Abstract
Exacerbations of chronic obstructive pulmonary disease (COPD) are episodes of worsening of symptoms, leading to substantial morbidity and mortality. COPD exacerbations are associated with increased airway and systemic inflammation and physiological changes, especially the development of hyperinflation. They are triggered mainly by respiratory viruses and bacteria, which infect the lower airway and increase airway inflammation. Some patients are particularly susceptible to exacerbations, and show worse health status and faster disease progression than those who have infrequent exacerbations. Several pharmacological interventions are effective for the reduction of exacerbation frequency and severity in COPD such as inhaled steroids, long-acting bronchodilators, and their combinations. Non-pharmacological therapies such as pulmonary rehabilitation, self-management, and home ventilatory support are becoming increasingly important, but still need to be studied in controlled trials. The future of exacerbation prevention is in assessment of optimum combinations of pharmacological and non-pharmacological therapies that will result in improvement of health status, and reduction of hospital admission and mortality associated with COPD.
Introduction
Exacerbations of chronic obstructive pulmonary disease (COPD) impose a substantial burden on health-care systems worldwide; they are a major cause of morbidity, mortality, and reduced health status.1 COPD exacerbations are now the most common cause of medical hospital admission in the UK (accounting for 15·9% of hospital admissions), at a cost to the National Health System of over £253 million a year.2 Exacerbations are also important outcome measures in COPD, and thus a reduction in their frequency is a key target for intervention.
Although in half of community-treated exacerbations, patients recover to baseline symptoms by 7 days, a study of the time course showed that in 14% of these events patients had still not returned to baseline symptoms within 35 days of onset, and in a small proportion of exacerbations, symptoms never returned to the baseline level.3 Thus COPD exacerbations can be quite protracted, which accounts for some of the considerable morbidity associated with such an event. An audit of hospital admissions showed that around 30% of patients presenting with an index exacerbation will be seen again and possibly readmitted with another (or recurrent) event within 8 weeks.4 In a cohort of patients with moderate to severe COPD followed-up after exacerbation, 22% had a recurrent event within 50 days of the first (index) exacerbation. Thus, such events are complex, and an initial exacerbation seems to increase susceptibility to a subsequent one.5
Definition
An exacerbation of COPD is described as an acute worsening of respiratory symptoms associated with a variable degree of physiological deterioration.3 The guidelines of the WHO and US National Heart Lung and Blood Institute Global Initiative for Chronic Obstructive Lung Disease (GOLD)6 define an exacerbation as “an event in the natural course of the disease characterized by a change in the patient's baseline dyspnoea, cough, and/or sputum that is beyond normal day-to-day variations, is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD”.6
Definitions based on use of health care have also been proposed, eg, unscheduled physician visits, changes or increases in medication, use of antibiotics or oral steroids at exacerbation, and hospital admission.7 However, health-care use in COPD can vary depending on access, and thus there could be substantial difficulty in the standardisation of such a definition. Additionally, many COPD exacerbations are not reported to health-care professionals and are either self-treated or left untreated.1 The latest GOLD guidelines also indicate that exacerbations can be self-limiting, especially if of mild severity, and the phrase “may warrant a change in regular medication” has been incorporated.7 However, health-care use can be used to define exacerbation severity: often defined as mild if increases in regular inhaled medication are needed, moderate if courses of steroids or antibiotics are needed, and severe if the patient requires hospital admission.
Exacerbations are usually inflammatory events, with several airway and systemic inflammatory markers increasing.5 There has been substantial interest in developing a systemic biomarker as a diagnostic test for an exacerbation. Hurst and colleagues8 did a large study of plasma biomarkers for COPD exacerbations, with 36 candidate molecules assessed in paired baseline and exacerbation plasma samples, and tested against a standard definition, meeting both health-care use and symptom-based criteria. To confirm the diagnosis of exacerbation, the most selective biomarker was C-reactive protein (CRP) but this was neither sufficiently sensitive nor specific alone. However, the combination of CRP with any increased major exacerbation symptom on that day (dyspnoea, sputum volume, or sputum purulence) increased the sensitivity and specificity. Further research will aid in the understanding of mechanisms of COPD exacerbations and will lead to the development of more specific biomarkers.
Pathophysiological changes
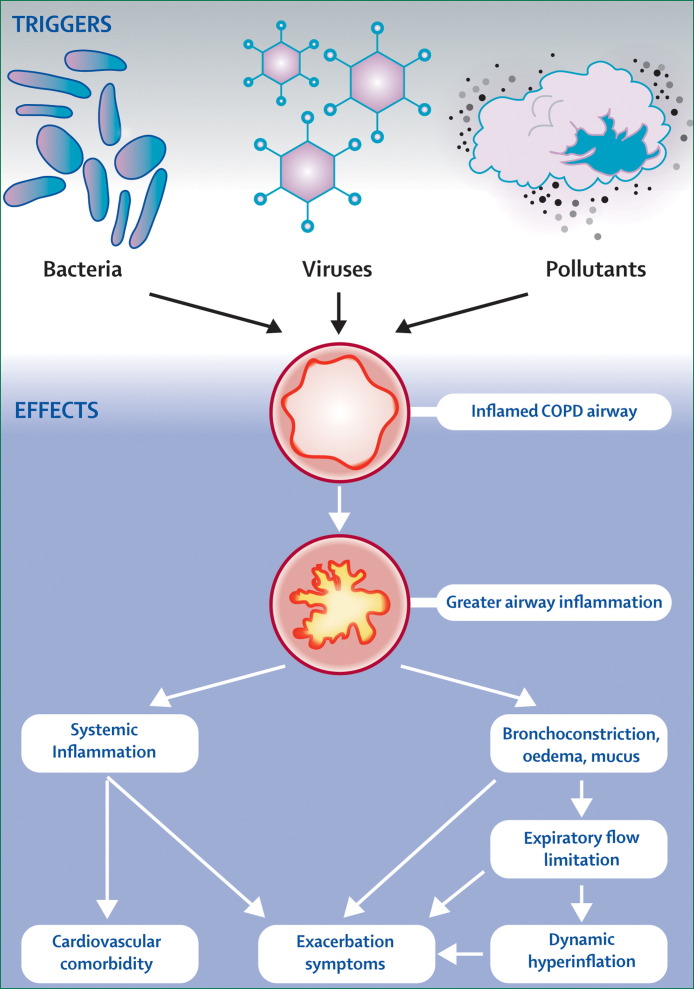
COPD exacerbations are associated with increased upper and lower airway and systemic inflammation (figure 1 ).9, 10, 11, 12, 13 There is little information available on the nature of the airway inflammatory changes especially when studied close to an exacerbation, because taking bronchial biopsies at exacerbation in patients with moderate to severe COPD is difficult. In stable COPD there is an increase in the CD8+ lymphocytes and macrophages in the bronchial mucosa and an increase in neutrophils with more severe disease.14 In one study, where biopsies were done at exacerbation in patients with chronic bronchitis, increased airway eosinophilia was reported, although the patients studied had only mild COPD.10 Modest increases were seen in neutrophils, T lymphocytes (CD3), and TNF alpha positive cells. However, in patients with more severe COPD, increases have been seen in airway neutrophils when stable that increase further at exacerbation.10, 11, 12 Qiu and colleagues11 have studied biopsies from patients with severe COPD, who were treated at exacerbation with tracheal intubation, and showed that there was pronounced airway neutrophilia, neutrophil elastase expression, and upregulation of neutrophil chemokine expression. However, studies in intubated patients with COPD are difficult, since the results can be complicated by secondary infection. Oxidative stress is a key factor in the development of airway inflammation in COPD. A study has shown that patients with severe exacerbations who needed hospital admission or assisted ventilation have evidence of increased large airway interleukin-8 (IL-8) levels and increased oxidative stress.12 Various markers of oxidative stress have been shown to rise in the airways with exacerbation such as hydrogen peroxide and 8-isoprostane and these markers can take some time to recover to baseline.15 Upper airway inflammation is increased in COPD patients, increases further at exacerbation, and is associated with lower airway inflammatory changes.13, 16
Figure 1.
Triggers of COPD exacerbations and associated pathophysiological changes leading to increased exacerbation symptoms
Systemic inflammation increases at exacerbation and although the causes of this response in COPD are not clear, there is probably a spill-over of inflammatory markers from the lungs. By contrast with stable disease,16 exacerbations seem to be associated with a direct correlation between the degree of airway inflammation and the size of the systemic acute-phase response.13 Systemic inflammation increases when the exacerbation is associated with bacterial and viral infection.17 Several inflammatory markers increase at exacerbation, such as plasma fibrinogen and CRP, that have been linked to increased cardiovascular risk. Respiratory infections have been associated with increased cardiac events18 and thus a COPD exacerbation, especially if triggered by an infection, might also be associated with increased cardiac morbidity.
The airway inflammatory responses during COPD exacerbations cause airway oedema, bronchospasm, and increased sputum production, leading to worsening airflow limitation and development of dynamic hyperinflation.19 Such hyperinflation is the main cause of dyspnoea, the most common symptom of an exacerbation, and has other effects including modulating gas exchange, mechanical, and cardiovascular effects.19 Generally the more severe the underlying disease, the greater the degree of physiological change at exacerbation leading to worsening of airflow limitation, and thus the more likely the patient is to develop respiratory failure.
Changes in airflow limitation result in changes in peak expiratory flow rate, but available data suggest that changes in peak flow are too small to be useful in assessing individual patients.3 This limitation might be because peak expiratory flow is mainly an assessment of large airway obstruction, although in COPD, most of the airflow limitation occurs in the small airways. Changes in exhaled nitric oxide also occur and are greater in the presence of viral infections, but are not suitable for monitoring of current exacerbations.20 Novel physiological methods to monitor exacerbations are being developed, and some data now suggest that inspiratory capacity measurements21 or within-breath forced oscillation measurements22 might be more useful to assess COPD exacerbations and indicate the degree of physiological impairment.
Causes of COPD exacerbations
COPD exacerbations are heterogeneous events that are now thought to be caused by complex interactions between the host, respiratory viruses, airway bacteria, and environmental pollution, leading to an increase in the inflammatory burden (panel ).23
Panel. Most common bacterial and viral pathogens isolated from patients with COPD exacerbations.
Bacteria
Haemophilus influenzae
Moraxella catarrhalis
Streptococcus pneumoniae
Pseudomonas aeruginosa
Viruses
Rhinovirus
Coronavirus
Influenza
Parainfluenza
Adenovirus
Respiratory syncytial virus
Viral infections
COPD exacerbations are frequently triggered by upper respiratory tract infections, which are more common in the winter months, when respiratory viral infections are prevalent in the community. Lung function also shows small but significant falls with reduction in outdoor temperature.24 Exacerbations triggered by respiratory viral infections are more severe and are associated with longer recovery times than those triggered by other factors.25, 26 Molecular diagnostic techniques have now enabled detection of respiratory viruses at exacerbation, which have been isolated in around half of exacerbations,27, 28 although this finding might be an underestimate due to difficulties in sampling at onset of symptoms. There have been few such studies in the developing world, but a study from Hong Kong detected viruses in only 22% of exacerbations.29 However, this study differed from others in the choice of assay and in that samples were taken from the upper airway rather than directly from the lower airway. A smaller study of 14 COPD patients admitted to hospital in Singapore reported that 64% of COPD exacerbations were associated with viruses although, like the Hong Kong study, most were associated with influenza virus,30 which was possibly due to less frequent use of the influenza vaccine.
The most common viruses isolated are human rhinoviruses (the most frequent viruses associated with exacerbations), and other viruses including coronavirus, respiratory syncytial virus, influenza, parainfluenza, and adenovirus. Since the introduction of influenza immunisation for patients with chronic lung disease, the virus has become a less prominent cause of exacerbation, though it is still likely to be an important factor at times of epidemics. Although respiratory syncytial virus infection has been seen at exacerbation,31 whether it was the sole cause is not entirely clear, since this virus has been detected in the airways of COPD patients when they are stable and is associated with increased airway inflammation in stable COPD.32 Latent expression of adenoviral E1A protein in alveolar epithelial cells can amplify the effects of lung inflammation induced by cigarette smoke.33 Thus, chronic viral infection might be linked to disease severity in COPD and further work is required on the relation between viruses detected in the stable state and at exacerbation.
With PCR techniques, rhinoviruses can be recovered from induced sputum more frequently than from nasal aspirates at exacerbation,26 suggesting that wild-type rhinoviruses can infect the lower airway and contribute to inflammatory changes at exacerbation. Low-dose experimental rhinovirus infection in patients with mild COPD has been shown produce symptoms that are typical of an exacerbation, confirming that respiratory viruses can infect the lower airway.34 Exacerbations triggered by respiratory viruses are also more severe, associated with longer recovery times,27 and have more chance of hospital admission than exacerbations where respiratory viruses were not detected.35
Bacterial infections
The precise role of bacteria at COPD exacerbations has been difficult to assess, since airway bacterial colonisation in the stable state is associated with the same organisms as those isolated at exacerbations, including Haemophilus Influenzae, Streptococcus Pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, and Pseudomonas aeruginosa.36 In one study in patients with moderate to severe COPD, bacteria were seen in 48·2% of patients in the stable state, rising to 69·6% at exacerbation, with an associated rise in airway bacterial load.37 Purulent sputum production has been regarded as a surrogate marker of bacterial infection, because COPD exacerbations associated with purulent sputum are more likely to produce positive bacterial cultures than those where the sputum production was mucoid.38, 39 Evidence for the involvement of bacteria has come from studies of antibiotic therapy, since exacerbations are often associated with increased sputum purulence and volume, and antibiotics have traditionally been used as first line therapy. A study investigating the benefit of antibiotics in more than 300 acute exacerbations showed a greater treatment success rate in patients treated with antibiotics, especially if their initial presentation was with the symptoms of increased dyspnoea, sputum volume, and purulence, than in patients who did not receive such treatment.40 Patients with mild COPD obtained less benefit from antibiotic therapy than those with more severe COPD. The results of a meta-analysis have shown that antibiotic treatment offered a small but significant benefit in treatment failure and mortality.41
Substantial progress has been made on investigating the role of bacterial infection at exacerbation, with the development of molecular typing methods allowing the detection of changes in bacterial strains, rather than species. Sethi and colleagues42 have suggested that isolation of a new bacterial strain in COPD patients who were regularly sampled was associated with an increased risk of an exacerbation. However, this finding does not conclusively prove that bacteria are direct causes of exacerbations, because not all exacerbations were associated with strain change, and not all strain changes resulted in exacerbation. The strain-specific immune responses to colonising bacterial species43 provide some further evidence that bacteria are not just innocent bystanders in the lower airways during exacerbations.
However, the situation with airway infection is further complicated, since in many COPD exacerbations both respiratory viruses and bacteria could be isolated. A greater systemic inflammatory response has been reported in those exacerbations associated with both H influenzae and rhinovirus isolation, and if the isolation of H influenzae was associated with new or worsening coryzal symptoms (a surrogate of viral infection), such infections were more severe as assessed by changes in symptoms and lung function at exacerbation onset.37 This change has been confirmed in a further study which reported greater lung function impairment and longer hospitalisations in patients with exacerbations associated with viral and bacterial coinfection than in those without coinfection.44 Thus bacterial coinfection with viruses might be of greater importance than bacterial infection alone at COPD exacerbation but consensus within the field has not yet emerged.
Atypical bacteria such as chlamydia, legionella, and mycoplasma have also been implicated at COPD exacerbation, although evidence on their role is conflicting, and these microorganisms might also interact with airway bacteria and viruses.45, 46, 47, 48 A recent study using real-time PCR detection methods found no role for these three atypical bacteria at COPD exacerbation.49
Pollution
COPD patients can have increased exacerbations and hospital admissions with increasing environmental pollution.50 In the APHEA study in six European cities, Anderson and colleagues51 reported a significant effect of air pollution levels on hospital admission for COPD, and similar results have also been seen in Taiwan52 and Brazil.53 However, common pollutants, especially nitrogen oxides and particulates, can interact with viral infection in asthma54 to precipitate exacerbation rather than acting alone, and a similar mechanism might occur in COPD. A study from Hong Kong has shown adverse effects of ambient concentrations of air pollutants (sulphur dioxide, nitrogen dioxides, ozone, and particulate matter with an diameter of less than 10 μg/m3 [PM10] and 2·5 μg/m3 [PM2·5]) on hospitalisation rates for COPD, especially during the winter season.55 Thus measures to improve air quality can have an effect on exacerbation frequency.
Effects
In general, exacerbations become both more frequent and more severe as the severity of the underlying COPD increases.56 However, there remain large differences in yearly exacerbation incidence rates between patients of similar COPD severity.1 There is no agreed definition of a patient with frequent exacerbations, but in several studies they were defined as those with yearly exacerbation rates of greater than the median for the study, usually around three symptom-defined exacerbations per year or two per year if the exacerbation is defined by the requirement for therapy with courses of antibiotics, corticosteroids, or both.
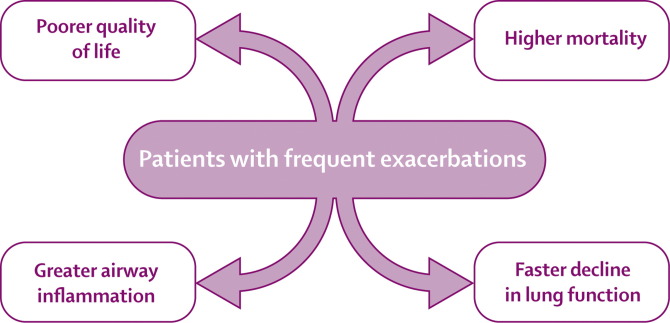
Patients with a history of frequent exacerbations have worse quality of life than patients with a history of less frequent exacerbations, and have consistent exacerbation frequencies when studied from year to year.1 These patients also have an increased risk of hospital admission57 and greater mortality (figure 2 ).58 A UK study suggested that COPD exacerbations do not affect lung function decline, though this study was done in men of working age with fairly good lung function.59 However, results of subsequent studies suggest that exacerbations do play a part in disease progression in patients who are active smokers60 and those who have frequent exacerbations,61 although this effect is fairly small, at around 25% of the total decline in lung function. This observation might be due to the fact that not all exacerbations recover to baseline levels of symptoms and lung function.3 The results of one audit showed that around 30% of patients seen at hospital with an index exacerbation will be seen again and possibly readmitted with a recurrent exacerbation within 8 weeks.62 Patients with a history of frequent exacerbations also have increased airway inflammation,9 which could also contribute to the disease progression.63 Why some patients have frequent exacerbations is not clear, although they possibly have increased susceptibility to respiratory viral infection.25 Thus this group of COPD patients is important for targeting interventions that have the potential of reducing exacerbation frequency.
Figure 2.
Effect of COPD exacerbations in the group with frequent exacerbations
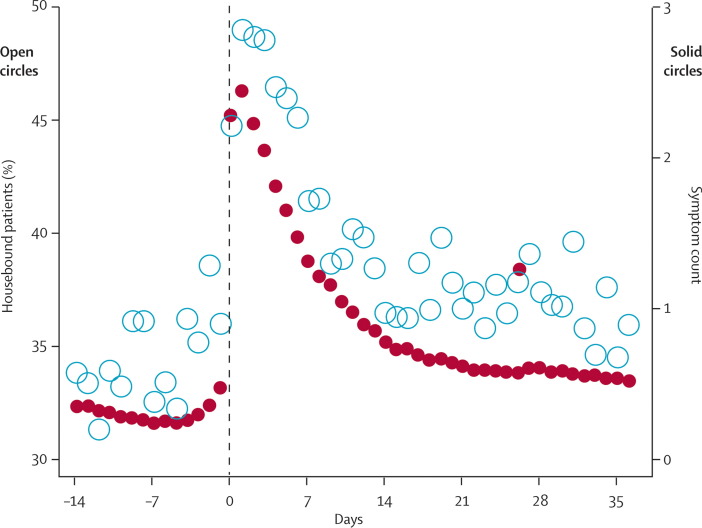
COPD exacerbations have functional consequences. Spruit and colleagues64 have shown that peripheral muscle weakness worsens during exacerbation, potentially contributing to reduced functionality and therefore to deconditioning (loss of fitness). Patients who do not improve their walking distance within a month after exacerbation are more prone to be readmitted to hospital.65 Donaldson and colleagues66 have also shown that exacerbations are associated with a decline in outdoor activity for up to 5 weeks after the onset of symptoms. Patients who have frequent exacerbations had a faster decline in functional status, as measured by time spent outdoors, than patients with infrequent exacerbations (figure 3 ). Thus, patients with frequent exacerbations are more likely to become housebound and are a subpopulation that needs targeting for pulmonary rehabilitation programmes.
Figure 3.
Time course of a COPD exacerbation over 51 days of time spent indoors (open circles) and symptom count (solid circles) for 136 patients who were monitored daily and had an exacerbation
Reproduced with permission from Fletcher and colleagues.59
COPD is associated with other comorbid conditions, and patients who are admitted to hospital are more likely to have associated comorbid conditions such as ischaemic heart disease, pneumonia, and diabetes than patients without a diagnosis of COPD.67 Increasing blood glucose concentrations have been shown to be associated with adverse clinical outcomes in patients with COPD exacerbations.68 Thus the disease burden of COPD exacerbations is likely to be much higher when these comorbidities are taken into account.67
There have been several reports of associations between pulmonary embolism, deep venous thrombosis, and COPD exacerbation.69, 70, 71 Exacerbations could trigger pulmonary embolic events, since acute infections are known to predispose to deep venous thrombosis and pulmonary embolism.72 There might also be diagnostic difficulties because both COPD exacerbations and pulmonary embolism might present solely with dyspnoea. However, a recent study has shown that pulmonary embolism is not a common feature in uncomplicated exacerbations,73 but some patients with exacerbations can have prolonged recovery periods, complicated by respiratory failure and comorbidity, when the risk of pulmonary embolism could become greater.
Prevention
Pharmacological therapies
A few studies have shown that a decreased exacerbation rate is associated with improved quality of life.74, 75, 76, 77 Thus lowering the exacerbation rate would be expected to decrease hospitalisations and have important health economic benefits. Several classes of drugs with potential for reducing exacerbations have been investigated, with variable evidence for their use: vaccines, bacterial extracts, inhaled steroids and long acting bronchodilators, phosphodiesterase inhibitors, and mucolytic agents.
Vaccines and immunostimulants
There are several studies of influenza and pneumococcal vaccinations, which are now routinely recommended for all patients with COPD of significant severity.78, 79, 80, 81 One study that reviewed the outcome of influenza vaccination in a cohort of elderly patients with chronic lung disease found that influenza vaccination is associated with significant health benefits with fewer outpatient visits, fewer hospitalisations, and reduced mortality.78
A Cochrane database review of four studies in COPD saw no evidence of efficacy for injectable anti-pneumococcal vaccines;82 however, in a study of the 23 serotype pneumococcal polysaccharide vaccine in COPD patients, Alfageme and colleagues83 showed that the vaccine was effective in the prevention of community acquired pneumonia, compared with placebo in patients younger than 65 years or those with severe airflow obstruction. However, no difference in mortality between the groups was seen in the study. Larger well designed studies are needed to examine the effects of pneumococcal vaccine in patients older than 65 years with COPD.
Immunostimulatory agents have also been reported to reduce COPD exacerbation frequency. 10 years ago, a study of the immunostimulant OM-85, a detoxified oral immunoactive bacterial extract, reported a reduction in the severe complications of exacerbations and hospital admissions in COPD patients, with a follow-up study confirming the economic benefits of using this agent.84 A recent randomised study has also found benefits but the patients had heterogeneous pathology.85 A systematic review of 13 trials involving 2066 patients saw no consistent evidence of a benefit though the agent is currently in use in Europe.86 Further study is needed to understand the mechanisms of action of this immunostimulant before its role in COPD can be defined.
Inhaled corticosteroids and long acting bronchodilators
The success of oral corticosteroids in the treatment of COPD exacerbations with reduction of hospital length of stay87, 88 has prompted much interest in the use of inhaled steroids to reduce exacerbation frequency in COPD. One of the early studies, the ISOLDE (Inhaled Steroid in Obstructive Lung Disease in Europe) study, was a 3-year study, powered to detect a significant change in FEV1 decline, was negative for the primary outcome but showed that exacerbation frequency can be reduced with inhaled steroids by about 25%.74 Generally the effect of inhaled steroids was greater in patients with more impaired lung function, suggesting that this is the group to target with long-term inhaled steroid therapy.89 In another study from the USA90 (The Lung Health Study), the inhaled steroid (triamcinolone) group had significantly fewer visits to a physician due to respiratory illness, suggesting that triamcinolone also reduced the frequency of COPD exacerbations.
Inhaled long-acting beta 2 agonist (LABA) therapy has been shown to cause small reductions in exacerbation frequency, although most studies involved short periods of therapy at 12 weeks.91, 92 Mahler and colleagues91 found that the time to the first exacerbation was longer with therapy with the long-acting beta agonist salmeterol, although the overall number of exacerbations during the study was small. The larger TORCH study93 of 6112 COPD patients reported that salmeterol reduced exacerbation frequency compared with placebo over a 3-year period. In another study comparing salmeterol with the short-acting ipratropium and placebo, there was no difference in the effect in either treatment arm on exacerbation frequency.94 Rossi and colleagues95 compared two different dosages of the inhaled bronchodilator formoterol with placebo or theophylline and concluded that formoterol was more effective and better tolerated than therapeutically appropriate doses of oral slow-release theophylline in symptomatic COPD patients. Furthermore, two studies reported that formoterol had no effect on total exacerbation frequency relative to placebo, though these studies did find that the rates of steroid-treated exacerbations were decreased in the formoterol group.96, 97
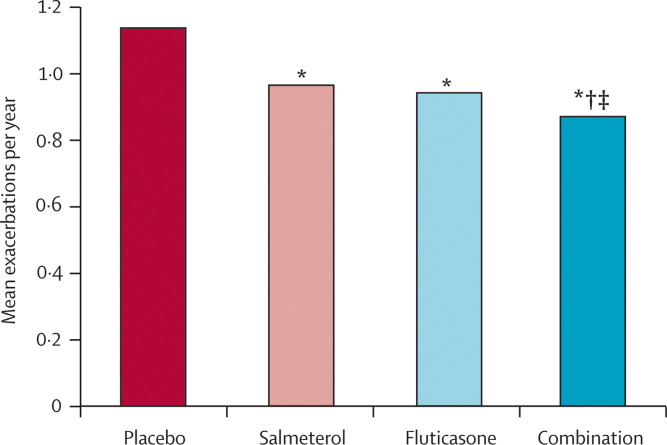
The balance of evidence favours a positive role for LABAs in decreasing exacerbation frequency, which might be the result of the inhibitory effect of β2-adrenoceptor agonists on plasma exudation and neutrophil migration, or possibly indicating an additional reduction in the expression of adhesion molecules. However, the likely pathway of action could be through a synergistic action on airway inflammatory cells in those patients already receiving inhaled corticosteroid therapy.98, 99 All of the major recent studies of combination therapy75, 93, 96, 97 have reported that the combination of inhaled steroid and LABA is more effective than either individual drug alone in reducing exacerbation frequency. Thus, inhaled steroids are unlikely to be used as sole therapy for COPD patients in the future. The data from the TORCH study have confirmed the effectiveness of the inhaled steroid-LABA combination in reducing exacerbation frequency, and the study has reported that the combination reduces hospital admission rates in COPD patients.93 However, most of these studies have been done in patients with more severe COPD with an FEV1 at less than 60% predicted (figure 4 ). The effect of the inhaled steroid-LABA combination on reducing exacerbations in patients with milder COPD is not clear, especially in those patients who have an increased exacerbation frequency.
Figure 4.
Effect of salmeterol (100 μg/day), fluticasone (1000 μg/day), both (combination), or placebo on the mean number of moderate and severe exacerbations of COPD per year, over 3 years
*p<0·001 vs placebo. †p=0,002 vs salmeterol. ‡p=0·024 vs fluticasone. Adapted with permission from Calverley and colleagues.93
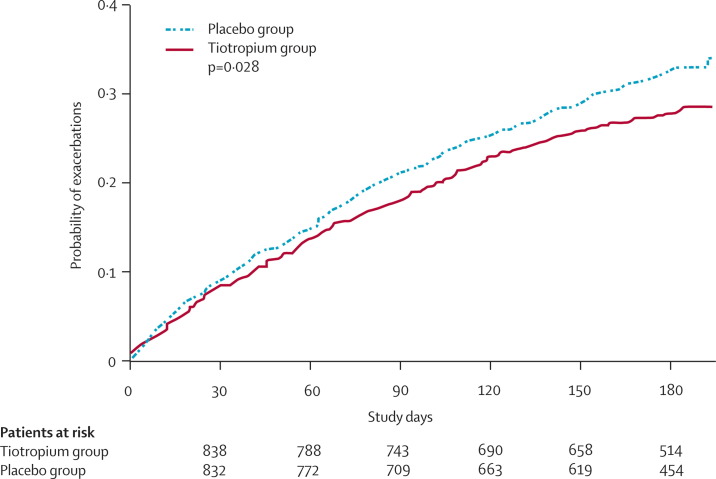
Long-acting anticholinergic agents such as tiotropium also reduce exacerbation frequency, and tiotropium has been shown to reduce exacerbations by 24%, compared with ipratropium when studied over a 1-year period.76, 77 Niewoehner and colleagues100 in a well designed study have confirmed the potential of tiotropium to reduce exacerbation frequency and also have shown a reduction in hospitalisation. Tiotropium does not have any known anti-inflammatory effect,101 and its effect on reducing exacerbations is most likely due to the reduction of dynamic hyperinflation that is the major cause of dyspnoea in COPD (figure 5 ).19 The combination of tiotropium with inhaled LABA and inhaled steroids has been explored in the Optimal Study.102 This randomised trial compared tiotropium-fluticasone-salmeterol versus tiotropium-salmeterol versus tiotropium-placebo, and the triple combination reduced hospitalisation as a result of exacerbation, but not the total number of exacerbations. However, a trend was seen in the reduction of the number of exacerbations with the triple combination, which failed to reach significance possibly due to the small size of the study and the high dropout rate. Triple therapy could be more effective than dual therapy, but further studies of these combinations are required with adequately powered studies.
Figure 5.
Kaplan-Meier estimates of the rate of first treatment failure at 6 months for the first COPD exacerbation treated with either tiotropium (continuous line) or placebo (dotted line)
Reproduced with permission from Niewoehner and colleagues.100
Phosphodiesterase inhibitors and other anti-inflammatory agents
The phosphodiesterase inhibitors now form a class of non-steroidal anti-inflammatory drugs that might be useful in the prevention of COPD exacerbations. Studies of the phosphodiesterase inhibitor theophylline have suggested that small reductions in exacerbation rates can be achieved with therapy,95, 103 though further studies are needed on the effect of low dose theophylline on exacerbation frequency in COPD. Two new receptor-specific phosphodiesterase inhibitors have been studied in COPD: cilomilast and roflumilast, which are both phosphodiesterase-4 inhibitors. A trial of cilomilast in COPD patients showed reduction in exacerbations in the cilomilast group.104 Two studies of roflumilast in COPD have been recently reported: Rabe and colleagues105 have reported a reduction in exacerbations after 24 weeks therapy with roflumilast, while Calverley and colleagues106 studied roflumilast in patients with GOLD stage III and IV in a 1-year trial and showed no overall effect on exacerbation rate, although patients with severe disease (GOLD stage IV) had fewer during the study period. Prevention of COPD exacerbations using this class of drugs is evolving and newer agents and more effective phosphodiesterase inhibitors will be developed with fewer adverse events.
Tumour necrosis factor (TNF)-α has an important role as a key mediator in COPD and has been a target for study. However, a trial of the anti-TNFα antibody, infliximab, showed no benefit on any of the main trial outcomes and no effect of the therapy on exacerbation rate.107
Mucolytic agents
The BRONCUS trial108 using N-acetylcysteine has shown no overall benefit of mucolytics on reduction of COPD exacerbations, except a small effect was noted in those patients who were not taking inhaled steroids. A follow-up meta-analysis of 26 randomised trials involving mucolytic therapy in COPD revealed a 20% reduction in exacerbations, with a large number of patients treated with mucolytics having no exacerbations.109 A study from North America has shown that small airways occluded with inflammatory exudate in COPD patients were associated with early death. This finding might stimulate further research into the role of mucolytic agents in COPD therapy.110 However, the consensus view is that the evidence for mucolytics preventing COPD exacerbations is not convincing; this view is supported by the GOLD guidelines, although mucolytics are still used quite widely in some parts of the world.
Long-term antibiotics
Long-term antibiotic treatment has been used by physicians in clinical practice previously in patients with very frequent exacerbations, either continuously or in rotation, though there is little evidence for their effectiveness. There are some problems with using long-term antibiotics as resistant bacteria could emerge and cause increased airway inflammation and exacerbations. However, in view of the presence of lower airway bacterial colonisation in these patients111 and data from Patel and colleagues112 that such colonisation is related to exacerbation frequency, there has been renewed interest in this type of intervention and some continuing studies will soon be reporting their findings.
Non-pharmacological therapies
Pulmonary rehabilitation and self management
Pulmonary rehabilitation is now an accepted intervention in COPD and although it has important benefits for patients, its effect on preventing exacerbations is less clear.113 In a randomised trial from South Wales, UK, Griffiths and colleagues114 reported that those patients treated with a pulmonary rehabilitation programme including exercise training and education had shorter hospital stays than the control group and fewer primary-care home visits. This finding suggests that a course of pulmonary rehabilitation might reduce exacerbation severity rather than frequency by increasing the patient's knowledge of COPD and how to access health care or self-manage during an exacerbation. Pulmonary rehabilitation could thus reduce hospital stay but also encourage early presentation for exacerbation therapy, which reduces exacerbation length and thus the severity of the event.115
Garcia-Aymerich and colleagues116 have also shown in a 1-year study that patients with high levels of usual physical activity were at reduced risk of readmission to hospital. An intensive disease-specific self-management programme done in Canada has been shown to reduce hospital admission rate,117 though a systematic review of nurse-led interventions failed to show a consistent effect on hospitalisation).118 Casas and colleagues119 have extended this approach by using a similar integrated care plan in two different environments (Barcelona, Spain, and Leuven, Belgium), with similar effects on decreased readmission rates for COPD exacerbation. Patients with COPD are elderly, often with a degree of cognitive impairment and might have difficulty with self-management at exacerbations. How optimum community support should be provided for patients who are at particular risk of hospital admission is not clear.
Home oxygen therapy and ventilatory support
Long-term oxygen therapy has several benefits in COPD patients who are chronically hypoxaemic, including reducing mortality, anxiety, and depression.120, 121, 122 In an epidemiological study, Garcia-Aymerich and colleagues52 noted that patients with hypoxaemia but not treated with long-term oxygen therapy had a higher risk of hospital admission. Another observational study from the Danish Oxygen Register123 has also suggested that long-term oxygen therapy reduces hospital admission rate. Controlled studies on home oxygen therapy and COPD exacerbations are difficult, since withholding therapy in a control hypoxaemic group would not be ethically justifiable.
COPD patients with chronic respiratory failure are particularly susceptible to exacerbations. After the early experience of domiciliary long-term non-invasive ventilation in patients with chest wall and neuromuscular disease, non-invasive ventilation has also been assessed in patients with hypercapnic COPD. Early observations on the effect of non-invasive ventilation in COPD showed a significant beneficial effect on health status, although data for exacerbations were not recorded.124 Because health status is such an important determinant of exacerbation frequency,1 improvement in health status could be due to a reduction of exacerbation frequency. In a controlled study lasting a year, no effect was seen of non-invasive ventilation on exacerbations but there was a reduction in hospital admission at the 3-month follow-up point.125 Thus, larger controlled studies are now required to assess the effect of non-invasive ventilation on exacerbation in hypercapnic COPD patients, particularly at risk of hospital admission.
Conclusion
COPD exacerbations are often triggered by airway infection and are an important cause of morbidity, impairment of health status, and mortality. Although many pharmacological and non-pharmacological interventions prevent exacerbations, the degree of reduction in exacerbation frequency is still restricted and we now need new interventions to be urgently developed and studied in well designed and adequately powered randomised trials. Combinations of these interventions will probably be most effective and this approach will need future development and assessment. One of the main objectives of therapy for COPD is to reduce the morbidity associated with exacerbations and thus improve the quality of life of patients with this disabling condition.
Search strategy and selection criteria
We searched Medline (1997–May, 2007), Embase (1997–May, 2007), and CINAHL (1956–April, 2006) with the search terms “acute exacerbation of COPD”, “COPD exacerbation prevention”, “COPD exacerbation impact”. Thus we largely selected publications in the past 10 years but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. Decisions to include specific references were based on authors' knowledge of published work, participation in expert meetings, and many years of research on the subject. Thus, this work is not an exhaustive systematic review of the burden and prevention of COPD exacerbations. However, it is an overview of current thinking on the importance of COPD exacerbations and how to prevent them based on research evidence and clinical practice
Acknowledgments
Acknowledgments
We are very grateful to John Hurst for assistance in the preparation of this Review.
Conflict of interest statement
JAW has received research grant funding from AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim, who all manufacture pharmacological therapies for prevention of COPD exacerbations. She has also received honoraria for lectures or attended advisory boards from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Bayer Healthcare, and Novartis Pharma. TARS has received honoraria for attendance of research meetings from AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim.
References
- 1.Seemungal TAR, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;151:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 2.British Thoracic Society. Burden of Lung Disease Report, 2nd edn 2006. http://www.brit-thoracic.org.uk/copd/pubs_frameset.html (accessed May 2, 2007).
- 3.Seemungal TAR, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 4.Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57:137–141. doi: 10.1136/thorax.57.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perera WR, Hurst JR, Wilkinson TMA. Inflammatory changes and recurrence at COPD exacerbations. Eur Respir J. 2007;29:527–534. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]
- 6.Rabe KF, Hurd S, Anzueto A. Global strategy for the diagnosis, management, and prevention of COPD—2006 Update. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200703-456SO. published online May 16. DOI: 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Roisin R. Towards a consensus definition for COPD exacerbations. Chest. 2000;117:398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 8.Hurst JR, Perera WR, Wilkinson TMA, Donaldson GC, Wedzicha JA. Utility of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 9.Bhowmik A, Seemungal TAR, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and physiological changes at COPD exacerbations. Thorax. 2000;55:114–200. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saetta M, Di Stefano A, Maestrelli P. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150:1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 11.Qiu Y, Zhu J, Bandi V. Biopsy neutrophilia, neutrophil chemokin and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:968–975. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- 12.Drost EM, Skwarski KM, Sauleda KM. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst JR, Perera WR, Wilkinson TMA, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:71–78. doi: 10.1164/rccm.200505-704OC. [DOI] [PubMed] [Google Scholar]
- 14.Hogg JC, Chu F, Utokaparch S. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 15.Biernacki W, Kharitonov SA, Barnes PJ. Increased leukotriene B4 and 8-isprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax. 2003;58:294–298. doi: 10.1136/thorax.58.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst JR, Wilkinson TMA, Perera WR, Donaldson GC, Wedzicha JA. Relationships among bacteria, upper airway, lower Airway, and systemic inflammation in COPD. Chest. 2005;127:1219–1226. doi: 10.1378/chest.127.4.1219. [DOI] [PubMed] [Google Scholar]
- 17.Wedzicha JA, Seemungal TAR, MacCallum PK. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemostasis. 2000;84:210–215. [PubMed] [Google Scholar]
- 18.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell DE, Parker CM. COPD exacerbations. 3: Pathophysiology. Thorax. 2006;61:354–361. doi: 10.1136/thx.2005.041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhowmik A, Seemungal TAR, Donaldson GC, Wedzicha JA. Effects of exacerbations and seasonality on exhaled nitric oxide In COPD. Eur Respir J. 2005;26:1009–1015. doi: 10.1183/09031936.05.00047305. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson NJ, Walker PP, Costello RW, Calverley PMA. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:1510–1516. doi: 10.1164/rccm.200504-595OC. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MK, Birch M, Carter R, Kinsella J, Stevenson RD. Measurement of physiological recovery from exacerbation of chronic obstructive pulmonary disease using within-breath forced oscillometry. Thorax. 2007;62:299–306. doi: 10.1136/thx.2006.061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapey E, Stockley RA. COPD exacerbations. 2: Aetiology. Thorax. 2006;61:250–258. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donaldson GC, Seemungal T, Jeffries DJ, Wedzicha JA. Effect of environmental temperature on symptoms, lung function and mortality in COPD patients. Eur Respir J. 1999;13:844–849. doi: 10.1034/j.1399-3003.1999.13d25.x. [DOI] [PubMed] [Google Scholar]
- 25.Hurst JR, Donaldson GC, Wilkinson TMA, Perera WR, Wedzicha JA. Epidemiological relationships between the common cold and exacerbation frequency in COPD. Eur Respir J. 2005;26:846–852. doi: 10.1183/09031936.05.00043405. [DOI] [PubMed] [Google Scholar]
- 26.Seemungal TAR, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16:677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seemungal TAR, Harper-Owen R, Bhowmik A. Respiratory viruses, symptoms and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 28.Rohde G, Wiethege A, Borg I. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58:37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan WC, Xiang X, Qiu D, Ng TP, Lam SF, Hegele RG. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am J Med. 2003;1154:272–277. doi: 10.1016/s0002-9343(03)00353-x. [DOI] [PubMed] [Google Scholar]
- 30.Ko FWS, Ip M, Chan PKS. Viral etiology of acute exacerbations of chronic obstructive pulmonary disease in Hong Kong. Chest. 2007 published online June 15. DOI: 10.1378/chest.07-0530 [Google Scholar]
- 31.Falsey AR, Formica MA, Hennessey PA. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:639–643. doi: 10.1164/rccm.200510-1681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson TM, Donaldson GC, Johnston SL, Openshaw PJ, Wedzicha JA. Respiratory syncytial virus, airway inflammation and FEV1 decline in patients with COPD. Am J Respir Crit Care Med. 2006;173:871–876. doi: 10.1164/rccm.200509-1489OC. [DOI] [PubMed] [Google Scholar]
- 33.Retmales I, Elliott MW, Meshi B. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 34.Mallia P, Message SD, Kebadze T, Parker HL, Kon OM, Johnston SL. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 36.Sapey E, Stockley RA. COPD exacerbations. 2: Aetiology. Thorax. 2006;61:250–258. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson TMA, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. Interactions between lower airway bacterial and rhinoviral infection at exacerbations of chronic obstructive pulmonary disease. Chest. 2006;129:317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stockley RA, O'Brien C, Pye A. Relationship to sputum colour to nature and out-patient management of acute exacerbations of COPD. Chest. 2000;117:1638–1645. doi: 10.1378/chest.117.6.1638. [DOI] [PubMed] [Google Scholar]
- 39.Soler N, Agustí C, Angrill J, De la Bellacasa JP, Torres A. Bronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary disease. Thorax. 2007;62:29–35. doi: 10.1136/thx.2005.056374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anthonisen NR, Manfreda J, Warren CPW, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–220. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 41.Ram FSF, Rodriguez-Roisin R, Granados-Navarrete A, Garcia-Aymerich J, Barnes NC. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;2 doi: 10.1002/14651858.CD004403.pub2. CD004403. [DOI] [PubMed] [Google Scholar]
- 42.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 43.Yi K, Sethi S, Murphy TF. Human immune response to nontypeable Hameophilus influenzae in chronic bronchitis. J Infect Dis. 1997;176:1247–1252. doi: 10.1086/514119. [DOI] [PubMed] [Google Scholar]
- 44.Papi A, Bellettato CM, Braccioni F. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 45.Mogulkoc N, Karakurt S, Isalska B. Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infection. Am J Respir Crit Care Med. 1999;160:349–353. doi: 10.1164/ajrccm.160.1.9809041. [DOI] [PubMed] [Google Scholar]
- 46.Blasi F, Damato S, Consentini R. C Pneumoniae and chronic bronchitis: association with severity and bacterial clearance following treatment. Thorax. 2002;57:672–676. doi: 10.1136/thorax.57.8.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seemungal TAR, Wedzicha JA, MacCallum PK, Johnston SL, Lambert PA. C Pneumoniae and COPD exacerbation. Thorax. 2002;57:1087–1088. doi: 10.1136/thorax.57.12.1087-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lieberman D, Lieberman D, Shmarkov O. Serological evidence of Legionella species infection in acute exacerbations of COPD. Eur Respir J. 2002;19:392–397. doi: 10.1183/09031936.02.00256702. [DOI] [PubMed] [Google Scholar]
- 49.Diederen BM, Van der Walk PD, Kluytmans JA, Peeters MF, Hendrix R. The role of atypical respiratory pathogens in exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;30:240–244. doi: 10.1183/09031936.00012707. [DOI] [PubMed] [Google Scholar]
- 50.Anderson HR, Limb ES, Bland JM, Ponce de Leon A, Strachan DP, Bower JS. Health effects of an air pollution episode in London, December 1991. Thorax. 1995;50:1188–1193. doi: 10.1136/thx.50.11.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson HR, Spix C, Medina S. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997;10:1064–1071. doi: 10.1183/09031936.97.10051064. [DOI] [PubMed] [Google Scholar]
- 52.Yang CY, Chen CJ. Air pollution and hospital admissions for chronic obstructive pulmonary disease in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A. 2007;70:1214–1229. doi: 10.1080/15287390701380880. [DOI] [PubMed] [Google Scholar]
- 53.Gouveia N, de Freitas CU, Martins LC, Marcilio IO. Respiratory and cardiovascular hospitalizations associated with air pollution in the city of Sao Paulo, Brazil. Cad Saude Publica. 2006;22:2669–2677. doi: 10.1590/s0102-311x2006001200016. [DOI] [PubMed] [Google Scholar]
- 54.Linaker CH, Coggon D, Holgate ST. Personal exposure to nitrogen dioxide and risk of airflow obstruction in asthmatic children with upper respiratory infection. Thorax. 2000;55:930–933. doi: 10.1136/thorax.55.11.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko FWS, Tam WWS, Chan DPS. The temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax. 2007 doi: 10.1136/thx.2006.076166. published online Feb 20. DOI: 10.1136/thx.2006.076166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J. 2003;41:46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58:100–105. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fletcher CM, Peto R, Tinker CM. The natural history of chronic bronchitis and emphysema. Oxford University Press; Oxford: 1976. [Google Scholar]
- 60.Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive lung disease: results from the Lung Health Study. Am J Respir Crit Care Med. 2001;164:358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 61.Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. The relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57:137–141. doi: 10.1136/thorax.57.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donaldson GC, Seemungal TAR, Patel IS. Airway and Systemic inflammation and decline in lung function, in chronic obstructive pulmonary disease. Chest. 2005;128:1995–2004. doi: 10.1378/chest.128.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spruit MA, Gosselink R, Troosters T. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58:752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 66.Donaldson GC, Wilkinson TMA, Hurst JR, Perera WR, Wedzicha JA. Exacerbations, and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:446–452. doi: 10.1164/rccm.200408-1054OC. [DOI] [PubMed] [Google Scholar]
- 67.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128:2005–2011. doi: 10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]
- 68.Baker EH, Janaway CH, Philips BJ. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61:284–289. doi: 10.1136/thx.2005.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winter JH, Buckier PW, Bautista AP. Frequency of venous thrombosis in patients with exacerbation of chronic obstructive lung disease. Thorax. 1983;38:605–608. doi: 10.1136/thx.38.8.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erelel M, Cuhadarogiu C, Ece T, Arseven O. The frequency of deep venous thormobosis and pulmonary embolus in acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2002;96:515–518. doi: 10.1053/rmed.2002.1313. [DOI] [PubMed] [Google Scholar]
- 71.Tillie-Leblond I, Marquette C-H, Perez T. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med. 2006;144:390–396. doi: 10.7326/0003-4819-144-6-200603210-00005. [DOI] [PubMed] [Google Scholar]
- 72.Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 73.Rutschmann OT, Cornuz J, Poletti P-A. Should pulmonary embolism be suspected in exacerbation of chronic obstructive pulmonary disease? Thorax. 2007;62:121–125. doi: 10.1136/thx.2006.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burge PS, Calverley PMA, Jones PW. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to sever chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calverley P, Pauwels R, Vestbo J. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 76.Vincken W, van Noord JA, Greefhorst APM. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002;19:209–216. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 77.Casaburi R, Mahler DA, Jones PA. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 78.Nichol KL, Baken L, Nelson A. Relation between influenza vaccination and out patient visits, hospitalisation and mortality in elderly patients with chronic lung disease. Ann Intern Med. 1999;130:397–403. doi: 10.7326/0003-4819-130-5-199903020-00003. [DOI] [PubMed] [Google Scholar]
- 79.Gorse GJ, O'Connor TZ, Young SL. Impact of a winter respiratory virus season on patients with COPD and association with influenza vaccination. Chest. 2006;130:1109–1116. doi: 10.1378/chest.130.4.1109. [DOI] [PubMed] [Google Scholar]
- 80.Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. 2004;25:1971–1972. doi: 10.1378/chest.125.6.2011. [DOI] [PubMed] [Google Scholar]
- 81.Wang CS, Wang ST, Lai CT, Lin LJ, Chou P. Impact of influenza vaccination on major cause-specific mortality. Vaccine. 2007;26:1196–1203. doi: 10.1016/j.vaccine.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 82.Granger R, Walters J, Poole PJ. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD001390.pub2. CD001390. [DOI] [PubMed] [Google Scholar]
- 83.Alfageme I, Vazquez R, Reyes N. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006;61:189–195. doi: 10.1136/thx.2005.043323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collet JP, Shapiro S, Ernst P. Effect of an immunostimulating agent on acute exacerbations and hospitalization in COPD patients. Am J Respir Crit Care Med. 1997;156:1719–1724. doi: 10.1164/ajrccm.156.6.9612096. [DOI] [PubMed] [Google Scholar]
- 85.Soler M, Mutterlein R, Cozma G, Swiss-German OM–85 Study Group Double-blind study of OM-85 in patients with chronic bronchitis or mild chronic obstructive pulmonary disease. Respiration. 2007;74:26–32. doi: 10.1159/000093933. [DOI] [PubMed] [Google Scholar]
- 86.Sprenkle MD, Niewoehner DE, MacDonald R, Rutks I, Wilt TJ. Clinical efficacy of OM-85 BV in COPD and chronic bronchitis: a systematic review. COPD. 2005;2:167–175. doi: 10.1081/copd-200050674. [DOI] [PubMed] [Google Scholar]
- 87.Niewoehner DE, Erbland ML, Deupree RH. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941–1947. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 88.Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354:456–460. doi: 10.1016/s0140-6736(98)11326-0. [DOI] [PubMed] [Google Scholar]
- 89.Jones PW, Willits LR, Burge PS, Calverley PMA. The influence of disease severity and the effect of fluticasone propionate on COPD exacerbations in the ISOLDE study. Eur Respir J. 2003;21:68–73. doi: 10.1183/09031936.03.00013303. [DOI] [PubMed] [Google Scholar]
- 90.The Lung Health Study research Group Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. NEJM. 2000;343:1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 91.Mahler DA, Donohue JF, Barbee RA. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–965. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 92.Rennard S, Anderson W, ZuWallack R. Use of long-acting inhaled beta2 adrenergic agonist salmeterol xinafoate in patients with COPD. Am J Respir Crit Care Med. 2001;163:1087–1092. doi: 10.1164/ajrccm.163.5.9903053. [DOI] [PubMed] [Google Scholar]
- 93.Calverley PM, Anderson JA, Celli B. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 94.Van Noord JA, de Munck DRAJ, Bantje ThA. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J. 2000;15:878–885. doi: 10.1034/j.1399-3003.2000.15e11.x. [DOI] [PubMed] [Google Scholar]
- 95.Rossi A, Kristufek P, Levine B. Comparison of the efficacy, tolerability and safety of formoterol dry powder and oral slow-release theophylline in treatment of COPD. Chest. 2002;121:1058–1069. doi: 10.1378/chest.121.4.1058. [DOI] [PubMed] [Google Scholar]
- 96.Szafranski W, Cukier A, Ramirez A. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 97.Calverley PM, Boonsawat W, Cseke Z. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912–918. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 98.Whelan CJ, Johnson M, Vardey CJ. Comparison of the anti-inflammatory properties of formoterol, salbutamol and salmeterol in guinea-pig skin and lung. Br J Pharmacol. 1993;110:613–618. doi: 10.1111/j.1476-5381.1993.tb13855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barnes PJ. Effect of beta-agonists on inflammatory cells. J Allergy Clin Immunol. 1999;104(suppl):S10–S17. doi: 10.1016/s0091-6749(99)70269-1. [DOI] [PubMed] [Google Scholar]
- 100.Niewoehner DE, Rice K, Cote C. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once daily inhaled anticholinergic bronchodilator: a randomised trial. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 101.Powrie DJ, Wilkinson TMA, Donaldson GC, et al. Effect of tiotropium on inflammation and exacerbations in chronic obstructive pulmonary disease Eur Respir J (in press).
- 102.Aaron SD, Vandemheen KL, Fergusson D. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 103.Zhou Y, Wang X, Zeng X. Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year. Respirology. 2006;11:603–610. doi: 10.1111/j.1440-1843.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 104.Rennard SI, Schachter N, Strek M, Rickard K, Amit O. Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4. Chest. 2006;129:56–66. doi: 10.1378/chest.129.1.56. [DOI] [PubMed] [Google Scholar]
- 105.Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbröker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 106.Calverley PM, Sanchez Toril F, McIvor A, Teichmann P, Bredenbroeker P, Fabbri LM. Effect of one year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 107.Rennard SI, Fogarty C, Kelsen S. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 108.Decramer M, Rutten van-Molken M, Dekhuijzen PN. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS) Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 109.Poole PJ, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD001287.pub2. CD001287. [DOI] [PubMed] [Google Scholar]
- 110.Hogg JC, Chu FSF, Tan WC. Survival following lung volume reduction in COPD: insights from small airway pathology. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200612-1772OC. published online June 7. DOI: 10.1164/rccm.200612-1772OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with chronic bronchitis. Am J Med. 2000;109:288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 112.Patel IS, Seemungal TAR, Wilks M, Lloyd Owen S, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character and severity of COPD exacerbations. Thorax. 2002;57:759–764. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scott CS, Walker P, Calverley PMA. COPD exacerbations. 4: Prevention. Thorax. 2006;61:440–447. doi: 10.1136/thx.2005.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Griffiths TL, Burr ML, Campbell IA. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation. Lancet. 2000;355:362–368. doi: 10.1016/s0140-6736(99)07042-7. [DOI] [PubMed] [Google Scholar]
- 115.Wilkinson TMA, Donaldson GC, Hurst JR, Seemungal TAR, Wedzicha JA. Impact of reporting and early therapy on outcome of exacerbations of COPD. Am J Respir Crit Care Med. 2004;169:1298–1303. doi: 10.1164/rccm.200310-1443OC. [DOI] [PubMed] [Google Scholar]
- 116.Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM, for the Estudi del Factors de Risc d'Agudització de la MPOC investigators Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58:100–105. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bourbeau J, Julien M, Maltais F. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 118.Taylor SJC, Candy B, Bryar RM. Effectiveness of nursing and nurse-led chronic disease management innovations for patients with chronic obstructive pulmonary disease: systematic review of evidence. BMJ. 2005;331:485. doi: 10.1136/bmj.38512.664167.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Casas A, Troosters T, Garcia-Aymerich J. Integrated care prevents hospitalisations for exacerbations in COPD. Eur Respir J. 2006;28:123–130. doi: 10.1183/09031936.06.00063205. [DOI] [PubMed] [Google Scholar]
- 120.Medical Research Council Working Party Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;i:681–686. [PubMed] [Google Scholar]
- 121.Nocturnal Oxygen Therapy Trial Group Continuous or nocturnal oxygen therapy in hypoxaemic chronic obstructive lung disease. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 122.Okubadejo AA, Paul EA, Jones PW, Wedzicha JA. Does long term oxygen therapy affect quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia? Eur Respir J. 1996;9:2335–2339. doi: 10.1183/09031936.96.09112335. [DOI] [PubMed] [Google Scholar]
- 123.Ringbaek TJ, Viskum K, Lange P. Does long-term oxygen therapy reduce hospitalisation in hypoxaemic chronic obstructive pulmonary disease? Eur Respir J. 2002;20:38–42. doi: 10.1183/09031936.02.00284202. [DOI] [PubMed] [Google Scholar]
- 124.Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD: a randomised controlled study. Am J Respir Crit Care Med. 1995;152:538–544. doi: 10.1164/ajrccm.152.2.7633704. [DOI] [PubMed] [Google Scholar]
- 125.Casanova C, Celli BR, Tost L. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest. 2000;118:1582–1590. doi: 10.1378/chest.118.6.1582. [DOI] [PubMed] [Google Scholar]