Abstract
Background and Purpose–
Enhancement of erythrophagocytosis by macrophages in a timely manner can limit the toxic effects of erythrocyte metabolites and promote brain recovery after intracerebral hemorrhage (ICH). In the current study, we investigated the therapeutic effect of retinoid X receptor agonist, bexarotene, in facilitating erythrophagocytosis and neurobehavioral recovery in two mouse models of ICH.
Methods–
Bone marrow-derived macrophages (BMDMs) and fluorescently labeled erythrocytes were used to study erythrophagocytosis in vitro with phenotypic changes quantified by gene expression. ICH was modeled in vivo using intrastriatal autologous blood and collagenase injection in mice with and without bexarotene treatment beginning 3 hours after ICH. In vivo phagocytosis ability and hematoma clearance were evaluated by erythrophagocytosis assays, flow cytometry, and histological analysis. Neurologic deficits and functional recovery were also quantified.
Results–
Bexarotene increased macrophage expression of phagocytosis receptors and erythrophagocytosis and reduced macrophage TNF production in vitro. In vivo, bexarotene treatment enhanced erythrophagocytosis, reduced hematoma volume, and ultimately improved neurological recovery after ICH in two distinct models of ICH.
Conclusions–
Bexarotene administration is beneficial for recovery after ICH by enhancing hemorrhage phagocytosis, modulating macrophage phenotype, and improving functional recovery.
Summary
Bexarotene treatment induces the expression of the phagocytic receptors Axl and CD36 in macrophages and promotes the clearance of erythrocytes and the hematoma from the brain after ICH. Moreover, bexarotene improves neurobehavioral outcomes in multiple preclinical models of ICH.
Keywords: intracerebral hemorrhage, bexarotene, phagocytosis, Axl receptor tyrosine kinase, macrophage, inflammation
Introduction
Intracerebral hemorrhage (ICH) is a severe form of stroke resulting from a rupture of brain parenchymal blood vessel for which there is currently no effective medical treatment.1, 2 Hematoma resolution occurs over weeks to months after ICH in patients and as a natural mechanism to remove the extravasated blood from the brain and reduce mass effect from the hematoma.3, 4 Although strategies advocating hematoma clearance for ICH therapeutics have been investigated for a decade,5–7 only one preclinical pharmacological approach has been successfully translated to clinical trial thus far.8 While the result of this clinical trial is still being determined, search of other potential pharmacological targets to accelerate hematoma resolution and brain recovery after ICH is in dire need. We and others have previously reported that promotion of macrophages (include tissue-resident and monocyte-derived macrophages) alternatively activated phenotype can accelerate hematoma clearance.3, 4, 9 We recently further demonstrated that monocyte-derived macrophages (MDMs) infiltration from periphery is an important contributor to hematoma resolution and long-term functional recovery after ICH.10 Activation of the phosphatidylserine receptors, including Axl, by erythrophagocytosis triggers brain infiltrating MDMs to shift their phenotype from proinflammatory to an ICH-specific reparative phenotype. High level of plasma cleaved (soluble)-Axl is correlated with worse 1-year outcome in ICH patients suggesting that Axl on MDMs is crucial for the process of erythrocyte phagocytosis and the acquisition of reparative phenotype in the ICH brain.
Bexarotene is a FDA approved selective retinoid X receptor (RXR) agonist for clinical use in patients with cutaneous T cell lymphoma.11 It has been shown to reduce Aβ deposition and behavioral deficits in the Alzheimer’s disease model,12 is the only drug that has been reported to increase Axl expression on myeloid cells13 and to rejuvenate aged-related declines in phagocytosis ability in human monocytes.14 These pharmacological properties of bexarotene could be beneficial for ICH brain recovery through the regulation of Axl-mediated macrophage erythrophagocytosis. In addition, upon activation, RXR can heterodimerize with PPAR-gamma, leading to enhanced expression of PPARG-gamma dependent genes (e.g. CD36) shown to be beneficial after ICH and provide protective effects on ICH-induced brain injury9, 15 leading to the hypothesis that RXR agonism should improve ICH outcomes. In this study, we utilized bone-marrow derived macrophages (BMDMs) and two preclinical mouse ICH models to evaluate the potential therapeutic role of bexarotene on hematoma clearance and beneficial effects on neurobehavioral outcome after ICH.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. All animal protocols were conducted in accordance with Guide for the Care and Use of Laboratory Animals from the NIH and ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments) and were approved by the Yale Institutional Animal Care and Use Committee. Mice were randomly allocated to experimental groups by coin flip approach by an investigator who then coded the treatments. Investigators who performed surgeries and outcome assessments were blinded to treatment assignments for all experiments until after data analysis. A total of sixty 10–12 week-old C57BL/6 (WT) male mice from Jackson laboratory were used in the in vivo studies and five six-week-old WT male mice were used for bone-marrow derived macrophages culture. Male mice were used to eliminate the effects of estrogen on the results of this first study of bexarotene. Three vehicle-treated collagenase ICH mice died at day 1 due to the natural mortality of this model, and one bexarotene-treated blood ICH mice died at day 2 after needle puncture to the vital organ during intraperitoneal (i.p.) injection. Hence, data from a total of four mice were excluded from the final statistical analysis. Sample size was based on previous experiments from our laboratory using the ICH models and neurobehavioral tests, powering for a robust effect size f (by repeated measures ANOVA) of 0.6 with α of 0.05, power of 0.8, and three repeated measurements of neurobehavior (computed in G*power).10, 16
ICH Models
Collagenase and autologous blood injection ICH models were performed as described previously.3, 10, 17 Briefly, under isoflurane anesthesia, 0.5 μl of 0.2 U type VII-S collagenase (Sigma-Aldrich) or 25 μl autologous blood was injected into the right striatum at the following coordinates relative to bregma: 2.5 mm lateral, and 3.0 mm deep at 5 ° angle. The rectal temperature was maintained at 37.0 ± 0.5 °C throughout the surgery and recovery periods.
Bexarotene Administration
For in vitro studies, 0.5 or 1 μM bexarotene (200499; Calbiochem) in Dulbecco’s phosphate-buffered saline (DPBS) or DPBS alone (vehicle) were added to macrophage cultures. For in vivo studies, bexarotene was injected intraperitoneally at 5 mg/kg in 400 μL of saline; vehicle control treated mice received 400 μL of saline. Mice were treated at 3 hours after ICH surgery and then once daily until euthanasia. The delivery route and dosing regimens18–20 and the in vitro concentrations14, 21, 22 of bexarotene were based on previous work.
Cell Culture
Bone marrow was harvested from the tibias and femurs of male mice and bone-marrow derived macrophages (BMDMs) were differentiated and maintained in recombinant murine M-CSF (50 ng/ml, R&D Systems) containing complete medium (BioWhittaker Lonza). BMDMs were used for experiments at 7 days in vitro (DIV) as described.10 In some experiments, BMDMs were stimulated with thrombin (10 U/ml) for 6 or 24 hours prior to quantification of gene expression by RT-qPCR.
In vitro and In vivo Erythrophagocytosis Assay
Macrophage erythrophagocytosis was evaluated in the BMDMs and perihematomal brains as previously described.10 Briefly, the RBCs were isolated from whole blood of donor mice, heat-shocked at 56 °C for 6 minutes to induce phosphatidylserine expression and labeled with lipophilic PKH-26 fluorescent probe (Sigma-Aldrich). The PKH-26-labeled RBCs for in vitro studies were counted and fed to BMDMs at 1:30 ratio of BMDMs and RBCs. After incubation at 37 °C for 15, 30, or 60 minutes, the unengulfed RBCs were removed by washing three times with DPBS followed by staining BMDMs with CD11b. The engulfed RBCs were observed by fluorescent microscopy and phagocytosis index was calculated by the mean fluorescence intensity of engulfed RBCs per cell.
For in vivo erythrophagocytosis assessment, the PKH-26-labeled RBCs were suspended in autologous plasma at 1:4 ratio of RBCs and plasma (20% hematocrit) and 25 μL labeled RBCs in plasma were intrastriatally injected in mice according to the procedure for autologous blood ICH model. Mice were euthanized at 3 days and the brain samples were prepared for flow cytometry analysis as described.10, 16 The RBCs-positive monocyte-derived macrophages (MDMs) in the ICH brains were defined as LIVE/DEAD-CD45hiCD11b+Ly6G-Ly6C+PKH-RBC+ population. MDMs isolated from the contralateral brains were used as control samples.
RT-qPCR
We extracted total RNA from BMDMs using QIAzol Lysis Reagent (miRNeasy Micro Kit; QIAGEN). The first strand of cDNA was synthesized from 100ng RNA with SuperScript VILO cDNA synthesis kit (Invitrogen), and then RT-qPCR performed on an ABI 7500 Fast real-Time PCR System (Applied Biosystem) using TaqMan universal PCR Master Mix II with UNG. The TaqMan Gene Expression Assay Mix for mouse Cd36 (Mm00432403_m1), Axl (Mm00437221_m1), Tnf (Mm00443258_m1) and Gapdh (Mm99999915_g1) were obtained from Thermo Fisher Scientific. The cycle time values of candidate gene were normalized to endogenous control Gapdh in the same sample. The expression level of mRNA was calculated using delta delta CT (∆∆CT) method as previously described.3, 10, 17
Hematoma Volume Measurement
Fresh 1-mm coronal slices across the whole brain were harvested from vehicle- and bexarotene-treated mice at days 3 and 7 after ICH based on our standard procedure.23 The brain slices were digitalized and analyzed, and the cubic volume of hematoma was calculated using ImageJ blinded to treatment as we described.3, 10
Behavioral Performance Evaluations
An investigator blinded to the treatment assignments evaluated functional and neurological deficits of mice by cylinder test, forelimb placing test, hindlimb adduction test, and neurologic severity scoring system as previously described.3, 10, 17 Briefly, for cylinder test, the mice were allowed to freely rear in a transparent glass cylinder. The laterality index from a total of 20 rears was calculated as (right-left) / (right+left+both). A score of zero indicates equal use of forelimbs, while the greater positive number indicates a more severe left hemiparesis. For the forelimb placing test, the vibrissae of mouse were brushed on the edge of a benchtop. Intact animals quickly place the ipsilateral forelimb onto the benchtop. For the hindlimb adduction test, the mouse was place on the edge of platform, and the hind paw was gently pulled down away from the edge. Intact animals rapidly retrieve and replace the hindlimb on the benchtop. In both tests, we recorded a total of 20 trials per day and the data were quantified as the percentage successful placing responses. In the neurologic severity scoring system, the mouse was evaluated for body symmetry, gait, climbing, circulating behavior, front limb symmetry, and compulsory circling. A score from 0 to 4 was graded for the individual test, establishing a maximum score of 24 as the most severe deficit.
Statistical analysis
Data are expressed as means ± standard deviation or individual mouse data with a line indicating the mean of the group. Statistical significance was determined by Student’s t test to analyze differences between two groups and by two-way repeated-measures ANOVA with post hoc Bonferroni’s multiple comparisons tests to compare behavioral recovery over time. P < 0.05 was considered to represent a significant difference. Statistical analysis was performed using SigmaStat 3.5 (Systat Software Inc.)
Results
Bexarotene promotes erythrocytes engulfment and reparative phenotype in BMDMs.
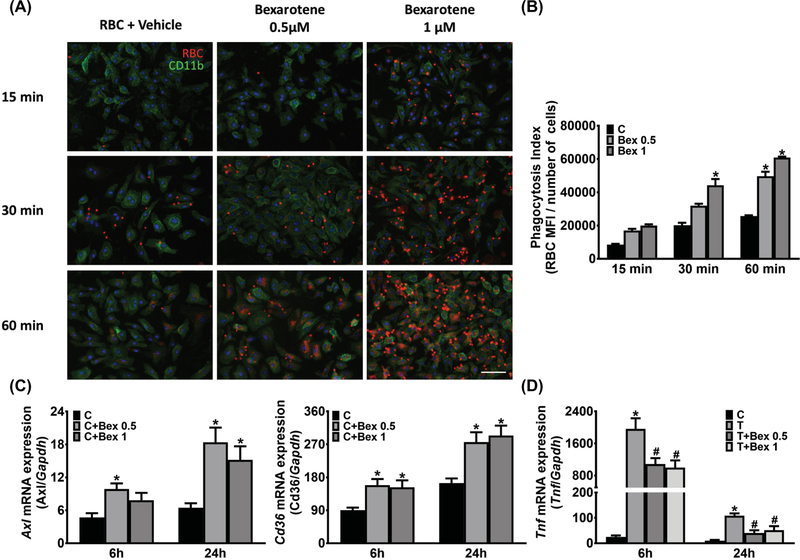
We previously demonstrated that the phosphatidylserine receptors Axl and Mertk mediate monocyte-derived macrophages (MDMs) erythrophagocytosis and resultant alternatively activated phenotypic changes in the ICH brain.10 Previous studies revealed that bexarotene treatment increased macrophages Axl expression and phagocytosis of polystyrene beads in the AD brain13 and reversed age-related decline in phagocytosis activity of human monocytes.14 To examine the effect of bexarotene treatment on macrophage erythrophagocytosis, BMDMs were treated with bexarotene for 24 hours. The BMDMs were then incubated with PKH-labeled erythrocytes for various durations in order to investigate the kinetics of erythrophagocytosis. We found that the engulfment of erythrocytes was increased by bexarotene 0.5 and 1 μM treatment (Figure 1A). A ~2.2 fold elevation of phagocytosis was observed after 30 min in the 1 μM bexarotene concentration. A longer erythrocytes incubation (60 min) showed that bexarotene-treated BMDMs significantly increased phagocytosis ability in both 0.5 μM (~1.9 fold) and 1 μM (~2.4 fold) conditions (Figure 1B). We next investigated the ability of bexarotene to regulate gene expression of receptors known to contribute to phagocytosis. Axl expression was increased by ~2.1 fold (0.5 μM; 6 h), ~2.8 fold (0.5 μM; 24 h), and ~2.4 fold (1 μM; 24 h) after bexarotene treatment (Figure 1C). In addition, expression of Cd36, another scavenger receptor implicated in erythrocyte phagocytosis4 and target of the PPAR-gamma-RXR heterodimer,24 was increased in BMDMs after bexarotene treatment for 6 and 24 h (Figure 1C). As reduction in the proinflammatory phenotype macrophages is associated with improved outcomes,10 we stimulated macrophages with thrombin and examined the effect of bexarotene treatment. Bexarotene treatment decreased Tnf expression (Figure 1D) in thrombin-stimulated BMDMs.
Figure 1.
Bexarotene promotes erythrophagocytosis and modulates macrophage phenotype. A, Representative immunofluorescence images show engulfment of PKH-26-labeled erythrocytes (Red) in BMDMs (Green) after erythrocyte incubation for 15, 30, and 60 min. BMDMs were incubated with bexarotene (0.5 or 1 μM) for 24 hours prior to treatment with erythrocytes. B, Quantification of erythrophagocytosis. n = 3 independent experiments/group; each experiment includes 2 technical replicates. *P ≤ 0.05 vs control group by 2-way repeated ANOVA and Bonferroni’s post hoc test. C, Gene expression of Axl and Cd36 in BMDMs after different concentrations of bexarotene treatment for 6 and 24 h. n = 3/group. *P ≤ 0.05 vs control group by 2-way ANOVA and Bonferroni’s post hoc test. D, Proinflammatory marker Tnf gene expression in thrombin-stimulated BMDMs after 0.1 or 1 μM bexarotene co-incubation for 6 and 24 h. n = 3/group. *P ≤ 0.05 vs control group by 2-way repeated ANOVA and Bonferroni’s post hoc test. C, control; Bex, bexarotene; T, thrombin.
Bexarotene enhances MDMs erythrophagocytosis, hematoma clearance, and functional recovery in acute ICH.
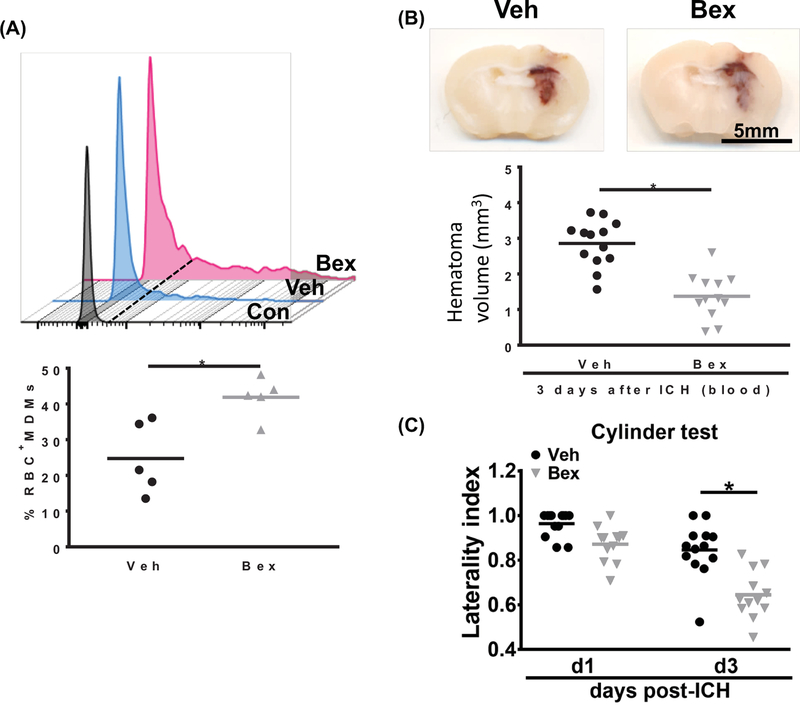
We and others have reported that macrophages are essential for hematoma clearance after ICH and macrophage alternative activation accelerates hematoma resolution and ICH recovery.3, 4, 10 Based on the in vitro findings that bexarotene promoted BMDMs erythrophagocytosis and reduced TNF production, we were interested determining whether bexarotene was beneficial in vivo. Mice were subjected to ICH induction by autologous injection of fluorescently-labelled erythrocytes followed by bexarotene or vehicle treatment beginning at 3 hours and continuing daily. At day 3 after ICH, the MDMs from the perihematomal brains were analyzed by flow cytometry for phagocytosis of labelled erythrocytes. Treatment with bexarotene resulted in increased MDM phagocytosis of erythrocytes compared to the vehicle-treated mice (41.9 ± 5.6 % vs. 24.7 ± 10.0 % positive MDMs, p<0.05; Figure 2A). Consistent with the flow cytometry results, bexarotene administration reduced hematoma volume compared with that of vehicle-treated mice on day 3 post-ICH (2.9 ± 0.7 mm3 vs. 1.4 ± 0.6 mm3 of hematoma; Figure 2B). Additionally, bexarotene-treated mice exhibited better functional performance on the cylinder test on day 3 after ICH (Figure 2C).
Figure 2.
Bexarotene treatment increases MDMs erythrophagocytosis, accelerates hematoma clearance, and improves behavioral function after autologous blood injection model of ICH. A, Top: representative histogram shows intensity of fluorescently-labeled erythrocytes in MDMs from vehicle- and bexarotene-treated mice at day 3 after ICH. Bottom: Quantification of percentage of MDMs that are positive for labeled erythrocytes. n = 5/group, *P < 0.05 vs vehicle group by Student’s t test. B, Top: representative coronal sections show visible hematoma in the vehicle- and bexarotene-treated mice at day 3 after blood injection. Bottom: Quantification of hematoma volume. n = 13 for vehicle group and n = 12 for bexarotene group, *P < 0.05 vs vehicle group by Student’s t test. C, Cylinder test results of vehicle- and bexarotene-treated mice at day 3 after blood injection. n = 13 for vehicle group and n = 12 for bexarotene group, *P < 0.05 vs vehicle group by 2-way repeated ANOVA and Bonferroni’s post hoc test. Veh, Vehicle; Bex, bexarotene.
Bexarotene reduces hematoma resolution and improves neurobehavioral outcomes at late phase of ICH.
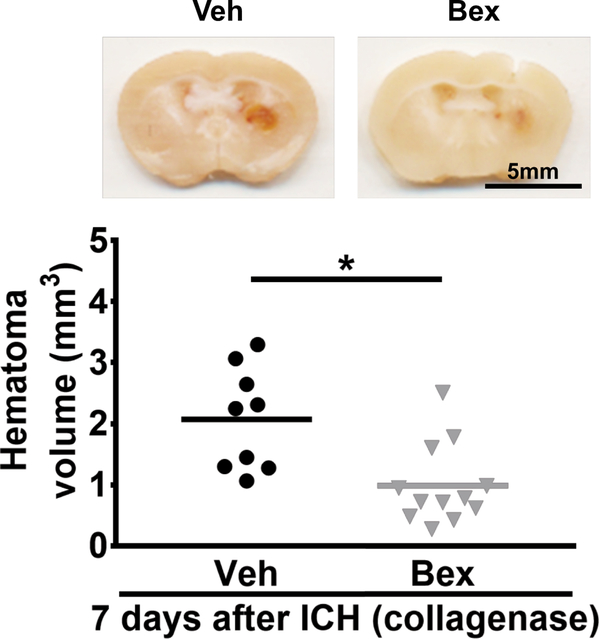
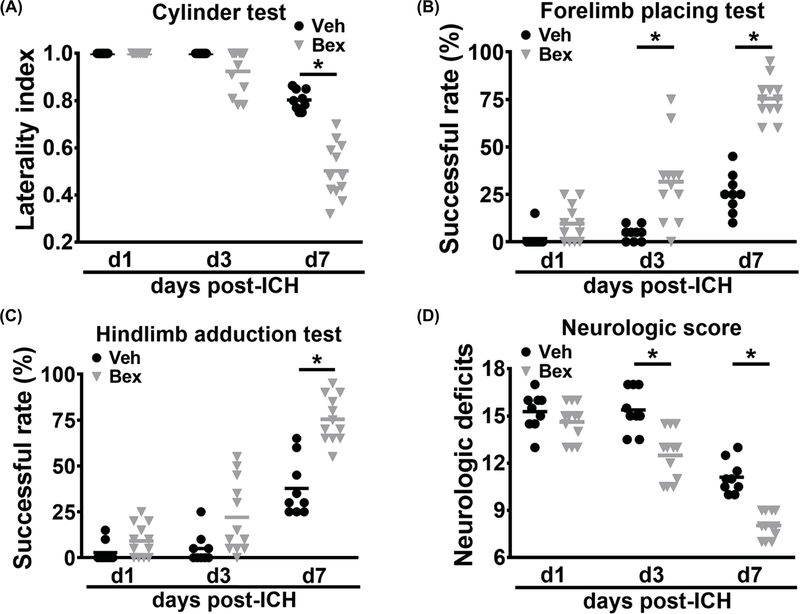
The autologous blood injection model is useful for studying early time points after ICH, but is limited in its ability to model longer term disability. Therefore, we took advantage of the larger hematoma volume and more severe and prolonged functional disability in the collagenase ICH model to examine the effect of bexarotene on hematoma resolution and neurobehavioral function recovery at a later time point after ICH. Bexarotene treatment began at 3 hours after ICH induction and continued daily for 7 days. Bexarotene-treated mice had smaller hematoma volumes compared with the vehicle group (0.9 ± 0.7 mm3 vs. 2.0 ± 0.8 mm3 of hematoma; Figure 3) on day 7 after ICH induction. Mice were subjected to behavioral testing on a battery of tests on days 1, 3, and 7 days after ICH. Mice treated with bexarotene began to show significant motor improvement at day 3. By day 7, bexarotene treatment led to improved performance on the cylinder test (0.5 ± 0.1 vs. 0.8 ± 0.04 laterality index, p<0.05), forelimb placing test (75.4 ± 10.5 % vs. 25.6 ± 10.4 % successful placements, p<0.05), hindlimb placing test (75.4 ± 12.5 % vs. 37.8 ± 15.4 % successful placements, p<0.05) at day 7, and neurologic scores (8.0 ± 0.8 vs. 11.1 ± 1.0 points, p<0.05) (Figure 4 A-D).
Figure 3.
Effect of bexarotene on long-term hematoma resolution after collagenase model of ICH. Top: representative coronal sections show visible hematoma in the vehicle- and bexarotene-treated mice at day 7 after collagenase injection. Bottom: Quantification of hematoma volume. n = 9 for vehicle group and n = 12 for bexarotene group, *P < 0.05 vs vehicle group by Student’s t test. Veh, Vehicle; Bex, bexarotene.
Figure 4.
Effect of bexarotene on neurological deficits after collagenase-induced ICH. The cylinder test (A), forelimb placing test (B), hindlimb adduction test (C), and neurologic deficit score (D) in the vehicle- and bexarotene-treated mice at days 1, 3, and 7 after collagenase ICH surgery. n = 9 for vehicle group and n = 12 for bexarotene group, *P < 0.05 vs vehicle group by 2-way repeated ANOVA and Bonferroni’s post hoc test. Veh, Vehicle; Bex, bexarotene.
Discussion
Pharmacological enhancement of hematoma clearance and reduction in proinflammatory responses are major goals for the treatment of ICH. In this proof-of-concept study, we have shown that bexarotene treatment leads to increased expression of key receptors responsible for erythrophagocytosis by macrophages after ICH, including Axl and CD36, and that erythrophagocytosis by macrophages can be augmented by bexarotene treatment. Using a translationally-relevant post-ICH treatment window in two different preclinical ICH models, we demonstrated that bexarotene administration accelerates hematoma clearance by MDMs and ameliorates neurobehavioral deficits at acute and later phases of ICH. In addition, bexarotene-mediated functional improvements were correlated with reduction in hematoma volume in both blood and collagenase ICH models. These observations are consistent with previous observations that behavioral outcomes can be improved through acceleration of hematoma clearance3, 4, 7, 9, 25 and the promotion of MDMs erythrophagocytosis and changes towards reparative MDM phenotypes in the ICH brain.10
Inflammatory responses are initiated by tissue injury, blood components, and cellular signals in the perihematomal region immediately after the ICH insult.1, 2, 26, 27 Converging lines of evidence support the notion that resolving the hematoma more rapidly and mitigating detrimental secondary inflammatory responses alleviates brain damage and reduces cell death after ICH.5, 28 With this in mind, we tested a therapy that targets both erythrophagocytosis and macrophage activation. MDMs contribute to acute disability29, 30 but also aid in brain repair at later stage of ICH through phenotypic changes induced by efferocytosis of erythrocytes.10 The present study describes a therapeutic intervention whereby MDMs in the ICH brain are induced to phagocytose erythrocytes and clear hematoma through the activation of retinoid X receptor (RXR) by bexarotene treatment. While several lines of study have shown that bexarotene is protective in traumatic, toxic, and ischemic brain injuries 19, 20, 22, 31, our work demonstrates for the first time that bexarotene improves ICH-induced functional deficits by modulating MDMs phenotype and function via enhancing erythrophagocytosis ability and speeding hematoma resolution. In addition, bexarotene has been shown to ameliorate CNS injury via acting on neurons, astrocytes and microglia,13, 32, 33 which could enhance the efficacy of the medication after ICH. Further work is needed to elucidate the contribution of bexarotene treatment on CNS resident cells after ICH.
Bexarotene is blood-brain barrier permeable and highly selective on RXR activation.12, 34 Upon RXR activation, it can interact with heterodimeric partners including RAR, PPAR, vitamin D receptors, and thyroid receptors.35 These nuclear receptors induce the expression of an array of genes that are specifically associated with reparative MDM functions and phagocytosis such as Axl, TREM2, CD300a, C1q and CD36.36, 37 In the AD brain, activation of the RXR/PPAR heterodimer enhance phagocytosis of amyloid-beta plaque in a CD36-dependent mechanism.24 We and others have shown that PPAR activation by rosiglitazone promotes macrophage reparative phenotype and hematoma resolution,3, 4, 8 and the activation of RXR/PPAR heterodimer is to be neuroprotective through modulating immune responses.18 Here, we provide direct evidence of enhanced phagocytic capacity and reduced pro-inflammatory cytokine production by macrophages after bexarotene treatment. Although bexarotene has been previously shown to acts as selective RXR agonist, studies also reveal that PPAR activation is partially involved in the beneficial effects that are triggered by bexarotene.18, 19, 21 We cannot out rule out that macrophages alternative activation after bexarotene treatment might be mediated by targeting multiple signaling pathways. The mechanism through which bexarotene induces phagocytic receptors such as Axl and CD36 expression should be further explored.
Despite extensive work, no medical or surgical therapy has been shown to improve ICH outcomes in large clinical trials. Recently, the results of two large trials relevant to this work were reported. I-DEF, the study of deferoxamine in reducing iron-mediated injury, failed to reach the primary endpoint of improved 90-day outcomes in treated patients. However, in a prespecified analysis, deferoxamine treatment was associated with improved outcomes between 90 and 180 days, suggesting that reducing erythrocyte-mediated injury could lead to better long-term recovery.38 The MISTIEIII trial tested the efficacy of more directly targeting hemorrhage volume reduction via minimally-invasive surgical removal.39 While this trial too failed to meet its primary endpoint of improved outcomes at 365 days, a prespecified exploratory analysis demonstrated that effective volume reduction to < 15 mL was associated with improved outcomes. Both trials support the common notion that erythrocyte-mediated injury leads to functional deficits in patients. Therapeutics that both enhance hematoma resolution and reduce inflammation-induced injury such as bexarotene may be useful in improving outcomes either alone or in combination with surgical evacuation.
Although our work provides proof-of-concept that bexarotene enhances erythrophagocytosis and improves outcome in two ICH models in male mice, the studies have several limitations. (1) Results were achieved using treatment beginning at 3h after ICH induction. Furthur efforts to discover the ideal delivery route, effects of a delayed treatment regimen and the optimal therapeutic window are cleared needed. (2) Sex, via estrogen, may effect ICH outcome.40, 41 Future work to discover the therapeutic efficacy of bexarotene in female mice following ICH is needed. (3) We evaluated the therapeutic potential of bexarotene administration in ICH until day 7 after injury, and demonstrated bexarotene treatment is benefitial for ICH recovery to this subacute time point. Additional studies of the optimal duration for treatment with bexarotene and effect on long term outcomes would advance our understanding of the therapeutic potential of bexarotene for the treatment of ICH. Our initial studies demonstrating a robust effect of bexarotene in the murine models that suggest that these additional studies to determine potential translational potential are warranted.
Supplementary Material
Acknowledgments
Sources of Funding
The work was supported by NIH R01NS095993 (LHS), the Ministry of Science and Technology of Taiwan (MOST 442 107-2320-B-002-063-MY2, CFC)) and the Excellent Translational Medicine Research Projects of National Taiwan University College of Medicine, and National Taiwan University Hospital (NSCCMOH-94-9, CFC)
Footnotes
Disclosures and Conflicts of Interest
LHS reports past consulting fees and non-financial support for Genentech unrelated to the subject of this work.
References
- 1.Hankey GJ. Stroke. Lancet. 2017;389:641–654 [DOI] [PubMed] [Google Scholar]
- 2.Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: Current approaches to acute management. Lancet. 2018;392:1257–1268 [DOI] [PubMed] [Google Scholar]
- 3.Chang CF, Wan J, Li Q, Renfroe SC, Heller NM, Wang J. Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol Dis. 2017;103:54–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362 [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson DA, Keep RF, Hua Y, Xi G. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: From macro to micro. J Cereb Blood Flow Metab. 2018;38:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Grotta J, Gonzales N, Aronowski J. Hematoma resolution as a therapeutic target: The role of microglia/macrophages. Stroke. 2009;40:S92–94 [DOI] [PubMed] [Google Scholar]
- 7.Ni W, Mao S, Xi G, Keep RF, Hua Y. Role of erythrocyte cd47 in intracerebral hematoma clearance. Stroke. 2016;47:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzales NR, Shah J, Sangha N, Sosa L, Martinez R, Shen L, et al. Design of a prospective, dose-escalation study evaluating the safety of pioglitazone for hematoma resolution in intracerebral hemorrhage (shrinc). Int J Stroke. 2013;8:388–396 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Chen Q, Tan Q, Feng Z, He Z, Tang J, et al. Simvastatin accelerates hematoma resolution after intracerebral hemorrhage in a ppargamma-dependent manner. Neuropharmacology. 2018;128:244–254 [DOI] [PubMed] [Google Scholar]
- 10.Chang CF, Goods BA, Askenase MH, Hammond MD, Renfroe SC, Steinschneider AF, et al. Erythrocyte efferocytosis modulates macrophages towards recovery after intracerebral hemorrhage. J Clin Invest. 2018;128:607–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gniadecki R, Assaf C, Bagot M, Dummer R, Duvic M, Knobler R, et al. The optimal use of bexarotene in cutaneous t-cell lymphoma. Br J Dermatol. 2007;157:433–440 [DOI] [PubMed] [Google Scholar]
- 12.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, et al. Apoe-directed therapeutics rapidly clear beta-amyloid and reverse deficits in ad mouse models. Science. 2012;335:1503–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage JC, Jay T, Goduni E, Quigley C, Mariani MM, Malm T, et al. Nuclear receptors license phagocytosis by trem2+ myeloid cells in mouse models of alzheimer’s disease. J Neurosci. 2015;35:6532–6543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nunez V, Johnson KR, et al. Retinoid x receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain. 2015;138:3581–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao XR, Gonzales N, Aronowski J. Pleiotropic role of ppargamma in intracerebral hemorrhage: An intricate system involving nrf2, rxr, and nf-kappab. CNS Neurosci Ther. 2015;21:357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor RA, Chang CF, Goods BA, Hammond MD, Mac Grory B, Ai Y, et al. Tgf-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest. 2017;127:280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CF, Cho S, Wang J. (−)-epicatechin protects hemorrhagic brain via synergistic nrf2 pathways. Ann Clin Transl Neurol. 2014;1:258–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Certo M, Endo Y, Ohta K, Sakurada S, Bagetta G, Amantea D. Activation of rxr/ppargamma underlies neuroprotection by bexarotene in ischemic stroke. Pharmacol Res. 2015;102:298–307 [DOI] [PubMed] [Google Scholar]
- 19.He J, Liu H, Zhong J, Guo Z, Wu J, Zhang H, et al. Bexarotene protects against neurotoxicity partially through a ppargamma-dependent mechanism in mice following traumatic brain injury. Neurobiol Dis. 2018;117:114–124 [DOI] [PubMed] [Google Scholar]
- 20.Zhong J, Cheng C, Liu H, Huang Z, Wu Y, Teng Z, et al. Bexarotene protects against traumatic brain injury in mice partially through apolipoprotein e. Neuroscience. 2017;343:434–448 [DOI] [PubMed] [Google Scholar]
- 21.Dheer Y, Chitranshi N, Gupta V, Abbasi M, Mirzaei M, You Y, et al. Bexarotene modulates retinoid-x-receptor expression and is protective against neurotoxic endoplasmic reticulum stress response and apoptotic pathway activation. Mol Neurobiol. 2018;55:9043–9056 [DOI] [PubMed] [Google Scholar]
- 22.Huuskonen MT, Loppi S, Dhungana H, Keksa-Goldsteine V, Lemarchant S, Korhonen P, et al. Bexarotene targets autophagy and is protective against thromboembolic stroke in aged mice with tauopathy. Sci Rep. 2016;6:33176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CF, Cai L, Wang J. Translational intracerebral hemorrhage: A need for transparent descriptions of fresh tissue sampling and preclinical model quality. Transl Stroke Res. 2015;6:384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. Ppargamma/rxralpha-induced and cd36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci. 2012;32:17321–17331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King MD, McCracken DJ, Wade FM, Meiler SE, Alleyne CH, Jr., Dhandapani KM. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J Neurosurg. 2011;115:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askenase MH, Sansing LH. Stages of the inflammatory response in pathology and tissue repair after intracerebral hemorrhage. Semin Neurol. 2016;36:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xi G, Strahle J, Hua Y, Keep RF. Progress in translational research on intracerebral hemorrhage: Is there an end in sight? Prog Neurobiol. 2014;115:45–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond MD, Ambler WG, Ai Y, Sansing LH. Alpha4 integrin is a regulator of leukocyte recruitment after experimental intracerebral hemorrhage. Stroke. 2014;45:2485–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, et al. Ccr2+ ly6c(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34:3901–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L, Cao F, Xu F, He B, Dong Z. Bexarotene reduces blood-brain barrier permeability in cerebral ischemia-reperfusion injured rats. PLoS One. 2015;10:e0122744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariani MM, Malm T, Lamb R, Jay TR, Neilson L, Casali B, et al. Neuronally-directed effects of rxr activation in a mouse model of alzheimer’s disease. Sci Rep. 2017;7:42270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J, Fu Y, Liu CC, Shinohara M, Nielsen HM, Dong Q, et al. Retinoic acid isomers facilitate apolipoprotein e production and lipidation in astrocytes through the retinoid x receptor/retinoic acid receptor pathway. J Biol Chem. 2014;289:11282–11292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landreth GE, Cramer PE, Lakner MM, Cirrito JR, Wesson DW, Brunden KR, et al. Response to comments on “apoe-directed therapeutics rapidly clear beta-amyloid and reverse deficits in ad mouse models”. Science. 2013;340:924-g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans RM, Mangelsdorf DJ. Nuclear receptors, rxr, and the big bang. Cell. 2014;157:255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific ppargamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roszer T, Menendez-Gutierrez MP, Lefterova MI, Alameda D, Nunez V, Lazar MA, et al. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid x receptor alpha deficiency. J Immunol. 2011;186:621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selim M, Foster LD, Moy CS, Xi G, Hill MD, Morgenstern LB, et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-def): A multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18:428–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (mistie iii): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393:1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura T, Hua Y, Keep RF, Park JW, Xi G, Hoff JT. Estrogen therapy for experimental intracerebral hemorrhage in rats. J Neurosurg. 2005;103:97–103 [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: Model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004;24:487–494 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.