Abstract
Background:
High blood pressure (BP) is a known risk factor for mobility and cognitive impairment in older adults. This study tested the association of cumulative BP exposure from young adulthood to midlife with gait and cognitive function in midlife. Furthermore, we tested whether these association were modified by cerebral white matter hyperintensity (WMH) burden.
Methods:
We included 191 participants from the Coronary Artery Risk Development in Young Adults study, a community-based cohort of young individuals followed over 30 years. Cumulative BP was calculated as the area under the curve (mmHg×years) from baseline up to year 30 examination (Y30). Gait and cognition were assessed at Y30 examination. Cerebral WMH was available at Y30 in a subset of participants (n=144) who underwent MRI. Multiple linear regression models were used to assess the association of cumulative BP exposure with gait and cognition. To test effect modification by WMH burden, participants were stratified at the median of WMH and tested for interaction.
Results:
Higher cumulative systolic and diastolic BP were associated with slower walking speed (both p=0.010), smaller step length (p=0.011 and 0.005, respectively) and higher gait variability (p=0.018 and 0.001, respectively). Higher cumulative systolic BP was associated with lower cognitive performance in the executive (p=0.021), memory (p=0.015) and global domains (p=0.010); and higher cumulative diastolic BP was associated with lower cognitive performance in the memory domain (p=0.012). All associations were independent of socio-demographics and vascular risk factors (body mass index, smoking, diabetes and total cholesterol). The association between cumulative BP and gait was moderated by WMH burden (interaction p<0.05). However, the relation between cumulative BP and cognitive function was not different based on WMH burden (interaction p>0.05).
Conclusions:
Exposure to higher BP levels from young to midlife is associated with worse gait and cognitive performance in midlife. Furthermore, WMH moderates the association of cumulative BP exposure with gait, but not with cognitive function in midlife. The mechanisms underpinning the impact of BP exposure on brain structure and function must be investigated in longitudinal studies using a life course approach.
Keywords: blood pressure, middle-aged, young, cognition, gait, white matter disease
INTRODUCTION
The detrimental impact of hypertension on human health is rooted in the multi-organ injury that silently erupts long before any intervention takes place1. The negative impact of hypertension on the brain structure and function is particularly devastating2. Observational studies have established a strong link between elevated blood pressure (BP) and cognitive impairment3 and increased risk of dementia4 in late life. High BP has been also linked to functional disability5, 6, slow gait speed7, and falls8 in older adults. Several studies have shown that gait slowing precedes cognitive decline, suggesting that gait changes may be an earlier marker of brain dysfunction than cognitive impairment9, 10. The cumulative impact of hypertension on brain structure and function is supported by the strong association between midlife measures of BP and late-life cognitive impairment11. Therefore, a life-course approach may be necessary to prevent the harmful consequences of high BP on cerebrovascular structure and function11, 12. However, the association between high BP exposure in young adulthood with cognitive function and mobility in midlife is unknown.
Radiographic markers of cerebral small vessel disease that manifest on brain magnetic resonance imaging (MRI) scans have been well established as measures of hypertensive brain injury13. Subcortical white matter hyperintensities (WMH), as one such radiographic measure, are strongly associated with hypertension as well as mobility and cognitive impairment in late-life14. Elevated BP in late life is associated with a greater burden and faster progression of WMH11. Furthermore, progression of WMH burden has been shown to manifest as greater decline in mobility and cognitive function among older adults14. These observations suggest that hypertension may contribute to cognitive and mobility impairment by promoting the development and progression of subcortical white matter injury. However, how WMH affect the relation of BP with mobility and cognitive function among middle-age individuals remains unclear.
Building on this background, in this study we examined the longitudinal link between cumulative exposure to BP from young adulthood to midlife with gait and cognitive function in midlife. In addition, we tested whether cerebral WMH burden moderates the link between cumulative BP and gait or cognition. Data were collected in a subset of participants from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort, in which 30 years of longitudinal data on vascular health and other measures is available. We hypothesized that exposure to higher BP from young to middle adulthood would be associated with worse gait and cognitive function in midlife, and that these associations would be stronger in those with higher WMH burden.
MATERIALS AND METHODS
Participants
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (https://www.cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).
The CARDIA study began in 1985–1986 and recruited 5115 black and white adults aged 18 to 30 years from 4 U.S. field centers (Birmingham, AL; Chicago, IL; Minneapolis, MN and; Oakland, CA)15. These participants have been followed through serial in-person examinations after 2 (Y2), 5 (Y5), 7 (Y7), 10 (Y10), 15 (Y15), 20 (Y20), 25 (Y25) and 30 (Y30) years. Contact is maintained with participants via telephone, mail, or email every 6 months, with annual interim medical history ascertainment. Over the last 5 years, >90% of the surviving cohort members have been directly contacted, and follow up for vital status is virtually complete through related contacts and intermittent National Death Index searches. At Y30 examination, all participants from the Chicago field center were invited to participate in the Cerebral Small Vessels in Motor and Cognitive Decline ancillary study. A total of 202 participants were enrolled and underwent detailed assessment including brain magnetic resonance imaging (MRI), and gait and cognitive assessments. Separate written consent was obtained and the study was approved by the institutional review board of Northwestern University. Of a total of 202 participants recruited, 191 had complete gait and cognitive function assessment and 144 had available brain MRI data for analyses. All MRI images were reviewed with an expert neuroradiologist (A.J.N.) and participants with signs of brain infarcts were excluded (n=2). Compared to the rest of Chicago field centers’ participants attended Y30 examination (n=554), those included in this study were younger and more frequently male (Supplemental Table 1).
Blood Pressure and Other Vascular Risk Factors
Standardized protocols were used to measure systolic BP (SBP) and diastolic BP (DBP) at each CARDIA visit. The protocol and procedure have been described in detail previously16. Briefly, BP was measured in the brachial artery 3 times after the participant had been sitting in a quiet room for 5 minutes. The BP measures were taken at 1-minute intervals and the average of the second and third measurements was used for analysis. From Y0 to Y15, BP was measured using a Hawksley random-zero sphygmomanometer (Hawksley, Sussex, United Kingdom). From Y20 onwards, BP was measured with an automated oscillometric BP monitor (Omron HEM-907XL; Online Fitness, Santa Monica, CA) due to concerns about mercury contained in the sphygmomanometer apparatus. These BP measurements were calibrated and standardized to the sphygmomanometer measures to remove any potential machine bias17. Cumulative exposure to SBP and DBP were calculated for each participant from Y0 to Y30 as the sum of (average of BP measurements between consecutive visits × years between the visits) to determine exposure to BP from young adulthood to middle age18.
Other measures including height, weight, glucose levels, lipid levels, and education were collected using standardized protocols at each CARDIA visit16. Body mass index was calculated as weight (Kg) divided by height in meters squared. Total cholesterol was determined on a chemistry analyzer using comparable enzymatic procedures (Hitachi 912; Roche Diagnostics). Diabetes was defined as fasting plasma glucose ≥ 126 mg/dL or self-reported use of diabetes medication. Smoking status and antihypertensive medication use were self-reported. Physical activity was measured using CARDIA physical activity questionnaire19 and depression was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D).
Gait and Cognitive Function Assessment
Gait measures were collected during a 40-foot walk on a 20 × 2 foot Zeno™ mat (Protokinetics Zeno Walkway). The walking trial consisted of two passes over the Zeno Walkway mat. The turns were completed off the mat and were not included in the data analysis. Participants started to walk four feet before the mat and were asked to walk at their normal pace. Using the Protokinetics Movement Analysis software, several gait parameters including step velocity, step length, step time, stride width, gait variability index (GVI) and step length asymmetry were computed. It has been shown that these parameters represent a broad range of gait characteristics that assess different gait domains20. Step velocity (cm/sec) was measured by dividing distance by time. Step length (cm) was measured as the distance between corresponding successive heel points of opposite feet. Stride width (cm) was measured as the perpendicular distance between two successive heel strikes of the same foot and the contralateral heel contact between those events. Step time (sec) was measured as the time elapsed to complete one step (from the first contact of one foot to the first contact of the following other foot). GVI describes the variability during gait and was calculated based on the variability of nine spatiotemporal gait parameters (step length, stride length, step time, stride time, swing time, stance time, single support time, double support time, and velocity) as defined by Gouelle et al.21. GVI quantifies the difference between the amount of variability observed for a reference group (mean score and standard deviation [SD] of 100 ± 10) and the amount of variability observed for an individual (higher GVI represents worse gait)21. Step length asymmetry is a measure of gait symmetry with 0 representing perfect right/left symmetry. It was calculated using the following formula:
All gait variables were averaged over the whole walking trial for the right and left feet.
Cognition was assessed at Y30 examination using an extensive set of neuropsychological tests across different cognitive domains. These tests included Hopkins Verbal Learning Test-Revised (HVLT-R)22, Digit Span test (DS)23, Trail Making Test A and B24, Digit Symbol Substitution Test (DSST)25, Stroop test25, and the National Institute of Health (NIH)26 -Flanker Inhibitory test, -List Sorting Working Memory test (NIH-LSWM) and -Picture Sequence Memory test (NIH-PSM). To enhance the interpretation of data, we constructed 4 composite cognitive domains of executive, memory, attention, and global cognitive function. The executive function domain consisted of the average z-score of NIH-Flanker Inhibitory test, NIH-LSWM test, Trails Making test B, DSST test and Stroop test. The memory domain consisted of the average z-score of NIH-PSM test and HVLT-R tests (total and delayed recall). Attention domain included the z-score of the DS test and Trails Making Test A, and the global domain included the average z-score of all the cognitive tests27. The z-scores of Stroop and Trails Making tests were reversed to maintain consistency with the other cognitive tests.
White Matter Hyperintensity Measurement
A 3T Siemens Prisma MRI machine was used for brain MRI acquisition at the Center for Translational Imaging at Northwestern University. WMH volumes were derived from T2 sagittal fluid-attenuated inversion recovery (FLAIR) sequence. The FLAIR images were denoised using an adapative non-local means filter28 and intensity bias corrected using the N4 algorithm29. Cleaned images were input to the Lesion Prediction Algorithm30 of the Lesion Segmentation Toolbox (LST 2.0.15) for statistical parametric mapping (SPM). Resultant voxelwise lesion probability maps were thresholded at greater than zero percent probability to form masks. The automatically generated masks were manually corrected by a trained operator (S.K.) using Freeview in Freesurfer. This was completed for each participant, and each mask was reviewed a second time for quality assurance. Tools in AFNI (Analysis of Functional NeuroImages) were consequently used to generate volumetric data, i.e. intracranial volumes (ICV) and WMH volumes. All WMH volumes were normalized by their ICV to correct for individual head sizes. Freesurfer and AFNI are both widely used neuroimaging software packages which contain a number of tools for functional and structural MRI analyses. Freeview is a tool within Freesurfer which was used for performing the manual edits, as it allows users to simultaneously view all 3 image axes (axial/saggital/coronal), and easily flip between the two image contrasts (T1/FLAIR) while editing the WMH masks. It also allows for thresholding the drawing tool to only label voxels within certain intensity ranges, which considerably reduces the time needed to perform the manual segmentations. AFNI is a large command-line based set of tools, one of which is 3dBrickStat which can be used for extracting ROI averages of volumetric data31.
Statistical Analysis
Characteristics of participants are presented as mean ± SD, proportion, and median with interquartile ranges where applicable. We used multivariable linear regression models to assess the association between cumulative BP exposure during 30 years of follow-up and gait or cognitive function at Y30 examination (all as continuous variables). The primary outcome variables were gait measurements or the composite cognitive domains. The primary exposures were measures of cumulative SBP and cumulative DBP (z-scores). The adherence to the assumptions of multiple linear regression were examined by visual inspection of the distribution of residuals through histograms and normal probability plots. Furthermore, homoscedasticity was visually inspected by plotting the standardized residuals against standardized predicted values. All analyses were performed in two steps: In the first step (model 1), analyses were adjusted for age, sex, race, height (where gait was the primary outcome) or education (where cognitive function was the primary outcome). In the next step (model 2), analyses were additionally adjusted for vascular risk factors (body mass index, smoking status, prevalence of diabetes, and total cholesterol level), depression (where cognitive function was the primary outcome) and physical activity (where gait was the primary outcome) at Y30 examination. These covariates were selected as potential confounders based on their biological plausibility since they are known to be associated with BP, cognitive function, or gait12, 32. In a separate analyses, we assessed the association of cumulative BP with gait or cognition after adjustment for cumulative exposure to other vascular risk factors including body mass index (kg/m2 × years), total cholesterol (mg/dL × years), fasting glucose (mg/dL × years) and life-time pack years of cigarette smoking (cigarette packs smoked per day × years smoking). We also explored the relationship between tertile of cumulative BP with gait and cognitive function using analyses of covariance. To examine the associations of cumulative BP over 30 years compared to a single BP measurement, all analyses in model 1 were repeated using baseline (Y0) or Y30 BP levels as the independent variable. To test whether WMH moderate the association of cumulative BP with gait or cognition, we repeated all the analyses in model 2 after stratifying participants based on the median of WMH (n=72 per group) and tested for interaction (WMH load groups × cumulative exposure to BP)33. Similarly, effect modification by antihypertensive medication use was tested using interaction models. All statistical analyses were conducted with IBM SPSS Statistics version 25.0 and a p value <0.05 was considered as statistically significant.
RESULTS
Table 1 summarizes the demographic and clinical characteristics of the participants included in this study (total cohort, n=191) and those who had available brain MRI data (MRI sub-cohort, n=144), with no major differences between the groups. Overall, this was not a hypertensive cohort, with fewer than 30% on BP medications. The mean cumulative exposure to SBP and DBP in the total cohort was 3333 ± 276 mmHg×years (range 2688, 4108 mmHg×years) and 2105 ± 227 mmHg×years (range 1563, 2758 mmHG×years), respectively. Table 2 shows the baseline characteristics of participants who became hypertensive (n=70) vs. those who remained normotensive (n=121) during follow-up. Hypertensive participants were younger, more frequently black, had lower education, and had higher body mass index and SBP at baseline (all p<0.05).
Table 1.
Characteristics of participants
| Characteristics | Total cohort (n=191) | MRI sub-cohort (n=144) |
|---|---|---|
| Cumulative SBP, mmHg×years | 3333 ± 276 | 3291 ± 261 |
| Cumulative DBP, mmHg×years | 2105 ± 227 | 2067 ± 209 |
| SBP at Y0, mmHg | 108 ± 10 | 107 ± 10 |
| DBP at Y0, mmHg | 66 ± 10 | 65 ± 10 |
| Demographics at Y30 | ||
| Age, y | 56 ± 4 | 56 ± 4 |
| Female, % | 46 | 42 |
| Blacks, % | 44 | 39 |
| Education, y | 15 ± 3 | 15 ± 3 |
| Height, cm | 171 ± 10 | 172 ± 9 |
| Risk Factors at Y30 | ||
| Body mass index, kg/m2 | 30 ± 6 | 28 ± 5 |
| Smoking: | ||
| Former, % | 23 | 22 |
| Current, % | 14 | 13 |
| Total cholesterol, mg/dL | 192 ± 38 | 196 ± 38 |
| Prevalence of diabetes, % | 15 | 13 |
| SBP, mmHg | 120 ± 15 | 118 ± 15 |
| DBP, mmHg | 74 ± 11 | 72 ± 10 |
| Physical activity (exercise units), median (IQR) | 270 (128, 475) | 283 (133, 514) |
| Depression (CESD score), median (IQR) | 7 (3–12) | 7 (3–11) |
| Cognition at Y30 | ||
| HVLT-R, total recall | 26 ± 5 | 27 ± 5 |
| HVTL-R, delayed, median (IQR) | 10 (8, 11) | 10 (8, 11) |
| Digit Span, digits | 18 ± 4 | 18 ± 4 |
| Digit Span Substitution, symbols | 68 ± 16 | 69 ± 17 |
| Stroop, sec, median (IQR) | 20 (15, 28) | 19 (14, 28) |
| Trails A, sec, median (IQR) | 28 (24, 36) | 28 (24, 36) |
| Trails B, sec, median (IQR) | 69 (53, 95) | 65 (52, 91) |
| NIH-LSWM, number correct | 53 ± 10 | 54 ± 10 |
| NIH-PSM, number correct | 53 ± 12 | 53 ± 12 |
| NIH-Flanker, number correct | 43 ± 9 | 44 ± 9 |
| Gait at Y30 | ||
| Step velocity, cm/sec | 122 ± 21 | 125 ± 19 |
| Step length, cm | 68 ± 8 | 70 ± 7 |
| Stride width, cm | 8 ± 4 | 8 ± 3 |
| Step time, sec | 0.57 ± 0.06 | 0.56 ± 0.05 |
| Gait variability index | 107 ± 9 | 106 ± 8 |
| Step length asymmetry, cm | 0.10 ± 4.62 | 0.29 ± 4.39 |
Data are expressed as mean ± SD or %, unless otherwise stated. Abbreviations: Y30: year 30 of follow-up visit; IQR: interquartile range; SBP: systolic blood pressure; DBP: diastolic blood pressure; CESD=center for epidemiologic studies depression scale; HVLT-R: Hopkin’s verbal learning test revised, NIH-LSWM and -PSM: national institute of health-list sorting working memory test and -picture sequence memory test.
Table 2.
Baseline characteristics of participants that remained normotensive vs. those that became hypertensive during follow-up
| Characteristics | Hypertensive (n=70) | Normotensive (n=121) | p-value |
|---|---|---|---|
| Cumulative SBP, mmHg×years | 3523 ± 244 | 3223 ± 231 | <0.001 |
| Cumulative DBP, mmHg×years | 2250 ± 223 | 2022 ± 185 | <0.001 |
| Demographics at Y0 | |||
| Age, y | 25 ± 4 | 24 ± 4 | 0.631 |
| Female, % | 44 | 46 | 0.790 |
| Blacks, % | 66 | 31 | <0.001 |
| Education, y | 14 ± 3 | 15 ± 2 | 0.021 |
| Height, cm | 170 ±10 | 173 ± 9 | 0.046 |
| Risk Factors at Y0 | |||
| Body mass index, kg/m2 | 26 ± 5 | 24 ± 4 | 0.002 |
| Smoking: | |||
| Former, % | 10 | 12 | |
| Current, % | 24 | 31 | 0.433 |
| Total cholesterol, mg/dL | 183 ± 38 | 175 ± 30 | 0.099 |
| Prevalence of diabetes, % | 0 | 0 | - |
| SBP, mmHg | 111 ± 9 | 106 ± 10 | 0.002 |
| DBP, mmHg | 68 ± 10 | 65 ± 10 | 0.056 |
| Physical activity (exercise units), median (IQR) | 431 (195–668) | 468 (304–802) | 0.060 |
Normotensive was defined as BP <140/90 mmHg during follow-up. Hypertensive was defined as SBP≥140 mmHg or DBP≥90 mmHg or antihypertensive medication use during follow-up. Data are expressed as mean ± SD or %, unless otherwise stated. P-values were calculated using t-test or Mann-Whitney U test for continuous variables, and chi-squared test for categorical variables. Abbreviations: Y0: baseline visit; IQR: interquartile range; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Results from the linear regression models to examine the association between cumulative BP exposure and gait are summarized in Table 3. In model 1, higher exposure to cumulative SBP was associated with slower walking speed, smaller step length, longer step time, and higher GVI. Further adjustments in model 2 did not essentially change these associations, except that the relation between cumulative SBP and step time was somewhat attenuated (β [SE]: 0.009 [0.005], p=0.063). Similarly, higher cumulative DBP was associated with worse gait characteristics in all models, except that the association between cumulative DBP and step time was marginal in model 2 (β [SE]: 0.008 [0.004], p=0.065). Cumulative exposure to SBP or DBP were not associated with stride width and step length asymmetry in any of the models. After adjustment for cumulative exposure to other vascular risk factors instead of risk factors measured at Y30 examination, higher exposure to cumulative BP remained associated with worse gait performance (Supplemental Table 2). Figure 1 shows the adjusted mean (SE) of gait variables in tertile of cumulative SBP and DBP.
Table 3.
Cumulative systolic and diastolic blood pressure in relation to gait in midlife
| Gait | Cumulative SBP per 1 SD higher β (95% CI) | p-value | Cumulative DBP per 1 SD higher β (95% CI) | p-value |
|---|---|---|---|---|
| Step velocity, cm/sec | ||||
| Model 1 | −5.98 (−9.03, −2.93) | <0.001 | −5.71 (−8.55, −2.88) | <0.001 |
| Model 2 | −4.24 (−7.45, −1.02) | 0.010 | −3.93 (−6.92, −0.94) | 0.010 |
| Step length, cm | ||||
| Model 1 | −2.20 (−3.25, −1.15) | <0.001 | −2.21 (−3.19, −1.24) | <0.001 |
| Model 2 | −1.42 (−2.50, −0.34) | 0.011 | −1.44 (−2.45, −0.44) | 0.005 |
| Step time, sec | ||||
| Model 1 | 0.010 (0.002, 0.02) | 0.014 | 0.010 (0.002, 0.02) | 0.012 |
| Model 2 | 0.009 (−0.0005, 0.018) | 0.063 | 0.008 (−0.0005, 0.016) | 0.065 |
| Stride width, cm | ||||
| Model 1 | 0.16 (−0.44, 0.76) | 0.602 | 0.08 (−0.48, 0.64) | 0.781 |
| Model 2 | −0.31 (−0.92, 0.29) | 0.310 | −0.47 (−1.03, 0.10) | 0.103 |
| Gait variability index | ||||
| Model 1 | 2.07 (0.63, 3.51) | 0.005 | 2.49 (1.17, 3.81) | <0.001 |
| Model 2 | 1.90 (0.33, 3.48) | 0.018 | 2.47 (1.03, 3.91) | 0.001 |
| Step length asymmetry, cm | ||||
| Model 1 | −0.49 (−1.25, 0.28) | 0.215 | −0.33 (−1.05, 0.39) | 0.372 |
| Model 2 | −0.58 (−1.41, 0.25) | 0.171 | −0.46 (−1.23, 0.32) | 0.246 |
β represents unstandardized regression coefficients.
Model 1: Adjusted for age, sex, race and height.
Model 2: Adjusted for age, sex, race, height, body mass index, smoking status, prevalence of diabetes, total cholesterol and physical activity measured at Y30 examination.
Abbreviations: SBP: systolic blood pressure and DBP: diastolic blood pressure; SD: standard deviation.
Figure 1. Gait at Y30 examination in tertiles of cumulative blood pressure over 30 years.
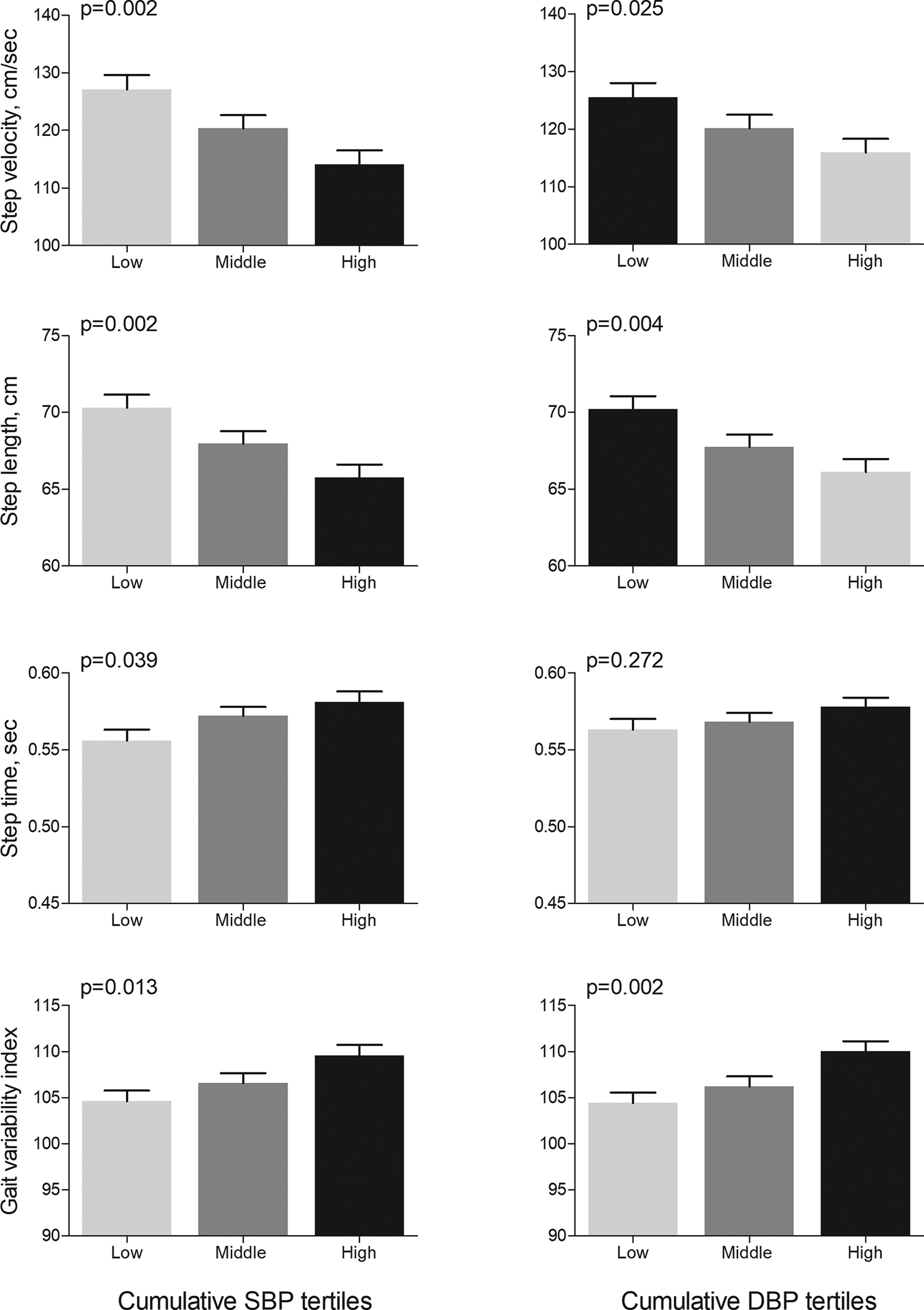
Bars represent means (standard error) calculated from ANCOVA after adjustment for age, sex, race and height. P values show p for trends from ANCOVA. Abbreviations: SBP: systolic blood pressure and DBP: diastolic blood pressure.
Table 4 shows the results for linear regression models examining the association between cumulative BP exposure and cognitive function across 4 domains. In model 1, higher exposure to cumulative SBP was associated with worse cognitive performance in the executive, memory, and global cognitive domains. Full adjustment in model 2 essentially did not change these associations. In contrast, higher exposure to cumulative DBP was only associated with worse performance in the memory domain. Adjustment for cumulative exposure to other vascular risk factors instead of risk factors measured at Y30 examination did not essentially change the association of cumulative BP with cognitive function (Supplemental Table 2). Figure 2 shows the adjusted mean (SE) of each cognitive domain in tertiles of cumulative SBP and DBP.
Table 4.
Cumulative systolic and diastolic blood pressure in relation to cognition in midlife
| Cognition | Cumulative SBP per 1 SD higher β (95% CI) | p-value | Cumulative DBP per 1 SD higher β (95% CI) | p-value |
|---|---|---|---|---|
| Memory domain | ||||
| Model 1 | −0.14 (−0.26, −0.02) | 0.022 | −0.13 (−0.24, −0.02) | 0.022 |
| Model 2 | −0.16 (−0.30, −0.03) | 0.015 | −0.16 (−0.28, −0.04) | 0.012 |
| Executive domain | ||||
| Model 1 | −0.12 (−0.21, −0.02) | 0.014 | −0.06 (−0.14, 0.03) | 0.213 |
| Model 2 | −0.12 (−0.22, −0.02) | 0.021 | −0.05 (−0.15, 0.04) | 0.263 |
| Attention domain | ||||
| Model 1 | −0.04 (−0.15, 0.07) | 0.451 | −0.02 (−0.12, 0.09) | 0.773 |
| Model 2 | −0.04 (−0.16, 0.08) | 0.548 | −0.03 (−0.14, 0.09) | 0.625 |
| Global domain | ||||
| Model 1 | −0.12 (−0.20, −0.03) | 0.007 | −0.08 (−0.16, 0.003) | 0.059 |
| Model 2 | −0.12 (−0.21, −0.03) | 0.010 | −0.08 (−0.17, 0.002) | 0.057 |
β represents unstandardized regression coefficients.
Mean ± SD of executive, memory, attention and global domains were −0.01±0.7, −0.01±0.8, 0.02 ±0.8 and −0.02±0.6, respectively.
Model 1: Adjusted for age, sex, race and education.
Model 2: Adjusted for age, sex, race, education, body mass index, smoking status, prevalence of diabetes, total cholesterol and depression measured at Y30 examination.
Abbreviations: SBP: systolic blood pressure and DBP: diastolic blood pressure; SD: standard deviation.
Figure 2. Cognition at Y30 examination in tertiles of cumulative blood pressure over 30 years.
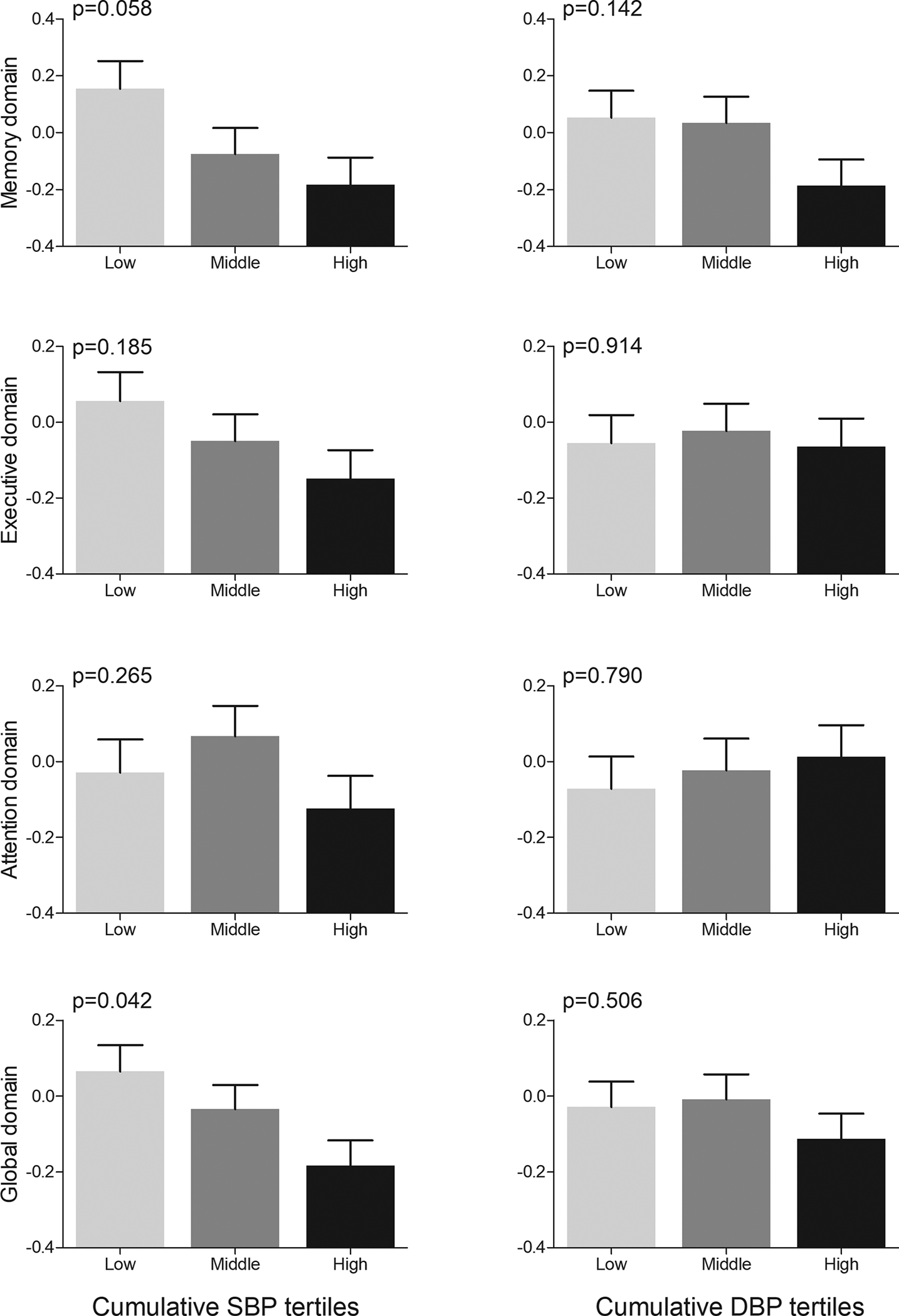
Bars represent means (standard error) calculated from ANCOVA after adjustment for age, sex, race and education. P values show p for trends from ANCOVA. Abbreviations: SBP: systolic blood pressure and DBP: diastolic blood pressure.
At baseline (Y0), the mean age of the participants was 24 ± 4 years, mean SBP was 108 ± 10 mmHg, and mean DBP was 66 ± 10 mmHg. Table 5 shows the associations of cumulative BP over 30 years compared to a single BP measurement (at baseline or Y30) with gait or cognition at Y30. Higher baseline (Y0) SBP and DBP values were only associated with slower walking speed and smaller step length. Higher baseline SBP was not associated with any of the cognitive domains; and higher baseline DBP was only associated with worse memory performance. SBP measured at Y30 was not associated with gait or cognitive function; and DBP measured at Y30 was only associated with shorter step length. All effect estimates for baseline or Y30 BP values were smaller than cumulative BP over 30 years (unstandardized β in Table 5).
Table 5.
Relationships of cumulative exposure to blood pressure over 30 years or Y0 (baseline) or Y30 blood pressure with gait and cognitive function
| SBP | DBP | |||||
|---|---|---|---|---|---|---|
| Gait | Cumulative SBP per 1 SD higher β (SE) | Y30 SBP per 1 SD higher β (SE) | Y0 SBP per 1 SD higher β (SE) | Cumulative DBP per 1 SD higher β (SE) | Y30 DBP per 1 SD higher β (SE) | Y0 DBP per 1 SD higher β (SE) |
| Step velocity | −5.98 (1.55)* | −1.29 (1.50) | −3.69 (1.46)§ | −5.71 (1.44)* | −2.73 (1.46) | −3.79 (1.42)‡ |
| Step length | −2.20 (0.53)* | −0.83 (0.52) | −1.24 (0.51)§ | −2.21 (0.49)* | −1.25 (0.51)§ | −1.58 (0.49)‡ |
| Step time | 0.01 (0.004)§ | −0.0001 (0.004) | 0.007 (0.004) | 0.01 (0.004)§ | 0.004 (0.004) | 0.006 (0.004) |
| Step width | 0.16 (0.30) | 0.23 (0.28) | 0.25 (0.28) | 0.08 (0.28) | 0.19 (0.28) | −0.02 (0.27) |
| GVI | 2.07 (0.73)‡ | 0.21 (0.70) | 0.63 (0.69) | 2.49 (0.67)* | 1.13 (0.68) | 0.85 (0.67) |
| Step asymmetry | −0.49 (0.39) | 0.02 (0.37) | −0.40 (0.36) | −0.33 (0.36) | 0.08 (0.36) | −0.21 (0.35) |
| SBP | DBP | |||||
| Cognition | Cumulative SBP Per 1 SD higher β (SE) | Y30 SBP per 1 SD higher β (SE) | Y0 SBP per 1 SD higher β (SE) | Cumulative DBP Per 1 SD higher β (SE) | Y30 DBP per 1 SD higher β (SE) | Y0 DBP per 1 SD higher β (SE) |
| Memory domain | −0.14 (0.06)§ | −0.07 (0.06) | −0.07 (0.06) | −0.13 (0.06)§ | −0.07 (0.06) | −0.14 (0.06)§ |
| Executive domain | −0.12 (0.05)§ | −0.05 (0.05) | −0.07 (0.04) | −0.06 (0.04) | −0.07 (0.04) | −0.01 (0.04) |
| Attention domain | −0.04 (0.06) | −0.05 (0.05) | −0.09 (0.05) | −0.01 (0.05) | −0.05 (0.05) | −0.02 (0.05) |
| Global domain | −0.11 (0.04)‡ | −0.08 (0.04) | −0.08 (0.04) | −0.08 (0.04) | −0.07 (0.04) | −0.05 (0.04) |
Analyses were adjusted for age, race, sex, and education or height.
β represents unstandardized regression coefficients.
Abbreviations: SE = standard error; SD: standard deviation; Y0: baseline visit; Y30: year 30 of follow-up visit; GVI: gait variability index.
P<0.0001;
P<0.01;
P<0.05
In the MRI sub-cohort (n=144), WMH load ranged from 0.0002 to 0.77 percentage of ICV. In the fully adjusted model (model 2), higher exposure to cumulative SBP and DBP were associated with higher WMH load (β [95% CI] = 0.31 [0.06, 0.56] and 0.25 [0.006, 0.50], respectively) (Supplemental Table 3). To test for effect modification by WMH on the observed associations, participants were stratified at the median into two WMH groups (n = 72 per group). The amount of WMH in the low WMH group ranged from 0.0002 to 0.02 percentage of ICV, and in the high WMH group from 0.02 to 0.77 percentage of ICV. Figure 3 shows the association of cumulative BP exposure with gait and cognition in those with high and low WMH burden. The association between cumulative exposure to SBP and step velocity, step length and GVI was strongest in participants with higher WMH burden (all p for interaction <0.05). Similarly, the association between cumulative exposure to DBP with step length and GVI were strongest in those with higher burden of WMH (all p for interaction <0.05). However, the association between cumulative BP exposure and cognitive function did not vary across the two WMH groups (all p for interactions > 0.05, Figure 3). Adjustment for WMH in model 2 did not change the association of cumulative BP with gait or cognition (β coefficients attenuated <10%, Supplemental Table 4). Finally, there was no significant interaction between cumulative BP exposure and antihypertensive medication use, or becoming hypertensive during follow-up, in relation to gait or cognitive function (n=61 on antihypertensive medication, all p-for interactions >0.05) (Supplemental Table 5 and 6).
Figure 3. Association between cumulative blood pressure during young adulthood with gait and cognition at midlife, stratified for those with low and high loads of white matter hyperintensities.
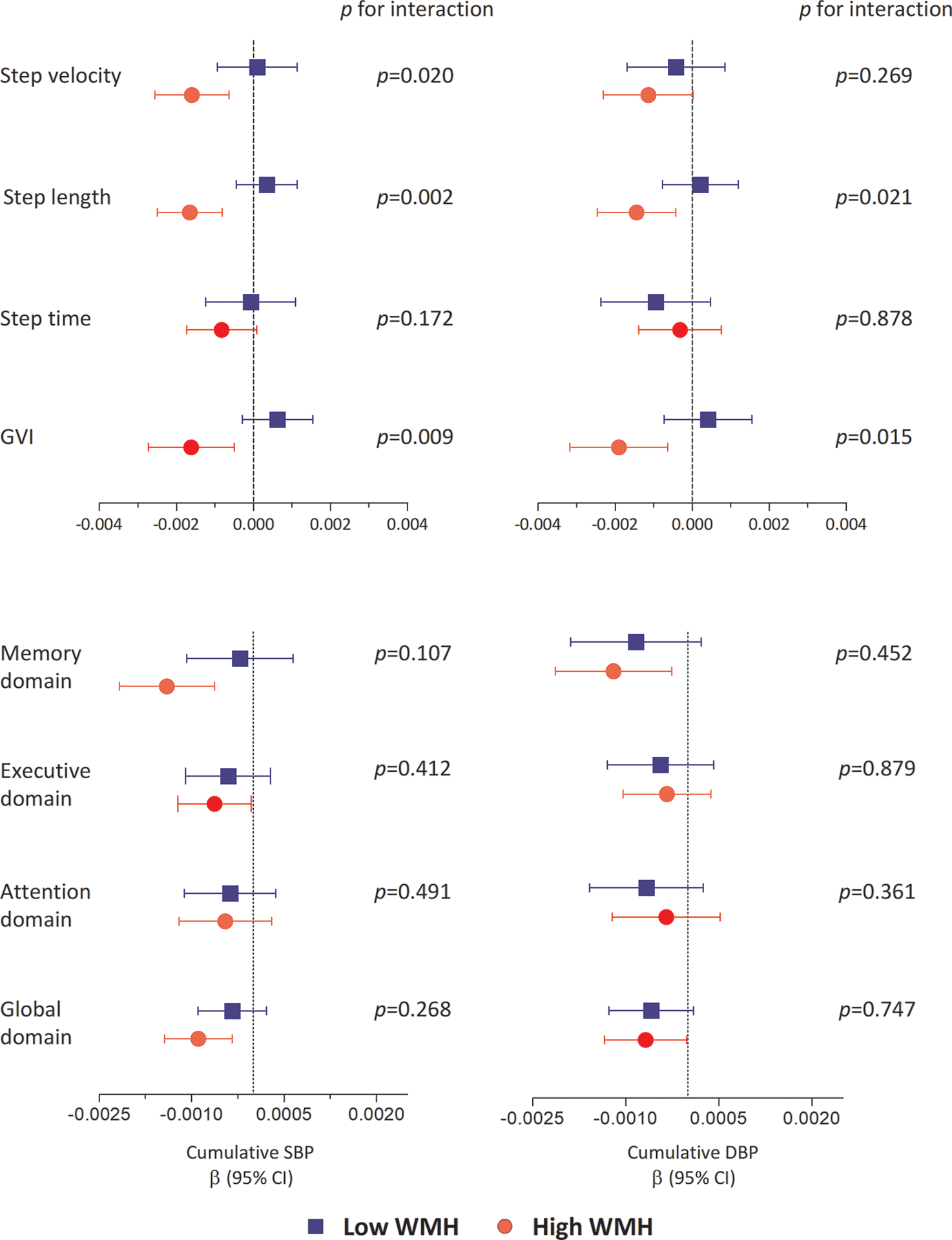
The β represents unstandardized regression coefficients. Gait variables were transformed to z-scores. GVI and step time were multiplied by −1 for consistency in interpretation. All analyses were adjusted for age, sex, race, height or education, smoking status, diabetes prevalence, total cholesterol level and body mass index measured at Y30 examination. p values show p for interaction. WMH was divided in low and high groups at the median of WMH (n=72 per group). Abbreviation: SBP: systolic blood pressure; DBP: diastolic blood pressure; WMH: white matter hyperintensity and GVI: gait variability index.
DISCUSSION
The main finding of this study is that exposure to higher BP starting in young adulthood, even at levels below the “hypertension” threshold34, is associated with worse gait and cognitive performance in midlife, independent of other vascular risk factors. Furthermore, we found that higher exposure to BP during young adulthood was consistently associated with worse gait performance in those with higher WMH burden, whereas the association of cumulative BP with cognitive performance was not affected by WMH burden.
The association of higher BP with cognitive and gait impairment is consistent with previously published work in the elderly population. Mounting evidence suggests that hypertensive individuals are at increased risk of dementia and mobility impairment in late life, independent of other conventional vascular risk factors and stroke11, 14. The Cardiovascular Health Study of older Americans, for example, has shown that slow gait speed, cognitive impairment, and depressive symptoms cluster together in individuals over the age of 74, and that this cluster of impairments is strongly linked to hypertension35. Much research has focused on examining the link between hypertension and functional impairment in older adults, the group that is at the highest risk of dementia and disability11. Recent findings suggest that individuals who develop hypertension in midlife are also susceptible to the deleterious health effect of hypertension for many decades2, 36. In line with this, observational studies suggest that elevated BP in the 4th and 5th decade of life is strongly linked to cognitive impairment 20–30 years later37. On the other hand, elevated BP in the 8th, 9th or 10th decade life have shown weak and conflicting results in relation to cognitive function38. Therefore, the chronicity and duration of BP exposure in early life may be especially important for functional impairments in later stages of life11. The findings from this study extend the previous research by showing that even in young individuals without clinically evident neurological disorders, higher cumulative exposure to BP during the 2nd, 3rd and 4th decade of life is associated with poorer gait and cognitive impairment in the 5th decade of life. This is consistent with previous studies on CARDIA population, where it has been shown that higher cumulative BP associates with worse cognitive performance during 25 years of follow-up39. Our results add to the previous work from the CARDIA cohort and show that cumulative BP exposure during young adulthood is also associated with several gait measures and that cerebral WMH burden likely moderates this association. Furthermore, our results suggest that long term exposure to higher BP during young adulthood may be more powerful in predicting midlife mobility and cognitive impairment than a single BP measurement.
The underlying pathological mechanism linking hypertension and adverse health outcomes is complex and not fully understood. Studies suggest that features of cerebral small vessel disease, such as WMH on brain MRI, may underlie the link between hypertension and cognitive or mobility decline40, 41. This premise is supported by studies showing a close link between WMH and hypertension, dementia, and mobility impairment in older adults14. The findings from the present study support a moderating effect of WMH on the link between higher cumulative BP and gait. However, at least in midlife, the link between cumulative BP exposure and cognitive function was not moderated by WMH burden. One explanation for these findings could be that other anatomical changes in the brain such as hippocampal atrophy42 may be more critical for cognition in midlife. Alternatively, gait may more vulnerable to the deleterious effects of overall white matter injury in early stages of life than cognitive function. In line with this, prior studies have shown that gait abnormalities may precede and predict cognitive decline in older adults43. In line with this, it has been show that in 204 participants from the Oregon Brain Aging Study evaluated for up to 20 years, slowing of gait speed was accelerated up to 12 years prior to development of mild cognitive impairment9. Similarly, in 1478 participants from the Mayo Clinic Study of Aging, slowing of gait preceded cognitive decline in all domains10. This is further supported by the shared neurochemical and pathological mechanisms between gait and cognition44, 45. Given the low-cost and noninvasive nature of gait measurements, gait characteristics may be useful clinical biomarkers predictive of cognitive decline in ageing46. Furthermore, our results do not support a mediating effect of WMH on the observed associations since inclusion of WMH in our models did not change the association between cumulative BP and cognition or gait. In this setting, other microstructural and morphological changes of the white matter may precede development of WMH and better explain the observed associations. Measures of white matter integrity, demyelination, and axonal degeneration from advanced brain MR modalities may provide a more nuanced understanding of hypertensive cerebrovascular damage in early life13.
Given that high BP is a modifiable risk factor, it continues to represent a potential target for prevention and/or delaying the progression of cognitive impairment and functional disability in the aging population2. Although many large randomized clinical trials (RCTs) have concluded the protective effect of BP treatment against cognition and dementia in older adults, many other RCTs have failed to replicate these findings11. Recent results from the SPRINT-MIND trial showed no significant reduction in the risk of probable dementia after treating ambulatory adults (mean age 68 years) to a SBP of less than 120 mmHg compared to less than 140 mmHg27. Furthermore, results from the same trial did not show any benefit from intensive BP treatment on mobility and gait speed in older adults aged 75 years or older47. Such negative findings could be due to enrollment of elderly individuals with irreversible cerebrovascular damage and short duration of follow-up. On the other hand, primordial prevention of high BP during early adulthood may be more effective than primary prevention of subsequent adverse outcomes48. In fact, sustained interventions through early stages of life may be required to reduce the onset or progression of gait and cognitive abnormalities in later stages of life48. In line with this, it has been shown that reducing the sodium intake of newborns for the first 6 months of life (n=466) reduces SBP levels by 2.1 mmHg compared to controls at 25 weeks of age49. The same participants followed at the age of 15 years (n=167) had 3.6 mmHg lower SBP and 2.2 mmHg lower DBP compared to controls50. Future studies are needed to investigate the impact of primordial high BP prevention during early adulthood, and possibly during infancy, on later cognitive and mobility outcomes.
The strengths of this study include the cohort of young individuals with few comorbidities, long duration of follow-up (30 years of BP assessment), and application of a comprehensive set of cognitive tests and gait characteristics that allowed us to assess different domains of cognition and gait. In addition, extensive phenotyping regarding vascular risk factors enabled us to correct for several potential confounders. We acknowledge the limitations of our study as well. Our cohort consisted of biracial black and white population and the results may not be generalizable to individuals from other race/ethnicity cohorts. Furthermore, cognition and gait were assessed at the end of study and we were not able to address the changes in cognition or gait over time. Longitudinal studies are essential to understanding the mechanisms underpinning the relationship between BP exposure and cognitive or gait decline over time.
In summary, the present study shows that higher BP exposure during young adulthood, even at levels below the clinical definition of hypertension, is associated with worse gait and cognition in mid-life, independent of other vascular risk factors. Furthermore, WMH burden moderated the association of cumulative BP with gait, but not with cognitive function. These results emphasize the impact of early life BP levels on brain structure and function and underscore the need for primordial prevention of high BP during early adulthood. Finally, the predictive value of gait as an early biomarker for cerebral small vessel disease will need to be further explored in younger cohorts with earlier gait measures and validated as a biomarker to monitor therapeutic efficacy of early interventions.
Supplementary Material
Clinical Perspective.
What is new?
Cumulative exposure to higher blood pressures from young adulthood to midlife, even at levels below the clinical definition of hypertension, associates with worse gait and cognitive function in midlife.
The impact of cumulative levels of blood pressure exposure was independent of other vascular risk factors during a follow-up period of over 30 years.
Higher burden of midlife cerebral white matter hyperintensity on magnetic resonance imaging moderates the association of cumulative blood pressure exposure with gait, but not with cognitive function.
What are the clinical Implications?
The deleterious effect of elevated blood pressure on brain structure and function may begin during early adulthood, emphasizing the need for primordial prevention of high blood pressure, but also reconsidering individual levels of blood pressure for diagnosis of hypertension.
Gait may be an earlier measure of hypertensive brain injury than cognition.
FUNDING SOURCES
The Cerebral Small Vessel in Motor and Cognitive Decline study is supported by National Institute of Health (NIH, R01NS085002). The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content. S. Sedaghat is supported by a Rubicon fellowship of the Netherlands Organization for Scientific Research (NWO).
Non-standard Abbreviations and Acronyms
- CARDIA
Coronary Artery Risk Development in Young Adults study
- Y1, Y2, …, Y30
year 1, year 2, …, year 30 of follow-up visit
- CES-D
Center for Epidemiologic Studies Depression Scale
- GVI
Gait Variability Index
- HVLT-R
Hopkins Verbal Learning Test-Revised
- DS
Digit Span test
- DSST
Digit Symbol Substitution Test
- NIH-Flanker Inhibitory test
National Institute of Health-Flanker Inhibitory test
- NIH-LSWM
National Institute of Health-List Sorting Working Memory test
- NIH-PSM
National Institute of Health-Picture Sequence Memory test
- FLAIR
Fluid-Attenuated Inversion Recovery
- SPM
Statistical Parametric Mapping
- LST
Lesion Segmentation Toolbox
- AFNI
Analysis of Functional NeuroImages
- ICV
Intracranial Volume
- SPRINT-MIND
Systolic Blood Pressure Intervention Trial - Memory and Cognition in Decreased Hypertension
Footnotes
CONFLICT OF INTEREST DISCLOSURES
None.
REFERENCES
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension. 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington F, Saxby BK, McKeith IG, Wesnes K and Ford GA. Cognitive performance in hypertensive and normotensive older subjects. Hypertension. 2000;36:1079–1082. [DOI] [PubMed] [Google Scholar]
- 4.Kennelly SP, Lawlor BA and Kenny RA. Blood pressure and dementia - a comprehensive review. Ther Adv Neurol Disord. 2009;2:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubert HB and Fries JF. Predictors of physical disability after age 50. Six-year longitudinal study in a runners club and a university population. Ann Epidemiol 1994;4:285–294. [DOI] [PubMed] [Google Scholar]
- 6.Pinsky JL, Branch LG, Jette AM, Haynes SG, Feinleib M, Cornoni-Huntley JC and Bailey KR. Framingham Disability Study: relationship of disability to cardiovascular risk factors among persons free of diagnosed cardiovascular disease. Am J Epidemiol. 1985;122:644–656. [DOI] [PubMed] [Google Scholar]
- 7.Rosano C, Longstreth WT Jr., Boudreau R, Taylor CA, Du Y, Kuller LH and Newman AB. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. J Am Geriatr Soc. 2011;59:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein D, Nagel G, Kleiner A, Ulmer H, Rehberger B, Concin H and Rapp K. Blood pressure and falls in community-dwelling people aged 60 years and older in the VHM&PP cohort. BMC Geriatr. 2013;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buracchio T, Dodge HH, Howieson D, Wasserman D and Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, Pankratz VS, Geda YE, Machulda MM, Ivnik RJ, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker KA, Power MC and Gottesman RF. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: a Review. Curr Hypertens Rep. 2017;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen DR, Shaaban CE, Wiley CA, Gianaros PJ, Mettenburg J and Rosano C. A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am J Physiol Heart Circ Physiol. 2018;314:H1117–H1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White WB, Marfatia R, Schmidt J, Wakefield DB, Kaplan RF, Bohannon RW, Hall CB, Guttmann CR, Moscufo N, Fellows D, et al. INtensive versus standard ambulatory blood pressure lowering to prevent functional DeclINe in the ElderlY (INFINITY). Am Heart J. 2013;165:258–265 e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR Jr., Liu K, Orden S, Pirie P, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8:68S–73S. [DOI] [PubMed] [Google Scholar]
- 16.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K and Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs DR Jr., Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG and Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P and Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz KH, Jacobs DR Jr., Leon AS, Schreiner PJ and Sternfeld B. Physical activity and body weight: associations over ten years in the CARDIA study. Coronary Artery Risk Development in Young Adults. Int J Obes Relat Metab Disord. 2000;24:1475–1487. [DOI] [PubMed] [Google Scholar]
- 20.Lord S, Galna B, Verghese J, Coleman S, Burn D and Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68:820–827. [DOI] [PubMed] [Google Scholar]
- 21.Gouelle A, Megrot F, Presedo A, Husson I, Yelnik A and Pennecot GF. The gait variability index: a new way to quantify fluctuation magnitude of spatiotemporal parameters during gait. Gait Posture. 2013;38:461–465. [DOI] [PubMed] [Google Scholar]
- 22.Benedict RH, Schretlen D, Groninger L and Brandt J. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 23.Reese CS, Suhr JA and Riddle TL. Exploration of malingering indices in the Wechsler Adult Intelligence Scale-Fourth Edition Digit Span subtest. Arch Clin Neuropsychol. 2012;27:176–181. [DOI] [PubMed] [Google Scholar]
- 24.Llinas-Regla J, Vilalta-Franch J, Lopez-Pousa S, Calvo-Perxas L, Torrents Rodas D and Garre-Olmo J. The Trail Making Test. Assessment. 2017;24:183–196. [DOI] [PubMed] [Google Scholar]
- 25.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR Jr., Zhu N, Lloyd-Jones DM, He K and Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D and Wallner-Allen K. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Group SMIftSR, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjon JV, Coupe P, Marti-Bonmati L, Collins DL and Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging. 2010;31:192–203. [DOI] [PubMed] [Google Scholar]
- 29.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA and Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt P Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging. [Dissertation]. Munich, Germany: München Universitätsbibliothek der Ludwig-Maximilians-Universität; 2017. [Google Scholar]
- 31.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 32.Pinter D, Ritchie SJ, Doubal F, Gattringer T, Morris Z, Bastin ME, Del CVHM, Royle NA, Corley J, Munoz Maniega S, et al. Impact of small vessel disease in the brain on gait and balance. Sci Rep. 2017;7:41637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes AF and Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther. 2017;98:39–57. [DOI] [PubMed] [Google Scholar]
- 34.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484–e594. [DOI] [PubMed] [Google Scholar]
- 35.Hajjar I, Yang F, Sorond F, Jones RN, Milberg W, Cupples LA and Lipsitz LA. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J Gerontol A Biol Sci Med Sci. 2009;64:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Launer LJ, Masaki K, Petrovitch H, Foley D and Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 38.Reitz C and Luchsinger JA. Relation of Blood Pressure to Cognitive Impairment and Dementia. Curr Hypertens Rev. 2007;3:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH and Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhaaren BF, Vernooij MW, de Boer R, Hofman A, Niessen WJ, van der Lugt A and Ikram MA. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61:1354–1359. [DOI] [PubMed] [Google Scholar]
- 41.Hajjar I, Quach L, Yang F, Chaves PH, Newman AB, Mukamal K, Longstreth W Jr., Inzitari M and Lipsitz LA. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the Cardiovascular Health Study. Circulation. 2011;123:858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yano Y, Reis JP, Levine DA, Bryan RN, Viera AJ, Shimbo D, Tedla YG, Allen NB, Schreiner PJ, Bancks MP, et al. Visit-to-Visit Blood Pressure Variability in Young Adulthood and Hippocampal Volume and Integrity at Middle Age: The CARDIA Study (Coronary Artery Risk Development in Young Adults). Hypertension. 2017;70:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montero-Odasso M, Verghese J, Beauchet O and Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchman AS and Bennett DA. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother. 2011;11:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson J, Allcock L, Mc Ardle R, Taylor JP and Rochester L. The neural correlates of discrete gait characteristics in ageing: A structured review. Neurosci Biobehav Rev. 2019;100:344–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amboni M, Barone P and Hausdorff JM. Cognitive contributions to gait and falls: evidence and implications. Mov Disord. 2013;28:1520–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Odden MC, Peralta CA, Berlowitz DR, Johnson KC, Whittle J, Kitzman DW, Beddhu S, Nord JW, Papademetriou V, Williamson JD, et al. Effect of Intensive Blood Pressure Control on Gait Speed and Mobility Limitation in Adults 75 Years or Older: A Randomized Clinical Trial. JAMA Intern Med. 2017;177:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillman MW. Primordial prevention of cardiovascular disease. Circulation. 2015;131:599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofman A, Hazebroek A and Valkenburg HA. A randomized trial of sodium intake and blood pressure in newborn infants. Jama. 1983;250:370–373. [PubMed] [Google Scholar]
- 50.Geleijnse JM, Hofman A, Witteman JC, Hazebroek AA, Valkenburg HA and Grobbee DE. Long-term effects of neonatal sodium restriction on blood pressure. Hypertension. 1997;29:913–917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


