Abstract
Aging impairs circadian clock function, leading to disrupted sleep-wake patterns and a reduced capability to adapt to changes in environmental light conditions. This makes shift work or the changing of time zones challenging for the elderly and, importantly, is associated with the development of age-related diseases. However, it is unclear what levels of the clock machinery are affected by aging, which is relevant for the development of targeted interventions. We found that naturally aged mice of >24 months had a reduced rhythm amplitude in behavior compared with young controls (3-6 months). Moreover, the old animals had a strongly reduced ability to adapt to short photoperiods. Recording PER2::LUC protein expression in the suprachiasmatic nucleus revealed no impairment of the rhythms in PER2 protein under the 3 different photoperiods tested (LD: 8:16, 12:12, and 16:8). Thus, we observed a discrepancy between the behavioral phenotype and the molecular clock, and we conclude that the aging-related deficits emerge downstream of the core molecular clock. Since it is known that aging affects several intracellular and membrane components of the central clock cells, it is likely that an impairment of the interaction between the molecular clock and these components is contributing to the deficits in photoperiod adaptation.
Keywords: circadian rhythm, clock gene, behavior, seasonal adaptation, suprachiasmatic nucleus, PERIOD 2, neuronal plasticity
The continuing rise in life expectancy over the past decades has increased the attention on research on healthy aging. Even in healthy humans, aging is associated with fragmented sleep-wake patterns and declined circadian rhythms in eating patterns and hormone secretion, which in turn diminishes quality of life (Dijk and Duffy, 1999; Dijk et al., 1999; Carvalho-Bos et al., 2007; Froy, 2011). Aging reduces the capability of adapting to changes in light regimes, and exposure to abrupt changes in light-dark (LD) cycles even leads to a higher mortality rate in rodents (Davidson et al., 2006; Azzi et al., 2014). Aging humans show reduced season-associated changes in behavior, and there is a seasonal effect on medical care needs and mortality in the elderly (Rolden et al., 2015; Cepeda et al., 2018). Moreover, several studies have suggested a negative interaction between age-related neurodegenerative diseases and disturbances in circadian rhythmicity (Leng et al., 2019). It has been suggested that improving circadian rhythms in the elderly—for example, with light therapy or melatonin treatment—can have beneficial effects on sleep-wake patterns. This will likely improve the quality of life and overall health and, moreover, could slow down the progression of neurodegenerative diseases (Most et al., 2010; Gaikwad, 2018).
The central circadian clock in mammals, the suprachiasmatic nucleus (SCN), is an interesting target for restoring circadian rhythms in the elderly. Age-related disruptions of rhythms in behavior and physiology can be restored by transplanting fetal SCN tissue near the hypothalamus of aged mice (Van Reeth et al., 1994; Cai et al., 1997). Moreover, it has been shown that disrupting circadian rhythms in the SCN (e.g., by knockout of some core clock genes) induces various symptoms of premature aging in mice and rats (Kondratov et al., 2006; Dubrovsky et al., 2010). Knowledge on the functioning of the aging circadian clock will benefit the design of strategies for targeted interventions to enhance circadian rhythms in the elderly, improving their health and overall well-being.
A combination of molecular (e.g., clock gene expression), cellular (e.g., electrical activity), and network (e.g., neurotransmitters) elements underlie the proper functioning of the SCN. Electrical activity and neurotransmitters are important for synchronizing the SCN network, as well as for the output of the SCN to other brain areas. Aging is associated with reduced synchronization and amplitude of electrical activity rhythms in SCN neurons (Nakamura et al., 2011; Farajnia et al., 2012; Leise et al., 2013), partially because of the age-related decline in expression of important neurotransmitters, such as vasoactive intestinal peptide (VIP) and γ-aminobutyric acid (GABA; Kawakami et al., 1997; Nygard and Palomba, 2006; Palomba et al., 2008). Despite numerous studies, it is still unclear how aging affects the molecular clock in the SCN and to what extent core clock genes, such as Per2, Cry1, and Clock, are affected, while behavior is invariably been found to be affected (Asai et al., 2001; Weinert et al., 2001; Kolker et al., 2003; Wyse and Coogan, 2010; Nakamura et al., 2011; Chang and Guarente, 2013; Bonaconsa et al., 2014). Recent discoveries of small molecules with the potential to directly influence molecular clock components (Chen et al., 2018) increase the urgency to identify the best suitable components of the aging clock as targets for successful restoration of rhythmicity.
To study how aging affects circadian rhythms at the level of both the whole organism and the central circadian clock, we performed behavioral recordings of old (~24 months) and young (~5 months) PER2::LUC mice and, afterward, recorded PER2::LUC gene expression characteristics in slices of the SCN. Under a 12:12 LD cycle (LD 12:12), we found that aging did not affect the molecular clock, as evidenced by unaltered PER2::LUC peak time and phase synchrony. Next, we investigated if challenging the circadian system by exposing mice to different photoperiods would induce differences at the behavioral level as well as the level of the molecular clock. We found that aging affected circadian behavior: old mice had reduced rhythm strength (LD 12:12) and were less able to adapt to short photoperiod (SP; LD 8:16). However, old mice showed similar single-cell PER2::LUC rhythm characteristics after adaptation to long photoperiod (LP) and SP as compared with young mice. These results suggest that the molecular clock of the aged SCN is still intact, while the behavioral phenotype is clearly affected.
Methods
Animals and Housing
The experiments performed in this study were conducted in accordance with the Dutch law on animal welfare. The permit (DEC 13198/PE. 16.039.001) was granted by the animal experiments committee Leiden. The homozygous PERIOD2::LUCIFERASE (PER2::LUC) mice were bred at the Leiden University Medical Center animal facility (see Buijink et al., 2016). We used young (4-8 months) and old (22-28 months) male PER2::LUC mice. The animals were kept in climate-controlled cabinets with full-spectrum diffused lighting with an intensity between 50 and 100 lux (Osram truelight TL) and ad libitum access to food and water throughout the experiment. Mice older than 20 months received, in addition to the regular food, hydration and nutritional gels as supportive care. Prior to behavioral assessment, mice were kept in groups of 2 to 5 mice in a 12 h:12 h LD (LD 12:12) cycle. During behavioral recordings, mice were kept in individual cages equipped with a passive infrared (PIR) sensor.
Behavioral Analysis
Home cage activity was recorded with a PIR sensor throughout the experiment. First, behavior was recorded under LD 12:12 for at least 10 days. Then mice were exposed to either LD 16:8 or 8:16 for 28 days, which is referred to as photoperiod 1 (PP1). This was followed by a period of constant darkness (DD) for 11 to 14 days, before mice were exposed again to either LD 16:8 or 8:16 for at least 14 days, referred to as photoperiod 2 (PP2), until the start of the bioluminescence recording of PER2::LUC. The photoperiod to which the mice were exposed was the same before and after DD. For the behavioral analysis, we used (1) the last 10 days of the LD 12:12 recordings, (2) the last 10 days of the first photoperiod exposure, (3) both 5 and 10 days from the second day of DD (marked in figures), and (4) the last 10 days of the second photoperiod exposure, before the start of the PER2 recordings. For these time segments, we determined the rhythm strength, the duration of activity (alpha) and resting (rho), and relative activity level during alpha and rho. In addition, we determined the period (tau) in DD over 10 days (free-running period). Time is expressed in projected external time (ExT), with ExT 0 being the middle of the dark phase and ExT12 the middle of the light phase.
We defined rhythm strength as the power of the F periodogram (p = 0.05 to peak). Alpha is defined by the interval between activity onset and offset. For this determination, activity recorded with the PIR sensors was averaged over 10 consecutive days (in DD, 5 days were used, and the period was corrected for tau, which was calculated over 10 days) and clustered in 10-min bins. Activity onset and offset are less distinct in PIR recordings than in recordings of wheel running, on which standard methods for calculating alpha are developed. Therefore, we used the following characteristics to determine onset and offset for our recordings: the values of the highest average activity in a 10-h bin of activity (M10) and lowest 5-h bin (L5) was calculated (Witting et al., 1990), activity was then smoothed for 2-h bins, activity onset was defined as the first instance in which the smoothed activity passed the ¾ value between L5 and M10 after the period with least activity, and activity offset was defined as the last instance in which the smoothed activity passed the ½ value between L5 and M10 before the period with least activity (Suppl. Fig. S2).
To study the adaptation to photoperiod, we used the change in alpha from LD 12:12 to DD: Δ alpha. To obtain Δ alpha, the length of alpha for the period in LD 12:12 was subtracted from the length of alpha in DD (5 days).
Bioluminescence Imaging and Analysis
Slice cultures of the SCN were prepared as previously described (Buijink et al., 2016). In brief, mice were killed by decapitation within 1 to 3 h before lights-off. The brain was dissected and placed in ice-cold artificial cerebrospinal fluid (ACSF) with low Ca2+ and high Mg2+. The ACSF contained the following (in mM): NaCl (116.4), KCl (5.4), NaH2PO4 (1.0), MgSO4 (0.8), CaCl2 (1.0), MgCl2 (4.0), NaHCO3 (23.8), D-glucose (15.1), and 5 mg/L gentamicin (Sigma-Aldrich, Munich, Germany), saturated with 95% O2–5% CO2 and pH 7.4. From each brain, the hypothalamus, containing the SCN, was isolated and sliced in 200-µm-thick coronal slices with a VT 1000S vibrating microtome (Leica Microsystems, Wetzlar, Germany). The SCN was optically identified and cut out, and both an anterior and posterior slice were placed on a Millicell membrane insert (PICMORG50, Merck-Milipore, Burlington, MA) in a 35-mm petri dish. The dish contained 1.2 mL Dulbecco’s Modified Eagle’s Medium supplemented with 10 mM HEPES buffer (Sigma-Aldrich), 2% B-27 (Gibco, Landsmeer, the Netherlands), 5 U/mL penicillin, 5 µg/mL streptomycin (0.1% penicillin-streptomycin; Sigma-Aldrich), and 0.2 mM D-luciferine sodium salt (Promega, Leiden, the Netherlands) and was adjusted to pH 7.2 with NaOH.
The dish containing the slices was sealed with a glass cover slip and transferred to a temperature-controlled (37 °C) and light-tight chamber (Life Imaging Services, Reinach, Switzerland), equipped with an upright microscope and a cooled charge-coupled device camera (ORCA–UU-BT-1024, Ham-amatsu Photonics Europe, Herrsching am Ammersee, Germany). Bioluminescence images from the anterior and posterior slices were acquired consecutively with an exposure time of 29 min, resulting in an image series with 1-h time resolution.
The bioluminescence image series was analyzed using a custom-made, MATLAB-based (Mathworks, Natick, MA, USA) program, as described in Buijink et al. (2016). In brief, we identified groups of pixels (regions of interest; ROIs) that showed characteristics of single cells. Therefore, these ROIs are referred to as single cells. The average bioluminescence was calculated for all pixels comprising the ROIs, for the image series, resulting in bioluminescence traces representing PER2::LUC expression for each single-cell ROI. The raw traces were smoothed for further analysis of rhythm characteristics, such as peak time and period. Phase distribution is defined as the standard deviation (SD) of peak time per slice of the first cycle in vitro. The cycle-to-cycle interval is defined as the time difference between 2 consecutive half-maximum values of the rising edge of the PER2::LUC expression rhythm. The period variability is defined as the SD of the cycle interval of individual cells, calculated for the first 3 cycles in vitro and averaged per slice.
Community Detection
We employed the community detection method we previously used for the identification of neuronal clusters in the SCN (Buijink et al., 2016; Almog et al., 2019). In short, a cross-correlation matrix was constructed from the multiple time series of PER2::LUC bioluminescence intensity traces, followed by filtering out the local (neuron-specific) noise and global (SCN-wide) dependencies from the correlation matrix, using random matrix theory. The resulting communities have a positive overall correlation within communities and negative overall correlation between communities, relative to the overall SCN activity. From a few slices, the cluster locations could not be determined; this was the case for 3 slices in the LP and 1 in the SP (too few cells) in the anterior SCN and 1 slice in the posterior SCN in LP.
Statistical Analysis
For the analysis of the data, we used GraphPad Prism (San Diego, CA). For comparing data from old and young mice, we used a 2-way analysis of variance (ANOVA), followed by a Tukey’s post hoc test. For the analysis of the data in which we compared both old and young mice as well as LP and SP exposure, we used a 1-way ANOVA, followed by Sidak’s multiple comparisons correction. Differences with p < 0.05 were considered significant.
Results
PER2::LUC Expression Is Not Altered with Aging under LD 12:12
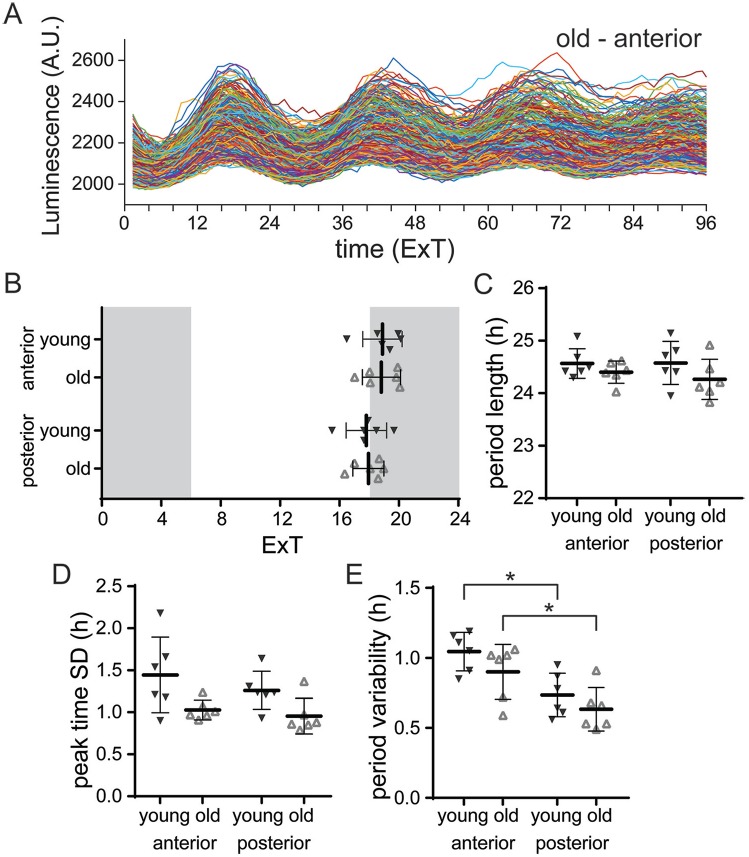
Circadian rhythms in single-cell PER2::LUC expression were measured in slice cultures from old (21-28 months) and young (4-8 months) PER2::LUC mice maintained in LD 12:12 (for an example, see Fig. 1A). We determined peak times and period of PER2::LUC rhythms from smoothed bioluminescence intensity traces of single SCN neurons. The average peak time was similar in slices from the SCN of young and old mice. Moreover, for both groups, peak time and period length did not differ between anterior and posterior slices (Fig. 1B,C; Tukey’s test, n.s.; Suppl. Table S1). Next, we tested whether aging affects the synchronization of PER2::LUC rhythms between SCN cells by using the SD of peak times as a measure of phase distribution within slices. In slices of the SCN from both young and old mice, PER2::LUC rhythms were synchronized to a similar degree (Fig. 1D; Tukey’s test, n.s.; Suppl. Table S1). The fluctuation of the cycle-to-cycle period was higher in the anterior SCN compared with the posterior SCN, in slices from both young and old mice (Fig. 1E; Tukey’s test, young: anterior v. posterior: p < 0.05, old: anterior v. posterior: p < 0.05; Suppl. Table S1). Combined, these results show that the PER2 expression in the SCN is unaltered by aging in the LD 12:12 light regime. Therefore, we sought to challenge the SCN in old mice using different photoperiods.
Figure 1.
PER2::LUC expression is not altered with aging under LD 12:12. (A) Examples of raw traces of bioluminescence intensity representing PER2::LUC expression from single cells in the anterior SCN of an old mouse (anterior, n = 242 cells; other examples in Suppl. Fig. S3). (B) Average peak time of PER2::LUC rhythms per slice of the anterior and posterior SCN from young and old mice, plotted as external time (ExT). Shaded areas represent the projected dark phase. (C) The average period length of the first 3 cycles in vitro is shown for the anterior and posterior SCN from young and old mice. (D) Phase distribution is defined as the standard deviation (SD) of peak time of the first cycle in vitro and was calculated per slice. Phase distribution is shown for the anterior and posterior SCN from young and old mice. (E) Period variability of single cells, averaged per slice from the anterior and posterior SCN from young and old mice. Filled triangles represent young mice (n = 6), and open triangles represent old mice (n = 6). Bars indicate mean ± SD. *p < 0.05, 2-way analysis of variance, corrected for multiple comparison with Tukey’s test.
Ability to Behaviorally Adapt to Photoperiod Is Compromised in Old Mice
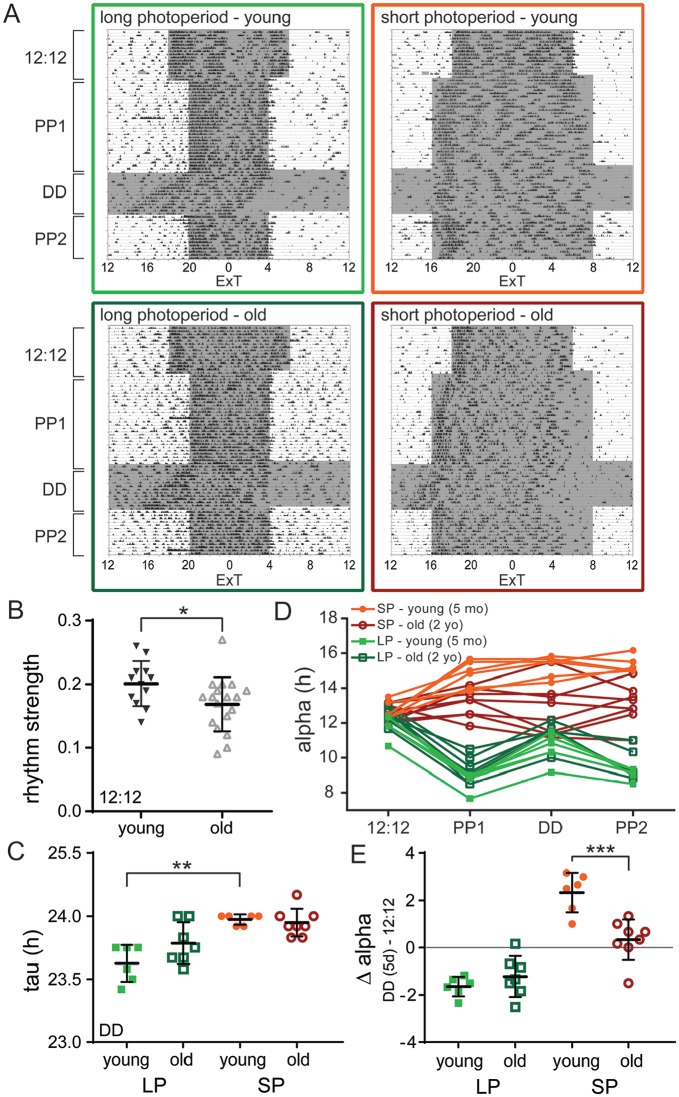
Both young and old mice were exposed to a seasonal adaptation protocol for either LP or SP (Fig. 2A; Suppl. Fig S2). Activity patterns in old mice were more fragmented, showing more activity during the day and a lower activity/rest-ratio compared with young mice (Suppl. Fig. S1C). As a consequence, the behavioral rhythm strength in old mice was reduced in LD 12:12 when compared with young mice (Fig. 2B; t test: p < 0.05; Suppl. Table S2). Moreover, the free-running period in DD was longer after exposure to SP than after LP for young mice, and this known after-effect of photoperiod (Pittendrigh and Daan, 1976) was absent in old mice (Fig. 2C; Sidak’s test, young: LP v. SP, p < 0.01, old: LP v. SP, n.s; Suppl. Table S2). To examine if the mice were able to adapt to either LP or SP, we determined the duration of locomotor activity (alpha) for the different light regime segments (Fig. 2D) and subtracted the length of alpha in DD from its length in LD 12:12 as a measure of photoperiod-induced change in alpha (Δ alpha; Fig. 2E). As expected, young mice showed an expansion of their activity profile under SP and a compression under LP, with an after-effect in the subsequent DD period (Fig. 2D; Suppl. Fig. S1; Refinetti et al., 2007). Old mice, however, were less capable of adapting to a different photoperiod. The alpha of old mice did not change after transition from LD 12:12 to SP nor from SP to DD, suggesting that old mice do not adapt to SP (Fig. 2D,E; Suppl. Table S2). In contrast to the SP condition, old mice did show adaptation to LP, with their average alpha in DD following LP being lower than in LD 12:12 (Fig. 2E; Suppl. Fig. S1). The level of adaptation to LP (Δ alpha) did not significantly differ between old and young mice (Fig. 2E; Suppl. Fig. S1; Sidak’s test, LP: young v. old, n.s.; Suppl. Table S2); however, the data from the old mice show a high variation, with some mice displaying no change of alpha in DD. This suggests that some old individuals were less able to adapt to LP. Taken together, the ability to adapt locomotor behavioral patterns to changing photoperiods is reduced in old mice.
Figure 2.
The ability to behaviorally adapt to photoperiod is reduced in old mice. (A) Single-plotted actograms showing representative passive infrared recordings from activity of young (upper panels) and old (lower panels) mice, adapted to long photoperiod (LP; left) and short photoperiod (SP; right). Shaded areas represent the dark period. The time on the x-axis is given in external time (ExT). (B) Rhythm strength of the LD 12:12 period for young (filled triangles; n = 12) and old (open triangles; n = 18) mice. (C) Period (tau) of free-running behavioral rhythm during the period of constant darkness (DD; first 10 days) for young and old mice after adaptation to LP and SP. (D) Activity period (alpha) for each segment of the entrainment protocol: LD 12:12, photoperiod 1 (PP1; LP or SP), constant darkness (DD; first 5 days), and PP2 (LP or SP), with each trace representing 1 mouse. (E) Degree of adaptation to photoperiod represented by Δ alpha, which is determined by calculating the difference in alpha between LD 12:12 and the DD (5-day) period. Δ alpha is given for young and old mice adapted to LP and SP. Filled circles represent SP, young (n = 6); open circles represent SP, old (n = 8); filled squares represent LP, young (n = 6); and open squares represent LP, old (n = 7). Bars indicate mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, 1-way analysis of variance, corrected for multiple comparison with Sidak’s test.
Response of the Molecular Clock Network to Photoperiod Is Unaltered in the Aged SCN
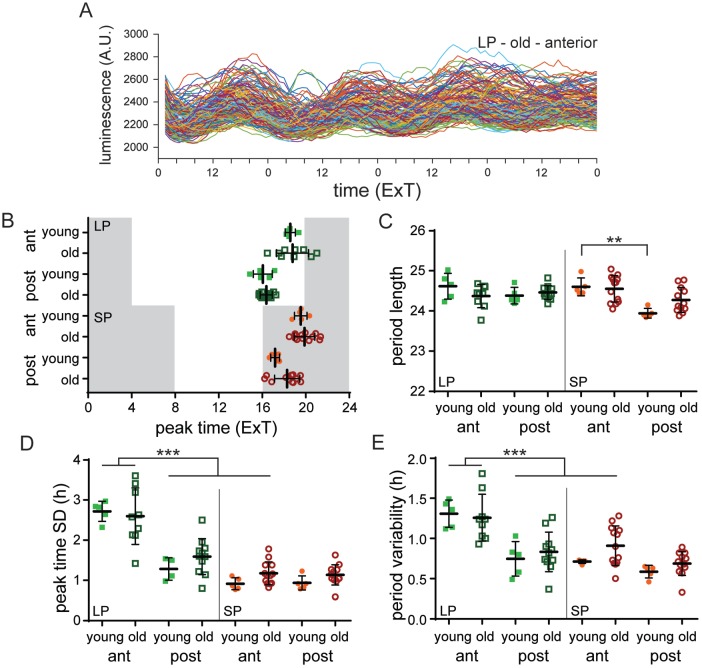
We next investigated whether the reduced capability for seasonal adaptation in old mice is the result of a more rigid, less adaptive, molecular clock. Therefore, we measured PER2::LUC expression in SCN cultured slices of old and young mice after the mice were reexposed for at least 2 weeks to either LP or SP following the DD period. We have previously shown that exposing young mice to LP (LD 16:8) causes a wider phase distribution of peak times and a higher cycle-to-cycle period variability of single-cell PER2::LUC rhythms in the anterior part of the SCN compared with SP (LD 8:16; Buijink et al., 2016). Since we see deficits in behavioral adaptation to photoperiod in old mice, and photoperiod can affect PER2 rhythm distribution, we may expect that the reduction in the capability to adapt to photoperiod is also seen at the molecular level. Surprisingly, the PER2::LUC rhythms were remarkably similar in the SCN of young and old mice. The average peak time was about 2 h later in the anterior slice compared with the posterior slice for all photoperiods in the SCN of both young and old mice (Fig. 3B; Sidak’s test, young v. old: n.s.; Suppl. Table S3). After adaptation to SP, we observed in the SCN of young mice a significantly shorter period length of PER2::LUC rhythms in the posterior part compared with the anterior part (Fig. 3C; Sidak’s test, young SP: anterior v. posterior, p < 0.01; Supp. Table S3), and this was similar in the SCN of aged mice (Fig. 3C; Sidak’s test, posterior SCN SP: young v. old, n.s.; Suppl. Table S3). Consistent with previous studies, the anterior SCN of young mice adapted to LP showed a wider phase distribution of peak times compared with the SP condition (Fig. 3D; Sidak’s test, young anterior SCN: LP v. SP, p < 0.001; Suppl. Table S3). This difference between LP and SP was also present in SCN slices from old mice (Fig. 3D; Sidak’s test, old anterior SCN: LP v. SP, p < 0.001; Suppl. Table S3). Furthermore, in both the anterior and posterior part, there was no significant difference in phase distribution between SCN slices from young and old mice (Fig. 3D; Sidak’s test, LP anterior: young v. old, n.s., LP posterior: young v. old, n.s., SP anterior: young v. old, n.s., SP posterior: young v. old, n.s.; Suppl. Table S3). Thus, even in the SCN of old mice, photoperiod still had a clear effect on the phase distribution of peak times.
Figure 3.
The molecular clock in the SCN of old mice can still adapt to different photoperiods. (A) Examples of raw traces of bioluminescence intensity representing PER2::LUC expression from single cells from the anterior SCN of an old mouse (n = 130 cells). (B) Average peak time of PER2::LUC rhythms per slice of the anterior and posterior SCN from young and old mice, adapted to long photoperiod (LP) and short photoperiod (SP) plotted as external time (ExT). Shaded areas represent the projected dark phase. (C) Average period length of the first 3 cycles in vitro is shown for the anterior and posterior SCN from young and old mice entrained to LP and SP. (D) Phase distribution of peak times per slice in the anterior and posterior SCN of young and old mice adapted to LP and SP. (E) Single-cell period variability per slice in the anterior and posterior SCN of young and old mice adapted to LP and SP. LP: anterior: young: n = 5, old: n = 9, posterior: young: n = 5, old: n = 11; SP: anterior: young: n = 5, old: n = 13, posterior: young: n = 5, old: n = 12. Filled circles represent SP, young; open circles represent SP, old; filled squares represent LP, young; open squares represent LP, old. Bars indicate mean ± SD. **p < 0.01, ***p < 0.001, 1-way analysis of variance, corrected for multiple comparison with Sidak’s test.
We analyzed the fluctuations in period from cycle to cycle of individual cells in SCN slices from old and young mice as a measure for coupling strength (Herzog et al., 2015). The SD of the cycle intervals of the first 3 cycles of PER2::LUC rhythms was determined for individual cells and averaged per slice. Consistent with our previous study (Buijink et al., 2016), the average variability in single-cell period in the SCN of young mice adapted to LP was increased in the anterior part compared with the posterior part (Fig. 3E; Sidak’s test, young LP: anterior v. posterior, p < 0.01; Suppl. Table S3) as well as to the (anterior) SCN of mice adapted to SP (Fig. 3E; Sidak’s test, young anterior SCN: LP v. SP, p < 0.01; Suppl. Table S3). This increase in single-cell cycle-to-cycle variability in the anterior SCN was similar in slices from old mice (Fig. 3E; Sidak’s test, old LP: anterior v. posterior, p < 0.001, old anterior SCN: LP v. SP, p < 0.01; Suppl. Table S3). There was no difference in the magnitude of increase in the single-cell period variability between SCN slices from young and old mice (Fig. 3E; Sidak’s test, LP anterior SCN: young v. old, n.s.; Suppl. Table S3).
Degree of Behavioral Adaptation to Photoperiod Is Related to PER2 Period Variability
So far, we have shown that old mice have a reduced ability to adapt to changing photoperiods, while on the other hand, they are still capable of adjusting their molecular clock as do young mice. However, there is a higher level of variability in both behavioral and PER2::LUC data from the old compared with young mice (Figs. 2E and 3D). We wondered whether old mice that showed little adaptation in their alpha to LP also exhibited a smaller distribution in PER2::LUC peak times. Therefore, we correlated PER2::LUC period variability in the anterior SCN with the adaptation to SP or LP (Δ alpha). For the individual groups, there is not a clear correlation between behavior and PER2::LUC expression rhythms (Suppl. Fig. S4). However, when all groups are plotted together, there appears to be an association between period variability and the adaptation of alpha to photoperiod (Suppl. Fig. S4; R2 = 0.74).
Small Changes in the Level of Functional Clusters of Neurons in the Aged SCN
We have previously shown that there is a difference in functional cluster characteristics between the SCN of mice exposed to SP and LP (Buijink et al., 2016). We wondered if aging would affect any aspects of these clusters of SCN neurons, in either their spatial pattern or their rhythm characteristics. Functional clusters were defined from time series data of PER2::LUC rhythm by an unbiased community detection algorithm (see the Methods section). We found that there is no difference in the location of the clustered cells in the SCN; there is a clear spatial distribution in the anterior and posterior SCN in both old and young mice, similar to that of our previous study. In LP, in both the young and old SCN, the ventromedial (VM) and medial (M) cluster peaked earlier than the dorsolateral (DL) and lateral (L) cluster respectively, while in the SP, this was the case only in the old SCN (Fig. 4B; Sidak’s test, VM cluster v. DL cluster, p < 0.05; Suppl. Table S4). Interestingly, as in our previous study, there is a significant difference between the VM and DL cluster in young mice exposed to LP. However, this difference is absent in old mice (Fig. 4D; Sidak’s test, anterior SCN LP: in young, DL v. VM cluster, p < 0.01; in old, DL v. VM cluster, n.s.; Suppl. Table S4). Taken together, it seems that there are small changes in PER2 rhythm characteristics of clusters of neurons in the old compared with the young SCN.
Figure 4.
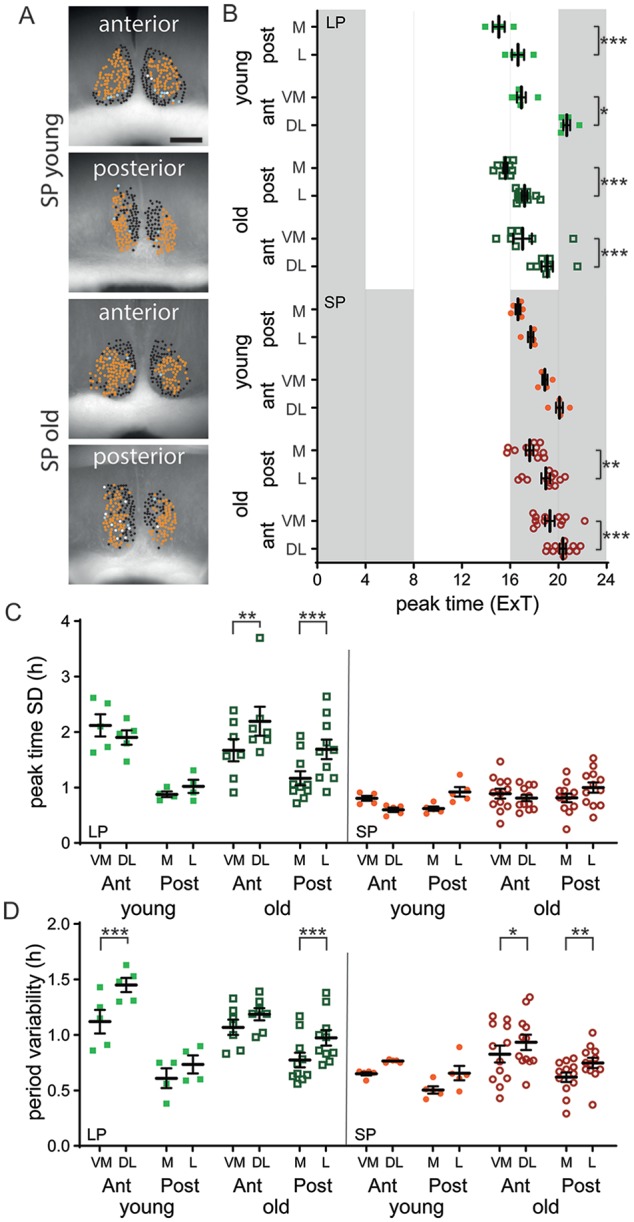
Functional cluster characteristics are similar in the old and young SCN. (A) Cell cluster location projected on bright-field image of the SCN. The different shades represent the different clusters. Examples are given for the short photoperiod (SP) of young and old mice of both the anterior and posterior slice. Scale bar marks 200 µm. (B) Average peak time of PER2::LUC rhythms for the ventromedial (VM) and medial (M), as well as the dorsolateral (DL) and lateral (L) cluster of the SCN of young and old mice entrained to either the long photoperiod (LP) or SP, plotted as external time (ExT). (C) Phase distribution of peak times for the VM/M and DL/L cluster in the anterior and posterior SCN of young and old mice adapted to the LP and SP. (D) Single-cell period variability for the VM/M and DL/L cluster in the anterior and posterior SCN of young and old mice adapted to the LP and SP. LP: anterior: young: n = 5, old: n = 7, posterior: young: n = 4, old: n = 10; SP: anterior: young: n = 5, old: n = 12, posterior: young: n = 5, old: n = 12. Filled circles represent SP, young; open circles represent SP, old; filled squares represent LP, young; open squares represent LP, old. Bars indicate mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, 1-way analysis of variance, corrected for multiple comparison with Sidak’s test.
Discussion
It is well known that with aging, sleep patterns and circadian rhythms get increasingly disturbed (Dijk and Duffy, 1999; Dijk et al., 1999). Moreover, elderly humans seem less capable of adjusting to shift work and changing of the seasons (Harma et al., 1994; Cepeda et al., 2018). However, it remains unclear which processes regulating circadian rhythms underlie this decreased flexibility. Therefore, we investigated the plasticity of both behavior and PER2::LUC expression rhythms in the young and old SCN under different lighting conditions. We show that aging does not affect PER2::LUC expression rhythms in an equinoctial light regime, while—in line with previous findings—behavioral rhythm strength is affected (Asai et al., 2001; Yamazaki et al., 2002; Kolker et al., 2003; Nakamura et al., 2011; Sellix et al., 2012; Leise et al., 2013). Considering that the old SCN neuronal network might be more rigid, we wanted to assess its adaptability by exposure to changes in day length, since this requires plasticity of the SCN network (VanderLeest et al., 2007; Porcu et al., 2018). We show that aged mice are less capable of adapting their locomotor behavioral pattern to changes in photoperiod compared with young mice. Surprisingly, the PER2::LUC expression rhythms in the SCN of old mice show similar levels of phase distribution and single-cell period variability as in young mice, with a wider phase distribution and higher period variability under LP compared with SP. We do see more variability in these parameters in aging mice and a general relationship between behavioral adaptation to photoperiod and PER2::LUC period variability. These results indicate that most of the plasticity of the molecular clock remains intact in the old SCN, and deficits in photoperiod adaptation arise downstream from the molecular clock.
Aging Affects Behavioral Adaptation to Photoperiod
To our knowledge, this is the first study to investigate the effect of exposure to LP and SP on the behavior and PER2 expression in old mice. Our results on locomotor behavior in different photoperiods corroborate the findings of previous studies that reported impairment of circadian entrainment in aged rodents. Scarbrough and colleagues (1997) showed that in Syrian hamsters, aging attenuates the effect of SP, as it did for the mice in our study. Aging is shown to reduce the sensitivity to light, which might explain the impairment in circadian entrainment (Zhang et al., 1996; Benloucif et al., 1997; Biello et al., 2018) and in reentrainment to changes in the LD cycle that we and others report (Valentinuzzi et al., 1997; Farajnia et al., 2012; Sellix et al., 2012).
Aging Has Little Effect on PER2::LUC Rhythms in the SCN in Different Photoperiods
In line with our results, previous studies have found no effect of aging on the peak time of PER2 rhythm in the SCN (Asai et al., 2001; Yamazaki et al., 2002; Kolker et al., 2003; Nakamura et al., 2011; Sellix et al., 2012; Leise et al., 2013) and no effect on peak time distribution (Sellix et al., 2012). Studies that examined PER2 expression in both the SCN and peripheral clocks have found that although the SCN retains its phase, organs such as the spleen and thymus peak significantly earlier in old compared with young mice (Sellix et al., 2012; Leise et al., 2013). In addition, the SCN of old mice still responds relatively similarly to a phase shift in the LD cycle as young mice, while their behavioral response, as well as PER2 expression in peripheral tissue, is markedly delayed (Sellix et al., 2012; Leise et al., 2013). The molecular clock of the SCN started to show signs of decay only when exposed to constant darkness or constant light (Nakamura et al., 2015; Polidarova et al., 2017). Therefore, in this study, we wanted to provoke the SCN of old mice by exposure to a naturally recurring challenge of the SCN network, namely, the seasonal changes in photoperiod (Buijink et al., 2016). We found no effect of this challenge in overall rhythm characteristics of PER2::LUC. In young and old mice, and in both LP and SP, the PER2::LUC expression in the anterior SCN peaks later than in the posterior SCN, and the peak time SD and period variability show a similar increase in the anterior SCN for both young and old mice (Fig. 3).
Studies on the aging clock have previously revealed a reorganization of the neuronal (Farajnia et al., 2012) and molecular networks (Chen et al., 2016). We therefore performed a detailed analysis of clusters of neurons in the SCN, using an unbiased method we used previously to show differences in neuronal networks in the SCN (Buijink et al., 2016; Almog et al., 2019). This analysis reconfirms the results from our previous study and also reveals some small differences in peak time and period variability between the young and old SCN. These data suggest that there are some small changes on the network level of the SCN. Taken together, both our and previous studies on PER2 expression in the SCN have not found rigorous effects of aging, which remains surprising, given that there are strong effects of aging on other SCN neuronal components as well as on PER2 expression in peripheral clocks.
Diverse Effects of Aging on the Molecular Clock of the SCN
Previous studies investigating other core clock genes in the SCN have failed to decisively show deficits in the molecular clock due to aging. Studies consistently show no age-related changes in the expression of the clock gene Per1 and a decay in expression of the clock gene Bmal1 (Asai et al., 2001; Weinert et al., 2001; Yamazaki et al., 2002; Kolker et al., 2003; Wyse and Coogan, 2010; Chang and Guarente, 2013; Bonaconsa et al., 2014). On the other hand, these studies have reported contradicting results on the effect of aging on the expression level of other clock genes, such as Per2, Cry1, Cry2, and Clock (see Banks et al., 2016, for a detailed account). For PER2::LUC, there are 2 recent studies that did find an effect of aging on PER2::LUC expression. Nakamura and colleagues (2015) found that constant darkness results in a faster decay of the amplitude and a spread of single-cell phases of PER2 expression rhythms in the SCN of old compared with young mice, although only after more than 24 h in vitro. Polidarova and colleagues (2017) found that after exposure to constant light, there was a higher incidence in arrhythmicity in PER2::LUC expression. However, only 7 of 15 old animals showed arrhythmic patterns (up from 3 of 15 in young animals) and only in 1 of the 2 slices extracted per animal. Interestingly, these 2 studies found no aging-induced deficits in PER2 rhythms in the regular LD 12:12, only after challenging the system with constant light or constant darkness. These data suggest that the molecular clock in the old SCN is robust enough to adapt to the substantial changes in photoperiod we used; nonetheless, the unaffected molecular clock does not alleviate the deficits seen in behavior.
Aging Affects Cellular and Network Properties of the SCN
Despite the apparent lack of effects of aging on the molecular clock we have reported here, there is mounting evidence that the SCN plays an important role in aging-related disruptions of circadian behavior on multiple levels. (1) The observation that age-related alterations in behavioral rhythmicity can be reversed by transplanting fetal SCN tissue in aged animals suggests that the SCN plays an important role in inducing aging-associated behavioral changes (Van Reeth et al., 1994; Cai et al., 1997; Hurd and Ralph, 1998). (2) The amplitude of the SCN multiunit electrical activity signal, both in vivo and ex vivo, decreases in aged mice (Watanabe et al., 1995; Nakamura et al., 2011; Farajnia et al., 2012). This decrease in amplitude can be the result of a change in phase synchrony, since a subpopulation of SCN neurons peaks in antiphase to the main activity peak in SCN slices of old mice (Farajnia et al., 2012). (3) Other electrical properties in SCN neurons also change with age, with alterations in the circadian regulation of ionic currents and cellular membrane deficits, which likely add to the decrease in the SCN’s electrical rhythm amplitude (Farajnia et al., 2012; Farajnia et al., 2015). (4) The number of SCN neurons in aged rats remains the same (Roozendaal et al., 1987; Miller et al., 1989; Madeira et al., 1995), which is in line with our results (Suppl. Fig. S3). However, several morphological changes have been reported, such as reduced dendritic thickness and a loss of synapses (Machado-Salas et al., 1977; Palomba et al., 2008). (5) Despite the overall number of neurons being unaffected in the old SCN, the number of arginine vasopressin– and VIP-expressing neurons is decreased (Roozendaal et al., 1987; Chee et al., 1988), and the functionality of the main neurotransmitter in the SCN, GABA, is reduced with aging (Palomba et al., 2008; Farajnia et al., 2012). If we assume that the molecular clock is the least affected by aging, as the data here and in previous studies suggest, the age-related deficits in clock function leading to the circadian phenotype seem to comprise an impairment of communication between the molecular clockwork and other intracellular clock components of SCN neurons. This may subsequently lead to neuronal network alterations, resulting in the observed deficits in the circadian behavior.
Weakened Link between the Molecular Clock and the SCN Network in Aging
The available data suggest that with aging, the molecular clock continues to function normally in SCN neurons, while the SCN network is weakened at the level of electrical activity and neurotransmitters. The lower-amplitude output signal of the SCN reduces its ability to drive peripheral circadian rhythms. However, this raises the question as to how the molecular clock can become dissociated from the other clock components of the SCN and at what level communication between the SCN network and the molecular clock is altered in aging. Interestingly, a recent study showed a functional dissociation between the molecular clock in the SCN and its downstream targets: in lactating mice, rhythms in electrical activity within the SCN as well as in the periphery were dampened, while molecular oscillations were unchanged, retaining the ability for circadian timekeeping (Abitbol et al., 2017). In addition, a modeling study of the SCN neuronal network predicts that differential GABAergic signaling can dissociate the electrical activity of SCN neurons and their molecular clock (DeWoskin et al., 2015). Other recent studies show that with the loss of the SCN network, circadian rhythms in electrical activity, calcium, and the molecular clock can become dissociated from each other (Enoki et al., 2017a, 2017b; Noguchi et al., 2017). When the network strength is reduced between SCN neurons by either blocking action potentials or a lack of connections in low-density neuronal cultures, electrical activity has less influence on calcium and PER2 expression rhythms (Noguchi et al., 2017).
In SCN neurons, calcium is an important mediator of signals from the membrane to the molecular clock and vice versa (Lundkvist et al., 2005; Enoki et al., 2017b). We have previously shown that aging reverses the rhythm in intracellular calcium levels in SCN neurons, with higher values in the night instead of the day (Farajnia et al., 2015). Therefore, we suggest that calcium homeostasis is disturbed in the aged SCN, leading to a weakening of the link between the molecular clock and the SCN network in both directions. However, the question remains as to how the SCN neurons are still able to adjust their phase distribution when their main modes of communication are disrupted. Further studies will be needed to elucidate the effect of aging on communication between the network and the molecular clock and its effect on calcium homeostasis to determine if this could be a target for strengthening circadian rhythmicity.
Supplemental Material
Supplemental material, supplemental_tables_and_figures for Aging Affects the Capacity of Photoperiodic Adaptation Downstream from the Central Molecular Clock by M. Renate Buijink, Anneke H. O. Olde Engberink, Charlotte B. Wit, Assaf Almog, Johanna H. Meijer, Jos H. T. Rohling and Stephan Michel in Journal of Biological Rhythms
Acknowledgments
We thank Gabriella Lundkvist for providing PER2::LUC knock-in mice and Mayke Tersteeg for technical support and animal care. This study was supported by funding from the Netherlands Foundation of Technology (STW; ONTIME 12191) and the Velux Foundation (project grant 1029 to S.M.).
Supplementary material is available online for this article.
Footnotes
Conflict of Interest Statement: The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: M. Renate Buijink  https://orcid.org/0000-0002-0904-139X
https://orcid.org/0000-0002-0904-139X
Jos H. T. Rohling  https://orcid.org/0000-0001-5721-2715
https://orcid.org/0000-0001-5721-2715
Stephan Michel  https://orcid.org/0000-0001-5506-5037
https://orcid.org/0000-0001-5506-5037
References
- Abitbol K, Debiesse S, Molino F, Mesirca P, Bidaud I, Minami Y, Mangoni ME, Yagita K, Mollard P, Bonnefont X. (2017) Clock-dependent and system-driven oscillators interact in the suprachiasmatic nuclei to pace mammalian circadian rhythms. PLoS One 12:e0187001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog A, Buijink MR, Roethler O, Michel S, Meijer JH, Rohling JHT, Garlaschelli D. (2019) Uncovering functional signature in neural systems via random matrix theory. PLoS Comput Biol 15:e1006934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. (2001) Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res 66:1133-1139. [DOI] [PubMed] [Google Scholar]
- Azzi A, Dallmann R, Casserly A, Rehrauer H, Patrignani A, Maier B, Kramer A, Brown SA. (2014) Circadian behavior is light-reprogrammed by plastic DNA methylation. Nat Neurosci 17:377-382. [DOI] [PubMed] [Google Scholar]
- Banks G, Nolan PM, Peirson SN. (2016) Reciprocal interactions between circadian clocks and aging. Mamm Genome 27:332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Masana MI, Dubocovich ML. (1997) Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res 747:34-42. [DOI] [PubMed] [Google Scholar]
- Biello SM, Bonsall DR, Atkinson LA, Molyneux PC, Harrington ME, Lall GS. (2018) Alterations in glutamatergic signaling contribute to the decline of circadian photoentrainment in aged mice. Neurobiol Aging 66:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaconsa M, Malpeli G, Montaruli A, Carandente F, Grassi-Zucconi G, Bentivoglio M. (2014) Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Exp Gerontol 55:70-79. [DOI] [PubMed] [Google Scholar]
- Buijink MR, Almog A, Wit CB, Roethler O, Olde Engberink AH, Meijer JH, Garlaschelli D, Rohling JH, Michel S. (2016) Evidence for weakened intercellular coupling in the mammalian circadian clock under long photoperiod. PloS One 11:e0168954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai A, Lehman MN, Lloyd JM, Wise PM. (1997) Transplantation of fetal suprachiasmatic nuclei into middle-aged rats restores diurnal Fos expression in host. Am J Physiol 272:R422-R428. [DOI] [PubMed] [Google Scholar]
- Carvalho-Bos SS, Riemersma-van der Lek RF, Waterhouse J, Reilly T, Van Someren EJ. (2007) Strong association of the rest-activity rhythm with well-being in demented elderly women. Am J Geriatr Psychiatry 15:92-100. [DOI] [PubMed] [Google Scholar]
- Cepeda M, Koolhaas CM, van Rooij FJA, Tiemeier H, Guxens M, Franco OH, Schoufour JD. (2018) Seasonality of physical activity, sedentary behavior, and sleep in a middle-aged and elderly population: the Rotterdam study. Maturitas 110:41-50. [DOI] [PubMed] [Google Scholar]
- Chang HC, Guarente L. (2013) SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153:1448-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee CA, Roozendaal B, Swaab DF, Goudsmit E, Mirmiran M. (1988) Vasoactive intestinal polypeptide neuron changes in the senile rat suprachiasmatic nucleus. Neurobiol Aging 9:307-312. [DOI] [PubMed] [Google Scholar]
- Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E, McClung CA. (2016) Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A 113:206-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yoo SH, Takahashi JS. (2018) Development and therapeutic potential of small-molecule modulators of circadian systems. Annu Rev Pharmacol Toxicol 58:231-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. (2006) Chronic jet-lag increases mortality in aged mice. Curr Biol 16:R914-R916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWoskin D, Myung J, Belle MD, Piggins HD, Takumi T, Forger DB. (2015) Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping. Proc Natl Acad Sci U S A 112:E3911-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF. (1999) Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med 31:130-140. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. (1999) Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol 516:611-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky YV, Samsa WE, Kondratov RV. (2010) Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging 2:936-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki R, Oda Y, Mieda M, Ono D, Honma S, Honma KI. (2017. a) Synchronous circadian voltage rhythms with asynchronous calcium rhythms in the suprachiasmatic nucleus. Proc Natl Acad Sci U S A 114:E2476-E2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki R, Ono D, Kuroda S, Honma S, Honma KI. (2017. b) Dual origins of the intracellular circadian calcium rhythm in the suprachiasmatic nucleus. Sci Rep 7:41733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Meijer JH, Michel S. (2015) Age-related changes in large-conductance calcium-activated potassium channels in mammalian circadian clock neurons. Neurobiol Aging 36:2176-2183. [DOI] [PubMed] [Google Scholar]
- Farajnia S, Michel S, Deboer T, vanderLeest HT, Houben T, Rohling JH, Ramkisoensing A, Yasenkov R, Meijer JH. (2012) Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci 32:5891-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O. (2011) Circadian rhythms, aging, and life span in mammals. Physiology 26:225-235. [DOI] [PubMed] [Google Scholar]
- Gaikwad S. (2018) The biological clock: future of neurological disorders therapy. Neural Regen Res 13:567-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harma MI, Hakola T, Akerstedt T, Laitinen JT. (1994) Age and adjustment to night work. Occup Environ Med 51:568-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Kiss IZ, Mazuski C. (2015) Measuring synchrony in the mammalian central circadian circuit. Methods Enzymol 552:3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MW, Ralph MR. (1998) The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms 13:430-436. [DOI] [PubMed] [Google Scholar]
- Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K, Ibata Y. (1997) Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett 222:99-102. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. (2003) Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythm 18:159-169. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20:1868-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leise TL, Harrington ME, Molyneux PC, Song I, Queenan H, Zimmerman E, Lall GS, Biello SM. (2013) Voluntary exercise can strengthen the circadian system in aged mice. Age (Dordr) 35:2137-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. (2019) Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 18:307-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. (2005) A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci 25:7682-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Salas J, Scheibel ME, Scheibel AB. (1977) Morphologic changes in the hypothalamus of the old mouse. Exp Neurol 57:102-111. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Santer RM, Paula-Barbosa MM, Gundersen HJ. (1995) Age and sex do not affect the volume, cell numbers, or cell size of the suprachiasmatic nucleus of the rat: an unbiased stereological study. J Comp Neurol 361:585-601. [DOI] [PubMed] [Google Scholar]
- Miller MM, Gould BE, Nelson JF. (1989) Aging and long-term ovariectomy alter the cytoarchitecture of the hypothalamic-preoptic area of the C57BL/6J mouse. Neurobiol Aging 10:683-690. [DOI] [PubMed] [Google Scholar]
- Most EI, Scheltens P, Van Someren EJ. (2010) Prevention of depression and sleep disturbances in elderly with memory-problems by activation of the biological clock with light—a randomized clinical trial. Trials 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS, Block GD. (2015) Age-related changes in the circadian system unmasked by constant conditions. eNeuro 2:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. (2011) Age-related decline in circadian output. J Neurosci 31:10201-10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Leise TL, Kingsbury NJ, Diemer T, Wang LL, Henson MA, Welsh DK. (2017) Calcium circadian rhythmicity in the suprachiasmatic nucleus: cell autonomy and network modulation. eNeuro 4:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard M, Palomba M. (2006) The GABAergic network in the suprachiasmatic nucleus as a key regulator of the biological clock: does it change during senescence? Chronobiol Int 23:427-435. [DOI] [PubMed] [Google Scholar]
- Palomba M, Nygard M, Florenzano F, Bertini G, Kristensson K, Bentivoglio M. (2008) Decline of the presynaptic network, including GABAergic terminals, in the aging suprachiasmatic nucleus of the mouse. J Biol Rhythms 23:220-231. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. (1976) Functional-analysis of circadian pacemakers in nocturnal rodents. 1. Stability and lability of spontaneous frequency. J Comp Physiol 106:223-252. [Google Scholar]
- Polidarova L, Sladek M, Novosadova Z, Sumova A. (2017) Aging does not compromise in vitro oscillation of the suprachiasmatic nuclei but makes it more vulnerable to constant light. Chronobiol Int 34:105-117. [DOI] [PubMed] [Google Scholar]
- Porcu A, Riddle M, Dulcis D, Welsh DK. (2018) Photoperiod-induced neuroplasticity in the circadian system. Neural Plast 2018:5147585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R, Cornélissen G, Halberg F. (2007) Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 8:275-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolden HJ, Rohling JH, van Bodegom D, Westendorp RG. (2015) Seasonal variation in mortality, medical care expenditure and institutionalization in older people: evidence from a dutch cohort of older health insurance clients. PLoS One 10:e0143154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. (1987) Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res 409:259-264. [DOI] [PubMed] [Google Scholar]
- Scarbrough K, Losee-Olson S, Wallen EP, Turek FW. (1997) Aging and photoperiod affect entrainment and quantitative aspects of locomotor behavior in Syrian hamsters. Am J Physiol 272:R1219-R1225. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M, Davidson AJ. (2012) Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci 32:16193-16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. (1997) Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol 273:R1957-1964. [DOI] [PubMed] [Google Scholar]
- VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, Meijer JH. (2007) Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol 17:468-473. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Zhang Y, Zee PC, Turek FW. (1994) Grafting fetal suprachiasmatic nuclei in the hypothalamus of old hamsters restores responsiveness of the circadian clock to a phase shifting stimulus. Brain Res 643:338-342. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Shibata S, Watanabe S. (1995) Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res. 695:237-239. [DOI] [PubMed] [Google Scholar]
- Weinert H, Weinert D, Schurov I, Maywood ES, Hastings MH. (2001) Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int 18:559-565. [DOI] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. (1990) Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry 27:563-572. [DOI] [PubMed] [Google Scholar]
- Wyse CA, Coogan AN. (2010) Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res 1337:21-31. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. (2002) Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A 99:10801-10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. (1996) Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience 70:951-961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplemental_tables_and_figures for Aging Affects the Capacity of Photoperiodic Adaptation Downstream from the Central Molecular Clock by M. Renate Buijink, Anneke H. O. Olde Engberink, Charlotte B. Wit, Assaf Almog, Johanna H. Meijer, Jos H. T. Rohling and Stephan Michel in Journal of Biological Rhythms