Summary
Background
Human infection with avian influenza A H7N9 virus emerged in eastern China in February, 2013, and has been associated with exposure to poultry. We report the clinical and microbiological features of patients infected with influenza A H7N9 virus and compare genomic features of the human virus with those of the virus in market poultry in Zhejiang, China.
Methods
Between March 7 and April 8, 2013, we included hospital inpatients if they had new-onset respiratory symptoms, unexplained radiographic infiltrate, and laboratory-confirmed H7N9 virus infection. We recorded histories and results of haematological, biochemical, radiological, and microbiological investigations. We took throat and sputum samples, used RT-PCR to detect M, H7, and N9 genes, and cultured samples in Madin-Darby canine kidney cells. We tested for co-infections and monitored serum concentrations of six cytokines and chemokines. We collected cloacal swabs from 86 birds from epidemiologically linked wet markets and inoculated embryonated chicken eggs with the samples. We identified and subtyped isolates by RT-PCR sequencing. RNA extraction, complementary DNA synthesis, and PCR sequencing were done for one human and one chicken isolate. We characterised and phylogenetically analysed the eight gene segments of the viruses in the patient's and the chicken's isolates, and constructed phylogenetic trees of H, N, PB2, and NS genes.
Findings
We identified four patients (mean age 56 years), all of whom had contact with poultry 3–8 days before disease onset. They presented with fever and rapidly progressive pneumonia that did not respond to antibiotics. Patients were leucopenic and lymphopenic, and had impaired liver or renal function, substantially increased serum cytokine or chemokine concentrations, and disseminated intravascular coagulation with disease progression. Two patients died. Sputum specimens were more likely to test positive for the H7N9 virus than were samples from throat swabs. The viral isolate from the patient was closely similar to that from an epidemiologically linked market chicken. All viral gene segments were of avian origin. The H7 of the isolated viruses was closest to that of the H7N3 virus from domestic ducks in Zhejiang, whereas the N9 was closest to that of the wild bird H7N9 virus in South Korea. We noted Gln226Leu and Gly186Val substitutions in human virus H7 (associated with increased affinity for α-2,6-linked sialic acid receptors) and the PB2 Asp701Asn mutation (associated with mammalian adaptation). Ser31Asn mutation, which is associated with adamantane resistance, was noted in viral M2.
Interpretation
Cross species poultry-to-person transmission of this new reassortant H7N9 virus is associated with severe pneumonia and multiorgan dysfunction in human beings. Monitoring of the viral evolution and further study of disease pathogenesis will improve disease management, epidemic control, and pandemic preparedness.
Funding
Larry Chi-Kin Yung, National Key Program for Infectious Diseases of China.
Introduction
Influenza A virus is subtyped on the basis of two surface proteins, haemagglutinin (H) and neuraminidase (N), which govern the viral lifecycle at cellular entry and release of virions. All subtypes of influenza A virus, from H1 to H16 and N1 to N9, are detected in wild water birds; H17N10 is found in bats.1 Although most infections with these subtypes are mild or asymptomatic in avian species, outbreaks in wild birds and poultry have been associated with highly pathogenic avian influenza H5, and outbreaks in poultry have been associated with H7 subtypes.1, 2 Human infections are generally confined to H1, H2, and H3 subtypes, because these subtypes have affinity for host cell receptors containing α-2,6-linked sialic acid (which occur in human beings), whereas other avian influenza viruses generally preferentially attach to avian host cell receptors, which contain α-2,3-linked sialic acid. Direct transmission of avian influenza viruses from domestic poultry to people have been documented only for the H5N1, H7N2, H7N3, H7N7, H9N2, and H10N7 subtypes.1, 3, 4, 5, 6, 7 Human infections due to these subtypes were generally mild and manifested as conjunctivitis and upper-respiratory-tract infections, except for the H5N1 subtype, which was associated with mortality of greater than 50%, and the H7N7 subtype, which has caused one death.1, 4 Since February, 2013, a novel reassortant H7N9 virus associated with human deaths but no apparent outbreaks in poultry and wild birds has emerged in eastern China. We report on four patients with severe infection due to this H7N9 virus. We sequenced, characterised, and compared viral genomes from a patient and an epidemiologically linked wet market chicken isolate.
Methods
Patients and associated procedures
Between March 7 and April 8, 2013, we included hospital inpatients if they had new-onset respiratory symptoms, unexplained radiographic infiltrate, and laboratory-confirmed H7N9 virus infection at the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou; Xiaoshan People's Hospital, Hanzhou; or Huzhou Central Hospital, Huzhou (all in China). This study was approved by the institutional review board of the First Affiliated Hospital, College of Medicine, Zhejiang University (reference number 2013-131). We entered history; physical examination; and haematological, biochemical, radiological, and microbiological investigation results into a predesigned database. We recorded patients' acute physiology and chronic health evaluation II (APACHE-II) scores8 and defined acute respiratory distress syndrome and multiorgan dysfunction syndrome on the basis of standard criteria.9, 10 Presumed incubation period was defined as the time between last poultry exposure and onset of symptoms.
All laboratory procedures for respiratory secretions have been previously reported.11 Briefly, we used Taqman real-time RT-PCR under standard thermocycling conditions to detect M, H7, and N9 genes. The primers that we used were M-forward (GAGTGGCTAAAGACAAGACCAATC), M-reverse (TTGGACAAAGCGTCTACGC), and M-probe (FAM-TCACCGTGCCCAGTGAGCGAG-BHQ1); H7-forward (AGAGTCATTRCARAATAGAATACAGAT), H7-reverse (CACYGCATGTTTCCATTCTT), and H7-probe (FAM-AAACATGATGCCCCGAAGCTAAAC-BHQ1); and N9-forward (GTTCTATGCTCTCAGCCAAGG), N9-reverse (CTTGACCACCCAATGCATTC) and N9-probe (HEX-TAAGCTRGCCACTATCATCACCRCC-BHQ1). The detection limit of the M, H7 and N9 RT-PCR assays was about 100 copies of RNA per mL. All samples were cultured with trypsin in the Madin-Darby canine kidney cell line for 7 days. We did immunofluorescent antigen staining for influenza A nucleoprotein (D3 ultra 8 DFA, respiratory virus screening and identification kit, Diagnostic Hybrid, OH, USA) under ultraviolet microscopy (Eurostar III plus, Euroimmune AG, Lubeck, Germany) in cell cultures with positive cytopathic changes. RT-PCR was used to subtype for H1, H3, H5, H9, and H7.
We assessed patients' respiratory tract samples on admission by multiplex PCR (Luminex 200 System, Luminex, TX, USA); did ResPlex II v2.0 assays (Qiagen, Germany) to detect co-infection with respiratory syncytial virus, influenza B virus, parainfluenza viruses 1–4, human metapneumovirus, enteroviruses, rhinovirus, adenovirus, bocavirus, and coronaviruses NL63, HKU1, 229E, and OC43; and used PCR to detect co-infection with Mycoplasma pneumoniae and Chlamydophila pneumoniae. 12 We investigated blood, sputum, or endotracheal aspirates and urine samples bacteriologically, as clinically indicated. Initial urine samples were tested for pneumococcal and Legionella antigens by immunochromatographic enzyme immunoassay (Binax NOW Streptococcus pneumoniae Urinary Antigen Test and Binax NOW Legionella Urinary Antigen Test, Binax, ME, USA). We used the Luminex enzyme immunoassay (Luminex, TX, USA) to monitor six different serum cytokines or chemokines—namely, interferon γ, interleukins 2, 4, 6, and 10, and tumour necrosis factor α (TNFα)—as a measure of host immunological responses.
Procedures in poultry and genome characterisation
Cloacal swabs were collected from 20 chickens, four quails, five pigeons, and 57 ducks from six epidemiologically linked wet markets (four in Hanzhou City and two in Huzhou City, Zhejiang) and stored in viral transport medium. The collected samples were inoculated into embryonated chicken eggs and viral replication was detected by haemadsorption, which has been previously described.13 We identified and subtyped isolates by RT-PCR sequencing (we used H7-specific and N9-specific primers). RNA extraction, complementary DNA synthesis, and PCR sequencing were done for one human and one chicken isolate.13 Sequencing was done with the BigDye Terminator v3.1 Cycle Sequencing Kit on the 3130xL Genetic Analyzer (Applied Biosystems, NY, USA). We characterised and phylogenetically analysed all eight gene segments of the patient's and the chicken's isolates together with virus sequence data available from GenBank. All sequences were assembled and edited with Lasergene 6.0 (DNASTAR, WN, USA); Bioedit 7 was used for alignment and analysis of aminoacid residues. We used the MEGA software package v5.05 (Center for Evolutionary Medicine and Informatics, Biodesign Institute, AZ, USA) to construct the phylogenetic trees of H, N, PB2, and NS genes on the basis of the neighbour-joining method, with Tamura-Nei model of nucleotide substitution. The nucleotide of the HA1 region was used for analysis. Bootstrap values from 1000 replicates were calculated to assess the reliability of the phylogenetic tree. Our gene sequences are deposited in GenBank (accession numbers KC885955-62 [human isolate], KC899666-73 [chicken isolate]).
Role of the funding source
The sponsors had no role in study design; data collection, analysis, or interpretation; or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
All four patients had history of poultry contact (table 1 ). The presumed incubation period ranged from 3 to 8 days (mean 5·8 days). Mean age was 56 years (table 1). None of the patients were obese and none had upper-respiratory-tract symptoms or conjunctivitis. All patients had fever, and lower-respiratory-tract symptoms (including dyspnoea, cough, and sputum), and one had prominent myalgia (table 1). Chest radiography and CT of all patients showed multilobar patchy consolidation and diffuse alveolar opacities (figure 1A–1F ). CT of patients 1 and 4 showed ground glass changes in some areas. Mean time between onset of symptoms and respiratory failure was 9 days.
Table 1.
Epidemiological and clinical features of patients with avian influenza A H7N9 virus infection
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||
|---|---|---|---|---|---|
| Age (years) | 39 | 68 | 64 | 51 | |
| Sex | Male | Male | Male | Female | |
| Ethnic origin | Chinese (Han) | Chinese (Han) | Chinese (Han) | Chinese (Han) | |
| Place of residence | Zhejiang, China | Zhejiang, China | Zhejiang, China | Zhejiang, China | |
| Contact history with poultry | Occupational (chef) | Slaughtered and cooked market live poultry | Bought market live poultry | Bought market live poultry | |
| Underlying medical disorders | Chronic hepatitis B virus infection, gallstones | Hypertension | Chronic bronchitis | Chronic rheumatic heart disease with aortic and mitral valve replacements | |
| Chronic smoker | Yes | Yes | Yes | No | |
| Presumed incubation period (days)* | Uncertain | 8 | 3 | 6 | |
| Presenting symptoms | |||||
| Temperature (°C) | 39·5 | 39·5 | 39·4 | 39·7 | |
| Sore throat | – | – | – | – | |
| Rhinorrhoea | – | – | – | – | |
| Conjunctivitis | – | – | – | – | |
| Cough | + | + | + | + | |
| Sputum | + | + | + | + | |
| Haemoptysis | + | + | – | + | |
| Dyspnoea | + | + | + | + | |
| Nausea or vomiting | – | – | – | – | |
| Diarrhoea | + | – | – | – | |
| Abdominal pain | + | – | – | – | |
| Myalgia | – | – | – | + | |
| Fatigue | + | + | – | + | |
| Skin rash | – | – | – | – | |
| APACHE-II score | 14 | 14 | 16 | 18 | |
| Time between onset of symptoms and initiation of oseltamivir (days) | NA | 15 | 6 | 27 | |
| Time between onset of symptoms and onset of respiratory failure (days) | 14 | 9 | 3 | 10 | |
| Time between onset of respiratory failure and need for mechanical ventilation (days) | 2 | 4 | 0 | NA | |
| Time between mechanical ventilation and death (days) | 4 | NA | 4 | NA | |
| Antibiotics given | Piperacillin–tazobactam, moxifloxacin, imipenem–cilastatin, linezolid, sulfamethoxazole | Cefoperazone–sulbactam, fluconazole | Cefoperazone–sulbactam, levofloxacin, imipenem–cilastatin, linezolid | Imipenem–cilastatin, cefoperazone–sulbactam, azithromycin | |
| Days after onset of symptoms on which intravenous methylprednisolone given (dosage) | Days 15–18 (80 mg every 24 h) | Days 15–23 (80 mg every 24 h days 15–19 and 40 mg every 24 h days 20–23) | Days 4–7 (80 mg every 24 h) | Days 13–33 (40 mg every 24 h days 13–15 and 30 mg every 24 h days 16–33) | |
| Days after onset of symptoms on which intravenous immunoglobulin given (dosage) | NA | Days 17–21 (20 g every 24 h) | Days 6–7 (5 g every 24 h) | NA | |
+ indicates the presence of a symptom, and – the absence. APACHE=acute physiology and chronic health evaluation. NA=not applicable.
The presumed incubation period is defined as the time between the last exposure to poultry and onset of symptoms.
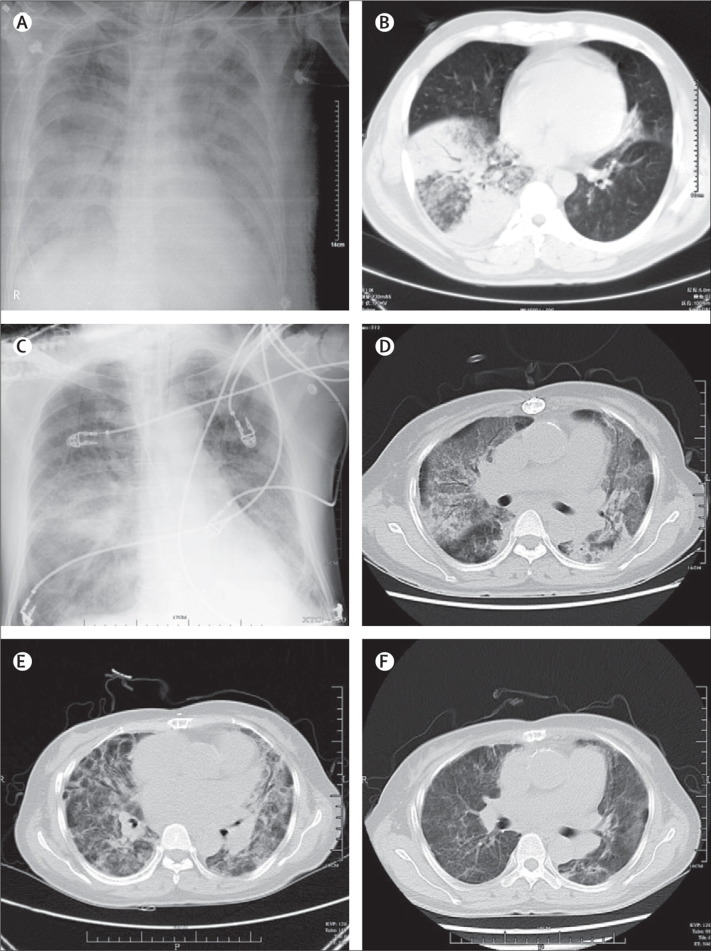
Figure 1.
Representative radiographic findings of H7N9 influenza
Chest radiograph of patient 1 taken 19 days after onset of symptoms, showing bilateral pulmonary infiltrates of airspace consolidation (A); CT of patient 1 taken 13 days after onset of symptoms, showing consolidation of right middle lobe (B); chest radiograph of patient 2 taken 14 days after onset of symptoms, showing bilateral interstitial infiltrate (C); and serial CTs of patient 4 taken 20 (D), 27 (E), and 35 (F) days after onset of symptoms, showing interval radiological improvement and resolution of bilateral ground glass changes.
Three patients were given 75 mg oral oseltamivir twice daily after tests for H7N9 virus were positive, starting a mean of 16 days after onset of symptoms onset (table 1). All patients required respiratory support—oxygen given through nasal cannulae at presentation. Two patients needed non-invasive ventilation by continuous positive airway pressure, and three subsequently received mechanical ventilation. Two patients received intravenous immunoglobulin and all received intravenous methylprednisolone (table 1). Two patients (patients 1 and 3) died 4 days after intubation. The other two patients were recovering clinically and radiologically and had been successfully extubated at the time of writing (figure 1). 303 household or workplace contacts and 82 health-care workers with unprotected exposure to the four patients were put under medical surveillance but none of them became symptomatic after 14 days.
Table 2 lists the results of laboratory investigations in the patients. All patients had pronounced lymphopenia at presentation. Total leucocyte counts were healthy or low at presentation, but leucocytosis with neutrophilia developed with disease progression. Three patients had thrombocytopenia at presentation. All patients' coagulation profiles were impaired and D-dimer concentrations substantially increased with disease progression. The patients who died had persistent lymphopenia, renal impairment, and rising aspartate transaminase and D-dimer concentrations. Hepatic aminotransferases, C-reactive protein, and creatine kinase or lactate dehydrogenase concentrations were increased in all patients at some stage of illness; derangement was worse in those who died.
Table 2.
Laboratory measurements in four patients with avian influenza A H7N9 virus infection
| Normal range | Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|---|
| Haemoglobin (g/dL) | 131·0–172·0 | 138·0; 122·0 | 108·0 | 127·0 | 114·0; 91·0 |
| Total white cells (×109 cells per L) | 4·0–10·0 | 2·2; 14·4 | 6·0; 13·4 | 5·6; 7·2 | 5·3; 37·3 |
| Neutrophils (×109 cells per L) | 2·0–7·0 | 1·8; 11·6 | 5·3; 12·6 | 5·3; 6·7 | 5·1; 34·5 |
| Lymphocytes (×109 cells per L) | 0·8–4·0 | 0·4 | 0·7; 0·5 | 0·2 | 0·1 |
| Platelets (×109 cells per L) | 83·0–303·0 | 55·0 | 212; 148 | 91·0 | 54·0 |
| Prothrombin time (s) | 10·0–13·5 | 15·0; 17·0 | 12·7; 11·2 | 14·4; 15·1 | 29·9; 65·5 |
| Activated thromboplastin time (s) | 22·0–36·0 | 34·1; 43·5 | 23·1; 44·3 | 75·6 | 107·5 |
| D-dimer (μg/L) | 0·0–700·0 | 3320·0; 23 000·0 | 5810·0; 17 490·0 | 288·0; 1235·0 | 5010·0; 6800·0 |
| Urea (mmol/L) | 2·9–8·2 | 6·4; 22·7 | 7·7; 8·6 | 5·4; 14·0 | 4·6; 10·2 |
| Creatinine (μmol/L) | 59·0–104·0 | 94·0; 470·0 | 45·0; 47·0 | 54·0; 148·0 | 63·0 |
| Bilirubin (μmol/L) | 0·0–21·0 | 43·8; 64·2 | 11·0 | 13·0; 28·7 | 16·0; 31·0 |
| Alanine aminotransferase (U/L) | 5·0–40·0 | 134·0 | 57·0; 89·0 | 33·0; 96·1 | 12·0; 30·0 |
| Aspartate aminotransferase (U/L) | 8·0–40·0 | 199·0; 319·0 | 62·0 | 48·0; 87·2 | 32·0; 128·0 |
| Lactate dehydrogenase (U/L) | 109·0–245·0 | 495·0; 1140·0 | 434·0; 466·0 | 535·0; 607·4 | 452·0; 2178·0 |
| Creatinine kinase (U/L) | 38·0–174·0 | 2533·0 | 44·0 | 109·0; 119·1 | 96·0; 119·0 |
| C-reactive protein (mg/L) | 0·0–8·0 | 74·9; 92·2 | 10·5; 11·7 | 175·3 | 56·5; 149·4 |
Results for when the patients presented and the patients' most abnormal result during disease progression are given. If the reading at presentation was the most abnormal reading, only one result is given.
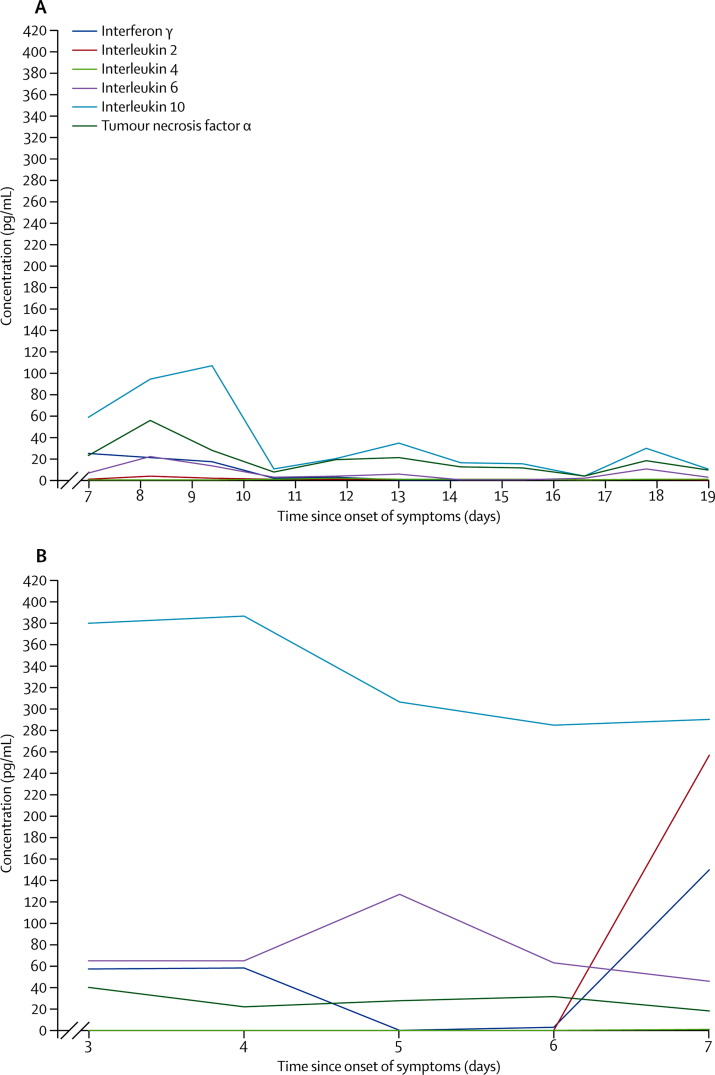
Overall, serum cytokine and chemokine concentrations were substantially higher in patient 3 (who died) than in patient 2 (who survived) (figure 2A, 2B ). Patient 3 had persistently high serum interleukin 10 concentrations (figure 2B) before death. RT-PCR assays of throat swab samples or sputum samples yielded positive results for H7N9 infection in all patients (table 3 ). Serial samples from patient 2 were tested; throat swab samples were consistently negative, but sputum samples were positive (table 3). H7N9 virus was isolated from respiratory specimens from patients 1, 3, and 4 in cell culture, and confirmed by RT-PCR (in which H7-specific and N9-specific primers were used). No viral co-infections were detected by multiplex PCR, and no bacterial or fungal co-infections were detected in 14 blood cultures and 16 respiratory secretion cultures. Two of five pigeons (40%), four of 20 chickens (20%), zero of four quails (0%), and zero of 57 ducks (0%) tested positive for the H7N9 virus.
Figure 2.
Serum cytokine and chemokine profile of patients 2 (A) and 3 (B)
Normal ranges: interferon γ (0·01–13·64 pg/mL); interleukin 2 (0·01–10·67 pg/mL); interleukin 4 (0·01–2·25 pg/mL); interleukin 6 (0·01–8·86 pg/mL); interleukin 10 (2·42–16·33 pg/mL); and tumour necrosis factor α (0·82–11·05 pg/mL).
Table 3.
Virological findings in patients with avian influenza A H7N9 virus infection
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| RT-PCR | Endotracheal aspirate | Sputum and throat swabs | Throat swab | Sputum |
| Time between onset of symptoms and collection of first positive specimen (days) | 17 | 15 | 6 | 11 |
| Time between onset of symptoms and first positive RT-PCR result (days) | 25 | 15 | 6 | 25 |
| M gene* | 27 | Sputum 34/32/32/34/32/32/−/−; throat −/−/− /− /− /− /− /− | 20 | 27 |
| H7 gene* | 25 | Sputum 32/30/32/30/30/30/−/−; throat −/−/− /− /− /− /− /− | 20 | 26 |
| N9 gene* | 25 | Sputum 34/34/36/34/34/34 /−/−; throat −/−/− /− /− /− /− /− | 24 | 28 |
| Viral culture in Madin-Darby canine kidney cells† | Yes | No | Yes | Yes |
Data are cycle threshold values. Patient 2's RT-PCR results for serial sputa and throat swabs taken on days 15–22 are presented.
Cytopathic effects appeared in Madin-Darby canine kidney cells at 48 h after inoculation.
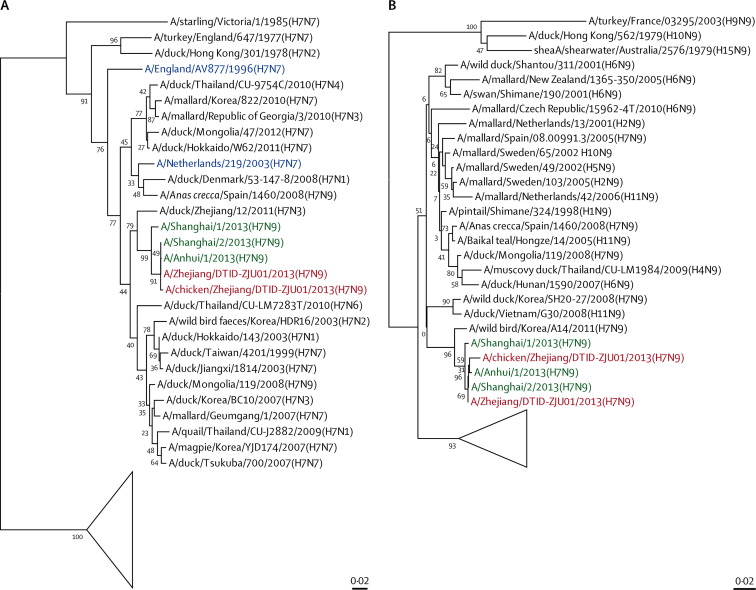
Sequence analysis of patient 3 and an epidemiologically linked chicken's H (1673 of 1683 bases [99·4%]) and N (1394 of 1398 bases [99·7%]) genes showed that the human H7N9 isolate was almost identical to chicken H7N9 isolate. The H7 in the isolates clustered with H of the H7N3 of ducks in Zhejiang, and the isolate N9 clustered with the N of the H7N9 of wild birds in Korea (figure 3 ). The six internal genes of the isolate H7N9 are closest to those of poultry H9N2 viruses of China (figure 4 ).
Figure 3.
Phylogenetic trees for the haemagglutinin (HA1) (A) and neuraminidase (N) (B) genes of H7N9 viruses isolated from a patient and a chicken in Zhejiang, China
Sequences of H7N9 viruses characterised in our study are red—A/Zhejiang/UTID-ZJU01/2013 (H7N9) is the human isolate and A/chicken/Zhejiang/DTID-ZJU01/2013 (H7N9) is the epidemiologically linked chicken isolate. H7N7 viruses that were reported to cause human infections are blue. Human isolates of H7N9 viruses described in a 2013 report14 are green. The other sequences (black) were derived from other subtypes of influenza viruses that were available in Genbank. The triangle represents viruses of North American (ie, Canadian, Mexican, and US) lineage.
Figure 4.
Phylogenetic trees for the PB2 (A) and NS (B) genes of H7N9 viruses isolated from a patient and a chicken in Zhejiang, China
Sequences of H7N9 viruses characterised in our study are red. Human isolates of H7N9 viruses described in a 2013 report14 are green. The other sequences (black) were derived from H9N2 influenza viruses characterised at different times and from different places.
Analysis of the H7 receptor binding site showed a Gln226Leu substitution in the human isolate and a Gly186Val substitution (H3 numbering) in both human and chicken isolates (table 4 ). No multibasic aminoacids were noted at the proteolytic cleavage site of this H7 in either the human or the chicken isolates. Although the PB2 Glu627Lys substitution frequently detected in human H5N1 isolates was not noted, an Asp701Asn substitution was noted in the human isolate. Deletion of five aminoacids in the stalk region of N9 at position 69–73 (N9 numbering) was noted in the human and chicken H7N9 isolates. We detected a premature stop codon near the C-terminus of NS1, leading to PDZ motif deletion, in all H7N9 isolates. A Ser31Asn substitution of the M2 gene associated with adamantane resistance was recorded in both isolates, but we noted no resistance mutations associated with neuraminidase inhibitors.
Table 4.
Substitutions of critical aminoacid residues in human and avian isolates of avian influenza A H7N9 virus, by residue
| Human (ZJ/DTID-ZJU01*) | Chicken (CK/ZJ/DTID-ZJU01*) | Human (Shanghai/1†) | Human (Shanghai/2†) | Human (Anhui/1†) | Function | |
|---|---|---|---|---|---|---|
| Haemagglutinin‡ | ||||||
| 186 | Val | Val | Gly | Val | Val | Gly186Val increases binding affinity for α-2,6-linked sialic acid receptor |
| 226 | Leu | Gln | Gln | Leu | Leu | Gln226Leu increases binding affinity for α-2,6-linked sialic acid receptor |
| Neuraminidase§ | ||||||
| 292 | Arg | Arg | Lys | Arg | Arg | Arg292Lys reduces susceptibility to oseltamivir and zanamivir |
| M2 | ||||||
| 31 | Asn | Asn | Asn | Asn | Asn | Ser31Asn causes resistance to adamantanes |
| PB2 | ||||||
| 627 | Glu | Glu | Lys | Lys | Lys | Glu627Lys results in mammalian host adaptation for viral RNA replication at 33°C |
| 701 | Asn | Asp | Asp | Asp | Asp | Asp701Asn enhances transmission in guinea pigs |
Discussion
We diagnosed avian influenza A H7N9 in all four patients (who were epidemiologically unlinked), two of whom died and two of whom were recovering at the time of writing (panel ). All patients had histories of occupational or wet market exposure to poultry. The genes of the H7N9 virus in patient 3's isolate were phylogenetically clustered with those of the epidemiologically linked wet market chicken H7N9 isolate. Human and chicken isolate H7 clustered with that of H7N3 of ducks in Zhejiang, and human and chicken isolate N9 clustered with that of H7N9 of wild birds in Korea, and the six internal genes of the isolate H7N9 are closest to those of poultry H9N2 viruses of China. These findings suggest sporadic poultry-to-person transmission.
Panel. Research in context.
Systematic review
We searched PubMed on April 11, 2013, with the terms “influenza”, “avian”, “H5”, “H5N1”, “H7”, “H9”, and “H10” for articles published in English. Our search did not reveal any reports of avian influenza A H7N9 virus infection in human beings before 2013. We noted only reports of poultry outbreaks caused by some H7N9 virus strains, which were usually weakly pathogenic for avian species. All reported human infections with avian influenza A virus were caused by H5N1, H7N2, H7N3, H7N7, H9N2, and H10N7 subtypes. Severe community-acquired pneumonia and multiorgan dysfunction due to H5N1 infection was associated with a mortality rate of more than 50%, and one death due to the H7N7 subtype has been reported.4 However, most H7 viruses and other subtypes caused mild respiratory illness or conjunctivitis in people. Most cases were attributed to avian-to-person transmission, and there was little evidence of first generation person-to-person transmission.
Interpretation
Our study showed that human infections with the avian influenza A H7N9 virus were acquired from live poultry markets in China (evidenced by the phylogenetic relatedness between viruses in isolates from a patient and an epidemiologically linked chicken). Markers of mammalian adaptation were found in the human virus isolate. Clinical manifestation of this new emerging infection is similar to that of H5N1 infection and can be fatal in patients with substantial cytokine activation and multiorgan dysfunction. Further virological study is important to establish diagnosis and allow early treatment with neuraminidase inhibitors and infection control. Rising poultry and human populations will increase the emergence of novel avian influenza viruses infecting human beings.
As was the case with influenza A H5N1 virus in 1997,2 severe acute respiratory syndrome (SARS) coronavirus in 2003,15 and human coronavirus EMC in 2012,16, 17 infection with a novel virus was suspected because the pneumonia of these patients did not respond to typical and atypical antibiotic coverage. The four patients were clustered within Zhejiang, China, within a few weeks (when migratory birds were moving north and transiting at the Yangtze River Delta). However, unlike other types of avian influenza affecting human beings, no increase in poultry deaths was noticed before the onset of human infections. After diagnosis of influenza A H7N9 infection was confirmed in patient 1 by RT-PCR, additional and retrospective testing of 486 patients between March 7 and April 8, 2013, led to the discovery of three further infected patients.
Similar to those infected with H5N1, our patients had few upper-respiratory-tract symptoms.1 They presented with high fevers, lower-respiratory-tract symptoms (especially dyspnoea), and radiological features of consolidation and ground glass changes. Multiorgan involvement was shown by abnormal results of liver and renal function tests, myalgia (with high creatine kinase concentrations suggestive of myositis), impaired coagulation, and severe lymphopenia. Gastrointestinal symptoms were noted only in patient 1—a profile that differs from that in previous reports of H5N1 or severe A H1N1 pdm09 infections.1 Respiratory failure progressed within 3–14 days and death 1–3 weeks after onset of symptoms. However, none of these clinical, radiological, or laboratory findings was pathognomonic. Oseltamivir was begun late (mean 16 days after symptom onset) because of the late presentation of respiratory failure and delay in virological diagnosis.
Similar to the cytokine storm noted in H5N1 infection, the substantially increased concentrations of proinflammatory and anti-inflammatory serum cytokines and chemokines in patient 3 (who died) were compatible with the clinical severity of this novel H7N9 infection.18 In particular, the serum concentrations of interleukin 10 were persistently increased in patient 3—a finding similar to that for severe A H1N1 pdm09 infection.12 Although serial RT-PCR assays of sputum samples from patient 2 were positive for viral infection, cycle thresholds were low 15–20 days after onset of symptoms and corroborated well with decreasing serum cytokine and chemokine concentrations.
Besides the likely absence of protection by pre-existing neutralising antibodies in the general population, the internal genes from the H9N2 virus might also contribute to the severe pathogenesis of this novel infection. Double and even triple reassortant avian H9N2 viruses were well reported,19 and the six internal genes of the 1997 H5N1 virus originated from avian H9N2 virus. Furthermore, H9N2 and H5N1 viruses both induced prominent cytokine and chemokine activation in human macrophages and epithelial cells compared with that induced by seasonal influenza A H1N1 virus.20 In previous studies, treatment with convalescent plasma or hyperimmune γ globulin seemed to improve survival of patients and therefore hyperimmune γ globulin should be considered in the treatment of severe H7N9 infection.21 Immunomodulatory agents such as celecoxib (but not corticosteroids) improve outcomes of H5N1 infections in mice.22
Other host factors, such as smoking and obesity, are risk factors for severe influenza.23, 24 Findings from murine models challenged with influenza viruses suggest that smoking worsens the response of proinflammatory chemokine and cytokines and histological changes of inflammatory infiltrates and lung damage, increases viral titres, and impairs pulmonary adaptive T-lymphocyte responses to the virus.25
Rapid virological diagnosis was established by RT-PCR of the M, H7, and N9 genes and confirmed by viral culture in cell lines. Similar to H5N1 infection, which mainly affected the lower respiratory tract, sputum and endotracheal aspirates might be better than nasopharyngeal and throat swabs for detection of influenza A H7N9.1 The predilection of influenza A H7N9 for the lower respiratory tract suggests that the virus might replicate more efficiently there, where both α-2,3-linked and α-2,6-linked sialic acid receptors are noted.1 α-2,3-linked sialic acid was detected on non-ciliated cuboidal bronchiolar cells at the junction between the respiratory bronchiole and alveolus. A substantial number of cells lining the alveolar wall also expressed this receptor.
The emergence of a new reassortant avian H7N9 virus causing human infections without preceding or concomitant outbreak in poultry was quite unexpected. This occurrence could be attributed to the absence of the multibasic aminoacid motif at the proteolytic cleavage site of H, which is associated with broad tissue tropism and organ dissemination and is therefore a key virulence marker of H5 or H7 subtypes of highly pathogenic avian influenza.1 This multibasic cleavage site is a virulence marker in birds but is not confirmed as a virulence marker in people. No pandemic virus has had this multibasic aminoacid motif. Wild waterfowl such as ducks and geese were the original natural reservoir (for H1–16, N1–9)26 and provide new genes for making new virus reassortants that then infect domestic poultry, such as chickens, ducks, and geese, which are in close contact with human beings. Further adaptive genetic changes of such viruses in domestic poultry can enable transmission to people.
The key concerns about the current outbreak of influenza A H7N9 virus are how the virus crosses the species barrier and whether it will further adapt to enable efficient person-to-person transmission. Sequence analysis showed that the human H7 had aminoacid substitutions associated with increased affinity for the human α-2,6-linked sialic acid receptor.27 However, binding to the α-2,3-linked sialic acid receptor is likely to be retained, allowing the virus to circulate in poultry and infect human lower-respiratory-tract mucosae, which contain both types of receptor.1
Shortening of the N stalk region of H5N1 viruses enhanced adaptation to land-based poultry.28 An important virus protein, PB2, in combination with two other viral proteins, PB1 and PA, comprise the viral RNA polymerase complex. PB2 is an important determinant of the host range and virulence of influenza viruses. Two aminoacids in PB2, 627Lys and 701Asn, have previously been detected in H5N1 viruses isolated from people.18 Coupled 627Lys–701Asp or 627Glu–701Asn substitutions were thought to be important for efficient transmission.29 Although we did not note Glu627Lys substitution in PB2 in our human isolate, we did record Asp701Asn substitution. Another study14 of the current H7N9 outbreak detailed three cases of infection in Shanghai and Anhui, China; the viral isolates showed Glu627Lys mutation. We noted neither genetic signature for mammalian adaptation—ie, Gln226Leu and Asp701Asn—in the chicken isolate, which suggests that this genetic adaptation might have occurred after the virus jumped from the chicken to the patient.
Ser31Asn mutation (associated with adamantane resistance) was noted, but neuraminidase inhibitors, including oseltamivir, zanamivir, and peramivir, should still be active if given early in the course of illness. One patient did not receive any antivirals and three patients received oseltamivir more than 5 days after onset of symptoms. We do not know whether oseltamivir resistance will emerge (as was the case with the H5N1 virus) because the last H7N9-positive sample from patient 2 was not tested for oseltamivir resistance after 5 days of oseltamivir treatment. Delayed initiation of oseltamivir treatment and use of corticosteroids have been associated with slow decreases in viral load and poor outcomes.23
In 2003, an H7N7 virus, which contained the multibasic aminoacid motif associated with virulence and the genetic marker for mammalian adaptation (Glu627Lys) caused one fatal infection in the Netherlands.4 Other severe human infections with the H7 subtype had not been reported before the current H7N9 outbreak. Previous surveillance studies have shown the H7N3 virus to be present in domestic ducks in Zhejiang.30 Avian H7 subtype viruses are likely to have become established in domestic poultry in Zhejiang. Interaction between newly established H7 subtypes and other avian influenza viruses, such as the H9N2 subtype, might have resulted in the current H7N9 strain, which has gained some ability to infect human beings. Further adaptation could lead to less symptomatic infection and more efficient person-to-person transmission. Aggressive intervention to block further animal-to-person transmission in live poultry markets, as has previously been done in Hong Kong, should be considered. Temporary closure of live bird markets and comprehensive programmes of surveillance, culling, improved biosecurity, segregation of different poultry species, and possibly vaccination programmes to control H7N9 virus infection in poultry seem necessary to halt evolution of the virus into a pandemic agent.
Acknowledgments
Acknowledgments
A donation from Larry Chi-Kin Yung and the National Key Program for Infectious Diseases of China (2012ZX10004210) funded the manpower and laboratory testing for this study. HC is partly supported by the Areas of Excellence of the University Grants Committee (Grant AoE/M-12/06). K-YY is partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease of the Department of Health, Hong Kong Special Administrative Region, China. We acknowledge the contributions of Lingxiang Ruan, Qibing Pu, Zhiping Chen, the technical staff of the Department of Microbiology at the University of Hong Kong, and Yuelong Shu for permitting us to use the data he deposited in Global Initiative on Sharing All Influenza Data (GISAID) EpiFlu.
Contributors
LL and K-YY were coprincipal investigators, designed and supervised the study, and wrote the grant application (assisted by YC). WL, SY, HG, JS, QF, YL, XY, YuZ, and SX had roles in recruitment, data collection, and clinical management. NW, HY, JW, DC, HW, SZ, HD, YaZ, K-HC, H-WT, and JL-LT did clinical laboratory testing and analysis. WS, PW, S-YL, MZ, and HC did the genome sequencing and analysis. JF-WC, KK-WT, HC, K-YY, and LL drafted the Article, and all authors contributed to review and revision and have seen and approved the final version.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.To KK, Ng KH, Que TL. Avian influenza A H5N1 virus: a continuous threat to humans. Emerg Microbes Infect. 2012;1:e25. doi: 10.1038/emi.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuen KY, Chan PKS, Peiris M. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 3.Arzey GG, Kirkland PD, Arzey KE. Influenza virus A (H10N7) in chickens and poultry abattoir workers, Australia. Emerg Infect Dis. 2012;18:814–816. doi: 10.3201/eid1805.111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koopmans M, Wilbrink B, Conyn M. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 5.Ostrowsky B, Huang A, Terry W. Low pathogenic avian influenza A (H7N2) virus infection in immunocompromised adult, New York, USA, 2003. Emerg Infect Dis. 2012;18:1128–1131. doi: 10.3201/eid1807.111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tweed SA, Skowronski DM, David ST. Human illness from avian influenza H7N3, British Columbia. Emerg Infect Dis. 2004;10:2196–2199. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng VC, Chan JF, Wen X. Infection of immunocompromised patients by avian H9N2 influenza A virus. J Infect. 2011;62:394–399. doi: 10.1016/j.jinf.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 9.Bernard GR, Artigas A, Brigham KL. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 10.Estenssoro E, Reina R, Canales HS. The distinct clinical profile of chronically critically ill patients: a cohort study. Crit Care. 2006;10:R89. doi: 10.1186/cc4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li IW, Chan KH, To KW. Differential susceptibility of different cell lines to swine-origin influenza A H1N1, seasonal human influenza A H1N1, and avian influenza A H5N1 viruses. J Clin Virol. 2009;46:325–330. doi: 10.1016/j.jcv.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 12.To KK, Hung IF, Li IW. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis. 2010;50:850–859. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Smith GJ, Li KS. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci USA. 2006;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peiris JSM, Lai ST, Poon LLM. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JF, Li KS, To KK, Cheng VC, Chen H, Yuen KY. Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic? J Infect. 2012;65:477–489. doi: 10.1016/j.jinf.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 17.De Jong MD, Simmons CP, Thanh TT. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li KS, Xu KM, Peiris JS. Characterization of H9 subtype influenza viruses from the ducks of southern China: a candidate for the next influenza pandemic in humans? J Virol. 2003;77:6988–6994. doi: 10.1128/JVI.77.12.6988-6994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law AH, Lee DC, Yuen KY, Peiris M, Lau AS. Cellular response to influenza virus infection: a potential role for autophagy in CXCL10 and interferon-alpha induction. Cell Mol Immunol. 2010;7:263–270. doi: 10.1038/cmi.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung IF, To KK, Lee CK. Hyperimmune intravenous immunoglobulin treatment: a multicentre double-blind randomized controlled trial for patients with severe A(H1N1)pdm09 infection. Chest. 2013 doi: 10.1378/chest.12-2907. published online Feb 28. [DOI] [PubMed] [Google Scholar]
- 21.Zheng BJ, Chan KW, Lin YP. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci USA. 2008;105:8091–8096. doi: 10.1073/pnas.0711942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng VC, To KK, Tse H, Hung IF, Yuen KY. Two years after pandemic influenza A/2009/H1N1: what have we learned? Clin Microbiol Rev. 2012;25:223–263. doi: 10.1128/CMR.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang AJ, To KK, Li C. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270–1280. doi: 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Kong Y, Barnes PF. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect Immun. 2011;79:229–237. doi: 10.1128/IAI.00709-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturm-Ramirez KM, Hulse-Post DJ, Govorkova EA. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79:11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen LM, Blixt O, Stevens J. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology. 2012;422:105–113. doi: 10.1016/j.virol.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou H, Yu Z, Hu Y. The special neuraminidase stalk-motif responsible for increased virulence and pathogenesis of H5N1 influenza A virus. PLoS One. 2009;4:e6277. doi: 10.1371/journal.pone.0006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steel J, Lowen AC, Mubareka S, Palese P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 2009;5:e1000252. doi: 10.1371/journal.ppat.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao R, Cao B, Hu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013 doi: 10.1056/NEJMoa1304459. published online April 11. [DOI] [PubMed] [Google Scholar]
- 30.Hai-bo W, Ru-feng L, En-kang W. Sequence and phylogenetic analysis of H7N3 avian influenza viruses isolated from poultry in China in 2011. Arch Virol. 2012;157:2017–2021. doi: 10.1007/s00705-012-1370-3. [DOI] [PubMed] [Google Scholar]