Abstract
BACKGROUND
A growing body of research has shown that people living in neighborhoods with more severe socioeconomic deprivation may have higher risks for colorectal cancer (CRC). However, previous studies have only examined neighborhood socioeconomic status (SES) at one point in time, so it is unclear whether changes in neighborhood SES can also influence the risks of CRC.
METHODS
Cox regression were used to examine different trajectories of change in neighborhood SES over 10-years in relation to the incidence of CRC among 266,804 participants (age 51–70) in the NIH-AARP Diet and Health Study. Eligible participants reported living in the same neighborhood at baseline (1995–1996) and in 2004–06 via a follow-up questionnaire. Changes in neighborhood SES were measured between 1990 and 2000 by SES indices derived from Census data. Neighborhoods were grouped into four categories based on median SES indices in 1990 and 2000 (low-low, low-high, high-low, high-high).
RESULTS
Compared to residents whose neighborhoods were in the higher SES group at both time-points (reference), those whose neighborhoods were consistently in the low SES group had an 7% higher risk [HR (95% CI), 1.07 (1.00, 1.14)] of developing CRC. Moreover, CRC risk was 15% higher (1.15 (1.02, 1.28)) when living in neighborhoods with decreasing SES (high-low) over time.
CONCLUSIONS
Our findings suggest that exposure to consistently low SES neighborhoods and/or a decrease in neighborhood SES over a period of time may be associated with higher risks of CRC.
Keywords: Neighborhood socioeconomic status, cancer risk, colon cancer, rectal cancer, long-term trajectory
Precis
Using data from NIH-AARP cohort study, the present manuscript employed cox regression to examine the associations between changes in neighborhood SES and risk of CRC. Findings suggest that changes in SES of the neighborhoods in which they lived were associated with the risk of CRC.
Introduction
Although the overall incidence for colorectal cancer (CRC) has been declining for several decades, it remains the third most common cancer in both men and women and second leading cause of cancer death in the US. In 2018, it is projected that there will be 140,250 new cases of CRC with an estimated 50,630 deaths.1
In recent decades, there has been a growing interest in understanding the impact of neighborhood socioeconomic status (SES) on health and health disparities, including the risk of developing CRC. 2–5 Findings from earlier studies in three large cohorts, the Nurses’ Health Study, the Black Women’s Health Study and the NIH-AARP Diet and Health study, suggested that living in lower SES neighborhoods was associated with a higher incidence of cancer, including CRC.6–8 However, to the best of our knowledge, no study has examined long-term changes in neighborhood SES in relation to CRC risk.
Long-term trajectories of neighborhood exposure may have a unique impact on health outcomes. Earlier studies have suggested that long-term trajectories of neighborhood SES may be stronger predictors of health disparities than single-time measures. 9,10 Moreover, several previous studies linked long-term trajectories or changes in neighborhood conditions with CRC risk factors. For example, improvement in neighborhood safety was linked to decrease in body-mass index (BMI),11 and loss of neighborhood supermarkets were related to worsening glycemic control.12 Several studies have suggested indirectly that improved neighborhood environment, such as improved physical environment or exposure to better socioeconomic environment with moving, may affect BMI13–15. Therefore, it is plausible to hypothesize that changes in neighborhood conditions may play a role in CRC risk.
More longitudinal studies are needed to focus on changes of exposure to neighborhood environment, as such studies may help better characterize health disparities in the population and identify vulnerable groups that are at high risk of adverse health outcomes. Moreover, they may also provide evidence in support of interventions to reduce health disparities.
Our study aimed to investigate the association between changes in neighborhood SES and incidence of CRC. Using a large cohort of middle-to-old aged men and women from multiple states in the nation who lived in the same neighborhood during the study period, we examined 10-year trajectories of neighborhood SES in relation to CRC incidence over 11 years of follow up. We hypothesized that, compared to people living in long-term high SES neighborhoods, people living in neighborhoods with long-term low SES or decreasing SES would have higher risk of developing CRC. Additionally, we hypothesized that those living in neighborhoods with increasing SES would have lower risk of developing CRC.
Materials and Methods
Study Population
Participants were from the NIH-AARP Diet and Health study, which was established in 1995–1996 by recruiting AARP members (aged 50–71 years) residing in one of six U.S. states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan). Details of the study have been previously described.16 The study was approved by the National Cancer Institute Special Studies Institutional Review Board.
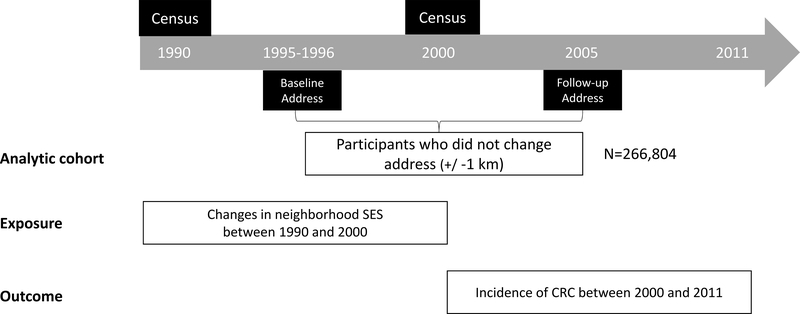
Based on information from the baseline and the follow-up (2004–2006) questionnaires, we focused our study on participants who reported living in the same neighborhood. Residential addresses were reported at the baseline and since then have been regularly updated using the National Change of Address database. In 2004, in order to prepare for mailing a follow-up questionnaire, a list of most up-to-date addresses was constructed for the entire cohort, including those who died before 2004. To determine moving status, we compared the addresses in 2004 with those at baseline and defined non-movers as those whose 2004 address was within 1km from their baseline address. Of the 566,398 participants who satisfactorily completed the baseline questionnaire, a total of 303,174 participants met these criteria and were eligible for this study. Of those, we further excluded participants who died or developed cancer before 2000 (N=34150) or those whose cancer diagnosis was not confirmed in the cancer registry (N=8737). The final analytic cohort included 160,210 men and 106,594 women. An outline of the study design is presented in Figure 1.
Figure 1:
Study Design
Neighborhood SES
Participants’ addresses were geocoded to latitude/longitude and linked to the 1990 and 2000 US census tracts. In total, 17,850 census tracts were included in our study. We generated an empirical index of neighborhood SES for both census time-points using the procedure developed by Messer et al.17 and Major et al..18 In brief, 14 census tract-variables presented in both 1990 and 2000 censuses were selected. These variables were related to seven components of the neighborhood environment, including age, education, employment status, housing characteristics, residential stability, poverty, and racial composition. Principal component analysis (PCA) were done on these variables, stratified by state, and we retained variables with consistently high loadings across states and in both census years. More specifically, a variable is retained when at least one loading was in the upper 20% of all the 224 variable loadings (>0.33) and with no loading lower than 90% (<0.06). Six variables were kept for the final analysis, including % total with less than high school, % total unemployed, % households with income below poverty, % households with an income <$22,500 (1990) or <30,000 (2000), % households on public assistance, and % households with no car. Using the retained variables, we re-ran the PCA for both cohorts in 1990 and 2000 respectively. We then used the final item loadings to weight each of the six variables’ contribution and derive the neighborhood SES index for census tracts included in our study.
To characterize trajectories of neighborhood conditions, we used median-split in both 1990 and 2000 based on the index to define low (<median) and high (≥median) neighborhood SES. We further classified the census tracts into four categories: (1) long-term high neighborhood SES (SES index ≥median in both 1990 and 2000); (2) decrease in neighborhood SES (SES index ≥ median in 1990 and SES index < median in 2000); (3) increase in neighborhood SES (SES index < median in 1990 and SES index ≥median in 2000); and (4) long-term low neighborhood SES (SES index <median in both 1990 and 2000).
CRC ascertainment
Cancer incidence were identified through cancer registry databases from the original eight locations and additional three states’ (Arizona, Nevada, and Texas) databases. Data obtained include information such as cancer diagnosed, diagnosis date, morphology code, grade, and stage information. To maintain temporal sequence between the SES exposure and CRC risk, for this analysis we examined CRC incidence between January 1, 2000 and the end of 2011. New CRC cases and date of diagnosis were obtained from participating state tumor registries using codes from the third edition of the International Classification of Diseases for Oncology (ICD-O-3, codes C180–189, C199 and C209). As shown in previous validation study, approximately 90% of cancers were identified through registry linkage 19.
Statistical analysis
Multivariable-adjusted hazard ratios (HRs) and two-sided 95% confidence intervals (CIs) were calculated using Cox proportional hazards models via the SAS PROC PHREG procedure (SAS 9.3; SAS Institute, Cary, North Carolina). As shown in earlier study, cox proportional hazard models (without prescribed frailties) yielded almost identical results as in frailty models for this cohort.20 Person-years were calculated from Jan 1, 2000 until the date of cancer diagnosis, relocation from the registry areas, death, or the end of the follow up (December 31, 2011), whichever occurred first. Clustering across census tracts were accounted for using robust variance estimation for standard error estimation. 21
In all of our models, we adjusted for potential confounders including demographic characteristics (age, sex, marital status and race/ethnicity), and education as an individual-level SES indicator. State of residence was included as random effect. In a separate model, we further considered a wide range of variables that could serve as both confounders and mediators, including lifestyle and medical history (smoking, healthy-eating index (HEI), BMI, physical activity, history of diabetes and aspirin use). Finally, because a large proportion (44%) of the baseline cohort were excluded primarily due to moving out of the neighborhood or death before 2000, we compared study characteristics between those who were included and those excluded. Although results showed that the study characteristics appeared to be largely comparable between the excluded and included groups, some differences were noted (Supplementary table 1). In addition, sensitivity analysis using inverse probability weighting to account for the potential impact of exclusions were also conducted. Results shown were also largely comparable between those who were included and those excluded.22
Results
Baseline characteristics according to the four trajectories of neighborhood SES between 1990 and 2000 are presented in Table 1. In our cohort, 43% of the respondents lived in census tracts with long-term high SES, 43.2% lived in tracts with long-term low SES, 6.9% of the respondents lived in tracts with decreased SES, and 7% lived in tracts with increased SES. Overall, when compared to participants living in neighborhoods with long-term high SES, those living in neighborhoods with decreased SES reported more current smoking, lower physical activity, and prolonged TV watching; participants living in neighborhoods with increased SES were less likely to have college degree, more likely to report current smoking, higher BMI and poor self-reported health; while participants in neighborhoods with long-term low SES were more likely to be women and non-Hispanic white, and they exhibited a less healthy lifestyle with more current smoking, lower physical activity, prolonged TV watching, less night time sleep, higher BMI, and lower HEI score.
Table 1.
Baseline (1995–96) study characteristics according to median-splits of SES index in 1990 and 2000 among 266,804 participants in the National Institutes of Health-AARP Diet and Health Study
| Neighborhood SES trajectory a | ||||
|---|---|---|---|---|
| Baseline characteristics | Long-term high SES | Increasing SES | Decreasing SES | Long-term Low SES |
| N (% of total) | 114447 (43.0) | 18579 (7.0) | 18419 (6.9) | 114940 (43.2) |
| Age, mean (SD) | 61.9 (5.4) | 62.2 (5.3) | 62.2 (5.3) | 62.3 (5.3) |
| Female, % | 36.1 | 38.4 | 41.2 | 43.8 |
| White, non-Hispanic, % | 94.0 | 94.4 | 91.5 | 85.4 |
| College and post college, % | 48.8 | 32.5 | 38.2 | 27.5 |
| Married, % | 76.2 | 72.8 | 68.8 | 65.8 |
| Current smoker, % | 9.2 | 11.2 | 11.3 | 13.2 |
| Physical activity >= 5 times/wk, % | 20.6 | 20.0 | 19.7 | 18.9 |
| TV viewing ≤ 2 hr/d, % | 24.3 | 19.5 | 30.6 | 17.9 |
| Nighttime sleep 7–8 hr/d, % | 39.6 | 37.6 | 36.9 | 34.2 |
| Body mass index, kg/m2, mean (SD) | 26.7 (4.7) | 27.1 (4.8) | 26.9 (4.9) | 27.5 (5.4) |
| alcohol consumption, g/d, mean (SD) | 13.8 (35.6) | 13.8 (39.8) | 13.0 (38.5) | 12.0 (39.4) |
| HEI-2005 total score | 67.3 (11.2) | 66.1 (11.6) | 67.0 (11.4) | 65.9 (11.7) |
| Self-reported health, excellent, % | 20.2 | 15.4 | 17.2 | 13.5 |
| Chronic conditions | ||||
| Heart disease | 12.6 | 14.2 | 13.3 | 14.1 |
| Stroke | 1.6 | 2.1 | 1.8 | 2.5 |
| Cancer | 21.9 | 21.3 | 20.8 | 20.5 |
| Diabetes | 7.2 | 8.7 | 8.6 | 10.6 |
Abbreviations: SD, standard deviation; SES, socioeconomic status; HEI, health eating index.
Trajectories were characterized based on neighborhood SES index in 1990 and 2000: long-term high SES, SES index value < median in both 1990 and 2000; increasing SES, SES index value < median in 1990 but ≥ median in 2000; decreasing SES, SES index value ≥ median in 1990 and < median in 2000; long-term low SES, SES index value ≥ median in both 1990 and 2000
We found that when compared to long-term high neighborhood SES, both long-term low neighborhood SES and a decrease in neighborhood SES were associated with a higher risk of CRC (Table 2). The patterns remained after controlling for individual-level sociodemographic factors (age, sex, marital status, education and race/ethnicity): a decrease in neighborhood SES was associated with a 15% higher risk of CRC (HR (95% CI), 1.15 (1.02, 1.28), while long-term low neighborhood SES was associated with an 7% higher risk of CRC (1.07, (1.00, 1.14). In sex-specific analyses, we found that the relationship between a decrease in neighborhood SES and a higher risk of CRC appeared to be stronger in women than in men (1.23 (1.02, 1.48) for women, 1.10 (0.95, 1.26) for men), although the p-for-interaction with sex was not statistically significant (p=0.794). Next, we further controlled for established risk factors for CRC (i.e. colonoscopy or sigmoidoscopy screening, BMI, alcohol use, smoking status, HEI score, physical activity, diabetes status. Supplementary Table 2) and found that the association between the risk of CRC and changes in neighborhood SES remained largely unchanged, especially for gender specific analysis. However, the results for long-term low neighborhood SES were attenuated and became statistically non-significant. In addition, very similar results were found after we adjusted for neighborhood SES in 1990 or used inverse weighting to control for the probability of being excluded from the analytical sample (data not shown).
Table 2.
Incidence of colorectal cancer according to neighborhood SES in 1990 and 2000
| Neighborhood SES trajectory | No. of colorectal cancer | Multivariable-adjusted HR (95% CI) a | Multivariable-adjusted HR (95% CI) b |
|---|---|---|---|
| All | |||
| Long-term high SES | 1988 | ref | ref |
| Decreasing SES | 366 | 1.16 (1.04, 1.29) | 1.15 (1.02, 1.28) |
| Increasing SES | 352 | 1.09 (0.97, 1.22) | 1.05 (0.93, 1.17) |
| Long-term low SES | 2185 | 1.12 (1.05, 1.19) | 1.07 (1.00, 1.14) |
| Women | |||
| Long-term high SES | 600 | ref | ref |
| Decreasing SES | 137 | 1.24 (1.03, 1.49) | 1.23 (1.02, 1.48) |
| Increasing SES | 117 | 1.12 (0.91, 1.37) | 1.09 (0.89, 1.33) |
| Long-term low SES | 824 | 1.12 (1.00, 1.24) | 1.07 (0.96, 1.19) |
| Men | |||
| Long-term high SES | 1388 | ref | ref |
| Decreasing SES | 229 | 1.12 (0.97, 1.29) | 1.10 (0.95, 1.26) |
| Increasing SES | 235 | 1.07 (0.94, 1.23) | 1.03 (0.89, 1.18) |
| Long-term low SES | 1361 | 1.12 (1.04, 1.21) | 1.07 (0.99, 1.16) |
Abbreviations: CI, confidence interval; HR, hazard ratio; SES, socioeconomic status.
Adjusted for age (50–<55, 55–<60, 60–<65, ≥65), for men and women, plus sex for all.
Adjusted for race/ethnicity (Non-Hispanic White, Non-Hispanic Black, other), education (<12 yrs, high school graduate, some college, college and post graduate), marital status (married, non-married) and the factors in model a. State of residence (CA, FL, GA, LA, MI, NC, NJ, PA) was included as a random effect.
We further investigated the relationship between trajectories of neighborhood SES and CRC by tumor location (Table 3). Overall, we found a significant relationship between decrease in neighborhood SES and a higher incidence of colon cancer (1.18 (1.04, 1.34), and the relationship was stronger in women (1.29 (1.05, 1.59) for women and 1.12 (0.95, 1.31) for men). The results for rectal cancer were largely null, with the exception of long-term low neighborhood SES, which was associated with an increase in rectal cancer risk in men with borderline statistical significance (1.14 (0.98, 1.32)).
Table 3.
Incidence of colon cancer and rectal cancer according to neighborhood SES in 1990 and 2000
| Neighborhood SES trajectory | Incidence of colorectal cancer |
|||
|---|---|---|---|---|
| Colon cancer | Rectal cancer | |||
| No. of cases | HR (95% CI) a | No. of cases | HR (95% CI) a | |
| ALL | ||||
| Long-term high SES | 1484 | ref | 504 | ref |
| Decreasing SES | 280 | 1.18 (1.04, 1.34) | 86 | 1.05 (0.83, 1.32) |
| Increasing SES | 265 | 1.06 (0.93, 1.22) | 87 | 0.99 (0.79, 1.25) |
| Long-term low SES | 1606 | 1.06 (0.99, 1.15) | 579 | 1.09 (0.96, 1.24) |
| WOMEN | ||||
| Long-term high SES | 458 | ref | 142 | ref |
| Decreasing SES | 110 | 1.29 (1.05, 1.59) | 27 | 1.02 (0.68, 1.54) |
| Increasing SES | 96 | 1.17 (0.94, 1.46) | 21 | 0.82 (0.52, 1.30) |
| Long-term low SES | 643 | 1.10 (0.97, 1.25) | 181 | 0.97 (0.77, 1.22) |
| MEN | ||||
| Long-term high SES | 1026 | ref | 362 | ref |
| Decreasing SES | 170 | 1.12 (0.95, 1.31) | 59 | 1.05 (0.80, 1.39) |
| Increasing SES | 169 | 1.02 (0.86, 1.20) | 66 | 1.05 (0.81, 1.37) |
| Long-term low SES | 963 | 1.05 (0.95, 1.15) | 398 | 1.14 (0.98, 1.32) |
adjusted for age (50–<55, 55–<60, 60–<65, ≥65), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, other), education (<12 yrs, high school graduate, some college, college and post graduate) and marital status (married, non-married). State of residence (CA, FL, GA, LA, MI, NC, NJ, PA) was included as a random effect.
Finally, since race/ethnicity and location play important roles in neighborhood SES and health,23,24 we further examined neighborhood SES conditions across different race/ethnicity groups (Supplementary Table 3) and different geographic locations (Supplementary Table 4). In terms of race, both White and non-White groups showed similar trends. Results for White participants were largely similar to those in the overall population, while among non-White participants, these associations appeared to be stronger despite smaller sample size: when compared to the reference group, decreasing SES and long-term low SES were associated with 63% (1.63 (1.06, 2.50)) and 35% increase in CRC risk (1.35 (1.04, 1.76)), respectively. Concerning geographical locations, most of the trends were consistent with the overall sample, however, there were a few interesting exceptions that worth noting. For example, the relationship between long-term low neighborhood SES and increased risk in CRC was only observed in Atlanta, Detroit, Louisiana, and Pennsylvania, but not in California, Florida, North Carolina, and New Jersey.
Discussion
In a large cohort of middle-to-old aged US men and women who lived in the same location for at least 10-years, we found that changes in SES of the neighborhoods in which they lived were associated with the risk of CRC. Specifically, compared to those living in neighborhoods with long-term high SES, those living in neighborhoods with consistently low SES, or neighborhoods that experienced a decrease in SES over 10-years had a higher risk of CRC. Interestingly, the higher risk associated with decreased neighborhood SES appeared to be stronger in women and for colon cancer.
To our knowledge, the current study is the first to extend what has been done in previous studies by examining the association between long-term trajectory of SES and incidence of CRC. Specifically, we found that long-term exposure to low levels of neighborhood SES was associated with a higher risk of CRC, which is consistent with previous findings using cross-sectional data from NIH-AARP study2 and Nurse’s Health Study8. Although no study has examined long-term trajectories of neighborhood SES in relation to CRC, several studies have examined its associations with other health outcomes. Kravitz-Wirtz found that growing up in neighborhoods characterized by long-term low SES was associated with worse self-rated health.25 Margerison-Zilko et al. reported that long-term high poverty in the neighborhood was associated with higher odds of preterm birth.26 In addition, Sheehan et al. showed that people living in census tracts with long-term high poverty had higher odds of being obese and had higher BMI values than those who lived in census tracts with long-term low poverty.10 Our findings, together with these studies, highlight the potential importance on examining health risks associated with exposure to long-term low neighborhood SES, which may be stronger predictors of health disparities than single-time measures.9
Another important contribution of our study is the finding of association between worsening neighborhood conditions (decrease in neighborhood SES) over time and a higher risk of CRC. A handful of studies reported an association between changes in neighborhood conditions and various physical or health outcomes. For example, Powell-Wiley and colleagues using data from the Multi-Ethnic Study of Atherosclerosis reported that improvement in neighborhood safety was linked to decreasing BMI for men.11 Moreover, Zhang et al. found that loss of neighborhood supermarkets was related to worsening glycemic control after examining the data from Kaiser Permanente Northern California Diabetes Registry.12 Although the studies mentioned above did not examine the incidence of CRC, their findings suggested that changes in neighborhood conditions may be related to important risk factors for CRC, such as obesity, cardiometabolic health and diet.27
Contrary to our original hypothesis, we found that an increase in neighborhood SES was not associated with a lower risk of CRC. One possible explanation is that for participants in our study the subsequent improvements in neighborhood conditions may be modest and the associated benefits relatively small. In other words, more drastic improvement in neighborhoods with more severe low SES at baseline may lead to larger benefits. Another possibility is that a longer latency period is needed to observe the benefit of neighborhood improvement compared to that is needed to observe the harmful effect associated with deteriorating neighborhood conditions on CRC risk. We encourage future studies to examine such possibilities in more diverse populations living in a wider range of neighborhood conditions and with a longer follow-up period.
Our stratified analysis by sex, race/ethnicity and region showed considerable heterogeneity in the association between neighborhood SES trajectories and CRC risk. First, we observed stronger associations among female as compared to male participants. Possible explanations for this gender heterogeneity might be that women are more sensitive to environmental change compared to men, and they may be more interactive with their neighborhood environment (communication with neighbors, shopping, and etc.), hence more likely to be affected by adverse neighborhood factors.28 As a result, they might be more vulnerable to stress and depression coming from the relative low SES neighborhood which might lead to CRC.29 Second, the associations for non-White groups also seemed to be stronger than those for White participants, suggesting that minority groups were particularly vulnerable to health disparities related to long-term low SES and worsening neighborhood conditions. Although no previous studies have examined the relationship between neighborhood conditions and CRC specifically in non-White populations, our results were consistent with some of the earlier studies that focused on other health outcomes. For example, in studies of patients with kidney disease, the effects of low neighborhood SES on total and cause-specific mortality were stronger in minority groups.30–32 In addition, we also showed that the associations between neighborhood trajectories and CRC risks can differ by region. Such heterogeneity could be driven by differences in environmental characteristics or different composition of population across different regions, and it may also reflect variations in Medicare, Medicaid, Temporary Assistance for Needy Families (TNAF) and other safety net policies by states. Future studies are needed to pinpoint the sources of heterogeneity to better understand the factors that may influence the effects of neighborhood conditions on CRC risk.
One unique strength of our study is the examination of long-term trajectories of exposure to neighborhood environment on incidence of CRC. Our study seems to suggest that policy makers should consider allocating recourses to help neighborhoods experiencing long-term low SES and neighborhoods with a deteriorating trend in SES. Another strength of our study is the use of longitudinal data with large numbers of participants and neighborhoods. Like many forms of solid cancers, CRC has a relatively long latency period. We were able to benefit from the relatively long follow-up time in the NIH-AARP Diet and Health Study, which allowed us to better evaluate the long-term impacts of neighborhood conditions, although as mentioned before, even longer follow-up may be needed to study the benefit associated with improving neighborhood conditions.
One limitation of the current study is that the cohort was predominantly Caucasian, and most of the study participants resided in relatively high SES neighborhoods. Earlier studies have shown that race/ethnicity plays an important role in modifying cancer disparities and may modify the effects of neighborhood SES or other contextual-level variables on CRC.31–34 As a result, our findings might not be generalizable to other populations, such as those who are non-White, younger or living in relatively lower SES neighborhoods. We encourage future studies to utilize cohorts with more diverse racial/ethnic and socioeconomic backgrounds to examine the relationship between long-term neighborhood trajectories and CRC incidence disparities. Another limitation is that we only had residential addresses at two different time-points (baseline and 2004), ten years apart. As a result, we were not able to distinguish participants who lived in the same neighborhood all the time across the ten-year period from those who had moved out of and back in the same neighborhood during this period. The former group were more likely to experience gradual change in the neighborhood environment, while the latter group may have experienced more sudden and dramatic changes through their moving processes. Our study cannot distinguish between these two groups and future studies with more detailed residential histories are highly encouraged to address this issue. In addition, we did not account for change in census tract boundaries. Although census tracts are relatively stable, changes in boundaries did happen in a small fraction of tracts. Such changes may also lead to misclassifications of neighborhood trajectories. Meanwhile, we may not have enough time to detect the changes. Since conditions in most neighborhoods tend to be stable over a period of time and it may take a long lag time for neighborhood effects on CRC to emerge, future studies should require large populations, geographically diverse neighborhoods and a sufficiently long follow up. Finally, we lacked information on personal income and individual-level SES indicators other than educational attainment at baseline, so we were unable to assess SES trajectories at individual-level. It would be interesting to assess whether SES experienced at the neighborhood- and individual-level may have an independent effect on the incidence of CRC.
In conclusion, our findings suggested that residents of neighborhoods with long-term low SES or a decrease in SES may be at higher risk of CRC. We encourage further research to examine the effects of long-term neighborhood SES on CRC risk in more diverse populations and to identify the underlying mechanisms.
Supplementary Material
Acknowledgments
Grant sponsor: Cancer Center Support Grant (P30 CA086862) from Cancer Epidemiology Population Science Program of the Holder Comprehensive Cancer Center
Footnotes
Conflict of interest: Authors have no potential conflicts of interest.
Contributor Information
Dong Zhang, Department of Health and Human Physiology, University of Iowa.
Charles E. Matthews, Department of Cancer Epidemiology and Genetics, National Cancer Institute
Tiffany M. Powell-Wiley, National Heart, Lung, and Blood Institute; National Institute on Minority Health and Health Disparities
Qian Xiao, Department of Health and Human Physiology, Department of Epidemiology, University of Iowa
Reference
- 1.American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 2.Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer. Cancer. 2012;118(14):3636–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu X, Pawlish KS, Roche LM. Cancer survival disparities by race/ethnicity and socioeconomic status in New Jersey. Journal of health care for the poor and underserved. 2010;21(1):144–160. [DOI] [PubMed] [Google Scholar]
- 4.Manser CN, Bauerfeind P. Impact of socioeconomic status on incidence, mortality, and survival of colorectal cancer patients: a systematic review. Gastrointestinal endoscopy. 2014;80(1):42–60. e49. [DOI] [PubMed] [Google Scholar]
- 5.Doubeni CA, Major JM, Laiyemo AO, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. Journal of the National Cancer Institute. 2012;104(18):1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bethea TN, Palmer JR, Rosenberg L, Cozier YC. Neighborhood socioeconomic status in relation to all-cause, cancer, and cardiovascular mortality in the Black Women’s Health Study. Ethnicity & disease. 2016;26(2):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian M, Schootman M, Doubeni CA, et al. Geographic variation in colorectal cancer survival and the role of small-area socioeconomic deprivation: a multilevel survival analysis of the NIH-AARP Diet and Health Study Cohort. American journal of epidemiology. 2011;174(7):828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D, Masyn KE, Kawachi I, Laden F, Colditz GA. Neighborhood socioeconomic status and behavioral pathways to risks of colon and rectal cancer in women. Cancer. 2010;116(17):4187–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do DP. The dynamics of income and neighborhood context for population health: do long-term measures of socioeconomic status explain more of the black/white health disparity than single-point-in-time measures? Social science & medicine. 2009;68(8):1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheehan CM, Cantu PA, Powers DA, Margerison-Zilko CE, Cubbin C. Long-term neighborhood poverty trajectories and obesity in a sample of california mothers. Health & place. 2017;46:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell-Wiley TM, Moore K, Allen N, et al. Associations of Neighborhood Crime and Safety and with Changes in Body Mass Index and Waist Circumference: The Multi-Ethnic Study of Atherosclerosis. American journal of epidemiology. 2017;186(3):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YT, Mujahid MS, Laraia BA, et al. Association between neighborhood supermarket presence and glycated hemoglobin levels among patients with type 2 diabetes mellitus. American journal of epidemiology. 2017;185(12):1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell-Wiley TM, Cooper-McCann R, Ayers C, et al. Change in neighborhood socioeconomic status and weight gain: Dallas Heart Study. American journal of preventive medicine. 2015;49(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch JA, Moore KA, Clarke PJ, et al. Changes in the built environment and changes in the amount of walking over time: longitudinal results from the multi-ethnic study of atherosclerosis. American journal of epidemiology. 2014;180(8):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrientos-Gutierrez T, Moore KA, Auchincloss AH, et al. Neighborhood physical environment and changes in body mass index: results from the Multi-Ethnic Study of Atherosclerosis. American journal of epidemiology. 2017;186(11):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. American journal of epidemiology. 2001;154(12):1119–1125. [DOI] [PubMed] [Google Scholar]
- 17.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. Journal of Urban Health. 2006;83(6):1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Major JM, Doubeni CA, Freedman ND, et al. Neighborhood socioeconomic deprivation and mortality: NIH-AARP diet and health study. PLoS One. 2010;5(11):e15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaud DS MD, Hermansen S, Leitzmann M, Harlan L, Kipnis V, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J of Registry Manage. 2005;32(2):70–75. [Google Scholar]
- 20.Doubeni CA, Schootman M, Major JM, et al. Health status, neighborhood socioeconomic context, and premature mortality in the United States: the National Institutes of Health–AARP Diet and Health Study. American journal of public health. 2012;102(4):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied logistic regression. Vol 398: John Wiley & Sons; 2013. [Google Scholar]
- 22.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Statistical methods in medical research. 2013;22(3):278–295. [DOI] [PubMed] [Google Scholar]
- 23.Williams DR, Jackson PB. Social sources of racial disparities in health. Health affairs. 2005;24(2):325–334. [DOI] [PubMed] [Google Scholar]
- 24.Robert SA, Reither EN. A multilevel analysis of race, community disadvantage, and body mass index among adults in the US. Social science & medicine. 2004;59(12):2421–2434. [DOI] [PubMed] [Google Scholar]
- 25.Kravitz-Wirtz N Cumulative effects of growing up in separate and unequal neighborhoods on racial disparities in self-rated health in early adulthood. Journal of health and social behavior. 2016;57(4):453–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margerison-Zilko C, Cubbin C, Jun J, Marchi K, Fingar K, Braveman P. Beyond the cross-sectional: neighborhood poverty histories and preterm birth. American journal of public health. 2015;105(6):1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DH, Keum N, Giovannucci EL. Colorectal cancer epidemiology in the nurses’ health study. American journal of public health. 2016;106(9):1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stafford M, Cummins S, Macintyre S, Ellaway A, Marmot M. Gender differences in the associations between health and neighbourhood environment. Social science & medicine. 2005;60(8):1681–1692. [DOI] [PubMed] [Google Scholar]
- 29.Bassett E, Moore S. Gender differences in the social pathways linking neighborhood disadvantage to depressive symptoms in adults. PloS one. 2013;8(10):e76554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johns TS, Estrella MM, Crews DC, et al. Neighborhood socioeconomic status, race, and mortality in young adult dialysis patients. Journal of the American Society of Nephrology. 2014:ASN. 2013111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. American journal of kidney diseases. 2010;55(6):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkova N, McClellan W, Klein M, et al. Neighborhood poverty and racial differences in ESRD incidence. Journal of the American Society of Nephrology. 2008;19(2):356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24(9):1342–1349. [DOI] [PubMed] [Google Scholar]
- 34.Albain KS, Unger JM, Crowley JJ, Coltman CA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. JNCI: Journal of the National Cancer Institute. 2009;101(14):984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.