Abstract
Purpose of review.
We review studies demonstrating lowered levels of insulin-like growth factors (IGFs) in patients with recent-onset type 1 diabetes (T1D) and discuss their potential roles in the disorder’s pathogenesis.
Recent findings.
IGFs have long been recognized as a class of hormones that promote growth, development and cellular metabolism throughout the human body. More recently, studies have noted an association between reduced pancreatic weight/volume and T1D. Thus, we believe it is important to understand pancreatic regulation of IGF expression and bioavailability, as well as the impact of IGFs on pancreatic growth and islet health. Additional studies of IGFs have been extended to their influence on the inflammatory/regulatory balance of monocytes, B cells, and T cells; features which have been previously established to show dysregulation in settings of T1D.
Summary.
These data suggest that IGFs may prevent known impairments in the pancreas and immune system in T1D and underscore the need to extend these studies, some of which were performed in health or other autoimmune diseases, toward T1D specifically. Collectively, the work emphasized here support the potential therapeutic use of IGFs in T1D prevention efforts as pancreatic growth factors and/or immunoregulatory agents.
Keywords: Insulin-like Growth Factors, Type 1 Diabetes, Pancreas, Immunology, Immunoregulation
INTRODUCTION
Insulin-like Growth Factors (IGFs) represent a family of related hormones, comprised of the ligands IGF1 and IGF2, which orchestrate cellular proliferation and metabolism (1). IGFs have historically garnered interest in the field of type 1 diabetes (T1D) due to their structural homology to insulin, although important distinctions exist between the two classes of hormones with regard to their regulation and signaling (2). IGFs are produced mainly by the liver in response to growth hormone and are secreted into the bloodstream to act on targets throughout the body in an endocrine or paracrine fashion (1). Additional cells relevant to T1D, including those comprising the pancreatic islets (3) and macrophages (4), can also produce IGFs for autocrine maintenance. IGFs are temporally regulated such that IGF1 drives postnatal growth and peaks during puberty, in contrast to IGF2, which promotes fetal development (5).
IGF action is regulated by a series of at least seven IGF binding proteins (IGFBP1–7) that modulate bioavailability in terms of the ability of IGFs to bind their main signaling receptor, the IGF1 receptor (IGF1R). IGF2R serves mainly as a decoy receptor for IGF2 uptake and degradation (1, 2). Though insulin can also bind IGF1R, both IGF1 and IGF2 possess relatively higher affinities to this receptor. IGF1R signaling is known to induce the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and Ras/mitogen-activated protein kinase (MAPK) signaling pathways, similar to those activated downstream of the insulin receptor (IR); although, IGF1R is thought to favor mitogenic signaling in comparison to IR’s tendency toward metabolic effects. Signaling becomes quite complex, as IR and IGF1R can also heterodimerize to create receptors with intermediate affinity for IGFs and cellular impacts between those of either homodimer (2).
IGF1 has been extensively characterized in adolescents and adults with new onset and established T1D (6, 7) and animal models of the disease (8), wherein serum or plasma levels are known to be decreased in comparison to healthy controls; however, whether this leads to defective growth remains inconclusive (9). It is also unknown whether IGF1 or IGF2 are dysregulated prior to disease onset and if a scarcity of these factors contributes to the development of T1D. Since C-peptide levels are positively correlated with IGF1 levels and measures of glycemia are negatively correlated with IGF1 (10), it remains unclear whether defective IGF regulation might be primary or secondary to the loss of insulin associated with T1D. The purpose of this Opinion is to highlight recent findings regarding the impact of IGFs on the pancreas and immune system to infer how low IGF levels or bioavailability may promote T1D pathogenesis.
IMPACTS OF IGF ON THE PANCREAS
Due to the relatively ubiquitous presence of IGF1R (1), we will discuss IGF action at the level of the whole pancreas and on various cell types within the endocrine and exocrine regions of the organ (Fig. 1).
Fig. 1. Impact of IGFs on the exocrine and endocrine pancreas.
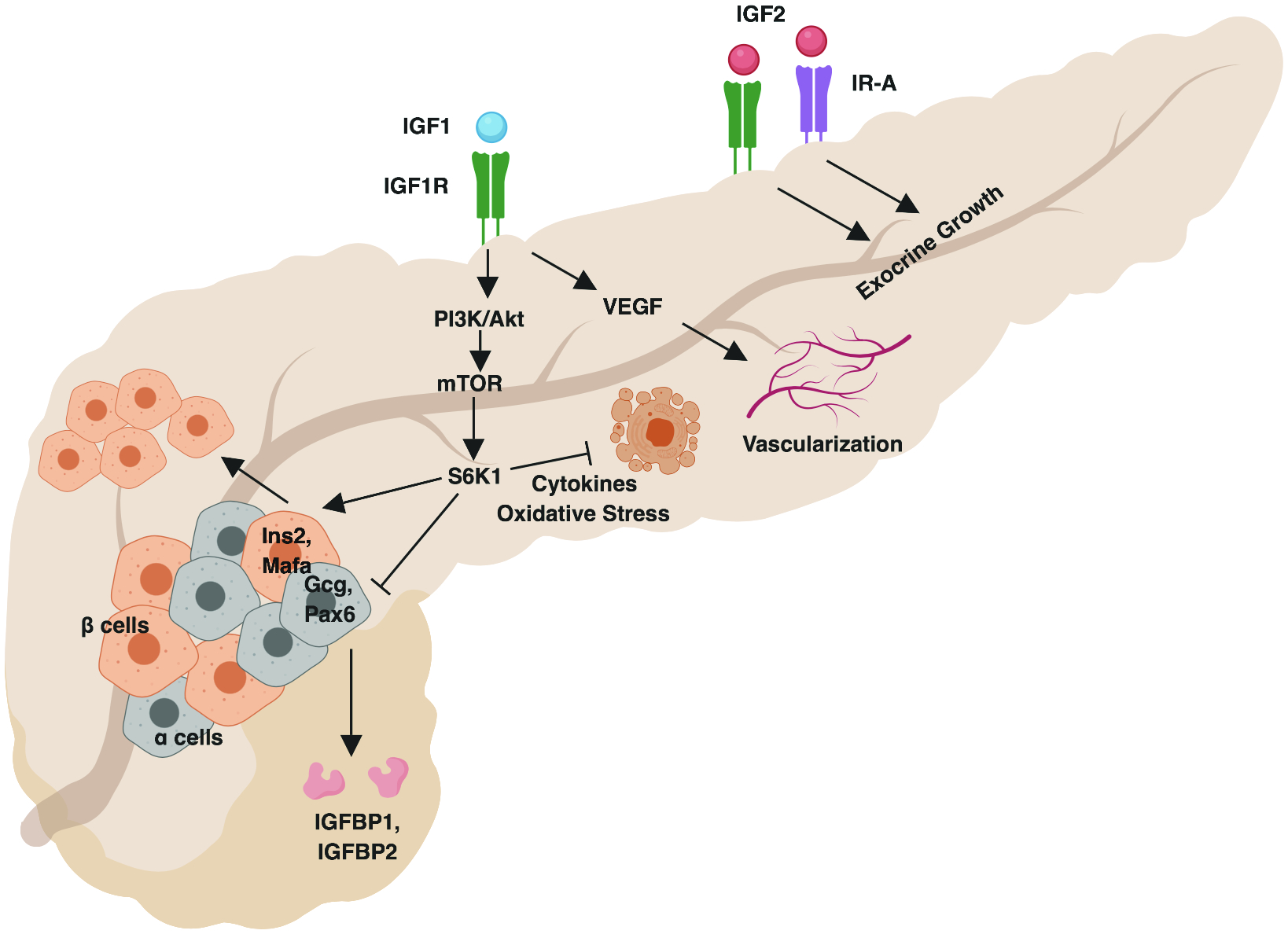
IGF2 drives exocrine growth in early life via IGF1R and IR-A. IGF1 induces IGF1R-mediated PI3K/Akt signaling, which promotes β-cell and inhibits α-cell related gene expression and enhances β-cell proliferation. PI3K/Akt signaling also inhibits β-cell apoptosis in the context of inflammatory cytokines and oxidative stress. α-cell-produced glucagon promotes IGFBP1 and IGFBP2 expression, limiting IGF availability. IGF1 also drives VEGF production to promote islet vascularization. Created with BioRender.
Pancreatic Organogenesis and Exocrine Development
Pancreatic weight, normalized for age and body mass index, and pancreas volume are significantly decreased in organ donors and living patients with T1D (11, 12) as well as at-risk subjects with T1D-predictive autoantibodies (AAb) (13). This disease-associated organ weight loss cannot be accounted for solely by the autoimmune-mediated loss of β-cells, as these comprise only approximately 1–2% of pancreatic mass (14). Recent studies have extrapolated these findings to show that pancreas volume, measured from living subjects via magnetic resonance imaging, is reduced even in first-degree relatives of T1D subjects whom are more likely to progress to T1D than the general population (15) and in patients with non-traditional forms of T1D, such as latent autoimmune diabetes in adults (LADA) (16). Evidence of reduced exocrine function in T1D patients may be reflective of decreased pancreatic size (15), and studies to characterize this possible association are currently ongoing. T1D subjects show downregulated levels of acinar-produced enzymes involved in protein digestion such as elastase (17) and the zymogen, trypsinogen, which is not only significantly decreased in T1D patients but also, in pre-T1D subjects with two or more AAb (18). Together, these data suggest that the entire pancreas, including the endocrine and exocrine compartments, may be functionally and volumetrically impaired in T1D.
IGFs may influence the development and growth of the pancreatic organ as a whole. While mouse models with either Insr or Igf1r knockout show normal pancreas size, combined ablation leads to a loss of the majority of the exocrine pancreatic mass, yet spares the endocrine compartment. Combined inactivation of Igf1 and Igf2 shows a similar loss of exocrine pancreas size at early time points prior to IGF1 dependency (19). Since IGF2 and insulin share a similar affinity for the specialized isoform of IR known as IR-A (2), these data are thought to suggest that IGF2 signaling via IGF1R and IR-A acts as a major driver of embryonic development of the exocrine pancreas. A new study utilizing a rat model of intrafetal IGF2 administration showed that digestive organ sizes were augmented, with significantly increased pancreatic weight in male offspring and a trend toward an increase in females upon IGF2 treatment (20). Whether low IGF2 levels during fetal development may account for reduced pancreatic volume in T1D remains unknown as similar studies have yet to be performed using early IGF2 treatment in rodent models of T1D or correlative observational studies in young human cohorts.
Pancreatic Islet Niche
β-cell loss in T1D may be accounted for by mechanisms including but not limited to apoptosis as well as de- or trans-differentiation. When human β-cell lines were stressed with factors thought to contribute to T1D onset, such as a virus or type I interferon, β-cell-specific gene expression was shown to decrease, giving way for increased expression of progenitor genes (21) or those of other endocrine cell types (22). Interestingly, a signaling mediator downstream of the PI3K/Akt pathway, ribosomal protein S6 kinase beta-1 (S6K1), is required for maintenance of β-cell identity. S6K1 inactivation leads to decreased expression of the β-cell markers, Ins2 and Mafa, and upregulated expression of α-cell genes, including Gcg and Pax6, in murine pancreatic tissue due to epigenetic modifications (23). Though debate is ongoing regarding whether similar de-differentiation and trans-differentiation events occur in human islets, these data imply that IGF-mediated PI3K signaling could drive endocrine cells toward the β-cell lineage.
PI3K/Akt signaling also plays an essential role in the regulation of β-cell apoptosis and proliferation. Physiological stimulation of the PI3K/Akt pathway in a variety of murine β-cell lines— either at rest or in the presence of inflammatory cytokines— protects against apoptosis, induces proliferation, and increases insulin secretion (24, 25). Likewise, stimulation of the PI3K/Akt pathway protects porcine β-cells from oxidative stress-related death, allowing for rescue from diabetes after transplantation in streptozotocin-treated mice (26). Enhancement of downstream mTOR signaling also increases the proliferation of β-cells in zebrafish as well as insulin secretion in murine and primary human islets (27). These data imply that compromised IGF signaling could potentially lead to impairments in β-cell proliferation, survival, or insulin secretory function. However, many of these findings remain to be validated in human islets and with direct IGF stimulation, and it is unclear whether there could be redundancy with autocrine insulin signaling.
Current reports on human T1D pancreata have shown that in addition to a loss of β-cells, there is an apparent loss in α-cell number and function, resulting in altered gene expression and lowered glucagon secretion (28, 29). Glucagon is historically recognized to counter-regulate insulin action and thereby, decrease IGF production (30), and it was recently uncovered that glucagon can also decrease IGF1 bioavailability, independently of insulin action, through a mechanism involving induction of IGFBP1 and IGFBP2 to block IGF action (31). These studies highlight the need to assess the impact of lowered glucagon responses, as seen in T1D, on IGF bioavailability.
Proper vascularization is necessary for islet health (32). Treatment of fetal sheep with an IGF1 analog increased pancreatic islet vascularity due to enhanced vascular endothelial growth factor (VEGF) expression. This accordingly augmented pancreatic insulin content in early life, suggesting that paracrine insulin and/or IGF1 signaling might locally promote islet growth and development (33). Interestingly, endothelial cell proliferation in situ relies on anti-inflammatory M2 macrophage-produced IGF1 (34); thus, an immunoregulatory environment could promote islet angiogenesis. However, enhanced islet vascularity might also augment immune infiltration, so the protective effect of IGFs in regard to angiogenesis may be restricted to early life and requires further validation in human tissues.
IMMUNOREGULATORY EFFECTS OF IGF
IGFs are known to regulate innate and adaptive immune responses, as has been previously reviewed (35), and deficiencies in immune regulation are generally accepted to contribute to the pathogenesis of T1D. Recent findings suggesting a potential role for IGFs in promoting immune tolerance in T1D and other autoimmune disorders will be discussed below (Fig. 2).
Fig. 2. Immunoregulatory effects of IGFs.
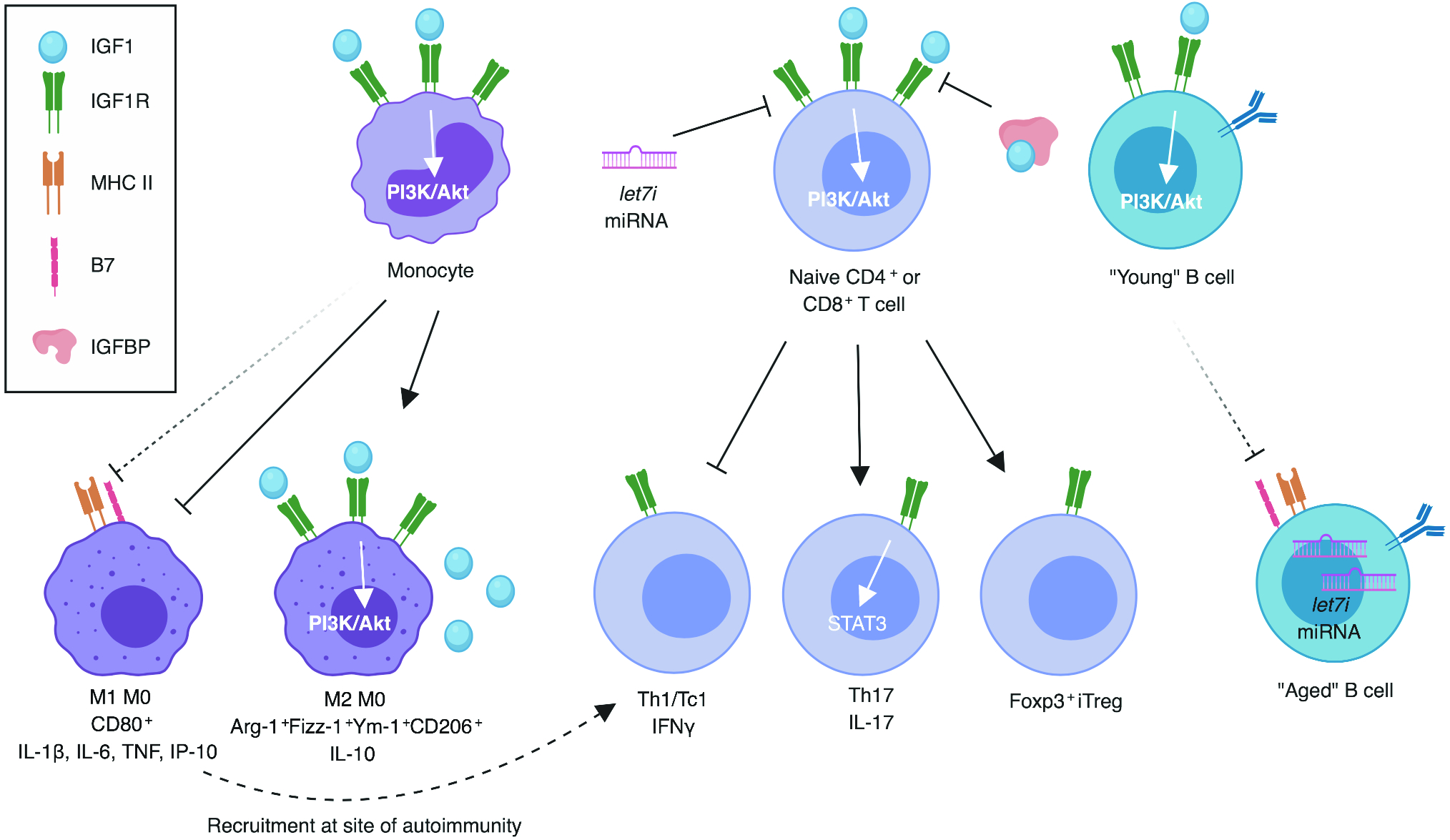
Known associations denoted by filled arrows, presumptive associations denoted by dashed arrows. IGF1 promotes M2 at the expense of M1 macrophage phenotype. Naïve T cells are skewed toward Th17 and iTreg and away from Th1 in the presence of IGF1. IGF1/IGF1R signaling inhibited by IGFBPs and let-7i miRNA. Let-7 class miRNAs overexpressed in aged B cells, presumably impeding the IGF1-signaling induced regulation of molecules associated with antigen presentation. Created with BioRender.
Macrophage and Monocyte Skewing
Macrophages and monocytes express high levels of IGF1R (35), and M2 macrophages can produce IGF1 for autocrine signaling to reinforce their regulatory phenotype (4). In vitro, IGF1 was shown to decrease the transcription of inflammatory cytokines, such as IL-1β, IL-6 and TNF, and the expression of the M1 macrophage marker CD80 in human macrophages and simultaneously, increased their transcription of the regulatory cytokine IL-10 and M2 markers, including Arg-1, Fizz-1, Ym-1 and CD206 (36). Ex vivo, splenocytes from a macrophage-specific Igf1 knockout mouse model exhibited an elevated percentage of inflammatory M1 macrophages, leading to increased inflammatory T cell skewing (37). These data suggest that IGFs shift macrophages and monocytes toward an anti-inflammatory M2 phenotype and away from inflammatory M1 subsets, and that this might indirectly promote a regulatory adaptive immune profile.
B Cell Function and Aging
B cell pathogenicity in T1D is thought to be due to their role as antigen-presenting cells, promoting the activation of autoreactive T cells (38). Non-obese diabetic (NOD) mice with excess β-cell-specific Igf1 expression showed decreased islet expression of mRNA associated with antigen presentation, including MHC class II and costimulatory B7 family receptors (39), although it remains unclear whether these changes are associated with B cells specifically or more generally affecting antigen presentation in the islet as a whole. Additionally, autoreactive B cells, such as those present in patients with T1D, can demonstrate an “aged” phenotype with aberrant transcription factor expression (40). Importantly, B cell aging is associated with epigenetic modifications that lead to decreased transcription of IGF1R signaling related genes, including IRS1, and increased expression of let-7 class microRNAs that inhibit IGF1R expression (41). Together, defective IGF1R signaling may contribute to autoimmune B cell pathogenicity, but there remains an outstanding need to evaluate these observations in the NOD mouse model and samples obtained from human subjects with T1D.
Peripheral Tolerance of T Cells
Emerging evidence indicates that IGFs can directly stimulate the PI3K/Akt pathway in activated primary human T cells (42). Concepts surrounding how IGFs promote thymocyte development and regulate central tolerance, particularly in regard to insulin, have been the subject of a recent extensive review (43). Here we will focus on studies highlighting the role of IGFs in peripheral tolerance.
There is a growing body of evidence that T cell skewing may be influenced by IGFs, either directly or indirectly, through immunoregulatory modulation of monocytes and macrophages, as described above. NOD mice genetically overexpressing Igf1 in β-cells or administered virally-encoded Igf1 targeted to the pancreas showed lower transcription of the diabetogenic T helper 1 (Th1)-related inflammatory cytokine, Ifnγ, and the chemokine, Ifnγ-induced protein 10 (Ip-10), within the pancreatic islets (39). The costimulatory Cd86 (B7–2) knockout model of spontaneous autoimmune peripheral polyneuropathy (SAPP) on the NOD background showed decreased neural T cell recruitment with Igf1 gene therapy and likewise, lowered proportions of IFNγ-producing CD4+ and CD8+ T cells ex vivo (44). Although this study noted a generalized decrease in splenocyte transcription of proinflammatory cytokines, including IL-17, after in vitro stimulation with IGF1 (44), others have suggested that IGF1 signaling promotes human and murine Th17 cell formation (45). Here, an inhibitor of insulin receptor substrate (IRS1/2) phosphorylation, downstream of IGF1R and IR, was shown to prevent IGF1R and Th17-related STAT3 phosphorylation in the synovia of an experimental arthritis mouse model, thereby reducing IL-6 production. Hence, a shift away from Th17 and toward regulatory T cell (Treg) lineage was observed, and many of these observations were subsequently replicated in ex vivo rheumatoid arthritis patient samples (45). Overall, IGF1 may shift T cell phenotype away from Th1 and toward other T helper subsets, including but not limited to Th17.
In addition to limiting inflammatory T cell skewing, increasing evidence suggests that IGFs promote the peripheral induction of Tregs (iTregs). An increased percentage of Foxp3+ Tregs were observed in the islets of NOD mice overexpressing Igf1 (39). Similarly, IGF1R signaling in conjunction with TGFβ has recently been shown to support the skewing of human naïve CD4+ T cells into iTregs (46, 47). Neutralization of IGFBP likewise aided in the iTreg generation (46). Multiple sclerosis (MS) patients showed lowered expression of IGF1R on naïve CD4+ T cells due to the overabundance of an exosomal microRNA, let-7i, which is thought to inhibit IGF1R translation (47). Although this mechanism has yet to be evaluated in the context of T1D, inhibition of let-7i warrants investigation as a potential therapeutic approach to restore immunoregulatory IGF/IGF1R signaling.
While excess inflammatory skewing or a defect in Treg skewing may contribute to numerous autoimmune disorders (48), it is interesting to note that various forms of monogenic diabetes of the young (MODY) result from mutations in both the Th17-related transcription factor, STAT3, and the Treg-related transcription factor, STAT5B (49). While there is controversy over whether Th17 cells promote or inhibit T1D, the effect of IL-17 seems to be highly context-dependent such that in combination with inhibited Th1 responses, Th17 skewing could restrain T1D pathogenesis (50). Thus, Th17 and iTreg may have protective roles in T1D, and their expansion with IGF1 could be beneficial.
CONCLUSIONS
The current literature supports the concept that IGFs are notable modulators of pancreas and islet health and innate and adaptive inflammation. However, many outstanding questions remain for the field. Further study is necessary to determine whether the impacts on pancreas growth and islet health, as discussed here, will translate from the non-diabetic pancreas to the stressed, dysfunctional T1D pancreas or whether the influence of IGFs on the balance of inflammatory and regulatory skewing observed from other autoimmune pathologies are also common to the pathogenesis of T1D. An in-depth characterization of IGF and IGFBP levels in human subjects with pre-T1D has yet to be performed, both at the system level and locally in disease-relevant tissues, such as the pancreas and thymus. An important question remaining for the field involves whether the impacts of IGFs on the T1D pancreas are primary or secondary to the loss of insulin. There is also a need to further understand the mechanisms behind IGF-mediated antigen presenting cell (i.e., macrophage/monocyte and B cell) modulation and peripheral T cell skewing in human subjects. The studies summarized herein highlight the potential use of low IGF levels or bioavailability as biomarkers in T1D and possible translational applications modulating IGF axis signaling for pancreatic or immunological benefit in subjects at-risk for T1D.
KEY POINTS.
Insulin-like growth factors (IGFs) may be scarce in type 1 diabetes.
The pancreas modulates local IGF availability to regulate growth, β-cell identity and survival, and angiogenesis.
IGFs drive regulatory phenotypes in monocytes, T cells, and B cells.
Acknowledgements
The authors thank Amanda Posgai (University of Florida) for editorial assistance and critical review of the manuscript.
Financial Support and Sponsorship
All authors are supported by grants from the National Institutes of Health (F31 DK117548 to M.R.S.; T32 DK108736 to M.A.A.; P01 AI42288 to M.A.A. and T.M.B.; and R01 DK106191 to T.M.B.).
Footnotes
Conflicts of Interest
The authors report no potential conflicts of interest relevant to the contents of this manuscript.
REFERENCES AND ANNOTATIONS
- 1.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14(5):329–41. [DOI] [PubMed] [Google Scholar]
- 2.van Beijnum JR, Pieters W, Nowak-Sliwinska P, Griffioen AW. Insulin-like growth factor axis targeting in cancer and tumour angiogenesis - the missing link. Biol Rev Camb Philos Soc 2017;92(3):1755–68. [DOI] [PubMed] [Google Scholar]
- 3.Al-Salam S, Hameed R, Parvez H, Adeghate E. Pattern of distribution of IGF-1 and EGF in pancreatic islets of type 2 diabetic patients. Islets 2009;1(2):102–5. [DOI] [PubMed] [Google Scholar]
- 4▪▪. Spadaro O, Camel CD, Bosurgi L, Nguyen KY, Youm YH, Rothlin CV, et al. IGF1 Shapes Macrophage Activation in Response to Immunometabolic Challenge. Cell Rep 2017;19(2):225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]; Macrophages demonstrated as a non-liver-derived source of IGF1 and IGF1R signaling to reinforce anti-inflammatory M2 phenotype.
- 5.Dupont J, Holzenberger M. Biology of insulin-like growth factors in development. Birth Defects Res C Embryo Today. 2003;69(4):257–71. [DOI] [PubMed] [Google Scholar]
- 6▪. Chisalita SI, Ludvigsson J. Insulin-Like Growth Factor-1 at Diagnosis and during Subsequent Years in Adolescents with Type 1 Diabetes. J Diabetes Res 2018;2018:8623560. [DOI] [PMC free article] [PubMed] [Google Scholar]; Replicated earlier findings of low IGF1 in new onset T1D patients and positive feedback of insulin on IGF1.
- 7.Gutefeldt K, Hedman CA, Thyberg ISM, Bachrach-Lindström M, Spångeus A, Arnqvist HJ. Dysregulated growth hormone-insulin-like growth factor-1 axis in adult type 1 diabetes with long duration. Clin Endocrinol (Oxf) 2018. [DOI] [PubMed] [Google Scholar]
- 8.Derakhshanian H, Javanbakht MH, Zarei M, Djalali E, Djalali M. Vitamin D increases IGF-I and insulin levels in experimental diabetic rats. Growth Horm IGF Res. 2017;36:57–9. [DOI] [PubMed] [Google Scholar]
- 9.Nambam B, Schatz D. Growth hormone and insulin-like growth factor-I axis in type 1 diabetes. Growth Horm IGF Res. 2018;38:49–52. [DOI] [PubMed] [Google Scholar]
- 10.Holt RI, Simpson HL, Sönksen PH. The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med. 2003;20(1):3–15. [DOI] [PubMed] [Google Scholar]
- 11.Campbell-Thompson ML, Kaddis JS, Wasserfall C, Haller MJ, Pugliese A, Schatz DA, et al. The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59(1):217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AJ, Thrower SL, Sequeiros IM, Ward A, Bickerton AS, Triay JM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;97(11):E2109–13. [DOI] [PubMed] [Google Scholar]
- 13.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308(22):2337–9. [DOI] [PubMed] [Google Scholar]
- 14.Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984;26(6):456–61. [DOI] [PubMed] [Google Scholar]
- 15▪. Campbell-Thompson ML, Filipp SL, Grajo JR, Nambam B, Beegle R, Middlebrooks EH, et al. Relative Pancreas Volume Is Reduced in First-Degree Relatives of Patients With Type 1 Diabetes. Diabetes Care 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Finding of lowered pancreas size extended beyond T1D subjects to populations at-risk for T1D.
- 16.Sasamori H, Fukui T, Hayashi T, Yamamoto T, Ohara M, Yamamoto S, et al. Analysis of pancreatic volume in acute-onset, slowly-progressive and fulminant type 1 diabetes in a Japanese population. J Diabetes Investig. 2018;9(5):1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondrashova A, Nurminen N, Lehtonen J, Hyöty M, Toppari J, Ilonen J, et al. Exocrine pancreas function decreases during the progression of the beta-cell damaging process in young prediabetic children. Pediatr Diabetes. 2018;19(3):398–402. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Campbell-Thompson M, Wasserfall CH, McGrail K, Posgai A, Schultz AR, et al. Serum Trypsinogen Levels in Type 1 Diabetes. Diabetes Care. 2017;40(4):577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kido Y, Nakae J, Hribal ML, Xuan S, Efstratiadis A, Accili D. Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and beta-cell compensation to insulin resistance. J Biol Chem. 2002;277(39):36740–7. [DOI] [PubMed] [Google Scholar]
- 20▪. White V, Jawerbaum A, Mazzucco MB, Gauster M, Desoye G, Hiden U IGF2 stimulates fetal growth in a sex- and organ-dependent manner. Pediatr Res 2018;83(1–1):183–9. [DOI] [PubMed] [Google Scholar]; Growth of the fetal pancreas influenced by intrafetal IGF2 levels.
- 21.Oshima M, Knoch KP, Diedisheim M, Petzold A, Cattan P, Bugliani M, et al. Virus-like infection induces human β cell dedifferentiation. JCI Insight 2018;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter CS, Stein RW. Evidence for Loss in Identity, De-Differentiation, and. Front Genet 2017;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪. Yi SA, Lee J, Park JW, Han J, Lee MG, Nam KH, et al. S6K1 controls epigenetic plasticity for the expression of pancreatic α/β cell marker genes. J Cell Biochem 2018;119(8):6674–83. [DOI] [PubMed] [Google Scholar]; Signaling downstream of IGF1R enforces beta cell identity.
- 24.Wong JC, Vo V, Gorjala P, Fiscus RR. Pancreatic-β-cell survival and proliferation are promoted by protein kinase G type Iα and downstream regulation of AKT/FOXO1. Diab Vasc Dis Res 2017;14(5):434–49. [DOI] [PubMed] [Google Scholar]
- 25.You W, Wang K, Yu C, Song L. Baicalin prevents tumor necrosis factor-α-induced apoptosis and dysfunction of pancreatic β-cell line Min6 via upregulation of miR-205. J Cell Biochem 2018;119(10):8547–54. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Yang B, Xu Z, Boivin E, Black M, Huang W, et al. Protective effect of cyanidin-3-O-glucoside on neonatal porcine islets. J Endocrinol 2017;235(3):237–49. [DOI] [PubMed] [Google Scholar]
- 27▪. Xu J, Jia YF, Tapadar S, Weaver JD, Raji IO, Pithadia DJ, et al. Inhibition of TBK1/IKKε Promotes Regeneration of Pancreatic β-cells. Sci Rep 2018;8(1):15587. [DOI] [PMC free article] [PubMed] [Google Scholar]; Signaling downstream of IGF1R promotes beta cell proliferation.
- 28▪. Bonnet-Serrano F, Diedisheim M, Mallone R, Larger E Decreased α-cell mass and early structural alterations of the exocrine pancreas in patients with type 1 diabetes: An analysis based on the nPOD repository. PLoS One 2018;13(1):e0191528. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated decreased alpha cell mass in human cadaver pancreata.
- 29▪. Brissova M, Haliyur R, Saunders D, Shrestha S, Dai C, Blodgett DM, et al. α Cell Function and Gene Expression Are Compromised in Type 1 Diabetes. Cell Rep 2018;22(10):2667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]; Remaining alpha cells dysfunctional with decreased glucagon secretion in human subjects with T1D.
- 30.Bereket A, Lang CH, Blethen SL, Gelato MC, Fan J, Frost RA, et al. Effect of insulin on the insulin-like growth factor system in children with new-onset insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1995;80(4):1312–7. [DOI] [PubMed] [Google Scholar]
- 31▪. Sarem Z, Bumke-Vogt C, Mahmoud AM, Assefa B, Weickert MO, Adamidou A, et al. Glucagon Decreases IGF-1 Bioactivity in Humans, Independently of Insulin, by Modulating Its Binding Proteins. J Clin Endocrinol Metab 2017;102(9):3480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]; New mechanism for glucagon regulating IGF bioavailability independently of insulin.
- 32.Duvillié B Vascularization of the pancreas: an evolving role from embryogenesis to adulthood. Diabetes. 2013;62(12):4004–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪. White A, Louey S, Chang EI, Boehmer BH, Goldstrohm D, Jonker SS, et al. A 1 week IGF-1 infusion decreases arterial insulin concentrations but increases pancreatic insulin content and islet vascularity in fetal sheep. Physiol Rep 2018;6(17):e13840. [DOI] [PMC free article] [PubMed] [Google Scholar]; Development of islet vascularity in early life depends upon local IGF1.
- 34▪. Talior-Volodarsky I, Mahou R, Zhang D, Sefton M. The role of insulin growth factor-1 on the vascular regenerative effect of MAA coated disks and macrophage-endothelial cell crosstalk. Biomaterials 2017;144:199–210. [DOI] [PubMed] [Google Scholar]; Macrophage-derived IGF1 shown to drive vascularization.
- 35.Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62(2):199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪. Wang J, Xie L, Wang S, Lin J, Liang J, Xu J Azithromycin promotes alternatively activated macrophage phenotype in systematic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death Dis 2018;9(11):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that human macrophages are skewed toward M2 and away from M1 phenotype by IGF1.
- 37▪. Yoon IS, Park H, Kwak HW, Woo Jung Y, Nam JH. Macrophage-derived insulin-like growth factor-1 affects influenza vaccine efficacy through the regulation of immune cell homeostasis. Vaccine 2017;35(36):4687–94. [DOI] [PubMed] [Google Scholar]; Extended the notion of autocrine IGF1 signaling skewing macrophages toward anti-inflammatory profile to this influencing T cell skewing.
- 38.Felton JL, Maseda D, Bonami RH, Hulbert C, Thomas JW. Anti-Insulin B Cells Are Poised for Antigen Presentation in Type 1 Diabetes. J Immunol. 2018;201(3):861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪▪. Mallol C, Casana E, Jimenez V, Casellas A, Haurigot V, Jambrina C, et al. AAV-mediated pancreatic overexpression of. Mol Metab. 2017;6(7):664–80. [DOI] [PMC free article] [PubMed] [Google Scholar]; Overexpression of Igf1 in NOD islets showed wide-ranging immunoregulatory effects both in the innate and adaptive compartments.
- 40.Phalke S, Marrack P. Age (autoimmunity) associated B cells (ABCs) and their relatives. Curr Opin Immunol. 2018;55:75–80. [DOI] [PubMed] [Google Scholar]
- 41▪. Koohy H, Bolland DJ, Matheson LS, Schoenfelder S, Stellato C, Dimond A, et al. Genome organization and chromatin analysis identify transcriptional downregulation of insulin-like growth factor signaling as a hallmark of aging in developing B cells. Genome Biol. 2018;19(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]; Determined an epigenetic mechanism of how IGF signaling preferentially supports immature B cell phenotype.
- 42▪▪. Mirdamadi Y, Bommhardt U, Goihl A, Guttek K, Zouboulis CC, Quist S, et al. Insulin and Insulin-like growth factor-1 can activate the phosphoinositide-3-kinase/Akt/FoxO1 pathway in T cells. Dermatoendocrinol. 2017;9(1):e1356518. [DOI] [PMC free article] [PubMed] [Google Scholar]; First paper showing that IGF1 induces PI3K signaling in CD3-activated primary human T cells.
- 43▪▪. Mendes-da-Cruz DA, Lemos JP, Passos GA, Savino W Abnormal T-Cell Development in the Thymus of Non-obese Diabetic Mice: Possible Relationship With the Pathogenesis of Type 1 Autoimmune Diabetes. Front Endocrinol (Lausanne) 2018;9:381. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review covers the expression of IGFs in the thymus and their roles in thymocyte development in the NOD mouse.
- 44.Gao T, Bogdanova N, Ghauri S, Zhang G, Lin J, Sheikh K. Systemic IGF-1 gene delivery by rAAV9 improves spontaneous autoimmune peripheral polyneuropathy (SAPP). Sci Rep 2018;8(1):5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪. Erlandsson MC, Töyrä Silfverswärd S, Nadali M, Turkkila M, Svensson MND, Jonsson IM, et al. IGF-1R signalling contributes to IL-6 production and T cell dependent inflammation in rheumatoid arthritis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(9):2158–70. [DOI] [PubMed] [Google Scholar]; T cell skewing toward Th17 was shown to be induced by IGF1R signaling due to STAT3 phosphorylation.
- 46▪. Miyagawa I, Nakayamada S, Nakano K, Yamagata K, Sakata K, Yamaoka K, et al. Induction of Regulatory T Cells and Its Regulation with Insulin-like Growth Factor/Insulin-like Growth Factor Binding Protein-4 by Human Mesenchymal Stem Cells. J Immunol 2017;199(5):1616–25. [DOI] [PubMed] [Google Scholar]; Initial demonstration of IGF-mediated skewing toward Treg and blockade of this effect by IGFBPs.
- 47▪. Kimura K, Hohjoh H, Fukuoka M, Sato W, Oki S, Tomi C, et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun 2018;9(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mechanistic insight into regulation of IGF1R expression in autoimmunity with consequences for Treg induction.
- 48.Bluestone JA, Bour-Jordan H, Cheng M, Anderson M. T cells in the control of organ-specific autoimmunity. J Clin Invest. 2015;125(6):2250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson MB, Patel KA, De Franco E, Houghton JAL, McDonald TJ, Ellard S, et al. A type 1 diabetes genetic risk score can discriminate monogenic autoimmunity with diabetes from early-onset clustering of polygenic autoimmunity with diabetes. Diabetologia. 2018;61(4):862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solt LA, Burris TP. Th17 cells in Type 1 diabetes: a future perspective. Diabetes Manag (Lond) 2015;5(4):247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


