Abstract
Background:
Few studies have assessed the duration of humoral immunity following yellow fever (YF) vaccination in a non-endemic population. We evaluated seropositivity among US resident travellers based on time post-vaccination.
Methods:
We identified serum samples from US travellers with YF virus-specific plaque reduction neutralization testing (PRNT) performed at CDC from 1988 to 2016. Analyses were conducted to assess the effect of time since vaccination on neutralizing antibody titer counts.
Results:
Among 234 travellers who had neutralizing antibody testing performed on a specimen obtained ≥1 month after vaccination, 13 received multiple YF vaccinations and 221 had one dose of YF vaccine reported. All 13 who received more than one dose of YF vaccine had a positive PRNT regardless of the amount time since most recent vaccination. Among the 221 travellers with one reported dose of YF vaccine, 155 (70%) were vaccinated within 10 years (range 1 month–9 years) and 66 (30%) were vaccinated ≥10 years (range 10–53 years) prior to serum collection. Among the 155 individuals vaccinated, <10 years prior to serum collection, 146 (94%) had a positive PRNT compared with 82% (54/66) of individuals vaccinated ≥10 years prior to serum collection (P = 0.01). Post-vaccination PRNT titers showed a time-dependent decrease. Individuals with immunocompromising conditions were less likely to have a positive PRNT (77%) compared with those who were not immunocompromised (92%; P = 0.04).
Conclusion:
Although the percentage of vaccinees with a positive PRNT and antibody titers decreased over time, a single dose of YF vaccine provided long-lasting protection in the majority of US travellers. A booster dose could be considered for certain travellers who are planning travel to a high risk area based on immune competence and time since vaccination.
Keywords: Yellow fever, vaccination, antibodies
Introduction
Yellow fever (YF) is a mosquito-borne viral disease that is endemic to sub-Saharan Africa and tropical South America. Clinical disease ranges from a mild, undifferentiated febrile illness to severe disease with jaundice and haemorrhage. The case-fatality rate for severe YF is 30–60%.1,2 Because no specific treatment exists for YF, prevention is critical to reduce disease morbidity and mortality. The most effective measure to prevent for YF is vaccination.
YF vaccine is recommended for persons aged ≥9 months who are travelling to or living in areas with risk for YF virus transmission.3 In addition, International Health Regulations allow countries to require proof of YF vaccination for travellers entering their country.4 These requirements are intended to minimize the potential importation and spread of YF virus. Proof of YF vaccination is recorded on the International Certificate of Vaccination or Prophylaxis (i.e. yellow card).
From 1970 through 2015, a total of 11 cases of YF were reported in travellers from the USA and Europe who travelled to West Africa or South America.1 Only one traveller had a documented history of YF vaccination; that patient survived. Starting in 2016, the number of traveller-associated YF cases increased substantially, primarily due to outbreaks in Angola and Brazil. From 2016 through mid-2018, more than 35 travel-associated cases have been reported in unvaccinated travellers who were residents of non-endemic areas or countries, including at least 13 European travellers and one American traveller to Peru.5–12
From 1965 through 2016, International Health Regulations considered a dose of YF vaccine to provide protection for 10 years. This interval was established based on limited evidence, and more recent studies suggest immunity is longer lasting.13–15 In 2013, the World Health Organization Strategic Advisory Group of Experts (SAGE) on immunization concluded that a single dose of YF vaccine is sufficient to confer sustained immunity and lifelong protection against YF disease, and a booster dose of the vaccine is not needed.9 This conclusion was based on a systematic review of published studies on the duration of immunity following a single dose of YF vaccine, and on data that suggest vaccine failures are extremely rare and do not increase in frequency with time since vaccination.15–28 In 2014, the World Health Assembly adopted the recommendation to remove the 10-year booster dose requirement from revised International Health Regulations, which was enacted in 2016.29 Based on the available data, the US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) also concluded that a single primary dose of YF vaccine provides long-lasting protection and is adequate for most travellers. Both SAGE and ACIP noted that further data were needed on the long-term immunogenicity in certain groups such as children and HIV-infected individuals. ACIP currently recommends a booster dose for selected individuals who might not have as robust or sustained immune response to YF vaccine or who are at increased risk for YF disease. We evaluated YF neutralizing antibody titers of US travellers based on time post-vaccination, and assessed the impact of factors (e.g. number of vaccine doses received, age and immune status) on titer levels.
Methods
Data collection
We retrospectively identified serum samples previously tested by YF virus-specific plaque reduction neutralization test (PRNT) at CDC from 1988 through 2016. Neutralizing antibody titers were determined using PRNT90, which is the reciprocal of the endpoint serum dilution that reduces the challenge virus plaque count by 90%. Testing methodology was consistent over the included time period though potentially included a few different passage levels of the viral seed used in the assay. Although serological correlates of protection for YF have not been established, the presence of neutralizing antibodies has been used as a surrogate to indicate a protective immune response.18–20
Overall, a total of 2004 specimens from 1224 individuals had PRNT at CDC. If multiple samples were submitted for an individual, only the most recent sample (i.e. the sample furthest from vaccination) was included in the analysis. Because we wanted to limit our analysis to US residents receiving YF vaccine for travel, we excluded 90 specimens collected from non-US residents (i.e. submitted from another country or from individuals noted to reside in another country), 356 specimens collected during research studies and 116 specimens collected for YF vaccine adverse event investigations. The time period from last vaccination to serum collection was calculated for each sample. If the date of vaccination was not provided on the test request form, we excluded the specimen (n = 398). If the specimen collection date was missing on the test request form, the date of specimen received at CDC was used as a surrogate. Thirty samples collected within 1 month of vaccination were excluded as there might not have been enough time elapsed to produce antibodies. Samples were considered to have originated from an immunocompromised traveller if the test request form submitted with the sample noted that the person was infected with HIV, had received an organ transplant within 10 years, or was specifically noted to be immunocompromised.
Data analysis
Categorical variables were described as counts and proportions and compared using Fisher’s exact test. Continuous variables were described as medians and ranges and compared using the Wilcoxon rank sum test. To assess the effect of time since vaccination on neutralizing antibody titers, data were analyzed using a hurdle model, which models the probability of a person having a positive neutralizing antibody titer and the level of the neutralizing antibody titer among those for whom it is positive. The binary part of the hurdle model used logistic regression to model the probability that a traveller would have a positive YF PRNT (titer ≥10) as a linear function of time since vaccination. For those with positive neutralizing antibody titers, the second part of the hurdle model used zero-truncated Poisson regression to model the expected magnitude of titers as a function of time since vaccination. Bootstrapping was used to calculate 95% confidence intervals (CI) for the estimates. The model was summarized graphically, with bootstrapped 95% pointwise confidence bands. Statistical analyses were conducted using SAS® version 9.3 (SAS Institute, Cary, North Carolina) and the ‘countreg’ and ‘boot’ packages in R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
We identified 234 US resident travellers with YF virus-specific PRNTs performed ≥1 month after vaccination. Of these, 120 (51%) were collected from females, 107 (46%) from males and 7 (3%) were from persons of unknown sex. Of the 206 travellers with known age, the median age was 44 years (range 2–87 years). Overall, 221 (94%) individuals were noted to have one previous vaccination, 12 (5%) had received two doses of YF vaccine, and 1 (<1%) had received three doses of YF vaccine. Among the 13 individuals with multiple YF vaccinations, 100% had a positive PRNT regardless of the time since most recent vaccination [median 17 years prior to testing (range 5 months–26 years)]. We focused the remainder of our analysis on the 221 individuals who reportedly received only one previous vaccination.
Of the 221 travellers with only one previous vaccination, 155 (70%) were vaccinated <10 years prior to serum collection (median 4 months, interquartile range [IQR] 2 months–3 years) and 66 (30%) were vaccinated ≥10 years prior to serum collection (median 15 years, IQR 12–25 years). Individuals with YF vaccinations ≥10 years prior were significantly older and more likely to be immunocompromised (Table 1). Among the 155 individuals whose last vaccination was <10 years prior to serum collection, 146 (94%) had a positive PRNT, compared with 54 (82%) of the 66 individuals who had received YF vaccination ≥10 years prior (P = 0.01, Table 1). Post-vaccination neutralizing antibody titers showed a significant time-dependent decrease (Table 2, Figure 1). Both parts of the hurdle model showed a statistically significant decrease in YF neutralizing antibody titers over time with the odds of having a positive PRNT decreasing by a factor of 0.95 (95% CI: 0.92–0.99) and the estimated positive antibody titers decrease by a factor of 0.97 (95% CI: 0.96–0.98) with each year post-vaccination. Among persons who received YF vaccine ≥10 years before serum collection, there was no difference in the proportion with a positive PRNT by age or sex.
Table 1.
Characteristics of US travellers with one reported dose of yellow fever vaccine and yellow fever virus plaque reduction neutralization test (PRNT) results by time since last vaccination
| Vaccination <10 years prior to serum collection N = 155 No. (%) |
Vaccination ≥10 years prior to serum collection N = 66 No. (%) |
P-valuea | |
|---|---|---|---|
| Femaleb | 80 (52) | 35 (53) | 0.88 |
| Median agec (range) | 37 (2–78) | 57 (14–77) | <0.01d |
| Immunosuppressione | 8(5) | 14 (21) | <0.01 |
| YF titer ≥1:10f | 146 (94) | 54 (82) | 0.01 |
Fisher exact unless otherwise noted.
Sex missing for five individuals.
Age missing for 26 individuals.
Wilcoxon rank sum.
Travellers were considered immunosuppressed if infected with HIV, had received an organ transplant within 10 years, or was specifically noted to be immunosuppressed on the test request form.
Titers ≥1:10 are considered positive using PRNT with a 90% cutoff.
Table 2.
Yellow fever neutralization titers among US travellers with one reported dose of vaccine by years post-vaccination
| Years post-vaccination | Seropositivea/tested | (%) | Geometric mean titer of reactive sera |
|---|---|---|---|
| 0–4 | 128/136 | 94 | 298 |
| 5–9 | 18/19 | 95 | 84 |
| 10 −19 | 34/42 | 81 | 62 |
| 20–29 | 13/16 | 81 | 58 |
| >30b | 7/8 | 88 | 24 |
Titers ≥1:10 are considered positive using plaque reduction neutralization test with 90% cutoff.
Maximum years post-vaccination was 53 years.
Figure 1.
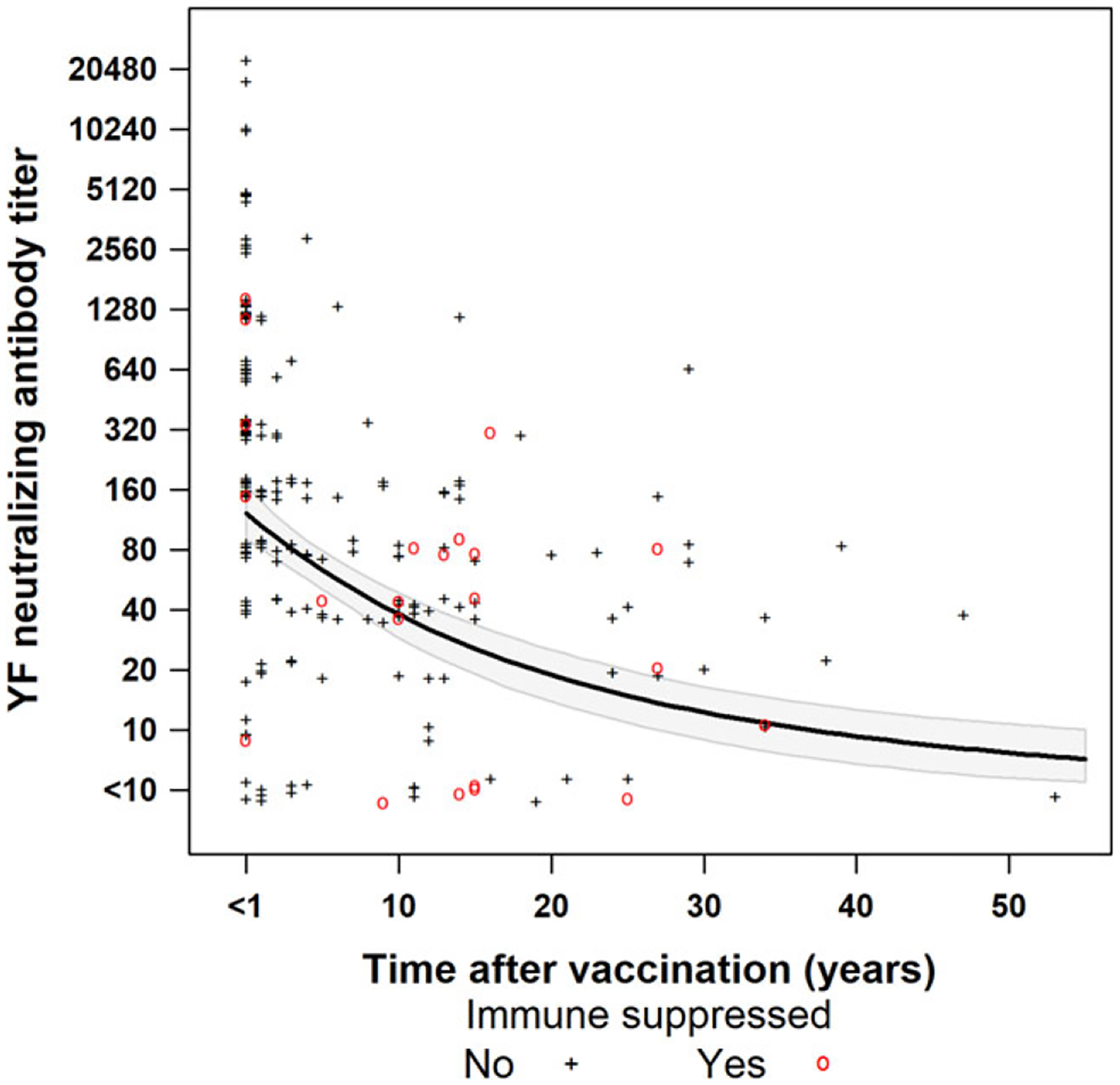
Yellow fever neutralizing antibody titers* among US travellers with one reported dose of yellow fever vaccine by years post-vaccination. The black line shows the expected titers computed from the two-part hurdle model with the 95% confidence bands shaded in grey. Data are jittered about the y-axis for visibility. *Titers determined by plaque reduction neutralization with a 90% cutoff.
There were 22 individuals noted to have an immunocompromising condition (nine taking immune suppressive medications or therapies, seven infected with HIV and six for whom the specific reason for immune compromise was not noted) who received their dose of YF vaccine a median of 13 years prior (range: 1 month–34 years). Overall, individuals with immunocompromising conditions were less likely to have a positive PRNT (77%; n = 17/22) compared with those who were not immunocompromised (92%; n = 183/199; P = 0.04). Finally, if the immunocompromised individuals were excluded, the difference in seropositivity between those receiving one dose <10 years ago (139/147; 95%) and one dose ≥10 years prior (44/52, 85%) remained significant (P = 0.04).
Discussion
Estimating the duration of immunity after YF vaccination can help determine the need for and timing of re-vaccination for travellers. Our analysis shows that a single dose of YF vaccine provides long-lasting protection for most US resident travellers; however, the proportion of persons with detectable antibodies decreased with increasing time since vaccination, as have been reported in other studies.30 This decrease was not observed among the small number of persons who had previously received two or more YF vaccinations; all of whom had a positive PRNT regardless of the time since most recent vaccination.
There have been a number of previous studies on the duration of immunity following a single dose of YF vaccine in non-endemic populations, but most included small numbers of vaccinees and some included only military members, who are more likely to be young, healthy men than the general population of travellers and might be more likely to be exposed to YF during service and therefore have a natural booster. Poland reported that neutralizing antibodies persisted for 30–34 years in 91 (78%) of 116 World War II veterans.18 Another retrospective study of 24 Marine and Navy personnel, of whom nine had received at least two doses of YF vaccine, found that 100% had neutralizing antibodies detected by log neutralization index after 16–19 years.17 Reinhardt et al. reported that 5/5 (100%) of persons had neutralizing antibodies ≥10 years after last vaccination.19 A study of German vaccinees reported neutralizing antibody titers in 38 (75%) of 51 persons tested 11–38 years after immunization.20 Another study reported neutralizing antibodies in 80 (95%) of 84 persons aged >60 years in France who were vaccinated 11–60 years previously.23 Methodological differences across studies, such as the included population, assays used (e.g. PRNT80 and mouse protection assay) and criteria for determining seropositivity, likely contributed to the variability among the reported results. Although these studies generally support sustained immunity following YF vaccine, there have been concerns noted about whether a single dose of YF vaccine is adequate.31–35 However, if the current studies are taken together, the overall long-term neutralizing antibody rate is 85% (238/280), which is comparable to the proportion we report here.
In this study, 94% of persons receiving YF vaccine within the last 10 years were seropositive. This is slightly lower than the estimates from clinical trials where 99% seropositivity rates were typical within 28 days of vaccination.1 One explanation for this difference could be the high PRNT cutoff (90%) used in the CDC Arbovirus Diagnostic Laboratory as part of the diagnostic algorithm. This test and cutoff has a lower sensitivity that those typically used in clinical trials. However, the 94% seropositivity rate might also represent the expected seroresponse rate among a more diverse population with antibody titers measured 1 month–10 years post-vaccination compared with traditionally healthy volunteers included in clinical trials where antibodies are typically measured one month post-vaccination.
The proportion of immunocompromised patients with detectable antibodies was lower than the proportion among persons without such conditions. This observation has been noted previously for persons with HIV and with other live viral vaccines in hematopoietic stem cell transplant recipients.36–37 Overall these findings suggest that re-vaccination of these individuals might be considered if their condition is not a contraindication to YF vaccination. ACIP currently recommends (1) persons who are HIV-infected should be revaccinated every 10 years if they continue to be at risk for YF virus infection; and (2) persons who received a hematopoietic stem cell transplant after receiving a dose of YF vaccine and who are sufficiently immunocompetent to be safely vaccinated should be revaccinated before their next travel to a YF risk area.
There are multiple limitations of this analysis. First, the data included in the analysis were limited to persons whose samples were submitted to CDC for testing and their vaccination date was provided. These individuals may not be representative of all US travellers or travellers from other areas. The factors that might have influenced specimen submission to CDC for testing (traveller age, medical conditions, concerns about a prior vaccination) are those that might decrease seropositivity rates. Therefore, we might expect that the seropositivity seen among this group underestimates that of the larger population of travellers. The available data did not include history of travel to or residence in YF-endemic areas and, therefore, did not account for potential re-exposure to YF virus, which could have positively influenced the seropositivity rate. Complete medical histories were also not available for patients, so the number of previous vaccinations, immune status, co-administration of other vaccines, and other variables could not be definitively ascertained and could impact the precision of the estimate of seroprotection. Additionally, some proportion of those individuals that were seronegative >10 years post-vaccination could have been primary non-responders to vaccination. Although we did have more than one sample collected over time on a limited number of the individuals included here, none of the individuals who were seronegative >10 years post-vaccination had more than one sample collected that would allow us to assess non-response. Due to the number of individuals included in this dataset, we were unable to adjust for age. Persons ≥10 years post-vaccination were significantly older than those <10 years, which could have impacted their immune response, as has been documented by others.38 Additionally, older persons in our cohort might have developed a precaution or contraindication to receiving a booster dose of the vaccine (i.e. why their sample was sent for testing prior to the change in the booster dose recommendation in 2015), which could have negatively impacted their long-term immunity. Data on timing of the immunocompromising condition was not available and patients may not have been immunocompromised at the time of their initial vaccination. Variable types of immunocompromising conditions were grouped together and might have different effects on immune response. Using a PRNT90 is a conservative approach as it likely underestimates those who might still have a detectable titer. In addition, it is unclear for those individuals who have an undetectable titer if they are truly not protected as other components of the immune system (e.g. CD8 T cells) contribute to long-term immunity.39 Finally, there were relatively few samples available to include in the analysis for some of the subsets (e.g. underlying medical conditions) under investigation which might have limited our ability to determine potential factors associated with waning long-term immunity.
These data support previous findings that a single dose of YF vaccine provides long-lasting protection and is adequate for the majority of travellers. However, a booster dose of vaccine could be considered for certain travellers whose immunologic response to the vaccine might be suboptimal or who received their last dose of YF vaccine at least 10 years previously and will be in a higher-risk setting based on season, location, activities, and duration of their travel.40–41 Additionally, for travellers with no financial barriers or medical contraindications or precautions, YF re-vaccination is likely to be an attractive option for decreasing risk of a life-threatening infection. Additional research is needed to further assess the long-term immunity following YF vaccine in certain populations whose immune response to their primary vaccination might wane more rapidly or be less robust.
Acknowledgements
We would like to thank previous staff of the Arboviral Disease Diagnostic Laboratory and Mark Delorey for their contributions to this work.
Footnotes
Conflict of interest: None declared.
References
- 1.Staples JE, Monath TP, Gershman MD, Barrett AD. Yellow fever vaccines. In: Plotkin’s Vaccines 7th edition Philadelphia, PA: Saunders Elsevier, 2017. [Google Scholar]
- 2.Johansson MA, Vasconcelos PF, Staples JE. The whole iceberg: estimating the incidence of yellow fever virus infection from the number of severe cases. Trans R Soc Trop Med Hyg 2014; 108:482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2010; 59:1–27. [PubMed] [Google Scholar]
- 4.World Health Organization. International Health Regulations, 3rd edn Geneva Switzerland: World Health Organization Press, 2005. Available at http://www.who.int/ihr/publications/9789241580496/en/ (17 July 2017, date last accessed). [Google Scholar]
- 5.Hamer DH, Angelo K, Caumes E et al. Fatal yellow fever in travelers to Brazil, 2018. MMWR Morb Mortal Wkly Rep 2018; 67:340–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman AP, Becraft R, Dean AB et al. Notes from the field: fatal yellow fever in a traveler returning from Peru—New York, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:914–5. September 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Yellow fever among travellers returning from South America—14 March 2017 Stockholm, Sweden: European Centre for Disease Prevention and Control, 2017. Available at: https://ecdc.europa.eu/en/publications/Publications/14-03-2017-RRA-Yellow%20fever,%20Flaviviridae-Suriname,%20Southern%20America.pdf (16 October 2018, date last accessed). [Google Scholar]
- 8.Wang L, Zhou P, Fu X et al. Yellow fever virus: increasing imported cases in China. J Infect 2016; 73:377–80. [DOI] [PubMed] [Google Scholar]
- 9.Gossner CM, Haussig JM, de Bellegarde de Saint Lary C, Kaasik Aaslav K, Schlagenhauf P, Sudre B. Increased risk of yellow fever infections among unvaccinated European travellers due to ongoing outbreak in Brazil, July 2017 to March 2018. Euro Surveill 2018; 23:pii=18–00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliosi E, Serero Corcos C, Barroso PF et al. Yellow fever in two unvaccinated French tourists to Brazil, January and March, 2018. Euro Surveill 2018; 23:1800240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanna A, Andrieu A, Carvalho L et al. Yellow fever cases in French Guiana, evidence of an active circulation in the Guiana Shield, 2017 and 2018. Euro Surveill 2018; 23:1800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan American Health Organization. Epidemiological Update: Yellow Fever. 20 March 2018. Available at: https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=yellow-fever-2194&alias=44111-20-march-2018-yellow-fever-epidemiological-update-111&Itemid=270&lang=en (16 October 2018, date last accessed).
- 13.Courtois G [Duration of immunity following yellow fever vaccination.]. Ann Soc Belg Med Trop 1954; 34:9–12. [PubMed] [Google Scholar]
- 14.Dick GWA, Gee FL. Immunity to yellow fever nine years after vaccination. Trans Roy Soc Trop Med Hyg 1952; 46:449–58. [DOI] [PubMed] [Google Scholar]
- 15.Gotuzzo E, Yactayo S, Cordova E. Review article: efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg 2013; 89:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groot H, Riberiro RB. Neutralizing and haemagglutinationinhibiting antibodies to yellow fever 17 years after vaccination with 17D vaccine. Bull World Health Organ 1962; 27:699–707. [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenzweig EC, Babione RW, Wisseman CL Jr. Immunological studies with group B arthropod-borne viruses. IV. Persistence of yellow fever antibodies following vaccination with 17D strain yellow fever vaccine. Am J Trop Med Hyg 1963; 12:230–5. [PubMed] [Google Scholar]
- 18.Poland JD, Calisher CH, Monath TP et al. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull World Health Organ 1981; 59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhardt B, Jaspert R, Niedrig M et al. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol 1998; 56:159–67. [DOI] [PubMed] [Google Scholar]
- 20.Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health 1999; 4:867–71. [DOI] [PubMed] [Google Scholar]
- 21.Gomez SY, Ocazionez RE. [Yellow fever virus 17D neutralizing antibodies in vaccinated Colombian people and unvaccinated ones having immunity against dengue.]. Rev Salud Publica (Bogota) 2008; 10:796–807. [DOI] [PubMed] [Google Scholar]
- 22.de Melo AB, da Silva Mda P, Magalhães MC et al. Description of a prospective 17DD yellow fever vaccine cohort in Recife, Brazil. Am J Trop Med Hyg 2011; 85:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulange Bodilis H, Benabdelmoumen G, Gergely A et al. Long-term persistence of yellow fever neutralizing antibodies in persons aged 60 years and older. Bull Soc Pathol Exot 2011; 104:260–5. [DOI] [PubMed] [Google Scholar]
- 24.Elliott M Yellow fever in the recently inoculated. Trans Roy Soc Trop Med Hyg 1944; 38:231–4. [Google Scholar]
- 25.Ross RW, Haddow AJ, Raper AB, Trowell HC. A fatal case of yellow fever in a European in Uganda. East Afr Med J 1953; 30:1–11. [PubMed] [Google Scholar]
- 26.Nolla-Salas J, Saballs-Radresa J, Bada JL. Imported yellow fever in vaccinated tourist. Lancet 1989; 334:1275. [DOI] [PubMed] [Google Scholar]
- 27.Tuboi SH, Costa ZG, da Costa Vasconcelos PF, Hatch D. Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998–2002. Trans Roy Soc Trop Med Hyg 2007; 101:169–75. [DOI] [PubMed] [Google Scholar]
- 28.de Filippis AM, Nogueira RMR, Jabor AV et al. Isolation and characterization of wild type yellow fever in cases temporally associated with 17DD vaccination during an outbreak of yellow fever in Brazil. Vaccine 2004; 22:1073–8. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Vaccines and vaccination against yellow fever: WHO Position Paper—June 2013. Wkly Epidemiol Rec 2013; 88:269–83. [PubMed] [Google Scholar]
- 30.Collaborative group for studies on yellow fever vaccines. Duration of post-vaccination immunity against yellow fever in adults. Vaccine 2014; 32:4977–84. [DOI] [PubMed] [Google Scholar]
- 31.Amanna IJ, Slifka MK. Questions regarding the safety and duration of immunity following live yellow fever vaccination. Expert Rev Vaccines 2016; 15:1519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasconcelos PF. Single shot of 17D vaccine may not confer life-long protection against yellow fever. Mem Inst Oswaldo Cruz 2018; 113: 135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campi-Azevedo AC, Costa-Pereira C, Antonelli LR et al. Booster dose after 10 years is recommended following 17DD-YF primary vaccination. Hum Vaccin Immunother 2016; 12:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grobusch MP, Goorhuis A, Wieten RW et al. Yellow fever revaccination guidelines change—a decision too feverish? Clin Microbiol Infect 2013; 19:885–6. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. International and Traveler Health: World – Yellow fever vaccination booster. Available at http://www.who.int/ith/updates/20140605/en/ (2 February 2015, date last accessed).
- 36.Rubin LG, Levin MJ, Ljungman P et al. Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–100. [DOI] [PubMed] [Google Scholar]
- 37.Veit O, Niedrig M, Chapuis-Taillard C et al. Swiss HIV Cohort Study. Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clin Infect Dis 2009; 48:659–6. [DOI] [PubMed] [Google Scholar]
- 38.Roukens AH, Soonawala D, Joosten SA et al. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PLoS One 2011; 6:e27753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed R, Akondy RS. Insights into human CD8 (+) T-cell memory using the yellow fever and smallpox vaccines. Immunol Cell Biol 2011; 89:340–5. [DOI] [PubMed] [Google Scholar]
- 40.Staples JE, Bocchini JA Jr, Rubin L, Fischer M. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine booster doses: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:647–50. [PMC free article] [PubMed] [Google Scholar]
- 41.Advisory Committee on Immunization Practices. Grading of Recommendations, Assessment, Development, and Evaluation for Use of Yellow Fever Vaccine Booster Doses. Available at: https://www.cdc.gov/vaccines/acip/recs/grade/yf-vac-boost.html (16 October 2018, date last accessed).


