Abstract
Lung cancer remains the overwhelmingly greatest cause of cancer death in the United States, accounting for more annual deaths than breast, prostate, and colon cancer combined. Accumulated evidence since the mid to late 1990s, however, indicates that low-dose CT screening of high-risk patients enables detection of lung cancer at an early stage and can reduce the risk of dying from lung cancer. CT screening is now a recommended clinical service in the United States, subject to guidelines and reimbursement requirements intended to standardize practice and optimize the balance of benefits and risks. In this review, the evidence on the effectiveness of CT screening will be summarized and the current guidelines and standards will be described in the context of knowledge gained from lung cancer screening studies. In addition, an overview of the potential advances that may improve CT screening will be presented, and the need to better understand the performance in clinical practice outside of the research trial setting will be discussed.
Keywords: CT, Screening, Thorax
© RSNA, 2020
Summary
The accumulated evidence and guidelines from 2 decades of clinical investigation have demonstrated that appropriate implementation of CT screening could prevent a substantial number of lung cancer deaths.
Essentials
■ Lung cancer has been the greatest cause of cancer mortality for decades, and this trend is expected to continue.
■ Low-dose CT screening can reduce lung cancer mortality.
■ Advances in detection and image analysis may improve the efficiency and effectiveness of low-dose CT screening.
■ Data on outcomes of CT screening across different clinical practice settings over time will be important for performance assessment and improvement.
Introduction
Despite gradual decreases in smoking rates and advances in treatment, lung cancer remains the most common cause of cancer death. Most lung cancers are still diagnosed at a late stage when they are difficult to cure, and there are millions of smokers and former smokers who are at increased risk and will be for decades. After more than 2 decades of research, evidence has accumulated that early detection by low-dose CT screening has the potential to improve these outcomes. Since the first reports on using low-dose CT to screen for lung cancer, the ability to reduce lung cancer mortality through CT screening has been verified, and evidence from carefully designed and conducted clinical investigations has been translated into everyday practice. Data and observations from screening trials have improved our understanding of the CT manifestations of early lung cancer and have provided information important to the development of guidelines for managing indeterminate lung nodules. Lung cancer CT screening has also provided much of the impetus for reducing radiation dose at chest CT and developing automated and quantitative analysis techniques. In this article, we review the history of lung cancer screening, explain how current screening guidelines are linked to findings from lung cancer screening trials, and describe best practices for optimizing performance. We also highlight technological advances that may improve efficiency and effectiveness. Finally, we note current shortcomings, particularly the need for outcome data from screening outside of the clinical trial setting.
Incidence and Mortality
Lung cancer is the second most frequent cause of cancer (after prostate cancer in men and breast cancer in women) and the most frequent cause of cancer death in the United States for both men and women, with more than 228 000 new cases and nearly 143 000 deaths expected in 2019 (1). The age-adjusted lung cancer mortality rates in the United States have been declining in men since around 1990 and in women since the early to mid-2000s (1), reflecting decreases in smoking rates over time with a lag of approximately 30 years (2). Projections show that if current smoking behaviors continue (ie, prevalence, age of initiation and cessation, and smoking intensity), lung cancer mortality will continue to fall in the coming decades; however, there may still be more than 100 000 U.S. lung cancer deaths in 2035, and more than 50 000 in 2065 (3). This is more than the current number of annual deaths due to breast, prostate, or colon cancer (1).
Worldwide, lung cancer is the most frequently diagnosed cancer (third after breast and colon cancer in women) and the most common cause of cancer death (second to breast cancer in women) overall, although rates and trends vary widely by region in relation to historic tobacco use patterns (4,5). Lung cancer was estimated to account for 388 000 deaths in Europe in 2018 (6) and 610 000 deaths in China in 2015 (7). Rates are highest in North America, Europe, Australia/New Zealand, Micronesia/Polynesia, and Eastern Asia, with the lowest rates in Central America and Eastern, Middle, and Western Africa (4).
Risk Factors
Cigarette smoking transformed lung cancer from a rarely diagnosed disease in the early 20th century (8) to the leading cause of cancer death in U.S. men since the mid-1950s and in U.S. women since the late 1980s (1) and is estimated to be responsible for 87% of lung cancer deaths (9). The risk of lung cancer increases with both the number of cigarettes per day and number of years smoked (10,11). Indoor radon is the second leading risk factor for lung cancer in the United States, accounting for a quarter of lung cancer deaths in never-smokers (12). Multiple other exposures and conditions also increase lung cancer risk, including chronic obstructive pulmonary disease (1.7- to 5.3-fold increase) (13,14) and emphysema (2.4-fold increase) (15), pulmonary fibrosis (eight- to 14-fold increase) (16,17), radiation therapy for Hodgkin lymphoma (2.6- to sevenfold increase) (18) or for breast cancer (10-fold increase) (19), previous tuberculosis (1.5-fold increase) (15) or pneumonia (1.6-fold increase) (15), a family history of lung cancer (twofold increase) (20), and occupational exposure to asbestos (particularly with asbestosis) and numerous other substances including arsenic, bis(chloromethyl) ether, chromium, formaldehyde, nickel, polycyclic aromatic hydrocarbons, hard metal dust, and vinyl chloride (21). A second lung cancer develops at a rate of 1%–2% per patient per year (22,23) or higher (24) in those who have had non–small cell lung cancer.
History of Lung Cancer Screening
Rationale
The 5-year relative survival for stage I disease, in which the tumor is confined to the primary site, ranges from approximately 50%–90%, depending on the size of the stage I tumor and the lung cancer registry used as the source of data; in contrast, 5-year survival for stage IV disease, in which distant metastases are present, is 3%–6% (25). This suggests the possibility that lung cancer deaths could be greatly reduced if the primary tumor could be found and treated before it has spread. From 2009 to 2015, however, 57% of patients had distant metastases at diagnosis, only 16% of patients had localized disease, and 5-year survival among all patients with lung cancer was 20.6% (26).
Chest Radiography
In the 1970s and 1980s, two randomized controlled trials, the Mayo Lung Project (27) and a Czechoslovakian trial (28), investigated whether early detection of lung cancer using chest radiography could reduce lung cancer mortality. Both trials found more cancers at an earlier stage that were more often resectable, and better survival in the screened groups; however, there was no reduction in the number of lung cancer deaths, and the Mayo trial reported no difference in the number of unresectable lung cancers. These findings demonstrate that improved disease detection and increased survival time after diagnosis, expected with screening due to lead time bias, are not equivalent to a reduction in mortality. While these studies had well-recognized limitations, such as low statistical power and intentional or unintentional screenings in the control groups, their conclusions were later supported by the findings of the Prostate, Lung, Colorectal, and Ovarian cancer screening trial (PLCO) conducted by the National Cancer Institute from 1994 to 2009, which found no mortality benefit from chest radiography screening (29).
Low-Dose CT
In the mid and late 1990s, investigators in Japan (30) and the United States (31) demonstrated the potential benefits of low-dose CT screening for lung cancer. Numerous Japanese, U.S., and European studies quickly followed (32–38). Lung cancer was found in 0.9%–2.7% at initial (prevalence) screening (30,31,33–35), three to four times more than with chest radiography (30,31). Importantly, over 50% and up to 93% of the detected lung cancers were stage I. Lung cancer was found in up to 0.6%–1.5% at annual repeat (incidence) screening, with reported stage I proportions of 48%–89% (39–42). While these early single-arm CT studies were highly encouraging, they lacked a control group and could not ascertain whether CT screening actually reduced lung cancer mortality. The randomized controlled U.S. National Lung Screening Trial (NLST), conducted by the National Cancer Institute from 2002 to 2009, thus was specifically designed to determine this (43).
In the NLST, 53 439 high-risk volunteer participants at 33 U.S. locations, age 55–74 years, who had smoked a minimum of 30 pack-years and were currently smoking or had quit within the last 15 years, were randomly assigned to undergo three annual screening examinations with either low-dose CT or posteroanterior chest radiography. After median follow-up of 6.5 years, there were 20% fewer deaths from lung cancer among those randomly assigned to the CT arm (P = .004) (44), with one lung cancer death prevented for every 320 screened with CT. The latter rate compares very favorably with the mammography estimate of one death prevented for every 519 women screened for 7 years (45). With a reduction in all-cause mortality in the CT arm of 6.7% (P = .02), the NLST is the only screening trial to find a statistically significant reduction in all-cause mortality, which was largely because a high proportion of deaths (25%) were due to lung cancer. After extending the median follow-up period to 11.3 years, subsequent analyses found that there were 10% fewer lung cancer deaths in the CT arm, with one death prevented for every 303 persons screened (46).
Several smaller randomized controlled trials were conducted contemporaneously with the NLST. The NELSON (Nederlans-Leuvens Longkanker Screenings Onderzoek) trial randomly assigned 15 792 participants in the Netherlands and Belgium to have CT screening (at baseline, 1, 3, and 5 years) or no screening (47). Lung cancer mortality with screening was 24% lower at 10 years overall (95% confidence interval: 6%, 39%), and 33%–59% lower in different years of follow-up in women (48). The Multicentric Italian Lung Detection randomized trial with 4099 participants reported a 39% reduction in cumulative risk of lung cancer mortality at 10 years with CT screening (95% confidence interval: 5%, 61%, P = .02), with one lung cancer death prevented for every 167 participants screened (49). Other randomized controlled trials in Italy (50) and Denmark (51) did not demonstrate a mortality benefit from CT screening, though they were underpowered and considered inconclusive.
Another large study, the International Early Lung Cancer Action Project (I-ELCAP), is a multicenter consortium initiated prior to the NLST and still ongoing. I-ELCAP uses longitudinal observational methods to study the diagnostic and treatment components of CT screening separately. Analyses focus on how frequently screening detects lung cancer at an early stage, and how frequently screen-detected lung cancers are cured, particularly those likely to be life-threatening without treatment, to assess the magnitude of the benefit associated with ongoing CT screening using metrics relevant to persons at risk. In one analysis, 1.5% of 31 567 persons at risk who were screened over a 12-year period were diagnosed with lung cancer, 85% of which were clinical stage I, with 10-year survival rate of 92% in those who underwent surgical resection (52).
Current Screening Guidelines in the United States
The multiple prospective single-arm observational studies demonstrated that CT screening is sensitive for detecting lung cancer at an early stage, while randomized controlled trials confirmed a mortality benefit. In 2013, CT screening for lung cancer was given a grade B recommendation by the United States Preventive Services Task Force (USPSTF), which concluded that there is moderate certainty of moderate net benefit in persons at high risk (53). Because of this, full coverage by private insurers is required under the Affordable Care Act. The 2015 decision memo by the Center for Medicare and Medicaid Services (CMS) also provides coverage for eligible individuals screened at centers that follow CMS requirements (54). CT screening has been endorsed by numerous medical and patient advocacy organizations (55–62), many of which have published lung cancer screening practice guidelines.
The results of screening trials were achieved with infrastructures that facilitated verification of participant eligibility based on age and smoking history, standardization of screening methods, follow-up after screening, and compliance with annual rescreening. Realizing similar benefits and minimizing harms in clinical practice will likely depend on how well these components can be replicated. Many of these important steps in the screening process have been recommended by professional societies or mandated by CMS. This discussion focuses on current guidelines in the United States, where screening has been implemented on a widespread basis as part of routine clinical practice, supplemented by data and approaches from European trials and recommendations.
Considerations prior to Screening
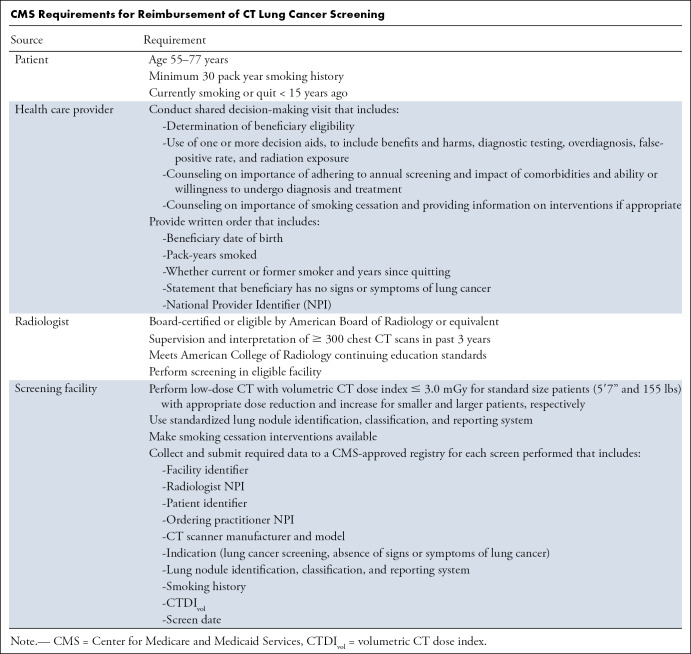
Eligibility.—Current USPSTF eligibility guidelines reflect those used in the NLST, including a minimum age of 55 years, minimum smoking history of 30 pack-years, and quit time of less than 15 years. For reimbursement of CT screening by CMS, health care providers, radiologists, and screening facilities must adhere to specific eligibility guidelines and fulfill multiple additional criteria (Table), many of which are also recommended by the USPSTF. A notable difference is that, based on studies modeling the expected benefit from screening by age (63), the USPSTF recommends annual screening up through 80 years of age, while CMS ends coverage at age 77 years, corresponding to the oldest age at the time of the final annual screen in the NLST.
CMS Requirements for Reimbursement of CT Lung Cancer Screening
Some organizations also recommend screening for persons as young as 50 years with a smoking history of 20 or more pack-years if there is another established risk factor for lung cancer (56,60,64), but this has not been recommended by the USPSTF, and thus insurance coverage for such individuals is not required. Persons with symptoms possibly due to lung cancer, such as new cough, hemoptysis, or weight loss, are not considered eligible for screening and should undergo a diagnostic CT scan instead. Screening is not advised for those with a limited life expectancy due to significant comorbidities, or for those unable or unwilling to undergo diagnosis and treatment. Unlike in the NLST, those with a history of lung cancer or treatment for cancer other than nonmelanoma skin cancer in the last 5 years are not specifically excluded.
Individual risk prediction.—Analyses of screening populations show substantial lung cancer risk variation among persons who are eligible under current age and smoking criteria. For example, the risk among NLST participants older than 65 years of age was more than double the risk of those younger than 65 (1.5% vs 0.7%) (65). A risk model developed from PLCO trial control group data (predictor variables included race, socioeconomic status, body mass index, history of chronic obstructive pulmonary disease and cancer, and family history of lung cancer, in addition to age and smoking variables) predicted lung cancer better in the PLCO chest radiography screening group than did NLST age and smoking criteria, missing 41% fewer cancers (66). A model predicting lung cancer mortality developed from the NLST radiography arm participant data found that the three highest risk quintiles in the NLST CT arm accounted for 88% of the deaths prevented by CT screening, while those in the lowest risk quintile accounted for only 1% of the prevented deaths (67). Another model showed that by screening only those above specific model-determined risk thresholds, more lung cancer deaths could be prevented by screening fewer persons than using USPSTF eligibility criteria (11).
A comparison of nine models found that lung cancer risk may vary by geographic region or in certain subpopulations, as models based on European data overestimated risk in U.S. populations, and some U.S. models overestimated risk in the heaviest smokers and some underestimated risk in Hispanics and Asian or other groups (68). Prospective studies in the United Kingdom (69) and in Canada (70) have demonstrated the feasibility of using risk models for determining CT screening eligibility, with higher lung cancer incidence and proportion of early stage cancers compared with previous studies observed in the latter. European societies have recommended individual risk assessment (58), and the USPSTF will consider the potential role of individual risk determination in their next guidelines update (71). Practical considerations may include determining which model and thresholds to use, implementation across different screening centers, and increased complexity compared with the current approach.
Shared decision making.—CMS requires a shared decision-making visit with a qualified health care provider before the first screening examination, a provision unique to lung cancer screening. Shared decision making involves disclosure of the risks and benefits of a course of action by the provider, and expression of personal preferences and values by the patient, to make health care decisions jointly (72,73). This process is also recommended by the USPSTF (53) and other organizations (57,61). Online tools to aid providers and patients have been made available by several sources, including the American College of Radiology (ACR) (74), Agency for Health Care Research and Quality (75), and the American Lung Association (76). The use and quality of shared decision making and tools are likely quite variable, which may contribute to differences in the perceived effectiveness, as both limitations (77,78) and success (79) have been reported. Financial reimbursement for this service is provided by CMS for Medicare and Medicaid beneficiaries, but is not required of private insurers.
Smoking cessation.—The USPSTF and other organizations recommend, and CMS requires, counseling on the importance of smoking cessation during shared decision making for lung cancer screening, and CMS stipulates that the screening facility make smoking cessation interventions available. Because the purpose of screening is to reduce the risk of lung cancer death, the importance of smoking cessation cannot be overemphasized. Smoking cessation gradually lowers the risk of lung cancer compared with continued smoking, though risk does not return to the level of the never smoker and continues to increase with age in all (80,81). Analyses of data from the NLST (82) and Nurses’ Health Study (83) found that smoking cessation reduced the risk of lung cancer death by 20% after 7 and 5 years, respectively, while the Framingham Heart Study found a 39% lower risk of lung cancer after 5 years (84).
Thus, after a relatively short time, the impact of smoking cessation on lung cancer risk may be similar to or greater than that of CT screening, and increases over time, at a fraction of the cost and risk. Quitting also reduces the risk of death from other smoking-related cancers and cardiovascular diseases (85). However, smoking cessation attempts are usually unsuccessful, even with behavioral and pharmacologic interventions. The quit rate in the general population of screening-age persons in the United States is around 5% (86), and no more than around 20% in most studies evaluating the effectiveness of individual or combined therapies (87). Higher quit rates have been observed among participants in lung cancer screening trials (11%–15%) (88,89) than in the general U.S. population, and in those with abnormal findings at screening (90), observations which may reflect greater motivation for risk reduction among clinical trial participants. Randomized trials to date, however, have not shown a benefit from counseling interventions at the time of screening compared with providing written information alone (89). Compared with the general smoking population, the screen-eligible population is older with longer and heavier smoking histories, and more likely to have medical comorbidities and greater involvement with the health care system, so more research is needed to determine the most effective interventions in this population in the screening setting (90).
Risks of Screening
The screening process can lead to adverse effects and costs that patients would not have incurred in the absence of screening, for no benefit. Guidelines recommend disclosing the potential harms of screening as part of the shared decision-making process (53,57,61). These risks are relatively small compared with the risk of lung cancer death in the screening-eligible population.
Diagnostic testing.—The most frequent subsequent test after CT screening is a surveillance CT scan to monitor indeterminate pulmonary nodules, resulting in additional radiation exposure. In the NLST (44), which classified CT studies with at least one noncalcified nodule larger than 4 mm in greatest transverse dimension as positive, 27% of initial CT screens were classified as positive, and 73% of these led to a follow-up CT scan before the next annual screen. However, under current guidelines (discussed below under Lung Nodule Management), in which the solid nodule size threshold for diagnostic follow-up is 6-mm average of length times width, the screen positivity rate would have been reduced by more than half in the NLST with a relatively much smaller decrease in sensitivity (91); the same effect was also found in an analysis of I-ELCAP data (92). More recent reports confirm these projections (93,94). The NELSON trial used a computer software program to measure nodule volume and classified screens as negative (largest nodule < 50 mm3 volume, or 4.6-mm diameter if spherical), positive (largest nodule > 500 mm3, or 9.8-mm diameter if spherical) with referral to clinician for workup, or indeterminate (largest nodule 50–500 mm3, or 4.8–9.8-mm diameter) with recommendation for a 3-month follow-up CT. With this algorithm, at the baseline screen of 7557 Dutch participants, 79.2% were negative, 1.6% were positive, and 19.2% were recommended for the 3-month scan, 5.3% of which were subsequently referred for additional workup due to a potentially malignant volume doubling time (1.0% of the whole cohort) (95).
Over all three screening years in the NLST (44), transthoracic biopsy was performed after a positive CT screen in 1.4% of participants, bronchoscopy in 3.8%, and thoracotomy or thoracoscopic surgery in 4.2%, while lung cancer was diagnosed after a positive CT screen in 3.6%. Among those who underwent an invasive procedure, a major complication of any type occurred in 12% of those with lung cancer, and in 0.1% of those in whom lung cancer was not confirmed. Death from any cause within 60 days of a procedure for a positive CT screen was rare, occurring in 1.5% of those with cancer and 0.1% of those without cancer. Diagnostic evaluation of NLST participants after screening, including both imaging and invasive procedures, was conducted at the discretion of the participants’ personal physicians who were not associated with the trial. Invasive testing rates for the entire NELSON trial have not been reported, but after two screening rounds in 7557 Dutch participants were 3.2% for bronchoscopy, 0.2% for transthoracic biopsy, and 2.0% for an invasive surgical procedure (95). Rates of invasive testing and adverse events in contemporary clinical screening practice are not yet known.
Radiation exposure.—At the highest recommended volumetric CT dose index (CTDIvol) of 3.0 mGy in a standard-size patient, a 25-cm standard scan length, and a k factor of 0.014 mSv ⋅ mGy-1 cm-1, a typical effective dose for screening CT would be 1.0 mSv or less (96). The average annual dose from environmental radiation in the United States is 3.1 mSv (97), about three times greater. Based on a linear–no threshold model of ionizing radiation effects, the lifetime attributable risk of any cancer developing due to an 8-mSv chest CT has been estimated as approximately 0.09% in women and 0.05% in men at 60 years of age (98), equivalent to 0.011% in women and 0.006% in men when scaled linearly to a 1-mSv chest CT. A single-center trial estimated a 0.05% additional risk of cancer after 10 years of screening and associated follow-up imaging, and one radiation-induced cancer for every 108 screen-detected lung cancers (99). According to the U.S. Food and Drug Administration, a CT with effective dose of 10 mSv may increase the possibility of developing a fatal cancer by 0.05% (100), which scales linearly to a risk of 0.005% for a 1-mSv lung cancer screening CT.
Based on these estimates, after 20 annual screening CT examinations, the increased risk of cancer would be 0.22% in women and 0.12% in men, and that of a fatal cancer 0.1%, from the screening CT examinations alone. These risks are extremely small relative to the estimated lifetime risk of developing lung cancer among all (smokers and nonsmokers) U.S. men of 6.7% and women of 5.9% (1), or the estimated risk for smokers of 15% or higher (101–103).
Overdiagnosis.—Overdiagnosis occurs when lung cancers are found that would never have been discovered in the absence of screening. Typically estimated as the difference in the number of cancers diagnosed in screened and control groups, analysis of the initial NLST data found that 18% of the screen-detected cancers in the CT arm may have been overdiagnosed, primarily adenocarcinomas classified at the time as bronchioloalveolar cell type (104). Statistical modeling studies that simulated longer follow-up estimated that 10% would be overdiagnosed (63). Subsequent analysis of data after the extended follow-up of NLST participants revealed an overdiagnosis rate of 3% overall, although it was 79% for adenocarcinomas previously classified as bronchioloalveolar cell type (46). It should be noted that once a cancer has been detected and treated as a result of screening, it cannot be known if it would have become clinically apparent in the absence of screening, so whether any individual cancer was overdiagnosed cannot be determined.
Missed lung cancer.—Diagnosis of lung cancer after a negative CT screening examination is infrequent but can occur. Of the 7155 Dutch CT arm participants in the NELSON trial, lung cancer was diagnosed within 2 years after a screen that had been interpreted as negative in 34 participants (0.5%); the cancer was visible in retrospect in 20, misinterpreted in two, and no abnormality was identified retrospectively in 12 participants (105). In the NLST, lung cancer was diagnosed in 44 of the 26 722 CT arm participants (0.16%) within a year after a negative CT screen (44). Retrospective review revealed abnormal findings of a positive screen in 40 of these, 22 of which were likely missed, 14 misinterpreted as clinically significant but not suspicious for lung cancer, and four with nodules originally considered to be less than 4 mm or stable for more than 2 years, and four patients with no or minor abnormalities not suspicious for lung cancer (105). It should be noted that the interval between screens in NELSON was 2 years after the first screen and 2.5 years after the second screen, while the interval in NLST was 1 year. In addition to missed peripheral pulmonary nodules, several types of abnormalities were identified retrospectively in these studies, including nodules or enlarged hilar lymph nodes abutting central pulmonary vessels or the mediastinum, endobronchial nodules, and enlarged mediastinal lymph nodes (106,107). Familiarity with these and other presentations of lung cancer, such as cystic or bubbly lucencies with thickened walls or nodules as initially recognized in the I-ELCAP cohort (108), and consolidation simulating pneumonia, is important in screening CT interpretation.
Incidental findings.—The reported frequency of clinically significant, actionable findings unrelated to lung cancer in most CT screening studies varies from about 1% to 20%, although there is no standard definition (109). The most common incidental findings at screening CT are the pulmonary abnormalities of emphysema, bronchitis, and interstitial lung disease (110) and cardiovascular disease including coronary artery calcification and thoracic aortic aneurysm (109,111). Being associated with heavy smoking, these findings are common in the screening population and the need for further evaluation is usually based on symptoms and clinical factors. However, neck, chest, or upper abdominal abnormalities may be identified that warrant additional imaging, other diagnostic evaluation, or treatment. The most commonly reported potentially significant abnormality categories in the NLST were cardiovascular (8.5%), renal (2.4%), hepatobiliary (2.1%), adrenal (1.2%), and thyroid (0.6%); the extrathoracic malignancy rate was 0.4% (111). As with screening for lung cancer, detection of incidental findings may reduce morbidity and mortality in some persons, but false-positive findings and overdiagnosis with unnecessary testing and treatment also may occur. Further study is needed to understand the net impact in clinical practice.
Low-Dose CT Scanning and Interpretation
CT Technical Specifications
The technical guidelines for CT lung screening are intended to provide image quality sufficient for detection and measurement of small pulmonary nodules while limiting radiation exposure as much as possible. Current guidelines of the ACR and American Association of Physicists in Medicine (96) recommend use of CT scanners with 16 or more detector rows and slice thickness of 2.5 mm or less, with 1-mm thickness preferred. These guidelines and CMS requirements limit the CTDIvol to 3.0 mGy for a standard size patient of 5ʹ7” (170 cm) and 155 lbs (70 kg), with appropriate reductions expected for smaller patients and increases for larger patients.
Newer radiation reduction technologies such as tin filtration may allow even greater dose reductions to less than 0.3 mGy (112,113). Automatic exposure control systems, which vary tube output at different anatomic locations during scanning depending on tissue attenuation, can be used to adjust dose for patient size. Care must be taken that appropriate manufacturer-specific settings are used, as some may inadvertently increase dose in some patients.
In addition to standard axial images, generating coronal and sagittal reconstructions and maximum intensity projection images are recommended (96). Multiplanar reconstructions can be helpful in determining whether certain solid or ground-glass opacities are truly nodules or have the linear or flat configuration of atelectasis and scars. Overlapping maximum intensity projections can improve nodule detection sensitivity by facilitating discrimination of small nodules from vessels (114–116).
Lung Nodule Management
Standardized methods for lung nodule management in CT screening, required by CMS, include the ACR Lung CT Screening Reporting and Data System (Lung-RADS) version 1.1 (117) and the similar guidelines of the National Comprehensive Cancer Network version 2.2019 (64). Lung-RADS 1.1 guidelines now require recording nodule measurements to one decimal point, rather than rounding up to the next highest integer. At the initial screen, no further evaluation before the next annual screen is recommended if there are no solid lung nodules with average diameter of 6.0 mm or larger, part-solid nodules with solid component of 6.0 mm or larger, nonsolid nodules of 30 mm or larger (20 mm in National Comprehensive Cancer Network version 2.2019 and previous Lung-RADS version 1.0), or other findings suspicious for lung cancer such as lobar collapse or mediastinal lymphadenopathy.
Follow-up is recommended for solid nodules that are greater than or equal to a 6.0-mm average diameter on initial screens. This is greater than the thresholds used in screening trials but is supported by I-ELCAP data, showing that such nodules can be safely followed for 1 year (118) and by the relationship between nodule size and frequency of malignancy in the NLST (119,120). In Lung-RADS 1.1, the size threshold for further evaluation of solid perifissural nodules with smooth borders and oval, lentiform, or triangular shape is larger than for other nodules, specifically an average diameter of 10 mm. This reflects observations that such nodules have a malignancy likelihood near zero (121,122). Interreader agreement on classifying nodules as perifissural may be only moderate at best, however, and the risk of misclassifying a cancer as a perifissural nodule may be increased if they have atypical features, are not attached to a fissure, or are located in the upper lobes (123).
For solid lung nodules that are greater than or equal to 6 mm on initial screens, management recommendations progress with increasing size of the largest nodule, from follow-up CT at 6 months (6 to < 8 mm), to follow-up CT at 3 months or PET/CT (8 to < 15 mm), to PET/CT or diagnostic CT or tissue sampling (≥15 mm). Nodules of any size can be given a higher classification (and more aggressive workup) if there are features that increase the suspicion of malignancy, a practice affirmed by a study that found an increased rate of malignancy when features such as internal nodule structure and border characteristics were considered (124). Lung-RADS also suggests using an individual prediction model (125) developed from Canadian lung cancer screening trial data to help guide management of suspicious nodules; this tool, which quantifies the likelihood that nodules at initial screening CT are malignant based on six CT (emphysema, larger size, upper lobe location, part-solid type, lower nodule number, and spiculation) and three patient (age, female sex, and family history of lung cancer) predictors, has been shown to have high predictive accuracy in several independent data sets (125–127), and better overall performance than Lung-RADS criteria (128).
A 30-mm threshold for further investigation of nonsolid (ground-glass) nodules is supported by I-ELCAP findings that nonsolid nodules of any size could be safely followed at yearly intervals until development of solid components (129). They also found that screen-detected nodules of nonsolid (ground-glass) or mixed nonsolid and solid (part solid) attenuation were less frequent than solid nodules, but more likely to be malignant (130), and advocated management of part-solid nodules based on the size of the solid component alone (131); in Lung-RADS, part-solid nodule management is more aggressive than for solid nodules, and is based on the size of the solid component with size thresholds lower than for pure solid nodules.
The actionable size threshold for new solid or part-solid nodules on annual repeat screens is smaller (4 mm if solid, any size if part solid) than on initial screens. This approach agrees with findings from the NELSON trial (132) and the NLST showing that new solid nodules had a higher rate of malignancy: 6% compared with an overall lung cancer rate of 3% during the first three screening rounds in NELSON (132), and 5.7% compared with 2.7% of all nodules detected on the baseline screen in the NLST (133). On follow-up scans or annual screens, an increase in average diameter of 1.5 mm or more should trigger further evaluation. Growing nodules should be rescanned in 3 months if solid and less than 8 mm or part solid with less than 4 mm solid component, and evaluated with diagnostic CT, PET/CT, or biopsy if solid and greater than or equal to 8 mm or part solid with greater than or equal to 4 mm solid component (solid component must be ≥ 8 mm for PET/CT).
Automated and Quantitative Techniques
Computer algorithms that analyze CT images have the potential to improve efficiency and increase consistency of interpretation in lung cancer screening. Automated methods for pulmonary nodule detection, size measurement, and tissue characterization have been developed and continue to be refined. With further development and study, these methods may assume an increasing role in future clinical practice.
Computer-aided Detection
Agreement among radiologists in lung nodule detection and measurements in lung cancer screening has been moderate (134–136). Nodule detection software increases detection rates at all experience levels, with sensitivity in the 80%–90% range, and improves interobserver agreement (137). False-positive rates generally range from three to eight per scan, but not all cancerous lesions are detected, so nodule detection software may be best used as a ”second reader” to improve sensitivity.
Computer-aided Diagnosis
Automated volumetry.—Computer-aided automated measurement of lung nodule volume is independent of nodule shape and orientation and sensitive to size change in all directions (138) and may be a more reliable indicator of nodule size and growth than diameter (139). The NELSON lung cancer screening trial, which used a nodule management algorithm based on automated volumetric measurements and volume doubling time calculations performed at a central reading site, demonstrated high sensitivity, specificity, and positive and negative predictive values with this approach (95). The volumetric approach has been used in other European trials (69,140,141) and is advocated by the European Society of Radiology and European Respiratory Society (58). Measurement variability appears to be lower with automated volumetry (142–145). Adoption in the United States has been limited, although the nodule volumes that correspond to specific diameter measurements have been provided for reference in the current version of LungRADS. Given the conceptual advantages of automated volumetry, studies focusing on direct comparisons of diameter and volume measurements, their impact on patient management, and interobserver variability for the same nodules are warranted.
The reliability of volumetric measurements depends on the use of appropriate and consistent CT acquisition and reconstruction parameters and analysis software (146–149), particularly for subcentimeter nodules (150). Volumetry is most reliable for solid nodules with aspect ratios that do not deviate excessively from spherical and are isolated or only in minimal contact with other structures. Both technical and anatomic factors can influence measurement accuracy and precision, which decrease as nodule size decreases; awareness of the potential error (ie, 95% confidence limits) associated with nodule volume measurements is paramount for their use in clinical management. Guidance on this issue is under development by the Radiological Society of North America’s Quantitative Imaging Biomarker Alliance (150). Subsolid nodules are potentially more challenging due to the reduced contrast between lesions and normal lung, though performance has been promising (151–154). A current drawback limiting the adoption of both detection and volumetry software is a lack of integration with the software used for clinical viewing and interpretation, requiring the launch of a separate application or use of a separate workstation.
Computer-aided diagnosis.—Computer-aided approaches also have been applied to lung nodules for tissue characterization. With radiomics, quantitative first-, second-, and higher-order statistical parameters are derived from measurements of nodule features such as size, shape, attenuation and its distribution (texture), and edge characteristics. These parameters are then used as variables in mathematical or machine learning models to predict, for example, whether a nodule is malignant (155–158), or the histopathologic features and prognosis of malignant nodules (159,160). Deep learning (161,162) also has been applied for nodule classification, through the use of convolutional neural networks with algorithms that directly analyze the spatial and attenuation features of images at the pixel level and self-modify the values of certain variables within the algorithm, to optimize the discrimination of different image classes such as benign versus malignant or low-grade versus high-grade malignancy.
Radiomics and deep learning have both achieved promising results, with the area under the receiver operating characteristic curve for predicting malignancy calculated at 0.8–0.9 in most studies (156–158,163–168). One particularly successful deep learning approach used the entire volume of more than 42 000 CT scans from nearly 15 000 participants in the NLST to develop a model that achieved an area under the curve of 0.94 on a separate NLST test set of 6716 cases, performed similarly on an independent clinical set of 1139 cases, and outperformed all six radiologists tested (169). These computer-aided methods could potentially allow even earlier diagnosis, reduce diagnostic evaluations and anxiety for benign nodules, and increase consistency and confidence in lesion management. In the future, studies comparing the use of quantitative methods to the current standard of care in patient management will be important for determining their impact on patient outcomes, and whether any performance benefits are worth the additional time and expense.
Utilization and Outcomes in Clinical Practice
Two decades of clinical investigation have produced solid, evidence-based guidelines for conducting lung cancer screening in an effective and safe manner. Still, a large-scale impact of screening on lung cancer mortality will not be achieved without widespread implementation. Thus far, adoption of the test in routine clinical practice has been limited, with estimated screening of less than 2% of the 7.6 million persons eligible in 2016 (170), and 14% of those eligible across 10 states in 2017 (171). There are likely multiple reasons for this, including fear, stigma, and cost concerns for patients; insufficient time or knowledge to conduct shared decision making and manage screening results for providers; and unfamiliarity with eligibility and insurance coverage criteria for both (172).
The effectiveness of CT screening in clinical practice outside of the controlled setting of a clinical trial has yet to be determined. While it may not be possible to directly evaluate the effect of screening on lung cancer mortality, important measures of performance and quality will include the risk levels of the screened populations; screening results; diagnostic evaluation rates, methods, and compliance; cancer detection rates; stage distribution; treatment; survival; and procedure-related adverse events. The ACR Lung Cancer Screening Registry, currently the only registry approved by CMS and in which screening center participation is required for Medicare reimbursement, should facilitate review of many screening outcomes of interest and their variability on a large scale.
Conclusions
The enormous challenge of reducing morbidity and mortality from lung cancer will continue for many years. The accumulated evidence and guidelines from 2 decades of rigorous study have increased confidence that appropriate implementation of CT screening can prevent a substantial number of lung cancer deaths, at low clinical risk. Now, more information is needed about patient outcomes in routine clinical practice across different settings. Continued refinements in patient selection and image analysis methods may further improve the efficiency and effectiveness of CT screening, but there is much room for other advances in the areas of prevention/risk reduction, early detection, and treatment.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: D.S.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: Received travel accommodation expenses to attend meetings on quantitative imaging lung cancer screening from RSNA and the International Association for the Study of Lung Cancer; editorial board member for Radiology: Imaging Cancer. Other relationships: disclosed no relevant relationships. W.C.B. disclosed no relevant relationships. C.C. disclosed no relevant relationships. P.F.P. disclosed no relevant relationships. D.F.Y. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: Serves on advisory board for GRAIL, is named as an inventor on numerous patents on applications related to the evaluation of diseases of the chest; some patents are owned by Cornell Research Foundation and are non-exclusively licensed to General Electric, and owns stock in Accumetra. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ACR
- American College of Radiology
- CMS
- Center for Medicare and Medicaid Services
- CTDIvol
- volumetric CT dose index
- I-ELCAP
- International Early Lung Cancer Action Project
- Lung-RADS
- Lung CT Screening Reporting and Data System
- NLST
- National Lung Screening Trial
- PLCO
- Prostate, Lung, Colorectal, and Ovarian cancer screening trial
- USPSTF
- United States Preventive Services Task Force
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Weiss W.Cigarette smoking and lung cancer trends. A light at the end of the tunnel? Chest 1997;111(5):1414–1416. [DOI] [PubMed] [Google Scholar]
- 3.Jeon J, Holford TR, Levy DT, et al. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Ann Intern Med 2018;169(10):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society . Global Cancer Facts & Figures. 4th ed. Atlanta, Ga: American Cancer Society, 2018. [Google Scholar]
- 6.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356–387. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 8.Adler I. Primary malignant growths of the lungs and bronchi; a pathological and clinical study. London, England: Longmans, Green, 1912. [Google Scholar]
- 9.United States Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. In: U.S. Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health. Atlanta, Ga: 2014. [Google Scholar]
- 10.Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003;95(6):470–478. [DOI] [PubMed] [Google Scholar]
- 11.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA 2016;315(21):2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawel DJ, Puskin JS. The U.S. Environmental Protection Agency’s assessment of risks from indoor radon. Health Phys 2004;87(1):68–74. [DOI] [PubMed] [Google Scholar]
- 13.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med 1987;106(4):512–518. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins RJ, Duan F, Chiles C, et al. Reduced Expiratory Flow Rate among Heavy Smokers Increases Lung Cancer Risk. Results from the National Lung Screening Trial-American College of Radiology Imaging Network Cohort. Ann Am Thorac Soc 2017;14(3):392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner DR, Boffetta P, Duell EJ, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol 2012;176(7):573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner-Warwick M, Lebowitz M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis and lung cancer. Thorax 1980;35(7):496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000;161(1):5–8. [DOI] [PubMed] [Google Scholar]
- 18.Lorigan P, Radford J, Howell A, Thatcher N. Lung cancer after treatment for Hodgkin’s lymphoma: a systematic review. Lancet Oncol 2005;6(10):773–779. [DOI] [PubMed] [Google Scholar]
- 19.Huang YJ, Huang TW, Lin FH, Chung CH, Tsao CH, Chien WC. Radiation Therapy for Invasive Breast Cancer Increases the Risk of Second Primary Lung Cancer: A Nationwide Population-Based Cohort Analysis. J Thorac Oncol 2017;12(5):782–790. [DOI] [PubMed] [Google Scholar]
- 20.Matakidou A, Eisen T, Houlston RS. Systematic review of the relationship between family history and lung cancer risk. Br J Cancer 2005;93(7):825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannino DM. Cigarette smoking and other possible risk factors for lung cancer. https://www.uptodate.com/contents/cigarette-smoking-and-other-possible-risk-factors-for-lung-cancer. Published 2019. Updated March 5, 2019. Accessed April 17, 2019.
- 22.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 1998;90(18):1335–1345. [DOI] [PubMed] [Google Scholar]
- 23.Ripley RT, McMillan RR, Sima CS, et al. Second primary lung cancers: smokers versus nonsmokers after resection of stage I lung adenocarcinoma. Ann Thorac Surg 2014;98(3):968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 2013;145(1):75–81; discussion 81–82. [DOI] [PubMed] [Google Scholar]
- 25.Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017;12(7):1109–1121. [DOI] [PubMed] [Google Scholar]
- 26.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. https://seer.cancer.gov/csr/1975_2016/. Accessed April 28, 2019. [Google Scholar]
- 27.Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR. Lung cancer screening: the Mayo program. J Occup Med 1986;28(8):746–750. [DOI] [PubMed] [Google Scholar]
- 28.Kubik A, Parkin DM, Khlat M, Erban J, Polak J, Adamec M. Lack of benefit from semi-annual screening for cancer of the lung: follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int J Cancer 1990;45(1):26–33. [DOI] [PubMed] [Google Scholar]
- 29.Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306(17):1865–1873. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201(3):798–802. [DOI] [PubMed] [Google Scholar]
- 31.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354(9173):99–105. [DOI] [PubMed] [Google Scholar]
- 32.Sone S, Takashima S, Li F, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet 1998;351(9111):1242–1245. [DOI] [PubMed] [Google Scholar]
- 33.Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20(4):911–920. [DOI] [PubMed] [Google Scholar]
- 34.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165(4):508–513. [DOI] [PubMed] [Google Scholar]
- 35.Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222(3):773–781. [DOI] [PubMed] [Google Scholar]
- 36.Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362(9384):593–597. [DOI] [PubMed] [Google Scholar]
- 37.MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW. Screening for lung cancer using low dose CT scanning. Thorax 2004;59(3):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126(1):114–121. [DOI] [PubMed] [Google Scholar]
- 39.Henschke CI, Naidich DP, Yankelevitz DF, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer 2001;92(1):153–159. [DOI] [PubMed] [Google Scholar]
- 40.Sone S, Li F, Yang ZG, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer 2001;84(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diederich S, Thomas M, Semik M, et al. Screening for early lung cancer with low-dose spiral computed tomography: results of annual follow-up examinations in asymptomatic smokers. Eur Radiol 2004;14(4):691–702. [DOI] [PubMed] [Google Scholar]
- 42.Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47(1):9–15. [DOI] [PubMed] [Google Scholar]
- 43.National Lung Screening Trial Research Team , Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258(1):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260(3):658–663. [DOI] [PubMed] [Google Scholar]
- 46.National Lung Screening Trial Research Team . Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial. J Thorac Oncol 2019;14(10):1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ru Zhao Y, Xie X, de Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging 2011;11(Spec No A):S79–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Koning HJ, van der Aalst CM, de Jong PA et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020; 382:503-513. [DOI] [PubMed] [Google Scholar]
- 49.Pastorino U, Silva M, Sestini S, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019;30(7):1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Infante M, Cavuto S, Lutman FR, et al. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am J Respir Crit Care Med 2015;191(10):1166–1175. [DOI] [PubMed] [Google Scholar]
- 51.Wille MM, Dirksen A, Ashraf H, et al. Results of the Randomized Danish Lung Cancer Screening Trial with Focus on High-Risk Profiling. Am J Respir Crit Care Med 2016;193(5):542–551. [DOI] [PubMed] [Google Scholar]
- 52.International Early Lung Cancer Action Program Investigators , Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355(17):1763–1771. [DOI] [PubMed] [Google Scholar]
- 53.Moyer VA; U.S. Preventive Services Task Force . Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 54.gov CMS.. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Published 2015. Accessed April 26, 2017.
- 55.Fehrenbach H. Commentaries on viewpoint: use of mean airspace chord length to assess emphysema. What does Lm tell us about lung pathology? J Appl Physiol (1985) 2008;105(6):1984–1985; author reply 1986–1987. [DOI] [PubMed] [Google Scholar]
- 56.Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144(1):33–38. [DOI] [PubMed] [Google Scholar]
- 57.Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63(2):107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kauczor HU, Bonomo L, Gaga M, et al. ESR/ERS white paper on lung cancer screening. Eur Radiol 2015;25(9):2519–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canadian Task Force on Preventive Health Care . Recommendations on screening for lung cancer. CMAJ 2016;188(6):425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedersen JH, Rzyman W, Veronesi G, et al. Recommendations from the European Society of Thoracic Surgeons (ESTS) regarding computed tomography screening for lung cancer in Europe. Eur J Cardiothorac Surg 2017;51(3):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzone PJ, Silvestri GA, Patel S, et al. Screening for Lung Cancer: CHEST Guideline and Expert Panel Report. Chest 2018;153(4):954–985. [DOI] [PubMed] [Google Scholar]
- 62.Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16(4):412–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160(5):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Comprehensive Cancer Network. Lung Cancer Screening Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. Published 2018. Updated August 27, 2018. Accessed April 17, 2019. [Google Scholar]
- 65.Pinsky PF, Gierada DS, Hocking W, Patz EF Jr, Kramer BS. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Ann Intern Med 2014;161(9):627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368(8):728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katki HA, Kovalchik SA, Petito LC, et al. Implications of Nine Risk Prediction Models for Selecting Ever-Smokers for Computed Tomography Lung Cancer Screening. Ann Intern Med 2018;169(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71(2):161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tammemagi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol 2017;18(11):1523–1531. [DOI] [PubMed] [Google Scholar]
- 71.United States Preventive Services Task Force. Draft research plan: lung cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-research-plan/lung-cancer-screening1. Published May 2018. Accessed April 17, 2019. [Google Scholar]
- 72.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med 2012;366(9):780–781. [DOI] [PubMed] [Google Scholar]
- 73.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27(10):1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.American College of Radiology. Lung cancer screening resources. https://www.acr.org/Clinical-Resources/Lung-Cancer-Screening-Resources. Accessed April 17, 2019.
- 75.Association for Healthcare Research and Quality . Lung cancer screening tools for patients and clinicians. https://effectivehealthcare.ahrq.gov/decision-aids/lung-cancer-screening/home.html. Accessed April 17, 2019. [Google Scholar]
- 76.American Lung Association . Lung cancer screening implementation guide. https://www.lungcancerscreeningguide.org/shared-decision-making/shared-decision-making-resources/. Accessed April 17, 2019.
- 77.Wiener RS, Koppelman E, Bolton R, et al. Patient and Clinician Perspectives on Shared Decision-making in Early Adopting Lung Cancer Screening Programs: a Qualitative Study. J Gen Intern Med 2018;33(7):1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brenner AT, Malo TL, Margolis M, et al. Evaluating Shared Decision Making for Lung Cancer Screening. JAMA Intern Med 2018;178(10):1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanner NT, Banas E, Yeager D, Dai L, Hughes Halbert C, Silvestri GA. In-person and Telephonic Shared Decision-making Visits for People Considering Lung Cancer Screening: An Assessment of Decision Quality. Chest 2019;155(1):236–238. [DOI] [PubMed] [Google Scholar]
- 80.Newcomb PA, Carbone PP. The health consequences of smoking. Cancer. Med Clin North Am 1992;76(2):305–331. [DOI] [PubMed] [Google Scholar]
- 81.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000;321(7257):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanner NT, Kanodra NM, Gebregziabher M, et al. The Association between Smoking Abstinence and Mortality in the National Lung Screening Trial. Am J Respir Crit Care Med 2016;193(5):534–541. [DOI] [PubMed] [Google Scholar]
- 83.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA 2008;299(17):2037–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tindle HA, Stevenson Duncan M, Greevy RA, et al. Lifetime Smoking History and Risk of Lung Cancer: Results From the Framingham Heart Study. J Natl Cancer Inst 2018;110(11):1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013;368(4):351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Centers for Disease Control and Prevention (CDC). Quitting smoking among adults--United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011;60(44)1513–1519. [PubMed] [Google Scholar]
- 87.Siu AL; U.S. Preventive Services Task Force . Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2015;163(8):622–634. [DOI] [PubMed] [Google Scholar]
- 88.Pedersen JH, Tønnesen P, Ashraf H. Smoking cessation and lung cancer screening. Ann Transl Med 2016;4(8):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tremblay A, Taghizadeh N, Huang J, et al. A Randomized Controlled Study of Integrated Smoking Cessation in a Lung Cancer Screening Program. J Thorac Oncol 2019;14(9):1528–1537. [DOI] [PubMed] [Google Scholar]
- 90.Joseph AM, Rothman AJ, Almirall D, et al. Lung Cancer Screening and Smoking Cessation Clinical Trials. SCALE (Smoking Cessation within the Context of Lung Cancer Screening) Collaboration. Am J Respir Crit Care Med 2018;197(2):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162(7):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Henschke CI, Yip R, Yankelevitz DF, Smith JP; International Early Lung Cancer Action Program Investigators*. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158(4):246–252. [DOI] [PubMed] [Google Scholar]
- 93.McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR Lung-RADS in a clinical CT lung screening program. J Am Coll Radiol 2015;12(3):273–276. [DOI] [PubMed] [Google Scholar]
- 94.Hsu HT, Tang EK, Wu MT, et al. Modified Lung-RADS Improves Performance of Screening LDCT in a Population with High Prevalence of Non-smoking-related Lung Cancer. Acad Radiol 2018;25(10):1240–1251. [DOI] [PubMed] [Google Scholar]
- 95.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361(23):2221–2229. [DOI] [PubMed] [Google Scholar]
- 96.American Association of Physicists in Medicine. Lung Cancer Screening Protocols Version 4.0. https://www.aapm.org/pubs/CTProtocols/documents/LungCancerScreeningCT.pdf. Published 2019. Accessed October 10, 2019. [Google Scholar]
- 97.Schauer DA, Linton OW. National Council on Radiation Protection and Measurements report shows substantial medical exposure increase. Radiology 2009;253(2):293–296. [DOI] [PubMed] [Google Scholar]
- 98.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169(22):2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.United States Food and Drug Administration . What are the Radiation Risks from CT? https://www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115329.htm. Updated December 5, 2017. Accessed April 17, 2019.
- 101.Brennan P, Crispo A, Zaridze D, et al. High cumulative risk of lung cancer death among smokers and nonsmokers in Central and Eastern Europe. Am J Epidemiol 2006;164(12):1233–1241. [DOI] [PubMed] [Google Scholar]
- 102.Mattson ME, Pollack ES, Cullen JW. What are the odds that smoking will kill you? Am J Public Health 1987;77(4):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Samet JM, Wiggins CL, Humble CG, Pathak DR. Cigarette smoking and lung cancer in New Mexico. Am Rev Respir Dis 1988;137(5):1110–1113. [DOI] [PubMed] [Google Scholar]
- 104.Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174(2):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15(12):1342–1350. [DOI] [PubMed] [Google Scholar]
- 106.Scholten ET, Horeweg N, de Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol 2015;25(1):81–88. [DOI] [PubMed] [Google Scholar]
- 107.Gierada DS, Pinsky PF, Duan F, et al. Interval lung cancer after a negative CT screening examination: CT findings and outcomes in National Lung Screening Trial participants. Eur Radiol 2017;27(8):3249–3256. [DOI] [PubMed] [Google Scholar]
- 108.Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol 2012;199(4):781–786. [DOI] [PubMed] [Google Scholar]
- 109.Tsai EB, Chiles C, Carter BW, et al. Incidental Findings on Lung Cancer Screening: Significance and Management. Semin Ultrasound CT MR 2018;39(3):273–281. [DOI] [PubMed] [Google Scholar]
- 110.Chung JH, Richards JC, Koelsch TL, MacMahon H, Lynch DA. Screening for Lung Cancer: Incidental Pulmonary Parenchymal Findings. AJR Am J Roentgenol 2018;210(3):503–513. [DOI] [PubMed] [Google Scholar]
- 111.Nguyen XV, Davies L, Eastwood JD, Hoang JK. Extrapulmonary Findings and Malignancies in Participants Screened With Chest CT in the National Lung Screening Trial. J Am Coll Radiol 2017;14(3):324–330. [DOI] [PubMed] [Google Scholar]
- 112.Gordic S, Morsbach F, Schmidt B, et al. Ultralow-dose chest computed tomography for pulmonary nodule detection: first performance evaluation of single energy scanning with spectral shaping. Invest Radiol 2014;49(7):465–473. [DOI] [PubMed] [Google Scholar]
- 113.Martini K, Higashigaito K, Barth BK, Baumueller S, Alkadhi H, Frauenfelder T. Ultralow-dose CT with tin filtration for detection of solid and sub solid pulmonary nodules: a phantom study. Br J Radiol 2015;88(1056):20150389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gruden JF, Ouanounou S, Tigges S, Norris SD, Klausner TS. Incremental benefit of maximum-intensity-projection images on observer detection of small pulmonary nodules revealed by multidetector CT. AJR Am J Roentgenol 2002;179(1):149–157. [DOI] [PubMed] [Google Scholar]
- 115.Valencia R, Denecke T, Lehmkuhl L, Fischbach F, Felix R, Knollmann F. Value of axial and coronal maximum intensity projection (MIP) images in the detection of pulmonary nodules by multislice spiral CT: comparison with axial 1-mm and 5-mm slices. Eur Radiol 2006;16(2):325–332. [DOI] [PubMed] [Google Scholar]
- 116.Kawel N, Seifert B, Luetolf M, Boehm T. Effect of slab thickness on the CT detection of pulmonary nodules: use of sliding thin-slab maximum intensity projection and volume rendering. AJR Am J Roentgenol 2009;192(5):1324–1329. [DOI] [PubMed] [Google Scholar]
- 117.American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS) Version 1.1. http://www.acr.org/Quality-Safety/Resources/LungRADS. Published 2019. Accessed May 31, 2019.
- 118.Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231(1):164–168. [DOI] [PubMed] [Google Scholar]
- 119.Yip R, Henschke CI, Yankelevitz DF, Smith JP. CT screening for lung cancer: alternative definitions of positive test result based on the national lung screening trial and international early lung cancer action program databases. Radiology 2014;273(2):591–596. [DOI] [PubMed] [Google Scholar]
- 120.Gierada DS, Pinsky P, Nath H, Chiles C, Duan F, Aberle DR. Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. J Natl Cancer Inst 2014;106(11):dju284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahn MI, Gleeson TG, Chan IH, et al. Perifissural nodules seen at CT screening for lung cancer. Radiology 2010;254(3):949–956. [DOI] [PubMed] [Google Scholar]
- 122.de Hoop B, van Ginneken B, Gietema H, Prokop M. Pulmonary perifissural nodules on CT scans: rapid growth is not a predictor of malignancy. Radiology 2012;265(2):611–616. [DOI] [PubMed] [Google Scholar]
- 123.Schreuder A, van Ginneken B, Scholten ET, et al. Classification of CT Pulmonary Opacities as Perifissural Nodules: Reader Variability. Radiology 2018;288(3):867–875. [DOI] [PubMed] [Google Scholar]
- 124.Chung K, Jacobs C, Scholten ET, et al. Lung-RADS Category 4X: Does It Improve Prediction of Malignancy in Subsolid Nodules? Radiology 2017;284(1):264–271. [DOI] [PubMed] [Google Scholar]
- 125.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369(10):910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Winkler Wille MM, van Riel SJ, Saghir Z, et al. Predictive Accuracy of the PanCan Lung Cancer Risk Prediction Model -External Validation based on CT from the Danish Lung Cancer Screening Trial. Eur Radiol 2015;25(10):3093–3099. [DOI] [PubMed] [Google Scholar]
- 127.White CS, Dharaiya E, Campbell E, Boroczky L. The Vancouver Lung Cancer Risk Prediction Model: Assessment by Using a Subset of the National Lung Screening Trial Cohort. Radiology 2017;283(1):264–272. [DOI] [PubMed] [Google Scholar]
- 128.White CS, Dharaiya E, Dalal S, Chen R, Haramati LB. Vancouver Risk Calculator Compared with ACR Lung-RADS in Predicting Malignancy: Analysis of the National Lung Screening Trial. Radiology 2019;291(1):205–211. [DOI] [PubMed] [Google Scholar]
- 129.Yankelevitz DF, Yip R, Smith JP, et al. CT Screening for Lung Cancer: Nonsolid Nodules in Baseline and Annual Repeat Rounds. Radiology 2015;277(2):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178(5):1053–1057. [DOI] [PubMed] [Google Scholar]
- 131.Henschke CI, Yip R, Smith JP, et al. CT Screening for Lung Cancer: Part-Solid Nodules in Baseline and Annual Repeat Rounds. AJR Am J Roentgenol 2016;207(6):1176–1184. [DOI] [PubMed] [Google Scholar]
- 132.Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17(7):907–916. [DOI] [PubMed] [Google Scholar]
- 133.Pinsky PF, Gierada DS, Nath PH, Munden R. Lung Cancer Risk Associated With New Solid Nodules in the National Lung Screening Trial. AJR Am J Roentgenol 2017;209(5):1009–1014. [DOI] [PubMed] [Google Scholar]
- 134.Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology 2008;246(1):265–272. [DOI] [PubMed] [Google Scholar]
- 135.Singh S, Pinsky P, Fineberg NS, et al. Evaluation of reader variability in the interpretation of follow-up CT scans at lung cancer screening. Radiology 2011;259(1):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pinsky PF, Gierada DS, Nath PH, Kazerooni E, Amorosa J. National lung screening trial: variability in nodule detection rates in chest CT studies. Radiology 2013;268(3):865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Al Mohammad B, Brennan PC, Mello-Thoms C. A review of lung cancer screening and the role of computer-aided detection. Clin Radiol 2017;72(6):433–442. [DOI] [PubMed] [Google Scholar]
- 138.Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;217(1):251–256. [DOI] [PubMed] [Google Scholar]
- 139.Heuvelmans MA, Walter JE, Vliegenthart R, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax 2018;73(8):779–781. [DOI] [PubMed] [Google Scholar]
- 140.Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21(3):308–315. [DOI] [PubMed] [Google Scholar]
- 141.Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67(4):296–301. [DOI] [PubMed] [Google Scholar]
- 142.Gietema HA, Wang Y, Xu D, et al. Pulmonary nodules detected at lung cancer screening: interobserver variability of semiautomated volume measurements. Radiology 2006;241(1):251–257. [DOI] [PubMed] [Google Scholar]
- 143.Gietema HA, Schaefer-Prokop CM, Mali WP, Groenewegen G, Prokop M. Pulmonary nodules: Interscan variability of semiautomated volume measurements with multisection CT-- influence of inspiration level, nodule size, and segmentation performance. Radiology 2007;245(3):888–894. [DOI] [PubMed] [Google Scholar]
- 144.Marchianò A, Calabrò E, Civelli E, et al. Pulmonary nodules: volume repeatability at multidetector CT lung cancer screening. Radiology 2009;251(3):919–925. [DOI] [PubMed] [Google Scholar]
- 145.Jeon KN, Goo JM, Lee CH, et al. Computer-aided nodule detection and volumetry to reduce variability between radiologists in the interpretation of lung nodules at low-dose screening computed tomography. Invest Radiol 2012;47(8):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goo JM, Tongdee T, Tongdee R, Yeo K, Hildebolt CF, Bae KT. Volumetric measurement of synthetic lung nodules with multi-detector row CT: effect of various image reconstruction parameters and segmentation thresholds on measurement accuracy. Radiology 2005;235(3):850–856. [DOI] [PubMed] [Google Scholar]
- 147.Ravenel JG, Leue WM, Nietert PJ, Miller JV, Taylor KK, Silvestri GA. Pulmonary nodule volume: effects of reconstruction parameters on automated measurements--a phantom study. Radiology 2008;247(2):400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Larici AR, Storto ML, Torge M, et al. Automated volumetry of pulmonary nodules on multidetector CT: influence of slice thickness, reconstruction algorithm and tube current. Preliminary results. Radiol Med (Torino) 2008;113(1):29–42. [DOI] [PubMed] [Google Scholar]
- 149.Wielpütz MO, Lederlin M, Wroblewski J, et al. CT volumetry of artificial pulmonary nodules using an ex vivo lung phantom: influence of exposure parameters and iterative reconstruction on reproducibility. Eur J Radiol 2013;82(9):1577–1583. [DOI] [PubMed] [Google Scholar]
- 150.Quantitative Imaging Biomarker Alliance. CT Small Lung Nodule Biomarker Committee. http://qibawiki.rsna.org/index.php/CT_Small_Lung_Nodule_Biomarker_Ctte. Accessed April 18, 2019. [Google Scholar]
- 151.Park CM, Goo JM, Lee HJ, Kim KG, Kang MJ, Shin YH. Persistent pure ground-glass nodules in the lung: interscan variability of semiautomated volume and attenuation measurements. AJR Am J Roentgenol 2010;195(6):W408–W414. [DOI] [PubMed] [Google Scholar]
- 152.Kim H, Park CM, Woo S, et al. Pure and part-solid pulmonary ground-glass nodules: measurement variability of volume and mass in nodules with a solid portion less than or equal to 5 mm. Radiology 2013;269(2):585–593. [DOI] [PubMed] [Google Scholar]
- 153.Scholten ET, de Hoop B, Jacobs C, et al. Semi-automatic quantification of subsolid pulmonary nodules: comparison with manual measurements. PLoS One 2013;8(11):e80249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lassen BC, Jacobs C, Kuhnigk JM, van Ginneken B, van Rikxoort EM. Robust semi-automatic segmentation of pulmonary subsolid nodules in chest computed tomography scans. Phys Med Biol 2015;60(3):1307–1323. [DOI] [PubMed] [Google Scholar]
- 155.McNitt-Gray MF, Hart EM, Wyckoff N, Sayre JW, Goldin JG, Aberle DR. A pattern classification approach to characterizing solitary pulmonary nodules imaged on high resolution CT: preliminary results. Med Phys 1999;26(6):880–888. [DOI] [PubMed] [Google Scholar]
- 156.Aoyama M, Li Q, Katsuragawa S, Li F, Sone S, Doi K. Computerized scheme for determination of the likelihood measure of malignancy for pulmonary nodules on low-dose CT images. Med Phys 2003;30(3):387–394. [DOI] [PubMed] [Google Scholar]
- 157.Way TW, Hadjiiski LM, Sahiner B, et al. Computer-aided diagnosis of pulmonary nodules on CT scans: segmentation and classification using 3D active contours. Med Phys 2006;33(7):2323–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gierada DS, Politte DG, Zheng J, et al. Quantitative Computed Tomography Classification of Lung Nodules: Initial Comparison of 2- and 3-Dimensional Analysis. J Comput Assist Tomogr 2016;40(4):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kawata Y, Niki N, Ohmatsu H, et al. Quantitative classification based on CT histogram analysis of non-small cell lung cancer: correlation with histopathological characteristics and recurrence-free survival. Med Phys 2012;39(2):988–1000. [DOI] [PubMed] [Google Scholar]
- 160.Raghunath S, Maldonado F, Rajagopalan S, et al. Noninvasive risk stratification of lung adenocarcinoma using quantitative computed tomography. J Thorac Oncol 2014;9(11):1698–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015;521(7553):436–444. [DOI] [PubMed] [Google Scholar]
- 162.Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine Learning for Medical Imaging. RadioGraphics 2017;37(2):505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li F, Aoyama M, Shiraishi J, et al. Radiologists’ performance for differentiating benign from malignant lung nodules on high-resolution CT using computer-estimated likelihood of malignancy. AJR Am J Roentgenol 2004;183(5):1209–1215. [DOI] [PubMed] [Google Scholar]
- 164.Way T, Chan HP, Hadjiiski L, et al. Computer-aided diagnosis of lung nodules on CT scans: ROC study of its effect on radiologists’ performance. Acad Radiol 2010;17(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dilger SK, Uthoff J, Judisch A, et al. Improved pulmonary nodule classification utilizing quantitative lung parenchyma features. J Med Imaging (Bellingham) 2015;2(4):041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hawkins S, Wang H, Liu Y, et al. Predicting Malignant Nodules from Screening CT Scans. J Thorac Oncol 2016;11(12):2120–2128 [Published correction appears in J Thorac Oncol 2018;13(2):280–281.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cheng JZ, Ni D, Chou YH, et al. Computer-Aided Diagnosis with Deep Learning Architecture: Applications to Breast Lesions in US Images and Pulmonary Nodules in CT Scans. Sci Rep 2016;6(1):24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Song Q, Zhao L, Luo X, Dou X. Using Deep Learning for Classification of Lung Nodules on Computed Tomography Images. J Healthc Eng 2017;2017:8314740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med 2019;25(6):954–961 [Published correction appears in Nat Med 2019;25(8):1319.]. [DOI] [PubMed] [Google Scholar]
- 170.Pham D, Bhandari S, Oechsli M, Pinkston CM, Kloecker GH. Lung cancer screening rates: Data from the lung cancer screening registry. J Clin Oncol 2018;36(15 Suppl):6504. [Google Scholar]
- 171.Zahnd WE, Eberth JM. Lung Cancer Screening Utilization: A Behavioral Risk Factor Surveillance System Analysis. Am J Prev Med 2019;57(2):250–255. [DOI] [PubMed] [Google Scholar]
- 172.Wang GX, Baggett TP, Pandharipande PV, et al. Barriers to Lung Cancer Screening Engagement from the Patient and Provider Perspective. Radiology 2019;290(2):278–287. [DOI] [PubMed] [Google Scholar]