Abstract
Background and Aim
In recent years, cognitive dysfunction (CD) in multiple sclerosis (MS) has received increased attention. Neuropsychological tests have been developed allowing to monitor changes in patients’ cognitive functions. Knowledge is lacking, however, about patients’ attitudes towards introducing routine cognitive testing. It was the aim of this qualitative study to explore this.
Materials and Methods
Based on a literature study, semi-structured interview guides were designed and used in qualitative interviews with 12 Danish patients. Participants were selected to represent different perspectives on CD and included patients with relapsing-remitting MS (RRMS) and secondary-progressive MS (SPMS), women and men with varying time since diagnosis and cognitive test scores using the Symbol Digit Modalities Test (SDMT). The data were analysed using a constructivist approach in order to identify significant relations between the quality of life (QoL) impact of CD, and attitudes towards routine cognitive testing.
Results
Most participants reported several subtypes of CD, yet objective CD did not coincide with subjective CD nor did it translate directly into poorer QoL. Overall, CD appeared to have larger impact on the QoL of patients with RRMS and higher SDMT scores, compared to patients with SPMS and lower SDMT scores. The QoL impact of CD manifested itself in the encounter between individual symptoms, expectations, coping and meaningful activities. All patients supported an introduction of routine cognitive testing, but patients with RRMS and SPMS had different main reasons to do so. These were related to supporting research, optimising treatment decisions, and providing documentation of this invisible MS symptom.
Conclusion
All aspects of MS patients’ QoL may be affected by CD. Introducing routine cognitive testing was widely supported by patients in all phases of MS calling for comprehensive care taking both physical and cognitive difficulties into account.
Keywords: multiple sclerosis, cognitive impairment, cognitive testing, quality of life, patient preferences
Plain Language Summary
Multiple sclerosis (MS) is mainly known to involve physical disability. However, many people with MS also experience another invisible symptom: cognitive difficulties such as poor memory, attention or slow thinking. Some feel more irritable or easily stressed than they used to; others find it hard to plan or socialise, for instance.
New cognitive tests will allow doctors to monitor if and which cognitive chances occur in patients with MS. But how do the patients feel about such testing? In this study, we explored MS patients’ experiences with cognitive difficulties and its impact on their quality of life. We examined if this affected their views on introducing routine cognitive testing. Is such testing mainly interesting for medical research purposes? Or do MS patients see a personal benefit in having this aspect of their disease examined?
From in-depth interviews with Danish MS patients, we learned that
Three-quarters of the patients experienced cognitive difficulties
Cognitive difficulties affected the patients’ emotional well-being, relations to other people and daily functioning
All patients’ endorsed routine cognitive testing
- The reasons behind their endorsement were related to
- their type of MS which determines their treatment options
- their adaptation to living with MS which was generally linked to the duration of their disease course
People with MS may find cognitive issues just as disabling as physical symptoms. They have a need for comprehensive care taking all their difficulties into account and support cognitive testing in line with physical examinations when visiting the hospital for routine controls.
Introduction
Cognitive dysfunction (CD) has been reported to occur in 40–70% of MS patients.1–5 CD may be seen at early disease stages and is not directly related to EDSS scores, yet tends to be more severe in progressive patients and to increase with disease duration. Even in mild CD, psychosocial, professional and daily functioning may be affected.3–7 MS-related CD may involve any of the six core functional domains: perceptual-motor function, language, learning and memory, executive function, complex attention and social cognition and emotion regulation.1–3,5,8-10 Sub-types may co-exist, and CD often interacts with physical and affective MS symptoms such as stress, depression, fatigue or pain, hampering a clear establishment of causalities and confounders.2,3,5,8,11-15 CD in MS is thus a complex research field and much is still unclear with respect to both the primary and secondary causes of CD, and the ability of coping and cognitive reserve to mitigate the impact of brain atrophy.1–5,7,11,12,16,17
Various neuropsychological tests have been developed to assess different domains of cognitive functioning.1,3-5,7,17-19 Composite testing using multidimensional outcome measures improves the chances of identifying all relevant deficits, but they are challenging to apply in clinical practice.7,17,19 The brief Symbol Digit Modalities Test (SDMT) is recommended in terms of its sensitivity and clinical applicability. Tablet-based testing will enable electronic transfer of data to patient records allowing to monitor changes over time.1,4,19-21 Consensus is lacking about when changing CD scores are clinically relevant and may lead to switch in treatment.1 It is considered likely that disease-modifying treatments (DMTs) have positive effects through the general reduction in disease activity. Yet cognition has rarely been the primary outcome in clinical trials, and high-quality studies have often been called for.1,3,16,17,19,22 Today, there is general agreement that cognitive functioning should be systematically monitored,1 but knowledge is lacking about MS patients’ attitudes towards introducing routine cognitive testing, which is crucial to successful implementation. Exploring patients’ views in relapsing and progressive phases on this possibility was therefore the main aim of this qualitative study set in Denmark.
As patient preferences are best understood in the context of their disease experiences, our secondary aim was to explore patient experiences with the quality of life (QoL) impact of CD. In later years, it has become increasingly clear that CD may affect MS patients’ social- and family lives, their self-perception and emotional well-being, daily activities and occupational functioning.2,3,5-9,11-16,23–27 Yet, most studies assessing CD-related QoL have focused either on one specific cognitive domain (eg, executive function), and/or the impact of CD on a specific function such as internet shopping.8,9,14,15,18,23,25,26 Only few have explored the perceived overall impact of CD on patients’ QoL from the patient perspective.11 In order to remedy this gap, we aimed to examine this in depth to set the context of patient attitudes to introducing routine cognitive testing.
Materials and Methods
In order to explore in-depth MS patient perceptions of CD and attitudes to routine cognitive testing, we applied qualitative research methods. Patients were included for qualitative interviews until saturation point from the neurology department at the University Hospital of Odense, Denmark.28 To gain different perspectives on MS-related CD, the purpose was to include an equal number of patients with relapsing-remitting MS (RRMS) and secondary-progressive MS (SPMS), a dispersion of women and men, patients with varying time since diagnosis and SDMT scores. Our aim was to include patients with SDMT scores ranging from approximately 20–60 since previous studies in MS cohorts have demonstrated values within this range.29,30 Patients with relevant co-morbidity, eg, depression, or legal/illegal drug use that might affect cognition and patients with current MS exacerbation were excluded.
Eligible patients coming in for check-ups were invited to participate in an individual or group interview according to their preference. Patients with RRMS were asked to carry out the SDMT and invited by a project nurse. Patients with SPMS were included by AT from a pre-existing study cohort using the BICAMS (Brief International Cognitive Assessment for MS) battery that includes the SDMT.18,31 Interested patients were given written study information and consented to be contacted by GLM, who received only their contact information, age, time of diagnosis, and SDMT score. Prior to the interviews, patients gave their written consent to participate. The study was carried out in compliance with the GDPR and did not require Ethics Committee approval in Denmark.
With respect to the primary study aim – patient attitudes towards routine cognitive testing – saturation point was reached after 12 patients had been interviewed: six with RRMS and six with SPMS. The patients were given the option of participating in either an individual or a group interview to ensure a situation that they would be comfortable with. The two interview types yield different types of data, ie, in-depth personal narratives and more socially negotiated accounts, that are both valuable and may supplement each other. Eight patients opted for individual interviews in their homes, one was interviewed in a hospital consultation room, and three participated in a group interview held in a conference room. A literature study formed the design of a semi-structured interview guide.28 Based on Wadel’s construct of focus and framework32 the interviews constituted a first (framework) part to explore patients’ experiences with CD and its QoL impact; followed by a second (focus) part centring on their attitudes towards introducing routine cognitive testing. The interviews thus began with background questions to the participants’ lives prior to their MS diagnosis and today. They were then asked about their MS symptoms and which currently had the highest impact on their QoL, and following this, to describe their cognitive difficulties. The questions moved on to descriptions of the impact of CD on their daily functioning and QoL. In the second part, the interviews first explored the patients’ experiences with cognitive testing; and then focused on their considerations regarding routine cognitive testing.
The interviews were transcribed verbatim and analysed using Nvivo 8 software (QSR International) and a constructivist approach to the relations between meaning and language.33 The participants’ statements were seen as reflecting an ongoing process of making sense of one’s experiences with MS-related CD and associated care needs. This involved an examination of the terminology used to speak about the topics and how they were associated with other issues, creating clusters of meaning. First, the data were coded into topics that were raised during the interviews. Secondly, main themes within each topic were identified, and finally, recurrent connections between topics and themes were analysed. This produced a pattern of the patients’ main experiences with CD and attitudes to regular cognitive testing.
Results
The cohort included eight women and four men aged between 25 and 60 years old. Overall, patients with SPMS had been diagnosed with MS for longer time; they were older, more often disability pensioners and single compared to patients with RRMS. Patients with SPMS had been diagnosed for 9–23 years (mean 18.5) compared to 1–15 years (mean 4.5) in patients with RRMS. All but one patient with SPMS (II-6) had SDMT scores of 19–37, whereas patients with RRMS scored 40–57. Four RRMS patients still worked, if at reduced hours, one was applying for disability pension, and one was on leave from her study due to CD (Table 1). These contextual factors turned out to be closely related to the QoL impact of CD.
Table 1.
Participant Characteristics
| MS Type | Interview Type and Patient No. | SDMT Score at Time of Inclusion | Gender | Age | Time of Diagnosis | Occupational Status | Family Status |
|---|---|---|---|---|---|---|---|
| Secondary progressive MS | II-1 | 19 | M | 56 | 2001 | Disability pension | Single Adult child |
| II-2 | 20 | M | 60 | 1996 | Disability pension | Single | |
| II-3 | 27 | F | 43 | 1999 | Disability pension | Single Child at home Adult child |
|
| II-4 | 34 | M | 53 | 1999 | Disability pension | Married Adult children |
|
| II-5 | 37 | F | 59 | 1998 | Flex job | Married Adult children |
|
| II-6 | 68 | F | 55 | 2010 | Disability pension | Single | |
| Relapsing-remitting MS | II-7 | 40 | F | 40 | 2004 | 16 h/w. flex job | Married |
| FG-8 | 43 | M | 32 | 2016 | Full-time work | Married 2 young children |
|
| II-9 | 45 | F | 55 | 2014 | 34 h/w. work | Co-hab. partner, Adult child |
|
| FG-10 | 48 | F | 46 | 2017 | Unemployed. Applying for disability pension | Single Adult child Teen at home |
|
| FG-11 | 55 | F | 45 | 2018 | Full-time work | Married 2 teens at home |
|
| II-12 | 57 | F | 25 | 2018 | Student (leave) | Partner (not cohabiting) |
Abbreviations: II, Individual interview; FG, focus group; MS, multiple sclerosis; SDMT, Symbol Digital Modalities Test; h/w, hours per week.
Quality of Life Impact of Cognitive Difficulties
During the interviews, it soon became clear that the patients’ understanding of the concept of “cognitive symptoms” were not comprehensive. When asked about their cognitive difficulties, they would typically mention issues with memory, attention, slow thinking or confusion, whereas problems with language, social cognition and emotion regulation only emerged when questioned in more detail. Ultimately, nine patients reported difficulties with memory, attention, expression, learning, spelling, overview, planning, slow thinking, confusion, multi-tasking, stress, coping with large gatherings, fast movement, and flexibility. Many also mentioned being emotionally sensitive, irritable or prone to anger (Table 2).
Table 2.
Participants’ Self-Reported Symptoms
| MS Type | Pt. no. |
All Current Self-Reported MS Symptoms: Shaded Cells Indicate Presence of Problems with the Specific Symptom | Current Self-Reported Cognitive Symptoms (Problems with) |
Subjective Main Symptom Affecting QoL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue | Gait/Balance | Muscle Weakness | Neuropathy/Pain | Spasms/Cramps | Incontinence | Hyperactive Bladder | Poor Sleep | Impaired Vision | Cognitive Difficulties | ||||
| Secondary progressive MS | II-1 | Gait | |||||||||||
| II-2 | Slow thinking, learning, memory, attention, language (straying), structuring, organizing, anger, frustration | Gait | |||||||||||
| II-3 | Confusion, attention, flexibility (changing plans), memory, language (expression), multitasking, mood/emotional sensitivity, anger, stress, overview | Gait | |||||||||||
| II-4 | Poor coping with crowds and noise, attention, irritability, anger | Gait/pain Hyperactive bladder |
|||||||||||
| II-5 | (Minor problems with language and slow thinking when tired) | Gait | |||||||||||
| II-6 | Slow thinking, memory, attention, excessive thoughts, language (expression, prosody), irritability, confusion, (understanding) | Fatigue Hyperactive bladder |
|||||||||||
| Relapsing-remitting MS | II-7 | Language (expression, straying), attention, sensitivity to sensory overload, overview (need for planning and structure), creativity (getting ideas), stress, irritability, memory, multitasking, learning, writing (spelling words and numbers) | Fatigue CD |
||||||||||
| FG-8 | Memory, attention, irritability, overview, (social gatherings) | Neuropathy (legs) | |||||||||||
| II-9 | (Minor memory issues) | Neuropathic pain (legs) | |||||||||||
| FG-10 | Memory, major anger (lacking control of expression), language (expression), attention, difficulties coping with noise and many people, difficulty with speed | Gait/pain (CD) |
|||||||||||
| FG-11 | Memory, planning, teaching, attention, difficulties coping with crowds (eg shopping) and movement around her (eg speed), mental fatigue, overview | Fatigue CD |
|||||||||||
| II-12 | Language (expression), analysis (seeing connections and patterns), overview, slow thinking, attention, memory, multitasking, emotional sensitivity, stress, anger | CD | |||||||||||
Abbreviations: II, Individual interview; FG, focus group; MS, multiple sclerosis; CD, cognitive difficulties.
Some patients had experienced CD from the onset of MS, but most felt their cognitive symptoms had gradually occurred and worsened since their MS diagnosis. One reported having had a cognitive attack (II-7). Cognitive symptoms were described as affecting each other, eg, with patients experiencing stress or anger when getting confused. Other factors aggravating CD were fatigue, time pressure, sensory overload, or loss of control. As such, the manifestation and QoL impact of CD depended on the demands and complexity of the context (eg, being at work or home) and particular situation, eg, caring for a sick child or playing complicated board games with friends.
Overall, CD affected the participants’ emotional well-being, partner and family relations, daily activities, as well as social and professional functioning. For instance, some patients reported that CD made them feel stupid and they worried about future worsening (II-7, II-12) or becoming an ill-tempered nuisance that “nobody wants to be with” (II-6). One had difficulties coordinating her care and poor relations to health-care professionals due to communication issues (FG-10). Most of those having a partner and family felt supported, though conflicts might arise from irritability, misunderstandings or forgetfulness (II-4, II-7, FG-8, FG-10). Two women said that CD was part of the reason they had postponed or decided not to have children as they feared not being up to the task (II-7, II-12). Most had reduced social participation and some were concerned about stigmatisation, eg, from appearing sluggish or dumb (II-7, II-9, II-12). Finally, some patients had lost their jobs due to CD (FG-10, II-2), or were struggling to remain professionally and educationally active (II-7, II-12, FG-11). It is noteworthy that the latter coincided with those reporting CD as a main symptom reducing their QoL. Occupational dysfunction affected patients’ self-esteem as well as their socio-economic situation with some having difficulties obtaining social support as CD is a concealed symptom.
I can’t get a flex [subsidised] job. The municipality won’t help me because I can’t prove that I have 50% disability. It [the CD] doesn’t show, as she [the case worker] said to me. There’s no visible proof. But at least she was honest about it. If I was to reduce my work hours, I had to pay for it myself. (FG-11)
There has to be more focus on the cognitive issues, not just the physical ones. The trouble is when part of what’s wrong with you is invisible to others. ’Oh, it’s probably not that bad’ [others might think]. (FG-10)
I had spoken to the neurologist about feeling slower and having difficulties learning new things and he suggested seeing a neuropsychologist. But I didn’t want to. That would just be another defeat, and I was still working my flex-job. But when I got laid off and needed to apply for a disability pension, I thought I’d better go. After the neuropsychological examination, I was granted pension. (II-2)
When the patients framed their experiences with CD, these were repeatedly described in connection with key activities and roles. In other words, to the patients, cognitive difficulties were part of clusters of meaning setting the context for the QoL impact of CD. The QoL impact depended on which functions were most individually important, eg, caring for children or working. Some patients had important (objective or subjective) CD, yet if this did not prevent them from meaningful activities, the QoL impact was reportedly minor. In contrast, if a patient struggled to hold on to a valued job or participate in social activities due to CD, the presence of cognitive symptoms felt substantial, irrespective of objective CD scores. This was particularly the case for patients with RRMS whose expectations were more often at odds with their capabilities. Impaired function in areas affecting self-perception, social role and identity appeared particularly burdensome.
The QoL impact of CD tended to change in time along with changed priorities and coping. While newly diagnosed RRMS patients often had difficulties accepting the disease, most experienced patients – typically with SPMS – expressed less impact on QoL despite increased CD due to improved adaptation of their everyday life to their capabilities. Hence, a general distinction between patients with RRMS and SPMS appeared.
Following the above, the subjective QoL impact of CD did not appear directly related to objective cognitive scores as measured using the SDMT. On the contrary, among patients reporting CD as an important symptom (II-6, II-7, FG-11, II-12) were three patients with the highest SDMT scores of all participants, including the two most recently diagnosed patients with RRMS. In contrast, none of the SPMS patients with SDMT scores below 40 considered CD to be the MS symptom mostly reducing their QoL; three said they experienced no CD at all (II-1, II-5, II-9). Instead, patients with SPMS considered gait and bladder problems to be main symptoms reducing their QoL.
Patient Attitudes Towards Cognitive Testing
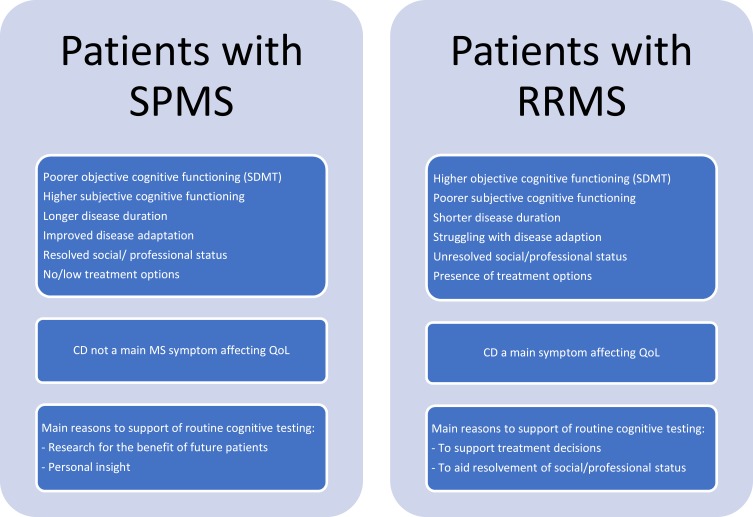
Across MS subtypes, all participants had positive experiences with taking the SDMT, and supported the introduction of routine cognitive testing. The overall position was that this would be relevant and reassuring given that cognitive symptoms are as integral to MS as physical symptoms. All participants had been comfortable being tested in an undisturbed consulting room in the presence of a nurse or neurologist. Albeit admittedly difficult to some, all patients found the SDMT pleasantly quick to carry out. Reasons given for supporting routine cognitive testing were related to treatment, research, financial support and psychological well-being, but overall, the considerations differed between patients with RRMS and SPMS. This was mainly associated with coping and the presence or lack of treatment options (Figure 1).
Figure 1.
Patient experiences with CD and attitudes toward cognitive testing.
Patients with RRMS, in particular, had ambivalent feelings with regard to knowing their cognitive test result and wondered if unawareness was more blissful. Four only wanted this information if it entailed recommended actions or new treatment decisions (II-6, II-7, FG-8, FG-11). They felt their own experiences with CD were more important than a test score. Yet, despite the risk of getting an upsetting result, most would want this information if cognitive testing becomes routine part of check-ups. The hope for cognitive testing to be able to substantiate treatment decisions took precedence to RRMS patients who often struggled with coping. Some explained that uncertainty about their future disease course was a difficult aspect of living with MS. All therefore supported any research providing knowledge about MS and improving predictions. The patients called for better understanding of which types of patients and location of plaques might be related to CD and which treatments might be associated with improved outcomes.
I think it [cognitive testing] is primarily relevant in relation to treatment. Are we on the right path, here? … The test isn’t of much use in itself. All right, now you know that you’ve declined, but what can you do about it? What if these pills aren’t good enough? I would like for them [the doctors] to be more proactive in that respect; that they’d say ‘let’s try these pills instead cause there’s too much activity going on there with you’. (FG-10)
I agree. Unless there are other people that it may be useful for, I mean for the research. But personally, it would make most sense to me if it has a purpose, I mean, to my treatment. (FG-8)
In comparison, long-term patients tended to have improved adaptation to MS. Due to lacking treatment options, patients with SPMS focused less than those with RRMS on treatment. They did support routine cognitive testing for research purposes and the benefit of future patients, however. Above all, patients with SPMS considered knowledge of their test result as a means for personal insight into their cognitive difficulties. Many wanted “evidence” to back their subjective experiences of CD:
I do it with a fear at the back of my head that ‘oh no, I’ll probably just get the documentation that it’s declined further’. But I want to know where I stand, that it’s not just me imagining things, that there’s proof of it. (II-3)
You can’t help getting upset if things are going downhill. I personally wouldn’t want to know if the [cognitive] test just shows that it gets worse and worse. But I want to contribute to the research into how this disease may develop and what can be done to treat it. I mean, if it turns out that all the patients getting one type of medicine have much better cognitive functions. But I don’t personally need to know that I’m doing really badly if there’s nothing to be done about it anyway. That is, unless I get in a situation where I’ll have to apply for disability pension and need to prove that it’s been going downhill. It has to serve some purpose. I mainly see it in relation to work; if at some stage you can’t work anymore. Then it’s nice to be able to say ‘well, this is part of the reason I can’t work, my cognitive problems aren’t getting any better’. (II-7)
Patients across MS subtypes hoped that documentation of CD might ease communication about their difficulties to others. This study suggested a particular need to support patients’ applications for disability pension or a subsidised job. Finally, one patient pointed out that regular testing would have a normalising effect if included in regular controls for all MS-patients (II-2).
If such tests were just standard, people wouldn’t be scared off. I mean, it is another defeat. First, some test shows that you can’t walk, and now, it will also be tested that it’s going downhill mentally or cognitively! But if this test becomes something all patients just do when they come in [for check-ups]… I mean, then it’s not just you. And it is incredibly relevant, just as relevant as a blood sample. (II-2)
No patients were against routine cognitive testing and patients with SPMS found the SDMT less demanding and tiring compared to the other tests included in the BICAMS. Still, three voiced concerns about whether such a short test would properly reflect their CD and whether a misleading test result might negatively affect treatment decisions. Also, some felt that their test performances had varied due to the time of day, fatigue or stress and worried how this might be interpreted. The patients therefore needed thorough information about how test results might be applied. Finally, many called for information about CD from the time of diagnosis including advice on cognitive training and coping strategies.
Discussion
MS-related CD is receiving increased attention and emphasis is growing on incorporating patient perspectives in the development of high quality of care. CD has been shown to constitute one of the most disabling MS symptoms reducing patients’ QoL and functioning.1,3,4 In one study, MS patients ranked cognitive functioning third of 11 important functions due to its impact on social functioning, especially.34 The present study applied qualitative research methods to explore in depth patients’ perspectives on cognitive testing in the context of the perceived impact of CD on their QoL.
Three-quarters of our participants reported a negative impact of CD on psychosocial QoL, daily activities and professional functioning. This confirms the findings of other studies that cognitive functioning is central to successful engagement in activities that give life meaning and to fulfil roles that ensure independence and life satisfaction.15,23,25 Ari and colleagues, for instance, described how patients’ experiences with impairment were not only related to their ability to participate in activities but more importantly to the significance of those activities to their well-being.15
In our sample of patients, subjective CD did not coincide with objective CD as measured using the SDMT.18,20,35 We chose the SDMT because of its high validity and clinical applicability, but clearly, composite testing would provide a more comprehensive objective measure of CD (3,4,7,17,19). Also, as the number of participants was small, our results should be interpreted with caution. They are, however, confirmed by other studies using larger cohorts and other neuropsychological tests. Rosti-Otajärvi and colleagues found that subjective and objective cognitive performance only coincided in approximately half of their sample of 196 MS patients. In their study, patients with RRMS tended to overestimate their CD while patients with more severe disability and progressive disease tended to underestimate their CD compared to objective CD as measured with the BRB-N (Brief Repeatable Battery of Neuropsychological Tests). Supporting our finding that QoL is mainly associated with subjective CD, they also found that mood was mainly associated with the patients’ subjective complaints.35 Along the same lines, Grech et al found that QoL as measured with the MSQOL-54 (Multiple Scleroses Quality of Life-54) in a sample of 107 patients with RRMS and SPMS was closely related with self-reported cognitive symptoms but not with objective CD assessed with various cognitive tests.14 Correspondingly in our cohort, CD primarily reduced the QoL of those with higher SDMT scores, while patients with lower SDMT scores felt relatively less concerned by CD – some not at all or with physical MS symptoms overshadowing CD. The former tended to be related to RRMS, younger age and a life context including children at home, cognitively demanding hobbies or social activities. Those struggling to remain professionally and educationally active were particularly affected by CD. Many patients with longer disease courses – mostly with SPMS, no children at home and disability pension – described improved adaptation of their activities to their capabilities over time, hence reducing the negative impact of CD symptoms on QoL. This supports the suggestion that coping may be more instrumental to QoL than symptoms.12
For our purposes, the key point is that it is subjective rather than objective CD that is associated with QoL.14,35 This study suggests that it is at the encounter between each patient’s preferences, expectations and functioning that the QoL impact of CD manifests itself. This is supported by an earlier explorative study in which the participants always reported MS-related dysfunctions in connection with environmental factors, ie, meaningful activities and participation. This highlights the complex relationship between functioning, disability, personal and contextual factors.13
MS patients’ health-related QoL have previously been shown to influence their care needs.6 In this study, the participants’ positive attitudes towards routine cognitive testing reflected their experiences with CD, disease stage and ensuing needs for comprehensive care taking both physical and cognitive difficulties into account. Therefore, introducing cognitive testing made sense for different reasons, depending especially on the patients’ treatment options and coping skills. Hence, participants with RRMS mainly supported any research into MS-related CD that might substantiate treatment decisions. Patients with SPMS were also supportive of research into MS-related CD and found the SDMT easily acceptable due to the simplicity of taking it. Still, lacking treatment options, they mainly endorsed cognitive testing as a means for personal insight and “proof” of their experienced cognitive difficulties. This study suggests an unmet need for documentation of this invisible symptom to support patients’ dealings with the social and health-care system, in particular. CD can reduce patients’ self-management skills including the ability to cope with MS and communicate relevantly with social and health-care professionals.3,6,12
Neuropsychological performance only partly explains patients’ real-world functioning, which remains a challenge to assess.1,13,23 It is our contention that qualitative research methods focusing on the ways patients make sense of their experiences may produce important insights into this complex research field. Hence, we focused on patient perceptions on the total impact of CD on their QoL in their environmental context, rather than assessing isolated cognitive domains or functions. This allowed us to explore the reasons behind patients’ attitudes towards introducing routine cognitive testing. While this approach allows for an in-depth understanding, it also has some limitations. Being a qualitative study with relatively few study participants, it allows for analytical but not statistical generalisation.36 We did, however, aim to include patients representing variation with respect to MS subtype, gender, time since diagnosis, and SDMT score. As all patients who came in for regular consultation and matched the inclusion criteria were invited and accepted to participate, the risk of inclusion bias is considered minor. There is also a minor risk that our cohort with SPMS held more positive views on cognitive testing as they had already volunteered for enrolment in an existing cognitive test study. Patients with diagnosed comorbidity such as depression were excluded from study participation yet as previously stated, the relationship between physical and affective symptoms and coping is complex. Confounding relations have yet to be established and this indistinctness also impacts the present study. It does, however, mirror patients’ disease experiences and is thus inseparable from their stated needs and preferences. It is subjectively experienced CD that is key to QoL, but the relationship between QoL and subjective and objective CD should be interpreted with caution due to the low number of participants. Still, this result is in line with other studies with a larger cohort. Also, the SDMT is a brief cognitive test with limitations, but we chose it as its validity has been demonstrated to be high and because it is clinically implementable.3,4,17,19
This study provides knowledge about patient perspectives on CD that may inform the introduction of routine cognitive testing of patients with MS. It shows that patients may need reassurance and information about its use, potential and limitations. Overall, the participants felt that cognitive symptoms should receive equal attention to physical symptoms with respect to research, treatment and support throughout the disease course. Many lacked knowledge about social, linguistic and emotional aspects of CD, in particular. CD may thus require HCPs to initiate patient communication about CD and comprehensive care taking all symptoms into account.
Conclusion
Routine cognitive testing was supported by the patients who called for comprehensive care taking both physical and cognitive difficulties into account.
Acknowledgments
The authors wish to thank Eva Kjølby Mortensen, project nurse at the Department of Neurology, Odense University Hospital, Odense, Denmark, for her help with including patients for this project. We are also indebted to PhD Lisbet Marstrand, neuropsychologist at the Department of Neurology, Danish Multiple Sclerosis Center, Rigshospitalet, Copenhagen, for sharing her insights into the field of MS-related CD in the initial phase of the study. Last but not least, we thank the patients who chose to share their thoughts and experiences with cognitive difficulties.
Funding Statement
This study was funded by a research grant from Biogen who did not participate in the study design, data collection, data analysis or manuscript preparations.
Disclosure
GLM has previously received speaker honoraria from Biogen, Sanofi Pasteur MSD, and Amgen, and support for congress participation from Sanofi Pasteur MSD. GLM has served on scientific advisory boards funded by Sanofi Pasteur MSD. AnthroConsult, owned by GLM, has received research support from Biogen, Sanofi Pasteur MSD, Amgen, Lundbeck, and Pfizer. AT has served on a scientific advisory board for Roche, received support for congress participation, and received research support from Biogen, Merck, Sanofi-Genzyme, Novartis, and Roche. TS has served on scientific advisory boards, received support for congress participation, received speaker honoraria and research support from Biogen and Novartis. ZI has served on scientific advisory boards, served as consultant, received support for congress participation, received speaker honoraria, and received research support from Biogen, Merck, Sanofi-Genzyme, Novartis, Teva, Roche and Lundbeck. The authors report no other conflicts of interest in this work.
References
- 1.Sumowski JF, Benedict R, Enzinger C, et al. Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology. 2018;90:278–288. doi: 10.1212/WNL.0000000000004977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalah MA, Ayache SS. Deficits in social cognition: an unveiled signature of multiple sclerosis. J Int Neuropsychol Soc (JINS). 2017;23(3):266–286. doi: 10.1017/S1355617716001156 [DOI] [PubMed] [Google Scholar]
- 3.Ruet A. Cognitive impairment in multiple sclerosis In: Brochet B, editor. Neuropsychiatric Symptoms of Inflammatory Demyelinating Diseases. Switzerland: Springer International Publishing; 2015:227–247. [Google Scholar]
- 4.Benedict RHB, DeLuca J, Enzinger C, et al. Neuropsychology of multiple sclerosis: looking back and moving forward. J Int Neuropsychol Soc (JINS). 2017;23:832–842. doi: 10.1017/S1355617717000959 [DOI] [PubMed] [Google Scholar]
- 5.Arnett PA, Strober LB. Cognitive and neurobehavioral features in multiple sclerosis. Mult Scler. 2011;17(11):1276–1281. doi: 10.1586/ern.11.12 [DOI] [PubMed] [Google Scholar]
- 6.Lee Mortensen G, Rasmussen PV. The impact of quality of life on treatment preferences in multiple sclerosis patients. Patient Prefer Adherence. 2017;11:1789–1796. doi: 10.2147/PPA.S142373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penner IK. Evaluation of cognition and fatigue in multiple sclerosis: daily practice and future directions. Acta Neurol Scand. 2016;134(Suppl 200):19–23. doi: 10.1111/ane.12651 [DOI] [PubMed] [Google Scholar]
- 8.Phillips LH, Henry JD, Nouzova E, et al. Difficulties with emotion regulation in multiple sclerosis: links to executive function, mood, and quality of life. J Clin Exp Neuropsychol. 2014;36(8):831–842. doi: 10.1080/13803395.2014.946891 [DOI] [PubMed] [Google Scholar]
- 9.Cotter J, Firth J, Enzinger C, et al. Social cognition in multiple sclerosis. A systematic review and meta-analysis. Neurology. 2016;87:1727–1736. doi: 10.1212/WNL.0000000000003236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. 2014;10:634–642. doi: 10.1038/nrneurol.2014.181 [DOI] [PubMed] [Google Scholar]
- 11.Benito- León J, Morales JM, Rivera-navarro J. Health-related quality of life and its relationship to cognitive and emotional functioning in multiple sclerosis patients. Eur J Neurol. 2002;9(5):497–502. doi: 10.1046/j.1468-1331.2002.00450.x [DOI] [PubMed] [Google Scholar]
- 12.Goretti B, Portaccio E, Zipoli V, et al. Coping strategies, cognitive impairment, psychological variables and their relationship with quality of life in multiple sclerosis. Mult Scler. 2011;17(5):623–629. doi: 10.1007/s10072-010-0372-8 [DOI] [PubMed] [Google Scholar]
- 13.Coenen M, Basedow-rajwich B, König N, et al. Functioning and disability in multiple sclerosis from the patient perspective. Chronic Illn. 2011;7(4):291–310. doi: 10.1177/1742395311410613 [DOI] [PubMed] [Google Scholar]
- 14.Grech LB, Kiropoulos LA, Kirby KM, et al. The effect of executive function on stress, depression, anxiety, and quality of life in multiple sclerosis. J Clin Exp Neuropsychol. 2015;37(5):549–562. doi: 10.1080/13803395.2015.1037723 [DOI] [PubMed] [Google Scholar]
- 15.Ari EB, Johansson S, Ytterberg C, et al. How are cognitive impairment, fatigue and signs of depression related to participation in daily life among persons with multiple sclerosis? Disabil Rehabil. 2014;36(23):2012–2018. doi: 10.3109/09638288.2014.887797 [DOI] [PubMed] [Google Scholar]
- 16.Chalah MA, Ayache SS. Alexithymia in multiple sclerosis: a systematic review of literature. Neuropsychologia. 2017;104:31–47. doi: 10.1016/j.neuropsychologia.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 17.Van Munster CE, Uitdehaag BM. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs. 2017;31(3):217–236. doi: 10.1007/s40263-017-0412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goverover Y, Chiaravalloti N, DeLuca J. Brief international cognitive assessment for multiple sclerosis (BICAMS) and performance of everyday life tasks: actual reality. Mult Scler J (MSJ). 2016;22(4):544–550. doi: 10.1177/1352458515593637 [DOI] [PubMed] [Google Scholar]
- 19.Ontaneda D, Cohen JA, Amato MP. Clinical outcome measures for progressive MS trials. Mult Scler. 2017;23(12):1627–1635. doi: 10.1177/1352458517729465 [DOI] [PubMed] [Google Scholar]
- 20.Sejbæk T, Blaabjerg M, Sprogøe P, et al. Reliability and validity of a Danish version of the multiple sclerosis neuropsychological screening questionnaire. Int J MS Care. 2018;(20):49–54. doi: 10.7241/1537-2073.2017-011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao SM, Losinski G, Mourany L, et al. Processing speed test: validation of a self-administered, iPad®-based tool for screening cognitive dysfunction in a clinic setting. Mult Scler. 2017;23(14):1929–1937. doi: 10.1177/1352458516688955 [DOI] [PubMed] [Google Scholar]
- 22.Niccolai C, Goretti B, Amato MP. Disease modifying treatments and symptomatic drugs for cognitive impairment in multiple sclerosis: where do we stand? Mult Scler Demyelinating Disord. 2017;2(1):8. doi: 10.1186/s40893-017-0025-3 [DOI] [Google Scholar]
- 23.Carotenuto A. Look beyond the door, not through the keyhole: evidence from a cognitive assessment including social cognition evaluation in multiple sclerosis. Eur J Neurol. 2018;25(2):205–206. doi: 10.1111/ene.13482 [DOI] [PubMed] [Google Scholar]
- 24.Goverover Y. Cognition and activities of daily living in multiple sclerosis In: DeLuca J, Sandroff BM, editors. Cognition and Behavior in Multiple Sclerosis. Washington (DC): American Psychological Association; 2018:171–190. [Google Scholar]
- 25.Sgaramella TM, Carrieri L, Stenta G, et al. Quality of life and adults with multiple sclerosis In: Merrick J, editor. Public Health: Some International Aspects. Hauppauge (NY): Nova Biomedical Books; 2016:211–219. [Google Scholar]
- 26.Valvano A, Floyd RM, Penwell-waines L, et al. The relationship between cognitive fusion, stigma, and well-being in people with multiple sclerosis. J Contextual Behav Sci. 2016;5(4):266–270. doi: 10.1016/j.jcbs.2016.07.003 [DOI] [Google Scholar]
- 27.Sgaramella TM, Carrieri L, Stenta G, et al. Self-reported executive functioning and satisfaction for quality of life dimensions in adults with multiple sclerosis. Int J Child Health Hum Dev. 2014;7(2):167–174. [Google Scholar]
- 28.Kvale S. Interviews. An Introduction to Qualitative Research Interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- 29.Kiely KM, Butterworth P, Watson N, et al. The symbol digit modalities test: normative data from a large nationally representative sample of Australians. Arch Clin Neuropsych. 2014;(29):767–775. doi: 10.1093/arclin/acu055 [DOI] [PubMed] [Google Scholar]
- 30.Roar M, Illes Z, Sejbæk T. Practise effect in Symbol Digit Modalities Test in multiple sclerosis patients treated with natalizumab. Mult Scler Rel Disord. 2016;10:116–122. doi: 10.1016/j.msard.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 31.Benedict HBR, Amato MP, Boringa J, et al. Brief International Cognitive Assessment for MS (BICAMS): international standards for validation. BMC Neurol. 2012;12:55. doi: 10.1186/1471-2377-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadel C. Feltarbeid I egen kultur. En innføring i kvalitativt orientert samfunnsforskning. SEEK a/s. Flekkefjord 1991:97–100.
- 33.Winther Jørgensen M, Phillips L. Diskursanalyse Som Teori Og Metode. Roskilde: Roskilde University Press; 1999. [Google Scholar]
- 34.Heesen C, Böhm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008:1–4. doi: 10.1177/1352458508088916 [DOI] [PubMed] [Google Scholar]
- 35.Rosti-otajärvi E, Ruutiainen J, Huhtala H, et al. Relationship between subjective and objective cognitive performance in multiple sclerosis. Acta Neurol Scand. 2014;130(5):319–327. doi: 10.1111/ane.12238 [DOI] [PubMed] [Google Scholar]
- 36.Malterud K. Kvalitative in Medisinsk Forskning: En Innføring. Universitetsforlaget. University of Copenhagen; 2011. [Google Scholar]