Abstract
Radiologic characterization of pancreatic lesions is currently limited. Computed tomography is insensitive in detecting and characterizing small pancreatic lesions. Moreover, heterogeneity of many pancreatic lesions makes determination of malignancy challenging. As a result, invasive diagnostic testing is frequently used to characterize pancreatic lesions but often yields indeterminate results. Computed tomography texture analysis (CTTA) is an emerging non-invasive computational tool that quantifies grey-scale pixels/voxels and their spatial relationships within a region of interest. In non-pancreatic lesions, CTTA has shown promise in diagnosis, lesion characterization, and risk stratification, and, more recently, pancreatic applications of CTTA have been explored. This review outlines the emerging role of CTTA in identifying, characterizing, and risk stratifying pancreatic lesions. While recent studies show clinical potential for CTTA of the pancreas, a clear understanding of which specific texture features correlate to high-grade dysplasia and predict survival has not yet been achieved. Further multidisciplinary investigations using strong radiologic-pathologic correlation are needed to establish a role for this non-invasive diagnostic tool.
Keywords: Texture analysis, imaging, pancreas, pancreatic cysts, pancreatic ductal adenocarcinoma (PDAC), pancreatic neuroendocrine tumor (PNET)
Introduction
Incidental detection of pancreatic lesions is increasing in frequency.1 Determining an accurate diagnosis of pancreatic lesions is crucial as medical and surgical management differ significantly for pancreatic cysts, pancreatic ductal adenocarcinomas (PDAC), pancreatic neuroendocrine tumors (PNET), and other lesions. Each lesion type has a varied malignant potential and spectrum of biologic behaviors, making it important to distinguish between different lesions and identify poor prognostic characteristics (i.e. presence of malignancy, higher histopathologic grade, etc.) to inform their management.
Although imaging technology continues to advance over time, clinicians rely on invasive and non-invasive testing to diagnose and characterize pancreatic lesions, a practice that remains challenging. Diagnostic uncertainty for pancreatic lesions influences the development of appropriate surveillance and management strategies. Recently, texture analysis—a radiomics technique that employs quantitative analysis of the distribution and spatial relationship of pixels/voxels within a region of interest (ROI) on an imaging study—has shown promise as a non-invasive diagnostic tool for a variety of non-pancreatic lesions and has been recently investigated for pancreatic applications. Commercially available texture analysis software with streamlined interfaces are making texture analysis more accessible and a more clinically relevant tool. This review will discuss the fundamentals of texture analysis and summarize the clinical evidence for its application for pancreatic lesions, including tumor characterization (diagnosis and grading) and risk stratification.
BACKGROUND
Texture Analysis
Clinicians evaluate the global attenuation on computed tomography (CT), signal intensity on magnetic resonance (MR), or standardized uptake volume (SUV) on positron emission tomography (PET) of a lesion to help guide diagnosis. However, beyond these and several other basic metrics including size, objective radiologic interpretation can be limited, and heterogeneous features can be challenging to quantify. A simple visualization of a lesion may miss subtle changes that may reflect histopathologic evidence of disease progression or regression. Genetic changes cause alterations in the histologic and gross appearance of pancreatic lesions, and small changes in the tumoral microenvironment can hold important clues about tumor biology.2,3 Texture analysis is a highly sensitive technique that can potentially capture and quantify minute details that can be missed by the naked-eye. Another potential advantage of CTTA is that this data can be retrospectively obtained on incidentally detected lesions on scans obtained for other indications.
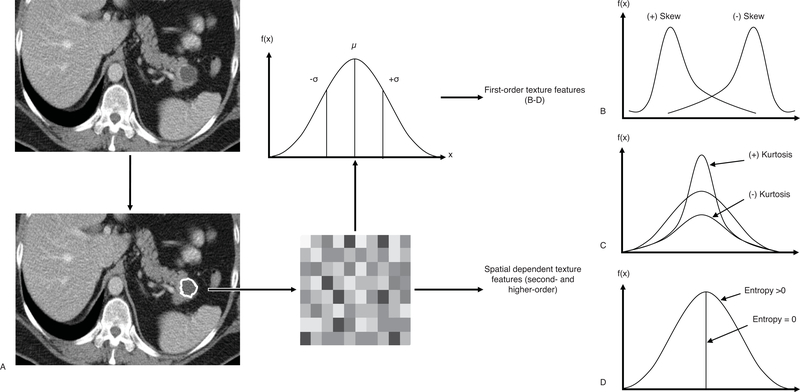
Texture analysis uses a well-established mathematical representation of the pixels and their spatial relationships in an image.4 For a selected ROI, texture analysis abstracts a signal of each pixel and represents the frequency of each signal value in a histogram. First-order texture features—mean gray level intensity, standard deviation, skewness, entropy, and kurtosis—provide information of the distribution of pixels in an ROI (Fig. 1). Whereas, second- and higher-order features describe the location and spatial relationship of pixels within an ROI.5
FIGURE 1.
Texture analysis work-flow and generation of first-order texture features. A, Computed tomographic imaging of a patient with a pancreatic lesion can be further evaluated with lesion analytic software. Lesion segmentation produces a well-defined area/volume of grey-scale pixels/voxels that may be represented as a frequency histogram including mean (µ) and standard deviation (σ). Further metrics that describe histogram skew (B), peakedness or kurtosis (C), or disorganization or entropy (D) may also be determined to represent first-order texture features. Advanced quantitative measures may be applied to the grey-scale region of interest to assess the spatial relationship between pixels/voxels to produce second and higher order texture features.
The generated frequency histogram of grey scale intensities, and subsequent second- and higher-order texture features are impacted by many radiologic imaging parameters. For instance, spatial scaling factors (SSFs) are graded stepwise improvements in CT imaging resolution that stabilize the pixel orientation within an ROI. Spatial scaling factors range from unfiltered (SSF 0) to fine resolution (SSF 2) to coarse resolution (SSF 6) based on feature size.6 Additional CT imaging parameters like current, voltage, slice thickness, and image reconstruction algorithm may impact the pixel intensity, image noise, and the spatial relationship between pixels.
The application of texture analysis has been more robustly studied in the evaluation of patients with lung, liver, and renal masses where success has been demonstrated in determining overall survival and discerning malignant from benign lesions.7,8,9 However, only a small number of studies to date have examined texture features of pancreatic lesions.
Pancreatic Lesions
Pancreatic lesions can be described based on their solid or cystic content. Solid pancreatic lesions are grossly categorized into PDACs arising from mutational events in pancreas progenitors, squamous cells of intercalated ducts, exocrine cells of pancreatic acini, and immune cells; and PNETs typically arising from cells that compose Islets of Langerhans.10,11 Cystic lesions are categorized into pseudocysts and cystic neoplasms of varying malignant potential. Pseudocysts tend to be sequelae of acute, acute-on-chronic, and chronic pancreatitis, and while they present risk for infection, they do not harbor malignant potential. Serous cystadenomas (SCAs), and simple, lymphoepithelial, and mucinous non-neoplastic cysts have the lowest malignancy rates. Cysts that harbor a higher degree of malignant potential include cystic PNETs, intraductal papillary mucinous neoplasms (IPMNs), and mucinous cystic neoplasms (MCNs).12
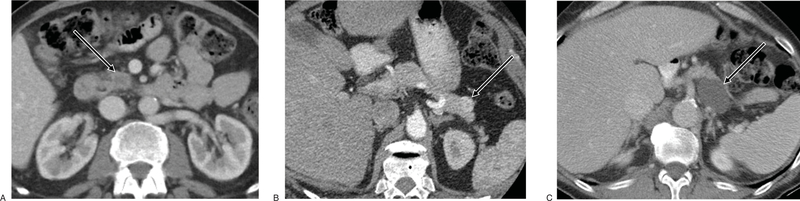
Given the diversity of possible pancreatic lesions, differentiation starts with CT imaging displaying characteristic radiologic features (Fig. 2). Imaging is helpful in the diagnosis and management of patients with pancreatic lesions; however, diagnostic uncertainty of pancreatic imaging persists, particularly for small lesions. Differentiation between PDACs and PNETs is aided by unique characteristics of each on CT and MR imaging. Pancreatic ductal adenocarcinoma lesions tend to be more fibrotic and less vascularized than PNETs. Therefore, detection of PDACs range from isoattenuating to hypoattenuating lesions on late arterial phase contrast-enhanced CT (CECT),13 whereas PNETs tend to be hyperattenuating on CECT arterial phase with portal venous washout (Fig. 2).14 Likewise, MR imaging depicts PDACs with low signal intensity on arterial phase T1 and T2-weighted sequences while PNETs are characteristically, moderately low signal intensity and high signal intensity on T1 and T2-weighted MR sequences, respectively.15 Pancreatic cysts are represented by hypoattenuating lesions on CECT and high signal intensity on T2-weighted MR sequences. Furthermore, calcifications, cyst wall thickening, non-enhancing mural nodules, and dilated pancreatic ducts are radiologic features suggestive of malignancy.
FIGURE 2.
Representative CT images of various pancreatic lesions. A, Pancreatic ductal adenocarcinoma in head/uncinate process of pancreas demonstrating rim enhancement with central hypoattenuation on contrast-enhanced computed tomography (CECT) portal venous phase. (B) Functional pancreatic neuroendocrine tumor (insulinoma) in tail of pancreas showing CECT arterial phase enhancement. (C) Pancreatic cyst in body/tail of pancreas demonstrating global hypoattenuation. Pancreatic lesions are denoted by white arrows.
Frequent deviations from these stereotypical patterns provide diagnostic uncertainty in pancreatic imaging. The presence of mixed cystic and solid components confers significant challenges in diagnosing and risk stratifying patients with pancreatic lesions. Gross features suggestive of malignancy may be absent in cysts with high-grade dysplasia. Many solid pancreatic lesions that are resected are indeterminate on pre-operative imaging due to poor sensitivity of imaging modalities. Overall, distinguishing lesions and characterizing their malignant potential with imaging alone remains a significant obstacle.
The poor prognosis of pancreatic cancer is particularly compounded by a lack of reliable image-based and endoscopic ultrasound-based screening tests.16 Moreover, pancreatic cancer is notorious for its absence of a sensitive and specific biomarker.17 According to the Surveillance, Epidemiology, and End Results Program, a review of cancer epidemiological data indicates that pancreatic cancer has the lowest 5-year survival rate at 9% for all stages.18 Although rates of metastasis at time of presentation have decreased by roughly 8% from 2004 to 2014, late detection is still likely a large contributing factor to high mortality for pancreatic cancer.19 Furthermore, resection of pancreas cancer requires major abdominal surgery with high risks of complications. Tools for determining a patient’s candidacy for surgery may be limited with current methodologies. Many patients receive neoadjuvant chemoradiotherapy and assessing response to therapy can be challenging for borderline resectable tumors. Taken together, there is a need to detect pancreatic cancer earlier in its pathogenesis.
Given these diagnostic gaps, there is clear potential for texture analysis to improve the diagnosis and characterization of lesions of the pancreas. In this review, we examine the findings of studies that have evaluated texture analysis of pancreatic lesions (summarized in Tables 1 and 2) and suggest methodologies that may improve texture analysis investigations of the pancreas.
TABLE 1.
Study Populations in Studies Investigating the Clinical Application of Texture Analysis on Pancreatic Lesions
| Publication | Inclusion Criteria | Exclusion Criteria | No. Patients | Institution |
|---|---|---|---|---|
| Hanania et al, 2016 | Patients with a pathologically confirmed IPMN imaged on a pre-operative pancreas protocol CT and surgically resected between March 2003- October 2011. | None provided | n = 53 | Texas MD Anderson Cancer Center |
| Permuth et al, 2016 | Patients with a pathologically confirmed IPMN, pre-resection CT imaging, and pre-operative matched miRNA expression data. | None provided | n = 38 | Moffitt Cancer Center and Research Institute |
| Attiyeh et al, 2019 | Patients with a pathologically confirmed BD-IPMN, pre-operative CT, and resection from 2005–2015. | Concurrent non-PDAC neoplasms, poor visualization on CT, and lack of portal venous phase. | n = 103 (Divided into 10 groups; 9 Training and 1 Testing groups) | Memorial Sloan Kettering Cancer Center |
| Chakraborty et al, 2018 | Patients with pathologically confirmed BD- IPMNs between 2005–2015. | None provided | n = 103 | Memorial Sloan Kettering Cancer Center |
| Cassinotto et al, 2017 | Patients with histopathologically confirmed PDAC, multi-phasic pancreas protocol CT imaging within 1 month of surgery, and resection from January 2009-June 2015. | Histology indicating anything other than PDAC, no pre-operative chemotherapy and/or radiotherapy, a tumor classified as borderline or locally advanced, incomplete histopathological evaluation, and patients with poor image quality | n = 99 | McGill University Health Centre at Montreal, Canada and University Hospital of Bordeaux in France |
| Canellas et al, 2018 | Patients with pathologically confirmed PNET assessed with dynamic CECT from February 2002-May 2016. | Pre-operative US, MRI, or non-CECT exams, no surgical resection of PNET, lack of diagnosis via FNA-EUS, and a pathology report of multiple tumors but no cyst identification if multiple | n = 101 | Massachusetts General Hospital |
| Choi et al, 2018 | Patients with pathologically confirmed PNET between January 2010-August 2014. | Lack of pre-operative CECT that included arterial and portal venous phase, and CT imaging on scanner performing <10 exams | n = 66 | Seoul National University Hospital |
| Hyun et al, 2016 | Patients with recently diagnosed PDAC and pre-operative 18F-FDG PET/CT imaging from January 2008-December 2010. | None provided | n = 137 | Samsung Medical Center in Seoul, Republic of North Korea |
| Eilaghi et al, 2017 | Patients with pathologically confirmed PDAC who underwent CECT prior to resection from 2007–2012. | PDAC associated with IPMN, and patients who died within 3 months of surgery | n = 30 | Sunnybrook Research Institute at University of Toronto |
| Chakraborty et al, 2017 | Patients older than 18 years of age who were enrolled in a Phase 2 clinical trial with newly diagnosed PDAC and pre-operative CT between July 2007-December 2011. |
Insufficient imaging, and borderline resectable or locally advanced | n = 35 | Memorial Sloan Kettering Cancer Center |
| Sandrasegaran et al, 2019 | Patients with unresectable PDACs treated with chemoradiotherapy between January 2007-December 2014. | Lack of pre-treatment CECT (IV), incomplete chemoradiotherapy, and surgical treatment | n = 60 | Indiana University School of Medicine |
| Yun et al, 2018 | Patients with histopathologically confirmed PDAC and surgical resection between January 2006-December 2014. | Pre-operative chemotherapy and/or radiotherapy, biliary stent placement, different CT imaging protocols, pancreatolith, and non-identifiable PDAC on imaging | n = 88 | Seoul National University Bundang Hospital |
| Attiyeh et al, 2018 | Patients with PDAC who had pre-operative CTA and surgical resection between 2009–12. | Pathological diagnosis of IPMN or pre-resection chemotherapy | n = 161 (113 in Training Group; 48 in Testing Group) | Memorial Sloan Kettering Cancer Center |
| Lin et al, 2019 | Patients with pathologically confirmed 1–3.0 cm grade ½ PNETs located in the pancreatic tail. Patients with pathologically confirmed IPASs with similar enhancement pattern to spleen and stable size and shape for 2 years. |
Lack of pre-treatment CECT | PNETs: n = 21; IPASs: n = 13 |
Affiliated Hospital and Yuying Children’s Hospital of Wezhou Medical University |
18F-FDG PET/CT indicates 18F-labeled Fluoro-2-deoxyglucose Positron Emission Tomography/ Computed Tomography; CECT: Contrast Enhanced Computed Tomography; CTA: Computed Tomography Angiography; FNA-EUS: Fine Needle Aspiration-Endoscopic Ultrasound; IPAS: Intrapancreatic Accessory Spleens; IPMN: Intraductal Papillary Mucinous Neoplasm; PDAC: Pancreatic Ductal Adenocarcinoma; PNET: Pancreatic Neuroendocrine Tumor.
TABLE 2.
Methodologies and Results of Studies Investigating the Clinical Application of Texture Analysis for Pancreatic Lesions
| Publication | Type of Pancreatic Lesions | Image Modality and Phase Used for Analysis | Software Program | ROI Segmentation | Primary Outcome | Secondary Outcome(s) | Significant Texture Features |
|---|---|---|---|---|---|---|---|
| Hanania et al, 2016 | IPMN | Arterial phase CECT | IBEX | For each patient, ROIs were semi-manually drawn around the cyst, cyst and pancreas, and the pancreas parenchyma. Solid and cystic components were segmented within ROI with exclusion of metal hardware. | Grade of dysplasia | --- | Fourteen GLCM features differentiated high-grade from low-grade IPMNs. |
| Permuth et al, 2016 | IPMN | Portal venous phase CECT angiography | Definiens/GE AWS Advanced Visualization software | An ROI was semi-manually drawn by a researcher and validated with the Definiens/GE AWS software to delineate segmentation boundaries. ROI included solid components. Arterial phase CECT and coronal slices were used as needed. |
Grade of dysplasia | Improved differentiation of IPMN grade when paired with miRNA genomic classifier data. | Texture (Fourier Descriptor Layer 1); Intensity histogram (Energy Layer 1 & Entropy Layer 1); Co-occurrence/Run-length (OF1 G1 CONTRAST Layer 1, G1 D0 HGRE Layer 1, and G1 D0 LGRE Layer 1), Laws (E5 E5 Energy Layer 1, L5 S5 Energy Layer 1, and R5 E5 Energy Layer 1); and Wavelet (P1 L3 C1 Layer 1 and P1 L3 C2 Layer 1) differentiated high-grade from low-grade IPMNs. |
| Attiyeh et al, 2019 | BD-IPMN | Portal venous phase CECT | Scout Liver and unnamed in-house software | An ROI of the largest cyst was manually segmented on each axial slice in which it appeared. | Grade of dysplasia | --- | Texture features including a novel mural nodule feature enhanced prediction of high-risk BD-IPMNs compared to clinical features alone. |
| Chakraborty et al, 2018 | BD-IPMN | Portal venous phase CECT | Scout Liver and MATLAB vR2015a | An ROI was manually drawn on the IPMN. | Grade of dysplasia | --- | A combination of EBF, EIF, FLCCF, and AWE better predicted high-risk IPMNs (AUC, 0.77) compared to standard texture features in combination (AUC, 0.74). |
| Cassinotto et al, 2017 | PDAC | Portal venous phase CECT | TexRAD | A 0.5cm2 ROI was placed in the most hypoattenuating segment of the PDAC; and an ROI was manually drawn on the largest cross-sectional area of the PDAC. Retained cysts and dilated bile ducts were omitted from ROI. |
Tumor grade | DFS | The average attenuation of the most hypoattenuating 0.5cm2 area was associated with high tumor grades. A value ≥62 HU was associated with a longer DFS. |
| Canellas et al, 2018 | PNET | Portal venous phase CECT | TexRAD v3.1 | An ROI was manually drawn around the largest cross-sectional area of the lesion. | Tumor grade | Progression-free survival | Second-order entropy (SSF 2–6) detected grade 1 from grades 2/3 PNET. Second-order entropy SSF2 >4.65 was associated with reduced progression-free survival. |
| Choi et al, 2018 | PNET | Arterial and portal venous phase CECT | Medical Imaging Solution for Segmentation and Texture Analysis (In-house) | An ROI was manually drawn around the largest axial cross-sectional area of the PNET to collect 2D texture features, and manual segmentation was propagated throughout the 3D structure of the PNET. | Tumor grade | --- | Increased skewness on arterial phase 3D, and decreased kurtosis on portal venous phase 2D analysis were predictors of grade 2/3 PNETs. |
| Hyun et al, 2016 | PDAC | Whole-body 18F-FDG PET/unenhanced CT | CGITA toolbox package of MATLAB v2012a | A volume of interest was drawn by contouring a line from center of PDAC and extending to surface. An automatic extension of 6 axes determines the edge of the PDAC based on sharp gradient. | Overall survival | --- | First-order entropy <3.77 HU is a better marker of prognosis compared to SUV. |
| Eilaghi et al, 2017 | PDAC | Portal venous phase CECT | Pro-CanVAS and MATLAB v8.5.0.197613- R2015a | ROIs were automatically drawn on each slice that contained PDAC; ROIs were also drawn to include parenchyma and 3 continuous slices toward upper and lower limits. | Overall survival | --- | Dissimilarity >16.311, and inverse difference normalized <0.969 are independently associated with improved PDAC survival. |
| Chakraborty et al, 2017 | PDAC | Portal venous phase CECT | Scout Liver and MATLAB vR2015a | An ROI was manually drawn around each axial slice of the PDAC. | Overall Survival (2-year) | --- | ACM2 was best able to predict >2-year survival in patients with PDAC with a >50% probability. |
| Sandrasegaran et al, 2019 | PDAC | Portal venous phase CECT | TexRAD Ltd | A polygonal ROI was optimally fitted to the PDAC in the slice with the largest cross-sectional area. Pixels of < −50 HU were excluded to omit gas and fat. |
Overall survival | --- | Increased MPP was associated with worse overall survival. Kaplan-Meier analyses found that MPP <31.625 and kurtosis <0.565 were associated with improved OS. |
| Yun et al, 2018 | PDAC | Pancreatic phase CECT | Unnamed in-house software | A polygonal ROI was manually drawn around the largest axial cross-sectional area that fits within the contours of the PDAC. Air and fatty infiltrates were excluded from ROI by excluding pixels with < 0 HU. |
DFS | --- | Average, standard deviation, contrast and correlation (SSF0/1/1.5/2), and average and contrast (SSF2.5) values were statistically different between patients with recurrent PDAC than those without. |
| Attiyeh et al, 2018 | PDAC | CECT | Scout Liver and MATLAB v2015a | An ROI was manually drawn on each axial slice of the PDAC which included necrotic areas. | Overall survival | --- | Features not indicated as this was a model-building investigation. |
| Lin et al, 2019 | PNET | Arterial phase CECT | MATLAB 2014a | 5 ROIs were manually drawn: the cross-sectional area with largest area and 2 continuous slices toward upper and lower limits. | Differential Diagnosis | --- | Uniformity at SSF1.5 and SSF2.5 differentiated PNETs from IPASs with AUC, 0.89 and 0.82, respectively. Entropy at SSF1.5 and SSF2.5 also differentiated PNETs from IPASs with AUC, 0.76 and 0.78, respectively. |
18F-FDG PET/CT: 18F-labeled Fluoro-2-deoxyglucose Positron Emission Tomography/ Computed Tomography; ACM: Angular Co-occurrence Matrix; AUC: Area Under the Curve; AWE: Average-Weighted Eccentricity; CECT: Contrast Enhanced Computed Tomography; CGITA: Chang-Gung Image Texture Analysis; DFS: Disease Free Survival; EBF: Enhanced Boundary Fraction; EIF: Enhanced Inside Fraction; FLCCF: Filled Largest Connected Component Fraction; GLCM: Grey Level Co-occurrence Matrix; HU: Hounsfield Units; IBEX: Imaging Biomarker Explorer; IPAS: Intrapancreatic Accessory Spleens; IPMN: Intraductal Papillary Mucinous Neoplasm; MPP: Mean of the Positive Pixels; OS: Overall Survival; PDAC: Pancreatic Ductal Adenocarcinoma; PNET: Pancreatic Neuroendocrine Tumor; SSF: Spatial Scale Filter; SUV: Standard Uptake Volume
GRADING PANCREATIC LESIONS
Texture evaluations of non-pancreatic lesions have shown promise in improving diagnostic accuracy by correlating radiologic features with histopathological grade.20 Determining the histopathologic grade of dysplasia in pancreatic lesions using non-invasive methods would be diagnostically valuable given the low sensitivity of biopsies for these lesions.21,22 As a result, several studies have investigated the use of texture analysis in grading various pancreatic lesion types.
Intraductal Papillary Mucinous Neoplasms
Hanania et al aimed to uncover whether texture analysis can be used to differentiate high-grade and low-grade dysplasia in IPMNs.23 They determined that 14 individual grey level co-occurrence matrices (GLCMs)—a subset of the second-order texture features—distinguish high-grade from low-grade IPMNs with significant accuracy. Grey level co-occurrence matricies area under the curve (AUC) statistics ranged from AUC, 0.64 to AUC, 0.82 (95% confidence interval [CI], 0.71–0.93). Furthermore, a panel of 10 GLCM metrics with the largest AUCs and true positive rates were selected from the 14 GLCMs to determine the validity of combining these metrics to enhance diagnostic accuracy. Differentiation of high-grade from low-grade dysplasia in IPMNs improved to AUC, 0.96 (95% CI, 0.92–0.99) when using the 10 GLCM metrics in combination.
Permuth et al conducted a similar study using texture analysis in conjunction with blood-based miRNA biomarkers to identify patients more likely to have pancreatic cysts concerning for malignancy.24 This study determined that 14 individual radiomic features (11 texture and 3 non-texture based) were predictive of high-grade dysplasia in IPMNs. Using AUC analyses of receiver operating characteristic (ROC) to determine diagnostic accuracy, Permuth et al demonstrated that using worrisome features per the Fukuoka Guidelines of IPMNs had an AUC, 0.54 (95% CI, 0.38–0.69).24 However, using their combined identified radiomic features increased the AUC to 0.77 (95% CI, 0.61–0.93), and using the 5-miRNA genomic classifier data increased the AUC to 0.83 (95% CI, 0.69–0.97). Overall, diagnostic accuracy was increased to an AUC, 0.93 (95% CI, 0.85–1.00) when worrisome features, radiomic features, and 5-miRNA genomic classifier were combined.
Both studies demonstrate that single texture features can be predictive of high-grade dysplasia in IPMNs and accuracy of detection improves when data are compounded. Additionally, Hanania et al determined GLCMs were most predictive of high-grade dysplasia, whereas, Permuth et al determined that first-order entropy (Odds Ratio [OR], 3.77; 95% CI, 1.34–10.63) and run-length texture features—a second-order texture feature (OR, 4.30; 95% CI, 1.37–13.49) were most predictive of high-grade dysplasia.23,24
While these two studies identified texture features predictive of high-grade dysplasia in IPMNs, low patient sample sizes in both studies were limitations, introducing potential overfitting into the proposed diagnostic models. Additionally, differences in imaging modalities used in the analysis make it challenging to compare these studies directly provided that imaging phase of contrast affects the attenuation values used in texture analysis.25 Hanania et al obtained data from CECT scans in the arterial phase,23 whereas Permuth et al obtained data from portal venous phase CT images.24 For these reasons, further examination of the performance of texture analysis for grading degree of dysplasia of IPMNs is needed.
One study examined the predictive value of texture features and a novel mural nodule texture feature in classifying branch duct IPMNs (BD-IPMNs) into high-risk (high-grade dysplasia and invasive carcinoma) and low-risk (low- and intermediate-grade dysplasia) lesions.26 Features significant for high-risk BD-IPMNs were determined with a univariate analysis of the texture and novel mural nodule features. Receiver operating characteristic analyses determined that quantitative texture features (AUC, 0.76) better predicted high-risk from low-risk BD-IPMNs compared to using clinical worrisome features (AUC, 0.67). The significance of the delineation between high- and low-risk disease further improved with combination of texture features and clinical evidence (AUC, 0.79). However, with the exception of the mural nodule texture feature, specific texture features were not reported in this investigation. A subsequent multivariate analysis using 90% of the patient sample as a training group and 10% as the testing group was conducted. The combined model of texture and clinical features was used to determine optimal sensitivity and specificity, or optimal positive predictive value and negative predictive value yielding 71%, 82%, 95%, and 79%, respectively. This study demonstrates that quantitative imaging features are superior to clinical data suggestive of high-risk disease, emphasizing its potential as a non-invasive assessment of the risk of BD-IPMNs. Additionally, Attiyeh, et al uniquely generates a predictive multivariate model to examine its validity against a small sub-set of patients to determine risk of BD-IPMNs with success.26 Further investigations that create models with unique texture features to predict risk of high-grade dysplasia of patients with BD-IPMNs are warranted.
A recent study examined the utility of risk-stratifying patients with BD-IPMNs to improve clinical management.27 Standard first- and second-order texture features were compared against radiographically inspired features (RiFs)—novel texture features associated with high-risk BD-IPMNs— in their ability to predict the degree of dysplasia of BD-IPMNs. Overall, they found RiFs and texture features are capable of identifying BD-IPMNs at increased risk of transforming to invasive cancer. Three RiFs were able to predict high-risk BD-IPMNs individually: enhanced boundary fraction (EBF; AUC, 0.703; P = 0.001), filled largest connected component fraction (FLCCF; AUC, 0.713; P = 0.001), and average-weighted eccentricity (AWE; AUC, 0.647; P = 0.024). Twelve independent texture features were able to delineate high-risk from low-risk BD-IPMNs (P < 0.05). The predictive power improved when all RiFs were combined to evaluate high-risk BD-IPMNs to an AUC, 0.77 (standard error of the mean [SEM] ± 0.037). The combination of texture features of cysts demonstrated an AUC, 0.70 (SEM ± 0.010). Additionally, collating standard texture features and RiFs achieved optimal association with high-risk BD-IPMNs (AUC, 0.78; SEM ± 0.006).
Univariate analysis of RiFs demonstrates that increased cyst wall enhancement (EBF), larger areas of enhancement within the cyst (FLCCF), and more areas of increased enhancement (AWE) were associated with high-risk BD-IPMNs. This association remained significant on multivariate analysis of RiFs. Chakraborty et al demonstrates that RiFs and standard texture features are capable of detecting BD-IPMNs at increased risk of transforming to invasive cancer with RiFs manifesting statistical superiority.27 Additional studies are warranted to develop novel quantitative markers that risk-stratify patients with BD-IPMN.
Pancreatic Ductal Adenocarcinomas
Pancreatic ductal adenocarcinoma grade is an important factor in determining survival and higher grade has been associated with reduced survival for increasing stage.28,29 Texture features predicting PDAC grade would be of obvious clinical utility. However, only one study so far has examined the potential for texture analysis grading of PDACs. In a retrospective analysis of 99 patients with PDAC, Cassinotto et al found that the degree of hypoattenuation of a PDAC correlates with histopathologically-determined grade by using a 0.5cm2 circular ROI to standardize measurements across the patient cohort.30 Specifically, lower mean attenuation of the most hypoattenuating area within the PDAC tumor was associated with high grade dysplasia (OR, 0.968; 95% CI, 0.94–0.998). The study group proposes two explanations: (1) increased hypoattenuation may correlate with the degree of necrosis, which is suggestive of PDAC aggressiveness, and (2) the tendency of PDACs to be highly fibrotic could result in delayed levels of contrast accumulation manifesting as hypoattenuation in PDACs with high-grade dysplasia. The group examined a total of 7 features (3 attenuation-based and 4 tumor heterogeneity-based) and found no other associations with PDAC grade.
Cassinotto et al also used a standardized ROI to determine the hypoattenuation of each lesion.30 While this does reduce variability imparted by PDAC size it may omit significant CECT-detectable signals in the periphery of the PDAC that may correlate to histopathological grade than solely the most hypoattenuating region. Further investigations in this area are needed, particularly with precise radiologic-pathologic correlations of the studied regions.
Pancreatic Neuroendocrine Tumors
Pancreatic neuroendocrine tumors have been another area of investigation for texture analysis given that the histopathological grading system is well-established. It is important to accurately identify PNET grade in order to predict survival since higher grades correlate with decreased overall survival.31 Canellas et al and Choi et al aimed to determine the CT texture features that are predictive of histopathological grade using CECT characterized PNETs prior to surgical resection. Despite similar methodologies in patient selection and image acquisition the two studies found differing results.32,33
Canellas et al examined 2D portal venous phase CECT images of 101 PNETs.32 Their analyses showed that decreased values of second-order entropy were predictive of high-grade (grades 2 and 3) PNETs across SSFs 2–6 (SSF2; OR, 3.7; P = 0.008). This association was maintained when SSFs 2–6 were pooled (X2 [degrees of freedom, 5], 7.3; P = 0.007). They described higher second-order entropy as a mathematical correlate to PNET tissue heterogeneity that may reflect advanced tumor grade.
Unlike the Canellas et al investigation, Choi et al examined similar texture features on both 2D and 3D arterial and portal venous phase CECT scans of 66 patients and did not observe statistical significance of second-order entropy being predictive of high-grade PNET dysplasia.32,33 Instead, Choi et al found skewness and kurtosis as texture features suggestive of PNET grade based on CECT phase.33 Grades 2/3 PNET showed higher skewness (OR, 1.972; 95% CI, 1.050–3.705; P = 0.035) on 3D arterial phase CECT, and lower kurtosis (OR, 0.436; 95% CI, 0.203–0.936; P = 0.033) on 2D portal venous phase CECT when compared to grade 1 PNETs. Choi et al suggest that the texture features predictive of higher grade PNETs may be explained by cystic degeneration altering the grey scale pixel/voxel attenuation of the generated intensity histogram.33
Discrepancies in results reported by Canellas et al and Choi et al may be due to sample size differences, use of SSFs in the Canellas et al study, and/or differences in CT scanning protocols and data collection tools (Table 2).32,33 Patients in the Canellas et al study received CECT with slice thickness of 5 mm, weight-based tube potential ranging 100–140, and automatic tube current modulation ranging 75–500 mAs.32 Patients in the Choi et al study received pre-operative CECT scans from various CT scanners (Brilliance 64-MDCT, Somatom Definition dual-source CT, and Sensation 16-MDCT).33 Collating data among these 3 CT scanners reveals more varied CT imaging parameters including slice thickness, weight-based tube potential, and automatic tube current modulation which has the potential to skew texture analysis data.33 Further investigations with standardized imaging parameters are needed. In addition, evaluating data from different phases of contrast may be capturing different information about the tumor best quantified by different types of texture features, making it possible that all the described features are useful in the appropriate imaging context.
RISK STRATIFICATION OF PANCREATIC LESIONS
Texture analysis has also been investigated as a way to risk stratify pancreatic lesions and to determine overall survivability (OS) and disease-free survival (DFS).
Pancreatic Ductal Adenocarcinoma
Several studies have employed standard CT texture analysis to address risk stratification in patients with possible PDAC. Positron emission tomography/computed tomography imaging employs the SUV of a lesion to determine the level of metabolic demand. A recent study of pre-operative PET/CT images were analyzed for first-order and second-order texture features in patients with newly diagnosed PDACs. The analysis of 137 patients with PDACs showed that first-order entropy <3.77 hounsfield unit (HU; P < 0.001) was better at predicting OS than SUV alone.34 Standardized uptake volume heterogeneity contributes to entropy and may be attributable to scattered pockets of more metabolically active PDAC tissue or from interspersed fibrosis leading to SUV segmentation. Hyun et al conclude that higher degrees of SUV homogeneity (reduced entropy) is indicative of improved OS of patients with PDAC.34
Another study determined that patients with PDAC had improved OS rates with the texture feature of dissimilarity >16.311 (AUC, 0.716; 95% CI, 0.528–0.903; P = 0.044) and <0.969 for inverse difference normalized (AUC, 0.716; 95% CI, 0.528–0.903; P = 0.044) when examined individually.35 Another study found that the most hypoattenuating 0.5 cm2 area of the lesion being ≥62 HU (AUC, 0.67; 95% CI, 0.53–0.77; P = 0.007) correlated to longer DFS.30 Chakraborty et al showed that the Angular Co-occurrence Matrix 2 (ACM2; AUC, 0.90; Accuracy, 82.86%) division of second-order texture features was most predictive of greater than 2-year survival associated with PDAC with a greater than 50% probability of prediction.36 Specifically, ACM2 sub-features: contrast, variance, sum average, difference variance, difference entropy, and inertia of patterns were most predictive of at least 2-year survival in patients with PDAC.
Additionally, Sandrasegaran et al performed an analysis of 60 patients with unresectable PDAC, primarily of the pancreatic head and aimed to determine the OS of this patient population and texture features associated with prognosis.37 Median survival in this patient cohort was 13.3 months. Multivariate Cox proportional hazard regression for OS showed that the mean value of positive pixels (MPP) had a hazard ratio of 1.04 (95% CI, 1.00–1.08; P = 0.042) on medium spatial filter (SSF 4). Additionally, MPP <31.625 (P = 0.0363) and kurtosis <0.565 (P = 0.0280), individually, were found in patients with better prognoses. Sandrasegaran et al suggested that increased MPP or kurtosis associated with worse OS of patients with unresectable PDAC may be due to more aggressive tumors associated with highly heterogenous tumor microenvironments including angiogenesis, hypoxia, and necrosis.37
Abdominal imaging can be subjected to motion artifact and poor delineation between soft tissues. Yun et al used SSFs without a filter (SSF0) to coarse filter (SSF6) to improve spatial resolution between malignant lesions and abdominal soft tissues and analyzed the performance of texture features for prediction of DFS at each SSF level.38 First-order texture analysis features including average, standard deviation, contrast, and correlation were significant predictors of DFS for SSF0,1,1.5,2; and average and contrast were significant predictors of DFS for SSF2.5 (P < 0.05 all).
One study used a patient cohort to create a model using carbohydrate antigen (CA) 19–9 levels, imaging texture features, and Brennan Score—a numerical representation of PDAC histopathology—to predict OS of patients undergoing PDAC resection.39 A training set of 113 patients generated a model using pre-operative CA 19–9 levels and CECT texture features which produced a c-index of 0.68 (95% CI, 0.62–0.73). Whereas, a mixed pre- and post-operative model using pre-operative CA 19–9 and CECT texture features, and post-operative Brennan Score produced a c-index of 0.73 (95% CI, 0.68–0.78). The validity of these models was examined using a smaller testing set of 48 patients. The former model of pre-operative CA 19–9 levels and CECT texture features, and latter model of pre-operative CA 19–9 and CECT texture features plus post-operative Brennan score produced c-indexes of 0.69 (95% CI, 0.62–0.77) and 0.74 (95% CI, 0.68–0.81), respectively. The group further reports that texture features suggestive of hypoattenuating PDAC lesions are associated with reduced OS. However, specific texture features were not reported in this investigation. Instead, a composite value of texture features was generated for each patient and applied to the model. To our knowledge, this investigation is the only study to generate a predictive multivariate model and examine its validity against a small sub-set of patients to determine OS of patients with PDAC; thus, addressing selection biases associated with retrospective studies. Further investigations that create models with unique texture features to predict OS of patients with PDAC are warranted.
Interpreting the existing literature that risk stratifies patients with pancreatic lesions using texture analysis is challenging given the heterogeneity of results and difficulty correlating texture features with specific histopathological features (i.e. limited precise radiologic-pathologic correlation). With these initial studies demonstrating associations between texture features with the prognosis of PDAC, additional studies and validation of models are needed to translate these findings into clinically useful quantitative imaging markers for PDAC. Furthermore, investigations of OS in patients with pancreatic cysts or PNETs are required given their varying malignant potential and absence from the literature.
DIFFERENTIAL DIAGNOSIS
Using texture analysis to help distinguish various types of pancreatic lesions would be helpful clinically. However, to date, investigations assessing the clinical utility of texture analysis of pancreatic lesions have focused on grading and risk stratifying patients by diagnosis. A single paper has demonstrated utility of texture analysis for differentiation of PNETs from intrapancreatic accessory spleens (IPASs) in the pancreatic tail.40 Included PNETs were pathologically confirmed, and patients with IPASs were included if the enhancement pattern matched that of the spleen and were stable in size for ≥2 years. In their ROC analysis of 21 patients with PNETs and 13 patients with IPASs, Lin et al determined that second-order entropy at SSF1.5 AND 2.5 were significantly different between PNETs and IPASs with AUC, 0.89 and AUC, 0.82 (both P < 0.01).40 Second-order entropy was also significantly different between the two lesion types generating an AUC of 0.76 and 0.78 for SSF1.5 and SSF2.5, respectively (P < 0.01). This study showed promise for utilizing texture analysis to differentiate two lesions with different management strategies that are often challenging to delineate with existing imaging interpretation strategies.
Patients with complex or indeterminant pancreatic lesions would benefit from further efforts to use texture analysis to differentiate between different types of cysts, PDACs, PNETs, and non-pancreatic tissue.
LIMITATIONS OF PRESENTED STUDIES AND FUTURE DIRECTIONS
The limitations of the studies presented include relatively small sample sizes that leave the data susceptible to overfitting statistical analyses and lack of direct correlation of texture features to pathologic features of pancreatic lesions (e.g. extent of calcifications, cystic and solid components, degree of metaplasia and dysplasia, etc.). All analyses to date for pancreatic lesions were acquired from CT imaging, while robust, detection and monitoring of pancreatic lesions is also conducted using non-CT imaging modalities such as MR. This limits the generalizability of CT texture analysis findings. Magnetic resonance texture analysis has shown to be effective in predicting survival outcomes in breast cancer and detecting high-grade dysplasia in prostate cancer.41,42 However, studies in MR texture are challenging given the variability in the numbers of series and image acquisition parameters. The utility of texture analysis of pancreatic lesions on MR remains to be seen.
From a technical perspective, most studies conducted texture analysis on a single-slice of pancreatic lesions. While this is standardized methodology in texture analysis, this protocol omits potentially significant data in the remaining imaging slices. It is still not clear whether a single slice analysis is sufficient or if a volumetric assessment is more robust. Another important limitation to consider are the contributions of healthy pancreas parenchyma to the texture landscape of a lesion. Varying degrees of fatty infiltrations of pancreas, microcalcifications, and/or free-fluid in the abdomen may have the potential to skew texture feature calculations. We recommend that future studies normalize texture feature data from lesions to that of healthy pancreas parenchyma.
SUMMARY
Texture analysis is a rapidly expanding field with implications for surgical and radiologic subspecialties. Advancements in technology and accessibility afford texture analysis the potential to serve as a diagnostic tool for a variety of pathologies. Although multiple studies summarized here show promise for the clinical utility of texture analysis of pancreatic lesions (Table 3), further studies are needed to broaden our understanding of which features are important and how the practice could be incorporated in the clinical setting.
TABLE 3.
Classification and Definition of Significant Texture Features in CTTA of Pancreatic Lesions
| Texture Feature Classification | Significant Texture Features | Definition | Lesion Studied and Publication |
|---|---|---|---|
| Non-textural features | Degree of hypoattenuation (standardized 0.5 cm2 area) | Degree of hypoattenuation (standardized 0.5 cm2 area) | PDAC: Cassinotto et al, 2017 |
| Degree of hypoattenuation | Measure of the extent of hypoattenuation. | PDAC: Attiyeh et al, 2018 | |
| First-order features | Skewness | Measure of the intensity histogram shift toward more positive or more negative pixel value (Fig. 1B) | PNET: Choi et al, 2018 & Lin et al, 2019 |
| Kurtosis | Measure of the peakedness of intensity histogram (Fig. 1C) | PNET: Choi et al, 2018 & Lin et al, 2019; PDAC: Sandrasegaran et al, 2019 | |
| Entropy | Measure of the uncertainty or disorganization among pixel intensities. | PDAC: Hyun et al, 2016 | |
| Mean of Positive Pixels | Measure of the mean of pixels above a value of zero. | PDAC: Sandrasegaran et al, 2019 | |
| Average | Measure of mean intensity of grey-level distribution. | PDAC: Yun et al, 2018 | |
| Standard deviation | Measure of the degree of dispersion around a mean. | PDAC: Yun et al, 2018 | |
| Second-order features | GLCMs: | Measure of the spatial relationship of grey scale pixels in an ROI. | Measure of the spatial relationship of grey scale pixels in an ROI. |
| -Entropy | Measure of the degree of stochastic spatial orientation of pixels. | PNET: Canellas et al, 2018 & Lin et al, 2019 | |
| -Dissimilarity | Measure of the sum of distance between paired pixels. | PDAC: Eilaghi et al, 2017 | |
| -Inverse difference normalized | Measure of the smoothness/homogeneity of pixels in an ROI. | PDAC: Eilaghi et al, 2017 | |
| -Contrast | Measure of the local level variations using the high attenuation pixels in a GLCM. | PDAC: Yun et al, 2018 | |
| -Correlation | Measure of the probability of pixels being adjacent in 2 different directions in a GLCM. | PDAC: Yun et al, 2018 | |
| -Uniformity | Measure of the spatial dispersion of pixels in a GLCM. | PNET: Lin et al, 2019 | |
| ACM2 | Matrix that characterizes directional edge patterns of all pixel-pairs. | PDAC: Chakraborty et al, 2017 | |
| Novel texture features | Enhanced Boundary Fraction | Measure of the cyst wall enhancement or infiltration of cyst into pancreas parenchyma. | IPMN: Chakraborty et al, 2018 |
| Filled Largest Connected Component Fraction | Measure of the largest enhanced area within the non-boundary region. | IPMN: Chakraborty et al, 2018 | |
| Average-Weighted Eccentricity | Measure of the collective area of enhanced regions that includes FLCC. | IPMN: Chakraborty et al, 2018 | |
| Enhancing mural nodules texture feature | Measure of the area of the largest enhanced region to the entire cyst area. | IPMN: Attiyeh et al, 2019 |
PDAC indicates pancreatic ductal adenocarcinoma; PNET, pancreatic neuroendocrine tumor
A summary of current gaps in CTTA of pancreatic lesions and proposed recommendations is outlined in Table 4. Specifically, we encourage multidisciplinary collaborations between surgeons, radiologists, and pathologists to achieve better radiologic-pathologic correlation, which is critical in this emerging field. Standardizing the data collection and statistical analysis methods and controlling for variability will provide more robust data to better understand texture features of pancreatic lesions. In addition, standardization of the available tools may allow for easier comparison of data and reproducibility of studies. Finally, condensing the results of multiple studies into clinically relevant tools will be needed. Ultimately, texture analysis has potential as a non-invasive diagnostic method to help patients with a variety of pancreatic lesions and other pathologies and deserves further multidisciplinary attention.
TABLE 4.
Summary of the Gaps in CTTA of Pancreatic Lesions and Recommendations for Improvement
| Gaps | Recommendations |
|---|---|
| Small sample sizes | Perform multi-institutional studies to increase sample size |
| Lack of MRI-based texture features | Repeat texture analysis investigations using MRI; Address differences from CTTA; Establish best series for MR texture analysis |
| Non-standard radiologic representation of pancreatic lesions being used for texture analysis | Standardize methodologies of image acquisition, data correction to control for background pancreas parenchyma, nature of ROI (eg, 2D vs 3D), and statistical analyses |
| Heterogeneity of significant texture features grading and risk stratifying pancreatic lesions | Conduct systematic reviews to collate data of multiple investigations |
| Lack of pathologic-radiologic correlation | Create multidisciplinary teams between surgeons, radiologists, and pathologists to correlate specific texture features with pathologic characteristics |
CTTA indicates computed tomography texture analysis; MRI, magnetic resonance imaging; ROI, region of interest.
ACKNOWLEDGMENTS
The authors thank Anthony D. Robinson for his assistance with design and creation of the figure in this manuscript.
Abbreviations:
- 18F-FDG PET/CT
18F-labeled Fluoro-2-deoxyglucose Positron Emission Tomography/ Computed Tomography
- ACM
Angular Co-occurrence Matrix
- AUC
Area Under the Curve
- AWE
Average-Weighted Eccentricity
- BD-IPMN
Branch Duct-Intraductal Papillary Mucinous Neoplasm
- CA19–9
Carbohydrate Antigen 19–9
- CECT
Contrast Enhanced Computed Tomography
- CGITA
Chang-Gung Image Texture Analysis
- CI
Confidence Interval
- CTA
Computed Tomography Angiography
- DFS
Disease-Free Survival
- EBF
Enhanced Boundary Fraction
- EIF
Enhanced Inside Fraction
- EUS/FNA
Endoscopic Ultrasound/Fine-Needle Aspiration
- FLCCF
Filled Largest Connected Component Fraction
- GLCM
Grey Level Co-occurrence Matrix
- HU
Hounsfield Unit
- IBEX
Imaging Biomarker Explorer
- IPAS
Intrapancreatic Accessory Spleens
- IPMN
Intraductal Papillary Mucinous Neoplasm
- MCN
Mucinous Cystic Neoplasms
- MPP
Mean of Positive Pixels
- MR
Magnetic Resonance
- OS
Overall Survivability
- PDAC
Pancreatic Ductal Adenocarcinoma
- PET
Positron Emission Tomography
- PNET
Pancreatic Neuroendocrine Tumor
- RiF
Radiographically-inspired Features
- ROC
Receiver Operating Characteristic
- ROI
Region of Interest
- SCA
Serous Cystadenoma
- SSF
Spatial Scaling Factor
- SUV
Standard Uptake Volume
Footnotes
Conflict of Interest:
V.R.R. has grant funding from NIH National Cancer Institute (F32 CA232352). M.G.L. has grant funding from Philips and Ethicon. For remaining authors none were declared.
REFERENCES
- 1.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Roentgenol. 2008;191:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davnall F, Yip CSP, Ljungqvist G, et al. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganeshan B, Skogen K, Pressney I, et al. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: Preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol. 2012;67:157–164. [DOI] [PubMed] [Google Scholar]
- 4.Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Transactions on Systems, Man, and Cybernetics. 1973;SMC-3:610–621. [Google Scholar]
- 5.Lubner MG, Smith AD, Sandrasegaran K, et al. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics. 2017;37:1483–1503. [DOI] [PubMed] [Google Scholar]
- 6.Yasaka K, Akai H, Abe O, et al. Quantitative computed tomography texture analyses for anterior mediastinal masses: Differentiation between solid masses and cysts. Eur J Radiol. 2018;100:85–91. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins SH, Korecki JN, Balagurunathan Y, et al. Predicting outcomes of nonsmall cell lung cancer using CT image features. IEEE Access. 2014;2:1418–1426. [Google Scholar]
- 8.Raman SP, Schroeder JL, Huang P, et al. Preliminary Data Using Computed Tomography Texture Analysis for the Classification of Hypervascular Liver Lesions. J Comput Assist Tomogr. 2015;39:383–395. [DOI] [PubMed] [Google Scholar]
- 9.Raman SP, Chen Y, Schroeder JL, et al. CT texture analysis of renal masses: Pilot study using random forest classification for prediction of pathology. Acad Radiol. 2014;21:1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Van Ness M, Guo Y, et al. Molecular pathology of pancreatic neuroendocrine tumors. J Gastrointest Oncol. 2012;3:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark A, Donahue TR, Reber HA, et al. Pancreatic cyst disease a review. JAMA - J Am Med Assoc. 2016;315:1882–1893. [DOI] [PubMed] [Google Scholar]
- 13.Wong JC, Raman S. Surgical resectability of pancreatic adenocarcinoma: CTA. Abdom Imaging. 2010;35:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappelli C, Boggi U, Mazzeo S, et al. Contrast enhancement pattern on multidetector CT predicts malignancy in pancreatic endocrine tumours. Eur Radiol. 2015;25:751–759. [DOI] [PubMed] [Google Scholar]
- 15.Al Ansari N. Role of magnetic resonance imaging in the detection and characterization of solid pancreatic nodules: An update. World J Radiol. 2015;7:361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corral JE, Mareth KF, Riegert-Johnson DL, et al. Diagnostic Yield From Screening Asymptomatic Individuals at High Risk for Pancreatic Cancer: A Meta-analysis of Cohort Studies. Clin Gastroenterol Hepatol. 2019;17:41–53. [DOI] [PubMed] [Google Scholar]
- 17.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute; [www.seer.cancer.gov]. January 2018. Available at: https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed March 16, 2019. [Google Scholar]
- 19.Salami A, Obaid T, Joshi ART. Trends in the clinical presentation, treatment, and survival for pancreatic adenocarcinoma. Am J Surg. 2019;217:103–107. [DOI] [PubMed] [Google Scholar]
- 20.Bae JM, Jeong JY, Lee HY, et al. Pathologic stratification of operable lung adenocarcinoma using radiomics features extracted from dual energy CT images. Oncotarget. 2017;8:523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang QX, Xiao J, Orange M, et al. EUS-Guided FNA for Diagnosis of Pancreatic Cystic Lesions: A Meta-Analysis. Cell Physiol Biochem. 2015;36:1197–1209. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt MJ, McPhail MJW, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–331. [DOI] [PubMed] [Google Scholar]
- 23.Hanania AN, Bantis LE, Feng Z, et al. Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget. 2016;7:85776–85784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Permuth JB, Choi J, Balarunathan Y, et al. Combining radiomic features with a miRNA classifier may improve prediction of malignant pathology for pancreatic intraductal papillary mucinous neoplasms. Oncotarget. 2016;7:85785–85797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubner MG, Stabo N, Abel EJ, et al. CT textural analysis of large primary renal cell carcinomas: Pretreatment tumor heterogeneity correlates with histologic findings and clinical outcomes. Am J Roentgenol. 2016;207:96–105. [DOI] [PubMed] [Google Scholar]
- 26.Attiyeh MA, Chakraborty J, Gazit L, et al. Preoperative risk prediction for intraductal papillary mucinous neoplasms by quantitative CT image analysis. HPB. 2019;21:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty J, Midya A, Gazit L, et al. CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas. Med Phys. 2018;45:5019–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YT, Huang ZP, Zhou ZW, et al. Equipping the American Joint Committee on Cancer staging for resectable pancreatic ductal adenocarcinoma with tumor grade: a recursive partitioning analysis. Med Oncol. 2016;33:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rochefort MM, Ankeny JS, Kadera BE, et al. Impact of tumor grade on pancreatic cancer prognosis: Validation of a novel TNMG staging system. Ann Surg Oncol. 2013;20:4322–4329. [DOI] [PubMed] [Google Scholar]
- 30.Cassinotto C, Chong J, Zogopoulos G, et al. Resectable pancreatic adenocarcinoma: Role of CT quantitative imaging biomarkers for predicting pathology and patient outcomes. Eur J Radiol. 2017;90:152–158. [DOI] [PubMed] [Google Scholar]
- 31.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canellas R, Burk KS, Parakh A, et al. Prediction of pancreatic neuroendocrine tumor grade based on CT features and texture analysis. Am J Roentgenol. 2018;210:341–346. [DOI] [PubMed] [Google Scholar]
- 33.Choi TW, Kim JH, Yu MH, et al. Pancreatic neuroendocrine tumor: prediction of the tumor grade using CT findings and computerized texture analysis. Acta radiol. 2018;59:383–392. [DOI] [PubMed] [Google Scholar]
- 34.Hyun SH, Kim HS, Choi SH, et al. Intratumoral heterogeneity of 18F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2016;43:1461–1468. [DOI] [PubMed] [Google Scholar]
- 35.Eilaghi A, Baig S, Zhang Y, et al. CT texture features are associated with overall survival in pancreatic ductal adenocarcinoma - a quantitative analysis. BMC Med Imaging. 2017;17:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakraborty J, Langdon-Embry L, Cunanan KM, et al. Preliminary study of tumor heterogeneity in imaging predicts two year survival in pancreatic cancer patients. PLoS One. 2017;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandrasegaran K, Lin Y, Asare-Sawiri M, et al. CT texture analysis of pancreatic cancer. Eur Radiol. 2019;29:1067–1073. [DOI] [PubMed] [Google Scholar]
- 38.Yun G, Kim YH, Lee YJ, et al. Tumor heterogeneity of pancreas head cancer assessed by CT texture analysis: Association with survival outcomes after curative resection. Sci Rep. 2018;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attiyeh MA, Chakraborty J, Doussot A, et al. Survival Prediction in Pancreatic Ductal Adenocarcinoma by Quantitative Computed Tomography Image Analysis. Ann Surg Oncol. 2018;25:1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin X, Xu L, Wu A, et al. Differentiation of intrapancreatic accessory spleen from small hypervascular neuroendocrine tumor of the pancreas: textural analysis on contrast-enhanced computed tomography. Acta radiol. 2019;60:553–560. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Ko ES, Lim Y, et al. Breast Cancer Heterogeneity: MR Imaging Texture Analysis and Survival Outcomes. Radiology. 2016;282:665–675. [DOI] [PubMed] [Google Scholar]
- 42.Niu XK, Chen ZF, Chen L, et al. Clinical application of biparametric MRI texture analysis for detection and evaluation of high-grade prostate cancer in zone-specific Regions. Am J Roentgenol. 2018;210:549–556. [DOI] [PubMed] [Google Scholar]