training intensity on VO2max in young healthy adults: a meta-regression and meta-analysis. Int J Exerc Sci. 2016;9(2):230-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milanovic Z Sporis G Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45(10):1469-1481. [DOI] [PubMed] [Google Scholar]