Abstract
Despite impressive success in molecular physiological understanding of photosynthesis, and preliminary evidence on its potential for quantum shifts in agricultural productivity, the question remains of whether increased photosynthesis, without parallel fine-tuning of the associated processes, is enough. There is a distinct lack of formal socio-economic impact studies that address the critical questions of product profiling, cost–benefit analysis, environmental trade-offs, and technological and market forces in product acceptability. When a relatively well understood process gains enough traction for translational value, its broader scientific and technical gap assessment, in conjunction with its socio-economic impact assessment for success, should be a prerequisite. The successes in the upstream basic understanding of photosynthesis should be integrated with a gap analysis for downstream translational applications to impact the farmers’ and customers’ lifestyles and livelihoods. The purpose of this review is to assess how the laboratory, the field, and the societal demands from photosynthesis could generate a transformative product. Two crucial recommendations from the analysis of the state of knowledge and potential ways forward are (i) the formulation of integrative mega-projects, which span the multistakeholder spectrum, to ensure rapid success in harnessing the transformative power of photosynthesis; and (ii) stipulating spatiotemporal, labour, and economic criteria to stage-gate deliverables.
Keywords: C3–C4, climate change, nutritious crops, photosynthesis, rice, sustainability, socio-economic frontier, translational products, yield
Achievements in photosynthesis research portend a capacity for transformative changes in food and nutritional security if integrated with water and nutrient use and the requirements of multiple stakeholders along the value chain.
Introduction
The human population grew from 1 to 2 billion in 123 years but from 5 to 6 billion in only 12 years (1987–1999), and, again in 12 years (1999–2011), from 6 to 7 billion (BBC News, https://www.bbc.com/news/world-15459643). Between 2025 and 2030, the global population is set to increase by at least another billion and may exceed 10 billion in around 2050 (UNDESA, https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html). Although population increase is the main reason to consider ways and means for producing more food, other critical factors necessitate a reappraisal of the agricultural practices, processes, and production. For example, the decreasing availability, accessibility, and affordability of one or more components of the land–water–labour–energy nexus affect agriculture globally. Similarly, the demand for increased production of seeds superior in agronomic and/or nutrition performance calls for paradigm shifts from increasing productivity for cheap food to sustainably intensifying agriculture to provide healthy food.
Asia is home to nearly 60% of the world’s population. Hence rice, Asia’s staple food, would be a target crop of high priority for research on increasing quantity and quality. Rice suffers more than the other crops from decreasing agricultural land, water, and labour, and is energy intensive. Also, its cultivation practices account for greater emission of greenhouse gases (GHGs), such as methane and nitrous oxide, than other cereals. It is particularly sensitive to other stresses such as drought, heat, cold, flooding, and salinity. Unlike wheat and maize, rice lacks the ‘futures market’ which underlies a comparatively less organized national and international rice seed industry. Consequently, the rice seed quality at input and its consequent output can suffer.
Rice is not only the staple food and lifeline for the majority of the world’s poor and hungry, but it is also key to improving economic stability and quality of life in most Asian countries due to national and international market and socio-economic forces. The demand for rice cultivation and consumption in Africa is also steadily increasing. These factors, when taken together, argue for improvements in productivity and quality, and in sustainable intensive cultivation, of rice in Asia and Africa.
Research agendas that address the increase in quantity and quality of rice and other crops for the future must be harmonized for a united global community effort to develop and utilize technological resources (Zaidi et al., 2019). Sufficient and nutritious food for the future is a global imperative that cannot be advanced sustainably without global transdisciplinary efforts. For example, problems in agriculture, associated with climate change, are still patchily understood. They are primarily geopolitical and/or ecoregional, and are currently addressed through local or regional solutions. Only with a global approach to the problems and solutions can sustainable productivity be achieved. Regional sea level rise and consequent salination, acidification, or eutrophication can be ameliorated for the long term if global players agree to support reduction of emissions, soil reclamation, availability of adapted varieties, and perhaps even consider relocation. Research on reduction of agricultural emissions on the global scale and salt-tolerant crop varieties at the regional scale, for example, can be helpful.
There are three major and interconnected research routes for increasing crop productivity for the future scenarios: phenotyping-based, genotyping-based, and physiological process-based. Historically, the phenotyping-based route would be the classical breeding and agronomy approach, and contemporarily the genotyping-based route would include all molecular approaches including various ‘omics’ methods of characterizing a genotype. However, the physiological process-based route would be the modern molecular physiology approach. Nevertheless, under the modern context of generating agricultural knowledge and products, the three routes cannot be independent of each other, and it is critical that the three routes are integrated to develop improved, adapted, productive, and consumer-preferred crop varieties. Keeping the farmers and consumers in mind, the need to maintain and increase nutritional quality applies to all cereals but is critical in rice for which the market forces work more around the grain attributes than around the flour attributes.
One particular plant trait, the understanding of which has progressed over time through an integrative course, is photosynthesis. Photosynthesis is the very basis of human existence. An extensive mechanistic understanding of this process over the years, from morphology and anatomy to biochemistry to molecular biology to biophysics and electrophysiology, makes photosynthesis an intuitively attractive and plausible avenue for increasing plant productivity. A relatively deep understanding of photosynthesis drives our efforts to manipulate it for increased crop productivity.
From the early to mid-18th century, discoveries revealed that plants derive nourishment from the atmosphere through their leaves and that the gas produced by plants is oxygen (Bonnet, 1754). Huzisige and Ke (1993) chronicled the timeline of landmark discoveries in understanding photosynthesis. That timeline revealed that studies in the 1830s and 1840s led to recognizing that light, chlorophyll, and chloroplasts are needed and that light energy converts into chemical energy. It took until the 1930s to determine that photosynthesis is a light-induced oxidation–reduction process and the Calvin–Benson cycle was elucidated only in the 1950s. Significant progress was made in biochemical and biophysical aspects underpinning photosynthesis in the 1960s. In the next decade, the carboxylase/oxidase functions of Rubisco (EC 4.1.1.39) were identified and the 3-D structures of Rubisco and mutants of Cyt b were available in the late 1980s and 1990s. Through the progressive mechanistic understanding of photosynthesis, the source–sink relationships between the photosynthetic assimilates also became clear (Ho et al., 1983), as did the unique connection between the sink and crop improvement for human consumption.
In the 1990s, ideas were proposed to manipulate (and improve) photosynthesis as a means to further improve crops generated during the ‘Green Revolution’, but the yields of which had started to plateau (Janaiah et al., 2006). Incremental success, since the Green Revolution, in improving the yield of cereals augmented crop productivity linearly by ~1.7% annually (Long et al., 2015). However, substantial yield increase did not always accompany suitable grain quality, and varieties with extremely high yield potential were rejected, leading to recent efforts in designing strategies to increase yield and quality simultaneously (Zeng et al., 2017). With molecular tools and plant transformation, the pipeline of gene identification, isolation, and characterization took centre stage. However, no single yield-related gene transformation led to a substantial yield increase. Gene combinations are starting to show promise, but this is only through traits of low heritability such as panicle number (Huang et al., 2018). Photosynthesis is the only frontier that is central to plant growth, development, and yield; relatively well understood at the mechanistic level, yet not fully harnessed in the downstream application for increased crop productivity and quality.
Can manipulation of photosynthesis lead to an increase in grain yield?
Atmospheric CO2 concentrations have risen by 30% over the last 60 years (https://www.esrl.noaa.gov/gmd/obop/mlo/), directly affecting photosynthesis. One of the predictions is an increase in yield of C3 plants by 2- or 3-fold. These predictions are based on studies that increased exogenous atmospheric CO2 by free-air CO2 enrichment (FACE; Ainsworth and Long, 2005; Gielen et al., 2005). The biomass of woody trees (e.g. poplar) increased by 15–30%. The technique was then tested with crops, including cereals, in field conditions in a crop rotation of barley, rye, sugar beet, and winter wheat, and yields increased by ~12% in barley, wheat, and sugar beet (Weigel and Manderscheid, 2012). However, the limited numbers of FACE experiments with a limited number of crops show considerable seasonal, geographical, and genotype variability for an increase in biomass and yield. The study of Weigel and Manderscheid (2012) also suggested a positive effect of elevated CO2 on yield under drought and also concluded that the variation in nitrogen content did not affect yield. However, there is considerable variability in the positive effect of elevated CO2 on yield depending on the water and nitrogen content available. In a FACE study with rice under different levels of nitrogen, elevated CO2 caused a similar increase in biomass under three different nitrogen regimes, but the yield increase was proportional to the nitrogen content (Kim et al., 2003). Similarly, the positive effects of elevated CO2 were more consistent in high-yielding cultivars with higher nitrogen use efficiency (NUE), thus once again highlighting the role of nitrogen (Hasegawa et al., 2013). The importance of nitrogen in preference to photosynthesis as a route to increasing crop yield was recently discussed (Sinclair et al., 2019). Vegetative biomass tends to increase during early growth and development under enhanced CO2 concentration, but this may not be maintained during reproductive development.
Most studies have assumed that with elevated CO2, a temperature increase occurs. At a lower temperature, the yield increase is much lower than at higher temperatures. Modelling studies predict that the beneficial effect of rising temperatures may not be sustained above certain temperature increases (Degener, 2015). Simultaneously, high temperature is known to negatively affect yield and grain quality (Krishnan et al., 2011). Maize cultivation is already under optimal temperatures and could be negatively affected by higher temperatures (Schlenker and Roberts, 2009). FACE studies conducted over time and across space have not used the same elevated CO2 concentrations. Thus, the interplay between CO2 and temperature, and combined effects on yields, remains ambiguous. Deciphering dependable patterns from the limited number of studies appears difficult and also inconclusive due to the multiplicity of variables in nutrient, water, temperature, edaphic, and soil microbial factors and their interactions under altered CO2 concentrations. For example, the yield of maize, a C4 plant, is not predicted to react substantially to elevated CO2, although it may improve under water stress (Kimball, 2011). Such an indirect sustainability effect through water saving is thought to be of a considerable proportion and could be equally, if not more, useful than the benefits to agriculture through elevated CO2 (Morgan et al., 2004). Also, recent FACE studies by Lv et al. (2020) revealed that temperature, nitrogen, and genotype variability have major contributions to the yield increase under FACE, once again stressing the interaction of multiple variables contributing to yield. Thus, modification of plants for improved photosynthesis, engineered or bred, for better capture or utilization of CO2 may not directly increase grain yield.
The prospects for the potential benefits to plant productivity from elevated CO2, as seen through FACE studies to date, raise some questions as well. First, considering the complex air–water–nutrient–soil–temperature nexus, how much of the beneficial effects of elevated CO2 are due to increased photosynthesis per se? In other words, do we have enough evidence that manipulation of the molecular and biochemical machinery of photosynthesis, to replicate the scenario of providing more CO2 to the plant, or a more efficient use or regeneration of CO2 inside the plant cells, leads to desirable levels of increase in agricultural productivity? Secondly, will the harvest index increase with the increase in photosynthesis; will the increase in biomass be preferentially partitioned to grain? Thirdly, with the possibility of the use of big data, machine learning, and artificial intelligence, would investments in the next-generation FACE studies be beneficial if they address the complex interactions among the critical variables of air, water, nutrients, soil, and temperature? Cai et al. (2016, 2018, 2020) conducted a series of studies of rice grown under FACE, showing that various biochemical and photosynthetic parameters were differentially affected by CO2 concentration, temperature, and growth stage. Such multiparametric studies can benefit from investments in machine learning.
As late as 2006, the argument was widespread, and is as yet unresolved, as to whether an increase in net photosynthesis can increase crop grain yield. Grain yield per unit ground area does not change much when panicle density or spikelets per panicle increase, because they are negatively correlated (Hasegawa et al., 2013). Theoretically, increased photosynthesis can lead to increased grain yield and this has been observed experimentally under elevated atmospheric CO2. To translate potential increases in photosynthetic capacity into increased yield, other molecular factors or pathways may need to be engineered. Some recent scientific outputs that take trail-blazing routes to increase photosynthesis look very promising. For example, manipulating the photorespiratory pathway has led to exciting results for a biomass increase in tobacco (South et al., 2019) and rice (Shen et al., 2019). These and similar studies outlined in Table 1 provide the hope that it is possible to increase plant photosynthesis and see its effect on increased biomass and grain yield. However, for such studies to be useful for the future, it is vital to report on grain yield traits of high heritability. In the study by Shen et al. (2019), for example, the increase in rice grain yield was through the number of panicles per plant, which is a yield trait with very low heritability (Kato, 1997; Kamara et al., 2017). The more dependable traits such as the 1000 grain weight and the number of filled grains per panicle were lower in transgenic plants engineered for the glycolate bypass compared with wild-type plants.
Table 1.
Achievements and gaps in photosynthesis research towards crop yield increase
| Progress | Quality of evidence | References | Gaps | |
|---|---|---|---|---|
| Upstream | Indirect selection | High | Zhu et al. (2008) | Efficient alleles |
| Enzyme engineering | High | Kromdijk et al. (2016) | Genetic networks | |
| Mutant screening | High | Kirst et al. (2017) | Protein interactions | |
| Omics data | Medium | Wang et al. (2012) | Source–sink relationships | |
| Bioinformatics | Medium | Burgess and Hibberd (2015) | Stomatal mechanisms | |
| Process modelling | Medium | Kubis and Bar-Even (2019 | Harvest index changes | |
| Gene cloning | High | Occhialini et al. (2016) | Trait heritability | |
| Gene editing | High | Khumsupan et al. (2019) | Nitrogen use efficiency | |
| Transgenic plants | High | Gu et al. (2015) | Carbon–nitrogen ratio | |
| Gene pyramiding | High | Kromdijk et al. (2016) | Biotic/abiotic stress | |
| Pathway engineering | High | South et al. (2019) | Microbiome changes | |
| Phenotyping | High | Keller et al. (2019) | Methane emission changes | |
| Translational | Root exudates | Weak | Olanrewaju et al., 2019 | Role of rhizobiome |
| Milling | Weak | Xu et al. (2015); Jing et al. (2016) | Grain quality | |
| Role of stomatal density | Weak | Way et al. (2014) | Scaled up analyses | |
| Biomass increase | Medium | López-Calcagno et al. (2019) | Commercial products | |
| Grain yield increase | Weak | Driever et al. (2017); Köhler et al. (2017) | ||
| Field test | Medium | Kromdijk et al. (2016) | ||
| Field phenotyping | Medium | Slattery et al. (2017) | ||
| Phenotypic changes in vascular bundle | High | Henry et al. (2017); Sedelnikova et al. (2018) | ||
| Carboxysome formation | High | Long et al. (2018) | ||
| Higher CO2 fixation | High | Yu et al. (2018) | ||
| FACE | Medium | Ainsworth and Long (2005); Gielen et al. (2005) | Extensive FACE studies | |
| Socio-economy and environment | Market surveys | |||
| Increased income | Weak | Klümper and Qaim (2014) | Cost–benefit analyses of alternative options | |
| Ex ante and ex post analyses | ||||
| Political economy of GE and seed systems | ||||
| Water use efficiency | ||||
| Land use efficiency | ||||
| Fertilizer/energy use efficiency | ||||
| Comparative technologies | ||||
| MEL processes | ||||
| Clear time scales of project termination |
The ‘quality of evidence’ column reflects the overall body of evidence on the matter; it does not reflect the quality of the reference paper.
Notwithstanding the lack of definitive evidence for an increase in grain yield by manipulating photorespiration, the point about a steady relationship between high photosynthesis and high yield is nevertheless made through a reverse argument. For example, the ‘Green Revolution’ rice variety IR8, a high-yielding variety, had a greater photosynthetic rate per unit leaf area than previous varieties (Hubbart et al., 2007). However, for rice, there is a biphasic pattern in variety development concerning photosynthetic efficiency. The varieties developed from 1966 to 1980 were better yielding due to better harvest index, while those developed after 1980 were better due to biomass increase (Hubbart et al., 2007). In the latter case, the selection was most probably based on traits of high heritability. Hence, an increase in biomass still holds the promise of an increase in grain yield if the relevant yield traits are duly considered.
Overall, photosynthesis is still below its biological potential (Long et al., 2006; Zhu et al., 2008), and can be improved to increase yield potential, through an increase in either harvest index or biomass. In the former case, the source–sink relationship for assimilation, transportation, and accumulation plays a vital role; but the physiological mechanism for the latter case is still not clear. Similarly, the physiological and biochemical basis of very high photosynthetic efficiencies at high solar irradiance in the so-called ‘superperformers’ such as Hirschfeldia incana are not known, although some comparative genomics results have been presented (Garassino, 2018). However, does this high photosynthetic efficiency lead to high grain/seed yield? Not all highly photosynthetically efficient plants achieve high yield.
For FACE studies, there is an understanding that the increase in yield, can only be partly indicative of the natural scenario of the high CO2 future. These studies indicate less yield benefit than expected, but they report improved production over long time scales, even with suboptimal Rubisco activity; improved NUE and water use efficiency (WUE) and stimulated dark respiration (Leakey et al., 2009). Studies taking other parameters of genotype, water, temperature, nutrients, and soil, and their interactions, into account under high CO2 are sparse. Since multivariable FACE studies are complex and expensive, modelling studies are used for insights and for informing field studies. However, comprehensive approaches that consider all the variables and their interactions do not yet exist. Degener (2015) used the BioSTAR crop model (https://www.uni-goettingen.de/en/431252.html) and considered different abiotic variables under two CO2 regimes to assess the quantitative and qualitative effect of CO2. Yield models were proposed over a century ago for 10 crops in a large agricultural area of Germany, spanning the maritime to continental climate. The study suggested that depending on the crop, season, and time scale of the model, one or more of the abiotic factors, or their interactions, become critical and lead to a reduction in crop yield. The beneficial effect of elevated CO2 may not be as high as predicted earlier, and whether CO2 levels in the future change in the predicted manner remains a key research question.
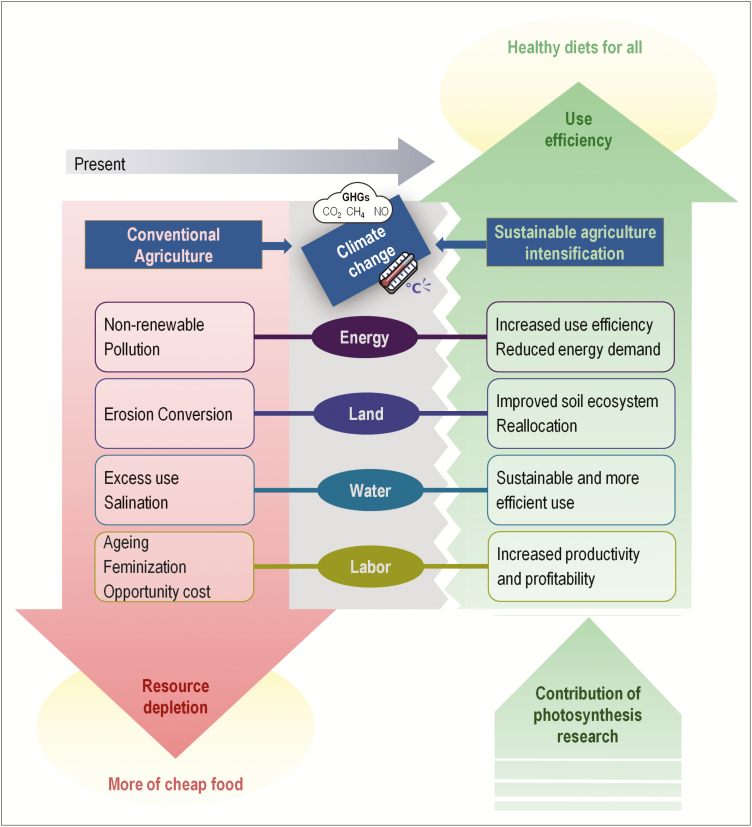
Overall, the data to date support photosynthesis improvement as a plausible means to accomplish a quantum increase in crop yields. Yield increases at present are achieved by mechanisms that provide for more CO2 and/or improved WUE. In the future, increasing atmospheric CO2 concentrations may help increase the yields of some crops. Importantly, improved photosynthesis as seen under FACE studies portends improved water, energy, land, and labour use efficiency (Fig. 1). Such environmentally benign and sustainable multipronged benefits of improved photosynthetic efficiency, as opposed to the exponentially increasing environmental damage of contemporary agricultural practices, should elicit broad research interest in photosynthesis. The ambitious project aiming to convert rice from C3 to C4 photosynthesis is one example of global efforts in this direction.
Fig. 1.
How can photosynthesis research support a paradigm shift toward higher food system efficiency? Conventional agriculture is causing incremental resource depletion. More land may be required to cater to future production scenarios. Land availability is constrained due to sprawling urbanization and other diverse land use. Agricultural soils are being eroded and depleted of minerals. Water is used indiscriminately and excessively. Heat- and drought-mediated, as well as seawater incursion-mediated, salinization of arable land restricts crop productivity. Excessive use of fertilizers and other chemical inputs also has consequences on pollution and energy consumption in a system that turns fossil fuel energy into food (Pimentel and Giampietro, 1994). At present, compared with the 1960s, more energy is needed to produce the same yield. Additionally, the working-age male population is leaving agriculture, leaving tasks in the fields to an older and female-dominated workforce. This scenario is compounded by the harmful effects of an anthropogenic climate change that is exacerbated by intensive agriculture. To break this paradigm, research for higher photosynthesis efficiency to obtain higher yields is one of the most promising avenues to develop more sustainable agricultural systems in the social, economic, and environmental contexts. Higher photosynthetic efficiency requires more carbon dioxide, which increases atmospheric carbon sequestration. Land systems re-balance by growing higher yielding crops which will free up land for other purposes. These crops will be more efficient in nutrient and water use, and be associated with a decrease in the labour to capital ratio. Similarly, reduced inputs will reduce the energy requirements from fossil fuels. Finally, higher yielding crops with high-efficiency photosynthesis and increased input use efficiency will increase productivity and reduce production costs, resulting in higher incomes for farmers. The additional income can be used for transformational changes such as child education, increased family welfare, and other necessities, such as better houses for better livelihoods.
C3 versus C4 photosynthesis
An extensive body of literature exists to show that photosynthesis by plants such as maize, sugarcane, and sorghum, where the first product of carbon fixation is a four-carbon (C4) oxaloacetate, is more efficient than plants such as rice where a three-carbon (C3) 3-phosphoglycerate is first made. The efficiency resides in photorespiration-mediated recovery and concentration of CO2 within the plant cells (Sage et al., 2012). The C4 plants can produce ~40–50% higher biomass and grain yields than C3 plants, due to the photosynthetic system that is 40% more efficient (Sage, 2016). Also, C4 plants have better NUE and WUE in the tropics and subtropics (Sage, 2016, and references therein). However, one advantage of C3 over C4 is the tolerance for shading, and that is a possible reason why C3 forests dominate over C4 plants (Sage, 2016). A modelling study by Gu et al. (2014) considered light-saturated and light-limited photosynthesis as key to scaling up the benefits of improved photosynthesis from leaf to canopy to crop level through genetic variation. Indeed the rich genetic diversity has not been queried for major differences in photosynthetic efficiency and there may be value in doing so. However, the question remains as to whether any of the unexplored rice that has higher photosynthetic efficiency will yield more than the top yielding rice varieties, because the purpose is to increase grain yield.
Most food crops are C3 plants; only maize, sugarcane, sorghum, and some millet are C4 plants that contribute to human nutrition. Therefore, with the potential of a much higher yield from C4 rice (Sheehy et al., 2008), efforts to convert C3 to C4 plants by engineering the photosynthesis system may well be justified. Scattered attempts at trying to convert C3 photosynthesis food crops to C4 photosynthesis concentrated on introducing critical C4 genes (Häusler et al., 2002; Miyao, 2003). For that, most researchers used the maize gene for phosphoenolpyruvate carboxylase (PEPC; Ku et al., 2001; Taniguchi et al., 2008). Due to the socio-economic importance of rice as a significant C3 grain crop, especially in the countries with food and nutrition insufficiency, it became a primary target from C3 to C4 conversion to increase productivity. Despite single-cell C4 mechanisms existing in aquatic and land plants to concentrate CO2 (von Caemmerer et al., 2014), the two-cell C4 system (mesophyll and bundle sheath) is the route chosen for the conversion of rice from C3 to C4 photosynthesis. This conversion is a complex and ambitious process of manipulating not just the biochemistry, but also the anatomical features. It will take a large number of genes (~40 core genes) to achieve this in rice, and high-level transcription factors are suggested as primary targets to control numerous downstream target genes (Burgess and Hibberd, 2015). Apart from C4, other biochemical or physiological pathways have been suggested for improved photosynthesis (Skillman et al., 2011). However, concomitant data on the effect on grain yield are lacking.
As costly and complicated as the C3 to C4 research may be, substantial financial and environmental gains are estimated once the final product is achieved (Sheehy et al., 2008; Sage and Zhu, 2011). Additionally, engineering C3 into C4 plants provides a model for developmental and functional engineering of organisms in the future. After nearly a decade of the ambitious C4 rice project, a major concern is that intermediate C3–C4 products do not bring any benefit over C3 because the photosynthetic efficiency is less than or equal to that of C3 plants (Vogan and Sage, 2012; Heckmann et al., 2014; Way et al., 2014). Another setback has been the realization that knowledge of the molecular basis of C4 versus C3 photosynthesis is incomplete and cryptic, as highlighted by recent transcriptomic and bioinformatic data (Sedelnikova et al., 2018).
One of the struggles of the C4 rice project has been the pressure to generate a product before the critical knowledge was available. Progress so far is therefore a combination of generating knowledge to fill the gaps in the mechanics of photosynthesis as a process, and harnessing available knowledge to develop the high-yielding product. For example, the catalytic turnover rate (kcat) and Km for CO2 (Kc) of Rubisco of C3 and C4 plants can be very different (Sage, 2002). The desirable high (kcat) Rubisco of C4 plants also has an undesirably high Kc. Yet, the Rubisco of C4Sorghum bicolor has a higher kcat to Kc ratio among C4 plants and may thus be a candidate gene to improve CO2 assimilation in rice (Ishikawa et al., 2009). In the last decade, progress towards a better molecular understanding has been phenomenal, thanks to the interdisciplinary and global nature of the consortium. However, a spillover effect has been the recognition of the research gaps that remain, such as the mechanisms for optimal nitrogen partitioning. These gaps must be filled in parallel if we are to be ready to optimally use the gains from the upstream, fundamental biology progress in understanding photosynthesis per se and not just the differences in C3 and C4 photosynthesis (Table 1). Some further details on the scientific gaps are given below.
Source–sink relationships
There has been much progress at the basic science level to understand how to improve the efficiency of photosynthesis. However, there remain gaps in how the additional assimilate must be transported and transformed into grain yield (source–sink and harvest index relationships). For example, there are numerous reports on genes, genomic regions, and genotypes for improved grain yield; most studies conclude that increasing leaf photosynthesis does not necessarily correlate to grain yield increase. However, there is a general agreement on the importance of the sink–source balance. The extent of changes in dry seed weight with changes in the amount of available assimilate in wheat, maize, and soybean suggests that grain yield is limited more by sink capacity than by the source (Borrás et al., 2004). Thus, while photosynthesis increases source capacity, the limiting factor is in the sink (Borrás et al., 2004; Long et al., 2006; Takai et al., 2011). Early seedling biomass determines later yield in many crops; therefore, by selecting for cultivars with desired sink and source traits, an increase in the rate of photosynthesis at early development stages leading to higher biomass can translate into higher grain yields as well (Long et al., 2006; Ohsumi et al., 2011; Takai et al., 2013; Li et al., 2014; Du et al., 2019). The genetic component of such plasticity in grain yield, whereby an increase in source at the seedling or early vegetative stage can prime increased sink capacity, has been shown in a study with two soybean lines under high CO2 levels (Long et al., 2006). Such plasticity in grain yield needs to be fully understood as a mechanism, and bred for, so that an engineered increase in photosynthesis under elevated CO2 is duly exploited and not limited by sink capacity. While the current drive to deliver the product quickly can address the issues of source–sink and harvest index relationships, other more critical gaps remain.
Genetic and regulatory networks
The intricate genetic and regulatory networks underlying increased photosynthetic efficiency are still elusive. Are there superior alleles for the core set of enzymes? What are the molecular networks for associated processes with the capacity to influence photosynthesis, for example WUE? Local genomic regulatory hubs such as the one discovered through multigene quantitative trait loci (QTLs; Dixit et al., 2015) can provide insights into sets of genes essential in such traits. Complex protein–protein interactions for how spatiotemporal alterations in protein products may engage with their known or new protein partners and how those, in turn, may affect the plant as a whole are yet to be fully understood. Nevertheless, this gap was known to exist, and an attempt was made to reconstruct the gene regulatory network for photosynthesis in Arabidopsis (Yu et al., 2014), with little progress. In any case, the gene-, protein-, or metabolite-based information gaps may be filled through results from mega-projects such as the C4 rice project, where deeper molecular understanding is the mandate.
Nutrient use efficiency
Despite clear evidence for improved WUE and nutrient use efficiency in C4 plants, there is little information on how improved C3 photosynthesis may affect resource use efficiency, including the carbon to nitrogen ratio and stomatal mechanisms.
Various factors influence the relationship between high photosynthesis and high grain yield, for example light response and genotypic variation (Long, 1993; Li et al., 2014; Du et al., 2019). Other factors of significant relevance when trying to increase yield by increasing photosynthesis are canopy architecture, leaf canopy size, NUE, and WUE. An increase in yield requires more water and nitrogen (fertilizer), and/or higher WUE and NUE (Mitchell and Sheehy, 2006). Several in-depth reviews of the plant nitrogen cost of photosynthesis, and nitrogen uptake and remobilization are available (Masclaux-Daubresse et al., 2011; Evans and Clarke, 2019). C4 plants achieve a greater rate of photosynthesis for a given unit of leaf nitrogen because Rubisco operates close to CO2 saturation and less protein is invested in it. Leaf nitrogen in C4 plants is 20% lower than in C3 plants (Schmitt and Edwards, 1981; Sage et al., 1987).
Yield gaps must be closed, to bring farmers’ yields closer to the assessed potential on experimental farms. Closing yield gaps can be achieved by increasing efficiencies of nitrogen and water, thus minimizing environmental impact associated with high demand, and through resilience to biotic and abiotic stresses (Simkin et al., 2019). There is a trade-off through transpiration between biotic and abiotic stress tolerance and increasing yield by increased photosynthesis. Plants reduce transpiration as a mechanism to tolerate water stress. Increasing the rate of photosynthesis, and thus transpiration, would therefore reduce tolerance to stresses such as drought (Collins et al., 2008; Driever and Kromdijk, 2013). Salinity-tolerant lines usually have reduced stomatal density, but this reduces photosynthesis and would potentially decrease crop yield (Liu et al., 2017).
With NUE and WUE as essential components of harvesting the benefit of increased photosynthesis, what is our understanding of the relationships of root morpho-anatomy and physiology to altered photosynthesis? A recent study to increase WUE by genetic modification of PSII in tobacco found that light-regulated reduction of stomatal opening resulted in a reduction in water loss in the field (Głowacka et al., 2018). Although this discovery should increase productivity, the authors found that plant size and dry matter decreased in non-water-limiting conditions. It was hypothesized that protein PsbS excess and the concomitant increase in non-photochemical quenching level could adversely affect the light use efficiency under fluctuating light. An increase in nitrogen content could improve photosynthesis, which also affected stomatal conductance and chlorophyll amount, and the total nitrogen content in the leaves increased (Głowacka et al., 2018). These parameters were negatively affected under drought, but, even in water-limiting conditions, an extensive root system combined with an increase of nitrogen would permit more dry matter to be accumulated. The positive correlation between biomass accumulation, NUE, and photosynthetic rate suggests that greater NUE could be a useful parameter for drought tolerance (Dinh et al., 2017). In their study, Saengwilai et al. (2014) reported a relationship between crown root number, nitrogen uptake, and increased photosynthesis.
Rhizobiome
We understand little about how the rhizobiome is affected by C3 or C4 photosynthesis, or if the rhizobiome affects grain yield or quality. A recent paper by Olanrewaju et al. (2019) reported that there is variation in the types of exudates released into the rhizosphere of C3 and C4 plants. This supported the earlier research by Chen et al. (2016) who reported that changes in above- and below-ground biomass, and photosynthesis, would alter respiration in the soil through changes in substrate availability below ground. They conclude that C4 plants produce higher amounts of exudates and would provide more underground substrate for the respiration of both roots and the rhizobiome. While C3 plants exude more carbohydrates and organic carbons, C4 plants exude more organic acids and amino acids. Exudates of C3 plants are mannose, maltose, and ribose (Vranova et al., 2013), and C4 plant exudates include inositol, erythritol, and ribitol (Olanrewaju et al., 2019). C4 species would therefore favour bacterial respiration as the exudates are more abundant in labile materials, whereas C3 plant species would intensify fungal respiration as the exudates contain more stable compounds (Chen et al., 2016). Nonetheless, the authors found that fungal respiration was predominant in both C3 and C4 species. Exudates of the C3 and C4 plants differ in pH and are hence mineralized differently (Tao et al., 2004), most probably by a different rhizobiome. Some of the rhizobiome changes in C3 and C4 plants may be related to the communities that populate the various root systems and their functions. While ectomycorrhiza obtain carbon from plants and participate in carbon exchange between plants, endomycorrhiza exude sugars to the root system (Sasse et al., 2018). Studies suggest that mutant plants that exude higher sugars than their wild types are more susceptible to disease. Therefore, alterations in the exudate composition of a particular crop could potentially have consequences on overall plant health and development. Greater understanding of plant–microbe interactions (e.g. substrate quality and quantity preferences) is needed to estimate the impacts of pests, pathogens, and tritrophic interactions on crops that exhibit altered photosynthetic efficiency. Plant root exudates are also more acidic from C4 plants (Tao et al., 2004), and acidic soils are known for having fewer pathogens, fungi, nematodes, and beneficial bacteria (as cited in Lareen et al., 2016). However, effects on the soil pH were minimal and thus the exudate changes between C3 and C4 plants may not have a major effect on root microbial communities. There have been studies of increasing seed yield by modifying the rhizobiome, but these alterations were related to specific inoculations known to apportion certain nutrients to the plant (e.g. the Sebacinales fungus in barley has been shown to increase yield and tolerance to stress, thus reducing yield losses; Waller et al., 2005).
What would be the effects of rhizobiome changes to stress tolerance (pH, exudate composition)? We could not find studies that analyse the influence of the rhizobiome on grain quality or vice versa, other than the effects of adding biostimulants to the soil. Effects on root form and function, root exudates, and hence the rhizobiome can drastically affect the methane emission values, particularly of rice. With agronomic practices such as alternate wetting and drying and direct-seeding becoming popular, the reaction of the rhizobiome to such practices may or may not remain the same for plants with altered photosynthesis.
Biotic stress
There is support in the literature for the attenuation of water-related abiotic stresses under elevated CO2 (Medina et al., 2016; Li et al., 2017) and increased photosynthesis (Yadav et al., 2018), which might also positively affect heat stress (Shanmugam et al., 2013), but there is little consideration for how the enriched biomass may react to the biotic stresses of pests and pathogens. On the one hand, there are reports that elevated CO2 can prime plant defences against fungal and bacterial pathogens (Mhamdi and Noctor, 2016). The adverse effect of elevated CO2 and temperatures on insect populations may be useful to plants, although altered feeding, fecundity, survival, and dispersal mechanisms may have a compensatory role (Trębicki et al., 2017). On the other hand, there are reports that increased biomass, due to increased photosynthesis, may be more prone to pests and pathogens (Ghini et al., 2008). There is indeed evidence that reducing photosynthesis may be a useful defence mechanism against biotrophic pathogens (Garavaglia et al., 2010). Importantly, biomass increase may not all be due to increased photosynthesis, and soil nutrients may have a critical role (Chatzistathis and Therios, 2013). In rice, in one study, an increase in biomass was seen to be inversely proportional to photosynthesis (Jahn et al., 2011). There is a potential trade-off between abiotic stress tolerance and responses to biotic stresses, and further studies are required.
Grain quality
No information could be found on how the improved photosynthesis, if it does translate into increased grain yield, may affect grain quality. These interactions should be modelled for better understanding because grain quality is critical for farmers and consumers. The rise in CO2 levels and the concomitant diminishing of photorespiration are both relevant for increased photosynthesis. However, climate change also comes with potentially adverse effects on crops, mainly through increased temperatures, decreased soil moisture, and a rise in phytotoxic tropospheric ozone (reviewed by Ort and Long, 2003; Long et al., 2005). How such integrated scenarios may affect grain quality remains an important question. An increase in rice grain productivity generally leads to a decrease in quality (Anderson et al., 1998; Dinh et al., 2017). Superior grain quality in rice implies not only more protein, micronutrients, and fibre, but also a range of amylose and amylopectin ratios which depend on regionally preferred sensory parameters of cooking, taste, aroma, and palatability. In rice, grain quality also includes the extent of chalkiness which affects milling. Xu et al. (2015) reported that chalkiness increased and thus the milling qualities decreased with an increase in all three yield components, namely panicle number, the number of grains per panicle, and 1000 grain weight. Similarly, the amylose to amylopectin ratio and protein content suffer with higher yield and elevated CO2, as reported by Jing et al. (2016). Therefore, despite optimizing the source–sink relationships and obtaining higher yields, the product may have poor grain quality and not be commercially viable. For example, grain protein content is sensitive to O3 and CO2 concentrations in the atmosphere. When wheat grain yield was affected by 10% increases in O3 and CO2, the grain protein was 8% and 7.5%, respectively. O3 strongly negatively affects harvest index (Pleijel and Uddling, 2011), whereas it is unaffected by CO2 concentration. The direct negative response in wheat grain protein under elevated CO2 reinforces the hypothesis of abnormal nitrate uptake or assimilation (Pleijel and Uddling, 2011).
With protein content, grain quality suffered a reduction in the content of minerals such as iron and zinc under high CO2, as seen through a modelling study on mineral and protein deficiency diseases in women and children (Smith and Myers, 2018). Such a mineral reduction was predicted to have a drastic effect on hundreds of millions of people globally, with the most affected regions being South and South-east Asia, Africa, and the Middle East. There is increasing evidence of elevated CO2 leading to a decrease in the content of minerals and protein in the grain (Ujiie et al., 2019). Thus, does a rice variety that has more efficient photosynthesis and a higher yield bear nutritionally inferior grains?
A newly developed rice variety, ‘Akiniwara’, is both high yielding and has good quality traits, particularly palatability. This variety has both an increased sink capacity and sink filling, demonstrating that these two traits are compatible. In their study, Yoshinaga et al. (2018) show that there was a minor reduction in perfect grain ratio related to increased sink capacity, and there was also a small increase in grain protein content compared with other high-yielding varieties and the control variety. There was also some evidence of lodging resistance in ‘Akiniwara’ (Yoshinaga et al., 2018).
Concerns around the different aspects of grain quality are a starting point in considering the gaps in the downstream translational and socio-economic aspects of ensuring the research reaches the end-users. Perhaps in the absence of the end-product to date, the downstream gaps may not be pressingly relevant. However, apart from the commercial success, the effects on the environment, and the concomitant translational and transformational value of the product, other downstream considerations must also be taken into account in parallel (Table 2).
Table 2.
Photosynthesis-related traits and pathways engineered for higher yield
| Pathway/ experiments | Genes | Effects | Crop/Plant | Conditions | Productivity increase | Reference |
|---|---|---|---|---|---|---|
| Pathway 1: Escherichia coli glycolate oxidation Pathway 2: glycolate oxidase and malate synthase from plants and catalase from E. coli Pathway 3: Plant malate synthase and a green algal glycolate dehydrogenase (w/o down-regulation of a native chloroplast glycolate transporter in the photorespiratory pathway) | Undisclosed | Pathway 1: increased biomass ~13%. Pathway 2: same as wild type. Pathway 3: increased biomass by 18% (24% with RNAi) | Tobacco | Field | 40% biomass increase | South et al. (2019) |
| TLA: truncated light-harvesting antenna | Su gene-aurea mutation (Su/su mutant) | Reduction of antenna size of the PS with decreased chlorophyll and carotenoids | Tobacco | Greenhouse | 25% increase in stem and yield | Kirst et al. (2017) |
| TLA: truncated light-harvesting antenna | Reduced chlorophyll synthesis (YL mutant) | Smaller antenna size, reduced chlorophyll synthesis, higher thylakoid membrane proteins, increased PSII efficency, high electron transport rate, Rubisco activity and regeneration enhanced | Rice | Field and greenhouse | Similar yield in shorter growth duration. Higher yield in high plant density field conditions | Gu et al. (2015) |
| TLA: truncated light-harvesting antenna | Reduced chlorophyll synthesis (Y11y11, y9y9 mutants) | Reduced chlorophyll content by 50%, increased photosynthetic efficiency and capacity early in the season. Capture less light and lower WUE by mutants impaired effects of lower chlorophyll under drought conditions suffered during the experiment | Soybean | Field | Same yield | Slattery et al. (2017) |
| Xanthophyll cycle and PSII | VDE-violaxanthin de-epoxidase ZEP-zeaxanthin epoxidase PsbS:PSII subunitS | Acceleration of NPQ relaxation and lower DES | Tobacco | Field | 15% increase productivity | Kromdijk et al., 2016 |
| CO2 transporter system | Bicarbonate transporter BicA | BicA transporter localized 75% to thylakoind membranes and 25% to chloroplast envelope. Transporter did not show activity. | Tobacco protoplasts | Controlled conditions | NA Piloting stage | Pengelly et al., 2014 |
| CO2 transporter system | Bicarbonate transporters BicA and StbA | Targeting bicarbonate transporters BicA and StbA to the chloroplast inner envelope membrane | Tobacco | Controlled conditions | NA Piloting stage | Rolland et al. (2016) |
| Rubisco enzyme | Cyanobacterial Rubisco (Gm-rbcL, Gm-rbcS) | Cyanobacterial Rubisco expression enhanced in tobacco under high CO2 Higher carboxylation rates Need to introgress genes coding for vertex proteins and metabolite pore shells for fully functional carboxysomes | Tobacco | Controlled conditions | NA Piloting stage | Occhialini et al. (2016) |
| Carboxysome biogenesis | Carboxysome protein (csoS1A, csoS2) and cyanobacterial Rubisco (cbbL, cbbS) | Assembly of carboxysomes in higher plants to compartmentalize Rubisco and carbonic anhydrase | Tobacco | Controlled conditions | NA Piloting stage | Long et al. (2018) |
| Improve carbon fixation with malyl-CoA–glycerate synthetic pathway (MCG) | Mcl (malyl-CoA lyase), gcl (glyoxylate carboligase), glxR (or GarR) as tartronate semialdehyde reductase, gark (glycerate kinase I), hyi (hydroxypyruvate isomerase), and ppc (PEPC) | Assimilation of glyocoylate to produce acetyl-CoA Enhances bicarbonate assimilation by 2-fold | Synechococcus elongatus | Controlled conditions | Effects not measured on PS or yield | Yu et al. (2018) |
| Procambium formation/auxin pathway | PIN1, MP/ARF5, HD-ZIP III | Induction of vascular formation by auxin maxima. Auxin accumulation at convergence point, auxin flow, maintain meristematic competence in the procambial centre preventing new procambium formation in neighbouring cells | Arabidopsis | Controlled conditions | NA Proof of concept stage | Sedelnikova et al. (2018) |
| Radial patterning/SHR–SCR pathway | NAKED ENDOSPERM1, ZmRVN1, r ZmSCR1, ZmSHR1, OsSHR1, and OsSHR2 | Specific pattern disposition of bundle sheath cells and mesophyll cells in the vascular bundles. Full characterization can take years. | Rice | Controlled conditions | NA Proof of concept stage | Henry et al. (2017); Sedelnikova et al. (2018) |
| Functionalization of vascular sheath cells | GOLDEN2, ZmG2-like1 | Chloroplast development in bundle and sheath cells, chloroplast biogenesis. Induction of sustained development of chloroplasts in the sheath cells with subsequent chlorophyll increase | Rice | Controlled conditions | NA Proof of concept stage | Wang et al. (2017) |
Translational successes and gaps in photosynthesis research
Research on increasing the efficiency of photosynthesis requires extensive laboratory and field facilities and complex equipment, and genetic engineering can be an essential component. Hence, while studies in controlled greenhouses are the most common, there have been a few field studies. Some tests of laboratory-based yield and biomass increase have been taken into field conditions. A recent study on tobacco (López-Calcagno et al., 2019) demonstrated that yield increase is possible by manipulating photorespiration. The biomass increase relative to the control in the greenhouse was stable, but there was high seasonal variability in the field where the biomass increase was between 27% and 47%. By altering photorespiration, glycolate metabolism was again shown to increase tobacco crop biomass in field conditions by 40% relative to the control (South et al., 2019). Although these are exciting developments, will the potential increase in biomass in cereals and other seed crops translate into an increase in grain yield? There are a few earlier studies in wheat (Driever et al., 2017) and soybean (Köhler et al., 2017) where sedoheptulose-1,7-bisphosphatase was overexpressed to enhance photosynthesis and growth. The study on soybean focused on the effect of elevated CO2 on yield in the future climate scenarios, and an 11–22% increase in seed yield in the field was achieved (Köhler et al., 2017). In wheat, there was an increase in both biomass and grains, the latter being 40% higher than in the control under greenhouse conditions. The increase in the number of grains was through an increased number of grains per ear or increase in the number of ears being produced per plant (Driever et al., 2017). These results validate the concept that, whether by elevated CO2 or by changing the mechanistic genetics, a change in photosynthetic efficiency can lead to increasing the grain yield. However, no enhanced varieties have crossed the pilot to commercialization barrier.
A few studies have looked at breaking the yield barrier in rice through altering photosynthesis and photorespiration, with minor success. One of these studies looked into the effects of co-overexpression of the genes of Rubisco and transketolase on photosynthesis in rice, which was greater by 35–53% and 39–84%, respectively, compared with the control (Suzuki et al., 2017). The changes in protein content were related to alterations in the mRNA content. However, increased irradiance and different concentrations of CO2 did not alter the rate of CO2 assimilation between the control plants and those co-overexpressing the genes. Thus, in rice, the co-overproduction of Rubisco and transketolase did not improve photosynthesis. The overproduction of transketolase alone by 80–94% did not affect photosynthesis, suggesting that transketolase does not limit photosynthesis. A recent paper reported a new approach to boost photosynthesis in rice through the glycolate oxidase, oxalate oxidase, and catalase (GOC) bypass, which works by enriching chloroplasts with CO2 that otherwise would be lost during photorespiration (Shen et al., 2019). The GOC bypass converts glycolate to CO2. Rice plants grown in the field showed higher photosynthetic efficiency, and increased biomass and nitrogen content. However, seed yield varied between seasons and even decreased when compared with control plants. Shen et al. (2019) claimed increased grain yield by increased panicle number, while the number of seeds per panicle and the 1000 grain weight was equal to, or less than, that of the plants not engineered in the glycolate metabolism pathway. Unfortunately, from the breeders’ perspective, panicle number per plant is a yield-related trait with the least heritability. Hence, one could argue whether the claimed grain yield increase in the transgenic plants is a stable trait.
Despite advances in research with novel strategies to increase grain yield in rice, the results are not conclusive and are somewhat disappointing for this crop. We still need more evidence about how the plant and components of the crop production and crop management system behave with modified photosynthesis because improving or altering one system has effects on other traits. For example, an increase in biomass in semi-dwarf rice varieties, which exhibit increased photosynthesis, led to an increase in the number of unproductive tillers, limiting the increase in yield (Khush, 2000). Questions remain around the trade-offs between photosynthesis increase and harvest index, NUE and WUE, the carbon:nitrogen ratio, and reactions to biotic and abiotic stresses.
An analysis of some high-yielding rice varieties, for example IR8, revealed that a high rate of photosynthesis was co-selected with high yield (Takai et al., 2013). Additionally, experiments in rice showed that the sink could still be filled 50% more (Sheehy et al., 2008). Therefore, increasing the source through photosynthesis in rice would not immediately be limited by the sink capacity per se. However, achieving the potential of the sink capacity is in turn dependent on other traits such as assimilate transportation and hormonal balances that facilitate assimilate transport, starch synthesis, starch component traits, and rate of dehydration.
Various studies identify related traits that support higher photosynthesis, such as thicker and greener leaves, stay-green top leaves, and flag leaf traits (Biswal and Kohli, 2013). However, an increase in biomass and photosynthesis (whether or not through C4) through higher leaf area would not necessarily provide either higher photosynthesis or higher yield, as the light intensity in the leaves would decrease due to canopy shading and the shade recovery processes. Alternatively, grain weight as a yield component is a trait of high heritability. It did not change much in the given varieties that showed increased yield under FACE study (Hasegawa et al., 2013), suggesting that the emphasis should be on increasing panicle number and spikelets per panicle in the elite varieties. However, there exists large variability in grain weight, underpinning the importance of the multiple variables involved (Xu et al., 2015; Li et al. 2019), and it can be positively or negatively affected in different genotypes, as shown in the case of wheat (Pleijel and Uddling, 2011; Broberg et al., 2019). The relationship between increasing photosynthesis and yield remains unclear in many of the crops of importance to human food and nutrition security in the future, especially when other production factors are suboptimal. Even the effect of elevated CO2 on photosynthesis, biomass increase, and soil nitrogen supply remains to be set on a permanent footing through long-term studies because Reich et al. (2018) noted that trends observed as a response of C3 and C4 grasses to elevated CO2 reversed after 10 years. Their findings challenged the prevailing paradigms and suggested that short-term results may not be predictive of long-term effects.
Potential socio-economic impacts and gaps in photosynthesis research
Research in upstream plant sciences is increasingly required to demonstrate its impact on people, the environment, the planet, and other species. How impactful has photosynthesis research been, or can be, for a farmer or consumer? Does an increase in photosynthesis add value towards creating viable and sustainable ecosystems? Building a strong case for photosynthesis research from these perspectives is more likely to attract resources. Ongoing upstream research on photosynthesis must be analysed to articulate a plausible impact pathway between investment and improved human and planetary prosperity.
Answering an array of questions may be necessary to demonstrate the socio-economic benefits of research on photosynthesis. The fundamental consideration would be the estimated net positive returns for farmers adopting the varieties with improved photosynthesis. This consideration, however, subsumes several questions. For example, in rice, can improvement in photosynthesis reduce farmers’ risk exposure? Can it reduce production costs? Can it generate premium grain quality traits, leading to the premium market price for the farmers? These questions, in turn, depend on dissemination and deployment strategies for the novel materials. For the most part, the programmes targeting material (technology) production and adoption tend to be rather linear and assume homogeneity of adopters. However, societies and economies are heterogeneous, and so are farm system vulnerabilities and exposure to risks (Lobell and Tebaldi, 2014). For example, remote locations limit access to markets (Barrett et al., 2015), specific ecosystems limit access to natural resources, and restrictive economic surroundings and systems limit the capacity to thrive in increasingly sophisticated and complex cultivation and market systems (Reardon and Timmer, 2012; Béné et al., 2019a, b). Hence, it is essential to consider policies that can maximize the positive effects, mitigate the negative consequences, and enable equitable distribution of additional wealth, especially among farmers. Farmers’ income can increase in three ways: (i) more production per unit and more sales at constant price; (ii) higher price per unit and/or savings through reduced input costs at constant volume; and (iii) change of the farming model by shifting to higher value crops or stepping out of farming altogether due to restrictive labour and input costs (Timmer, 1988; Dorward et al., 2009; Reardon and Timmer, 2014; Barrett et al., 2018). This third pathway is not relevant to the discussion on the socio-economic benefits of advances in photosynthesis.
The first option targets the production frontier. Constraints on land availability and the decreasing productivity of agricultural land (Huston, 2005; Mazoyer and Roudart, 2006) imply that increased production (and sales) must rely on filling the crop yield and labour productivity gap. However, unlike the Green Revolution paradigm of more food for more people on more land, today the market and social forces demand not just more food but also more nutritious food cultivated through sustainable and inclusive agricultural practices (Simelton et al., 2012; Pingali, 2015). The hope is that advances in photosynthesis may lead to farm-level increases in ‘healthier’ yield at no or reduced cost to the environment. Figure 1 captures how more efficient photosynthesis contributes to improved ‘use efficiencies’ for critical resources that are being depleted or contaminated through conventional agricultural practices.
The second pathway to higher income suggests operating on, or even moving beyond, the price frontier, perhaps through innovations in input cost reduction. For example, adopting genetically engineered (GE) plants for herbicide tolerance can save the manual labour costs associated with weeding such that on the same piece of land the same volume of yield is now produced more cheaply, but the produce sells at the same market price. Barrows et al. (2014) studied the impacts of GE technology on agricultural supply and land use. They found that maize prices would have been 5–19% higher without cultivating GE crops, soybean would have been 19–33% higher, and cotton 9–17% higher. By increasing yields and reducing pest and weed control input costs, GE crop production helps control market prices. Reducing losses and waste during the production cycle also adds to the income.
Research on climate-smart technologies and practices (Campbell et al., 2014; Steenwerth et al., 2014) indicates that it is possible to increase input efficiency while also improving the socio-economic outcomes for farmers (Branca et al., 2012; Karfakis et al., 2012; ). The FACE studies of improved photosynthesis with elevated CO2 demonstrate improved NUE and WUE. Engineering more efficient photosynthesis under ambient CO2 concentrations may thus contribute to reducing such input costs. However, there is growing political pressure to re-internalize the externalities associated with intensive agriculture and there needs to be a more in-depth and holistic cost–benefit analysis. Thus, proper costing of a diminishing local water-table, poor soil and air quality, and loss of biodiversity associated with agriculture (Pingali, 2012) will inevitably translate into higher production costs (Benton and Bailey, 2019). Alternatively, as an example, reduction in one or more of the GHGs (CO2, CH4, and NO2) emissions resulting from more photosynthetically efficient plants could prove extremely valuable from both economic and environmental standpoints. Extensive modelling studies on the multiple parameters feeding into making increased photosynthesis successful must be complemented by socio-economic models to improve policy recommendations for impact, as recently exemplified by the resource-constrained scenarios (Kruseman et al., 2020).
Since efficient photosynthesis can increase NUE, a parallel for fertilizer may be visualized by considering that between 1996 and 2014, herbicide and pesticide applications on GE crop fields were reduced by 8.2% (by active ingredient). The decrease in the Environmental Impact Quotient (Kovach et al., 1992) indicator was 18.5% (Brookes and Barfoot, 2018). With fewer chemicals entering the environment, there is less contamination of water bodies and less damage to the environment and biodiversity (Mannion and Morse, 2012). Other environmental benefits arising from higher photosynthetic crop adoption include reduced fossil fuel use from application of fewer inputs and probably reduced soil cultivation contributing to lower GHG emissions (Brookes and Barfoot, 2018).
Techno-economic development, and especially the predicted decrease in the numbers of poor, small-scale farmers, calls for a clear vision on the ‘who, when, why, and how’ of the consumer of crops with improved photosynthesis. Product profiles should be the guiding principle for the next phase of photosynthesis research to ensure socio-cultural, environmental, and economic relevance of the final product. In effect, photosynthesis is probably the best understood basic biological process with potentially the highest likelihood of delivering a useful product. However, it is a highly complex process and we may spend another few decades only understanding it better, without heading towards and delivering a tangible product. Thus, using the process of photosynthesis to make a quantum difference in agricultural productivity has to be embedded in a multidisciplinary and holistic, yet pragmatic, analysis to guide large-scale projects. An approach that consists of starting with upstream sciences and later on exploring the field agronomy, markets, and political forces is far too linear. Instead, data on such downstream aspects should be generated in parallel to maximize the chances of final commercial success of the research product and that the required quantum leap in agricultural productivity is achieved sooner rather than later.
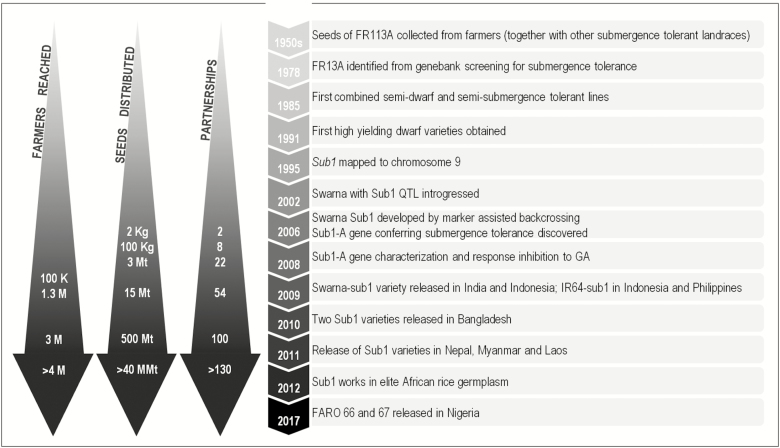
One good example to consider for how an integrative approach of parallel projects realized the vision of a product is submergence-tolerant rice, which is now a part of the farmer and customer base. Figure 2 shows a timeline for the development of the submergence-tolerant Sub-1 rice. It took nearly 40 years (1950–1990), since the identification of the tolerant phenotype in some rice accessions, to obtain a semi-dwarf, semi-tolerant rice line. However, within a little more than 20 years of the identification of the QTL, the downstream research and development could be achieved by involving a large array of stakeholders. Marker generation, marker-assisted backcrossing, marker-assisted selection, gene cloning, gene characterization, gene-based marker-mediated trait introgression, variety development, multienvironment testing, variety release, tolerant lines in elite local genotypes of multiple countries, seed dissemination in countries over three continents, and even impact analysis of the amounts of seed produced and number of farmers reached over time could be conducted thanks to an international collaboration network. Such progress was due to mega-projects involving multiple stakeholders right from genetics and genomics to local seed-distributing non-government organizations. The ex post analysis revealed an additional farmer income of nearly US$200 ha–1 (unpublished results). The additional income was used, among other expenses, to pay for children’s education—a transformative change. The important discriminator for success was not the search for a submergence-tolerant phenotype per se but for yield under submergence (David MacKill, personal communication). A similar approach of yield under drought has led to the identification of a number of large effect QTLs (Swamy et al., 2011) some of which have now been pyramided (Kumar et al., 2018), and the lines are a part of the breeding pipeline to develop drought-tolerant rice varieties. One of the QTLs has been extensively studied at the molecular level to highlight its complex functional nature (Dixit et al., 2015; Raorane et al., 2015a, b). The highlight in both submergence and drought tolerance research is the identification of QTLs for yield under stress. A similar approach for photosynthesis could target a panel of high- and low-yielding cereal genotypes with direct proportionality to photosynthesis (since there can be other avenues to yield increase; Jahn et al., 2011). With an extensive array of molecular–physiological understanding, available omics data, and the advantage of an expert upstream research consortium already operative, any quantitative genetics discovery could be quickly exploited for characterization and further utilization in the breeding pipeline. Hence, the two main factors contributing to success would be the investment in collaboration with downstream breeders, agronomists, and socio-economic experts, and the identification of clear time- and stage-bound deliverables to allocate resources optimally. For example, if no strong QTLs could be identified soon, it would be an indication that there is not much allelic variation in the genes involved. Such a result may suggest the need for higher order mechanisms such as the protein post-translational modification or protein–protein interaction networks. Simultaneously, physiologists and agronomists could be looking at the same diversity panel for clues to suggest target mechanisms such as NUE, WUE, and source–sink relationships to be more deeply explored. The product profile data for target eco-geographies, policy research, and ex ante socio-economic surveys would provide information on the scope and target scenarios for success of the product in the pipeline in order to maximize the impact of research. Collaboration with groups at the country level would take the product through local trials and prime the seed sector and other crucial partnership, while at the same time involving governments and other local organizations.
Fig. 2.
Timeline of the development of the Sub1 flood-tolerant varieties from experiment to scaling up to impact. As developments on the research aspect of the Sub1 gene mechanistics and technology progressed, various advances evolved at the partnership and capacity development levels. Beyond scientific partnerships collaborating for product research and development, in 2006 National Agricultural Research and Education Systems were involved to scale up the seed dissemination capacity. Partnerships increased not only in number, but also in kind, and, by 2008, NGOs, farmer organizations, and private seed companies were also on board. Soon to follow, national government programmes and state governments, public and private seed companies, and international partners were also actively participating in extension, capacity building, and seed delivery systems. The enterprise was such a success that in 2013, 40 000 t of seeds were produced and 4 million farmers were adopting Sub1 varieties in various countries in South and South-east Asia. In the figure: kg=kilograms, T=tonnes, MT=‘000 tonnes, k=1000, M=million. After Bailey-Serres et al. (2010) and Mackill et al. (2012).
Concluding remarks
Despite incremental evidence for its potential, research on mechanistically improving photosynthesis efficiency is still insufficient to ensure its value in creating more grain, seed, or fruit yield. Recent literature shows there needs to be an assessment of the pros and cons of improving the single or dual cell photosynthesis systems. Unlike the ‘Green Revolution’, technologies for increasing agricultural productivity now must factor in additional economic, social, cultural, and political aspects to ensure success. More importantly, whether it is converting C3 crops into C4 crops, or only increasing the photosynthetic efficiency of C3 plants, a parallel multistakeholder approach is more likely to succeed. The information on the mechanisms and pathways of photosynthesis, gained especially in the last decade when the upstream photosynthesis science is conducted with a vision of the downstream product, allows scientists to understand better how to mine our genetic resources to improve crop yields. It is also critical to assess if the elevated CO2 of the future may be the source for highly efficient photosynthetic plants, what the life cycle assessment route for the planetary boundaries would be in the altered eco-system, and how that would be sustainable. Now is the time to consider what the real benefits will be, and to whom they may accrue. There are apparent trade-offs, most obviously in what is known to be physiologically connected to photosynthesis improvements such as the NUE and WUE. How can these be balanced but, more importantly, are these the only connected parameters? Could other perturbations render the advancements commercially non-viable, such as grain quality, root structure, and function, rhizobiome, biotic and abiotic stress responses, etc.? Will grain quality diminish under a high CO2 and/or high temperature climate? How does that compare with a CO2 increase mechanistically within the plant cells? This information is not currently available. How does the present state-of-the-art in gene editing, with respect to technologies and regulatory needs now and in the future, factor into the acceptance or otherwise of the GE crops? Hence, what timelines and boundaries exist to assess the viability of current or future technical approaches? How valid and valuable is the translational value of the resources and efforts spent upstream? We cannot wait to have the full knowledge and understanding of an increasingly complex food system to start tinkering with it, but equally we do not have the luxury of continual research without linking it to socio-economically feasible and acceptable products.
Increased photosynthesis primarily translates into biomass increase but may increase grain or seed yield. Where the biomass is the product, the chances of success are high. Success with conventional mechanisms to further increase production in the field has, to some extent, plateaued. Based on FACE studies, a breakthrough seems plausible from the modification of photosynthesis by GE mechanisms. Such a breakthrough could have both positive and negative effects on the farmers as they should expect, for this increased productivity and profitability, to pay higher prices for seed of GE varieties as a result of the cost of stringent stewardship; they will need to become more vertically integrated to obtain GE seeds and may need to apply more fertilizer (mostly nitrogen) to feed the increased biomass.
Breakthroughs in upstream research such as improved photosynthesis need to be understood and assessed in the broader context. The success underpinning the ‘Green Revolution’ in improving the farmers’ livelihoods (Hazell, 2009; Pingali, 2012) is embedded not only in the improved plants, but also in the use of recommended crop management practices, increased use of fertilizer inputs, and improvements in infrastructure made by governments (Smale, 2016). Unfortunately, many farmers of the ‘Green Revolution’ have remained poor because at that time ‘poverty disaggregation’ was not considered broadly. Thus, improved photosynthetic research requires a phased, yet parallel, integrated approach, not just at the level of multidisciplinary science teams, but at the level of multiple stakeholders in both the public and private sectors. It needs to ensure that the breakthroughs from upstream photosynthesis research can translate into the form of increased crop productivity, more stable farm gate prices, increased farmers’ income, as well as improved consumer satisfaction.
Internalizing and transferring such technologies and products into national agricultural systems cannot happen without additional investments in both upstream and downstream research. Such direct participation in dissemination or deployment is necessary with the proactive review and negotiations for international legal instruments that facilitate an equitable distribution of financial value embedded in novel intellectual property or existing traditional knowledge (Rao, 2017). Public–private platforms show the way forward for the governments of developing countries to act in the interest of resource-poor farmers.
Acknowledgements
The authors thank Dr Russell Reinke for a review of the manuscript and useful comments to improve it. Reports on results from the C4 Rice Consortium at IRRI are acknowledged as helpful in formulating some ideas for the manuscript. Organizers of the Translational Photosynthesis Conference 2019, Brisbane, are acknowledged for the opportunity raised to come up with the idea of the manuscript. Reports from the CGIAR Research Program on rice agrifood systems which were used for Fig. 2 are acknowledged.
Glossary
Abbreviations
- FACE
free-air CO2 enrichment
- GE
genetically engineered
- GHG
greenhouse gas
- NUE
nitrogen use efficiency
- PEPC
phosphoenolpyruvate carboxylase
- WUE
water use efficiency
References
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–371. [DOI] [PubMed] [Google Scholar]
- Anderson WK, Shackley BJ, Sawkins D. 1998. Grain yield and quality: does there have to be a trade-off? In: Braun HJ, Altay F, Kronstad WE, Beniwal SPS, McNab A, eds. Wheat: prospects for global improvement. Developments in plant breeding 6. Dordrecht: Springer, 249–254. [Google Scholar]
- Bailey-Serres J, Fukao T, Ronald P, Ismail AM, Heuer S, Mackill D. 2010. Submergence tolerant rice: SUB1’s journey from landrace to modern cultivar. Rice 3, 138–147. [Google Scholar]
- Barrett CB, Christiaensen L, Sheahan M, et al. . 2015. The structural transformation of rural Africa: on the current state of African food systems and rural non-farm economies. Report prepared for the African Economic Research Consortium’s Biannual ResearchWorkshop. http://barrett.dyson.cornell.edu/files/papers/Barrett%20Christiaensen%20Sheahan%20Shimeles%20v2.pdf [Google Scholar]
- Barrett CB, Christian P, Shimeles A. 2018. The processes of structural transformation of African agriculture and rural spaces. World Development 105, 283–285. [Google Scholar]
- Barrows G, Sexton S, Zilberman D. 2014. Agricultural biotechnology: the promise and prospects of genetically modified crops. Journal of Economic Perspectives 28, 99–120. [Google Scholar]
- Béné C, Oosterveer P, Lamotte L, et al. . 2019. a When food systems meet sustainability—current narratives and implications for actions. World Development 113, 116–130. [Google Scholar]
- Béné C, Prager SD, Achicanoy HAE, Toro PA, Lamotte L, Cedrez CB, Mapes BR. 2019b Understanding food systems drivers: a critical review of the literature. Global Food Security 23, 149–159. [Google Scholar]
- Benton TG, Bailey R. 2019. The paradox of productivity: agricultural productivity promotes food system inefficiency. Global Sustainability 2, e6. [Google Scholar]
- Biswal AK, Kohli A. 2013. Cereal flag leaf adaptations for grain yield under drought: knowledge status and gaps. Molecular Breeding 31, 749–766. [Google Scholar]
- Bonnet C. 1754. Recherches sur l’usage des feuilles dans les plantes : et sur quelques autres sujets relatifs à l’histoire de la vegetation. Göttingen: E. Luzac, fils. [Google Scholar]
- Borrás L, Slafer GA, Otegui ME. 2004. Seed dry weight response to source–sink manipulations in wheat, maize, and soybean: a quantitative reappraisal. Field Crops Research 86, 131–146. [Google Scholar]
- Branca G, Tennigkeit T, Mann W, Lipper L. 2012. Identifying opportunities for climate-smart agriculture investments in Africa. Rome: FAO. [Google Scholar]
- Broberg MC, Hogy P, Feng Z, Pleijel H. 2019. Effects of elevated CO2 on wheat yield: non-linear response and relation to site productivity. Agronomy 9, 243–261. [Google Scholar]
- Brookes G, Barfoot P. 2018. Environmental impacts of genetically modified (GM) crop use 1996–2016: impacts on pesticide use and carbon emissions. GM crops & food. Biotechnology in Agriculture and the Food Chain 9, 109–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SJ, Hibberd JM. 2015. Insights into C4 metabolism from comparative deep sequencing. Current Opinion in Plant Biology 25, 138–144. [DOI] [PubMed] [Google Scholar]
- Cai C, Li G, Di L, et al. . 2020. The acclimation of leaf photosynthesis of wheat and rice to seasonal temperature changes in T-FACE environments. Global Change Biology 26, 539–556. [DOI] [PubMed] [Google Scholar]
- Cai C, Li G, Yang H, et al. . 2018. Do all leaf photosynthesis parameters of rice acclimate to elevated CO2, elevated temperature, and their combination, in FACE environments? Global Change Biology 24, 1685–1707. [DOI] [PubMed] [Google Scholar]
- Cai C, Yin X, He S, et al. . 2016. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Global Change Biology 22, 856–874. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Thornton P, Zougmoré R, van Asten P, Lipper L. 2014. Sustainable intensification: what is its role in climate-smart agriculture? Current Opinion in Environmental Sustainability 8, 39–43. [Google Scholar]
- Chatzistathis T, Therios I. 2013. How soil nutrient availability influences biomass and how biomass stimulation alleviates heavy metal toxicity in soils: the cases of nutrient use efficient genotypes and phytoremediators respectively. Biomass Now: Cultivation and Utilization. InTechOpen. [Google Scholar]
- Chen J, Wang Q, Li M, Liu F, Li. 2017 W. 2016. Does the different photosynthetic pathway of plants affect soil respiration in a subtropical wetland? Ecology and Evolution 6, 8010–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NC, Tardieu F, Tuberosa R. 2008. Quantitative trait loci and crop performance under abiotic stress: where do we stand? Plant Physiology 147, 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degener JF. 2015. Atmospheric CO2 fertilization effects on biomass yields of 10 crops in northern Germany. Frontiers in Environmental Science 3, 48. [Google Scholar]
- Dinh TH, Watanabe K, Takaragawa H, Nakabaru M, Kawamitsu Y. 2017. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Production Science 20, 412–422. [Google Scholar]
- Dixit S, Kumar Biswal A, Min A, et al. . 2015. Action of multiple intra-QTL genes concerted around a co-localized transcription factor underpins a large effect QTL. Scientific Reports 5, 15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward AR, Kirsten JF, Omamo SW, Poulton C, Vink N. 2009. Institutions and the agricultural development challenge in Africa. In: Kirsten JF, Dorward AF, Poulton C, Vink N, eds. Institutional economics perspectives on African agricultural development. Washington DC: International Food Policy Research Institute, 3–34. [Google Scholar]
- Driever SM, Kromdijk J. 2013. Will C3 crops enhanced with the C4 CO2-concentrating mechanism live up to their full potential (yield)? Journal of Experimental Botany 64, 3925–3935. [DOI] [PubMed] [Google Scholar]
- Driever SM, Simkin AJ, Alotaibi S, et al. . 2017. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philosophical Transactions of the Royal Society B: Biological Sciences 372, doi: 10.1098/rstb.2016.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q, Liu T, Jiao X, Song X, Zhang J Li J.. 2019. Leaf anatomical adaptations have central roles in photosynthetic acclimation to humidity. Journal of Experimental Botany 70, 4949–4962, [DOI] [PubMed] [Google Scholar]
- Evans JR, Clarke VC. 2019. The nitrogen cost of photosynthesis. Journal of Experimental Botany 70, 7–15. [DOI] [PubMed] [Google Scholar]
- Garassino F. 2018. First hints on the genetic basis of the extreme photosynthesis of Hirschfeldia incana. MSc thesis, Wageningen Agriculture University. [Google Scholar]
- Garavaglia BS, Thomas L, Gottig N, Zimaro T, Garofalo CG, Gehring C, Ottado J. 2010. Shedding light on the role of photosynthesis in pathogen colonization and host defense. Communicative & Integrative Biology 3, 382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghini R, Hamada E, Bettiol W. 2008. Climate change and plant diseases. Scientia Agricola 65, 98–107. [Google Scholar]
- Gielen B, Calfapietra C, Lukac M, et al. . 2005. Net carbon storage in a poplar plantation (POPFACE) after three years of free-air CO2 enrichment. Tree Physiology 25, 1399–1408. [DOI] [PubMed] [Google Scholar]
- Głowacka K, Kromdijk J, Kucera K, Xie J, Cavanagh AP, Leonelli L, Leakey ADB, Ort DR, Niyogi KK, Long SP. 2018. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nature Communications 9, 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Zhou T, Luo J, et al. . 2015. An-2 encodes a cytokinin synthesis enzyme that regulates awn length and grain production in rice. Molecular Plant 8, 1635–1650. [DOI] [PubMed] [Google Scholar]
- Gu J, Yin X, Stomph TJ, Struik PC. 2014. Can exploiting natural genetic variation in leaf photosynthesis contribute to increasing rice productivity? A simulation analysis. Plant, Cell & Environment 37, 22–34. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Sakai H, Tokida T, et al. . 2013. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Functional Plant Biology 40, 148–159. [DOI] [PubMed] [Google Scholar]
- Häusler RE, Hirsch HJ, Kreuzaler F, Peterhänsel C. 2002. Overexpression of C4-cycle enzymes in transgenic C3 plants: a biotechnological approach to improve C3-photosynthesis. Journal of Experimental Botany 53, 591–607. [DOI] [PubMed] [Google Scholar]
- Hazell PBR. 2009. The green revolution. IFPRI discussion paper 00911. Washington DC: International Food Policy Research Institute. [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber APM, Lercher MJ. 2014. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a mount Fuji fitness landscape. Cell 153, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Henry S, Dievart A, Divol F, Pauluzzi G, Meynard D, Swarup R, Wu S, Gallagher KL, Périn C. 2017. SHR overexpression induces the formation of supernumerary cell layers with cortex cell identity in rice. Developmental Biology 425, 1–7. [DOI] [PubMed] [Google Scholar]
- Ho LC, Shaw AF, Hammond JBW, Burton KS. 1983. Source–sink relationships and carbon metabolism in tomato leaves 1. 14C assimilate compartmentation. Annals of Botany 52, 365–372. [Google Scholar]
- Huang L, Zhang R, Huang G, et al. . 2018. Developing superior alleles of yield genes in rice by artificial mutagenesis using the CRISPR/Cas9 system. The Crop Journal 6, 475–482. [Google Scholar]
- Hubbart S, Peng S, Horton P, Chen Y, Murchie EH. 2007. Trends in leaf photosynthesis in historical rice varieties developed in the Philippines since 1966. Journal of Experimental Botany 58, 3429–3438. [DOI] [PubMed] [Google Scholar]
- Huston MA. 2005. The three phases of land-use change: implications for biodiversity. Ecological Applications 15, 1864–1878. [Google Scholar]
- Huzisige H, Ke B. 1993. Historical corner dynamics of the history of photosynthesis research. Photosynthesis Research 38, 185–209. [DOI] [PubMed] [Google Scholar]
- Ishikawa C, Hatanaga T, Misoo S, Fukayama H. 2009. Screening of high kcat Rubisco among Poaceae for improvement of photosynthetic CO2 assimilation in rice. Plant Production Science 12, 345–350. [Google Scholar]
- Jahn CE, Mckay JK, Mauleon R, Stephens J, McNally KL, Bush DR, Leung H, Leach JE. 2011. Genetic variation in biomass traits among 20 diverse rice varieties. Plant Physiology 155, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janaiah A, Otsuka K, Hossain M. 2006. Is the productivity impact of the green revolution in rice vanishing? Empirical evidence from TFP analysis. Economic and Political Weekly 40, 5596–5600. [Google Scholar]
- Jing LQ, Wu YZ, Zhuang ST, Wang YX, Zhu JG, Wang YL, Yang LX. 2016. Effects of CO2 enrichment and spikelet removal on rice quality under open-air field conditions. Journal of Integrative Agriculture 15, 2012–2022. [Google Scholar]
- Kamara N, Asante MD, Akromah R, Kamara CS. 2017. Genetic analysis of yield and yield components in Oryza sativa × Oryza sativa cross. African Crop Science Journal 2, 539–550. [Google Scholar]
- Karfakis P, Lipper L, Smulders M. 2012. The assessment of the socio-economic impacts of climate change at the household level and policy implications. Rome: Agricultural Development Economics Division FAO. [Google Scholar]
- Kato T. 1997. Selection response for the characters related to yield sink capacity of rice. Crop Science 37, 1472–1475. [Google Scholar]
- Keller B, Vass I, Matsubara S, Paul K, Jedmowski C, Pieruschka R, Nedbal L, Rascher U, Muller O. 2019. Maximum fluorescence and electron transport kinetics determined by light-induced fluorescence transients (LIFT) for photosynthesis phenotyping. Photosynthesis Research 140, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khumsupan P, Donovan S, McCormick AJ. 2019. CRISPR/Cas in Arabidopsis: overcoming challenges to accelerate improvements in crop photosynthetic efficiencies. Physiologia Plantarum 166, 428–437. [DOI] [PubMed] [Google Scholar]
- Khush GS. 2000. Strategies for increasing the yield potential of rice. In: Sheehy JE, Mitchell PL, Hardy B, eds. Redesigning rice photosynthesis to increase yield. Proceedings of the workshop on the quest to reduce hunger: redesigning rice photosynthesis. Amsterdam: Elsevier Science B.V., 207–212. [Google Scholar]
- Kim HY, Lieffering M, Kobayashi K, Okada M, Miura S. 2003. Seasonal changes in the effects of elevated CO2 on rice at three levels of nitrogen supply: a free-air CO2 enrichment (FACE) experiment. Global Change Biology 9, 826–837. [Google Scholar]
- Kimball B. 2011. Lessons from FACE: CO2 effects and interactions with water, nitrogen and temperature. In: Hillel D, Rosenzweig C, eds. Handbook of climate change and agroecosystems Vol. 1 of ICP series on climate change impacts, adaptation, and mitigation. London: Imperial College Press, 87–108. [Google Scholar]
- Kirst H, Gabilly ST, Niyogi KK, Lemaux PG, Melis A. 2017. Photosynthetic antenna engineering to improve crop yields. Planta 245, 1009–1020. [DOI] [PubMed] [Google Scholar]
- Klümper W, Qaim M. 2014. A meta-analysis of the impacts of genetically modified crops. PLoS One 9, e111629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler IH, Ruiz-Vera UM, VanLoocke A, Thomey ML, Clemente T, Long SP, Ort DR, Bernacchi CJ. 2017. Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. Journal of Experimental Botany 68, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J, Petzoldt C, Degni J, Tette J. 1992. A method to measure the environmental impact of pesticides. New York Food and Life Sciences Bulletin 192, 2–8. [Google Scholar]
- Krishnan P, Ramakrishnan B, Reddy KR, Reddy VR. 2011. High-temperature effects on rice growth, yield, and grain quality. Advances in Agronomy 111, 87–206. [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. [DOI] [PubMed] [Google Scholar]
- Kruseman G, Bairagi S, Komarek AM, Milan AM, Nedumaran S, Petsakos A, Prager S, Yigezu YA. 2020. CGIAR modeling approaches for resource constrained scenarios: IV Models for analyzing socio-economic factors to improve policy recommendations. Crop Science doi: 10.1002/csc2.20114. [DOI] [Google Scholar]
- Ku MSB, Cho DH, Li X, Jiao DM, Pinto M, Miyao M, Matsuoka M. 2001. Introduction of genes encoding C4 photosynthesis enzymes into rice plants: physiological consequences. In: Goode JA, Chadwich D, eds. Rice biotechnology: improving yield, stress tolerance and grain quality. New York: John Wiley & Sons, 100–116. [DOI] [PubMed] [Google Scholar]
- Kubis A, Bar-Even A. 2019. Synthetic biology approaches for improving photosynthesis. Journal of Experimental Botany 70, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Sandhu N, Dixit S, Yadav S, Swamy BPM, Shamsudin NAA. 2018. Marker-assisted selection strategy to pyramid two or more QTLs for quantitative trait-grain yield under drought. Rice 11, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lareen A, Burton F, Schäfer P. 2016. Plant root–microbe communication in shaping root microbiomes. Plant Molecular Biology 90, 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey AD, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. 2009. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany 60, 2859–2876. [DOI] [PubMed] [Google Scholar]
- Li H, Wang G, Zheng Q, Li B, Jing R, Li Z. 2014. Genetic analysis of biomass and photosynthetic parameters in wheat grown in different light intensities. Journal of Integrative Plant Biology 56, 594–604. [DOI] [PubMed] [Google Scholar]
- Li R, Li M, Ashraf U, Liu S, Zhang J. 2019. Exploring the relationships between yield and yield-related traits for rice varieties released in China from 1978 to 2017. Frontiers in Plant Science 10, 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Li XN, Yu JJ, Liu FL. 2017. Effect of the transgenerational exposure to elevated CO2 on the drought response of winter wheat: stomatal control and water use efficiency. Environmental and Experimental Botany 136, 78–84. [Google Scholar]
- Liu X, Fan Y, Mak M, et al. . 2017. QTLs for stomatal and photosynthetic traits related to salinity tolerance in barley. BMC Genomics 18, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell DB, Tebaldi C. 2014. Getting caught with our plants down: the risks of a global crop yield slowdown from climate trends in the next two decades. Environmental Research Letters 9, 074003. [Google Scholar]
- Long BM, Hee WY, Sharwood RE, et al. . 2018. Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts. Nature Communications 9, 3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP. 1993. The significance of light-limited photosynthesis to crop canopy carbon gain and productivity—a theoretical analysis. In: Abrol YP, Mohanty P, Govindjee, eds. Photosynthesis—photoreactions to plant productivity. Dordrecht: Kluwer, 547–560. [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Morgan PB. 2005. Global food insecurity. Treatment of major food crops with elevated carbon dioxide or ozone under large-scale fully open-air conditions suggests recent models may have overestimated future yields. Philosophical Transactions of the Royal Society B: Biological Sciences 360, 2011–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Marshall-Colon A, Zhu XG. 2015. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66. [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. 2006. Can improvement in photosynthesis increase crop yields? Plant, Cell & Environment 29, 315–330. [DOI] [PubMed] [Google Scholar]
- López-Calcagno PE, Fisk S, Brown KL, Bull SE, South PF, Raines CA. 2019. Overexpressing the H-protein of the glycine cleavage system increases biomass yield in glasshouse and field-grown transgenic tobacco plants. Plant Biotechnology Journal 17, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Huang Y, Sun W, Yu L, Zhu J. 2020. Response of rice yield and yield components to elevated [CO2]: a synthesis of updated data from FACE experiments. European Journal of Agronomy 112, 125961. [Google Scholar]
- Mackill DJ, Ismail AM, Singh US, Labios RV, Paris TR. 2012. Development and rapid adoption of submergence-tolerant (Sub1) rice varieties. Advances in Agronomy 115, 299–352. [Google Scholar]
- Mannion AM, Morse S. 2012. Biotechnology in agriculture: agronomic and environmental considerations and reflections based on 15 years of GM crops. Progress in Physical Geography 36, 747–763. [Google Scholar]
- Masclaux-Daubresse C, Chardon F. 2011. Exploring nitrogen remobilization for seed filling using natural variation in Arabidopsis thaliana. Journal of Experimental Botany 62, 2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer M, Roudart L. 2006. A history of world agriculture. from the neolithic age to the current crisis. London: Earthscan. [Google Scholar]
- Medina S, Vicente R, Amador A, Araus JL. 2016. Interactive effects of elevated [CO2] and water stress on physiological traits and gene expression during vegetative growth in four durum wheat genotypes. Frontiers in Plant Science 7, 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A, Noctor G. 2016. High CO2 primes plant biotic stress defences through redox-linked pathways. Plant Physiology 172, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PL, Sheehy JE. 2006. Supercharging rice photosynthesis to increase yield. New Phytologist 171, 688–693. [DOI] [PubMed] [Google Scholar]
- Miyao M. 2003. Molecular evolution and genetic engineering of C4 photosynthetic enzymes. Journal of Experimental Botany 54, 179–189. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Pataki DE, Körner C, et al. . 2004. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140, 11–25. [DOI] [PubMed] [Google Scholar]
- Occhialini A, Lin MT, Andralojc PJ, Hanson MR, Parry MA. 2016. Transgenic tobacco plants with improved cyanobacterial Rubisco expression but no extra assembly factors grow at near wild-type rates if provided with elevated CO2. The Plant Journal 85, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi A, Takai T, Ida M, Yamamoto T, Arai-Sanoh Y, Yano M, Ando T, Kondo M. 2011. Evaluation of yield performance in rice near-isogenic lines with increased spikelet number. Field Crops Research 120, 68–75. [Google Scholar]
- Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO. 2019. Plant health: feedback effect of root exudates–rhizobiome interactions. Applied Microbiology and Biotechnology 103, 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort RD, Long PS. 2003. Converting solar energy into crop production. In: Chrispeels MJ, Sadava DE, eds. Plant, genes, and crop biotechnology. 2nd edn American Society of Plant Biologists and ASPB Education Foundation, 240–269. [Google Scholar]
- Pengelly JJ, Förster B, von Caemmerer S, Badger MR, Price GD, Whitney SM. 2014. Transplastomic integration of a cyanobacterial bicarbonate transporter into tobacco chloroplasts. Journal of Experimental Botany 65, 3071–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D, Giampietro M. 1994. Food, land, population and the U.S. Economy. Carrying Capacity Network; http://www.dieoff.com/page55.htm. [Google Scholar]
- Pingali PL. 2012. Green revolution: impacts, limits, and the path ahead. Proceedings of the National Academy of Sciences, USA 109, 12302–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingali PL. 2015. Agricultural policy and nutrition outcomes—getting beyond the preoccupation with staple grains. Food Security 7, 583–591. [Google Scholar]
- Pleijel H, Uddling J. 2011. Yield vs quality trade-offs for wheat in response to carbon dioxide and ozone. Global Change Biology 18, 596–605. [DOI] [PubMed] [Google Scholar]
- Rao C. 2017. Biotechnology for farmers welfare and poverty reduction: technologies, impact and policy framework. Agricultural Economics Research Review 30, 241–256. [Google Scholar]
- Raorane ML, Pabuayon IM, Miro B, et al. . 2015. a Variation in primary metabolites in parental and near-isogenic lines of the QTL qDTY12.1: altered roots and flag leaves but similar spikelets of rice under drought. Molecular Breeding 35, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raorane ML, Pabuayon IM, Varadarajan A, et al. . 2015. b Proteomic insights into the role of the large-effect QTL qDTY12.1 for rice yield under drought. Molecular Breeding 35, 139. [Google Scholar]
- Reardon T, Timmer CP. 2012. The economics of the food system revolution. Annual Review of Resource Economics 4, 225–264. [Google Scholar]
- Reardon T, Timmer CP. 2014. Five inter-linked transformations in the Asian agri-food economy: Food security implications. Global Food Security 3, 108–117. [Google Scholar]
- Reich PB, Hobbie SE, Lee TD, Pastore MA. 2018. Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science 360, 317–320. [DOI] [PubMed] [Google Scholar]
- Rolland V, Badger MR, Price GD. 2016. Redirecting the cyanobacterial bicarbonate transporters BicA and SbtA to the chloroplast envelope: soluble and membrane cargos need different chloroplast targeting signals in plants. Frontiers in Plant Science 7, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Tian X, Lynch JP. 2014. Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology 166, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. 2002. Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. Journal of Experimental Botany 53, 609–620. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2016. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. Journal of Experimental Botany 67, 4039–4056. [DOI] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW, Seemann JR. 1987. The nitrogen use efficiency of C3 and C4 plants: III. Leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiology 85, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Sage RF, Zhu XG. 2011. Exploiting the engine of C4 photosynthesis. Journal of Experimental Botany 62, 2989–3000. [DOI] [PubMed] [Google Scholar]
- Sasse J, Martinoia E, Northen T. 2018. Feed your friends: do plant exudates shape the root microbiome? Trends in Plant Science 23, 1360–1385. [DOI] [PubMed] [Google Scholar]
- Schlenker W, Roberts MJ. 2009. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. Proceedings of the National Academy of Sciences, USA 106, 15594–15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MR, Edwards GE. 1981. Photosynthetic capacity and nitrogen use efficiency of maize, wheat and rice: a comparison between C3 and C4 photosynthesis. Journal of Experimental Botany 32, 459–466. [Google Scholar]
- Sedelnikova OV, Hughes TE, Langdale JA. 2018. Understanding the genetic basis of C4 Kranz anatomy with a view to engineering C3 crops. Annual Review of Genetics 52, 249–270. [DOI] [PubMed] [Google Scholar]
- Shanmugam S, Kjaer KH, Ottosen CO, Rosenqvist E, Kumari Sharma D, Wollenweber B. 2013. The alleviating effect of elevated CO2 on heat stress susceptibility of two wheat (Triticum aestivum L.) cultivars. Journal of Agronomy and Crop Science 199, 340–350. [Google Scholar]
- Sheehy JE, Ferrer AB, Mitchell PL, Elmino-Mabilangan A, Pablico P, Dionora MJA. 2008. How the rice crop works and why it needs a new engine. In: Sheehy JE, Mitchell PL, Hardy B, eds. Charting new pathways to C4 rice. Los Baños, Philippines: International Rice Research Institute, 3–26. [Google Scholar]
- Shen BR, Wang LM, Lin XL, et al. . 2019. Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Molecular Plant 12, 199–214. [DOI] [PubMed] [Google Scholar]
- Simelton E, Fraser EDG, Termansen M, et al. . 2012. The socioeconomics of food crop production and climate change vulnerability: a global scale quantitative analysis of how grain crops are sensitive to drought. Food Security 4, 163–169. [Google Scholar]
- Simkin AJ, López-Calcagno PE, Raines CA. 2019. Feeding the world: improving photosynthetic efficiency for sustainable crop production. Journal of Experimental Botany 70, 1119–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Rufty TW, Lewis RS. 2019. Increasing photosynthesis: unlikely solution for world food problem. Trends in Plant Science 24, 1032–1039. [DOI] [PubMed] [Google Scholar]
- Skillman JB, Griffin KL, Earll S, Kusama M. 2011. Photosynthetic productivity: can plants do better? In: Moreno-Pirajan JC, ed. Thermodynamics—systems in equilibrium and nonequilibrium. IntechOpen. [Google Scholar]
- Slattery RA, VanLoocke A, Bernacchi CJ, Zhu XG, Ort DR. 2017. Photosynthesis, light use efficiency, and yield of reduced-chlorophyll soybean mutants in field conditions. Frontiers in Plant Science 8, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale M. 2016. GMOs and poverty: the relationship between improved seeds and rural transformations. Canadian Journal of Development Studies 38, 139–148. [Google Scholar]
- Smith MR, Myers SS. 2018. Impact of anthropogenic CO2 emissions on global human nutrition. Nature Climate Change 8, 834–839. [Google Scholar]
- South PF, Cavanagh AP, Liu HW, Ort DR. 2019. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363, eaat9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwerth KL, Hodson AK, Bloom AJ, et al. . 2014. Climate-smart agriculture global research agenda: scientific basis for action. Agriculture and Food Security 3, 11. [Google Scholar]
- Suzuki Y, Kondo E, Makino A. 2017. Effects of co-overexpression of the genes of Rubisco and transketolase on photosynthesis in rice. Photosynthesis Research 131, 281–289. [DOI] [PubMed] [Google Scholar]
- Swamy BP, Vikram P, Dixit S, Ahmed HU, Kumar A. 2011. Meta-analysis of grain yield QTL identified during agricultural drought in grasses showed consensus. BMC Genomics 12, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Adachi S, Taguchi-Shiobara F, et al. . 2013. A natural variant of NAL1, selected in high-yield rice breeding programs, pleiotropically increases photosynthesis rate. Scientific Reports 3, 2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Kondo M, Yano M, Yamamoto T. 2011. A quantitative trait locus for chlorophyll content and its association with leaf photosynthesis in rice. Rice 3, 172–180. [Google Scholar]
- Taniguchi Y, Ohkawa H, Masumoto C, et al. . 2008. Overproduction of C4 photosynthetic enzymes in transgenic rice plants: an approach to introduce the C4-like photosynthetic pathway into rice. Journal of Experimental Botany 59, 1799–1809. [DOI] [PubMed] [Google Scholar]
- Tao S, Liua WX, Chena YJ, et al. . 2004. Evaluation of factors influencing root-induced changes of copper fractionation in the rhizosphere of calcareous soil. Environmental Pollution 129, 5–12. [DOI] [PubMed] [Google Scholar]
- Timmer CP. 1988. The agricultural transformation. In: Chenery H, Srinivasan TN, eds. Handbook of development economics. Elsevier, 275–331. [Google Scholar]
- Trębicki P, Dáder B, Vassiliadis S, Fereres A. 2017. Insect–plant–pathogen interactions as shaped by future climate: effects on biology, distribution, and implications for agriculture. Insect Science 24, 975–989. [DOI] [PubMed] [Google Scholar]
- Ujiie K, Ishimaru K, Hirotsu N, et al. . 2019. How elevated CO2 affects our nutrition in rice, and how we can deal with it. PLoS One 14, e0212840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan PJ, Sage RF. 2012. Effects of low atmospheric CO2 and elevated temperature during growth on the gas exchange responses of C3, C3–C4 intermediate, and C4 species from three evolutionary lineages of C4 photosynthesis. Oecologia 169, 341–352. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Edwards GE, Koteyeva N, Cousins AB. 2014. Single cell C4 photosynthesis in aquatic and terrestrial plants: a gas exchange perspective. Aquatic Botany 118, 71–80. [Google Scholar]
- Vranova V, Rejsek K, Skene KR, Janous D, Formanek P. 2013. Methods of collection of plant root exudates in relation to plant metabolism and purpose: a review. Journal of Plant Nutrition and Soil Science 176, 175–199. [Google Scholar]
- Waller F, Achatz B, Baltruschat H, et al. . 2005. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proceedings of the National Academy of Sciences, USA 102, 13386–13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Guo L, Li Y, Wang Z. 2012. Systematic comparison of C3 and C4 plants based on metabolic network analysis. BMC Systems Biology 6 Suppl 2, S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Khoshravesh R, Karki S, Tapia R, Balahadia CP, Bandyopadhyay A, Quick WP, Furbank R, Sage TL, Langdale JA. 2017. Re-creation of a key step in the evolutionary switch from C3 to C4 leaf anatomy. Current Biology 27, 3278–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way DA, Katul GG, Manzoni S, Vico G. 2014. Increasing water use efficiency along the C3 to C4 evolutionary pathway: a stomatal optimization perspective. Journal of Experimental Botany 65, 3683–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel HJ, Manderscheid R. 2012. Crop growth responses to free air CO2 enrichment and nitrogen fertilization: rotating barley, ryegrass, sugar beet and wheat. European Journal of Agronomy 43, 97–107. [Google Scholar]
- Xu Q, Chen W, Xu Z. 2015. Relationship between grain yield and quality in rice germplasms grown across different growing areas. Breeding Science 65, 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SK, Khatri K, Rathore MS, Jha B. 2018. Introgression of UfCyt c6, a thylakoid lumen protein from a green seaweed Ulva fasciata Delile enhanced photosynthesis and growth in tobacco. Molecular Biology Reports 45, 1745–1758. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S, Heinai H, Ohsumi A, Furuhata M, Ishimaru T. 2018. Characteristics of growth and quality, and the factors contributing to high yield in newly developed rice variety ‘Akidawara’. Plant Production Science 21, 186–192. [Google Scholar]
- Yu H, Li X, Duchoud F, Chuang DS, Liao JC. 2018. Augmenting the Calvin–Benson–Bassham cycle by a synthetic malyl-CoA–glycerate carbon fixation pathway. Nature Communications 9, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zheng G, Shan L, Meng G, Vingron M, Liu Q, Zhu XG. 2014. Reconstruction of gene regulatory network related to photosynthesis in Arabidopsis thaliana. Frontiers in Plant Science 5, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SS, Vanderschuren H, Qaim M, Mahfouz MM, Kohli A, Mansoor S, Tester M. 2019. New plant breeding technologies for food security. Science 363, 1390–1391. [DOI] [PubMed] [Google Scholar]
- Zeng D, Tian Z, Rao Y, et al. . 2017. Rational design of high-yield and superior-quality rice. Nature Plants 3, 17031. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. 2008. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Current Opinion in Biotechnology 19, 153–159. [DOI] [PubMed] [Google Scholar]