Abstract
Purpose
Lipid-lowering medications are often prescribed to decrease the risk of micro- and macro-cardiovascular complications related to dyslipidaemia. Despite widespread prescription of lipid-lowering drugs, including statins, adherence to therapy is a challenge worldwide. This systematic review of reviews aimed to conduct a critical appraisal and synthesis of review findings and to provide an overview of the factors that were found to affect adherence to lipid-lowering drugs, focusing on statins, in the reviews.
Patients and Methods
A systematic review methodology was used. MEDLINE, Embase, and Epistemonikos databases were searched for relevant publications. AMSTAR 2 criteria were used to assess the quality of the selected publications.
Results
From a total of 763 screened publications, 9 met all inclusion criteria and were included in this synthesis. Several factors were identified as being associated with adherence to lipid-lowering agents. Among them, high socio-economic and educational position, and middle age had a positive effect on adherence to lipid-lowering agents. Contrary, female sex, older and younger age, non-white race, low socio-economic position, high co-payments, being a new statin user, comorbidities, side effects, regimen complexity, type and intensity of statin dose, smoking, alcohol consumption, imperceptible benefits, and medical distrust contributed to non-adherence. The overall quality of the included reviews was considered critically low to moderate.
Conclusion
This review of reviews has evaluated the impact of factors on adherence statins. Further research related to modifiable predictors for non-adherence is warranted.
Keywords: nonadherence, adherence, statins, dyslipidaemia, lipid-lowering drugs, review
Introduction
Dyslipidemia is a critical predisposing factor for the development of cardiovascular diseases. Globally, one third of ischemic heart diseases are attributed to dyslipidemia, and it is estimated to cause 2.6 million deaths annually.1 As such, managing dyslipidemia is essential in reducing cardiovascular complications. Lipid-lowering medications, such as the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or statins, are widely prescribed to decrease the risk of micro- and macrocardiovascular complications related to dyslipidemia.2–5
The supposed benefits of statins can only be obtained if the patient takes the drug. Despite the widespread prescription of statins, adherence to statin therapy is a major challenge worldwide. Although the exact rate of nonadherence to statins is difficult to determine because it depends greatly on the setting, enrolled patients, and measurement methods used in research, numerous studies have documented differing but systematically high rates of nonadherence to statin therapy. It has been demonstrated that nonadherence is influenced by the high rate of discontinuation immediately after therapy has been initiated.6 Further, 25–50% (the figures vary across studies) of patients discontinue their statin therapy within the first year after treatment initiation, and the consistency of use decreases over time.6–11
Recognizing the issue of nonadherence to statins, researchers have shown great interest in assessing factors that influence patients’ adherence to statin therapy. Systematic reviews synthesizing such factors have been published, however with widely different focuses and findings. While most reviews have concentrated on reviewing specific predictors of either adherence or nonadherence to statins – eg, gender, age, race, drug costs, etc. – others have focused on patients’ attitudes toward statins and their reasoning for adhering or not adhering to the therapy. Owing to the variation in the focus of the reviews, there is a need to synthesize and critically appraise the available evidence in the field in a single source to provide cumulative, reliable and accessible information to researchers, healthcare professionals, decisionmakers and patients. The objective of the present systematic review of reviews was thus to undertake a systematic search, conduct critical appraisal and synthesis of review findings, and provide an overview of all of the factors that have been found, in the reviews, to either promote or hinder adherence to statins.
Materials and Methods
The methodology used to conduct this systematic review of reviews involved the same methodological processes used in systematic reviews of primary research.12 However, the unit of analysis was reviews rather than primary research. The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) checklist was used to guide the process as well as in reporting the present systematic review of reviews.13
Inclusion Criteria
The search strategy and eligibility criteria were organized in accordance with the Patient, Intervention, Comparison and Outcome (PICO) search framework.14 Reviews obtained from the systematic search were eligible for inclusion if the target review: 1) was labeled as (or the methods described were in concordance with) a systematic review, 2) included studies that investigated factors affecting adherence and/or nonadherence to statins, 3) one or more lipid-lowering agents, including statins, were prescribed for the patient population, and 4) adherence and/or nonadherence was a measured outcome. Reviews that reported adherence to statins in combination with other lipid-lowering agents or other medications (eg, anti-hypertensive therapy) were included, even if the entire cohort was not exposed to statins. The research question posed in the present review of reviews was likely to be addressed by both quantitative and qualitative primary research that would be synthesized by different types of systematic reviews, and as such, all types of systematic reviews were included in this review of reviews.
Search Strategy
An electronic literature search for systematic reviews published from database inception to February 2019 was conducted in MEDLINE, Embase and Epistemonikos. The search strategy combined various relevant medical subject headings (MeSH), keywords, and word variants for “hyperlipidemias” (eg, lipidemias, hypercholesterolmias, etc.) in both UK and US spellings, “lipid-lowering agents” (eg, antihyperlipidemics, hydroxymethylglutaryl-coenzyme, HMG CoA reductase inhibitor, etc.) and “adherence” (eg, compliance, discontinuation, concordance, persistence, etc.). A built-in filter was used to limit the search results to systematic reviews. The literature search was conducted by authors THA and ON, and the full search strategy for MEDLINE is available in Appendix 1. Additional reviews were also searched using a manual reference search in the selected reviews.
Data Extraction and Synthesis
Findings were summarized according to the PRISMA guidelines.13 From each eligible systematic review, data were extracted on authors, year of publication, aim(s), review type, search strategy, number of studies included, design of included studies, risk of bias/quality assessment tool, sources of funding, and authors’ key findings. To address differences in terminology across reviews, we extracted information on any factors reported, in the reviews, to have an impact on adherence to statin therapy. MVI conducted data extraction for all of the included reviews. Data extraction was verified by KO. Any disagreements were discussed until consensus was reached. The data synthesis was narrative due to the heterogeneity across reviews in PICO elements, study designs, quality assessment and measurement techniques. The data synthesis is presented in Table 1, supplemented by summary evidence presented in Table 2.
Table 1.
Review Characteristics
| First Author (Year) | Aim(s) | Type of Review | Search Period | Search Strategy | Inclusion Criteria | Number of Included Studies | Design of Studies | Quality Assessment Tool | Funding/Disclosures | |
|---|---|---|---|---|---|---|---|---|---|---|
| Banerjee (2016)24 | To identify health system features, programs or strategies which act as barriers or facilitators to adherence to evidence-supported medications for CVD secondary prevention. | Systematic review | From inception to October 2015 | MEDLINE, Embase, Cochrane Library, PsychINFO, Health Systems Evidence, HMIC, LILACS, Africa-Wide Information and Google Scholar were searched. Conference proceedings and reference lists of relevant research articles were also searched. Searched terms were provided. | Quantitative and qualitative studies reporting associations of local, national, regional or international health system level factors, interventions, policies or programs with adherence to medications for the secondary prevention of CVD. Included studies had analyses of barriers and facilitators to adherence or persistence to at least one of β blockers, statins, angiotensin–renin system blockers and aspirin. | 25 | 11 RCTs, 1 non-randomized trial, 11 cohort studies, 1 cross-sectional, 1 case-control. | The Cochrane Risk of Bias tool | Funding was World Heart Federation Emerging Leaders Programme. The authors declared that no competing interests exist. | |
| Chee (2014)21 | To determine patients’ perceptions of statins, as well as the impact these had on statin use and adherence. | Literature review | October 1991 to May 2012 | PubMed, Medscape, the Cochrane Database and the Western Pacific Region Index Medicus were searched. Search terms were provided. Additional studies were identified using a manual reference search for included citations. | Studies that reported factors affecting adherence or interventions that target to improve adherence. | 58 | Unspecified | Unspecified | Unspecified | |
| Hope (2019)17 | To identify predictors of statin adherence for the primary prevention of CVD. | Systematic review | January 1984 to May 2017 | Embase, MEDLINE, CINAHL and PsychINFO were searched. Search terms were provided. | Articles were included if they reported on: 1) people receiving treatment for the primary prevention of CVD, 2) statins were prescribed, 3) adherence was defined as the extent to which patients followed their statins regimen during the period of prescription, 4) predictors of adherence were defined and measured, and 5) if the study was original research. | 19 | 3 cross-sectional studies, 11 retrospective cohort studies, 3 prospective cohort studies, and 2 RCTs. | As reported by Sanderson4 | The authors received no specific funding for this work. The authors declared that no competing interests exist in relation to this systematic review. After review of the journal policy the authors of this manuscript have the following competing interests: Prof. George Kitas and Prof. Deborah Symmons. | |
| Ju (2018)25 | To provide a comprehensive synthesis of qualitative studies on patient perspectives on statins for CVD prevention. | Systematic review | From inception to October 2016 | The ENREQ framework was followed. MEDLINE, Embase, PsycINFO, and CINAHL were searched. Google Scholar and reference lists of relevant studies and reviews were also searched. PhD dissertations were searched on ProQuest Dissertations and Theses database, British Library Electronic Digital Thesis Online Service, and the Europe E-theses Portal. It is unclear whether search terms were provided. | Qualitative studies that reported patients’ perspectives on statins were included. Studies involving adult patients at risk of CVD and patients receiving statins as primary or secondary preventive therapy for CVD were eligible. Articles that only included patients with familial hypercholesterolemia or perspectives from health professionals were excluded. | 32 | 19 qualitative studies, 6 mixed methods studies, 1 ethnomethodologic study, 2 phenomenological studies, and 2 ethnographic studies. The 2 last studies were unspecified. | Not applicable | This work was supported by a National Health and Medical Research Council Partnership Grant (NHMRC) (1092674), including support from the National Heart Foundation of Australia, and an NHMRC Program Grant (1092597). Two authors are supported by NHMRC Fellowships (1106716 and 1042717). The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. | |
| Lemstra (2012)22 | To quantify the proportion of adherence to statin medications by study design, and to provide estimates of risk indicators associated with nonadherence to statin medications. | Meta-analysis | From inception to June 2011 | PubMed, PsycINFO, CINAHL, Cochrane CENTRAL, DARE, NHSEED, HTA Database, and Embase were searched. Search terms were provided. Reference sections of each article were reviewed for additional papers. | Articles were included if they: 1) determined the proportion of adherence to statin therapy during a defined period, 2) were observational cohort studies or RCTs, and 3) used a validated tool for measurement of adherence. Articles were restricted to English language. The review excluded studies with fewer than 50 participants. | 67 | 53 cohorts and 14 RCTs. | The Delphi list for RCTs. As reported by Sanderson4 for observational studies. | One author was funded by an unconditional research grant from the Ministry of Health in the Province of Saskatchewan which obtained an unconditional research grant from Merck Frosst/Schering Pharmaceuticals. Another author had educational financial support from the Province of Saskatchewan’s Ministry of Health, AstraZeneca Canada, Merck Frosst/Schering, and Pfizer Canada. Two authors had no conflicts of interest to disclose. None of the sponsors were involved in developing this study or writing the article. | |
| Lewey (2013)18 | To evaluate the effect of race/ethnicity and gender on adherence to statin therapy for primary or secondary prevention. | Systematic review and meta-analysis | From inception to April 2010 | MEDLINE, Embase, ClinicalTrials.gov, and the Cochrane Database of Systematic Reviews were searched. Search terms were provided. | Studies that evaluated adherence to statins and reported on gender, race, or ethnicity as a predictor of adherence in univariate or multivariable analysis. Studies were excluded if they did not: 1) present quantitative measures of adherence, 2) present original data, 3) evaluate gender, race, or ethnicity as a predictor of adherence, or 4) evaluate statin use. | 53 | 47 cohort studies, 2 RCTs and 4 cross-sectional. | Newcastle Ottawa Quality Assessment Scale. | The authors received research support to study medication adherence through unrestricted grants from Aetna, CVS Caremark, the Robert Wood Johnson Foundation, and the Commonwealth Fund. The authors were solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents. One author is a consultant to Mercer Health and Benefits, Inc. Another author is a consultant on research methodology to United Healthcare. A third author is an employee of CVS Caremark. |
|
| Mann (2014)23 | To determine the association between drug insurance and patient cost sharing strategies on medication adherence, clinical and economic outcomes in those with chronic diseases. | Literature review | From inception to March 2013 | MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials, and Current Controlled Trials were searched. Search terms were provided. | Included studies that examined various cost sharing strategies including co-payments, coinsurance, fixed co-payments, deductibles and maximum out-of-pocket expenditures. | 11 | 2 separate reports of 1 RCT, 4 interrupted time series, and 5 controlled before-after studies. | The Cochrane Risk of Bias tool for RCTs, and the Cochrane EPOC taxonomy for non-randomized trials. | This study was funded by a team grant from Alberta Innovates – Health Solutions (AI-HS) to the Interdisciplinary Chronic Disease Collaboration. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. One author was supported by an AI-HS Clinician Fellowship award. Another author was supported by an AI-HS Trainee award. Two authors were supported by career salary support awards from AI-HS. Another author was supported by the Roy and Vi Baay Chair in Kidney Research. One author was supported by a Government of Canada Research Chair. Three authors were also supported by an alternative funding plan from the Government of Alberta and the Universities of Calgary and Alberta. |
|
| Mann (2010)20 | To identify reliable predictors of non-adherence to statins. | Review of literature and meta-analysis | From inception to February 2009 | MEDLINE, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, National Health Service Economic and Evaluation Database, Health Technology Assessment Database, Embase, PsycINFO. Searched terms were provided. | Articles were included if they (1) included prospective or retrospective observational cohorts in which statins were evaluated as an outcome measure, (2) included adults above 18 years, (3) used either a validated self-report scale or an objective measure with more than 50 participants, (4) had a description of the study design and the analysis reported on at least 2 predictors of adherence to statins in a multivariable analysis with relative risks. Studies with fewer than 50 participants were exclude. Articles were restricted to English language. | 22 | 22 cohort studies. | Quality assessment applied, but not specified. | This research was supported by grant lK23DK081665, a Patient-Oriented Mentored Scientist Award through the National Institute of Diabetes, Digestive, and Kidney Diseases. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have declared that no competing interests exist. | |
| Ofori-Asenso (2018)19 | To identify factors associated with non-adherence and discontinuation among older statin users (≥ 65 years) | Systematic review | From inception to December 2016 | Followed PRISMA guidelines. MEDLINE, Embase, CINAHL, PsycINFO, NHSEED, DARE, and Cochrane Central Register of Controlled Trials were searched. Search terms provided. | Articles were eligible if they: 1) reported on predictors of nonadherence and/or discontinuation among older statin users, 2) adopted objective adherence measurements, 3) adopted validated scales (in case of utilized self-reports), 4) measured adherence via the medication possession ratio, proportion of days covered (PDC), or proportion of doses taken, only those employing an 80% cutoff to dichotomize adherence were considered. Articles were restricted to English language. | 22 | Unspecified | NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. | The first author was supported by a Monash Graduate Scholarship and Monash International Postgraduate Research Scholarship for his doctoral studies. One author was funded by a National Health and Medical Research Council Senior Research Fellowship. No other funding has been received to undertake the work. The last author reports past participation in advisory boards and/or receiving honoraria from: Amgen Australia; AstraZeneca/Bristol-Myers Squibb Australia; Janssen-Cilag; Merck, Sharp, and Dohme (Australia); Novartis Australia; Novo Nordisk; Sanofi; Servier Laboratories; Takeda Australia; and Monash University (undertaking contract work for AstraZeneca Pty Limited/Bristol- Myers Squibb Australia Pty Limited) for work unrelated to this study. |
Table 2.
Key Findings According to the Overall AMSTAR 2 Quality Assessment Score
| Overall Score (AMSTAR 2) | Review | Key Findings |
|---|---|---|
| Moderate | Banerjee et al (2016)24 | Co-payment was associated with lower persistence with statins. Non-persistence to secondary prevention medications, including statins, was less likely with private insurance and prescription cost assistance. Institutional living was correlated with persistence to statins. Stroke unit care was associated with persistence for statins. |
| Hope et al (2019)17 | Older age predicted statin adherence. Men were more adherent than women. People with diabetes and hypertension were more likely to adhere to statins. Being an ex-smoker predicted adherence. Higher income and higher level of educations associated with adherence. Hispanic and Black Americans were less likely to adhere to statins compared to white Americans. People with comorbidities were more likely to be adherent to statins. Depression inversely associated with statin adherence. Obesity was associated with lower odds of being adherent in women. Alcoholism nearly doubled the risk of non-adherence. People were more likely to adhere to fluvastatin and rosuvastatin than to lovastatin. Men on a moderate daily dose and men on a high daily dose were less likely to adhere compared to men on a low daily dose of statins. Women on a high daily dose of statins were less likely to adhere compared to women on a low daily dose. | |
| Low | Lemstra et al (2012)22 | Patients who were dispensed statin medications for primary prevention, in comparison with secondary prevention, were 52% more likely to be nonadherent. New statin users, in comparison with experienced or previous statin users, were 46% more likely to become nonadherent. Patients required to make a co-payment when their statin medications were dispensed were 28% more likely than others to be nonadherent. Patients who were of lower income status were 26% more likely to become nonadherent than those who were not of lower income status. Patients without hypertension were 16% more likely to be non-adherent than patients with hypertension. |
| Lewey et al (2013)18 | Rates of nonadherence were higher in women than men (53% vs 50%). Women were 10% more likely to be nonadherent than men. Crude rates of nonadherence were higher in patients of nonwhite race as compared with white race (50% vs. 45%). Nonwhite patients were 53% more likely to be nonadherent to statin therapy. | |
| Mann et al (2014)23 | In patients aged 65 or older, drug insurance increased the odds of adherence to guideline-recommended medications by 19–136% compared to those without drug insurance coverage. In hypertensive patients aged 65 or older, drug insurance was associated with a two-fold increase in the odds of using an antihypertensive agent compared to those without drug insurance. Full drug insurance led to higher use of antihypertensive medications at the exit screening examination (20% absolute increase). Increasing co-payment by USD 5 per prescription, with or without an annual maximum out-of-pocket expenditure, resulted in a 30–40% lower adjusted odds of adherence across a variety of measures. Small co-payment (up to 25%) did not appear to impact adherence, while large co-payments (95% co-pay) had a substantial impact on medication adherence. A 100% co-payment was associated with a two-fold reduction in drug adherence. | |
| Mann et al (2010)20 | Age was a statistically significant predictor of adherence. Young adults (<50 years) and those ≥70 years of age had higher rates of nonadherence compared to middle-aged adults. Women were less likely to be adherent compared to men. A history of comorbid diabetes, hypertension, or cardiovascular disease was associated with better adherence, as was higher income and increased testing of lipid levels. People with depression were less likely to be adherent. Racial minorities were less likely to be adherent. First-time statin users were less likely to be adherent as compared to experienced statin users. Higher out-of-pocket costs were associated with lower statin adherence. | |
| Ofori-Asenso et al (2018)19 | Women were more likely to be nonadherent to statin therapy than men. Black or non-white race had a 66% higher likelihood of nonadherence than white populations. Being a smoker was associated with a higher likelihood of nonadherence and a 14% higher likelihood of discontinuation. In comparison to prevalent users, new users were more likely to be nonadherent. Higher co-payment increased the likelihood of nonadherence and had an adverse impact on statin continuation; higher co-payment was associated with a 61% higher likelihood of discontinuation. Lower income status was associated with a 20% higher likelihood of discontinuation. A history of CVD was associated with higher adherence and lower discontinuation; patients receiving statins for primary prevention had a 49% higher likelihood of nonadherence; patients taking statins for primary prevention had a 66% higher likelihood of discontinuation. Taking other cardiovascular medications was associated with 4% higher likelihood of statin discontinuation. Having hypertension or diabetes had a positive impact on statin continuation; patients without hypertension had a 13% higher likelihood of discontinuing statin therapy, and patients without diabetes had 9% increased odds of discontinuation. Having dementia, cancer or respiratory disorders were associated with a higher likelihood of discontinuation. | |
| Critical low | Chee et al (2014)21 | Factors found to have a negative impact on adherence to statins include female sex, patients questioning their personal needs for statins due to absence of symptoms, imperception benefits of statins, lack of communication between the physician and the patient during the consultation, side effects, preference for diet control and exercise over pharmacy, and costs. Factors that were found to have a positive influence on adherence to statins include older age, high income, a high number of comorbidities, and previous cardiovascular events. |
| Ju et al (2018)25 | Reported patient perceptions that had a negative influence on people’s adherence to statins include questioning the utility/efficacy of statins, having uncertainties about pharmacological mechanisms, medical distrust, valuing other priorities over the perceived benefits of statins, experiencing debilitating side effects and toxicity to the body, fearing perpetual dependence, not considering themselves to be ill enough to take statins, and financial strains. Reported patient perceptions that can had a positive influence on adherence to statins include trust in the efficacy and benefit of statins, that statins provide a sense of control and ease anxiety about high cholesterol. |
Quality Assessment
The methodological quality of the included reviews was appraised by the AMSTAR 2 tool (Assessing the Methodological quality of Systematic Reviews), which is a validated 16-item tool for critically appraising systematic reviews of randomized or non-randomized studies of healthcare interventions, or both.15 Two reviewers (MVI and KO) independently assessed each review using the AMSTAR 2 tool. Any disagreements were discussed until consensus was reached. To assess the quality of the overall body of evidence within the reviews, we relied on the review authors’ conclusions regarding the quality of the primary studies included in each review.
As suggested by the authors of AMSTAR 2, critical items of the tool were identified, for evaluating and classifying the included reviews, as well as a total score.15 Critical items where errors or biases would seriously affect the validity of conclusions of the included reviews were identified as follows:
A protocol was registered before commencement of the review (item 2)
Comprehensive literature searching (item 4)
Risk of bias from individual studies was included in the review (item 9)
Appropriateness of meta-analytical methods (if meta-analysis) (item 11)
Consideration of risk of bias in interpretation of the results of the review (item 13)
Assessment of presence and likely impact of publication bias (item 15).
The overall quality of the included reviews was rated in accordance with AMSTAR 2 guidelines.15 According to these guidelines, item 7 (ie list of excluded studies) also constitutes a critical item; however, none of the reviews presented such a list and the author group did not consider this item to be critical. Hence, in the overall rating of reviews, item 7 was not appraised as a critical item. The overall confidence ratings were as follows (Table 3): “High” methodological quality if the review did not contain any of the critical items and no or one non-critical weakness; “Moderate” if the review had more than one non-critical weakness; “Low” if the review had one critical weakness with or without non-critical weaknesses; and “critically low” if the review had more than one critical item with or without non-critical weaknesses.
Table 3.
Results of the Review Quality Assessment (AMSTAR 2)
| Assessment Question (Item) | 1. Were the Components of PICO Included? | 2. Protocol Reported? Any Deviations Justified? | 3. Study Design Justified? | 4. Comprehensive Literature Search? | 5. Study Selection Performed in Duplicate? | 6. Data Extraction in Performed in Duplicate? | 7. List of Excluded Studies? Were These Justified? | 8. Characteristics of Studies Provided in Detail? | 9. Risk of Bias Assessed? | 10. Sources of Funding of Included Studies? | 11. Methods Used to Combine the Finding of Studies Appreciate? Test on Heterogeneity? | 12. Was RoB Accounted for if Meta-Analysis Was Performed? | 13. Was RoB Discussed in Individual Studies? | 14. Discussion of Any Heterogeneity Observed in the Results? | 15. If Quantitative Synthesis, was Publication Bias Investigated and Discussed in Relation to the Results? | 16. Conflicts of Interest Stated? | Overall Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | |||||||||||||||||
| Banerjee (2016)24 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No MA | No MA | Yes | Yes | No MA | Yes | Moderate |
| Chee (2014)21 | Yes | No | Yes | PY | No | No | No | No | No | No | No MA | No MA | No | No | No MA | No | CL |
| Hope (2019)17 | Yes | PY | Yes | PY | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Moderate |
| Ju (2018)25 | Yes | No | Yes | No | No | Yes | No | Yes | No | No | No MA | No MA | No | No | No MA | Yes | CL |
| Lemstra (2012)22 | Yes | No | Yes | PY | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Low |
| Lewey (2013)18 | Yes | No | Yes | PY | Yes | Yes | No | Yes | PY | No | Yes | No | No | Yes | Yes | Yes | Low |
| Mann (2014)23 | Yes | No | Yes | PY | Yes | Yes | No | Yes | Yes | No | No MA | No MA | No | Yes | No MA | Yes | Low |
| Mann (2010)20 | Yes | PY | Yes | PY | Yes | Yes | No | Yes | No | No | Yes | No | No | Yes | No | Yes | Low |
| Ofori-Asenso (2018)19 | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | Yes | No | No | Yes | Yes | Yes | Low |
Notes: High = No or one none-critical weakness: the systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest. Moderate = More than one non-critical weakness: the systematic review has more than one weakness but no critical flaws. It may provide an accurate summary of the results of the available studies that were included in the review. Low = One critical flaw with or without non-critical weaknesses: the review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest. Critically low = More than one critical flaw with or without non-critical weaknesses: the review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies.
Abbreviations: PY, partial yes; MA, meta-analysis; RoB, risk of bias; CL, critically low.
Results
Study Selection
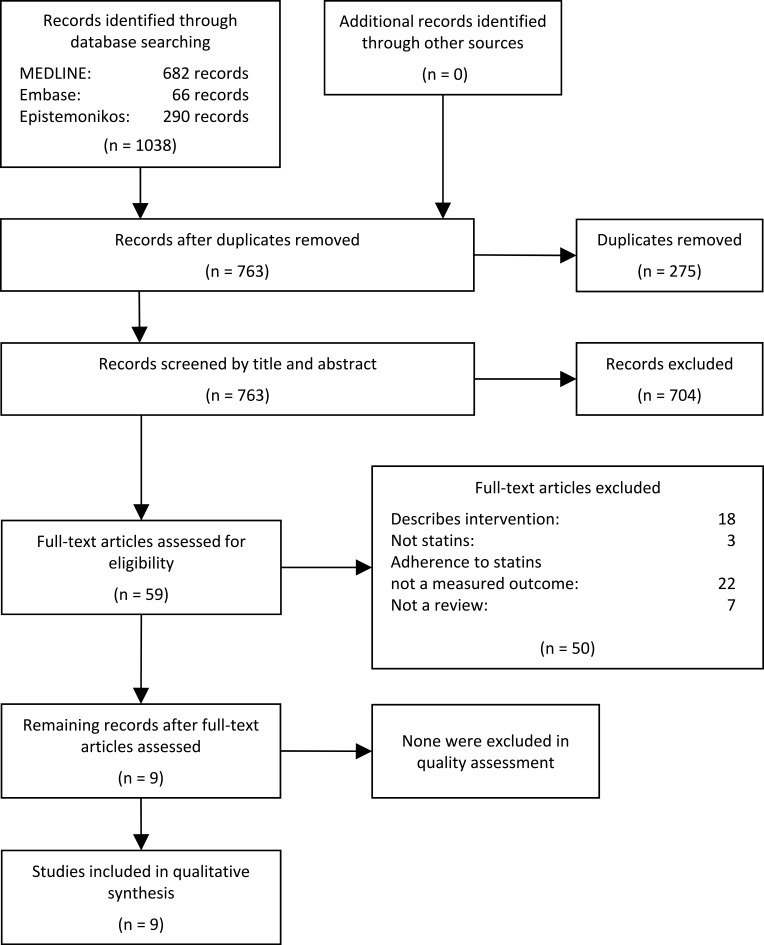
Initial database searches retrieved 1038 records. All records were uploaded to EPPI-Reviewer 4,16 checked for duplicates and configured for screening. After duplicate removal (n=275), titles and abstracts of the 763 identified records were independently screened in accordance with the eligibility criteria by reviewers MVI and KO. An inter-rater reliability value of 93% was achieved. Any discrepancies concerning the potential relevance of the reviews were resolved by consensus. Based on title and abstract screening, 59 reviews seemed potentially relevant. Full-text versions of the publications were retrieved and screened in detail independently by MVI and KO. The authors compared their results and an inter-rater reliability value of 83% was found. Disagreements were resolved through discussion with a third author (DG), and consensus was reached on which reviews to include. Nine reviews met all of the eligibility criteria. Most articles were excluded because they reported on adherence to a clinical intervention with statins or other lipid-lowering medications, described an intervention designed to improve adherence to lipid-lowering agents, or because they did not include adherence to statins as a measured outcome. Reference lists of included reviews were manually searched to identify additional reviews, but no additional systematic reviews were found. No reviews were excluded due to their AMSTAR 2 ratings. Overall, nine reviews from 2011 to 2019 met the eligibility criteria and were included in our review. The process of study selection is illustrated in a flow chart in Figure 1. A list of excluded studies from the full-text screening is provided in Appendix 2.
Figure 1.
The process of study selection.
Methodological Quality of Included Reviews
All included reviews provided their research question or objectives following the PICO search framework (item 1). Four reviews provided a study protocol prior to the conduct of the review; however, only two studies had registered the protocol a priori. Most of the included reviews conducted a comprehensive literature search and performed study selection and data extraction in duplicate (items 3–6). All reviews failed to provide a list and justification of excluded studies (item 7), but most reviews provided summarized reasons for excluding studies illustrated in flow charts. No reviews reported on the source of funding for the included primary studies (item 10). The critical AMSTAR 2 items that most of the reviews failed to meet were protocol registration (item 2) and discussion of risk of bias in individual studies (item 13). Only two reviews assessed the publication bias using funnel plot or statistical test. Based on the proposed rating scheme of AMSTAR 2, the overall methodological quality rating of included reviews was classified as “moderate” for two reviews, five were rated “low”, and two were considered to have a “critically low” confidence on the results. Summarized methodological quality assessments of the reviews are presented in Table 3.
Socio-Demographic Factors
Sex
In four reviews, patients’ sex was identified as an important factor influencing adherence to statins.17–20 All five reviews concluded that women were more likely than men to be nonadherent to statins. The most comprehensive review dealing with the association between sex and adherence was conducted by Lewey et al.18 They found that crude rates of nonadherence were higher in women than in men (53% and 50%, respectively). Pooled across the included studies, women were 10% more likely to be nonadherent to statins (OR 1.10, 95% CI 1.07–1.13). The increased relative risk for women persisted in studies using multivariable methods and did not differ meaningfully in large studies as compared with small studies or based on the indication for the prescribed statin (eg, primary vs. secondary prevention). Similarly, Mann et al observed 18 studies that examined sex as a predictor of adherence. The majority of these showed that women were less likely than men were to be adherent. Comparing women to men, the relative risk values for low adherence, discontinuation, and overall were 1.07 (95% CI 1.02–1.12), 1.07 (95% CI 1.03–1.12), and 1.07 (95% CI 1.04–1.11), respectively.20 Hope et al found that, in one large high-quality study and four low-quality studies, men were more likely to be adherent to statins than women were, while the opposite was the case in one high- and one low-quality study.17
Age
Three reviews reported on the influence of age on adherence to statins.17,19,20 In a meta-analysis conducted by Mann et al, age was found to be a significant predictor of adherence in all included studies. However, the relationship was not monotonic. People younger than 50 years were significantly more likely to have lower adherence (proportion of days covered <80%) or to have discontinued their statin therapy compared to people between 50 and 65 years. However, older people (≥70 years) also displayed lower adherence and discontinuation compared to people aged 50–65 years. For instance, in one included study using adults aged 18–34 years as the reference, a U-shaped relationship was observed with an odds for nonadherence of 0.68 (95% CI 0.56–0.85) among 35- to 44-year-olds, 0.41 (95% CI 0.34–0.50) among 45- to 54-year-olds, 0.34 (95% CI 0.28–0.41) among 55- to 64-year-olds, 0.44 (95% CI 0.36–0.53) among 65- to 74-year-olds, and 0.46 (95% CI 0.38–0.57) among those 75 years or older. As such, age had a U-shaped association with adherence; the oldest (≥70 years) and youngest (<50 years) people had lower adherence than those in the middle age groups (50–59). In total, 10 of the 11 studies examining the effect of age in this review were consistent with the U-shaped relationship.20
In Hope et al’s review, there was evidence that older age predicted statin adherence. In four studies (three high and one low quality), including a total of 662,638 participants, that adjusted for confounders, and six studies (two high and four low quality) with unadjusted effects that included 496,921 participants, it was found that adherence increased with older age. Two of the included studies did not find any association between age and adherence. One of the included studies found that the adjusted odds of adherence increased by up to a factor of two per five-year increase in age in both female and male cohorts aged 40–65 years. In the same cohort study, the odds of being adherent decreased by up to 60% per five-year increase in age in female and male cohorts aged 65–80 years.17
Ofori-Asenso et al’s review identified factors associated with nonadherence and discontinuation among older statin users (aged ≥65 years), showing that the associations between age and nonadherence and discontinuation were not reported in a consistent manner across the included studies. In three studies, increasing age was associated with higher nonadherence and discontinuation, two studies reported an inverse association between age and nonadherence, and five studies found no association between age and nonadherence, while two studies found no association between increasing age and discontinuation. Thus, the association between age and nonadherence and discontinuation was equivocal among statin users aged ≥65 years.19
Race
Three reviews illustrated racial differences in statin adherence.17–19 Lewey et al’s review reported that nonadherence was higher in non-white as compared with white patients (50% vs. 45%). Pooled across studies, non-white patients were 53% more likely to be nonadherent to statin therapy (OR 1.53; 95% CI 1.25–1.87), with significant heterogeneity in the pooled estimate (I2 0.98, P value for heterogeneity 0.001), however with less heterogeneity in studies measuring adherence by self-report (I2 0.53, P value for heterogeneity 0.09) but with a similar risk of nonadherence among non-white patients (OR 1.56; 95% CI 1.08–2.24). The odds of nonadherence were lower but still significantly increased among non-white people treated for secondary prevention (OR 1.28; 95% CI 1.04–1.59), where there was little between-study heterogeneity (I2 0.06, P value for heterogeneity 0.36).18 In five studies that had adjusted for socioeconomic status, insurance status, or co-payment amount, non-white patients continued to have an increased risk of nonadherence (OR 1.51; 95% CI 1.19–2.02).
Ofori-Asenso et al reported that black or non-white patients aged ≥65 years had a 66% higher likelihood of being nonadherent than white populations aged ≥65 years (OR 1.66; 95% CI 1.39–1.98).19
The effect of race was also reported by Hope et al. One included study reported that being Hispanic American reduced the odds of adherence compared to being White American (OR 0.26; 95% CI 0.07–1.00). Another study found that the relative risk of being Hispanic American reduced the likelihood of adherence, even after adjustment for other factors (RR 0.77; 95% CI 0.72–0.84). The same study also found that Black Americans were less likely to be adherent to statins compared to White Americans (RR 0.77; 95% CI 0.70–0.86).17
Income
Four reviews reported effects of income level on adherence.17,20-22 Analyzing 11 studies with a total sample size of 1,194,722 patients, Lemstra et al reported that patients of lower-income status were 26% more likely to become nonadherent than those who were of lower-income status (OR 1.26; 95% CI 1.16–1.37).22
Five studies in the Mann et al review found that higher income was significantly associated with adherence; however, four studies offered equivocal results. Overall, patients with higher income were more likely to be adherent (OR of nonadherence 0.85; 95% CI 0.78–0.91).20
Hope et al’s review highlighted a study showing that participants in higher income quintiles were more likely to be adherent to statin therapy compared to participants in the lowest income quintile, after adjustment for age, income, education and hypertension. This effect was observed in men and women of middle and post-retirement age, the strongest effects being observed for men of middle age (OR 1.56, 95% CI; 1.54–1.56). Another included study found that income had a strong positive effect on the odds of adherence in men; men in the lower-income quintiles were less likely to adhere to statin therapy compared to men in the highest income quintile, the strongest effect being observed for men in the lowest income quintile (OR 0.74; 95% CI 0.68–0.79). Similar findings were observed in women; however, the strength of these associations was weakened in the cohort of women. Women in the lowest income quintile were less likely to be adherent compared to women in the highest income quintile (OR 0.93; 95% CI 0.86–1.00).17
Education
Hope et al’s review reported that a higher level of education was associated with statin adherence.17 In studies included in this review where more than 50% of participants were men, a higher level of education increased the likelihood of adherence (OR 1.07; 95% CI 1.04–1.10); whereas in studies where 50% or more of participants were women, a higher education reduced the likelihood of adhering to statins (OR 0.92; 95% CI 0.89–0.95). These estimates included two studies that were of high quality and adjusted for other confounders.17 Hope et al also highlighted two additional studies: one showing that the likelihood of being adherent was lower for men if they had a basic or secondary level of education compared to men with a higher level of education, and the other concluding that men who had at least 12 years of education were more likely to be adherent compared to those with 7–12 years of education. Again, the opposite effect was observed in women: Increasing level of education was associated with lower odds of being adherent. These effects remained after controlling for other covariates.17
New Users
Two reviews reported that new users of statins were more likely to be nonadherent than prevalent users.19,22 Lemstra et al reported that for seven of the included studies, with a total sample size of 857,155 patients, new statin users were 46% more likely to become nonadherent compared with experienced or previous statin users (OR 1.46; 95% CI 1.33–1.60).22 In Ofori-Asenso et al’s meta-analysis, new statin users aged ≥65 years were likely to be more nonadherent than prevalent users aged ≥65 years (OR 1.58; 95% CI 1.21–2.07).19
Cost, Co-Payment and Insurance
In four of the reviews, the cost of statins, co-payment and insurance coverage were found to affect adherence to statins.17,21,23,24 The most comprehensive review dealing with these factors was by Mann et al.23 This review focused on determining the impact of drug insurance and cost sharing on medication adherence, including statins; however, it did not focus on statins exclusively. The review found that providing drug insurance to people with chronic diseases who have no drug insurance increased adherence to drugs. For instance, three of the included studies found that drug insurance increased the odds of adherence in patients aged 65 years or older by 19–136% compared to having no drug insurance coverage. It was also reported that being subject to a 100% co-payment, for those who had not yet reached their deductible, was associated with a two-fold increase in the risk of discontinuing statins. Further, non-persistence and discontinuation were less likely with private insurance and prescription cost assistance.23
Banerjee et al’s review reported that a co-payment of ≥USD 20 was associated with lower persistence at 1 year post first prescription of stations, compared to a co-payment of <USD 10 (OR 0.42; 95% CI 0.36–0.49). Additionally, according to an US-based prospective cohort study that included 7955 patients with myocardial infarction (MI) from 216 hospitals, non-persistence to secondary prevention medications, including statins, was less likely with private insurance (OR 0.85; 95% CI 0.76–0.95) and prescription cost assistance (OR 0.63; 95% CI 0.54–0.75). Another study including 5855 individuals post-MI found that full adherence was higher with full prescription coverage for all included medication classes, including statins (OR 1.41; 95% CI 1.18–1.67).24
One review referred to a large study reporting that an increase in cost sharing was associated with lower rates of drug treatment, lower adherence among existing users and more frequent discontinuation of therapy. With a 10% increase in cost-sharing, medication use decreased by 2–6%.22
Comorbidities and Disease History
The presence of different comorbidities can contribute to both adherence and nonadherence to statins.17,19-21 Comorbidities that contribute to nonadherence include depression,17 dementia,19 cancer,19 respiratory disorder,19 renal disease,19 and obstructive pulmonary disease.17 By contrast, diabetes,17,19,20 overweight or obesity,17 and a history of stroke20 or myocardial infarction contribute to adherence.20,21 For instance, Ofori-Asenso et al reported an 11% and 17% higher likelihood of nonadherence among older statin users who had depression (OR 1.11; 95% CI 1.06–1.16) or respiratory disorder (OR 1.17; 95% CI 1.12–1.23), respectively.11 Similarly, Hope et al’s review reported that, in one included study, depression was inversely associated with good adherence after adjustment for other covariates (OR 0.85; 95% CI 0.79–0.93 in men and OR 0.91; 95% CI 0.85–0.95 in women).
In Hope et al’s review, eight studies examined the relationship between diabetes and being adherent. These studies found evidence that people with diabetes were more likely to adhere to statins. Similarly, in the meta-analysis conducted by Ofori-Asenso et al, older patients without diabetes had 9% increased odds of discontinuation of statins (OR 1.09; 95% CI 1.04–1.15). Similarly, Mann et al reported that, in 7 of the 13 included studies, people with diabetes were found to be more likely to be adherent to statins.20
A history of myocardial infarction (MI) or stroke was associated with higher adherence and lower discontinuation in Ofori-Asensio et al’s review; older patients receiving statins for primary prevention had a 49% higher likelihood of nonadherence (OR 1.49; 95% CI 1.40–1.59) and a 66% higher likelihood of discontinuation (OR 1.66; 95% CI 1.24–2.22).19 Similar findings were reported by Mann et al, where 9 of the included studies showed that patients with a history of MI, cardiovascular disease (CVD) or stroke were more likely to be adherent to statins (odds of nonadherence in patients with a history of CVD were 0.68; 95% CI 0.68–0.71).20 Additionally, Lemstra et al reported that, for 18 studies with a total sample size of 982,487, patients who were dispensed statins for primary prevention, in comparison with secondary prevention, were 52% more likely to be nonadherent22 and had a 66% higher likelihood of discontinuation.22
Treatment-Related Factors
Side Effects
Across three reviews, the occurrence of side effects, including myalgia, tiredness, muscle weakness and pain, was reported as causes of nonadherence or discontinuation of statin therapy.17,21,25 Chee et al reported that myalgia accounts for up to 25% of all adverse events related to statins use, resulting in reduced adherence and increased station discontinuation.21 Another included study in this review showed from a wide survey on people with dyslipidaemia that 19.8% of participants discontinued statin therapy and 16.7% reduced their statin dose due to muscular side effects.21
The narrative synthesis conducted by Ju et al showed that some patients felt they had not been properly informed about side effects before starting statin therapy; they indicated this had caused them to speculate that statins caused adverse effects and symptoms as well as to cease the medication to determine whether it was causing the side effects.25
Hope et al’s review reported that, in a large survey, 15% of the patient population with diabetes did not adhere to statin therapy because of the side effects, while another survey concluded that 5% of the patient population had discontinued statin therapy for this reason.17
Treatment Complexity
Across three of the reviews, the treatment regimen complexity and the number of other medications taken concurrently were reported to influence adherence to statins.17,20,24 However, these factors were ambiguous, acting as both facilitators of and barriers to adherence to statins. For instance, Mann et al found a strong relationship between increasing number of non-cardiovascular medications and low statin adherence in seven of eight studies; however, the opposite was shown in one study.20 Similar conclusions were reported in Banerjee et al’s review, which showed that taking more than 10 pills was associated with nonadherence, while fewer medications and primary care follow-up were associated with adherence.24 In Hope et al’s review, four studies investigated the association between the total number of medications a person received and adherence to statins. One study analysis found that patients who took more medications were 10% to 20% more likely to be adherent; however, the remaining three studies found no increased likelihood of adherence per additional medication for men and women, after adjustment for all other variables.17
Different Types of Statins
One review suggested that patients have concerns about the medication class of statins.17 In Hope et al’s review, four studies examined the type of statin, and the effect for particular statins varied greatly across studies. In one included study, it was found that people were more likely to adhere to fluvastatin and rosuvastatin than to simvastatin, and less likely to adhere to lovastatin compared to simvastatin.17
Intensity of Statin Dose
One review reported that, in one of the included studies, the intensity of the statin dose was inversely associated with adherence; men on a moderate daily dose (OR 0.89; 95% CI 0.84–0.94) and men on a high daily dose were less likely to adherence to statin therapy compared to men on a low daily dose of statins (OR 0.70; 95% CI 0.54–0.92). Similar and larger effects were observed in the cohort of women; women on a high daily dose of statins were 60% less likely to adhere compared to women on a low daily dose.17
Health Behavior and Lifestyle Factors
Smoking
One review reported that being a smoker was associated with nonadherence,19 while another review found that being an ex-smoker was associated with adherence.17 Ofori-Asenso et al found that among people ≥65 years of age, being a smoker meant a 12% higher likelihood of nonadherence (OR 1.12; 95% CI 1.03–1.21) and a 14% higher likelihood of discontinuation (OR 1.14; 95% CI 1.06–1.23).19 In Hope et al’s review, one large high-quality study found that being an ex-smoker predicted good adherence (OR 1.20; 95% CI 1.0–1.3).17
Alcohol Consumption
Four studies in Hope et al’s review evaluated the association between alcohol consumption and statin adherence. Two of these studies reported that severe alcohol misuse nearly doubled the risk of nonadherence, after adjustment for other factors.17
Patient Perceptions
Lack of Knowledge About Statins
The regularity of statin use also depends on patients’ knowledge about statins and about the benefits statins can have for prevention of adverse health outcomes. Two reviews reported that some patients did not fully understand why statins were prescribed for them, while others had difficulty understanding the preventive effects of statins, consequently questioning the need to take them, or resulting in forgetting or not prioritizing taking the medication.17,21 Thus, the amount and quality of information on statins transmitted to the patient can be a contributing factor to adherence.21 In Chee et al’s review, patients reported not having heard about the details related to statins from their physicians, or not being satisfied with the information their primary physician had provided about cholesterol-lowering medications, including statins. For instance, discussions regarding the duration of statin therapy rarely occurred in new consultations, and some patients were unaware of the need for long-term therapy, which can increase the likelihood of statin nonadherence.21
Imperceptible Benefits
Reported barriers to adherence also include imperceptible benefits of consuming statins.25 Due to the absence of symptoms linked to high lipidemic levels or of visible improvements in patients’ health condition, it was reported that some patients did not feel ill enough to consume statins, or felt uncertain as to the value of statins, causing them to discontinue statin therapy.25
Questioning the Necessity and Utility of Statins
Two reviews reported that patients’ low perceived susceptibility to dyslipidemia-related complications had led to low perceived need for statins, and consequently poor adherence.21,25 Further, some patients regarded statins as medications indicated only for patients with serious health conditions, and they did not consider themselves ill enough to take statins. Other patients felt their cholesterol levels were close enough to target thresholds, concluding that statins would not be of much benefit to them.22 Some patients were also confused about how statins work in the body and uncertain about the relationship between any residual clogging and using statins. Uncertainty concerning how such a powerful medication is absorbed into the body to target cholesterol caused some to be wary about taking statins. Some formed their own theories about taking statins, and these theories made them nervous about committing to the regimen.25
Medical Distrust
Patients’ suspicions about caretakers overprescribing statins were also found to influence adherence to statins.2,6 Ju et al reported that some patients suspected their healthcare professional might have prescribed statins unnecessarily as an automatic response to slightly elevated cholesterol level, rather than based on a detailed review and consideration of the individual’s clinical characteristics and cardiovascular disease risk profile. Other patients feared perpetual dependence on statins and expressed concerns about having to take statins indefinitely.6
Discussion
The present systematic review of reviews aimed to provide a comprehensive overview of factors that may influence adherence to statins. It was based on literature searches in February 2019. We identified nine relevant reviews that met the inclusion criteria.
Of the sociodemographic factors, high income, high level of education, and being between 50 and 65 years of age seem to have a positive effect on adherence to statins. On the contrary, female sex, non-white race, age younger than 50 years and older than 70, low income, low level of education in men, and high level of education in women, being a new statin user, and having a high co-payment all seem to contribute to nonadherence to statins. Various comorbidities also seem to contribute to nonadherence to statin therapy, including depression, cancer, respiratory disorder, renal disease, and obstructive pulmonary disease, while having a diagnosis of diabetes or hypertension, being overweight or obese, and having a history of stroke or myocardial infarction contribute to better adherence to statins. Furthermore, indicators of regimen complexity have been shown to have a negative effect on adherence whenever the amount of medication being taken concurrently with statins exceeded 10 pills, while consuming fewer than 10 pills seemed to contribute to adherence. The occurrence of various side effects and moderate to high doses of statins also contributed to statin nonadherence. Smoking and alcohol consumption had a negative effect on adherence. Patients’ lack of knowledge, imperceptible benefits, questioning the necessity and utility of statins, and medical distrust contributed to nonadherence.
The use of the AMSTAR 2 tool provided detailed quality assessment as its items involved rigorous and transparent steps for conducting a systematic review of reviews and for assessing biases to the results. In this review of reviews, however, the overall quality of included reviews was generally assessed as low (ranged from critically low to moderate) according to the AMSTAR 2 combined score. This raises questions about the validity of the review conclusions. Lack of research protocol statement and registration may affect the transparency and integrity of the review results. Lack of a list of excluded studies may leave some information missing, while lack of revealing funding sources of included primary studies may make readers ignore factors affecting objectivity and reliability of reviews. As such, the findings of the review should be interpreted in view of the sub-optimal quality. Altogether, the current methodological and reporting quality of reviews about statin adherence needs to be improved, and review authors should strive to comply with contemporary guidelines to make the results more transparent and scientific. However, the suboptimal quality assessment of the included reviews may also imply that many reviews, in general, fail to meet the ideal quality of methodological appraisals suggested by the AMSTAR 2 guidelines. Particularly older reviews that were initiated before the relatively new AMSTAR 2 guidelines became available. In this study, the two highest rated reviews17,24 were both relatively new and future review studies within the field are more likely to be of high quality.
Authors of included reviews generally concluded that the reliability of the included primary studies was low to moderate or did not comment on the quality of primary studies. The low to moderate quality of primary studies may further affect reliability of the evidence of reviews on statin adherence. Thus, more high-quality research in this field is warranted.
Strengths and Limitations
The present review of reviews has several methodological strengths. We conducted a broad and comprehensive literature search to identify reviews in three electronic databases that index biomedical systematic reviews. No limits on publication year or language were applied. Each phase of the screening process was performed independently by at least two authors. Moreover, we used the widely acknowledged tool AMSTAR 2 to appraise the quality of the included systematic reviews.
Our review was also subject to limitations. The included reviews were widely heterogeneous and the findings covered a wide range of diverse predictors of statin nonadherence, which led to difficulties in synthesizing any overall findings. Focusing on a selected type of predictors would offer better opportunities to conduct a thorough analysis. Although we carried out a thorough literature search, some relevant reviews may not have been included in the present study. The quality assessment indicated sub-optimal quality of the included reviews which may also compromise findings in this review. Future review studies in this field should adhere to contemporary quality standards to ensure high quality of their work. The findings of this review must be interpreted in the context of its limitations.
Conclusion
The present review identifies some key indicators of nonadherence to statins. The bulk of evidence for nonadherence to statins is growing, although much of the evidence is related to factors that are not easily modifiable. The present findings could be of benefit to healthcare professionals, researchers, decisionmakers, and patients. The review may provide a framework to guide initiatives aiming to improve adherence among statin users.
Disclosure
All authors are employed at Steno Diabetes Center Copenhagen, a hospital and research institution under the Capital Region of Denmark, which is partly funded by a grant from the Novo Nordisk Foundation. The funder had no role in any part of this article. Mr Ole Norgaard reports grants from Novo Nordisk Foundation, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.World Health Organization. Global Status Report on Noncommunicable Diseases 2010 2011. ISBN 978 92 4 156422 9.
- 2.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilbert JJ. The World Health Report 2002 - Reducing Risks, Promoting Healthy Life. World Health Organization. Vol. 16 2003. doi: 10.1080/1357628031000116808 [DOI] [PubMed] [Google Scholar]
- 4.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease (Review). Cochrane Database Syst Rev. 2013;1:1–97. doi: 10.1002/14651858.CD004816.pub5.www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–1389. doi: 10.1016/S0140-6736(94)90566-5 [DOI] [PubMed] [Google Scholar]
- 6.Lemstra M, Blackburn D. Nonadherence to statin therapy: discontinuation after a single fill. Can J Cardiol. 2012;28(5):567–573. doi: 10.1016/j.cjca.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry HJ, McDermott B. Recognizing and improving patient nonadherence to statin therapy. Curr Atheroscler Rep. 2008;10(1):19–24. doi: 10.1007/s11883-008-0004-4 [DOI] [PubMed] [Google Scholar]
- 8.Helin-salmivaara A, Lavikainen P, Korhonen M, et al. Patterns of statin use among 10 cohorts of new users from 1995 to 2004: a register-based nationwide study. Am J Manag Care. 2010;16(2):116–122. [PubMed] [Google Scholar]
- 9.Casula M, Tragni E, Catapano AL. Adherence to lipid-lowering treatment: the patient perspective. PatientPreferAdherence. 2012;6(1177–889X(Electronic)):805–814. doi: 10.2147/PPA.S29092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perreault S, Blais L, Dragomir A, et al. Persistence and determinants of statin therapy among middle-aged patients free of cardiovascular disease. Eur J Clin Pharmacol. 2005;61(9):667–674. doi: 10.1007/s00228-005-0980-z [DOI] [PubMed] [Google Scholar]
- 11.Ofori-Asenso R, Zomer E, Curtis A, et al. Patterns and predictors of adherence to statin therapy among older patients: protocol for a systematic review. JMIR Res Protoc. 2017;6(3):e39. doi: 10.2196/resprot.7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1). doi: 10.1186/1471-2288-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(7716):332–336. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller SA, Forrest JL. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Based Dent Pract. 2001;1(2):136–141. doi: 10.1067/med.2001.118720 [DOI] [Google Scholar]
- 15.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas J, Brunton J, Graziosi S. EPPI-Reviewer 4.0: Software for Research Synthesis. London: Social Science Research Unit, Institute of Education, University of London; 2010. [Google Scholar]
- 17.Hope HF, Binkley GM, Fenton S, Kitas GD, Verstappen SMM, Symmons DPM. Systematic Review of the Predictors of Statin Adherence for the Primary Prevention of Cardiovascular Disease. PLoS One. 2019;14(1):1-38. doi: 10.1371/journal.pone.0205138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewey J, Shrank WH, Bowry ADK, Kilabuk E, Brennan TA, Choudhry NK. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J. 2013;165(5):665–678.e1. doi: 10.1016/j.ahj.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 19.Ofori-Asenso R, Jakhu A, Curtis AJ, et al. A systematic review and meta-analysis of the factors associated with nonadherence and discontinuation of statins among people aged ≥65 years. Journals Gerontol Ser A. 2018:1–8. doi: 10.1093/gerona/glx256 [DOI] [PubMed] [Google Scholar]
- 20.Mann DM, Woodward M, Munther P, Falzon L, Kronish I. Predictors of non-adherence to statins: a systematic review and meta-analysis. Ann Pharmacother. 2010;44(9):1410–1421. doi: 10.1345/aph.1P150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chee Y, Chan V, Tan N. Understanding patients’ perspective of statin therapy: can we design a better approach to the management of dyslipidaemia? A literature review. Singapore Med J. 2014;55(8):416–421. doi: 10.11622/smedj.2014099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can J Cardiol. 2012;28(5):574–580. doi: 10.1016/j.cjca.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 23.Mann BS, Barnieh L, Tang K, et al. Association between drug insurance cost sharing strategies and outcomes in patients with chronic diseases: a systematic review. PLoS One. 2014;9(3):e89168. doi: 10.1371/journal.pone.0089168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee A, Khandelwal S, Nambiar L, et al. Health system barriers and facilitators to medication adherence for the secondary prevention of cardiovascular disease: a systematic review. Open Hear. 2016;3(2):e000438. doi: 10.1136/openhrt-2016-000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju A, Hanson CS, Banks E, Korda R, Craig JC, Usherwood T. Patient beliefs and attitudes to taking statins: systematic review of qualitative studies. Br J Gen Pract. 2018;68(671):e408–e419. doi: 10.3399/bjgp18X696365 [DOI] [PMC free article] [PubMed] [Google Scholar]