Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
To assess the effects of pharmacological interventions for the treatment of asymptomatic carotid stenosis, to prevent neurological impairment, stroke, disability, death, and other complications.
Background
See Table 1 for a glossary of terms.
1. Glossary of terms.
| Term | Definition |
| Amaurosis fugax | Transient monocular visual loss associated with vascular thromboembolic events arising from the internal carotid arterial system |
| Anticoagulants | Drugs that suppress, delay, or prevent blood clots |
| Antiplatelet agents | Drugs which prevent blood clots by inhibiting platelet function |
| Atherosclerosis | A disease characterised by a build‐up of abnormal fat, cholesterol and platelet deposits on the inner wall of the arteries |
| Atheromatous plaques | A fatty deposit in the inner lining (intima) of an artery, resulting from atherosclerosis |
| Atherosclerotic debris | Pieces of atheromatous plaque that can break off and be carried by the bloodstream |
| Body mass index (BMI) | Body mass divided by the square of the body height, universally expressed in units of kg/m2 |
| Computed tomography angiography (CTA) | Computed tomography scanning that uses an injection of contrast material into the blood vessels to help diagnose and evaluate blood vessel disease or related conditions |
| Digital subtraction angiography (DSA) | Fluoroscopy technique used in interventional radiology to clearly visualise blood vessels in a bony or dense soft tissue environment |
| Direct thrombin inhibitors | A drug that acts as anticoagulant by directly inhibiting the enzyme thrombin (factor IIa) |
| Duplex ultrasound | Non‐invasive evaluation of blood flow through the arteries and veins by ultrasound devices |
| Dyslipidemia | Abnormal concentration of fats (lipids or lipoproteins) in the blood |
| Embolism | Obstruction of an artery or vein, typically by a clot of blood or an air bubble |
| Fator Xa inhibitors | A type of anticoagulant that works by selectively and reversibly blocking the activity of clotting factor Xa, preventing clot formation |
| Heparin | A drug which is used to prevent blood clotting (anticoagulant, blood thinner) |
| Ipsilateral encephalic territories | The same side of the brain |
| Low molecular weight heparin | A drug which is used to prevent blood clotting (anticoagulant) |
| Magnetic resonance angiography (MRA) | A group of techniques based on magnetic resonance imaging (MRI) to image blood vessels |
| Obesity | A condition where the amount of body fat is beyond healthy conditions (BMI greater than 30 kg/m2) |
| Oedema | Excess watery fluid which collects in tissues of the body, causing swelling when fluid leaks out of the body's vessels |
| Overweight | Where body fat is over that of the average population, but less than unhealthy conditions (BMI between 25 kg/m2 and 30 kg/m2) |
| Placebo | Substance or treatment with no active effect, like a sugar pill |
| Randomised controlled trial (RCT) | A study in which the participants are divided randomly into separate groups to compare different treatments |
| Stroke | Neurological deficit attributed to an acute focal injury of the central nervous system by a vascular cause, persisting ≥ 24 hours or until death |
| Thrombosis | Local coagulation of blood (clot) in a part of the circulatory system |
| Transient ischaemic attack (TIA) | A transient episode (less than 24 hours) of neurological dysfunction caused by focal brain, spinal cord, or retinal ischaemia without acute infarction |
| Unfractionated heparin (UFH) | A mixture of heparins obtained from animals which is used to prevent blood coagulation. Used to prevent and treat clotting disorders |
| Vascular | Relating to blood vessels (arteries and veins) |
| Vitamin K antagonists (VKAs) | Substances that reduce blood clotting by reducing the action of vitamin K |
Description of the condition
Strokes, characterised by brain tissue injury due to stenosis or arterial occlusion, can cause death or permanent neurological disability and approximately 90% are ischaemic. This largely occurs as a result of carotid stenosis, hypertension, or cardiac arrhythmia (Brott 2013; Flumignan 2017; Mozaffarian 2016). Carotid artery stenosis (narrowing of the carotid arteries) is an important cause of cerebrovascular disease and transient ischaemic attack (TIA), underlying almost 15% of strokes (Easton 2009). The cumulative risk of stroke related to severe carotid stenosis is nearly 12% in the first year (approximately 15% to 18% in one year and 26% over two years (NASCET 1991), and approximately 30% over five years (Moore 1995; NASCET 1991). Significant stenosis (of more than 50% of vessel diameter) is usually responsible for 8% of all strokes and increases the risk of recurrence after the first episode to 16% over five years (Hillen 2003), mostly due to cerebral embolisms caused by biological changes to the atherosclerotic plaque (Flaherty 2013).
Ischaemic stroke is a major global public health problem. Nearly 800,000 events are reported annually in the USA, approximately 610,000 of which represent the first attack in the patient. Stroke is the second most common cause of death, accounting for nearly 5.5 million deaths worldwide in 2016 (De Waard 2017; Feigin 2014; Naylor 2018; NICE 2019; Venkatachalam 2014).
Furthermore, stroke is a significant cause of permanent neurological disability in Europe; out of approximately 1.2 million stroke survivors in the UK, 60% are discharged with some impairment (CDC 2001; NICE 2019; Strong 2007).
The direct costs of stroke alone amounted to approximately USD 28 billion between the years 2014 and 2015 in the USA, and this cost is estimated to more than double in the next 20 years (Benjamin 2019; Feigin 2016; Gorelick 1999). It is expected that by 2030 there will be 80 million strokes worldwide, with 12 million deaths (an increase of 50% compared with 2012), and 200 million disability‐adjusted life years lost worldwide (Benjamin 2019; Feigin 2014).
Extracranial carotid stenosis may be asymptomatic or symptomatic. The embolisation of atherosclerotic debris or thrombotic material from plaques of arterial stenoses are most frequently associated with cerebrovascular symptoms such as stroke, TIA in the ipsilateral encephalic territories, and amaurosis fugax. People with asymptomatic carotid stenosis (ACS) are at risk not only of stroke or related symptoms but also of other cardiovascular episodes, such as myocardial infarction (heart attack) and peripheral artery disease (Divya 2015; Flumignan 2017).
Asymptomatic carotid stenosis is a common condition in clinical practice, affecting about 3% to 7% of the general population. It is more prevalent in older people (over 60 years of age), and can evolve into a stroke in 0.3% to 2% of patients each year (De Weerd 2010; Park 2019). An atherosclerotic lesion, a diffuse and degenerative disease of the arteries, usually provokes ACS which narrows the vessel wall. A sudden rupture of atheromatous plaques from significant asymptomatic stenosis of the carotid artery can lead to thromboembolism, which causes 10% to 15% of all strokes (Bulbulia 2017). Thus, for people with extracranial carotid disease, it is important to identify risk factors, the degree of stenosis of the artery, and the characteristics of the plaque, such as ulcerations, intra‐plaque haemorrhage, and lipid content, that may increase the likelihood of a cerebrovascular event (De Waard 2017; Derdeyn 2007; Naylor 2018; Ricotta 2011).
The modifiable risk factors associated with ACS — such as hypertension, smoking, dyslipidaemia, diabetes, obesity, a sedentary lifestyle, alcoholism, inadequate diet quality, and psychosocial factors — can vary in importance according to region, ethnic group, gender, age, and family history. However, together these factors consistently contribute towards increasing the risk of cerebrovascular disease, making them targets for general approaches to preventing cerebrovascular events worldwide (Arnett 2019; Guzik 2017; O'Donnel 2016).
In order to diagnose and classify ACS there are some complementary imaging tests: duplex ultrasound (DUS) and angiography by magnetic resonance imaging (MRI), computed tomography angiography (CTA), or digital subtraction angiography (DSA). DSA was practically discontinued at the end of the 20th century as a diagnostic method, especially in asymptomatic patients, as it is associated with a 1.2% risk of neurological events (ACAS 1995). On the other hand, DUS is affordable, non‐invasive, and inexpensive. It also does not bring the additional risks associated with DSA, MRA, and CTA, such as the use of iodinated or paramagnetic contrast, X‐ray exposure, and embolisation risks. Thus, DUS is widely used as the first diagnostic method for detecting carotid stenosis in both symptomatic patients and those with risk factors for asymptomatic stenosis (Cassola 2018; Daolio 2019; Naylor 2018; Ricotta 2011; Wardlaw 2006).
The European Carotid Surgery Trial (ECST) and the North American Symptomatic Carotid Endarterectomy Trial (NASCET) applied different techniques to measure the percentage of stenosis in DSA (Figure 1), and identified those patients who would benefit from revascularisation. While ECST used residual lumen diameter as a denominator, NASCET used disease‐free diameter in a segment of the carotid artery above the stenosis. Using NASCET measurement standards, other studies (namely the Asymptomatic Carotid Atherosclerosis Study (ACAS) and the Asymptomatic Carotid Surgery Trial 1 (ACST‐1)) have shown that surgical intervention would also benefit some asymptomatic patients with carotid stenosis greater than 60% of diameter on DSA (ACAS 1995; ECST 1998; Halliday 2004; NASCET 1991; Naylor 2018; Ricotta 2011).
1.
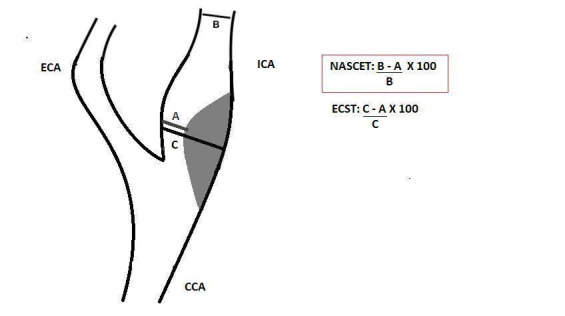
Longitudinal view of carotid bifurcation with methods of measuring carotid stenosis at angiography.
A: narrowest ICA diameter B: normal distal cervical ICA diameter C: estimated original diameter at the site of the most stenosis CCA: common carotid artery ECA: external carotid artery ECST: European Carotid Surgery Trial ICA: internal carotid artery NASCET: North American Symptomatic Carotid Endarterectomy Trial
Description of the intervention
It is important to ensure that people with ACS receive the best therapeutic option to avoid cerebral ischaemia, and this includes: the control of hypertension; use of lipid‐lowering drugs to reduce cholesterol levels in order to regress plaque(s), decrease the risk of plaque accident, and for anti‐inflammatory purposes; use of hypoglycaemic drugs; and the use of antiplatelet and anticoagulant agents.
Antihypertensive therapy
High blood pressure is one of the most powerful risk factors, and its decrease seems to be directly related to a lower incidence of stroke. A reduction of 5 mmHg to 10 mmHg blood pressure is associated with a 30% to 40% reduced risk of stroke compared with placebo. Despite a lack of randomised controlled trials (RCTs) assessing the effects of antihypertensives in people with ACS, the European Society for Vascular Surgery (ESVS) recommends a target blood pressure for people with ACS below 140/90 mmHg and, more radically, the American Heart Association (AHA) lowered these ideal blood pressure levels to close to 130/80 mmHg, with diastolic blood pressure less than 85 mmHg for people with diabetes in both guidelines (Arnett 2019; Brott 2013; Lawes 2004; Naylor 2018; Ricotta 2011).
Maintaining blood pressure may reduce stenosis and prevent lesion progression. Calcium channel blockers and angiotensin‐converting enzyme inhibitors are associated with plaque reduction to a greater extent than diuretics and beta‐blockers (Arnett 2019; Naylor 2018; Ricotta 2011).
Lipid‐lowering drugs
At the end of the 20th century, less than 10% of people with carotid stenosis regularly used statins to treat dyslipidaemia. At the start of the 21st century there was an increase in statin use as studies showed a decrease in cardiovascular events in symptomatic patients by more than one‐third when low‐density lipoprotein (LDL) cholesterol levels were below 70 mg/dL. Systematic reviews observed a significant reduction in cardiovascular mortality (including stroke) when statins, mainly atorvastatin 80 mg daily, were used in primary prevention, for instance in people with ACS. However, ezetimibe or PCSK9 inhibitors may be an alternative treatment for high‐risk patients who cannot tolerate statins (Amarenco 2006; Brott 2013; Herder 2012; Naylor 2018; Ricotta 2011; Taylor 2002; Taylor 2013; Wilson 2019; Zhan 2018).
Management of diabetes
Diabetes mellitus is an independent predictor of moderate and severe carotid stenosis and can contribute for doubling the chances of stroke. Medications used for glycaemic control include oral hypoglycaemic agents (metformin or sulfonylureas, or both), insulin therapy, or the new glucose‐lowering medications such as the analogue of human glucagon‐like peptide 1, dipeptidyl peptidase 4 inhibitors, sodium glucose cotransporter 2 inhibitors, and thiazolidinediones. Strong control of glycaemic levels is not directly related to a decreased risk of stroke, but glycosylated haemoglobin levels lower than 7% may contribute to a reduction in other related events, such as microangiopathy. Meanwhile, systematic reviews indicated that strict control in people with a body mass index above 30 kg/m2 was effective in reducing the risk of cerebrovascular disease (Chiquete 2014; De Weerd 2010; Holman 2014; Naylor 2018; Ricotta 2011; Zhang 2013).
Antiplatelet drugs
There is weak evidence for the use of antiplatelet drugs in people with ACS for reducing the risk of stroke, but there is more robust evidence for their use in secondary prevention. However, the use of aspirin at doses between 75 mg and 325 mg (or clopidogrel 75 mg when aspirin is intolerable) is recommended in asymptomatic patients to prevent other cardiovascular events (Murphy 2019; Naylor 2018; Ricotta 2011).
Anticoagulant agents
Anticoagulant therapy is known to prevent stroke in people with atrial fibrillation, but warfarin has not been shown to be more effective compared to antiplatelet therapy for secondary prevention in people without atrial fibrillation. However, recent studies have indicated that the use of low‐dose rivaroxaban together with aspirin may decrease the risk of stroke in both symptomatic and asymptomatic patients (Ricotta 2011; Sharma 2019).
How the intervention might work
As carotid atherosclerosis is an important aspect in stroke pathophysiology, proper management of the diseases that lead to its increase may correspond to key targets for stroke prevention. The approaches discussed above work together to control the risk factors that increase atherosclerosis, avoiding irregular and ulcerated plaques, microembolic particles, and preventing carotid artery disease from progressing (Naylor 2018).
The ACAS and ACST‐1 studies used an initial pharmacological therapy which has significantly changed in recent decades. For instance, only around 10% to 20% of ACAS and ACST‐1 participants regularly used lipid‐lowering drugs. There was a decline in annual stroke rates of approximately 60% between 1995 and 2004, which strongly correlates with improved pharmacological treatment associated with the increased use of aspirin, antihypertensive drugs, and statins, in that decade. Control of hypertension can reduce the risk of stroke by up to 30%, while control of cholesterol can reduce this risk by 15%. In addition, people with diabetes who, associated with glycaemic control, were taking statins, antiplatelet, and antihypertensive drugs, showed a 60% reduction in the risk of cardiovascular disease and death (ACAS 1995; Halliday 2004; Naylor 2018; Ricotta 2011).
Why it is important to do this review
Some randomised controlled trials (RCTs) have evaluated the use of pharmacological interventions, and topical guidelines currently recommend triple medical therapy (e.g. antiplatelet agents, antihypertensive therapy, and statins) in addition to lifestyle interventions to reduce the risk of stroke. Routine carotid endarterectomy or stenting is not reasonable in asymptomatic patients, except in particular high‐risk patients on medical therapy (Naylor 2018). However, the optimal therapeutic management strategy remains unclear (Raman 2013). Additionally, recent studies suggest that a more effective antithrombotic strategy (e.g. direct oral anticoagulants plus antiplatelet agents) may be more effective than antiplatelet agents alone for decreasing the risk of major vascular events (Abbott 2007; Sharma 2019).
Stroke continues to be the main cause of permanent disability and one of the most important causes of death in the world, and its impact leads to considerable socioeconomic impairment, not only to the patient and family, but also to society as a whole. In this context, pursuing the best pharmacological strategies may be useful to decrease ACS‐related mortality and permanent neurological disability (Naylor 2018).
Objectives
To assess the effects of pharmacological interventions for the treatment of asymptomatic carotid stenosis, to prevent neurological impairment, stroke, disability, death, and other complications.
Methods
Criteria for considering studies for this review
Types of studies
We will include all RCTs with parallel (e.g. cluster or individual) or cross‐over design. We will only use data from the first phase of cross‐over studies to avoid the risk of carry‐over effects, as described in Section 23.2.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a). We will include studies reported as full text, those published as abstract only, and unpublished data. Quasi‐randomised trials (i.e. studies in which participants are allocated to intervention groups based on methods that are not truly random, such as hospital number or date of birth) will not be considered.
Types of participants
We will consider for inclusion participants of any gender and any age with ACS. Carotid stenosis will be defined as a narrowing of the internal or common carotid artery (or both), diagnosed by at least one valid objective test (e.g. DUS or angiography by tomography, magnetic resonance or digital subtraction). We will use the classification of carotid stenosis with the use of ultrasound as defined by Grant 2003 for the participant classification (Table 2). We will consider participants as asymptomatic if they were without ipsilateral neurological symptoms (e.g. amaurosis fugax, TIA or stroke) in the previous six months (Naylor 2018). All trials involving participants with ACS will be considered, irrespective of the degree of stenosis or the method of determining the degree of stenosis.
2. DUS criteria for internal carotid stenosis.
| Consensus panel based on Grant 2003 | ||||
| Degree of stenosis (%) | Primary parameters | Additional parameters | ||
| ICA PSV (cm/sec) | Plaque estimate (%)* | ICA/CCA PSV ratio | ICA EDV (cm/sec) | |
| Normal | < 125 | None | < 2.0 | < 40 |
| < 50% | < 125 | < 50 | < 2.0 | < 40 |
| 50% to 69% | 125 to 230 | ≥ 50 | 2.0 to 4.0 | 40 to 100 |
| ≥ 70% but less than near occlusion | > 230 | ≥ 50 | > 4.0 | > 100 |
| Near occlusion | High, low or undetectable | Visible | Variable | Variable |
| Total occlusion | Undetectable | Visible, no detectable lumen | Not applicable | Not applicable |
| *Plaque estimate (diameter reduction) based on DUS B‐mode and on additional colour mode ultrasound. | ||||
CCA: common carotid artery DUS: duplex ultrasound EDV: end diastolic velocity ICA: internal carotid artery PSV: peak systolic velocity
If we find studies with mixed populations, and only a subset of the participants meets our inclusion criteria, we will attempt to obtain data for the subgroup of interest from the trialists in order to include the study. For studies with mixed populations where we cannot obtain data on the subgroup of interest, but at least 50% of the study population are of interest, we will include all participants in our analysis. Moreover, we will explore the effect of this decision in a sensitivity analysis. We will exclude studies in which less than 50% of the population are of interest and data on the subgroup of interest are not available.
Types of interventions
We will include trials comparing one pharmacological intervention (agent or drug) with placebo, no treatment, or another pharmacological intervention. We will include trials of any combination of interventions, providing co‐treatments are balanced between the treatment and control arms for the ACS treatment. We will also include studies that compare different doses of drugs.
We will consider the following interventions:
anticoagulants (unfractionated heparin (UFH) and low molecular weight heparins (LMWHs); vitamin K antagonists (VKA); direct oral anticoagulants (DOAC), factor Xa inhibitors and direct thrombin inhibitors; pentasaccharides);
antiplatelet agents (e.g. aspirin, clopidogrel);
antihypertensive drugs (e.g. angiotensin‐converting enzyme inhibitors, beta blockers);
glycaemic‐lowering agents (e.g. biguanides, sulphonylureas);
lipid‐lowering agents (e.g. statins).
The possible comparisons are:
anticoagulants plus antiplatelet agents versus antiplatelet agents;
one class of antiplatelet versus a combination of antiplatelets from two classes;
one class of antiplatelet versus another class of antiplatelet;
anticoagulants versus antiplatelet agents;
one class of lipid‐lowering versus another class of lipid‐lowering;
one class of antihypertensive versus another class of antihypertensive;
one class of glycaemic‐lowering versus another class of glycaemic‐lowering;
any combination of the above treatments versus any combination, with or without placebo.
Types of outcome measures
Primary outcomes
Neurological impairment, assessed using clinical outcome measures or any validated international scales (e.g. the National Institutes of Health Stroke Scale (NIHSS), the modified Rankin Scale (mRS), the Barthel Index (BI)). If we identify both dichotomous and continuous variables related to neurological impairment, we will report them separately as independently outcomes.
Ipsilateral major or disabling stroke, related to the extracranial carotid stenosis and confirmed by any objective additional test (e.g. computerised tomography, angiography) other than only clinical examination.
Secondary outcomes
Stroke‐related mortality.
Major bleeding: defined by a haemoglobin concentration decrease of 2 g/dL or more, a retroperitoneal or intracranial bleed, a transfusion of two or more units of blood, or fatal haemorrhagic events, as defined by the International Society on Thrombosis and Haemostasis (Schulman 2010). We will also consider the definition stipulated by the included study.
Progression of carotid stenosis (any increase in extracranial carotid stenosis), evaluated by change in range of stenosis, i.e. less than 50%, 50% to 69%, 70% or more, near occlusion or occlusion. We will consider the carotid stenosis if it was evaluated by any valid objective method (e.g. duplex ultrasound (Grant 2003), or angiography by tomography, magnetic resonance or digital subtraction (NASCET 1991)).
Adverse events, such as all‐cause mortality, gastrointestinal, allergic reaction, renal failure, or minor bleeding.
Quality of life, analysed by any validated questionnaire (e.g. SF‐36 (Ware 1992)) or participant's subjective perception of improvement (yes or no) as reported by the study authors. If we are unable to pool data on quality of life due to the use of different measurements, we will attempt to extract data on improvement.
We will present the outcomes at the following two time points after the start of the intervention, if data are available:
early outcomes (at six months or less after the start of the intervention);
long‐term outcomes (more than six months after the start of the intervention.
Search methods for identification of studies
See the methods for the Cochrane Stroke Group Specialised register. We will search for trials in all languages and arrange for the translation of relevant articles where necessary.
Electronic searches
We will search the Cochrane Stroke Group trials register and the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library; latest issue);
MEDLINE Ovid (from 1946) (Appendix 1);
Embase Ovid (from 1974);
Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS) (from 1982), via Virtual Health Library;
Indice Bibliográfico Español de Ciencias de la Salud (IBECS), via Virtual Health Library.
We will model the subject strategies for databases on the search strategy designed for MEDLINE by the Cochrane Stroke Group's Information Specialist (Appendix 1). We have opted to write a highly‐sensitive search strategy and have eliminated the pharmacological interventions component of the search entirely. The reasons for this are as follows. The problem component 'asymptomatic carotid stenosis' is already well defined and, when combined with Cochrane's verified RCT filter, retrieved a low number of results during test searches in MEDLINE Ovid. Pharmacological interventions search blocks can help improve recall when included in search strategies. However, because the initial test search recall is relatively low, as suggested above, we elected not to include them in the enclosed search but we will select the relevant interventions manually. We will combine all search strategies deployed with subject strategy adaptations of the highly‐sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2019).
We will search the following ongoing trials registers:
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov/);
World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/ictrp/en/);
Stroke Trials Registry (www.strokecenter.org/trials/).
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we will:
check the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials, and search Google Scholar to forward‐track relevant references (scholar.google.co.uk/);
contact original trial authors for clarification and further data if trial reports are unclear;
where necessary, contact experts/trialists/organisations in the field to obtain additional information on relevant trials, using a standard letter template (Appendix 2); and
-
conduct a search of various grey literature sources, dissertation and theses databases, and databases of conference abstracts, including:
British Library EThOS (UK E‐Theses Online Service);
ProQuest Dissertation and Theses Global;
Repositório UNIFESP (thesis repository of Universidade Federal de Sao Paulo, Brazil).
Data collection and analysis
Selection of studies
Two review authors (CNBC, NC) will independently screen titles and abstracts of the references obtained as a result of our searching activities and will exclude obviously irrelevant reports using the Covidence tool (Covidence). We will retrieve the full‐text articles for the remaining references and two review authors (CNBC, NC) will independently screen these, identify studies for inclusion and record reasons for exclusion of the ineligible studies. We will resolve any disagreements through discussion or, if required, we will consult a third review author (RLGF). We will collate multiple reports of the same study so that each study, not each reference, is the unit of interest in the review. We will record the selection process and complete a PRISMA flow diagram (Moher 2010).
Data extraction and management
We will use a data collection form for study characteristics and outcome data, which we will pilot on at least one study in the review. Two review authors (CNBC, NC) will independently extract data from the included studies. We will extract the following study characteristics.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting and date of study.
Participants: number randomised, number lost to follow‐up/withdrawn, number analysed, number of interest, mean age, age range, gender, severity of condition, diagnostic criteria, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
We will resolve disagreements by consensus or by involving a third review author (RLGF). One review author (CNBC) will transfer data into Review Manager (RevMan 2014). We will double‐check that data are entered correctly by comparing the data presented in the systematic review with the data extraction form. A second review author (NC) will spot‐check study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (CNBC, NC) will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We will resolve any disagreements by discussion or by involving another review author (RLGF). We will assess the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
In cluster‐randomised trials, we will consider particular biases as recommended in section 8.15.1.1 of the Cochrane Handbook for Systematic Reviews of Interventions: 1) recruitment bias; 2) baseline imbalance; 3) loss of clusters; 4) incorrect analysis; and 5) comparability with individually randomised trials (Higgins 2017). We will grade each potential source of bias as high, low, or unclear and provide a quote from the study report, together with a justification for our judgement in the 'Risk of bias' table. We will summarise the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on risk of bias relates to unpublished data or correspondence with a trialist, we will note this in the 'Risk of bias' table.
When considering treatment effects, we will take into account the risk of bias for the studies that contribute to that outcome.
Assessment of bias in conducting the systematic review
We will conduct the review according to this published protocol and report any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We will analyse dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We will analyse continuous data using the mean difference (MD) when the same scale/score is used, or standardised mean difference (SMD) when different scales/scores are used, with 95% CIs. We will enter data presented as a scale with a consistent direction of effect. We will narratively describe skewed data reported as medians and interquartile ranges.
Unit of analysis issues
Individuals will be our unit of analysis. If trials include multiple intervention arms, we will consider only the arms relevant to the scope of our review. Where a study includes multiple intervention groups, we will combine groups to create a single pair‐wise comparison. Where a study includes repeated observations, we will follow Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a).
Crossover trials
We do not anticipate identifying any cross‐over RCTs. However, if we identify any such studies, we will only use data from the first phase to avoid the risk of carry‐over effects, as described in Section 23.2.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a).
Cluster‐randomised trials
We do not anticipate identifying any cluster‐RCTs. However, if we identify any such studies, we will include them in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in Section 23.1.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both types of trials if there is little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
We will contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study is identified as an abstract only). Where possible, we will use the Review Manager calculator to calculate missing standard deviations using other data from the trial, such as confidence intervals. Where this is not possible, and the missing data are thought to introduce serious bias, we will explore the impact of including such studies in the overall assessment of results by a sensitivity analysis. For all outcomes, we will follow intention‐to‐treat (ITT) principles to the greatest degree possible, that is, we will analyse participants in their randomised group regardless of what intervention they actually received. We will use available‐case data for the denominator if ITT data are not available.
We will present study‐level data so that missing and unclear data are clearly indicated and to make available any unpublished data acquired from investigators.
Assessment of heterogeneity
We will inspect forest plots visually to consider the direction and magnitude of effects and the degree of overlap between confidence intervals. We will use the I2 statistic to measure heterogeneity among the trials in each analysis; we acknowledge that there is substantial uncertainty in the value of I2 when there is only a small number of studies. If we identify substantial heterogeneity, we will report it and explore possible causes by pre‐specified subgroup analysis.
As strict thresholds for interpretation of I2 are not recommended, we will follow the guide to interpretation in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
When the I2 value lies in an area of overlap between two categories (e.g. between 50% and 60%), we will consider differences in participants and interventions among the trials contributing data to the analysis (Deeks 2019).
Assessment of reporting biases
We will use funnel plots to investigate reporting biases if we identify 10 or more studies, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b).
Data synthesis
We will synthesise the data using Review Manager 5.3 (RevMan 2014). We will undertake meta‐analysis only where this is meaningful, that is if the treatments, participants, and the underlying clinical question are similar enough for pooling to be appropriate.
If we are confident that trials are estimating the same underlying treatment effect, i.e. the included studies are homogenous (considering population, interventions, comparators, and outcome characteristics), we will use a fixed‐effect meta‐analysis. If clinical heterogeneity is sufficient to expect that underlying treatment effects differ between trials or if at least substantial heterogeneity is identified, we will use a random‐effects meta‐analysis. If there is substantial clinical, methodological, or statistical heterogeneity across trials that prevents the pooling of data, we will use a narrative approach to data synthesis (Deeks 2019).
We will address all outcomes listed in the Types of outcome measures section in the Results section of the review under the heading 'Effects of interventions', with outcomes addressed in the order in which they are shown in Types of outcome measures. In addition, we will present one 'Summary of findings' table for each comparison, in which we will summarise the main outcomes. We will include the results of individual studies and any statistical summary of these in 'Data and analyses' tables in the review.
GRADE and 'Summary of findings' table
We will create 'Summary of findings' tables of comparisons that have included studies. In these tables, we will use the following outcomes:
neurological impairment;
ipsilateral major or disabling stroke;
stroke‐related mortality;
major bleeding;
progression of carotid stenosis;
adverse events;
quality of life.
We will use the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We will use methods and recommendations described in Chapter 15 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019), and GRADEproGDT software (GRADEproGDT 2015). We will create a separate 'Summary of findings' table for each comparison, for example: 1) anticoagulants plus antiplatelet agents versus antiplatelet agents; 2) one class of antiplatelet versus another class of antiplatelet, etc. We will justify all decisions to downgrade the quality of studies using footnotes, and we will make comments to aid the reader's understanding of the review where necessary.
Two review authors (CNBC, NC), working independently, will make judgements about evidence quality, with disagreements resolved by discussion or involving a third review author (RLGF). We will justify, document, and incorporate judgements into the reporting of results for each outcome.
We plan to extract study data, format our comparisons in data tables, and prepare 'Summary of findings' tables before writing the results and conclusions of our review. A template 'Summary of findings' table is included as Table 3.
3. Template for 'Summary of findings' table.
| Anticoagulants plus antiplatelet agents versus antiplatelet agents for asymptomatic carotid stenosis | ||||||
|
Patient or population: adults with asymptomatic carotid stenosis Settings: community Intervention: anticoagulants plus antiplatelet agents Comparison: antiplatelet agents | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with antiplatelet agents | Risk with anticoagulants plus antiplatelet agents | |||||
| [control] | [experimental] | |||||
| Neurological impairment | [mean difference] (CI) | [mean difference] (CI) | _ | [value] ([value]) |
⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
|
| Ipsilateral major or disabling stroke | [value] per 1000 | [value] per 1000 ([value] to [value]) | RR [value] ([value] to [value]) | [value] ([value]) |
⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
|
| Stroke‐related mortality | [value] per 1000 | [value] per 1000 ([value] to [value]) | RR [value] ([value] to [value]) | [value] ([value]) |
⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
|
| Major bleeding | [value] per 1000 | [value] per 1000 ([value] to [value]) | RR [value] ([value] to [value]) | [value] ([value]) |
⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
|
| Progression of carotid stenosis | [value] per 1000 | [value] per 1000 ([value] to [value]) | RR [value] ([value] to [value]) | [value] ([value]) |
⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
|
| Adverse events | [value] per 1000 | [value] per 1000 ([value] to [value]) | RR [value] ([value] to [value]) | [value] ([value]) |
⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
|
| Quality of life | [value] per 1000 | [value] per 1000 ([value] to [value]) | RR [value] ([value] to [value]) | [value] ([value]) |
⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. We are very uncertain about the estimate. | ||||||
Subgroup analysis and investigation of heterogeneity
We will perform subgroup analyses for the following factors, if sufficient data are available.
-
Participant characteristics:
age (e.g. adults (18 years to 74 years) and older people (75 years and over));
race;
comorbidities (e.g. tobacco addiction);
degree of baseline stenosis as defined by Grant 2003 and available at Table 2.
-
Intervention characteristics:
doses of drugs;
types of drugs (e.g. UFH, LMWH, VKA, DOAC among anticoagulants; aspirin, clopidogrel among antiplatelet agents);
route of administration (e.g. oral, intravenous, subcutaneous);
pre‐specified target achieved (e.g. low‐density lipoprotein level below 70 mg/dL).
We will use the following outcomes (i.e. the primary outcomes) in the subgroup analyses:
neurological impairment;
ipsilateral major or disabling stroke.
We will use the formal test for subgroup differences in Review Manager 5.3 (RevMan 2014) and base our interpretation on this.
Sensitivity analysis
We plan to carry out the following sensitivity analyses, to test whether key methodological factors or decisions have affected the main result. These analyses will be grouped according to study design (individual, cross‐over, or cluster).
Only including studies with a low risk of bias. We will consider a study to have a low risk of bias overall if there is no high‐risk judgement in any of the four main domains, i.e. random sequence generation, allocation concealment, incomplete outcome data, and selective reporting.
We will examine both the fixed‐effect model and random‐effects model meta‐analyses, and we will explore the differences between the two estimates.
If we identify studies with missing data that are unobtainable, we will repeat analyses excluding these studies to determine their impact on the primary analyses.
We will use the following outcomes (i.e. the primary outcomes) in the sensitivity analyses:
neurological impairment;
ipsilateral major or disabling stroke.
Reaching conclusions
We will base our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We will avoid making recommendations for practice and our implications for research will suggest priorities for future research and outline what the remaining uncertainties are in the area.
Acknowledgements
We thank Cochrane Stroke, Cochrane Brazil, Joshua David Cheyne (Cochrane Stroke's Information Specialist), and the Division of Vascular and Endovascular Surgery of Universidade Federal de Sao Paulo, Brazil, for their methodological support.
Also, we thank Peter Langhorne, Daniel Bereczki, Aryelly Rodriguez, Joshua David Cheyne, and Dominick McCabe for the peer review comments.
Parts of the methods section of this protocol are based on a standard template established by Cochrane.
Appendices
Appendix 1. MEDLINE search strategy
1. carotid artery diseases/ or carotid artery thrombosis/ or carotid stenosis/ 2. carotid arteries/ or carotid artery, common/ or carotid artery, external/ or carotid artery, internal/ 3. (carotid adj5 (stenosis or thrombo$ or disease$ or narrow$ or plaque$ or arterioscler$ or atheroscler$)).tw. 4. or/1‐3 5. exp Asymptomatic Diseases/ 6. asymptomatic.tw. 7. 5 or 6 8. 4 and 7 9. randomized controlled trial.pt. 10. controlled clinical trial.pt. 11. randomized.ab. 12. placebo.ab. 13. randomly.ab. 14. trial.ab. 15. groups.ab. 16. or/9‐15 17. 8 and 16
Appendix 2. Enquiry letter
Dear Doctor
I am currently conducting a systematic review entitled 'Pharmacological interventions for asymptomatic carotid stenosis' with the Cochrane Stroke Group based in the University of Edinburgh. To ensure that the results are valid, it is essential that all relevant trials are included.
Cochrane was established to ensure all forms of health care will be subject to critical evaluation using standard criteria and specialised software.
As a [manufacturer/expert/trialist] of [drug/intervention name], it is possible that a trial of this or a similar agent has been conducted in patients with asymptomatic carotid stenosis. If so, we would be grateful if you could supply us with copies of any relevant protocols, reports or publications in the first instance; later it may become necessary to obtain the raw data. If the trial is eligible for inclusion in the review, [Pharmaceutical company/specialist name] will be cited in the final report which will be published electronically within the Cochrane Database of Systematic Reviews, and in standard medical journals
I would be grateful if you could fill in the accompanying form, and forward any information which you feel may be appropriate.
Thank you for your help.
Yours faithfully
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Form for reply from Pharmaceutical Company/Trialist/Expert
Trials that fulfil the following criteria will be eligible for inclusion in the review:
Types of participants:
Treatment regimen:
A valid randomisation method:
For example: a centralised scheme, e.g. by telephone or scheme controlled by pharmacy, e.g. pre‐coded or numbered containers or on‐site computer system where allocations are in a locked unreadable file or assignment envelopes ‐ sequentially numbered, sealed and opaque or other combinations which provide assurance of adequate concealment. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Name of Pharmaceutical Company/Trialist/Expert
Name (person to whom any future correspondence should be addressed):
Trials fulfilling the above criteria:
Have not been conducted ( ) Are currently underway * ( ) Have been conducted in the past * ( )
* Please enclose relevant protocols, citations, reports or other publications
Thank you for your valuable help.
Please complete and return to:
Dr Caroline NB Clezar, MD Department of Surgery, Division of Vascular and Endovascular Surgery Universidade Federal de São Paulo Rua Borges Lagoa, 754 São Paulo Brazil
e‐mail: caroline.bessa@gmail.com
Contributions of authors
CNBC drafted the protocol and is the guarantor of the review. NC drafted the protocol. CDQF drafted the protocol. LCUN drafted the protocol. VFMT drafted the protocol. RLGF drafted the protocol. All authors reviewed and approved the protocol content prior to submission.
Sources of support
Internal sources
New Source of support, Other.
External sources
-
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
This study was financed in part by CAPES, finance code 001.
Declarations of interest
CNBC: none known. NC: none known. CDQF: none known. LCUN: none known. VFMT: none known. RLGF: none known.
New
References
Additional references
Abbott 2007
- Abbott AL, Bladin CF, Levi CR, Chambers BR. What should we do with asymptomatic carotid stenosis?. International Journal of Stroke 2007;2(1):27‐39. [PUBMED: 18705984] [DOI] [PubMed] [Google Scholar]
ACAS 1995
- Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, et al. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273(18):1421‐8. [PUBMED: 7723155] [PubMed] [Google Scholar]
Amarenco 2006
- Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High‐dose atorvastatin after stroke or transient ischemic attack. New England Journal of Medicine 2006;355(6):549‐59. [PUBMED: 16899775] [DOI] [PubMed] [Google Scholar]
Arnett 2019
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596‐646. [PUBMED: 30879355] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2019
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics ‐ 2019 update: a report from the American Heart Association. Circulation 2019;139(10):e56‐e528. [PUBMED: 30700139] [DOI] [PubMed] [Google Scholar]
Brott 2013
- Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Developed in collaboration with the American Academy of Neurology and Society of Cardiovascular Computed Tomography. Catheterization and Cardiovascular Interventions 2013;81(1):E76‐123. [PUBMED: 23281092] [DOI] [PubMed] [Google Scholar]
Bulbulia 2017
- Bulbulia R, Halliday A. The Asymptomatic Carotid Surgery Trial‐2 (ACST‐2): an ongoing randomised controlled trial comparing carotid endarterectomy with carotid artery stenting to prevent stroke. Health Technology Assessment 2017;21(57):1‐40. [PUBMED: 29019319] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassola 2018
- Cassola N, Baptista‐Silva JCC, Flumignan CDQ, Sesso R, Vasconcelos V, Flumignan RLG. Duplex ultrasound for diagnosing symptomatic carotid stenosis in the extracranial segments. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.CD013172] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2001
- Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults‐United States, 1999. Morbidity and Mortality Weekly Report 2001;50(7):120‐5. [PUBMED: 11393491] [PubMed] [Google Scholar]
Chiquete 2014
- Chiquete E, Torres‐Octavo B, Cano‐Nigenda V, Valle‐Rojas D, Dominguez‐Moreno R, Tolosa‐Tort P, et al. Characterisation of factors associated with carotid stenosis in a population at high risk [Caracterizacion de factores asociados con estenosis carotidea en una poblacion de alto riesgo]. Revista de Neurologia 2014;58(12):541‐7. [PUBMED: 24915030] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Melbourne, Australia: Veritas Health Innovation, accessed at 25 March 2020.
Daolio 2019
- Daolio RM, Cassola N, Flumignan C, Nakano L, Guedes H, Amorim J, et al. PC126. Accuracy of vascular ultrasound compared with computed tomography angiography for extracranial carotid stenosis imaging. Journal of Vascular Surgery 2019;69(6):e239‐40. [DOI: 10.1016/j.jvs.2019.04.356] [DOI] [Google Scholar]
De Waard 2017
- Waard DD, Morris D, Borst GJ, Bulbulia R, Halliday A. Asymptomatic carotid artery stenosis: who should be screened, who should be treated and how should we treat them?. Journal of Cardiovascular Surgery 2017;58(1):3‐12. [PUBMED: 27901325] [DOI] [PubMed] [Google Scholar]
De Weerd 2010
- Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta‐analysis. Stroke 2010;41(6):1294‐7. [PUBMED: 20431077] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta‐analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6 (updated July 2019). Cochrane , 2019. Available from www.training.cochrane.org/handbook. Cochrane.
Derdeyn 2007
- Derdeyn CP. Carotid stenting for asymptomatic carotid stenosis: trial it. Stroke 2007;38(2 Suppl):715‐20. [PUBMED: 17261723] [DOI] [PubMed] [Google Scholar]
Divya 2015
- Divya KP, Sandeep N, Sarma S, Sylaja PN. Risk of stroke and cardiac events in medically treated asymptomatic carotid stenosis. Journal of Stroke and Cerebrovascular Diseases 2015;24(9):2149‐53. [PUBMED: 26142257] [DOI] [PubMed] [Google Scholar]
Easton 2009
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40(6):2276‐93. [PUBMED: 19423857] [DOI] [PubMed] [Google Scholar]
ECST 1998
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; Vol. 351, issue 9113:1379‐87. [PUBMED: 9593407] [PubMed]
Feigin 2014
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990‐2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383(9913):245‐54. [PUBMED: 24449944] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feigin 2016
- Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurology 2016;15(9):913‐24. [PUBMED: 27291521] [DOI] [PubMed] [Google Scholar]
Flaherty 2013
- Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013;40(1):36‐41. [PUBMED: 23075828] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2017
- Flumignan CDQ, Flumignan RLG, Navarro TP. Extracranial carotid stenosis: evidence based review [Estenose de carotida extracraniana: revisao baseada em evidencias]. Revista do Colegio Brasileiro de Cirurgioes 2017;44(3):293‐301. [PUBMED: 28767806] [DOI] [PubMed] [Google Scholar]
Gorelick 1999
- Gorelick PB, Sacco RL, Smith DB, Alberts M, Mustone‐Alexander L, Rader D, et al. Prevention of a first stroke: a review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. JAMA 1999;281(12):1112‐20. [PUBMED: 10188663] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Grant 2003
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray‐scale and Doppler US diagnosis ‐ Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229(2):340‐6. [PUBMED: 14500855] [DOI] [PubMed] [Google Scholar]
Guzik 2017
- Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum 2017;23(1):15‐39. [PUBMED: 28157742] [DOI] [PubMed] [Google Scholar]
Halliday 2004
- Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363(9420):1491‐502. [PUBMED: 15135594] [DOI] [PubMed] [Google Scholar]
Herder 2012
- Herder M, Johnsen SH, Arntzen KA, Mathiesen EB. Risk factors for progression of carotid intima‐media thickness and total plaque area: a 13‐year follow‐up study: the Tromso Study. Stroke 2012;43(7):1818‐23. [PUBMED: 22550052] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019a
- Higgins JPT, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. Cochrane.
Higgins 2019b
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hillen 2003
- Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke 2003;34(6):1457‐63. [PUBMED: 12750544] [DOI] [PubMed] [Google Scholar]
Holman 2014
- Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose‐lowering drugs or strategies in type 2 diabetes. Lancet 2014;383(9933):2008‐17. [PUBMED: 24910232] [DOI] [PubMed] [Google Scholar]
Lawes 2004
- Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke 2004;35(3):776‐85. [PUBMED: 14976329] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al. (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Moher 2010
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. International Journal of Surgery 2010;8(5):336‐41. [PUBMED: 20171303] [DOI] [PubMed] [Google Scholar]
Moore 1995
- Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott T, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation 1995;91(2):566‐79. [PUBMED: 7805271] [DOI] [PubMed] [Google Scholar]
Mozaffarian 2016
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics ‐ 2016 update: a report from the American Heart Association. Circulation 2016;133(4):e38‐360. [PUBMED: 26673558] [DOI] [PubMed] [Google Scholar]
Murphy 2019
- Murphy SJX, Naylor AR, Ricco JB, Sillesen H, Kakkos S, Halliday A, et al. Optimal antiplatelet therapy in moderate to severe asymptomatic and symptomatic carotid stenosis: a comprehensive review of the literature. European Journal of Vascular and Endovascular Surgery 2019;57(2):199‐211. [PUBMED: 30414802] [DOI] [PubMed] [Google Scholar]
NASCET 1991
- Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis. New England Journal of Medicine 1991;325(7):445‐53. [PUBMED: 1852179] [DOI] [PubMed] [Google Scholar]
Naylor 2018
- Naylor AR, Ricco JB, Borst GJ, Debus S, Haro J, Halliday A, et al. Editor's choice ‐ Management of atherosclerotic carotid and vertebral artery disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2018;55(1):3‐81. [PUBMED: 28851594] [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Clinical Excellence (NICE). Impact stroke. NICE practice guidelines 2019. Available from www.nice.org.uk/Media/Default/About/what‐we‐do/Into‐practice/measuring‐uptake/NICE‐Impact‐stroke.pdf (accessed 09 July 2019).
O'Donnel 2016
- O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang X, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet 2016;20(388):761‐75. [DOI] [PubMed] [Google Scholar]
Park 2019
- Park MS, Kwon S, Lee MJ, Kim KH, Jeon P, Park YJ, et al. Identification of high risk carotid artery stenosis: a multimodal vascular and perfusion imaging study. Frontiers in Neurology 2019;10:765. [PUBMED: 31379719] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raman 2013
- Raman G, Moorthy D, Hadar N, Dahabreh IJ, O'Donnell TF, Thaler DE, et al. Management strategies for asymptomatic carotid stenosis: a systematic review and meta‐analysis. Annals of Internal Medicine 2013;158(9):676‐85. [PUBMED: 23648949] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3. Copenhagen: Cochrane, 2014.
Ricotta 2011
- Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. Journal of Vascular Surgery 2011;54(3):e1‐31. [PUBMED: 21889701] [DOI] [PubMed] [Google Scholar]
Schulman 2010
- Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. Journal of Thrombosis and Haemostasis 2010;8(1):202‐4. [PUBMED: 19878532] [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou Petal. Chapter 15: Interpreting results and drawing conclusions. Draft version (29 January 2019) for inclusion in: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6 (updated January 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook (accessed 24 September 2019). [Google Scholar]
Sharma 2019
- Sharma M, Hart RG, Connolly SJ, Bosch J, Shestakovska O, Ng KKH, et al. Stroke outcomes in the COMPASS Trial. Circulation 2019;139(9):1134‐45. [PUBMED: 30667279] [DOI] [PubMed] [Google Scholar]
Strong 2007
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurology 2007;6(2):182‐7. [PUBMED: 17239805] [DOI] [PubMed] [Google Scholar]
Taylor 2002
- Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: ARterial Biology for the Investigation of the Treatment Effects of Reducing cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 2002;106(16):2055‐60. [PUBMED: 12379573] [DOI] [PubMed] [Google Scholar]
Taylor 2013
- Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD004816.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venkatachalam 2014
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting?. Current Cardiology Reports 2014;16(1):440. [PUBMED: 24258207] [DOI] [PubMed] [Google Scholar]
Wardlaw 2006
- Wardlaw JM, Chappell FM, Stevenson M, Nigris E, Thomas S, Gillard J, et al. Accurate, practical and cost‐effective assessment of carotid stenosis in the UK. Health Technology Assessment 2006;10(30):iii‐iv, ix‐x, 1‐182. [PUBMED: 16904049] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JEJ, Sherbourne CD. The MOS 36‐item Short‐form Health Survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] [PubMed] [Google Scholar]
Wilson 2019
- Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139(25):e1144‐61. [PUBMED: 30586775] [DOI] [PubMed] [Google Scholar]
Zhan 2018
- Zhan S, Tang M, Liu F, Xia P, Shu M, Wu X. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.CD012502.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang C, Zhou YH, Xu CL, Chi FL, Ju HN. Efficacy of intensive control of glucose in stroke prevention: a meta‐analysis of data from 59,197 participants in 9 randomized controlled trials. PLoS One 2013;8(1):e54465. [PUBMED: 23372729] [DOI] [PMC free article] [PubMed] [Google Scholar]


