Abstract
Introduction
The biological changes after irradiation in lung cancer cells are important to reduce recurrence and metastasis of lung cancer. To optimize radiotherapy of lung adenocarcinoma, our study systematically explored the mechanisms of biological behaviors in residual A549 and XWLC-05 cells after irradiation.
Methods
Colony formation assay, cell proliferation assay, cell migration assay, flow cytometry, BALB/C-nu mice xenograft models and Western blot of pan-AKT, p-Akt380, p-Akt473, PCNA, DNA-PKCS, KU70, KU80, CD133, CD144, MMP2 and P53 were used in our study to assess biological changes after irradiation with 0, 4 and 8 Gy at 0–336 hr after irradiation in vitro and 20 Gy at transplantation group, irradiated transplantation group, residual tumor 0, 7, 14, 21, and 28 days groups in vivo.
Results
The ability of cell proliferation and radiosensitivity of residual XWLC-05 cells was better than A549 cells after radiation in vivo and in vitro. MMP-2 has statistical differences in vitro and in vivo and increased with the migratory ability of cells in vitro. PCNA and P53 have statistical differences in XWLC-05 and A549 cells and the changes of them are similar to the proliferation of residual cells within first 336 hr after irradiation in vitro. Pan-AKT increased after irradiation, and residual tumor 21-day group (1.5722) has statistic differences between transplantation group (0.9763, p=0.018) and irradiated transplantation group (0.8455, p=0.006) in vivo. Pan-AKT rose to highest when 21-day after residual tumor reach to 0.5 mm2. MMP2 has statistical differences between transplantation group (0.4619) and residual tumor 14-day group (0.8729, p=0.043). P53 has statistical differences between residual tumor 7-day group (0.6184) and residual tumor 28 days group (1.0394, p=0.007). DNA-PKCS has statistical differences between residual tumor 28 days group (1.1769) and transplantation group (0.2483, p=0.010), irradiated transplantation group (0.1983, p=0.002) and residual tumor 21 days group (0.2017, p=0.003), residual tumor 0 days group (0.5992) and irradiated transplantation group (0.1983, p=0.027) and residual tumor 21 days group (0.2017, p=0.002). KU80 and KU70 have no statistical differences at any time point.
Conclusion
Different proteins regulated apoptosis, proliferation and metastasis of lung adenocarcinoma after radiotherapy at different times. MMP-2 might regulate metastasis ability of XWLC-05 and A549 cells in vitro and in vivo. PCNA and P53 may play important roles in proliferation of vitro XWLC-05 and A549 cells within first 336 hr after irradiation in vitro. After that, P53 may through PI3K/AKT pathway regulate cell proliferation after irradiation in vitro. DNA-PKCS may play a more important role in DNA damage repair than KU70 and KU80 after 336 hr in vitro because it rapidly rose than KU70 and KU80 after irradiation. Different cells have different time rhythm in apoptosis, proliferation and metastasis after radiotherapy. Time rhythm of cells after irradiation should be delivered and more attention should be paid to resist cancer cell proliferation and metastasis.
Keywords: lung adenocarcinoma, XWLC-05 cells, biological, radiation
Introduction
Lung cancer is one of the most malignant tumors with the highest incidence, the mortality of which is the highest among all tumors.1,2 Surgery, Radiation therapy and chemotherapy are the main treatment strategies for advanced NSCLC.
Radiotherapy killed cancer cells by breaking DNA damage directly or indirectly.3 Radiotherapy is an important treatment for early or advanced lung cancer patients.2 With the development of radiotherapy, radiotherapy becomes more and more accurate. However, the recurrence and metastasis of lung cancer still exist after irradiation.
Zheng et al4 reported that X-ray irradiation improved the metastasis of tongue squamous cell carcinoma (TSCC) cells increasing dose-dependently with exposure to X-ray radiation. Residual tumors after chemotherapy grew slower than before, but the metastatic ability was significantly enhanced in MHCC97L and HepG2 cells of hepatocellular carcinoma and transplanted tumors in nude rats.5 The biological behavior of lung cancer cells after radiotherapy may change, but the specific changes and mechanisms need to be studied.
Our study chose XWLC-05 and A549 human adenocarcinoma cell line to compare the differences in the biological behavior of XWLC-05 and A549 residual cells after irradiation. By adding different doses of X-ray irradiation to XWLC-05 and A549 cells, we recorded and analyzed the changes in radiation sensitivity, cell proliferation, migration, cell cycle regulation, apoptosis, adenocarcinoma stem cells and protein expression at different times. This study aims to guide the comprehensive treatment of lung cancer, especially individualized radiotherapy.
PI3K/AKT pathway and PCNA play an important role in cancer metabolism and proliferation.6 DNA-PKCS, KU70 and KU80 play important roles during the repair of DNA damage. CD133, CD144, MMP2 and P53 can affect cancer stem cells and metastasis. Most reports did not explore the dynamic protein changes at different time after irradiation. The mechanism of lung cancer recurrence and metastasis are unknown. If the time rhythm of cancer cells proliferation and metastasis after irradiation were confirmed, we can choose the best time to reduce cancer cells proliferation or transformation by irradiation. So, we chose pan-AKT, p-Akt380, p-Akt473, PCNA, DNA-PKCS, KU70, KU80, CD133, CD144, MMP2 and P53 to assess the mechanism of recurrence and metastasis during lung cancer cell growth, metastasis and stem cells after irradiation. We found that different proteins play important roles in apoptosis, proliferation and metastasis of lung adenocarcinoma after radiotherapy at different times. The time of delivery of radiotherapy should be long enough to resist cancer cell proliferation. Pathway inhibitor combined with radiotherapy should be used at a reasonable time which may improve the effect of pathway inhibitor combined with radiotherapy.
Methods
A549 and XWLC-05 Cell Culture and Irradiation
The XWLC-05 cell was a kind of human lung adenocarcinoma cell line, supported by the first No.1 Affiliated Hospital of Kunming Medical University.7–10 The A549 cell line (Cell center, Yunnan, China) and XWLC-05 cell line were maintained in RPMI-1640 (HyClone, USA) containing 10% fetal bovine serum (FBS; Gibco, USA). Cells were incubated at 37°C in a humidified incubator with 5% CO2 and treated with different radiation doses of 4 and 8 Gy. Irradiation dose rate of X-rays was 2.2 Gy/min in the Irradiation (IR) Center of the Third Affiliated Hospital of Kunming Medical University.
A549 and XWLC-05 Xenograft Models and Tissue Cell Suspension
Male BALBc-nu mouse (aging 3–4 weeks old and weighting 10–15g) were obtained from Hunan SJA Laboratory Animal Company Limited (Hunan, China). All mice were lacking T cells and kept in a specific pathogen-free (SPF) animal laboratory of Kunming Medical University. After 7-day adaptive feeding, we concentrated A549 or XWLC-05 cells at the logarithmic phase and adjusted with PBS to 2×108 cells/mL by Moxi Z mini automated cell counter kit (Orflo, America). We injected 100ul (2×107cells) A549 or XWLC-05 cells under the left axillary skin of a mouse. The diameters of tumors were observed every day (Tumor volume (cm3)=ab2/2, a: maximum diameter; b: minimum diameter11). Transplantation tumor mice were killed or irradiated when tumor volume reached 1cm3 (transplantation group and irradiated transplantation group). Irradiated transplantation tumor mice were killed when the tumor diameters grew up to 1cm again. Then, Irradiated transplantation tumors were separated and cut into 1mm3. We injected 2 pieces of irradiated transplantation tumor specimens under the left axillary skin of another 3–4-week male BALBc-nu mouse by mouse trocars. In this way, we made residual tumor mice models. Residual tumor mice were killed on days 0, 7, 14, 21, 28 after tumors reached 0.5cm3. All tumors were separated and weighted. We had a pre-experiment to find out the irradiation dose that mouse models could bear. We tried 4 Gy, 6Gy, 10Gy, 15Gy and 20Gy local irradiation. Because higher doses cause more significant changes in vitro, we chose 20 Gy to get outcomes to hypofractionated radiation therapy that would be more similar to those in humans.
We separated and cut tumors into specimens, and then we used 0.5% IV collagenase (biosharp, China) at 37°C for 1.5 hr. After being filtered by sterilized nylon networks (200 mesh, Huaihe, China), cell suspensions were centrifuged (1000 rpm, 5 min) and resuspended 3 times with phosphate-buffered saline solution (PBS, Hyclone, USA). Red blood cells were removed with 7μm pre-separation filter (Mitenyi Biotec GmbH, Bergisch Gladbach, Germany). In this way, we made tissues into cell suspensions for flow cytometry. All of our operations were in sterile environment and instruments and containers were sterilized also.
The mouse experiments were approved by the ethics committee of Kunming Medical University. Animal experiments followed the guidelines for the welfare of the national standard “Laboratory Animal-Requirements of Environment and Housing Facilities” (GB 14925-) and conformed to “Yunnan Administration Rule of Laboratory Animal”. Mice were randomly assigned into transplantation group, irradiated transplantation group, and residual tumor 0-, 7-, 14-, 21-, 28-day groups, with 5 mice in each group.
Cell Proliferation Assay
The cell proliferation was detected by the Cell Counting Kit-8 (CCK8, Dojindo, Japan) assay at 24, 48, 72, 96, 336, 360, 384, and 408 hr after 4 Gy and 8 Gy irradiation. The cells were seeded in a 96-well plate at a density of 3 × 103 cells per well.
Briefly, 20 μL of the CCK8 solution (5 mg/mL) was added to each well, and the cells were incubated for another 4 hr at 37°C. The optical density was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA). The viability index was calculated as the experimental OD value/the control OD value. Three independent experiments were performed in quadruplicate.
Colony Formation Assay
To analyze colony formation, single cells were plated in a 10 cm dish before 0, 0.5,1, 2, 4, 6, and 8 Gy 6MV X-ray irradiation. The number of cells was 100, 100, 200, 400, 1000, 4000, and 8000, respectively. The cells were removed after being cultured under a static condition for 13 days. The colonies containing 50 or more cells were counted. Colonies were fixed with 99% methanol for 30 min and then stained with 0.1% crystal violet for 20 min. SF (surviving fraction) = Number of colonies/(cells inoculated × plating efficiency). We used SF to calculate D0 (mean lethal dose) and α/β of cells by single-hit multitarget model and liner quadric (LQ) model.
Cell Apoptosis Analysis by Flow Cytometry
An annexin V-FITC /PI double staining assay (BD, USA) was used to detect the apoptosis of A549 and XWLC-05 cells 12, 24, 48, 96, and 192 hr after 4 Gy and 8Gy (respectively) 6MV X-ray irradiation. The cells were washed twice with cold PBS and then resuspended in Annexin V Binding Buffer at a concentration of 1 × 106 cells/mL. Then, 100 μL of the solution (1 × 105 cells) was transferred to a 5 mL culture tube.
Next, 5 μL of FITC-annexin V and 5 μL PI were added with gentle vortexing and incubation for 15 min at room temperature (25°C) in the dark. Then, 400 μL of Binding Buffer was added to each tube and analyzed by flow cytometry within 1 hr, so as in tumor suspensions.
Cell Cycle Analysis by Flow Cytometry
Attached cells were harvested at 12, 24, 48, 96, and 192 hr after 4 Gy and 8 Gy radiation for cycle measurement using DAPI (SYSMEX PARTEC Germany). The cells were washed twice with cold PBS and 1 mL of DAPI was added to each tube and analyzed by flow cytometry within 1 hr, so as in cell suspensions of tumor tissues.
CD44 and CD133 Surface Expression by Flow Cytometry
Attached A549 cells and XWLC-05 cells were collected at 12, 24, 48, 96, and 192 hr after either single 4 Gy or 8 Gy irradiation for CD44 and CD133 surface expression detection. For CD133 staining, 1 × 106 cells were stained with 20 μL CD133 mouse mAb (CST, USA). The antibody was incubated for 60 min at room temperature in the dark. Then, 2.5 μL of anti-mouse IgG Fab2 Alexa Fluor (R) 647 Molecular Probes were added. The antibody was incubated for 30 min at room temperature in the dark. Then, samples were washed 3 times with PBS. For single CD133 staining of tumor tissue suspension cells, we put 20ul Human CD133 PE-conjugated Antibody (R&D, USA) into 1 × 106 cells (100ul) tumor suspensions for 30 min at RT, then samples were washed for 3 times with PBS.
For CD44 staining, 1 × 106 cells were stained with 20 μL Hu CD44 APC G44-26 (BD, USA) and incubated for 30 min at RT in the dark. The samples were washed 3 times with PBS, so as in tumor tissue suspension cells for single CD44 staining.
For CD133 and CD44 co-staining of tumor tissue suspension cells, we put 20ul Human CD133 PE-conjugated Antibody (R&D, USA) and 20ul Hu CD44 APC G44-26 into 1 × 106 cells (100ul) tumor suspensions for 30 min at RT, then samples were washed for 3 times with PBS. All stained samples analyzed by flow cytometry (Beckman, USA) within 1 hr.
Cell Migration Assay
The cell migration was measured using a transwell assay 24, 48, 96, and 192 hr after either single 4 Gy or 8 Gy radiation. Irradiated A549 and XWLC-05 cells were seeded in transwells (Corning Incorporated, NY, USA) at a density of 6 × 104 cells/well. Cells with 200 μL of RPMI1640 supplemented with 10% fetal bovine serum (FBS; Gibco, USA) were incubated for 24 hr at 37°C. Cells were fixed via incubation with 100% methanol for 30 min and stained with 0.1% crystal violet for 20 min. Migrated cells on the lower surface of the filter were counted per filter in random microscopic fields at a 40-fold magnification. The reported values are the means of three independent experiments.
Western Blot Analysis
The cells were harvested at 12, 24, 48, 96, and 192 hr after either single 4 Gy or 8 Gy irradiation and transplantation group, irradiated transplantation group, residual tumor 0-, 7-, 14-, 21-, 28-day group tumors respectively used in RIPA lysis buffer (Solarbio, China). The protein was collected at 12,000 rpm and 4°C. Proteins were resolved on 8% SDS polyacrylamide gels, transferred onto nitrocellulose membranes, blocked with 5% nonfat dry milk, and then incubated with the primary antibodies: 1:1000 for p53 (Elabscience, China), 1:1000 for PCNA (2586s, CST, USA), 1:1000 for MMP2 (4022s, CST, USA), 1:1000 for MS-ku80 (Ab119935, abcam, USA), 1:1000 for KU70 (D10A7)(4588s, CST, USA), 1:1000 for ATK (Pan) (4691s, CST, USA), 1:1000 for P-AKT (Thr308) (5106S, CST, USA), 1:1000 for P-AKT (Ser473) (12694s, CST, USA), 1:1000 for DNA-PKCS (123113, CST, USA), 1:3000 for GAPDH (Elabscience, China) and 1:2000 forβ-actin (AF0501, Elabscience, China). The membrane was incubated with primary antibody overnight at 4°C. The blots were washed with 1% PBST and then incubated with rabbit-anti goat IgG (SAB) or goat anti-rabbit IgG (SAB) conjugated to horseradish peroxidase for 2 hr at room temperature. The immunoreactive protein bands were developed by enhanced chemiluminescence, and the immunoreactive bands were analyzed by a densitometer.
Statistical Analysis
The data are depicted as the mean ± SD for normal distribution parameters and the median for non-normal distribution parameters. The multivariate analysis of variance for normal distribution parameters and Kruskal–Wallis H text for non-normal distribution parameters was used to compare independent samples. All analyses were performed on SPSS 22.0, and differences were considered significant if p < 0.05.
Results
Different Effects on Cell Proliferation and Apoptosis of Residual A549 or XWLC-05 Cells During Radiation
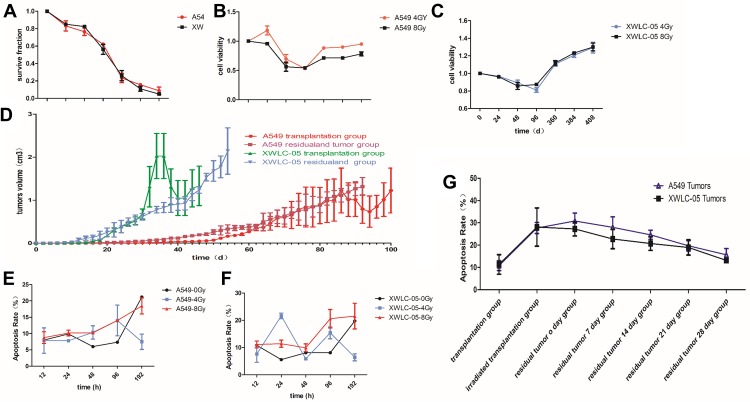
We calculated SF (SF (surviving fraction) = Number of colonies/(cells inoculated × plating efficiency)); then, we used SF to calculate D0 (mean lethal dose) by single-hit multitarget model (S=extrapolation number×e−k×Dose) and α/β of cells by and liner quadric (LQ) model (BED=n×dose×[1+dose/(α/β)]). With the increasing radiation dose, SF decreased gradually. The survival fraction of A549 cells was higher than that of XWLC-05 cells in vitro (Figure 1A). D0 is a reflection of radiosensitivity in cells. Higher value of D0 means worse radiosensitivity. D0 of A549 cells was 3.224Gy while XWLC-05 cells were 2.447Gy, A549 cells have worse radiosensitivity than XWLC-05 cells. Radiation causes reversible sublethal damage in cancer cells, less value of α/β represents the ability to repairing cell sublethal damage is better. The α/β of A549 is 19.92 while XWLC-05 is 9.18.
Figure 1.
Cell proliferation and apoptosis of residual XWLC-05 higher than A549 cells. (A) survival fraction of A549 cells and XWLC-05 cells. (B) Proliferation viability of A549 and XWLC-05 cells. (C) Cell viability of XWLC-05 cells after 4Gy and 8Gy irradiation. (D) Tumor volumes of A549 and XWLC-05 cells. (E) Apoptosis rate of A549 cells. (F) Apoptosis rate of XWLC-05 cells. (G) Apoptosis rate of A549 and XWLC-05 tumors.
Radiation suppressed the proliferation of A549 cells and XWLC-05 cells within 96 hr in a time-dependent manner. There were no significant differences in the proliferation between the 8 Gy radiation and 4 Gy radiation (p>0.05, Figure 1B and C).
In vitro, radiation made the volumes of tumors decreased for several days, then it increased again. XWLC-05 tumors grow faster than A549 cell tumors before and after irradiation (Figure 1D). A549 transplantation group (0.196, p=0.000) and A549 residual and tumor group (0.075, p=0.033) have statistical differences with XWLC-05 residual and tumor group (0.547).
There were no statistical differences in every vivo A549 and XWLC-05 groups (p>0.05). However, there had been statistical differences in total (A549 plus XWLC-05) irradiated transplantation group (10.305) with transplantation group (29.625, p=0.018) and residual tumor 0-day group (30.224, p=0.007). Moreover, there had been statistical differences in residual tumor 0-day group (30.224) with residual tumor 28-day group (13.7, p=0.043). This suggests that A549 and XWLC-05 tumor cell apoptosis rates increased after irradiation and then decreased to baseline during 0–7days after residual tumor reached 1cm3.
In vivo and vitro, A549 and XWLC-05 tumor cell apoptosis rates increased after irradiation (Figure 1E–G). The cell apoptosis rates of the two irradiated groups were increased after radiation and was higher with 8 Gy radiation than 4 Gy radiation. The highest cell apoptosis rate in A549 cells and XWLC-05 cells was seen at 192 hr and 48 hr after radiation. 48-h group (8.56) presented a statistical difference with 192h group (18.4950, p=0.041) after irradiation. In vivo, A549 and XWLC-05 tumor cell apoptosis rates increased after irradiation. However, there were no statistical differences in the similar tumor groups (P>0.05).
Different Effects on Cells Cycle of Residual A549 or XWLC-05 Cells During Radiation
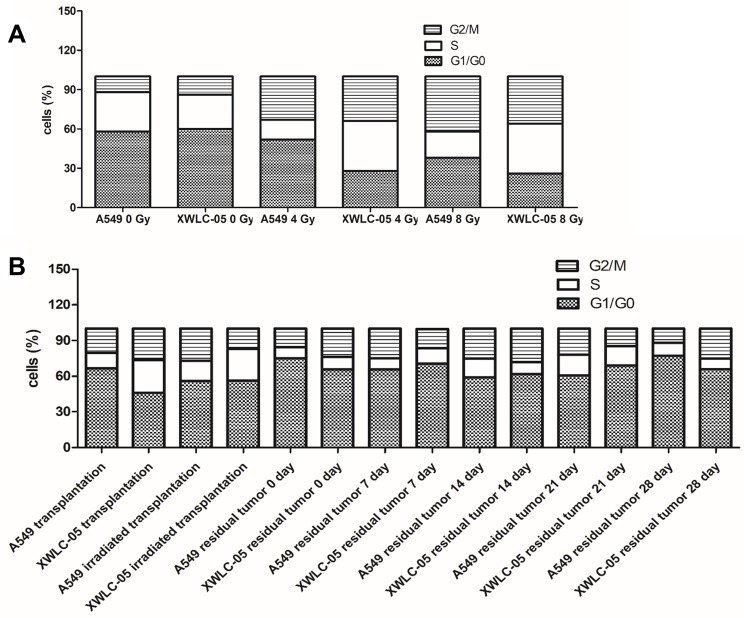
The effect of radiation on the cell cycle was assessed using a DAPI assay. The cell cycle of A549 and XWLC-05 cells (Figure 2A) and cell suspensions of tumor tissues (Figure 2B) were changed to different degrees after radiation.
Figure 2.
Cell cycle of residual XWLC-05 and A549 cells has little differences. (A) Cell cycle of A549 and XWLC-05 cells. (B) Cell cycle of of A549 and XWLC-05 tumor tissues.
G1, S, G2/M phase of 4Gy and 8Gy presented no statistical differences (P>0.05) in XWLC-05 and A549 cells. The proportion of G1 phase in A549 and XWLC-05 increased at first. Compared with the unirradiated control group, the proportion of G2/M phase in irradiated A549 and XWLC-05 cells was increased after radiation. The highest proportion of G2/M phase in A549 cells was observed 12 hr after 8 Gy radiation (P<0.05), but in XWLC-05 cells, the highest peak of G2/M phase was observed 48 hr after 8 Gy radiation (P<0.05). Considering time point affection, 192-hr group (16.3) presented statistic difference with 48-hr (19.51, p=0.049) or 96-hr group (21.28, p=0.034) after irradiation. Highest peak of G2/M phase may occur at 96h after irradiation.
In vivo, the percent of G1, S, G2/M phase cells of XWLC-05 and A549 tumor increased after irradiation had no statistical differences (p=0.213). G1, S or G2/M phase cells of different time presented no statistical difference after irradiation (p≥0.05).
Differences in Stem Cells of XWLC-05 or A549 Cells During Radiation
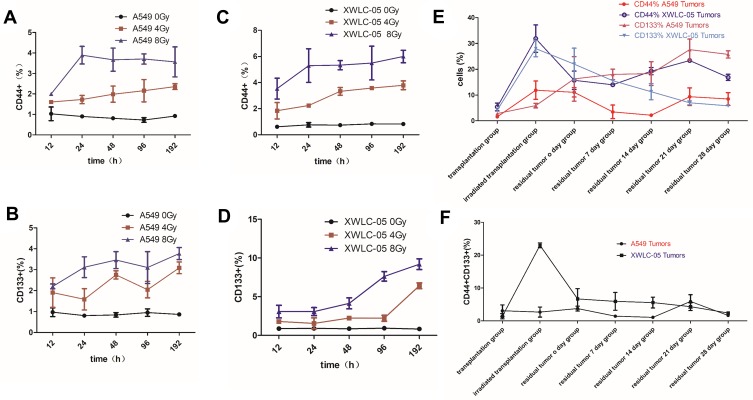
CD44 and CD133 are biomarkers of lung cancer stem cells. CD44 or CD133 expression in A549 cells and XWLC-05 were increased with time (Figure 3A–F).
Figure 3.
Differences in stem cells of XWLC-05 or A549 cells during radiation. (A) CD44 expression in A549 cells with time after 0, 4, 8 Gy irradiation. (B) CD44 expression in XWLC-05 with time after 0, 4, 8 Gy irradiation. (C) CD133 expression in A549 cells with time after 0, 4, 8 Gy irradiation. (D) CD133 expression in XWLC-05 with time after 0, 4, 8 Gy irradiation. (E) CD444+ and CD133+ cells in irradiated transplantation group A549 and XWLC-05 tumors. (F) CD444+CD133+ cells in irradiated transplantation group A549 and XWLC-05 tumors.
The percentages of CD44+ or CD133+ cells in 8-Gy groups were higher than 4-Gy groups, but there are no statistical differences between every group (P>0.05) (Figure 3A–D). The percentage of CD44+, CD133+ and CD444+CD133+ cells (Figure 3E and F) in XWLC-05 tumor increased after irradiation while A549 group increased little; however, there are no statistic differences between every vivo A549 and XWLC-05 groups (p>0.05). CD44+ cells has statistic differences between transplantation group (18.7150) and irradiated transplantation group (2.6645, p=0.034), CD133+ cells have statistic differences between irradiated transplantation group (3.3860) and residual tumor 0-day group (15.795, p=0.034). But there have been no statistical differences between CD44+CD133+ cells. Considering CD44+CD133+ cells were more representative than CD44+ or CD133+ cells, these results suggest a greater possibility that differences in stem cells of vivo XWLC-05 or A549 Cells during radiation may not be significant.
Differences in Migration Ability of Residual A549 or XWLC-05 Cells in vivo
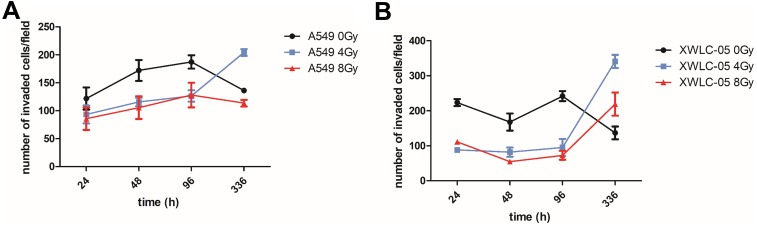
The migration ability of A549 and XWLC-05 (Figure 4A and B) cells was changed after radiation in a time-dependent manner. The migratory ability of A549 cells was stronger than that of XWLC-05 cells during 24 to 96 hr after 4 Gy radiation. A 336 hr after 4-Gy or 8-Gy radiation, the migratory ability of XWLC-05 cells was stronger than that of A549 cells. The migratory ability of XWLC-05 cells and A549 cells after 8 Gy radiation was significantly weaker than 4 Gy radiation at 336 hr after radiation. The migration ability of residual A549 or XWLC-05 cells was increased in vivo, but because of there different time rhythm, there were no statistical differences at every time point.
Figure 4.
Differences in migration ability of residual A549 or XWLC-05 Cells in vivo. (A) Migration ability of A549 cells. (B) Migration ability of XWLC-05 cells.
P53/PI3K/AKT Ability of Residual A549 or XWLC-05 Cells in vivo and vitro
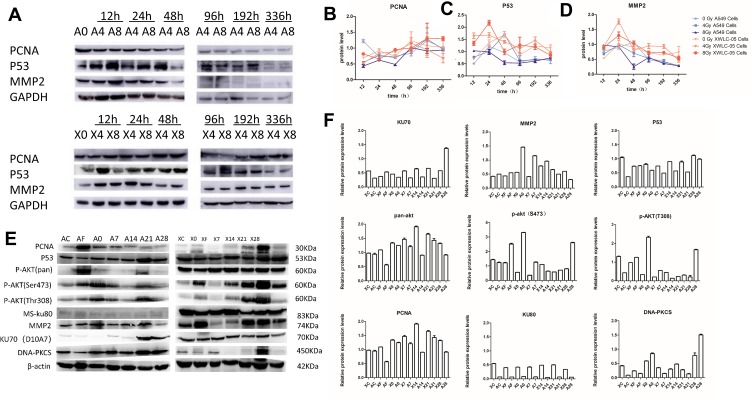
The unirradiated and irradiated A549 and XWLC-05 cells were collected 12, 24, 48, 96, 192 and 336 hr after radiation. The changes in PCNA expression were not consistent with the proliferation of A549 and XWLC-05 cells after radiation. The expressions of p53 in the irradiated A549 and XWLC-05 groups were consistent with the changes of cell apoptosis. The unirradiated and irradiated A549 and XWLC-05 cells were collected 12, 24, 48, 96, 192 and 336 hr after radiation. MMP-2 might regulate metastasis ability of XWLC-05 and A549 cells in vitro. PCNA, P53 may play important roles in proliferation of vitro residual XWLC-05 and A549 cells within 336 hr in vitro. The expression of MMP-2, PCNA, P53 has no statistical differences at every group (p>0.05). PCNA, P53, MMP2 raised after irradiation and then decreased. PCNA, P53, MMP2 reached highest at 192h (1.15), 24h (1.27) and 24h (1.00) (Figure 5A–D).
Figure 5.
P53/PI3K/AKT Ability of Residual A549 or XWLC-05 Cells in Vivo. (A) Western blot of A549 and XWLC-05 cells. (B) Expression of PCNA. (C) Expression of P53. (D) Expression of MMP2. (E) Western blot of A549 and XWLC-05 tumors. (F) Expression of P53 of pan AKT, DNA – PKCS, MMP2, Ku70, Ku80 and P53 changed little. PCNA, p-AKT (Thr308) and p-AKT (ser473) in A549 and XWLC-05 tumors.
PCNA has no statistical differences at every time point in vivo. AKT has statistical differences at every time point in vivo. DNA-PKCS may take a more important role than KU70 and KU80 in DNA damage repair in vivo. MMP-2 might regulate metastasis ability of XWLC-05 and A549 cells in vivo (Figure 5E and F). Pan AKT increased after irradiation, and residual tumor 21-day group (1.5722) has statistic differences between transplantation group (0.9763, p=0.018) and irradiated transplantation group (0.8455, p=0.006). pAkt (thr380) and pAkt (ser407) have no statistical differences at every time point. MMP2 has statistical differences between transplantation group (0.4619) and residual tumor 14-day group (0.8729, p=0.043). P53 has statistical differences between residual tumor 7-day group (0.6184) and residual tumor 28-day group (1.0394, p=0.007). P53 pathway may regulate tumor apoptosis by PI3K/AKT pathway after irradiation in vivo. DNA-PKCS has statistic differences between residual tumor 28-day group (1.1769) with transplantation group (0.2483, p=0.010), irradiated transplantation group (0.1983, p=0.002) and residual tumor 21-day group (0.2017, p=0.003), residual tumor 0-day group (0.5992) with irradiated transplantation group (0.1983, p=0.027) and residual tumor 21-day group (0.2017, p=0.002). KU80 and KU70 have no statistical differences at every time point.
Discussion
This is the first study to compare the biological behavior and molecular biology between Xuanwei lung adenocarcinoma XWLC-05 and normal lung adenocarcinoma A549 cell in vitro after radiation. In our study, we found that the capacity of cell proliferation and radiosensitivity of residual XWLC-05 cells were better than A549 cells after radiation in vivo and vitro. MMP-2 might regulate metastasis ability of XWLC-05 and A549 cells in vitro and vivo. PCNA, P53 may play important roles in proliferation of residual XWLC-05 and A549 cells within the first 336 hr after irradiation in vitro. PCNA has no statistical differences at every time point in vivo. AKT plays an important role in vivo cells proliferation. DNA-PKCS may take more important role than KU70 and KU80 in DNA damage repair in vivo. Some researchers have found that the proliferation and migration potentials of cancers (cervical cancer C33A and CaSki cells,12 colorectal cancer SW480, SW620 and HCT116 cells,13 hepatocellular carcinoma MHCC97L, HepG2, Hep3B and Huh7 cells,14 glioblastoma U87 cells,15 glioma U251 cells,16 and tongue squamous cell carcinoma Tca-8113 cells4) were enhanced after radiation. p53 promotes apoptosis and regulates cell cycles in response to cellular stress signals. Transcriptional activation by p53 is critical for the induction of apoptosis.17 Transcriptional activation by p53 upregulates BAD during DNA damage-induced radiation. p53 can translocate from nucleus into cytoplasm and interact with BAD. p53 translocates to mitochondrial outer membrane and increases its permeability, inducing cell apoptosis.18 Targeted therapies for p53-deficient genotype lung cancer are feasible and significant.19 In our study, we found that after radiation p53 expression in XWLC-05 and A549 cells was enhanced, which was consistent with the cell apoptosis observed after radiation. PCNA, due to its role in proliferation, has been widely used as a tumor marker for cancer cell progression and patient prognosis.20–22 Our study found that the PCNA expression in A549 cells decreased below the unirradiated control levels after radiation, whereas 192 hr after radiation, the PCNA expression in A549 cells was enhanced. The change of PCNA expression in A549 cells was not completely consistent with cell proliferation, which was suppressed 12 to 336 hr after radiation. The change of PCNA expression in XWLC-05 cells was not consistent with the proliferation of XWLC-05 cells, which was suppressed within 96 hr after radiation and increased 360 hr after radiation. It is well known that proteins can be expressed in different parts of the cell. Different protein expression sites in cells have different effects on cell biological behavior. Proliferation of lung adenocarcinoma cells after radiation was not consistent with the changes in PCNA expression.
CSCs are known to be radiation resistant and chemoresistant. CSCs also cause tumor metastasis and relapse. Surface markers of CSCs are expressed in a variety of human malignant tumors, including tumors of the lung, colon, pancreas, brain, breast, and others.23–27 In our study, we detected the expression of CD133 and CD44. We found that the expressions of both CD133 and CD44 on A549 and XWLC-05 cells were increased after radiation, but the percentages of CD133+ or CD44+ of A549 cells were not obvious change after radiation in vivo and vitro. The rates of CD44+ or CD133+ in 8-Gy groups were higher than 4-Gy groups. Further, the percentage of CD444+ and CD133+ cells in irradiated transplantation group XWLC-05 tumor cells presented a statistical difference (P<0.05) compared with A549 tumor cells. Mi Hyun Kim et al28 reported that the expressions of CD133 and CD44 in human breast cancer cells were increased after irradiation. Glioblastoma multiforme, after 50 Gy irradiation, displayed increased proliferation infiltration, and migration in mouse models, and cancer stem cell marker CD44 expression was enhanced.29 Some studies have found that increased expression of CD44 is associated with poor disease progression and poor prognosis in none small cell lung cancer.30 Clinical studies have found that the expression of CD133 in residual cancer cells after chemoradiotherapy is associated with distant recurrence and poor DFS.31 In our study, CD44 and CD133 expressions were not with migration of lung adenocarcinoma cells after radiation. In fact, the main effect of radiation is to kill the common cancer cells, but less to the tumor stem cells, so the proportion of cancer stem cells was relatively increased in the short term after radiation. The proliferation and differentiation of cancer stem cells lead to an absolute increase in the proportion of cancer stem cells in the long term after radiation. The CD133 and CD44 expressions of lung adenocarcinoma cells were significantly increased after radiation, which was correlated with cell migration.
Irradiated XWLC-05 cells showed higher metastatic than A549 cells in vitro, MMP-2 may connect with metastasis protein of residual XWLC-05 and A549 cells in our study. The migratory ability of cancer increased after radiation, not only in vitro but also in the clinical study. Radiotherapy of advanced carcinomas of the bladder and the uterine cervix led to increased metastatic disease.32,33 In contrast to other studies, we found that the proliferation and metastatic potential of residual tumors was decreased at first and then increased. Our result is consistent with Li Tao’s study,34 which found that the migration ability of hepatocellular carcinoma is decreased at first and then increased. Li Tao also found that the metastatic potential of the residual hepatocellular carcinoma was dependent on dose. However, in our study, we did not observe this phenomenon. As liver cancer cells and lung cancer cells have their own characteristics, the migration response to radiation and dose may be inconsistent. Speake35 found that the expression of MMP was increased in rectal cancer cells (HT1080 and HCT cell lines) after radiation. Cheng’s14 study also demonstrated a radiation-enhanced invasion capability in HCC cells through upregulation of MMP-9, which they hypothesized was correlated with metastasis due to radiotherapy in clinical practice. In our study, we found that radiation induced a temporary increase in the expression of MMP-2 in A549 and XWLC-05 cells. Whereas 336 hr after radiation, the expression of MMP-2 in A549 and XWLC-05 cells decreased to unirradiated control levels. The change in the expression of MMP-2 was significantly inhibited within 96 hr after radiation and increased 336 hr after radiation. Li Tao’s study34 found that the expression of MMP-2 in hepatocellular carcinoma was inconsistent with the migration potential. The migration of the tumor may be also affected by other factors, such as E-Cadherin, nm23, VEGF and so on, and the distant metastasis of adenocarcinoma may be more closely related to angiogenesis factors.
The changes in cell cycle in residual XWLC-05 and A549 cells were no significant differences. Shuning Yang36 reported that the highest rate of A549 cells arrested in G2/M phase was seen 48 hr after radiation, which then gradually reduced. In our study, A549 and XWLC-05 cells were arrested in G2/M phase after radiation, but the proportion of G2/M phase was decreased over time and was eventually even lower than that of the unirradiated control. However, we found that the highest proportion of G2/M of A549 was seen 12 hr after radiation, but that of XWLC-05 was seen 48 hr after radiation. These results were not completely consistent with Shuning Yang’s study, because there are some differences between the experimental conditions and experimental time points. In vivo, there are no statistical differences between two kinds of cells after irradiation. This phenomenon due to the time of testing cell cycle was too long to seen significant radiogenic changes of cell cycle.
XWLC-05 cells had better abilities of cell proliferation and metastasis than A549 cells after radiation. XWLC-05 cells also had higher radiosensitivity than A549 cells. It suggests that hyperfraction radiotherapy can be considerer to reduce radiation damage to normal tissues and increase the death of the Xuanwei adenocarcinoma cancer cells. Sufficient treatment should be ensured to kill more stem cells of Xuanwei adenocarcinoma cancer. Treatments, such as chemotherapy, radiotherapy, surgery, targeted therapy, biotherapy which inhibit Xuanwei lung cancer metastasis should be considered for suitable Xuanwei lung cancer patients in early stages. Our study is not sufficient about the proteins and pathways of biology behaviors in XWLC-05 and A549 cells after radiation. But the time of delivery radiotherapy should prolong enough to resist cancer cell proliferation. Pathway inhibitor combines with radiotherapy should be used at a reasonable time which may improve the effect of pathway inhibitor combine with radiotherapy. Pathway inhibitor combines with radiotherapy should be used at a reasonable time which may improve the effect of pathway inhibitor combine with radiotherapy. More exploration and study regarding the proteins and pathways associated with proliferation and migration after radiation.
Conclusion
Those results show systematic biological differences and changes after radiation between XWLC-05 cells and A549 cells. Different time has different protein take faction. MMP-2 might regulated metastasis ability of XWLC-05 and A549 cells. PCNA, P53 may play important roles in proliferation of residual XWLC-05 and A549 cells within the first 336 hr after irradiation in vitro. After that, P53 may through PI3K/AKT pathway regulate cell proliferation after irradiation in vitro. DNA-PKCS may play a more important role in DNA damage repair than KU70 and KU 80 after 336 hrs in vitro. Time rhythm of radiotherapy should be paid more attention to resist cancer cell proliferation and metastasis. Enough time of radiotherapy should be delivered.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81560488, 81660504, and 81860536), Yunnan Provincial Training Special Funds for High-level Health Technical Personnel (No. H-201624), Yunnan Health Science Foundation (No. 2016NS085), Key Project of Department of Education of Yunnan Province (2015Z090), and National Key Clinical Specialty (Oncology) Fund.
Disclosure
The authors declare no conflicts of interest regarding this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Balata H, Fong KM, Hendriks LE, et al. Prevention and early detection for non-small cell lung cancer: advances in thoracic oncology 2018. J Thorac Oncol. 2019. doi: 10.1016/j.jtho.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 3.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498–e509. doi: 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 4.Zheng Q, Liu Y, Zhou HJ, et al. X-ray radiation promotes the metastatic potential of tongue squamous cell carcinoma cells via modulation of biomechanical and cytoskeletal properties. Hum Exp Toxicol. 2015;34(9):894–903. doi:810.1177/0960327114561664 [DOI] [PubMed] [Google Scholar]
- 5.Xiong W, Ren ZG, Qiu SJ, et al. Residual hepatocellular carcinoma after oxaliplatin treatment has increased metastatic potential in a nude mouse model and is attenuated by Songyou Yin. BMC Cancer. 2010;10:219. doi: 10.1186/1471-2407-1110-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito A, Viale G, Curigliano G. Safety, tolerability, and management of toxic effects of phosphatidylinositol 3-kinase inhibitor treatment in patients with cancer: a review. JAMA oncol. 2019;5(9):1347–1354. doi: 10.1001/jamaoncol.2019.0034 [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Zhang L, Liu L, et al. Chemosensitizing effect of shRNA-mediated ERCC1 silencing on a Xuanwei lung adenocarcinoma cell line and its clinical significance. Oncol Rep. 2017;37(4):1989–1997. doi: 10.3892/or.2017.5443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z, Wang X, Yang Y, Zhao Y, Shen Z, Huang Y. MicroRNA expression profiling of lung adenocarcinoma in Xuanwei, China: a preliminary study. Medicine. 2019;98(21):e15717. doi: 10.1097/MD.0000000000015717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Li G, Huang Y, et al. Association of inorganics accumulation with the activation of NF-kappaB signaling pathway and the iNOS expression of lung tissue in Xuanwei lung cancer patients. Zhongguo Fei Ai Za Zhi. 2016;19(1):30–37. doi: 10.3779/j.issn.1009-3419.2016.01.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan FC, Wang QQ, Ruan YH, Ma LJ, Jia JT, Jin KW. Establishment and biological characteristics of lung cancer cell line XWLC-05. Ai Zheng. 2007;26(1):21–25. [PubMed] [Google Scholar]
- 11.Naito S, von Eschenbach AC, Giavazzi R, Fidler IJ. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986;46(8):4109–4115. [PubMed] [Google Scholar]
- 12.Su WH, Chuang PC, Huang EY, Yang KD. Radiation-induced increase in cell migration and metastatic potential of cervical cancer cells operates via the K-Ras pathway. Am J Pathol. 2012;180(2):862–871. doi:810.1016/j.ajpath.2011.1010.1018 [DOI] [PubMed] [Google Scholar]
- 13.Gnosa S, Capodanno A, Murthy RV, Ejby Jensen LD, Sun XF. AEG-1 knockdown in colon cancer cell lines inhibits radiation-enhanced migration and invasion in vitro and in a novel in vivo zebrafish model. Oncotarget. 2016;07(10):13155. doi: 10.18632/oncotarget.13155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006;25(53):7009–7018. doi: 10.1038/sj.onc.1209706 [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Zhou L, Han N, Zhang M, Lyu X. Wnt/beta-catenin pathway involvement in ionizing radiation-induced invasion of U87 glioblastoma cells. Strahlenther Onkol. 2015;191(8):672–680. doi:610.1007/s00066-00015-00858-00067 [DOI] [PubMed] [Google Scholar]
- 16.Zhao T, Wang H, Ma H, Wang H, Chen B, Deng Y. Starvation after Cobalt-60 gamma-ray radiation enhances metastasis in U251 glioma cells by regulating the transcription factor SP1. Int J Mol Sci. 2016;17(4):386 doi:310.3390/ijms17040386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao C, Saito S, Kang J, Anderson CW, Appella E, Xu Y. p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage. EMBO J. 2000;19(18):4967–4975. doi: 10.1093/emboj/19.18.4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang P, Du W, Heese K, Wu M. The bad guy cooperates with good cop p53: bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol Cell Biol. 2006;26(23):9071–9082. doi: 10.1128/MCB.01025-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turrell FK, Kerr EM, Gao M, et al. Lung tumors with distinct p53 mutations respond similarly to p53 targeted therapy but exhibit genotype-specific statin sensitivity. Genes Dev. 2017;31(13):1339–1353. doi: 10.1101/gad.298463.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padmanabhan V, Callas P, Philips G, Trainer TD, Beatty BG. DNA replication regulation protein Mcm7 as a marker of proliferation in prostate cancer. J Clin Pathol. 2004;57(10):1057–1062. doi: 10.1136/jcp.2004.016436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebina M, Steinberg SM, Mulshine JL, Linnoila RI. Relationship of p53 overexpression and up-regulation of proliferating cell nuclear antigen with the clinical course of non-small cell lung cancer. Cancer Res. 1994;54(9):2496–2503. [PubMed] [Google Scholar]
- 22.Zhao H, Lo YH, Ma L, et al. Targeting tyrosine phosphorylation of PCNA inhibits prostate cancer growth. Mol Cancer Ther. 2011;10(1):29–36. doi: 10.1158/1535-7163.MCT-1110-0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–514. doi: 10.1038/sj.cdd.4402283 [DOI] [PubMed] [Google Scholar]
- 26.Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol. 2009;568:161–173. doi: 10.1007/1978-1001-59745-59280-59749_59710 [DOI] [PubMed] [Google Scholar]
- 27.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- 28.Kim MH, Kim MH, Kim KS, et al. In vivo monitoring of CD44+ cancer stem-like cells by gamma-irradiation in breast cancer. Int J Oncol. 2016;48(6):2277–2286. doi:2210.3892/ijo.2016.3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietras A, Katz AM, Ekstrom EJ, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14(3):357–369. doi:310.1016/j.stem.2014.1001.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran TA, Kallakury BV, Sheehan CE, Ross JS. Expression of CD44 standard form and variant isoforms in non-small cell lung carcinomas. Hum Pathol. 1997;28(7):809–814. doi: 10.1016/S0046-8177(97)90154-4 [DOI] [PubMed] [Google Scholar]
- 31.Kawamoto A, Tanaka K, Saigusa S, et al. Clinical significance of radiation-induced CD133 expression in residual rectal cancer cells after chemoradiotherapy. Exp Ther Med. 2012;3(3):403–409. doi: 10.3892/etm.2011.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson P, Dische S. Local tumor control and the subsequent incidence of distant metastatic disease. Int J Radiat Oncol Biol Phys. 1981;7(12):1645–1648. doi: 10.1016/0360-3016(81)90186-3 [DOI] [PubMed] [Google Scholar]
- 33.Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992;24(2):197–204. doi: 10.1016/0360-3016(92)90671-4 [DOI] [PubMed] [Google Scholar]
- 34.Li T, Zeng ZC, Wang L, et al. Radiation enhances long-term metastasis potential of residual hepatocellular carcinoma in nude mice through TMPRSS4-induced epithelial-mesenchymal transition. Cancer Gene Ther. 2011;18(9):617–626. doi:610.1038/cgt.2011.1029 [DOI] [PubMed] [Google Scholar]
- 35.Speake WJ, Dean RA, Kumar A, Morris TM, Scholefield JH, Watson SA. Radiation induced MMP expression from rectal cancer is short lived but contributes to in vitro invasion. Eur J Surg Oncol. 2005;31(8):869–874. doi: 10.1016/j.ejso.2005.05.016 [DOI] [PubMed] [Google Scholar]
- 36.Yang S, Xu J, Shao W, et al. Radiation-induced bystander effects in A549 cells exposed to 6 MV X-rays. Cell Biochem Biophys. 2015;72(3):877–882. doi:810.1007/s12013-12015-10555-12012 [DOI] [PubMed] [Google Scholar]