Abstract
Rationale:
Excessive vasoconstriction in response to mental stress may be a potential mechanism by which acute psychological stress leads to adverse cardiac events.
Objectives:
We investigated whether excessive digital vasoconstriction during acute mental stress predicts adverse cardiovascular outcomes among patients with coronary artery disease (CAD).
Methods and Results:
549 patients with stable CAD (age 63±9, 76% male, 29% Black) underwent mental stress testing with a standardized public speaking stressor and followed prospectively for cardiovascular endpoints. Digital pulse wave amplitude was continuously measured using peripheral artery tonometry (PAT, Itamar Inc.). Stress/rest PAT ratio (sPAT) of pulse wave amplitude during mental stress/baseline was calculated and dichotomized by the median value into “low” and “high” sPAT ratio groups. Upon 3-year follow-up, Fine and Gray’s sub-distribution hazard ratios (sHR) were used to examine the association between sPAT ratio and the composite endpoint of cardiovascular death, myocardial infarction, revascularization, and hospitalization for heart failure. The median sPAT ratio was 0.68 (interquartile range 0.48 – 0.88), indicating 32% vasoconstriction with mental stress. Men were more likely to have low sPAT ratio than women (odds ratio [OR] 1.79, P=0.007) while those on beta blockers were less likely to have low sPAT ratio (OR 0.52, P=0.003). After adjusting for demographic and cardiovascular risk factors, medications, and rate-pressure product change during mental stress, those with low sPAT ratio were at significantly higher risk of adverse outcomes (sHR 1.77 [95% CI 1.12 – 2.80]).
Conclusions:
Greater peripheral vasoconstriction with mental stress, denoted by a low sPAT ratio, is associated with a higher risk of adverse cardiovascular outcomes in patients with CAD.
Keywords: Mental stress, vasomotion, cardiovascular outcomes, coronary artery disease, vasoconstriction, cardiovascular events, outcome
Subject Terms: Clinical Studies, Coronary Artery Disease, Vascular Biology
Graphical Abstract
INTRODUCTION
Observational studies have suggested that acute psychological stress is associated with adverse cardiovascular outcomes.1, 2 Yet, the mechanisms for this effect remain incompletely understood.2 Potential mechanistic pathways, particularly among patients with established atherosclerotic disease, include paradoxical vasoconstriction of the atherosclerotic epicardial arteries and coronary microvessels in response to mental stress,3–5 leading to myocardial ischemia,6, 7 plaque disruption,8 platelet aggregation,9 and/or cardiac arrhythmia10, 11, which would eventually result in myocardial infarction or death.
Abnormalities in coronary vasomotion are thought to play a critical role in precipitation of myocardial ischemia due to mental stress and mental stress-related cardiac events,7, 12 but are difficult to ascertain routinely because of the invasive nature of coronary vasomotor tests. To overcome these shortcomings, we and others have investigated peripheral arterial tonometry (PAT)-derived changes in the digital pulse amplitude during mental stress as a reflection of systemic vasomotor changes during mental stress.13–17 We calculated stress/rest PAT (sPAT) ratio as a ratio of the pulse wave amplitude of the digit during mental stress compared to the resting amplitude, where a value <1 signifies vasoconstriction. We found that peripheral vasoconstriction measured by sPAT is a strong predictor of developing myocardial ischemia during mental stress,13–17 and more importantly, it correlates with the coronary vasomotor responses to mental stress.18 Thus, the sPAT ratio may provide a readily available, non-invasive and feasible method to quantitate the coronary vasomotor responses during mental stress.
In the current investigation, using a large cohort of patients with stable coronary artery disease (CAD), we sought to identify the determinants of greater peripheral vasoconstriction in response to acute mental stress, and investigated whether peripheral vasoconstriction during acute mental stress have implications in predicting incident adverse cardiovascular events. We hypothesized that excessive vasoconstriction in response to mental stress would be a potential link between psychological stress and adverse cardiovascular events, and that greater peripheral vasoconstriction, as denoted by a lower sPAT ratio, would be associated with a greater risk of incident adverse cardiac events.
METHODS
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
Study design and population.
A total of 695 subjects were enrolled from the Mental Stress Ischemia Prognosis Study (MIPS),19 a prospective cohort study that enrolled patients with stable CAD between June 2011 and August 2014 from Emory University affiliated hospitals. Presence of CAD was defined either by an abnormal coronary angiogram showing evidence of atherosclerosis with at least luminal irregularities, previous percutaneous or surgical coronary revascularization, previous myocardial infarction (MI), or positive nuclear stress test. Exclusion criteria included acute coronary syndrome or decompensated heart failure (HF) in the previous two months, end-stage renal disease, and severe psychiatric conditions that would affect study assessments, such as schizophrenia. Clinical information, such as previous CAD events, risk factors, and current medication use were obtained using standardized questionnaires and chart reviews. The study protocol was approved by the Institutional Review Board of Emory University, and all subjects provided informed consent. Online Figure I illustrates the inclusion of final analytical sample involved in the study.
Mental stress test.
Details of the study protocol have been published elsewhere.19 Briefly, the testing procedure was performed in the morning after 12-hour fast, and any anti-anginal medications (beta blockers, calcium channel blockers, and long acting nitrates), xanthine derivatives, and caffeine-containing products were withheld for 24 hours prior to the mental stress testing procedure to reduce potential interference of their vasoactive properties. Subjects first spent a 30-minute rest period in a temperature controlled (21–23°C), quiet, and dimly lit room. Then, vital signs were measured and a standardized mental stress protocol was performed as previously published.19 Specifically, the subjects were asked to imagine a stressful situation in which a close relative had been mistreated in a nursing home. They were subsequently asked to prepare a statement for 2 minutes, and were given the following 3 minutes to present it in front of an evaluative audience wearing white coats. All mental stress testing procedures were conducted by trained and experienced staff to ensure standardization of the stress-provoking elements. Throughout the protocol, hemodynamic parameters, including blood pressure and heart rate were recorded using IntelliSense Professional Digital Blood Pressure Monitor (HEM-907EL, OMRON, Japan). The cuff was positioned over the upper arm of the contralateral side to the PAT device, and each hemodynamic parameter was measured every 5 minutes during the rest period and every 1 minute during the mental stress period, followed by every 5 minutes during the recovery period. Rate-pressure product (RPP) was calculated as systolic blood pressure × heart rate, and the change in RPP was quantified as the percentage difference between the maximum value during the speech and the minimum resting value at baseline.
Digital blood flow measurement via finger plethysmography.
The PAT (Itamar-Medical, Israel) device was used to measure digital arterial pulse wave amplitude continuously during rest and the mental stress test, as previously described.15, 16 Briefly, the PAT uses a modified form of plethysmography to measure pulsatile blood volume changes (Online Figure II). The probe was applied on the index finger of the contralateral side to blood pressure measurement. The PAT probe applies a constant sub-diastolic pressure over the distal two thirds of the finger to prevent distal venous blood stasis, unload arterial wall tension, and stabilize the probe to reduce noise. As a result, the changes in pulsatile volume only reflect changes in digital arterial blood perfusion. The device is also connected via thin tubing to an isolated volume reservoir to buffer within the probe itself. Pulsatile pressure changes from the probe are registered from a pressure transducer, and then fed into a specialized software which filters, amplifies, stores, and analyzes the signal in an operator-independent manner. The baseline pulse wave amplitude during rest was determined by averaging the last 3 minutes of recording that preceded the mental stress test. The pulse wave amplitude during the mental stress test was determined visually as the area of maximum vasoconstriction during the speaking period with a duration 30 second to 2 minutes. sPAT ratio during mental stress was calculated as the ratio of pulse wave amplitudes during mental stress over the resting baseline, such that a ratio <1 signifies peripheral arterial vasoconstriction during mental stress. Subjects were categorized into those with low sPAT ratio (≤ median) and those with high sPAT ratio (> median) for analysis. Illustrative examples of sPAT tracings for those with low sPAT ratio and high sPAT ratio are displayed in Online Figure III.
Catecholamine response, inflammatory biomarker, and endothelial function.
To explore potential mechanisms of sPAT response during mental stress, catecholamine response to mental stress was examined. As previously described,20 plasma levels of epinephrine and norepinephrine were measured at rest and 2 minutes into the speech task (EIA Kit; 2-CAT ELISA, Labor Diagnostika Nord as supplied by Rocky Mountain Scientific, Centennial, Co). The catecholamine response during mental stress was defined as the value during mental stress minus the value at baseline (N=416 for both epinephrine and norepinephrine). In addition, as an inflammatory biomarker, resting interleukin-6 (IL-6) levels were measured by a high-sensitivity assay using the MesoScale system (Meso Scale Diagnostics, Rockville, MD) with the SECTOR Imager 2400, as previously described (N=455).20 Finally, endothelial function was assessed at baseline by flow-mediated dilation (FMD) of brachial artery as previously described (N=495).17 Percent change in the brachial diameter during hyperemia in reference to the baseline diameter was used for analysis.
Follow-up.
Subjects were followed prospectively for adverse cardiovascular outcomes. Cardiovascular events including death, MI, coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting surgery), and hospitalization for HF were ascertained during face-to-face clinic visits at 1 and 2 years and by phone calls at 3 years, as well as through medical record review and by querying the Social Security Death Index. All events were adjudicated by study investigators (MH, AJS, and AQ) who were blinded to other study data. Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause (fatal MI), cardiac arrhythmia (including resuscitated), or HF. The endpoint of the study was cardiovascular death, MI, revascularization, or hospitalization for HF.
Statistical analyses.
Normal distribution of all continuous variables was tested with the parametric test Shapiro-Wilk. For comparison between those with low and high sPAT ratio based on the sample’s median value, two-sample t tests or Wilcoxon tests were used for continuous variables and chi-square tests were used for categorical variables. Logistic regression was used to identify independent predictors of low sPAT ratio during mental stress test. The covariates included demographic and medical history factors, including age, sex, race (Black vs. non-Black), body mass index (BMI), prior MI, hypertension, hyperlipidemia, diabetes, HF, smoking history, medication use (beta blocker and angiotensin converting enzyme [ACE] inhibitor use), and RPP change during mental stress. Multiple linear regression models were used to examine the association between sPAT ratio and catecholamine response / IL-6 / FMD while adjusting for the aforementioned covariates.
In survival analysis, the cumulative incidence function (CIF) of the study endpoint while treating non-cardiovascular death as competing risk was compared between those with low sPAT ratio and those with high sPAT using the CIF homogeneity test by Gray.21 Follow-up time was defined as the time from enrollment to one of the following: the first study endpoint, loss to follow-up, or end of follow-up. Then, Fine and Gray’s sub-distribution hazard model was used to investigate the association between low sPAT ratio and time to events while treating non-cardiovascular death as competing risk. Pre-defined covariates were included in a sequential fashion to the unadjusted model [Model 1] to assess the effect of covariate adjustments. First we added demographic variables (age, sex, race) [Model 2], followed by addition of baseline cardiovascular risk profile variables (BMI, hypertension, hyperlipidemia, diabetes, smoking history, prior MI, beta blocker / ACE inhibitor use, HF) [Model 3], and then addition of the percent change in RPP during mental stress testing [Model 4]. Lastly, we examined whether the association with the outcome differed with respect to the presence of pre-specified covariates (age, sex, race, BMI, prior BMI, diabetes, HF and beta blocker use) by including the interaction term between sPAT ratio and the corresponding covariate in the fully adjusted model (Model 4).
As a secondary analysis, the survival analysis was repeated in the same fashion while treating sPAT ratio as a continuous variable. We also explored the optimal cutoff of sPAT ratio that differentiated the cohort into high and low risk for the study endpoint. We performed log-rank tests at all the potential cutoff values within the data range, and the optimal sPAT ratio was selected as the cutoff that yielded the lowest P-value as previously described.22 Throughout all analyses, P-values < 0.05 were considered statistically significant, and all analyses were performed with SAS (version 9.4, Cary, NC) or R 3.4.1 (The R Foundation, Vienna, Austria).
RESULTS
Study population.
Among 695 participants recruited into the MIPS study, 2 participants were excluded due to ineligibility based on their history of lupus vasculitis and sickle cell disease. A total of 553 had sPAT readings of adequate quality at baseline and with mental stress to determine their sPAT ratio. Four additional subjects who were lost to follow-up were excluded, the study cohort comprised of 549 subjects with a mean age of 63 years, 76% male and 29% Black (Table 1). During mental stress testing, the median sPAT ratio was 0.68 [IQR 0.48 – 0.88] and the median RPP increase was 57% (interquartile range [IQR] 39 – 81).
Table 1.
Baseline demographic and clinical characteristics of the s stratified by the peripheral arterial tonometry (PAT) ratio*.
| Variables | All (N=549) | Low sPAT Ratio [≤median] (N=284) | High sPAT Ratio [>median] (N=265) | P-value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 62.7 ± 8.9 | 62.5 ± 9.0 | 62.9 ± 8.9 | 0.60 |
| Male | 417 (76) | 231 (81) | 186 (70) | 0.002 |
| Black race | 157 (29) | 82 (29) | 75 (28) | 0.88 |
| BMI, kg/m2 | 29.8 ± 5.3 | 30.1 ± 4.8 | 29.5 ± 5.7 | 0.20 |
| Ever smoking history | 330 (60) | 181 (64) | 149 (56) | 0.064 |
| Hypertension | 419 (76) | 228 (80) | 191 (72) | 0.024 |
| Diabetes mellitus | 181 (33) | 104 (37) | 77 (29) | 0.060 |
| Hyperlipidemia | 457(83) | 246 (87) | 211 (80) | 0.028 |
| Prior CABG | 185 (34) | 97 (34) | 88 (33) | 0.81 |
| Prior PTCA | 297 (54) | 150 (53) | 147 (56) | 0.53 |
| Prior MI | 203 (37) | 97 (34) | 106 (40) | 0.16 |
| Heart failure | 130 (24) | 68 (24) | 62 (23) | 0.88 |
| Ejection fraction†, % | 68 ± 14 | 66 ± 15 | 70 ± 13 | 0.059 |
| Mental stress testing related variables | ||||
| % RPP change‡ | 57 (39, 81) | 56 (39, 78) | 58 (38, 83) | 0.51 |
| Medication use | ||||
| Aspirin, % | 473 (86) | 246 (87) | 227 (86) | 0.75 |
| Beta blocker, % | 406 (74) | 197 (69) | 209 (79) | 0.011 |
| Clopidogrel, % | 184 (34) | 92 (32) | 92 (35) | 0.57 |
| Statin, % | 467 (85) | 241(85) | 226 (86) | 0.81 |
| ACE inhibitor, % | 243 (44) | 137 (48) | 106 (40) | 0.057 |
| ARB, % | 89 (16) | 42 (15) | 47 (18) | 0.35 |
| Calcium channel blocker, % | 123 (22) | 56 (20) | 67 (25) | 0.12 |
| Antidepressant, % | 117 (21) | 68 (24) | 49 (19) | 0.12 |
| Follow-up events | ||||
| All death | 24 (4) | 13 (5) | 11 (4) | 0.81 |
| CV death | 14 (3) | 9 (3) | 5 (2) | 0.34 |
| MI | 24 (4) | 13 (5) | 11 (4) | 0.81 |
| Coronary revascularization | 66 (12) | 39 (14) | 27 (10) | 0.20 |
| HF hospitalization | 20 (4) | 14 (5) | 6 (2) | 0.096 |
| CV death/MI/revascularization/HF hospitalization | 88 (16) | 56 (20) | 32 (13) | 0.015 |
Values are mean (SD), median (25th and 75th interquartile range), or n (%) unless specified otherwise.
Obtained from single-photon emission computerized tomography images as part of the parent study.19
RPP (rate-pressure product) was defined by systolic blood pressure × heart rate. % change was calculated as the maximum value during the mental stress test in comparison to the minimum value at rest.
Abbreviations: sPAT = Stress/rest Peripheral artery tonometry; BMI = body mass index; CABG = coronary artery bypass grafting, PTCA=percutaneous transluminal coronary angioplasty. RPP=rate pressure product; MSIMI = mental stress-induced myocardial ischemia; ACE = angiotensin converting enzyme, ARB = angiotensin II receptor blocker; CV=cardiovascular.
Association between sPAT Ratio and baseline characteristics.
In unadjusted analyses, subjects with low sPAT ratio (≤ median) were more likely to be male and to have a history of hypertension and hyperlipidemia, and less likely to be on a beta blocker than those with a high sPAT ratio (> median) (Table 1). In a multivariable logistic regression, male sex (odds ratio [OR] 1.70, 95% confidence interval [CI] 1.11 – 2.62) remained independently associated with a low sPAT ratio, and being on a beta blocker therapy (OR 0.53, 95% CI 0.34–0.81) remained inversely associated with a low sPAT ratio (Table 2).
Table 2.
Multivariable logistic regression of sPAT ratio (Low PAT ratio vs. High PAT ratio) as a function of baseline characteristics.
| Variables | OR, 95% C.I. | P-value |
|---|---|---|
| Age, per 10 years | 0.91 (0.74 – 1.12) | 0.35 |
| Male | 1.79 (1.17 – 2.75) | 0.007 |
| Black race | 1.06 (0.70 – 1.61) | 0.79 |
| BMI, per 5kg/m2 | 1.11 (0.92 – 1.33) | 0.27 |
| Prior MI | 0.85 (0.59 – 1.24) | 0.4 |
| Hypertension | 1.49 (0.95 – 2.34) | 0.084 |
| Hyperlipidemia | 1.44 (0.88 – 2.34) | 0.15 |
| Diabetes mellitus | 1.28 (0.86 – 1.89) | 0.23 |
| Ever smoking history | 1.30 (0.91 – 1.87) | 0.15 |
| Heart failure | 1.01 (0.66 – 1.55) | 0.97 |
| Beta blocker use | 0.52 (0.34 – 0.79) | 0.003 |
| ACE inhibitor use | 1.23 (0.85 – 1.77) | 0.27 |
| RPP change,* per 10% increase | 0.99 (0.94 – 1.05) | 0.82 |
RPP (rate-pressure product) was defined by systolic blood pressure × heart rate. % change was calculated as the maximum value during the mental stress test in comparison to the minimum value at rest.
Abbreviations: sPAT = stress/rest peripheral arterial tonometry; OR = odds ratio; C.I.= confidence interval; BMI = body mass index; MI = myocardial infarction; RPP = Rate pressure product.
Catecholamine response, inflammatory biomarker, and endothelial function.
We analyzed catecholamine response to mental stress, inflammatory biomarker, and endothelial function of those with low and high sPAT ratios as potential driving mechanisms of peripheral vasomotor response during mental stress. First, there was a significant inverse correlation between the change in norepinephrine levels and sPAT ratio (r=−0.22, P<0.001), such that those with low sPAT ratio had significantly higher change in norepinephrine levels (12.8 ± 191.9 pg/mL) than those with high sPAT ratio (−59.6 ± 256.8 pg/mL, P=0.001; Figure 1). In the multivariable model with adjustment for demographic / clinical factors, medication use (beta blocker and ACE inhibitor use), and RPP change during mental stress, 1 standard deviation increment of the change in norepinephrine level was significantly associated with 0.06 [−0.09 to −0.02] lower sPAT ratio, signifying 6% greater vasoconstriction (P=0.001). On the other hand, the change in epinephrine levels during mental stress was not significantly different between those with low and high sPAT ratio (23.0 ± 37.3 vs. 24.7 ± 38.7 pg/mL, P=0.65). The median level of IL-6 was lower among those with low PAT ratio than those with high sPAT ratio (1.3 [0.9, 1.9] pg/mL vs 1.4 [1.1, 2.2] pg/mL; P=0.009), but the association between sPAT ratio and IL-6 was no longer significant in the multivariable model with the aforementioned covariates (P=0.79). Similarly, FMD was also lower among those with low sPAT ratio than those with high sPAT ratio (4.1 [2.4, 6.7] % vs 3.3 [1.6, 5.7] %; P=0.005), but the association between sPAT ratio and FMD was not significant in the multivariable adjusted model (P=0.23).
Figure 1. Relationship between changes in circulating norepinephrine level and sPAT ratio during mental stress.
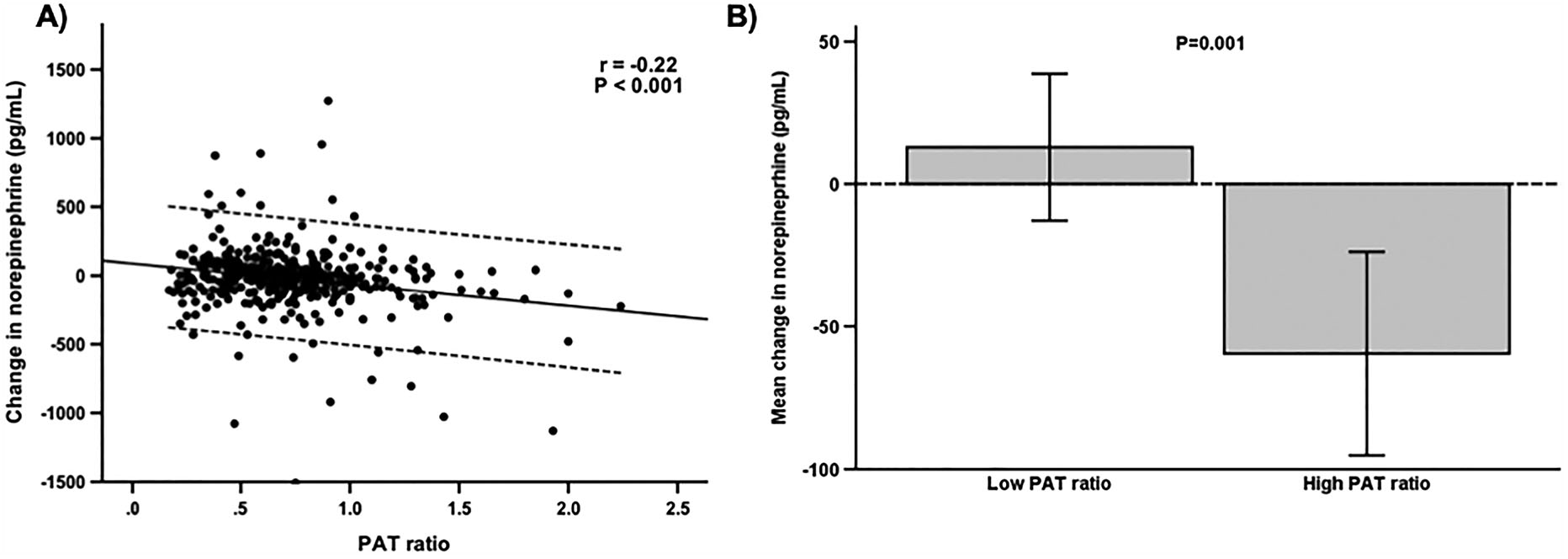
A) Change in plasma levels of norepinephrine during mental stress was inversely correlated with sPAT ratio. Solid line represents the line of best fit and dashed line represents 95% confidence interval. B) Those with low sPAT ratio had significantly greater change in circulation norepinephrine levels during mental stress. Error bars represents 95% confidence intervals of the mean values. Abbreviations: sPAT = peripheral arterial tonometry.
PAT ratio and adverse cardiovascular outcomes.
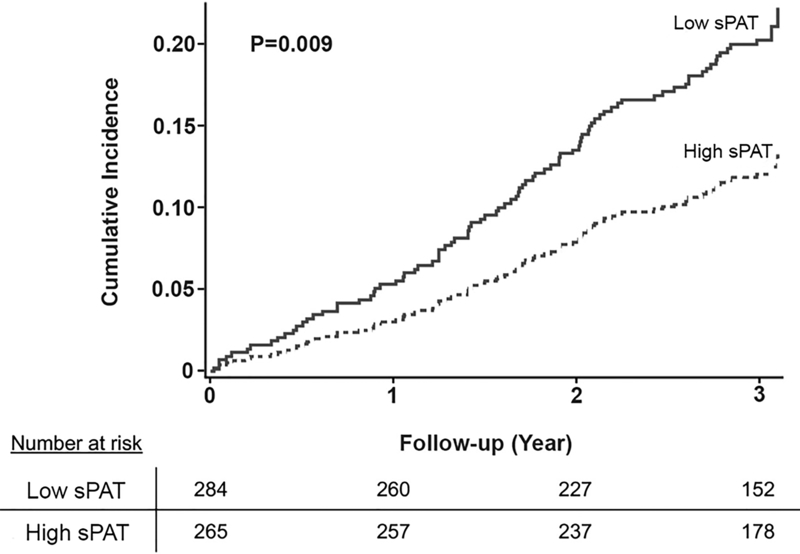
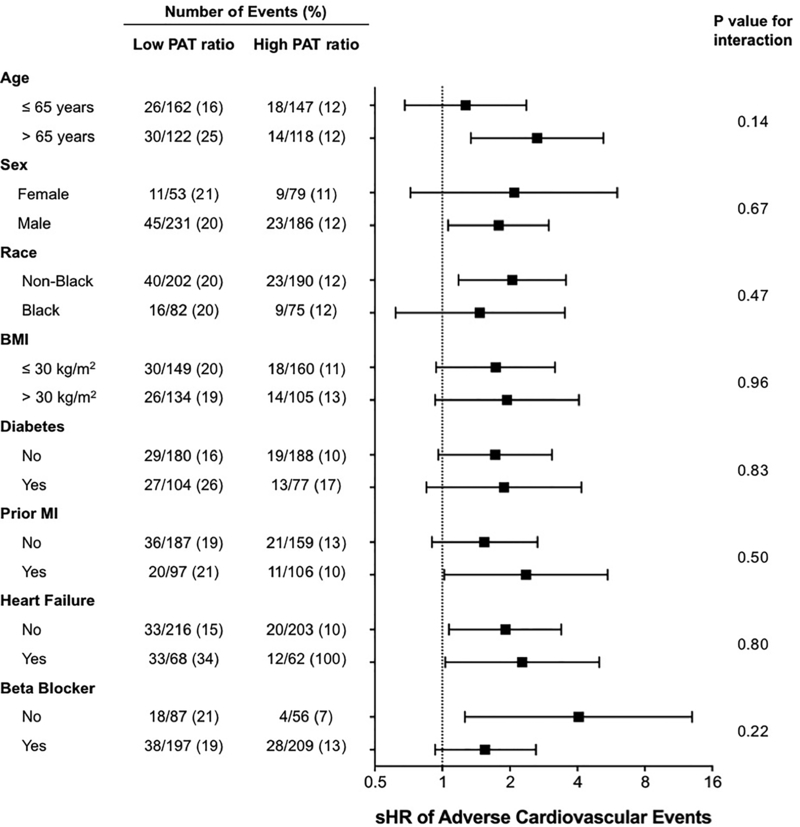
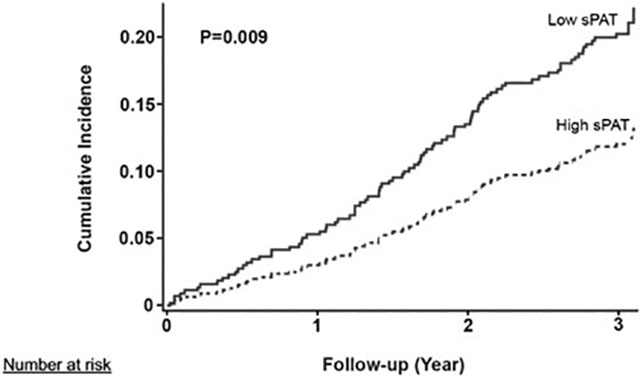
During a median follow-up duration of 3 years, 56 of 280 (20%) subjects with low sPAT ratio (≤median) and 32 of 265 (13%) with high sPAT ratio had adverse outcomes (Table 1). In unadjusted analysis using CIF homogeneity test of Gray, those with a low sPAT ratio had a significantly higher cumulative incidence of adverse cardiovascular events compared to those with a high sPAT ratio (P=0.009, Figure 2). In multivariate Fine and Gray’s models, a low sPAT ratio remained a significant predictor of adverse cardiovascular events after adjustment for demographic variables (sub-distribution hazard ratio [sHR] 1.77, 95% CI [1.14 – 2.76]; Model 2) and clinical variables (sHR 1.72 [1.08 – 2.73]; Model 3) (Table 3). Finally, adjusting for the percent change in RPP during mental stress did not change the results substantially either (sHR 1.77 [1.12 – 2.80]). Other independent predictors of adverse outcomes in the fully adjusted model (Model 4) included diabetes (sHR 1.77, P=0.012) and history of HF (sHR 2.24, P=0.0006; Online Table I). In sensitivity analyses, there was no statistically significant interaction with the covariates selected a priori (Figure 3). When the sPAT ratio was treated as a continuous variable, a similar association was observed, with higher risk associated with lower sPAT ratio, but it did not reach statistical significance (sHR 1.06 [0.99 – 1.14] per 0.1 decrease in sPAT ratio; Online Table II). We also explored the optimal cutoff for the sPAT ratio that best differentiated high- and low-risk groups in our sample with the lowest log-rank P values, and a sPAT ratio of 0.73 yielded the smallest P-value of 0.003 (Online Figure IV).
Figure 2. Cumulative incidence of adverse cardiovascular outcomes stratified by sPAT ratio (low vs. high).
P-values were generated from cumulative incidence function homogeneity test of Gray.
Table 3.
Association between sPAT ratio (low vs high) and the risk of adverse cardiovascular outcomes*
| Model | Covariates | sHR | 95% C.I. | P-value |
|---|---|---|---|---|
| Model 1 | Unadjusted | 1.77 | 1.15 – 2.73 | 0.010 |
| Model 2 | Model 1+ Demographic variables† | 1.77 | 1.14 – 2.76 | 0.011 |
| Model 3 | Model 2 + Clinical variables‡ | 1.72 | 1.08 – 2.73 | 0.022 |
| Model 4 | Model 3 + % RPP change§ | 1.77 | 1.12 – 2.80 | 0.014 |
Adverse cardiovascular outcomes were defined as cardiovascular death, myocardial infarction, revascularization, or hospitalization for heart failure while treating non-cardiovascular death as competing risk.
Age, sex, and race.
Body mass index, hypertension, hyperlipidemia, diabetes mellitus, smoking history, prior myocardial infarction, medication use (beta blocker, angiotensin converting enzyme inhibitor), and heart failure
RPP (rate-pressure product) was defined by systolic blood pressure × heart rate. % change was calculated as the maximum value during the mental stress test in comparison to the minimum value at rest.
Abbreviations: sPAT = stress/rest peripheral artery tonometry; sHR = sub-distribution hazard ratio; C.I. = Confidence intervals; RPP = rate-pressure product
Figure 3. Sensitivity analysis of the association between low sPAT ratio and adverse cardiovascular outcomes.
Adverse cardiovascular outcomes were defined as cardiovascular death, myocardial infarction, revascularization, or hospitalization for heart failure while treating non-cardiovascular death as competing risk. Abbreviations: sPAT = stress/rest peripheral arterial tonometry; sHR = sub-distribution hazard ratio; CI = confidence interval; BMI = body mass index; MI = myocardial infarction.
DISCUSSION
In a large cohort of patients with stable CAD, we demonstrated that the greater degree of peripheral vasoconstriction in response to acute mental stress, measured as the sPAT ratio, was associated with a higher risk of incident adverse cardiovascular events. To the best of our knowledge, this is the first investigation to demonstrate that peripheral vasoreactivity in response to acute psychological stress has prognostic implications in CAD. This relationship further advances our understanding of the linkage between acute psychological stress, vasoreactivity, and adverse outcomes in patients with CAD.
Cardiovascular reactivity to acute mental stress is believed to be mediated via the activation of the sympathetic nervous system and hypothalamus-pituitary-adrenal axis, leading to increases in heart rate, blood pressure, vasoconstriction, and vagal withdrawal.2, 23 Vasomotor responses to mental stress in both the coronary and peripheral circulations have been previously studied. Atherosclerotic epicardial arteries in patients with CAD and those with endothelial dysfunction tend to constrict in response to mental stress while normal coronary arteries dilate.3–5 We have previously demonstrated that patients with CAD had impaired vasodilation of the coronary microcirculation in response to mental stress that may be partly related to alpha-adrenergic activation.24 Similarly, blunted vasodilator response of peripheral conduit arteries25 as well as microvascular constriction leading to increases in peripheral vascular resistance26 in response to mental stress have been reported in patients with CAD. Lastly, we recently demonstrated by simultaneous measurements in the coronary and digital circulations that the peripheral vasomotor response during mental stress, expressed as the sPAT ratio, closely correlates with coronary vasomotion.18 Yet, to our knowledge, no study has specifically demonstrated the relationship between peripheral vasoconstrictor response to mental stress and incident adverse outcomes. Our current investigation uniquely contributes to the literature by demonstrating that greater peripheral vasoconstrictor response to mental stress, non-invasively measured as the sPAT ratio, is prognostic in CAD patients.
The relationship between the hemodynamic response to mental stress and outcomes has been previously reported. A greater hemodynamic response to acute mental stress involving an exaggerated rise in blood pressure or heart rate has been associated with incident hypertension27 and a higher mortality rate.28 Importantly, in our study, the prognostic value of peripheral vasoconstriction was independent of the hemodynamic response to mental stress that was assessed as the RPP change during mental stress. In fact, the RPP change was not a significant predictor of adverse outcomes in our study, suggesting that, in individuals with CAD, the peripheral vasoconstrictor response better predicts incident cardiovascular events than the hemodynamic reactivity to mental stress.
In the current investigation, we explored several potential mechanisms underlying the PAT ratio response to mental stress. While inflammation (IL-6), endothelial function (FMD), or epinephrine reactivity to mental stress were not independently associated with the sPAT ratio, we found a clear inverse relationship between norepinephrine reactivity to mental stress and sPAT ratio. Despite some inherent limitations of venous plasma norepinephrine measurements, such as presynaptic reuptake and rapid clearance of norepineprhine,29 our results suggest that peripheral vasoconstriction detected as a lower sPAT ratio, is associated with the release of norepinephrine in response to mental stress, leading to α-adrenergic activation. In other words, a pathologic vasoconstrictor response to mental stress could be a manifestation of maladaptive catecholamine reactivity, which has been previously linked to unfavorable cardiovascular outcomes, such as increased risk of incident hypertension.30 Interestingly, evidence also suggests that the catecholamine reactivity to mental stress is relatively stable within individuals up to 18 years.31 Thus, it would be pertinent to examine whether sPAT ratio response to mental stress is also similarly stable over time, which would further strengthen the value of sPAT ratio as a novel prognostic marker in patients with CAD.
Furthermore, though not examined directly in the current study, there are also other possible mechanisms that may mediate the association between excessive vasoconstriction during mental stress and adverse events. For example, coronary epicardial vasoconstriction with mental stress may trigger atherosclerotic plaque disruption and rupture due to changes in wall shear stress, ultimately leading to acute coronary events.32–35 Experimental studies have suggested that chronic intermittent mental stress results in more plaque formation with vulnerable features, such as greater inflammatory changes and thinner cap.36 In addition, coronary vasoconstriction could trigger malignant arrhythmia leading to adverse events. Endothelin, an important physiologic mediator of vasoconstriction with increased activity in atherosclerotic disease, has been suggested as a mechanistic link between vasoconstriction and arrhythmogenesis.37–41 Finally, pathologic vasoconstrictor response to mental stress may be also a manifestation of maladaptive autonomic response of brain circuits to psychological stress. We recently demonstrated that both peripheral vasoreactivity and transient myocardial ischemia in response to mental stress are associated with activation of specific brain areas involved in stress response and autonomic regulation.42, 43 It should be recognized that response to laboratory-provoked mental stress has been shown to reasonably approximate responses to stress in daily life,44, 45 and therefore, those with excessive vasoconstriction to the laboratory-provoked mental stress would have repeated, pathologic vasoconstrictor response during their daily lives, ultimately leading to adverse cardiac events via the aforementioned potential mechanisms.
We have also identified several subgroups that may be more predisposed to this phenomenon. First, we and others have previously reported that men tended to have greater peripheral vasoconstriction (low sPAT ratio) in response to mental stress than women.46, 47 Potential reasons for sex differences in sPAT ratio include vasodilatory effect of estrogen48 as well as differences in the sensitivity of adrenergic receptors in peripheral vasculature.49, 50 However, the increased risk from excessive vasoconstriction was observed in both sexes. Secondly, current use of beta blockers was associated with less peripheral vasoconstriction during mental stress. Previous studies have shown the beneficial effects of beta blockade on stress-mediated coronary vasoconstriction.51 It is also plausible that subjects using beta blockers may have experienced reduced stress reactivity related to altered stress perception. Nevertheless, beta blockers were withheld for 24 hours prior to the mental stress testing in our protocol, and there was no significant difference by the use of beta blocker therapy in the overall adverse impact of low sPAT ratio on outcomes.
Strength and limitations.
Using a non-invasive method for assessment of peripheral vasomotion during mental stress that we had previously shown to correlate with vasomotion in the coronary circulation, we demonstrate the value of this test as an independent determinant of outcomes in a large cohort of stable CAD patients. We have also minimized misclassification bias by excluding subjects with poor quality pulse recording at baseline or during mental stress. Limitations of this study include its single center nature and therefore the need for independent replication. In particular, a cut-off value for the risk associated with sPAT ratio, if one truly exists, needs to be confirmed in an independent sample. Finally, our study protocol utilized a single public speaking stressor. Whether other modalities of psychological stress testing or the use of multiple stressors can reproduce similar results needs to be investigated. Finally, our findings may not be applicable in subjects without CAD. Despite these limitations, our study is strengthened by the large size of well-characterized cohort of patients with CAD, the use of the novel technique to use sPAT ratio to quantify peripheral vasomotion, and its prospective design to study the risk of incident cardiovascular outcomes.
Conclusion.
In a large cohort of stable CAD patients, we demonstrate that a greater degree of peripheral digital vasoconstriction during mental stress is associated with a higher risk of adverse cardiovascular outcomes. These results demonstrate the importance of systemic vasoreactivity in response to psychological stress as a predictor of cardiovascular outcomes. Whether therapeutic interventions targeting peripheral vasomotor responses to stress will improve clinical outcomes requires further investigation.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Acute psychological stress is associated with adverse cardiovascular outcomes.
Potential mechanisms among patients with coronary artery disease include paradoxical vasoconstriction of the atherosclerotic epicardial and coronary microvessels leading to myocardial ischemia and/or infarction.
Peripheral vasoconstriction during acute psychological stress reflects similar changes in coronary blood flow.
The clinical significance of peripheral vasoconstriction in response to mental stress is unknown.
What New Information Does This Article Contribute?
Greater peripheral vasoconstriction is associated with greater norepinephrine release with stress.
Excessive peripheral vasoconstriction in response to acute mental stress is an independent predictor of future adverse cardiovascular events.
In a prospective cohort study, we explored the prognostic value of microvascular reactivity to acute psychological stress as a marker for adverse cardiovascular events. This study shows that greater peripheral microvascular constriction during acute psychological stress, mediated at least partly by alpha sympathetic nervous system activation, independently predicts adverse cardiovascular events in subjects with stable coronary artery disease. Overall, these findings illustrate the mechanistic link between psychological stress and adverse cardiovascular events. Peripheral microvascular constriction during psychological stress may be employed as a biomarker of cardiovascular stress vulnerability. Whether therapeutic interventions targeting peripheral vasoconstriction with stress will improve clinical outcomes requires further investigation.
ACKNOWLEDGEMENTS
We would like to thank Nancy Murrah, Joy Hartsfield, and Lucy Shallenberger for their contribution to the study with data collection, management, and study operation.
SOURCES OF FUNDING
This work was supported by the NIH (P01 HL101398, P20HL113451-01, P01HL086773-06A1, R56HL126558-01, R01 HL109413, R01HL109413-02S1, UL1TR000454, KL2TR000455, K23HL127251, K24HL077506, K24 MH076955, and K12HD085850). Also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR002378, TL1TR002382. The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Nonstandard Abbreviations and Acronysms:
- ACE
Angiotensin converting enzyme
- BMI
Body mass index
- CAD
Coronary artery disease
- CI
Confidence interval
- CIF
Cumulative incidence function
- EF
Ejection fraction
- FMD
Flow-mediated dilation
- HF
Heart failur
- IL-6
Interleukin-6
- MI
Myocardial infarction
- IQR
Interquartile range
- sPAT ratio
Peripheral arterial tonometry ratio (stress/rest)
- RPP
Rate-pressure product
- sHR
Sub-distribution hazard ratio
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Dimsdale JE. Psychological stress and cardiovascular disease. Journal of the American College of Cardiology. 2008;51:1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9:360. [DOI] [PubMed] [Google Scholar]
- 3.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. New England Journal of Medicine. 1991;325:1551–1556 [DOI] [PubMed] [Google Scholar]
- 4.Boltwood MD, Taylor CB, Burke MB, Grogin H, Giacomini J. Anger report predicts coronary artery vasomotor response to mental stress in atherosclerotic segments. The American Journal of Cardiology. 1993;72:1361–1365 [DOI] [PubMed] [Google Scholar]
- 5.Lacy CR, Contracta RJ, Robbins ML, Tannenbaum AK, Moreyra AE, Chelton S, Kostis JB. Coronary vasoconstriction induced by mental stress (simulated public speaking). The American Journal of Cardiology. 1995;75:503–505 [DOI] [PubMed] [Google Scholar]
- 6.Vaccarino V. Mental stress-induced myocardial ischemia. In: Baune BT, Tully PJ, eds. Cardiovascular diseases and depression: Treatment and prevention in psychocardiology. Cham: Springer International Publishing; 2016:105–121. [Google Scholar]
- 7.Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016;102:472–480 [DOI] [PubMed] [Google Scholar]
- 8.Stone PH. Triggering myocardial infarction. New England Journal of Medicine. 2004;351:1716–1718 [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Boyle SH, Ortel TL, Samad Z, Velazquez EJ, Harrison RW, Wilson J, Kuhn C, Williams RB, O’Connor CM, Becker RC. Platelet aggregation and mental stress induced myocardial ischemia: Results from the responses of myocardial ischemia to escitalopram treatment (remit) study. American heart journal. 2015;169:496–507.e491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg JS, Arshad A, Kowalski M, Kukar A, Suma V, Vloka M, Ehlert F, Herweg B, Donnelly J, Philip J, Reed G, Rozanski A. Increased incidence of life-threatening ventricular arrhythmias in implantable defibrillator patients after the world trade center attack. Journal of the American College of Cardiology. 2004;44:1261–1264 [DOI] [PubMed] [Google Scholar]
- 11.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106:1800–1805 [DOI] [PubMed] [Google Scholar]
- 12.Steptoe A, Brydon L. Emotional triggering of cardiac events. Neuroscience & Biobehavioral Reviews. 2009;33:63–70 [DOI] [PubMed] [Google Scholar]
- 13.Goor DA, Sheffy J, Schnall RP, Arditti A, Caspi A, Bragdon EE, Sheps DS. Peripheral arterial tonometry: A diagnostic method for detection of myocardial ischemia induced during mental stress tests: A pilot study. Clinical Cardiology. 2004;27:137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burg MM, Graeber B, Vashist A, Collins D, Earley C, Liu J, Lampert R, Soufer R. Non-invasive detection of risk for emotion provoked myocardial ischemia. Psychosomatic medicine. 2009;71:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan M, York KM, Li H, Li Q, Lucey DG, Fillingim RB, Sheps DS. Usefulness of peripheral arterial tonometry in the detection of mental stress‐induced myocardial ischemia. Clinical Cardiology. 2009;32:E1–E6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramadan R, Sheps D, Esteves F, Maziar Zafari A, Douglas Bremner J, Vaccarino V, Quyyumi AA. Myocardial ischemia during mental stress: Role of coronary artery disease burden and vasomotion. Journal of the American Heart Association. 2013;2:e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O’Neal WT, Sullivan S, Tahhan AS, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko YA, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, Quyyumi AA. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. International journal of cardiology. 2017;243:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammadah M, Kim JH, Al Mheid I, Samman Tahhan A, Wilmot K, Ramadan R, Alkhoder A, Khayata M, Mekonnen G, Levantsevych O, Bouchi Y, Kaseer B, Choudhary F, Gafeer MM, Corrigan FE, Shah AJ, Ward L, Kutner M, Bremner JD, Sheps DS, Raggi P, Vaccarino V, Samady H, Mavromatis K, Quyyumi AA. Coronary and peripheral vasomotor responses to mental stress. Journal of the American Heart Association. 2018;7:e008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA. The mental stress ischemia prognosis study (mips): Objectives, study design, and prevalence of inducible ischemia. Psychosomatic Medicine. 2017;79:311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan S, Kelli HM, Hammadah M, Topel M, Wilmot K, Ramadan R, Pearce BD, Shah A, Lima BB, Kim JH, Hardy S, Levantsevych O, Obideen M, Kaseer B, Ward L, Kutner M, Hankus A, Ko YA, Kramer MR, Lewis TT, Bremner JD, Quyyumi A, Vaccarino V. Neighborhood poverty and hemodynamic, neuroendocrine, and immune response to acute stress among patients with coronary artery disease. Psychoneuroendocrinology. 2019;100:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics. 1988:1141–1154 [Google Scholar]
- 22.Chang C, Hsieh M-K, Chang W-Y, Chiang AJ, Chen J. Determining the optimal number and location of cutoff points with application to data of cervical cancer. PloS one. 2017;12:e0176231–e0176231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. The Lancet. 2007;370:1089–1100 [DOI] [PubMed] [Google Scholar]
- 24.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. The American Journal of Cardiology. 1995;76:125–130 [DOI] [PubMed] [Google Scholar]
- 25.Cardillo C, Kilcoyne CM, Cannon RO, Panza JA. Impairment of the nitric oxide–mediated vasodilator response to mental stress in hypertensive but not in hypercholesterolemic patients. Journal of the American College of Cardiology. 1998;32:1207–1213 [DOI] [PubMed] [Google Scholar]
- 26.Jain D, Shaker SM, Burg M, Wackers FJT, Soufer R, Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. Journal of the American College of Cardiology. 1998;31:1314–1322 [DOI] [PubMed] [Google Scholar]
- 27.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. Hypertension. 2010;55:1026–1032 [DOI] [PubMed] [Google Scholar]
- 28.Carroll D, Ginty AT, Der G, Hunt K, Benzeval M, Phillips AC. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology. 2012;49:1444–1448 [DOI] [PubMed] [Google Scholar]
- 29.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. Journal of Pharmacology and Experimental Therapeutics. 2003;305:800–811 [DOI] [PubMed] [Google Scholar]
- 30.Flaa A, Eide IK, Kjeldsen SE, Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure. Hypertension. 2008;52:336–341 [DOI] [PubMed] [Google Scholar]
- 31.Hassellund SS, Flaa A, Sandvik L, Kjeldsen SE, Rostrup M. Long-term stability of cardiovascular and catecholamine responses to stress tests. Hypertension. 2010;55:131–136 [DOI] [PubMed] [Google Scholar]
- 32.Muller JE, Abela GS, Nesto RW, Tofler GH. Triggers, acute risk factors and vulnerable plaques: The lexicon of a new frontier. Journal of the American College of Cardiology. 1994;23:809–813 [DOI] [PubMed] [Google Scholar]
- 33.Tofler Geoffrey H, Muller James E. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863–1872 [DOI] [PubMed] [Google Scholar]
- 34.Fuster V, Fayad ZA, Badimon JJJTL. Acute coronary syndromes: Biology. Lancet. 1999;353:s5–s9 [DOI] [PubMed] [Google Scholar]
- 35.Maseri A, L’Abbate A, Baroldi G, Chierchia S, Marzilli M, Ballestra AM, Severi S, Parodi O, Biagini A, Distante A, Pesola A. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of “preinfarction” angina. The New England journal of medicine. 1978;299:1271–1277 [DOI] [PubMed] [Google Scholar]
- 36.Roth L, Rombouts M, Schrijvers DM, Lemmens K, De Keulenaer GW, Martinet W, De Meyer GR. Chronic intermittent mental stress promotes atherosclerotic plaque vulnerability, myocardial infarction and sudden death in mice. Atherosclerosis. 2015;242:288–294 [DOI] [PubMed] [Google Scholar]
- 37.Duru F, Barton M, Lüscher TF, Candinas R. Endothelin and cardiac arrhythmias: Do endothelin antagonists have a therapeutic potential as antiarrhythmic drugs? Cardiovascular Research. 2001;49:272–280 [DOI] [PubMed] [Google Scholar]
- 38.Lerman A, Holmes DR Jr., Bell MR, Garratt KN, Nishimura RA, Burnett JC Jr. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–2431 [DOI] [PubMed] [Google Scholar]
- 39.Ezra D, Goldstein RE, Czaja JF, Feuerstein GZ. Lethal ischemia due to intracoronary endothelin in pigs. American Journal of Physiology-Heart and Circulatory Physiology. 1989;257:H339–H343 [DOI] [PubMed] [Google Scholar]
- 40.Garjani A, Wainwright CL, Zeitlin IJ, Wilson C, Slee SJ. Effects of endothelin-1 and the eta-receptor antagonist, bq123, on ischemic arrhythmias in anesthetized rats. Journal of cardiovascular pharmacology. 1995;25:634–642 [DOI] [PubMed] [Google Scholar]
- 41.Szabo T, Geller L, Merkely B, Selmeci L, Juhasz-Nagy A, Solti F. Investigating the dual nature of endothelin-1: Ischemia or direct arrhythmogenic effect? Life sciences. 2000;66:2527–2541 [DOI] [PubMed] [Google Scholar]
- 42.Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Al Mheid I, Lima BB, Garcia EV, Nye J, Ward L, Kutner MH, Raggi P, Pearce BD, Shah AJ, Quyyumi AA, Vaccarino V. Brain correlates of mental stress-induced myocardial ischemia. Psychosomatic Medicine. 2018;80:515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah A, Chen C, Campanella C, Kasher N, Evans S, Reiff C, Mishra S, Hammadah M, Lima BB, Wilmot K, Al Mheid I, Alkhoder A, Isakadze N, Levantsevych O, Pimple PM, Garcia EV, Wittbrodt M, Nye J, Ward L, Lewis TT, Kutner M, Raggi P, Quyyumi A, Vaccarino V, Bremner JD. Brain correlates of stress-induced peripheral vasoconstriction in patients with cardiovascular disease. Psychophysiology. 2018;0:e13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman RE, Hanson M, Babyak M, Thyrum ET, Krantz DS, O’Connor C. Mental stress–induced ischemia in the laboratory and ambulatory ischemia during daily life: Association and hemodynamic features. Circulation. 1995;92:2102–2108 [DOI] [PubMed] [Google Scholar]
- 45.Gottdiener JS, Krantz DS, Howell RH, Hecht GM, Klein J, Falconer JJ, Rozanski A. Induction of silent myocardial ischemia with mental stress testing: Relation to the triggers of ischemia during daily life activities to ischemic functional severity. Journal of the American College of Cardiology. 1994;24:1645–1651 [DOI] [PubMed] [Google Scholar]
- 46.Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA, Vaccarino V. Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arterioscler Thromb Vasc Biol. 2018;38:473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan M, Li Q, Brumback B, Lucey DG, Bestland M, Eubanks G, Fillingim RB, Sheps DS. Comparison of peripheral arterial response to mental stress in men versus women with coronary artery disease. The American Journal of Cardiology. 2008;102:970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller VM, Duckles SP. Vascular actions of estrogens: Functional implications. Pharmacological Reviews. 2008;60:210–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res. 1987;61:581–585 [DOI] [PubMed] [Google Scholar]
- 50.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. 2000;36:1233–1238 [DOI] [PubMed] [Google Scholar]
- 51.Kaufmann PA, Mandinov L, Seiler C, Hess OM. Impact of exercise-induced coronary vasomotion on anti-ischemic therapy. Coronary Artery Disease. 2000;11:363–369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.