We highlight recent progress in understanding genes and processes in sources and sinks, and their integration in the context of crop ideotype and agricultural environment with promise in increasing crop yields.
Keywords: Carbohydrate allocation, crop yields, source–sink, traits, trehalose 6-phosphate, wheat
Abstract
Understanding processes in sources and sinks that contribute to crop yields has taken years of painstaking research. For crop yield improvement, processes need to be understood as standalone mechanisms in addition to how these mechanisms perform at the crop level; currently there is often a chasm between the two. Fundamental mechanisms need to be considered in the context of crop ideotypes and the agricultural environment which is often more water limited than carbon limited. Different approaches for improvement should be considered, namely is there genetic variation? Or if not, could genetic modification, genome editing, or alternative approaches be utilized? Currently, there are few examples where genetic modification has improved intrinsic yield in the field for commercial application in a major crop. Genome editing, particularly of negative yield regulators as a first step, is providing new opportunities. Here we highlight key mechanisms in source and sink, arguing that for large yield increases integration of key processes is likely to produce the biggest successes within the framework of crop ideotypes with optimized phenology. We highlight a plethora of recent papers that show breakthroughs in fundamental science and the promise of the trehalose 6-phosphate signalling pathway, which regulates carbohydrate allocation which is key for many crop traits.
Introduction
Improvement of crop yields has been a major technological achievement in the post-war period akin to other significant global advances such as the near elimination of some major contagious diseases, and has been a major factor in the alleviation of global hunger and poverty. Crop yield increases have been driven by absolute need through population growth as well as opportunity provided by new technology. In the 1960s, the Green Revolution was able to improve yields of cereals through stem shortening which increased yield through better harvest index and reduced losses due to lodging. Improved yields were then protected with selection of disease resistance genes. This was achieved through relatively straightforward breeding and utilization of two harvests in one year in north and south Mexico to speed up the selection process. Genetic modification (GM) in the 1990s provided a second revolution. Highlighting here major crops where GM has impacted, the ability to genetically modify crops to express Bacillus thuringiensis (Bt) endotoxins to protect them from the pernicious stem corn borer has led to yield improvements in maize and cotton. At the same time this has also improved farm profitability and lowered environmental impacts through reduced pesticide use. Herbicide-resistant soybean and canola also raised yields because of better weed control and enabled no tillage and often two crops in one season, meaning that soybean could be grown where it might not otherwise have been grown, with the added bonus of providing nitrogen to the soil. However, until recently, GM has not improved crop yields for commercial application through targeting intrinsic yield-determining genes and processes except for a genetically modified maize variety which performs slightly better under drought (Castiglioni et al., 2008). With the development of gene editing, promising signs are emerging that this could provide a new way forward. Molecular breeding has and will continue to be a staple of the improvement process. Both gene editing and molecular breeding require knowledge of the associated gene sequences and, even better, the function and importance of the gene(s) in determining yield. This requires advances in the fundamental science of gene and process function. There is also the need to consider how processes are integrated, such as source–sink balance within the whole plant in the agricultural environment. Whilst it is not likely that single gene candidates can provide a doubling or trebling of yields as seen in the 1960s, understanding genes and their functions in crop yield improvement is likely to provide a necessary basis for the current requirement to increase crop yields by 70% by 2050 for a projected 9 billion people. Such genes need to be considered in the light of projected ideotypes for enhancing crop yields. In this review, we summarize traits, with emphasis on cereals and particularly wheat, which are most likely to improve yields in coming years, with a particular focus on source–sink traits and their inter-relationship.
Traits for improvement of crop yields
A recent article (Senapati et al., 2019) highlighted traits providing a wheat ideotype that were most likely to give wheat yield improvements in high-yielding environments of the UK and New Zealand subject to largely mild intermittent drought. These characteristics were made up of phenological traits of leaf appearance and day length responses for flowering time, provision of photosynthate through maximization of flag leaf area and stay-green, with resilience of this photosynthetic capacity under drought, duration of the grain-filling period, and improvement of root water uptake. It was estimated that optimization of these traits could give rise to between 43% and 62% increases in yield in the likely climate of 2050 (Senapati et al., 2019). Within this framework of a wheat ideotype, it is the objective of many researchers to introduce further improvements in specific source and sink characteristics such as in photosynthesis and leaf gas exchange, grain number, and size. Ultimately, significant improvements in crop yields will require effective synchronization within and between optimized source and sink traits.
Source traits
Photosynthesis has been favoured in recent years for yield improvement. Several components of photosynthetic leaf gas exchange are currently being targeted (Simkin et al., 2019) in line with photosynthetic models of where limitations lie (Zhu et al., 2010). Photosynthetic models are very good at identifying where limiting steps may be as standalone mechanisms including field environments (Zhu et al., 2010). In the context of a crop canopy in an agricultural environment, linking specific steps of photosynthesis to crop yields is far more difficult (Paul et al., 2017). With this view in mind, Wu et al. (2019) developed a diurnal canopy photosynthesis stomatal conductance model that connected leaf photosynthesis to crop yield in a crop canopy typical of an Australian cropping region in spring and summer for wheat and sorghum considering both water-limited and water-unlimited conditions. The model used simulated canopy responses and data for crop biomass and yield for wheat and sorghum from diverse field data sets. The model included three major targets that have been proposed for enhancing C3 and C4 photosynthesis to improve crop yields: (i) maximum carboxylation rate of Rubisco; (ii) electron transport capacity; and (iii) mesophyll conductance for CO2. An outcome of the model is that it was estimated that improving each of these components by 20% individually would make no or only modest improvements in crop yield. Maximal improvements in crop yield were 7–8% for a 20% increase in the rate of electron transport in wheat and sorghum under full irrigation. Increasing all three components together lifted yield improvements to 9.2% and 12.2% for sorghum and wheat, respectively, under full irrigation, but with more modest improvement where water was limited. Rubisco has long been proposed as the bottleneck for photosynthetic improvement, but a 20% enhancement in maximum Rubisco carboxylation rate was found to have no impact on yield for both wheat and sorghum even under full irrigation (Wu et al., 2019). A recent study overexpressed Rubisco in maize, which resulted in a 15% increase in maximal assimilation rate which increased fresh weight, but yield was not measured. Interestingly, the authors comment that while no growth penalty was observed under optimal conditions, highly expressing Rubisco transgenes could negatively affect yield due to the increased metabolic load (Salesse-Smith et al., 2018). A very interesting recent study (Lobo et al., 2019) showed that expressing 2-carboxy-d-arabinitol-1-phosphate phosphatase in wheat to remove 2-carboxy-d-arabinitol-1-phosphate, a Rubisco inhibitor, also decreased Rubisco active sites and wheat yields in an opposite direction to what might be expected. This emphasizes the issue of understanding systems as a whole and that any modification of Rubisco activity to improve photosynthesis and yield is likely to be far from straightforward because of the many complexities of this enzyme. A second Calvin cycle enzyme, sedoheptulose 1,7-bisphosphatase (SBPase), shows promise in model species in a controlled environment (Driever et al., 2017) and in tobacco in the field under elevated CO2 (Rosenthal et al., 2011). It may be that combining enhanced activities of both Rubisco and SBPase could provide enhanced ribulose bisphosphate substrate for Rubisco in addition to improved carboxylation capacity.
A major conclusion of Wu et al. (2019) was that the effects of photosynthetic enhancement on crop water dynamics have not previously been considered in a rigorous way. Water use by the crop remains an over-riding caveat when considering increased carbon gain, as much of agricultural production is rainfed rather than irrigated, and hence subject to potential intermittent drought. The challenge faced by this is that in water-limited situations, enhanced photosynthesis leads to more rapid depletion of water. A critical new outcome of the cross-scale modelling system through the crop life cycle (Wu et al., 2019) is that enhanced photosynthesis improves biomass gain early in the season when soil water is more abundant, but results in greater soil water depletion, leaving less for later growth and development, impinging on yields in all but the most hydrated agricultural environments. Interestingly, a confirmation of this conclusion comes from a study by Borrell et al. (2014) who showed that a reduction in canopy size associated with stay-green sorghum reduced pre-flowering water demand, increasing water availability during grain filling and increasing grain yield.
Introducing C4 photosynthesis into rice may be a way to mitigate increased water loss associated with improved carbon gain, as C4 has improved water use efficiency. However, it is thought that C4 rice engineering may be many years away from realizing an improved crop because of the complexities of engineering this complex trait to combine both metabolic and anatomical changes, an ambitious goal, although interesting progress has been made (Wang et al., 2017). For crops such as wheat, except for areas such as northern Europe and New Zealand, improvement in photosynthesis, unless associated with improvements in water use efficiency, are unlikely to be beneficial. Most of agriculture is water limited to some degree, and most benefits for yield would come from increasing water availability and uptake and/or decreasing crop water loss rather than increasing CO2 fixation unless improved carbon uptake came with no water cost.
Interestingly, PSII overexpression increases tobacco water use efficiency by decreasing stomatal opening in response to light (Glowacka et al., 2018). However, plants had less dry weight at harvest, and further evaluation would be required under water-limited conditions which were not tested in the study. Other work from the Long group has shown that engineering tobacco for accelerated recovery from photoprotection gives large biomass improvements (15%) in the field (Kromdijck et al., 2016). Transgenic expression of Arabidopsis violaxanthin de-epoxidase, PSII subunit S, and zeaxanthin epoxidase in combination led to a marked acceleration of non-photochemical quenching relaxation on transfer of leaves from high light to shade. This resulted in more rapid recovery of the efficiency of photosynthetic CO2 assimilation in the shade. Such photosynthetic improvements, if they do not involve increased water loss, could be key to a photosynthetic engineering strategy. Integration of such benefits into yield would need to be evaluated in a major crop in the field.
Photorespiration is estimated to reduce yields by 20–50% in C3 crops. South et al. (2019) transformed tobacco chloroplasts with synthetic glycolate metabolic pathways thought to be more efficient than the native pathways. Synthetic pathway flux was maximized by inhibiting glycolate export from the chloroplast. The synthetic pathways improved photosynthetic quantum yield by 20% and increased biomass productivity by >40% in replicated field trials. These results show that engineering alternative glycolate metabolic pathways into chloroplasts while inhibiting glycolate export into the native pathway can drive increases in carbon gain in a C3 plant and increase biomass under agricultural field conditions. However, it would be important to replicate these results beyond tobacco in a food security crop with a significant sink (e.g. grain) to see that increased biomass translated into increased yield. In the eletters that accompanied this paper, leading experts question the extent of the biomass advantage conferred. Something other than photorespiration must be involved, as the biochemistry of glycolate cannot account for the biomass increase (Evans, 2019). Secondly, bias was introduced in the field assessment because the transgenics were shading the wild type due to the earlier growth vigour of the transgenics. This could easily explain the 40% biomass gain (Fischer et al., 2019). Fischer goes on to mention the difficulties of claims of such large growth effects. Another very recent study in rice with altered photorespiratory bypass showed that a CO2-concentrating effect of the alteration increased grain yield under certain conditions such as in the spring and with high light (Shen et al., 2019). However, the lack of a spectacular effect in rice comparable with that seen in tobacco indicates that improving yields by this route in major food security crops may be more challenging than for tobacco.
Selection for higher yields has increased stay-green in modern maize hybrids. A strategy to improve this further has been shown by Zhang et al. (2019). Transgenic maize lines where a nac7 gene was down-regulated by RNAi showed delayed senescence and increased both biomass and nitrogen accumulation in vegetative tissues, demonstrating that NAC7 functions as a negative regulator of the stay-green trait.
Recent work on regulation of stomatal function has also provided some exciting possibilities for crop yield enhancement, particularly as stomata offer promise in the improvement of crop water use efficiency so crucial in crop yield improvement. Papanatsiou et al. (2019) expressed the synthetic light-gated K+ channel gene BLINK1 in guard cells surrounding stomatal pores in Arabidopsis to enhance the solute fluxes that drive stomatal aperture. BLINK1 introduced a K+ conductance and accelerated both stomatal opening under light exposure and closure after irradiation. Integrated over the growth period, BLINK1 drove a 2.2-fold increase in biomass in fluctuating light, without cost in water use by the plant. This demonstrates the potential of enhancing stomatal kinetics to improve water use efficiency without penalty in carbon fixation. Further confirmation of the crucial effects of stomata have been published by Caine et al. (2019) who engineered rice by overexpressing the rice epidermal patterning factor OsEPF1, creating plants with substantially reduced stomatal density and correspondingly low stomatal conductance. Low stomatal density rice lines were more able to conserve water, using ~60% of the normal amount 4 and 5 weeks after germination. When grown at elevated atmospheric CO2, rice plants with low stomatal density were able to maintain their stomatal conductance and survive drought and high temperature (40 °C) for longer than control plants. Low stomatal density rice gave equivalent or even improved yields, despite a reduced rate of photosynthesis in some conditions. Emphasizing the need to study processes at a whole-plant level, reduced stomatal conductance induced the formation of increased root cortical aerenchyma (Mohammed et al., 2019). This may be because of inhibited oxygen diffusion to the root, creating an oxygen deficit and stimulating the formation of the aerenchyma, or the possible involvement of an unknown EPF signalling pathway. Another recent study (Gonzalez et al., 2019) shows improved water use efficiency and grain yield in transgenic wheat through expression of a HaHB4 transcription factor. The mode of action of this gene is not known, but enhanced water use efficiency could be due to an improved source trait which then leads to more tillers per plant and more spikelets per spike. Clearly, in this case, a coordination between enhanced water use efficiency of the source, if the source is the primary site of action in this case, and grain yield has occurred.
Canopy structure may also be a means to improve carbon capture. Erect canopies significantly increase yield in maize (Pendelton et al., 1968), but this trait does not appear to have been a target of selection in wheat. Recent work shows the promise of erectophile compared with planophile canopies (Richards et al., 2019). The advantage of the former is that more light penetrates the canopy to illuminate lower leaves and more light is deflected from erect leaves into the rest of the canopy. Leaf area index of wheat typically reaches a maximum soon after flag leaf emergence. This time just after flowering is the most crucial time for the determination of grain number, and hence sink strength and the yield of wheat (Fischer, 2007), when carbon is in greatest demand with stems and ears actively growing and fertile florets are being established. The period is considered a bottleneck for the determination of yield potential, so extra light capture and photosynthesis during this period is expected to increase grain number, yield, and biomass.
Sink traits
The example above (Richards et al., 2019) is a perfect case of interaction between source and sink. High photosynthesis around flowering establishes high sink potential, setting up the plant for high yield later on. The period 10–15 d before anthesis is most important for determination of grain number (Fischer, 2007). Knowledge of the molecular events (e.g. sugar supply and signalling) that are important for determining grain number during this period may be particularly important for yield enhancement. A combination of both carbon assimilate supply and nitrogen over this period is thought to be crucial in determining grain numbers (Sinclair and Jamieson, 2006). Genes controlling wheat spikelet arrangement and hence grain numbers have been discovered. However, it is unclear how these genes interact with carbon and nitrogen supply. A recent example from the literature is Ppd-1 which is a key regulator of inflorescence architecture and paired spikelet development in wheat (Boden et al., 2015). FRIZZY PANICLE drives supernumerary spikelets in bread wheat (Dobrovolskaya et al., 2015). Mutations in the TtBH-A1 meristem identity gene increase spikelet and grain number per spike (Poursarebani et al., 2015). Genetic modification of wheat spike architecture by introgressing the ‘Miracle wheat’ bht-A1 allele into an elite durum wheat cv. Floradur successfully developed near isogenic lines (NILs) with a modified spikelet arrangement, increasing spikelet and grain number per spike without compromising grain size (Wolde et al., 2019). Overexpression of an auxin receptor OsAFB6 significantly enhanced grain yield by increasing cytokinin and decreasing auxin concentrations in rice panicles (He et al., 2018). Auxin signalling F-Box 6 (OsAFB6) increased spikelets per panicle and primary branch number, enhancing grain yield by 50%. Beyond the crucial developmental period just before anthesis, there are a number of studies in wheat that conclude that sinks still exert a dominant effect on yield (Borras and Slafer, 2004; Serrago et al., 2013). To illustrate this, an interesting study by Borrill et al. (2015) showed that NAM RNAi wheat with delayed senescence and higher rates of photosynthesis during grain filling accumulated fructan in stems rather than filling grain, showing that the capacity to fill grain rather than flag leaf photosynthesis almost completely limits yield during grain filling. Hence the elucidation of genetic factors that control sink strength of grain is a priority. Wheat spikelets that make up the wheat spike or ear can bear more than one grain, unlike other cereals, and the number of grains per spikelet has increased through breeding (Thomas, 2017). Further enhancing spikelet number is a strategy as increasing spike fertility as an approach has been difficult (Guo et al., 2016).
For grain weight, quantitative trait loci (QTLs) have been identified, but no gene controlling grain weight in wheat has yet been cloned. Single loci may increase grain weight by <10%. In wheat, changes in grain size are more difficult to detect than changes in spike architecture. TaGW2 is a negative regulator of grain size through regulation of cell number. Mutations in all three homeologues have an additive effect on grain size up to 20% in wheat (Wang et al., 2019). The hexaploid nature of wheat may mean that single gene mutations have a lower impact that in other species such as rice. In rice, GW2 encodes a RING-type E3 ubiquitin ligase (Song et al., 2007), and a loss-of-function mutation produces 50% increased grain weight. This gene appears to be conserved across Arabidopsis, wheat, rice, and maize (Brinton and Uauy, 2019), providing an excellent example of how knowledge from model species can translate to crops. Another example is KLUH, an Arabidopsis cytochrome P450 gene, which promotes cell proliferation and seed size in Arabidopsis and wheat (Ma et al., 2016). Transcription factor genes such as SPL16 regulate grain width in rice promoting cell proliferation (Wang et al., 2012). The ubiquitin pathway regulates grain size in Arabidopsis and rice (Du et al., 2014; Huang et al., 2017; Shi et al., 2019). Expansins and cell wall genes can also regulate grain size through cell size (Munoz and Calderini, 2015). Phytohormones, G-protein signalling (Miao et al., 2019), and mitogen-activated protein kinase signalling can also regulate grain size (Brinton and Uauy, 2019). The miR1432–OsACOT (acyl-CoA thioesterase) module determines grain yield via enhancing the grain filling rate in rice through modification of auxin and abscisic acid levels mediated by changes in fatty acid biosynthesis (Zhao et al., 2019). An interesting study that supports the idea that final grain weight is determined by factors early during grain development (i.e. cell size and number) was published recently (Fahy et al., 2018). This study came to this conclusion after analysing the interaction between starch metabolism enzyme activities, ADP-glucose pyrophosphorylase and soluble starch synthase, and final grain yield, and concluded that yield is not determined or limited by the activities of starch-metabolizing enzymes during grain filling but by earlier factors that set the capacity for grain size. Interestingly, Tian et al. (2018) overexpressed starch synthase in wheat and found that this improved yield under heat stress. Starch synthesis could potentially limit yield under more extreme conditions. However, it is likely that significant selection for starch synthesis has already occurred over the course of wheat yield improvement. TaBT1 plays a vital role in starch synthesis and is significantly correlated with thousand grain weight (TGW) in wheat. BT1 is responsible for the transmembrane transportation of ADP-glucose in endosperm starch synthesis and universally exists in cereals (Denyer et al., 1996; Sikka et al., 2001; Tetlow et al., 2003). TaBT1 is concluded to have undergone strong selection during wheat improvement for starch synthesis and TGW (Wang et al., 2019). Another enzyme involved in starch synthesis is sucrose synthase which catalyses the first step in the conversion of sucrose to starch. Genotyping 1520 wheat accessions showed significant differences between sucrose synthase haplotypes and TGW. Frequency changes for favoured haplotypes showed gradual increases in cultivars released since the beginning of the last century in China, Europe, and North America. This work shows that the endosperm starch synthesis pathway has already been selected in global wheat breeding for higher yield (Hou et al., 2014). Starch synthesis capacity may currently not limit wheat yield.
Interactions of traits for yield improvement
The number of occasions when single genes can be selected to increase yield may be small. As already mentioned, genetic modification has not yet increased yield reproducibly in the field in an important crop to the point of commercial release, except for one case of drought-tolerant maize. However, recent papers in the last couple of years outlined above give much cause for hope. Gene editing of negative regulators of yield traits in rice may offer particular promise. However, ultimately large yield increases of more than a few percent are likely to require changes in more than one gene and process. This does necessitate knowledge of how processes are integrated. An example of such complexity is in the interaction of grain number with grain size. One strategy to increase both would be to combine mutations in negative regulators of grain size GS3 (Fan et al., 2006) and grain number GN1a (Ashikari et al., 2005). When this was attempted, increased grain number and size was observed in 10 different rice genotypes tested (Shen et al., 2016) but translated into greater yield in only three of the 10 cultivars because tillering was reduced in the other seven cultivars. In sorghum, 17 QTLs for TGW were identified in a cross between cultivated and wild sorghum (Tao et al., 2018). Eleven of these QTLs exhibited an opposing effect on grain number. The other six had smaller phenotypic effects and were not associated with grain number. They were found to segregate in cultivated material and provide scope for increasing grain size and yield. Both drought and heat stress reduce seed set (Dolferus et al., 2011; Singh et al., 2015). Abiotic stress resilience may come from increasing seed set under such conditions. Interestingly, Singh et al. (2015) showed, in a study of sorghum genetic variation in seed set under heat stress, that poor seed set was not compensated for by an increase in grain mass, again showing that this compensation mechanism (seed number and size) does not always operate.
Clearly there are strong interactions between grain size, number, and tillering. One such factor, although probably not an exclusive factor, is carbohydrate supply, which affects grain number, size, and tillering. A recent study showed that roots virtually ceased growing when the demand for carbon by the shoots during stem elongation and ear growth became large (Li et al., 2019). These studies underline the importance of whole-plant carbon allocation in regulating growth. If roots are engineered for better water uptake which requires greater investment of carbon in roots, carbon allocation to ears could be impinged. A new study by Ogura et al. (2019) reports a new regulatory gene and molecular mechanism that links auxin-dependent root angle regulation with improved plant fitness under variable rainfall conditions in Arabidopsis, which could underpin improving root architecture for yield in crops. Understanding how trade-offs in carbohydrate allocation between organs and processes and whether carbon or water are the factors limiting yield in the field are important considerations. One important underpinning factor is understanding how plant carbohydrate status and allocation is managed and integrated in crops. This may provide important fundamental science that could direct how crop yields could be improved.
The trehalose pathway, a central regulator of carbon allocation and integrator of source and sink
Source–sink interactions and coordination have been highlighted as key in yield enhancement either in stacking both beneficial source and sink characteristics (Sonnewald and Fernie, 2018) or in enhancing source–sink coordination for yield (Reynolds et al., 2012). Source–sink optimization is also identified as a means to deal with specific abiotic stresses such as drought and heat (Peleg et al., 2011; Abdelrahman et al., 2020). Interestingly, the breeding for yield in wheat has also improved performance under drought conditions (Cattivelli et al., 2008). It may be that the necessity for an improved source–sink is greater under abiotic stress, hence there are general benefits of improving the source–sink inter-relationship. This contradicts strategies for improving yield under drought that focus on stress protection mechanisms and survival (e.g. Khan et al., 2019) which usually produce yield penalties. It may be that focusing on an optimized source–sink balance is a better overall strategy for crop improvement in a range of environments. A more extreme xerophytic strategy involving stress protection mechanisms to improve plant survival may be suitable for more marginal environments. Sucrose has been postulated for a long time as a mediator of source–sink interactions (Farrar et al., 2000) perhaps more so than any other factor as sucrose is the direct end-product of photosynthesis and the starting point for growth, and is transported from the source to the sink. Potentially sucrose could feedforward-regulate sinks through up-regulating genes for sink strength, and feedback-regulate photosynthesis if supply of sucrose outstripped demand (Farrar et al., 2000). Sucrose does have some direct regulatory functions, such as in regulating translation (Hummel et al., 2009), but a powerful regulatory function as a signal of sucrose is through trehalose 6-phosphate (T6P), which performs at least some of the coordinating role between source and sink (Paul et al., 2017) at the same time as maintaining metabolic and sucrose homoestasis (Figueroa and Lunn, 2016). The potential of T6P in regulating photosynthesis was first documented by Paul et al. (2001) and Pellny et al. (2004). However, rather than direct regulation of photosynthesis by T6P, it is likely that T6P regulates photosynthesis through effects on sinks and their metabolism, which then integrates sinks through changes in sucrose demand with the source (Oszvald et al., 2018). A number of crop traits are associated with the T6P pathway genes trehalose phosphate synthase (TPS) and trehalose phosphate phosphatase (TPP), such as grain size in wheat (Zhang et al., 2017, TPP gene) anaerobic germination in rice (Kretzschmar et al., 2015, TPP gene), and other stresses such as salt stress (Vishal et al., 2019, TPS gene), and inflorescence architecture in maize (Claeys et al., 2019, TPP gene). All of these traits with the exception of those of Claeys et al. (2019) can be explained through the effect of T6P on carbohydrate allocation and metabolic homeostasis and regulation (Fig. 1). In the case of the study of Claeys et al. (2019), a role for T6P is yet to be proven and the TPP gene in this case may have an alternative function.
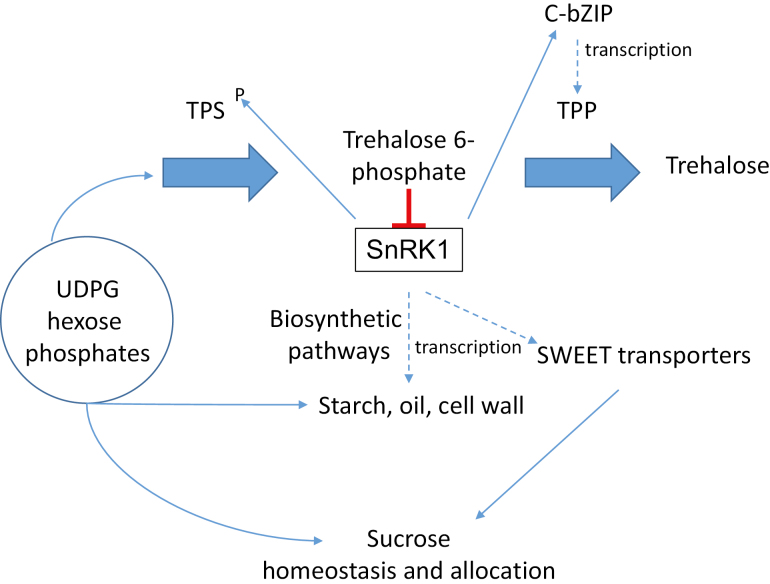
Fig. 1.
A summary of the trehalose biosynthetic pathway and its role in regulating resource allocation. TPSs (trehalose phosphate synthases) and TPPs (trehalose phosphate phosphatases) regulate the synthesis of trehalose 6-phosphate, a key metabolic signal and regulator of SnRK1 (SNF1-related kinase 1). SnRK1 regulates TPS by phosphorylation and activates TPP transcription through the C/S1 group bZIPs (Harthill et al., 2006; Ma et al., 2011). SnRK1 regulates biosynthetic pathways through regulation of gene expression (starch, oil, and cell wall) (Zhang et al., 2009; Figueroa and Lunn, 2016; Zhai et al., 2018) and sucrose allocation through regulation of SWEET transporter transcription (Oszvald et al., 2018).
Three very recent studies (Oszvald et al., 2018; Zhai et al., 2018; Li et al., 2019) have provided more detailed analysis of the mechanistic basis for the strong association of T6P with crop traits that centre around carbohydrate allocation. First, comparison of the metabolome and transcriptome of sweet and grain sorghum with contrasting sugar-accumulating phenotypes and a cross between these two genotypes showed different patterns of T6P accumulation (Li et al., 2019). Differential T6P signal between the lines was associated with the T6P regulators, TPPs and C-group bZIP transcription factors. These changes could explain the divergent sucrose, starch, and cell wall metabolism between the genotypes. The authors conclude for this study that this has helped identify genes that could be important in regulating sucrose allocation and accumulation into other end-products. Having a handle on a tangible mechanism of assimilate partitioning in crops has been long sought after, and is a major breakthrough. Secondly, confirming the crucial role of TPPs in the previous study, overexpression of a TPP gene with a MADS6 promoter active in the vasculature of maize reproductive tissue during the flowering period altered allocation of sucrose from the pith of the developing cob to the developing female florets (Nuccio et al., 2015; Oszvald et al., 2018). Lower T6P, particularly in pith and florets due to TPP expression, had similar effects on gene expression in both tissues but produced different metabolic outcomes, with a shift in sugars, sugar phosphates, and amino acids from pith to florets. Altered sucrose allocation from pith to florets was associated with up-regulation of SWEET transporters. The effect could be explained through T6P/SnRK1 regulation of SWEET expression. The altered allocation of sucrose enhanced yield particularly under drought. Normally, drought can cause a large reduction in seed number through restriction of sucrose supply (Boyer and Westgate, 2004). Modification of T6P could be a strategy to prevent loss of grain under drought and potentially increase seed number overall by changing sucrose allocation to florets. Interestingly, Oszvald et al. (2018) also showed higher photosynthesis as a consequence of increased sucrose allocation to florets through delayed developmental decline of the photosynthetic rate. This underlines the whole-plant nature of the regulation of photosynthesis. Improvements in photosynthesis that translate to greater crop yield may require coordination between source and sink because of the strong interaction with and regulation of photosynthesis by whole-plant processes. It is possible that the photosynthetic potential of crops could be masked by insufficient sink strength. In a third example, T6P was found to activate oil biosynthesis through WRINKLED1 (WRI1), the transcriptional activator of fatty acid synthesis. WRI1 was recently identified as a target of SnRK1 (Zhai et al., 2018). In these three examples, the accumulation and metabolism of all major end-products important in crops, namely sucrose, starch, cell wall, amino acids, and oil, are regulated by T6P (Fig. 1). These end-products themselves are directly related to crop yield potential—the capacity of crops to accumulate and partition these end-products in crop sinks. Hence, there is the prospect of being able to improve flow of sucrose to end-products and the partitioning between them in addition to altering sucrose flow to improve grain set and number. Alteration of these pathways can influence the performance of crops under abiotic stresses such as drought (Nuccio et al., 2015) and anoxia (Kretzschmar et al., 2015). The studies show that the effects of T6P are strongly context dependent, interacting with the regulation of gene expression patterns and potential of a particular cell and developmental stage. These three examples show how an understanding of fundamental science of crop processes may enable crop improvement through the development of strategies, markers, and target genes for intervention. In contrast, direct overexpression of metabolic enzymes of primary pathways has not yet resulted in the development of new crop varieties suited to field environments. This may be because such attempts to directly engineer metabolism are often confounded by the strong underlying regulatory processes of metabolic homeostasis such as that mediated by T6P.
It will be interesting and important for further improvements in crop yield and resilience to understand how the T6P pathway has been modified through breeding and what further changes can be made. Interventions that modify T6P through genetic modification in maize (Nuccio et al., 2015) and chemical application in wheat (Griffiths et al., 2016) show the potential of the pathway for further yield improvement and that the T6P pathway is not yet optimized in crops. T6P does not cross membranes; hence, for the development of a possible yield-enhancing spray, chemical modification of T6P is necessary to enable uptake by the crop. Chemical design that resulted in photorelease of T6P in planta once taken up when applied as a spray to wheat 10 days after anthesis (DAA) increased grain size by 10–20% (Griffiths et al., 2016). This is currently being developed as a biostimulant application for crop improvement. Encouraging results of a similar magnitude of yield enhancement have been achieved in field environments. If nothing else, this work does show that there are limiting factors at 10 DAA in the grain that currently restrict grain size, which can be removed through T6P. The work shows where one limitation to yield lies and how it can be improved in a field situation. Potentially for wheat, the T6P pathway could be involved in the determination of grain set as well as grain size, as it is thought that sugar supply is a factor in determining initiation of female reproductive primordia (Fischer, 2007) as well as maintenance of grain numbers once set (Nuccio et al., 2015). In other crops, the potential of T6P to regulate synthesis and partitioning between sucrose, starch, cell wall, and oil and interaction with abiotic stresses means that the T6P pathway is a dominant control point for crop traits and will probably feature strongly in future crop improvement programmes.
GWAS and QTL mapping for yield
As yield is such a multigenic process, as already discussed, there was early optimism that linkage mapping and genome-wide association studies (GWAS) would lead to large improvements in grain yield. However, this has not occurred due, in part, to the complexity of yield, both genetically and in its interaction with biotic and abiotic influences in field environments. QTL mapping is an invaluable tool to search for the underlying physiological and genetic mechanisms of important traits, typically being an early step in determining areas of interest within the genome. In the case of qualitative traits with few governing genes, mapping studies can identify key genomic regions with relative ease due to the clear relationship between the expressed phenotype and QTLs. Marker-assisted selection (MAS) with a strongly linked marker or the causative mutation itself may also enable more efficient selection for the desired phenotype. Eventual use of MAS for yield improvement has often been stated as the aim of GWAS in crops (Breseghello and Sorrells, 2006; Gao et al., 2015; Tadesse et al., 2015; Lozada et al., 2017; Lei et al., 2018), but the complex nature of yield makes this unlikely. As far as we are aware, no such markers have been used in yield selection. Brinton and Uauy (2019)—see earlier—highlighted the difficulties in phenotyping a trait that varies within genotypes and even across a single spike. The wheat homologue of the QTL, GRAIN WEIGHT2 (GW2), affecting grain width and weight in rice (Song et al., 2007) has been traced to an area of very low recombination, covering over half the chromosome where it is present (Sukumaran et al., 2018; Brinton and Uauy, 2019). The wheat genome is ~40 times larger than that of rice (Argumuganathan and Earle, 1991), which hampers identification of causative mutations. Furthermore, without a large population, QTL intervals can contain hundreds of genes, and any subsequent attempt to determine underlying polymorphism(s) is difficult.
The strong influence of genotype×environment interactions on yield often results in detected QTLs being unstable across environments. For example, in a double-haploid wheat mapping population (Bonneau et al., 2013), the allele that conferred increased TGW, usually a highly repeatable trait, differed between environments. In several trials in Mexico and Australia, the allele from one parent had a favourable effect on grain yield. However, in other trials in Australia, the allele from the other parent was associated with higher grain yield. The environmental factor behind the reversal of the allelic effects was unknown, although there were many possible contributors in addition to heat and drought stress, including sowing density and irrigation types. This illustrates the difficulty in identifying QTLs for yield and potential selection markers. There is no guarantee an allele will give a yield advantage, even if the target environment is similar to the one where the QTL was detected. This is even a concern within the same location where water availability and temperatures can fluctuate year to year. Gao et al. (2015) reported that the effect of an allele associated with an increase in TGW in a bi-parental spring wheat population one year was reversed the following year within the same location.
In rice, many genes originally identified through GWAS and QTL mapping with source-related traits have been cloned and characterized, although this has not translated into them being used to improve breeding programmes (reviewed by Li et al., 2018). Relatively large variation for stomatal and mesophyll conductance has been found in rice (Gu et al., 2012), indicating a potential target for yield improvement. In further work on wheat, Barbour et al. (2016) found a QTL responsible for 9% of mesophyll conductance variation in controlled growing conditions. Shahinnia et al. (2016) found no significant correlations between stomatal size or density and yield in wheat, although several stomatal traits co-located with previously found QTLs for yield. It was suggested that the lack of correlation could be associated with the indirect effect these traits have on yield through water use efficiency. While these QTLs may add important pieces to the puzzle, their direct use would be unlikely to elicit positive effects on yield without full understanding of all interacting factors, especially when phenotyping was not performed in the field.
Yield stability across a range of environmental conditions is needed, and therefore targeting strategic points within physiological mechanisms is more valuable than action based on specific QTLs. Examples of this include a narrow root angle in wheat and barley to reach water low in the soil profile in areas where terminal drought is common (Christopher et al., 2013; Robinson et al., 2018); stay-green to prolong photosynthetic activity, especially where drought may accelerate senescence (Gous et al., 2016); and anaerobic germination tolerance in rice, improving establishment in flooded rice fields (Kretzschmar et al., 2015). These traits are specific to certain environments rather than of general use to yield improvement. Many correlations between adaptive traits and yield in the field have been reported, although these vary greatly. For example, Robinson et al. (2018) reported genetic correlations of seminal root angle and subsequent field-based barley yield of between –0.21 and 0.36 across 20 field trials. Pyramiding of adaptive traits into ideotypes predicted to yield well through crop modelling may achieve better improvements in terms of yield; yet, for this to be possible, QTLs of large enough effect still need to be identified.
A holistic approach to genetic dissection of yield-related traits is required that includes a combination of technologies that link genetics, physiology, and metabolism to identify underlying mechanisms and their control. Field testing under realistic agricultural conditions needs to occur. Linkage mapping and GWAS are more beneficial when coupled with other techniques, and this is becoming easier with the introduction of online freely available databases and tools. High-throughput phenotyping is a pre-requisite for technologies such as GWAS and forward and reverse genetic approaches (Furbank et al., 2019). Phenomics needs to enhance understanding of traits that have effects at the canopy and yield level. Huge amounts of genetic data are available across many species, populations, developmental stages, and tissues. This will lead to a better understanding of mechanisms affecting yield development and allow more informed approaches to yield improvement.
Conclusion
Recent years have produced a number of exciting developments in understanding key genes and processes that underpin traits important for yield improvement such as grain numbers and size, stomatal function, leaf architecture, and carbohydrate allocation (Fig. 2). Much of this work has been demonstrated in food security crops, with some validation in field environments. Some interesting work on improving photosynthesis in tobacco and Arabidopsis has also been conducted. There is unlikely to be single gene or even single process modifications that will give rise to yield improvements beyond a few percent. The hexaploid nature of wheat means that single gene changes are muted unless all three homeologues can be targeted, such as by gene editing. GWAS and QTL mapping, which provide a more holistic approach to yield improvement, have yet to produce any major benefits to general yield improvement of crops except where adaptions to more specialized environments are required. Gene editing offers promise for cereals where negative regulators of yield can be targeted as a first step. Much of yield improvement will depend on balancing carbon gain with water losses from the crop as most of agriculture is rain fed and often water limited. Yield improvement will require that carbon allocation processes within the crop are optimized towards maximizing grain set and grain filling, and that other altered traits such as deeper roots do not divert carbon away from the grain. Again, biomass in shoots and roots need to be distributed to balance carbon gains to water losses. To achieve large increases in yield will require an understanding of how genes and processes interact to regulate yield in the field environment. Given the current blend of fundamental science and the promise of gene editing, there is much room for optimism.
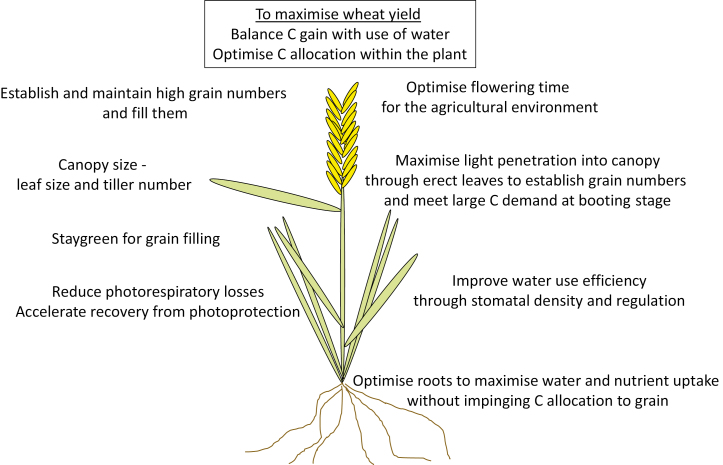
Fig. 2.
A summary of important traits for yield improvement of wheat that also apply to other crops. The balancing of carbon and water demands is seen as crucial, as is understanding and optimization of whole-plant carbon allocation to maximize grain numbers and size at harvest.
Acknowledgements
Rothamsted Research receives strategic funding from the Biotechnological Sciences Research Council of the UK. We acknowledge the Designing Future Wheat Strategic Programme (BB/P016855/1), Biotechnological and Biological Sciences Research Council Sustainable Agriculture Research and Innovation Club grant BB/N004205/1 and International Wheat Yield Partnership grant BB/S01280X/1.
Author contributions
MJP spoke at the conference on Translational Photosynthesis in Brisbane in July 2019 that precipitated this review article. MJP structured and wrote much of the article, and AW and CAG polished and contributed to the final version. AW wrote the section on GWAS and QTL mapping for yield.
References
- Abdelrahman M, Burritt DJ, Gupta A, Tsujimoto H, Tran LP. 2020. Heat stress effects on source–sink relationships and metabolome dynamics in wheat. Journal of Experimental Botany 71, 543–554. [DOI] [PubMed] [Google Scholar]
- Arumuganathan K, Earle ED. 1991. Estimation of nuclear DNA content of plants by flow cytometry. Plant Molecular Biology Reporter 9, 221–231. [Google Scholar]
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M. 2005. Cytokinin oxidase regulates rice grain production. Science 309, 741–745. [DOI] [PubMed] [Google Scholar]
- Barbour MM, Bachmann S, Bansal U, Bariana H, Sharp P. 2016. Genetic control of mesophyll conductance in common wheat. New Phytologist 209, 461–465. [DOI] [PubMed] [Google Scholar]
- Boden SA, Cavanagh C, Cullis BR, Ramm K, Greenwood J, Jean Finnegan E, Trevaskis B, Swain SM. 2015. Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat. Nature Plants 1, 14016. [DOI] [PubMed] [Google Scholar]
- Bonneau J, Taylor J, Parent B, Bennett D, Reynolds M, Feuillet C, Langridge P, Mather D. 2013. Multi-environment analysis and improved mapping of a yield-related QTL on chromosome 3B of wheat. Theoretical and Applied Genetics 126, 747–761. [DOI] [PubMed] [Google Scholar]
- Borras L, Slafer GA. 2004. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: a quantitative reappraisal. Field Crops Research 86, 131–146. [Google Scholar]
- Borrell AK, Mullet JE, George-Jaeggli B, van Oosterom EJ, Hammer GL, Klein PE, Jordan DR. 2014. Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. Journal of Experimental Botany 65, 6251–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrill P, Fahy B, Smith AM, Uauy C. 2015. Wheat grain filling is limited by grain filling capacity rather than the duration of flag leaf photosynthesis: a case study using NAM RNAi plants. PLoS One 10, e0134947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS, Westgate ME. 2004. Grain yields with limited water. Journal of Experimental Botany 55, 2385–2394. [DOI] [PubMed] [Google Scholar]
- Breseghello F, Sorrells ME. 2006. Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 172, 1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton J, Uauy C. 2019. A reductionist approach to dissecting grain weight and yield in wheat. Journal of Integrative Plant Biology 61, 337–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine RS, Yin X, Sloan J, et al. . 2019. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytologist 221, 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni P, Warner D, Bensen RJ, et al. . 2008. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiology 147, 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattivelli L, Rizza F, Badeck F-W, Mazzucotelli E, Mastrangelo AM, Francia E, Mare C, Tondelli A, Stanca AM. 2008. Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crops Research 105, 1–14. [Google Scholar]
- Christopher J, Christopher M, Jennings R, Jones S, Fletcher S, Borrell A, Manschadi AM, Jordan D, Mace E, Hammer G. 2013. QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theoretical and Applied Genetics 126, 1563–1574. [DOI] [PubMed] [Google Scholar]
- Claeys H, Vi SL, Xu X, et al. . 2019. Control of meristem determinacy by trehalose 6-phosphate phosphatases is uncoupled from enzymatic activity. Nature Plants 5, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Dunlap F, Thorbjørnsen T, Keeling P, Smith AM. 1996. The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial. Plant Physiology 112, 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaya O, Pont C, Sibout R, et al. . 2015. FRIZZY PANICLE drives supernumerary spikelets in bread wheat. Plant Physiology 167, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R, Ji X, Richards RA. 2011. Abiotic stress and control of grain number in cereals. Plant Science 181, 331–341. [DOI] [PubMed] [Google Scholar]
- Driever SM, Simkin AJ, Alotaibi S, Fisk SJ, Madgwick PJ, Sparks CA, Jones HD, Lawson T, Parry MAJ, Raines CA. 2017. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philosophical Transactions of the Royal Society B: Biological Sciences doi: 10.1098/rstb.2016.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Li N, Chen L, Xu Y, Li Y, Zhang Y, Li C, Li Y. 2014. The ubiquitin receptor DA1 regulates seed and organ size by modulating the stability of the ubiquitin-specific protease UBP15/SOD2 in Arabidopsis. The Plant Cell 26, 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. 2019. Where did the carbon go? Science 363, eaat9077 elletter. doi: 10.1126/science.aat9077. [DOI] [Google Scholar]
- Fahy B, Siddiqui H, David LC, Powers SJ, Borrill P, Uauy C, Smith AM. 2018. Final grain weight is not limited by the activity of key starch-synthesising enzymes during grain filling in wheat. Journal of Experimental Botany 69, 5461–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q. 2006. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theoretical and Applied Genetics 112, 1164–1171. [DOI] [PubMed] [Google Scholar]
- Farrar J, Pollock C, Gallagher J. 2000. Sucrose and the integration of metabolism in vascular plants. Plant Science 154, 1–11. [DOI] [PubMed] [Google Scholar]
- Figueroa CM, Lunn JE. 2016. A tale of two sugars: trehalose 6-phosphate and sucrose. Plant Physiology 172, 7–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RA. 2007. Understanding the physiological basis of yield potential in wheat. Journal of Agricultural Science 145, 99–113. [Google Scholar]
- Fischer RA, Richards RA, Sadras V. 2019. 40% increased growth with genetic engineering. Science 363, eaat9077 elletter. doi: 10.1126/science.aat9077. [DOI] [Google Scholar]
- Furbank RT, Jimenez-Berni JA, George-Jaeggli B, Potgieter AB, Deery DM. 2019. Field crop phenomics: enabling breeding for radiation use efficiency and biomass in cereal crops. New Phytologist 223, 1714–1727. [DOI] [PubMed] [Google Scholar]
- Gao FM, Wen WE, Liu JD, Rasheed A, Yin GH, Xia XC, Wu X, He ZH. 2015. Genome-wide linkage mapping of QTL for yield components, plant height and yield-related physiological traits in the Chinese wheat cross Zhou 8425B/Chinese Spring. Frontiers in Plant Science 6, 1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głowacka K, Kromdijk J, Kucera K, Xie J, Cavanagh AP, Leonelli L, Leakey ADB, Ort DR, Niyogi KK, Long SP. 2018. Photosystem II subunit S overexpression increases the efficiency of water use in a field-grown crop. Nature Communications 9, 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González FG, Capella M, Ribichich KF, Curín F, Giacomelli JI, Ayala F, Watson G, Otegui ME, Chan RL. 2019. Field-grown transgenic wheat expressing the sunflower gene HaHB4 significantly outyields the wild type. Journal of Experimental Botany 70, 1669–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gous PW, Hickey L, Christopher JT, Franckowiak J, Fox GP. 2016. Discovery of QTL for stay-green and heat-stress in barley (Hordeum vulgare) grown under simulated abiotic stress conditions. Euphytica 207, 305–317. [Google Scholar]
- Griffiths CA, Sagar R, Geng Y, et al. . 2016. Chemical intervention in plant sugar signalling increases yield and resilience. Nature 540, 574–578. [DOI] [PubMed] [Google Scholar]
- Gu J, Yin X, Stomph TJ, Wang H, Struik PC. 2012. Physiological basis of genetic variation in leaf photosynthesis among rice (Oryza sativa L.) introgression lines under drought and well-watered conditions. Journal of Experimental Botany 63, 5137–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Slafer GA, Schnurbusch T. 2016. Genotypic variation in spike fertility traits and ovary size as determinants of floret and grain survival rate in wheat. Journal of Experimental Botany 67, 4221–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harthill JE, Meek SE, Morrice N, Peggie MW, Borch J, Wong BH, Mackintosh C. 2006. Phosphorylation and 14-3-3 binding of Arabidopsis trehalose-phosphate synthase 5 in response to 2-deoxyglucose. The Plant Journal 47, 211–223. [DOI] [PubMed] [Google Scholar]
- He Q, Yang L, Hu W, Zhang J, Xing Y. 2018. Overexpression of an auxin receptor OsAFB6 significantly enhanced grain yield by increasing cytokinin and decreasing auxin concentrations in rice panicle. Scientific Reports 8, 14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Jiang Q, Hao C, Wang Y, Zhang H, Zhang X. 2014. Global selection on sucrose synthase haplotypes during a century of wheat breeding. Plant Physiology 164, 1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Wang D, Duan P, Zhang B, Xu R, Li N, Li Y. 2017. WIDE AND THICK GRAIN 1, which encodes an otubain-like protease with deubiquitination activity, influences grain size and shape in rice. The Plant Journal 91, 849–860. [DOI] [PubMed] [Google Scholar]
- Hummel M, Rahmani F, Smeekens S, Hanson J. 2009. Sucrose-mediated translational control. Annals of Botany 104, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Anwar S, Yu S, Sun M, Yang Z, Gao ZQ. 2019. Development of drought-tolerant transgenic wheat: achievements and limitations. International Journal of Molecular Science 20, 3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar T, Pelayo MA, Trijatmiko KR, et al. . 2015. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nature Plants 1, 15124. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. [DOI] [PubMed] [Google Scholar]
- Lei L, Zheng HL, Wang JG, Liu HL, Sun J, Zhao HW, Yang LM, Zou DT. 2018. Genetic dissection of rice (Oryza sativa L.) tiller, plant height, and grain yield based on QTL mapping and metaanalysis. Euphytica 214, 109. [Google Scholar]
- Li F, Wen W, He Z, et al. . 2018. Genome-wide linkage mapping of yield-related traits in three Chinese bread wheat populations using high-density SNP markers. Theoretical and Applied Genetics 131, 1903–1924. [DOI] [PubMed] [Google Scholar]
- Li X, Weiss M, Richards R. 2019. Does root growth slow during fast stem and ear growth in wheat? Proceedings of the 2019 Agronomy Australia Conference, 25–29 August 2019, Wagga Wagga, Australia.
- Li Y, Wang W, Feng Y, Tu M, Wittich PE, Bate NJ, Messing J. 2019. Transcriptome and metabolome reveal distinct carbon allocation patterns during internode sugar accumulation in different sorghum genotypes. Plant Biotechnology Journal 17, 472–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo AKM, Orr DJ, Gutierrez MO, Andralojc PJ, Sparks C, Parry MAJ, Carmo-Silva E. 2019. Overexpression of ca1pase decreases rubisco abundance and grain yield in wheat. Plant Physiology 181, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Marshall-Colon A, Zhu XG. 2015. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66. [DOI] [PubMed] [Google Scholar]
- Lozada DN, Mason RE, Babar MA, et al. . 2017. Association mapping reveals loci associated with multiple traits that affect grain yield and adaptation in soft winter wheat. Euphytica 213, 222. [Google Scholar]
- Ma J, Hanssen M, Lundgren K, et al. . 2011. The sucrose-regulated Arabidopsis transcription factor bZIP11 reprograms metabolism and regulates trehalose metabolism. New Phytologist 191, 733–745. [DOI] [PubMed] [Google Scholar]
- Ma M, Zhao H, Li Z, Hu S, Song W, Liu X. 2016. TaCYP78A5 regulates seed size in wheat (Triticum aestivum). Journal of Experimental Botany 67, 1397–1410. [DOI] [PubMed] [Google Scholar]
- Miao J, Yang Z, Zhang D, et al. . 2019. Mutation of RGG2, which encodes a type B heterotrimeric G protein γ subunit, increases grain size and yield production in rice. Plant Biotechnology Journal 17, 650–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed U, Caine RS, Atkinson JA, Harrison EL, Wells D, Chater CC, Gray JE, Swarup R, Murchie EH. 2019. Rice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Scientific Reports 9, 5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M, Calderini DF. 2015. Volume, water content, epidermal cell area, and XTH5 expression in growing grains of wheat across ploidy levels. Field Crops Research 173, 30–40. [Google Scholar]
- Nuccio ML, Paul M, Bate NJ, Cohn J, Cutler SR. 2018. Where are the drought tolerant crops? An assessment of more than two decades of plant biotechnology effort in crop improvement. Plant Science 273, 110–119. [DOI] [PubMed] [Google Scholar]
- Nuccio ML, Wu J, Mowers R, et al. . 2015. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nature Biotechnology 33, 862–869. [DOI] [PubMed] [Google Scholar]
- Ogura T, Goeschl C, Filiault D, Mirea M, Slovak R, Wolhrab B, Satbhai SB, Busch W. 2019. Root system depth in Arabidopsis is shaped by EXOCYST70A3 via the dynamic modulation of auxin transport. Cell 178, 400–412. [DOI] [PubMed] [Google Scholar]
- Oszvald M, Primavesi LF, Griffiths CA, Cohn C, Basu SS, Nuccio ML, Paul MJ. 2018. Trehalose 6-phosphate in maize reproductive tissue regulates assimilate partitioning and photosynthesis. Plant Physiology 176, 2623–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanatsiou M, Petersen J, Henderson L, Wang Y, Christie JM, Blatt MR. 2019. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 363, 1456–1459. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Oszvald M, Jesus C, Rajulu C, Griffiths CA. 2017. Increasing crop yield and resilience with trehalose 6-phosphate: targeting a feast–famine mechanism in cereals for better source–sink optimization. Journal of Experimental Botany 68, 4455–4462. [DOI] [PubMed] [Google Scholar]
- Paul M, Pellny T, Goddijn O. 2001. Enhancing photosynthesis with sugar signals. Trends in Plant Science 6, 197–200. [DOI] [PubMed] [Google Scholar]
- Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E. 2011. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnology Journal 9, 747–758. [DOI] [PubMed] [Google Scholar]
- Pellny TK, Ghannoum O, Conroy JP, Schluepmann H, Smeekens S, Andralojc J, Krause KP, Goddijn O, Paul MJ. 2004. Genetic modification of photosynthesis with E. coli genes for trehalose synthesis. Plant Biotechnology Journal 2, 71–82. [DOI] [PubMed] [Google Scholar]
- Pendelton JW, Smith GE, Winter SR, Johnston TJ. 1968. Field investigations of relationships of leaf angle in corn (Zea mays) to grain yield and apparent photosynthesis. Agronomy Journal 60, 422. [Google Scholar]
- Poursarebani N, Seidensticker T, Koppolu R, et al. . 2015. The genetic basis of composite spike form in barley and ‘Miracle-Wheat’. Genetics 201, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G. 2012. Achieving yield gains in wheat. Plant, Cell & Environment 35, 1799–1823. [DOI] [PubMed] [Google Scholar]
- Richards RA, Cavanagh CR, Riffkin P. 2019. Selection for erect canopy architecture can increase yield and biomass of spring wheat. Field Crops Research 244, 107649. [Google Scholar]
- Robinson H, Kelly A, Fox G, Franckowiak J, Borrell A, Hickey L. 2018. Root architectural traits and yield: exploring the relationship in barley breeding trials. Euphytica 214, 151. [Google Scholar]
- Rosenthal DM, Locke AM, Khozaei M, Raines CA, Long SP, Ort DR. 2011. Over-expressing the C3 photosynthesis cycle enzyme sedoheptulose-1-7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biology 11, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salesse-Smith CE, Sharwood RE, Busch FA, Kromdijk J, Bardal V, Stern DB. 2018. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nature Plants 4, 802–810. [DOI] [PubMed] [Google Scholar]
- Senapati N, Brown HE, Semenov MA. 2019. Raising genetic yield potential in high productive countries: designing wheat ideotypes under climate change. Agricultural and Forest Meteorology 271, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrago RA, Alzueta I, Savin R, Slafer GA. 2013. Understanding grain yield responses to source–sink ratios during grain filling in wheat and barley under contrasting environments. Field Crops Research 150, 42–51. [Google Scholar]
- Shahinnia F, Le Roy J, Laborde B, Sznajder B, Kalambettu P, Mahjourimajd S, Tilbrook J, Fleury D. 2016. Genetic association of stomatal traits and yield in wheat grown in low rainfall environments. BMC Plant Biology 16, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen BR, Wang LM, Lin XL, et al. . 2019. Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Molecular Plant 12, 199–214. [DOI] [PubMed] [Google Scholar]
- Shen L, Wang C, Fu Y, Wang J, Liu Q, Zhang X, Yan C, Qian Q, Wang K. 2018. QTL editing confers opposing yield performance in different rice varieties. Journal of Integrative Plant Biology 60, 89–93. [DOI] [PubMed] [Google Scholar]
- Shi C, Ren Y, Liu L, et al. . 2019. Ubiquitin specific protease 15 has an important role in regulating grain width and size in rice. Plant Physiology 180, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikka VK, Choi S, Kavakli IH, Sakulsingharoj C, Gupta S, Ito H, Okita TW. 2001. Subcellular compartmentation and allosteric regulation of the rice endosperm ADPglucose pyrophosphorylase. Plant Science 161, 461–468. [Google Scholar]
- Simkin AJ, López-Calcagno PE, Raines CA. 2019. Feeding the world: improving photosynthetic efficiency for sustainable crop production. Journal of Experimental Botany 70, 1119–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Jamieson PD. 2006. Grain number, wheat yield and bottling beer: an analysis. Field Crops Research 8, 60–67. [Google Scholar]
- Singh V, Nguyen CT, van Oosterom EJ, Chapman SC, Jordan DR, Hammer GL. 2015. Sorghum genotypes differ in high temperature responses for seed set. Field Crops Research 171, 32–40. [Google Scholar]
- Song XJ, Huang W, Shi M, Zhu MZ, Lin HX. 2007. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nature Genetics 39, 623–630. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Fernie AR. 2018. Next-generation strategies for understanding and influencing source–sink relations in crop plants. Current Opinion in Plant Biology 43, 63–70. [DOI] [PubMed] [Google Scholar]
- South PF, Cavanagh AP, Liu HW, Ort DR. 2019. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363, eaat9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Lopes M, Dreisigacker S, Reynolds M. 2018. Genetic analysis of multi-environmental spring wheat trials identifies genomic regions for locus-specific trade-offs for grain weight and grain number. Theoretical and Applied Genetics 131, 985–998. [DOI] [PubMed] [Google Scholar]
- Tadesse W, Ogbonnaya FC, Jighly A, Sanchez-Garcia M, Sohail Q, Rajaram S, Baum M. 2015. Genome-wide association mapping of yield and grain quality traits in winter wheat genotypes. PLoS One 10, e0141339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Mace E, George-Jaeggli G, Hunt C, Cruickshank A, Henzell R, Jordan D. 2018. Novel grain weight loci revealed in a cross between cultivated and wild sorghum. Plant Genome 11, 170089. [DOI] [PubMed] [Google Scholar]
- Tetlow IJ, Davies EJ, Vardy KA, Bowsher CG, Burrell MM, Emes MJ. 2003. Subcellular localization of ADPglucose pyrophosphorylase in developing wheat endosperm and analysis of the properties of a plastidial isoform. Journal of Experimental Botany 54, 715–725. [DOI] [PubMed] [Google Scholar]
- Thomas SG. 2017. Novel Rht-1 dwarfing genes: tools for wheat breeding and dissecting the function of DELLA proteins. Journal of Experimental Botany 68, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Talukder SK, Fu J, Fritz AK, Trick HN. 2018. Expression of a rice soluble starch synthase gene in transgenic wheat improves the grain yield under heat stress conditions. In Vitro Cellular & Developmental Biology 54, 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishal B, Krishnamurthy P, Ramamoorthy R, Kumar PP. 2019. OsTPS8 controls yield-related traits and confers salt stress tolerance in rice by enhancing suberin deposition. New Phytologist 221, 1369–1386. [DOI] [PubMed] [Google Scholar]
- Wang P, Khoshravesh R, Karki S, Tapia R, Balahadia CP, Bandyopadhyay A, Quick WP, Furbank R, Sage TL, Langdale JA. 2017. Re-creation of a key step in the evolutionary switch from C3 to C4 leaf anatomy. Current Biology 27, 3278–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wu K, Yuan Q, et al. . 2012. Control of grain size, shape and quality by OsSPL16 in rice. Nature Genetics 44, 950–954. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hou J, Liu H, Li T, Wang K, Hao C, Liu H, Zhang X. 2019. TaBT1, affecting starch synthesis and thousand kernel weight, underwent strong selection during wheat improvement. Journal of Experimental Botany 70, 1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolde GM, Mascher M, Schnurbusch T. 2019. Genetic modification of spikelet arrangement in wheat increases grain number without significantly affecting grain weight. Molecular Genetics and Genomics 294, 457–468. [DOI] [PubMed] [Google Scholar]
- Wu A, Hammer GL, Doherty A, von Caemmerer S, Farquhar GD. 2019. Quantifying impacts of enhancing photosynthesis on crop yield. Nature Plants 5, 380–388. [DOI] [PubMed] [Google Scholar]
- Zhai Z, Keereetaweep J, Liu H, Feil R, Lunn JE, Shanklin J. 2018. Trehalose 6-phosphate positively regulates fatty acid synthesis by stabilizing WRINKLED1. The Plant Cell 30, 2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fengler KA, Van Hemert JL, et al. . 2019. Identification and characterization of a novel stay-green QTL that increases yield in maize. Plant Biotechnology Journal doi: 10.1111/pbi.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, He Z, Tian X, et al. . 2017. Cloning of TaTPP-6AL1 associated with grain weight in bread wheat and development of functional marker. Molecular Breeding 37, 78. [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Mitchell R, Powers S, Schluepmann H, Delatte T, Wingler A, Paul MJ. 2009. Inhibition of Snf1-related protein kinase (SnRK1) activity and regulation of metabolic pathways by trehalose 6-phosphate. Plant Physiology 149, 1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YF, Peng T, Sun HZ, et al. . 2019. miR1432–OsACOT (acyl-CoA thioesterase) module determines grain yield via enhancing grain filling rate in rice. Plant Biotechnology Journal 17, 712–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology 61, 235–261. [DOI] [PubMed] [Google Scholar]