Abstract
The ileal apical sodium-dependent bile acid transporter (ASBT) is crucial for the enterohepatic circulation of bile acids. ASBT function is rapidly regulated by several posttranslational modifications. One reversible posttranslational modification is S-acylation, involving the covalent attachment of fatty acids to cysteine residues in proteins. However, whether S-acylation affects ASBT function and membrane expression has not been determined. Using the acyl resin-assisted capture method, we found that the majority of ASBT (∼80%) was S-acylated in ileal brush border membrane vesicles from human organ donors, as well as in HEK293 cells stably transfected with ASBT (2BT cells). Metabolic labeling with alkyne–palmitic acid (100 μm for 15 h) also showed that ASBT is S-acylated in 2BT cells. Incubation with the acyltransferase inhibitor 2-bromopalmitate (25 μm for 15 h) significantly reduced ASBT S-acylation, function, and levels on the plasma membrane. Treatment of 2BT cells with saturated palmitic acid (100 μm for 15 h) increased ASBT function, whereas treatment with unsaturated oleic acid significantly reduced ASBT function. Metabolic labeling with alkyne–oleic acid (100 μm for 15 h) revealed that oleic acid attaches to ASBT, suggesting that unsaturated fatty acids may decrease ASBT's function via a direct covalent interaction with ASBT. We also identified Cys-314 as a potential S-acylation site. In conclusion, these results provide evidence that S-acylation is involved in the modulation of ASBT function. These findings underscore the potential for unsaturated fatty acids to reduce ASBT function, which may be useful in disorders in which bile acid toxicity is implicated.
Keywords: bile acid, membrane transport, intestinal epithelium, protein acylation, intestine, apical sodium-dependent bile acid transporter (ASBT), bile acid transport, intestinal epithelial cells, posttranslational modification (PTM), SLC10A2, S-acylation, ileum, lipid modification
Introduction
The apical sodium-dependent bile acid transporter (ASBT)2 (SLC10A2) is responsible for the reclamation of bile acids from the lumen of the distal ileum and is thus crucial for maintaining the enterohepatic circulation of bile acids. A great deal of evidence has supported the importance of ASBT in intestinal bile acid absorption. For instance, inactivating mutations in ASBT have been linked to primary bile acid malabsorption (1) and mice with targeted deletion of the slc10a2 gene display loss of bile acids in the stool, decreased bile acid pool size, and enhanced bile acid synthesis (2). Conversely, enhanced ASBT function is associated with diseases such as hypercholesterolemia and necrotizing enterocolitis, and ASBT inhibition is being explored for the treatment of several of these disorders (3–6). Given the importance of ASBT as a therapeutic target, it is critical to understand the cellular processes that regulate the function of ASBT.
Previous studies have demonstrated that ASBT function is tightly regulated by several posttranslational mechanisms. ASBT has been shown to be ubiquitinated at lysine residues, which target the protein for proteasomal degradation (7, 8). ASBT is also glycosylated at asparagine residue 10, which stabilizes the protein and protects against degradation (9, 10). In addition, ASBT is subject to serine, threonine, and tyrosine phosphorylation, which regulates its trafficking between the plasma membrane and subapical compartments (11, 12) as well as its proteasomal degradation (7). Given that these posttranslational regulatory mechanisms rely on the covalent modification of amino acid residues, it is likely that additional modifications involving other amino acid residues may also be involved in regulating ASBT.
In this regard, cysteine residues can be subject to protein S-acylation, a lipid posttranslational modification that is an important regulatory mechanism for many membrane proteins (13). S-acylation is the reversible attachment of long chain fatty acids (FAs) to cysteine residues via a covalent thioester bond. The attachment of FAs is catalyzed by the zDHHC family of palmitoyl acyltransferases, named for their highly conserved zinc finger aspartic acid-histidine-histidine-cysteine domain (13–15). These enzymes are polytopic transmembrane proteins with a cytosolic catalytic domain. Although it is generally accepted that zDHHC proteins possess S-acyltransferase activity, relatively little is known with regard to how these enzymes identify their target proteins. Unlike many enzymes, including those responsible for other protein lipid modifications, there are few known consensus sequences for zDHHC enzymes (16). This has made prediction of protein S-acylation quite challenging, requiring experimental approaches to determine whether a protein is subject to S-acylation and which cysteine residues are involved. Interestingly, recent evidence has clearly demonstrated the importance of cysteine residues in ASBT function, although their potential role in maintaining ASBT function via S-acylation has yet to be fully investigated (17, 18). We hypothesized that posttranslational modification by S-acylation is critical for ASBT function and may modulate its rapid functional response to certain external cues, such as the availability of different types of fatty acids.
S-acylation has been shown to regulate the function of a wide variety of cellular proteins, including ion and solute transporters (13, 19). Plasma membrane trafficking, transport capacity, protein stability, and phosphorylation status of these transport proteins have been shown to be modulated by S-acylation. For instance, S-acylation of the β and γ subunits of the epithelial sodium channel was shown to modulate channel gating by increasing the open probability and reducing self-inhibition of the channel (20, 21). In addition to regulation of the epithelial sodium channel, it was recently shown that S-acylation of the cystic fibrosis transmembrane conductance regulator is crucial for its trafficking from the Golgi apparatus to the plasma membrane (22, 23). Additionally, S-acylation of the dopamine transporter (SLC6A3) was shown to regulate its phosphorylation status, transport kinetics, and stability (24, 25).
In the present study, we aimed to determine the S-acylation of ASBT in native intestine and a cell culture model, and also investigated the role of S-acylation in regulating ASBT function and subcellular localization. We demonstrated that S-acylation is crucial for the function of ASBT and may serve as an important regulatory role in bile acid homeostasis.
Results
ASBT is acylated in native intestinal cells and 2BT cells
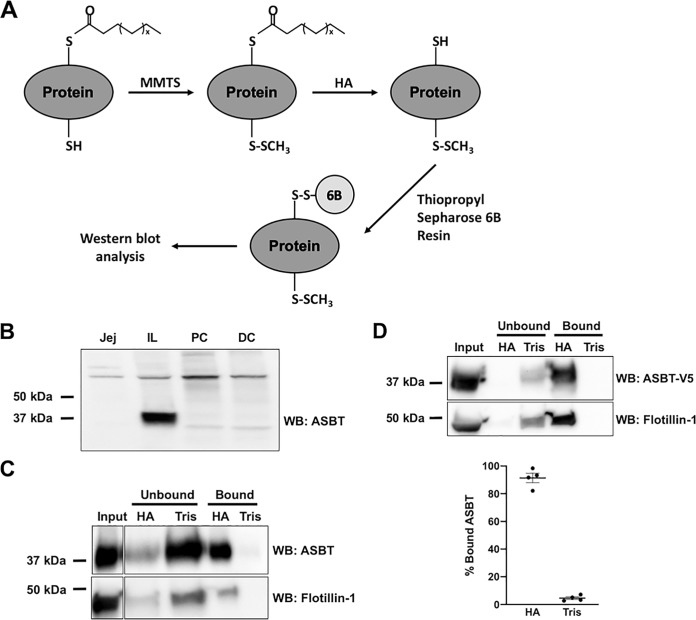
S-acylation is a crucial mechanism by which the localization and function of membrane proteins are regulated (13). We first aimed to determine whether ASBT protein was S-acylated in native intestinal tissues from human donors. We used acyl resin-assisted capture (acyl-RAC) to quantify the proportion of ASBT that was S-acylated. In this method, proteins are treated with S-methyl methanethiosulfonate (MMTS), which blocks free (nonacylated) thiols. Blocked proteins are then treated with hydroxylamine (HA), which cleaves the thioester bonds of S-acylated residues. Thus, cysteine residues that were initially nonacylated are blocked by MMTS, whereas residues that were initially acylated are cleaved to form free thiols. Proteins are then precipitated using thiopropyl Sepharose 6B resin, which binds to the initially acylated free thiols. This method results in S-acylated proteins binding to the resin, whereas nonacylated proteins remain unbound in the supernatant (26, 27) (Fig. 1A). For these experiments, treatment with Tris was used as a negative control for the HA reaction, such that in samples treated with Tris, no protein should bind to the resin as acyl groups are not cleaved. Anti-human ASBT antibodies were validated in human donor intestine, demonstrating a band at ∼38 kDa in Brush Border Membrane Vesicles (BBMVs) generated from ileum, but not in jejunum, proximal colon, or distal colon (Fig. 1B). When acyl-RAC was performed on BBMVs from human donor ileum, the majority of ASBT and flotillin-1 (a well-known S-acylated membrane protein) (28) were found in the bound fraction of HA-treated samples (Fig. 1C). These data strongly indicate that the majority of ASBT is acylated in human intestine.
Figure 1.
ASBT is S-acylated in native intestine. A, schematic of acyl-RAC method. Free cysteines are blocked with MMTS, followed by cleavage of thioesters with hydroxylamine. Acylated proteins are precipitated with thiopropyl Sepharose 6B resin. B, the expression of ASBT in brush border membrane vesicles prepared from different regions from the human gastrointestinal tract: jejunum (jej); ileum (IL), proximal colon (PC), and distal colon (DC). C, ASBT acyl-RAC in human ileal brush border membrane vesicles (representative Western blotting from three different donors with similar results). D, ASBT acyl-RAC in HEK293 cells stably transfected with ASBT-V5. n = 4.
We next investigated whether ASBT is acylated in HEK293 cells stably transfected with a human ASBT-V5 fusion protein (2BT cells). We have previously shown that ASBT-V5 in 2BT cells is functional and regulated in a similar manner to native ASBT (29, 30). When acyl-RAC was performed in these cells, ∼80% of ASBT-V5 bound to the resin, similar to what was seen in native tissues (Fig. 1D).
2-Bromopalmitate reduces ASBT acylation and function
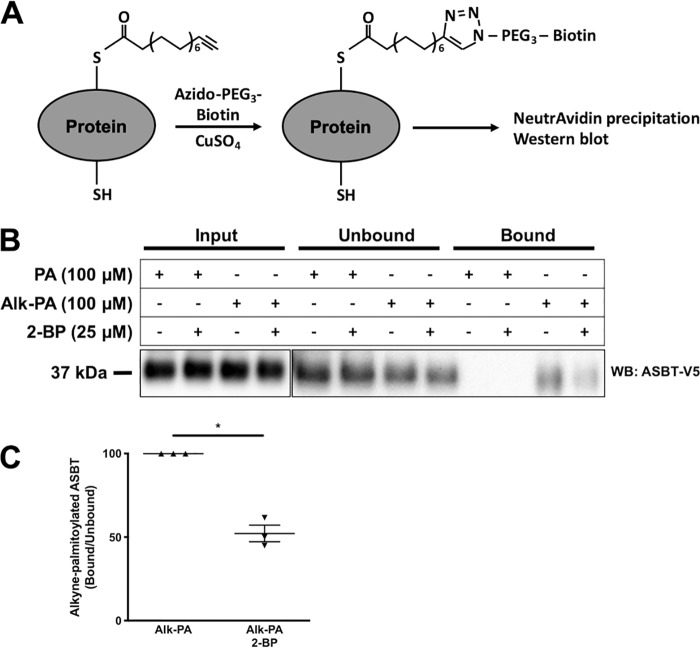
To further confirm that ASBT is acylated in 2BT cells, we performed metabolic labeling by incorporating alkyne–palmitic acid (alk-PA) into acylated proteins. Cells were deprived of lipids for 6 h in media containing 0.25% FA-free BSA, followed by 15 h incubation with either 100 μm PA or 100 μm alk-PA in the presence or absence of 25 μm 2-bromopalmitate (2-BP), an inhibitor of acyltransferases. Protein lysates were prepared, and alkyne–palmitoylated proteins were conjugated to azido-PEG3-biotin via copper-catalyzed azide-alkyne cycloaddition, also known as “click chemistry” (31, 32). Biotin-conjugated proteins were precipitated with NeutrAvidin agarose beads, followed by SDS-PAGE and Western blotting (Fig. 2A). As shown in Fig. 2B, ASBT-V5 was detected in the bound fraction from cells treated with alk-PA but not from those treated with PA (as expected), indicating that alk-PA was incorporated into ASBT-V5. The incorporation of alk-PA in ASBT-V5 was ∼50% reduced by treatment with the acyltransferase inhibitor 2-BP (Fig. 2, B–C), suggesting enzyme-dependent acylation of ASBT in 2BT cells.
Figure 2.
2-Bromopalmitate decreases ASBT acylation. A, schematic of metabolic labeling by alk-PA in 2BT cells. Alk-PA incorporates into acylated proteins and is conjugated to biotin by click chemistry. Biotinylated proteins are precipitated with NeutrAvidin agarose resin. B and C, incorporation of alk-PA in ASBT in the presence or absence of 25 μm 2-BP. n = 3, *, p < 0.05.
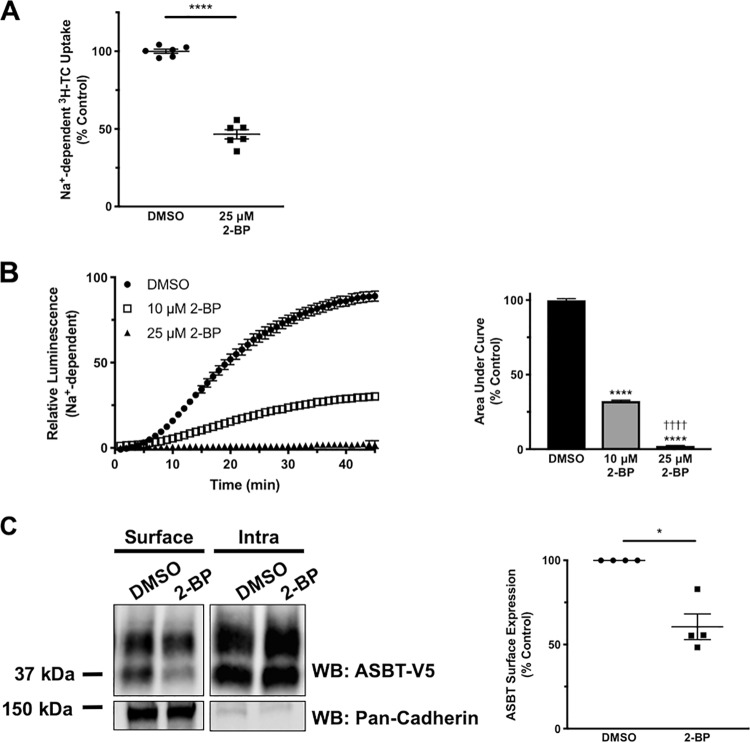
In addition to reducing ASBT acylation, 30-min treatment with 25 μm 2-BP was able to produce a robust ∼50% decrease in [3H]TC uptake (Fig. 3A). These findings were confirmed by measuring real-time luminescence from the bioluminescence bile acid tracer CA-SS-Luc. When 2BT cells were treated with 2-BP at the same time that CA-SS-Luc was added, the rate of CA-SS-Luc luminescence generation was markedly reduced when cells were treated with 10 or 25 μm 2-BP (Fig. 3B). The area under the curve measurement, which represents total CA-SS-Luc uptake, was ∼70% reduced by 10 μm 2-BP and nearly eliminated by 25 μm 2-BP. To investigate potential mechanisms by which 2-BP reduces ASBT function, we examined ASBT plasma membrane expression by cell surface biotinylation. As shown in Fig. 3C, 30-min treatment with 25 μm 2-BP reduced ASBT surface expression by ∼40%, suggesting that S-acylation may be important for targeting ASBT to the plasma membrane. These data indicate that reduced acylation by 2-BP may lead to decreased ASBT plasma membrane expression, with a corresponding decrease in ASBT function.
Figure 3.
Inhibition of S-acylation decreases ASBT activity and surface membrane expression. A, [3H]TC uptake in 2BT cells treated with 25 μm 2-BP for 30 min. n = 6; ****, p < 0.0001. B, bioluminescence and area under the curve calculations from 2BT cells treated with 1 μm CA-SS-Luc and 100 μm TC added simultaneously with either 10 μm or 25 μm 2-BP and imaged in real time. n = 4, ****, p < 0.0001 compared with DMSO, ††††, p < 0.0001 compared with 10 μm 2-BP. C, cell surface biotinylation of 2BT cells treated with 25 μm 2-BP for 30 min. n = 4; *, p < 0.05.
Incubation with unsaturated fatty acids decreases ASBT function
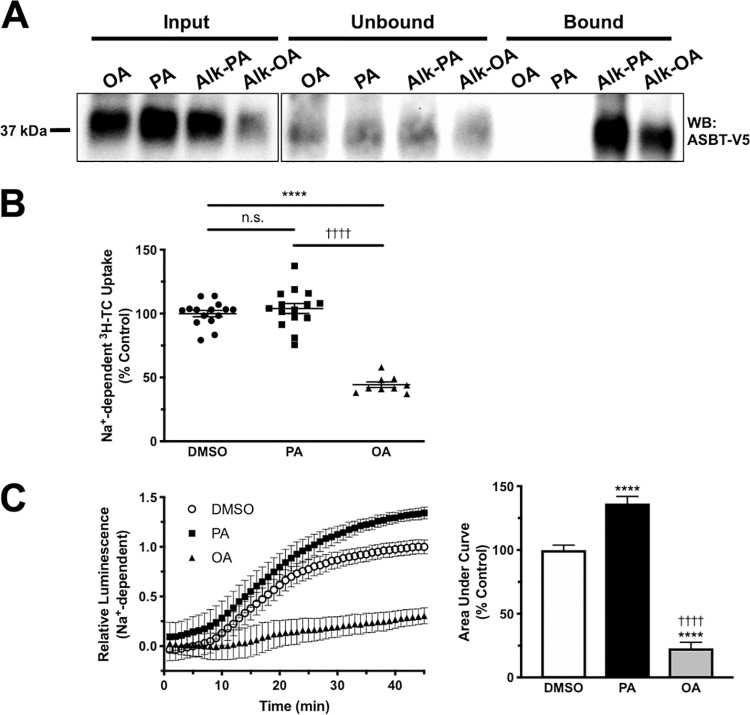
Although PA is the most common fatty acid seen in S-acylated proteins, other types of fatty acids can be incorporated into proteins by S-acylation (16, 33). To determine whether other fatty acids can be incorporated into ASBT, we performed metabolic labeling with alkyne–oleic acid (alk-OA). As shown in Fig. 4A, alk-OA was incorporated into ASBT, indicating that oleic acid may S-acylate ASBT. Interestingly, the type of fatty acyl moiety has been shown to affect the function of acylated proteins (34–36). We hypothesized that incubation with fatty acids other than PA would result in altered ASBT function. 2BT cells were incubated for 15 h with either vehicle (DMSO), 100 μm PA (16:0), or 100 μm OA (18:1). Following incubation, [3H]taurocholate (TC) uptake was performed and results were normalized to vehicle. Treatment with PA showed a slight increase in [3H]TC uptake. However, incubation with 18:1 unsaturated OA decreased uptake by ∼50% (Fig. 4B). Using the CA-SS-Luc bioluminescence approach for measuring ASBT function, the results showed that PA incubation significantly increased the bioluminescence by ∼30% compared with DMSO control, whereas OA incubation reduced bioluminescence by ∼75% after 45 min of imaging (Fig. 4C). These studies indicate that unsaturated fatty acids may decrease ASBT function via a mechanism dependent on ASBT acylation.
Figure 4.
Incubation with unsaturated fatty acids decreases ASBT function. A, metabolic labeling of ASBT by alk-OA in 2BT cells (representative Western blotting of three separate experiments). B, [3H]TC uptake in 2BT cells treated with either DMSO, 100 μm PA, or 100 μm OA. n = 6; ****, p < 0.0001 compared with DMSO; ††††, p < 0.0001 compared with PA. C, real-time uptake and area under the curve calculation of bioluminescent bile acid tracer CA-SS-Luc in 2BT cells treated for 15 h with DMSO, 100 μm PA, or 100 μm OA. n = 4; ****, p < 0.0001 compared with DMSO; ††††, p < 0.0001 compared with PA.
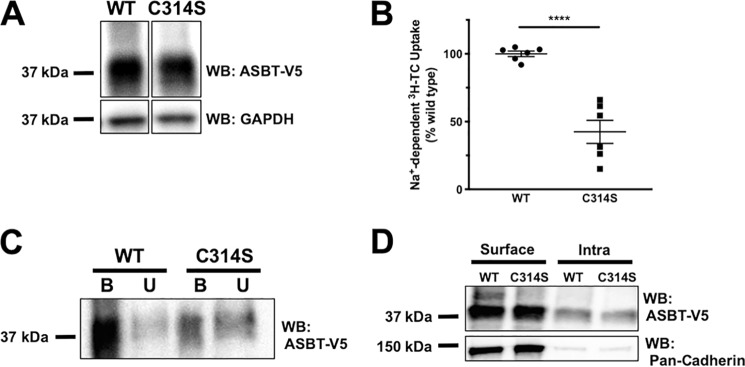
Cysteine 314 is important for ASBT S-acylation and transport function
To determine which cysteine residue(s) may be important for ASBT S-acylation, we generated a mutant ASBT with Cys-314 mutated to Ser. This site was chosen because its location in the cytoplasmic C-terminal domain of ASBT makes it accessible to zDHHC enzymes, which possess a cytosolic catalytic domain (17, 37). WT and C314S ASBT-V5 plasmids were transfected in HEK293 cells and Western blotting and [3H]TC uptake were performed. As shown in Fig. 5A, C314S ASBT-V5 retained expression in these cells. However, this mutant displayed a significant ∼50% decrease in its transport function relative to WT ASBT (Fig. 5B). Additionally, we performed acyl-RAC in these cells to determine the acylation status of C314S ASBT-V5. Interestingly, this mutant showed remarkably reduced acylation compared with WT ASBT (Fig. 5C). These data indicate that Cys-314 is acylated under basal conditions and that blocking acylation at this site may contribute to the reduction in ASBT transport function. However, as can be seen in Fig. 5C, there is a population of ASBT C314S that remains acylated, suggesting the involvement of additional cysteine residues. Of note, the surface level of C314S ASBT-V5 was not significantly different from the WT ASBT-V5 fusion protein (Fig. 5D).
Figure 5.
Cys-314 is important for ASBT S-acylation and transport function. A and B, cellular expression (A) and [3H]TC uptake (B) in HEK293 cells transfected with WT or C314S ASBT-V5. n = 3; ****, p < 0.0001 compared with WT. C, Western blotting of bound (S-acylated) and unbound (nonacylated) acyl-RAC fractions from HEK293 cells transfected with WT or C314S ASBT-V5 (representative of three Western blots with similar results). D, a representative Western blotting for cell surface biotinylation of WT and C314S ASBT-V5 fusion proteins.
Discussion
In this manuscript, we have demonstrated that S-acylation is an important posttranslational regulatory mechanism that modulates ASBT function. It is important to note that unlike many other posttranslational regulatory mechanisms, there are no known sequence motifs that are useful for predicting which proteins are likely to be S-acylated. Although there are some prediction tools available (38–41), it remains necessary to empirically identify S-acylated proteins. To date, no studies examining the global pool of S-acylated proteins in the intestine have been performed. There is preliminary evidence to suggest that ASBT is also S-acylated.3 In the present study, we demonstrated by acyl-RAC that ASBT is S-acylated in human ileum under basal conditions, as well as in HEK293 cells that overexpress human ASBT-V5 fusion protein (2BT cells). Furthermore, studies in 2BT cells showed that alk-PA incorporation into ASBT is reduced ∼50% by the acyltransferase inhibitor 2-BP, providing further evidence that ASBT is a substrate for zDHHC palmitoyl acyltransferases. Interestingly, incubation with 2-BP reduced ASBT S-acylation and ASBT plasma membrane expression, suggesting that S-acylation may contribute to targeting ASBT to plasma membranes. Importantly, these experiments also demonstrate that ASBT acylation is functionally relevant. Indeed, 25 μm 2-BP treatment for 30 min decreased transport function by ∼50%, suggesting that ASBT S-acylation may be subject to rapid and dynamic regulation. Similarly, real-time measurement of CA-SS-Luc bioluminescence was reduced by ∼70% in response to 10 μm 2-BP and almost completely abolished by 25 μm 2-BP over the course of 45-min imaging.
Interestingly, recent evidence has indicated that the effects of S-acylation may vary depending on the specific fatty acid bound to a protein. Originally, palmitic acid was thought to be the principal fatty acid attached to cysteine residues, which is why this posttranslational regulatory mechanism has traditionally been called palmitoylation. However, it has become understood that other fatty acids can also be substrates for S-acylation. Indeed, studies by Muszbek et al. (42) used GC/MS to show that in human platelets ∼74% of the pool of fatty acids incorporated into proteins were palmitic acid, ∼22% were stearic acid, and ∼4% were oleic acid. Importantly, they did note that intersubject variability was high, and that the composition of this pool could be altered by exogenous supplementation of fatty acids. These studies highlight the potential role of nonpalmitic fatty acids in regulating proteins via S-acylation. Other studies have demonstrated that zDHHC enzymes, which are the acyltransferases responsible for S-acylation, have a relatively broad substrate specificity that allows PA, OA, and long chain polyunsaturated fatty acids to be utilized as substrates and incorporated into proteins (43, 44). Indeed, numerous studies have identified distinct functional effects depending on the fatty acyl moiety attached to a protein (36). For example, studies demonstrated that polyunsaturated FAs can be attached to cysteine residues to reduce association with lipid rafts and affect protein function (34, 35). In the present study, we showed that 15-h incubation of 2BT cells with saturated PA increased ASBT function, whereas incubation with unsaturated OA decreased transporter activity. Using click chemistry–based metabolic labeling, we also showed that alkyne–oleic acid incorporates into ASBT, indicating that the effects of OA on ASBT function may be dependent on S-acylation. The measured increase in ASBT in response to treatment with PA was not remarkable when ASBT function was assessed by the traditional method using the radioactive labeled TC. However, there was a clear 30% significant increase in ASBT activity when determined by the novel bioluminescence-based approach. The differences in the results may be because the traditional method allows the determination of the function only at one time point whereas the bioluminescence-based method measures the overall activity of ASBT in real-time over a period of 45 min.
A seven-transmembrane model for ASBT protein has been determined and the effects of mutations in all cysteine residues were assessed (17, 18, 45, 46). In the current study, we chose to examine whether mutation in Cys-314 has any effect on ASBT S-acylation. This particular cysteine residue was chosen because it is the only cysteine residue found in the cytosolic C terminus of ASBT and is conserved among species. Given that the catalytic domain of zDHHC enzymes are present in the cytosol (17, 37), we would expect S-acylated residues to be located in cytosolic domains. Also, the Cys-314 was predicted as a potential site for S-acylation using GPS-Lipid 1.0 (41). Of note, some cysteine-to-alanine or -serine mutations were previously shown to abrogate ASBT function, whereas mutations in others including Cys-314 decreased ASBT function only by about 50% (17, 18, 45, 46). Our current data further confirmed these previous findings showing that the C314S mutation also decreased ASBT by about 50%, whereas the mutant was equally expressed when transfected in HEK293 cells. Acyl-RAC showed that a smaller pool of C314S ASBT was S-acylated compared with WT ASBT, indicating that Cys-314 is subject to S-acylation. However, the fact that a sizeable pool of ASBT C314S remains acylated suggests the involvement of additional acylation sites. We also found that the C314S mutation did not significantly alter ASBT expression on the plasma membrane as compared with WT ASBT. This observation is not in agreement with the conclusion made from the data presented in Fig. 3 that indicated a decrease in the level of ASBT on plasma membrane by the S-acylation inhibitor 2-BP. Several possible explanations could be considered to reconcile these seemingly incongruous results. One possibility is that ASBT harbors multiple sites for S-acylation including Cys-314 and blocking the S-acylation on Cys-314 alone (C314S mutant) is not sufficient to reduce the ASBT level on plasma membranes, in contrast with total inhibition of S-acylation (treatment with 2-BP). Another possibility is that 2-BP causes a decrease in ASBT S-acylation a well as in the acylation of additional cellular proteins involved in ASBT trafficking to plasma membranes. Further studies will be needed to determine which other cysteine residues are subject to S-acylation and also which acyltransferases and thioesterases are involved in ASBT acylation.
It also be interesting in future studies to determine whether ASBT S-acylation is important in the pathophysiology of diseases in which ASBT is implicated. It has been shown that several disease states, including obesity and metabolic syndrome, are associated with alterations in the global pool of S-acylated proteins, often referred to as the palmitoylome (47, 48). It is important to mention that the S-acylation of transport proteins has been implicated in the pathogenesis of metabolic diseases. For instance, it has been shown that the fatty acid transporter CD36 is subject to S-acylation, which is important for its endoplasmic reticulum and Golgi processing, as well as its targeting to lipid rafts (49). Importantly, Zhao et al. (50) recently demonstrated that hepatic CD36 is hyperpalmitoylated in nonalcoholic steatohepatitis patients, resulting in increased plasma membrane CD36 expression. This study also showed that overexpression of a nonacylated CD36 mutant in the liver of mice protected against nonalcoholic steatohepatitis development, implicating CD36 S-acylation in the pathogenesis of this disease.
In this regard, dietary lipids, insulin signaling, and ethanol metabolism can alter the palmitoylome (51–54). For instance, feeding of a high-fat diet (∼60% calories from saturated FAs) was associated with hyperpalmitoylation and dysregulation of AMPA glutamate receptor subunit GluA1 in the brain (54). These studies strongly suggest that consumption of FAs affects global S-acylation. However, it is not known whether changing the composition of dietary FAs will affect the fatty acyl moieties attached to proteins, which could be expected to show functional changes. Therefore, it will be important in future studies to examine the effects of individual dietary compounds as well as disease states on ASBT acylation status and transport function, in both cell culture and in vivo models.
In conclusion, we have demonstrated that ASBT function depends on its S-acylation. Inhibiting acylation with 2-BP or incubation with unsaturated FAs decreases ASBT function, whereas incubation with saturated PA increases ASBT function. We identified Cys-314 as a potential site of S-acylation, but there are likely other sites that remain unidentified to be determined in future studies. Our results provide insights into a novel molecular mechanism via which fatty acids affect the function of ASBT and bile acid absorption.
Experimental procedures
Cell culture and materials
HEK293 were purchased from ATCC and grown in plastic flasks at 37 °C in an atmosphere consisting of 5% CO2 and 95% air. Cells were cultured in Dulbecco's modified Eagle's medium containing 4 mm l-glutamine, 4.5 g/liter glucose, 1 mm sodium pyruvate, and 1.5 g/liter sodium bicarbonate, supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 mg/ml gentamicin (Invitrogen). HEK293 cells stably transfected with human ASBT-V5 (2BT) were cultured in the same media containing 7 μg/ml puromycin. All chemicals and reagents were purchased from Sigma-Aldrich unless otherwise specified.
Western blot analysis
Cells were lysed using commercially available cell lysis buffer (Cell Signaling Technologies) supplemented with 0.1% SDS, unless otherwise specified. Samples were prepared in Laemmli buffer (Bio-Rad) supplemented with 2.5% β-mercaptoethanol, boiled for 5 min, and resolved by SDS-PAGE. Proteins were transferred to nitrocellulose membranes and probed with antibodies. Anti-V5 tag antibodies were obtained from Invitrogen (catalogue no. R96025), Anti–flotillin-1 antibodies were obtained from Abcam (catalogue no. ab50671), and Anti–pan cadherin antibodies were obtained from Sigma-Aldrich (catalogue no. C1821). Human ASBT was detected using custom-made antibodies generated previously in our laboratory (29). Cell surface biotinylation was performed as previously described (11, 12).
Preparation of human intestinal brush border membrane vesicles
Small and large intestines from healthy adult organ donors were obtained from Gift of Hope (IL) immediately after harvest of organs for transplantation. The small intestine was divided into three segments of equal length. The proximal segment was designated as the jejunum and the distal segment was designated as the ileum. The large intestine was divided into proximal and distal segments of equal length. The intestine was cleaned, and mucosa were scraped from the seromuscular layer and stored at −80 °C. Purified BBMVs were prepared from frozen samples by the CaCl2 precipitation method as described previously (29, 55–57). Purified BBMVs were suspended in buffer containing 25 mm HEPES, pH 7.4, 25 mm NaCl, 1 mm EDTA, and 1% Triton X-100 for acyl-RAC analysis (see below).
Acyl resin-assisted capture (acyl-RAC)
Acyl-RAC was performed on crude membrane preparations as described previously (26, 27). Briefly, protein lysates were suspended in detergent-free buffer containing 25 mm HEPES, pH 7.4, 25 mm NaCl, and 1 mm EDTA. Lysates were subjected to ultracentrifugation at 136,000 × g for 1 h to prepare crude membranes, which were then solubilized in the lysis buffer supplemented with 1% Triton X-100. Membranes were subjected to thiol blocking by 1.5% S-methyl methanethiosulfonate (MMTS) in a buffer containing 100 mm HEPES, pH 7.4, 1 mm EDTA, and 2% SDS for 4 h at 40 °C. Blocked proteins were precipitated in acetone, washed several times, and resuspended in buffer containing 100 mm HEPES, pH 7.4, 1 mm EDTA, and 1% SDS. Hydrated thiopropyl Sepharose 6B resin was added to these proteins and mixed with HA, pH 7.5, at a final concentration of 0.5 m. As a negative control, a separate reaction was prepared with HA replaced by Tris-HCl. This solution was incubated overnight at room temperature with end-over-end mixing. The following day, supernatant (unbound fraction) was collected, the resin was washed several times, and bound proteins were eluted by boiling in Laemmli buffer supplemented with 2.5% β-mercaptoethanol for 10 min. The volumes of unbound and bound fractions were equalized and subjected to SDS-PAGE followed by Western blotting.
Metabolic labeling with alkyne-fatty acids
Metabolic labeling with 100 μm alk-PA (palmitic acid 15-yne; Avanti Polar Lipids) or 100 μm alk-OA (oleic acid 17-yne; Avanti Polar Lipids) was performed in DMEM supplemented with 0.25% fatty acid–free BSA. To prepare these solutions, 200 mm DMSO stocks of either unlabeled PA, OA alk-PA, or alk-OA were added to 20% fatty acid–free BSA to a concentration of 8 mm. 50 mm 2-bromopalmitate or vehicle were also added to a concentration of 2 mm. These solutions were sonicated briefly and incubated at 37 °C until the fatty acids had fully dissolved, followed by 80× dilution in 37 °C DMEM. These solutions were added to cells and incubated at 37 °C for 15 h to allow for incorporation of fatty acids. Following incubation, cells were lysed in buffer containing 150 mm NaCl, 50 mm HEPES, 2 mm MgCl2, 0.1% Triton X-100, 1% SDS, and 1× EDTA-free protease inhibitor. Up to 200 μg of protein lysate was subjected to copper-catalyzed azide-alkyne cycloaddition with azido-PEG3-biotin as described previously (58). Briefly, lysates were incubated for 1 h at room temperature with 50 μm azido-PEG3-biotin in a solution containing 100 μm CuSO4, 500 μm THPTA, and 1.5 mm sodium ascorbate. Proteins were precipitated in methanol, washed, and solubilized in RIPA buffer. They were then incubated with NeutrAvidin agarose beads (Thermo Fisher) overnight at 4 °C with end-over-end mixing. The following day, supernatant (unbound fraction) was collected, the resin was washed several times, and bound proteins were eluted by boiling in Laemmli buffer supplemented with β-mercaptoethanol for 10 min. Samples were then subjected to SDS-PAGE followed by Western blotting.
Supplementation with fatty acids
Fatty acid supplementation of 2BT cells with either 100 μm PA (16:0) or oleic acid (18:1) was performed in DMEM supplemented with 0.25% fatty acid–free BSA prepared as above. ASBT function was then assessed as described below.
Assessment of ASBT function by [3H]TC uptake
Following treatment with FAs or 2-BP, assessment of ASBT activity by [3H]TC uptake was performed as described previously (29). Briefly, cells were washed twice at room temperature with uptake buffer containing 110 mm NaCl (with sodium) or choline chloride (without sodium), 4 mm KCl, 1 mm MgSO4, 1 mm CaCl2, 45 mm mannitol, 5 mm glucose, and 10 mm HEPES, pH 7.4. Cells were then incubated with uptake buffer containing 1 μCi/ml of [3H]TC and 10 μm cold TC at room temperature for 5 min, washed with ice-cold PBS, and lysed in 0.5 m NaOH. Radioactivity was measured by liquid scintillation counting (Packard Tri-CARB 1600-TR, Packard Instruments) and total protein was measured by the method of Bradford (59). Results were calculated as pmol · mg protein−1 · 5 min−1 and normalized to control.
Assessment of ASBT function by bioluminescent bile acid tracer (CA-SS-Luc) uptake
Assessment of ASBT activity by CA-SS-Luc uptake was performed as described recently (60). Briefly, cells were washed twice at room temperature with uptake buffer containing 110 mm NaCl (with sodium) or choline chloride (without sodium), 4 mm KCl, 1 mm MgSO4, 1 mm CaCl2, 45 mm mannitol, 5 mm glucose, 10 mm HEPES, pH 7.4, and 100 μm TC. Cells were then incubated with uptake buffer containing 1 μm CA-SS-Luc and immediately imaged by a Xenogen IVIS Spectrum in vivo imaging system (Caliper Life Sciences) and bioluminescence was captured continuously with 1-min exposure times for 45 min. The luminescence produced from each well was quantified using Living Image software (PerkinElmer).
Plasmid construction
Human ASBT cDNA was previously generated in our laboratory and subcloned into pSF-CMV-Puro-COOH-TEV-V5 (Oxford Genetics) (60). To generate the ASBT C314S mutant, site-directed mutagenesis was performed with the QuikChange II XL kit (Agilent) as per the manufacturer's instructions using the primers: 5′-TCTGCCTTGTTTTTTCCATGACTTTTCTTGTATGCCACATAAAATC-3′ and 5′-GATTTTATGTGGCATACAAGAAAAGTCATGGAAAAAACAAGGCAGA-3′.
Transfection experiments
HEK293 cells were seeded into 24-well plates or 10-cm dishes and grown to ∼70% confluence. Cells were transfected with 1 μg/well or 12 μg/dish of ASBT-V5 WT or C314S plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. 48 h post transfection, ASBT function was assessed by [3H]TC uptake or CA-SS-Luc bioluminescence method and S-acylation was measured by acyl-RAC analysis.
Statistical analysis
Results were expressed as mean ± S.E. of at least three experiments performed on multiple occasions. Statistical analysis was performed by Student's t test (GraphPad Prism). p ≤ 0.05 was considered statistically significant.
Author contributions
A. L. T., C. R. M., R. K. G., and W. A. A. conceptualization; A. L. T., P. M., C. R. M., P. K. D., S. S., R. K. G., and W. A. A. data curation; A. L. T., P. M., C. R. M., P. K. D., S. S., R. K. G., and W. A. A. formal analysis; A. L. T., R. K. G., and W. A. A. funding acquisition; A. L. T., P. K. D., S. S., R. K. G., and W. A. A. validation; A. L. T., P. M., C. R. M., R. K. G., and W. A. A. investigation; A. L. T., P. M., C. R. M., S. S., R. K. G., and W. A. A. methodology; A. L. T. writing-original draft; A. L. T., P. M., C. R. M., P. K. D., S. S., R. K. G., and W. A. A. writing-review and editing; P. K. D., S. S., R. K. G., and W. A. A. resources; P. K. D., S. S., R. K. G., and W. A. A. project administration; R. K. G. and W. A. A. supervision.
This work was supported by the Veterans Affairs Research Career Scientist Award and Merit Award BX000152 (to W. A. A.). This work was also supported by NIDDK, National Institutes of Health, Grants DK109709 (to W. A. A.), F30 DK113703 (to C. R. M.), F30 DK117535 (to A. L. T.), DK054016 (to P. K. D.), DK98170 (to R. K. G.); by Veterans Affairs Merit Award BX002867 (to S. S.) and Veteran Affairs Merit Award BX002011 (to P. K. D.); and by a Veterans Affairs Senior Research Career Scientist Award (to P. K. D.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or reflect the position or policy of the Department of Veterans Affairs or the United States government.
P. Swaan et al., University of Maryland, personal communication.
- ASBT
- apical sodium-dependent bile acid transporter
- HA
- hydroxylamine
- FA
- fatty acid
- acyl-RAC
- acyl resin-assisted capture
- MMTS
- S-methyl methanethiosulfonate
- alk
- alkyne
- PA
- palmitic acid
- OA
- oleic acid
- 2-BP
- 2-bromopalmitate
- TC
- taurocholate
- BBMVs
- Brush Border Membrane Vesicles.
References
- 1. Oelkers P., Kirby L. C., Heubi J. E., and Dawson P. A. (1997) Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J. Clin. Invest. 99, 1880–1887 10.1172/JCI119355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dawson P. A., Haywood J., Craddock A. L., Wilson M., Tietjen M., Kluckman K., Maeda N., and Parks J. S. (2003) Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J. Biol. Chem. 278, 33920–33927 10.1074/jbc.M306370200 [DOI] [PubMed] [Google Scholar]
- 3. Chen L., Yao X., Young A., McNulty J., Anderson D., Liu Y., Nystrom C., Croom D., Ross S., Collins J., Rajpal D., Hamlet K., Smith C., and Gedulin B. (2012) Inhibition of apical sodium-dependent bile acid transporter as a novel treatment for diabetes. Am. J. Physiol. Endocrinol. Metab. 302, E68–E76 10.1152/ajpendo.00323.2011 [DOI] [PubMed] [Google Scholar]
- 4. Wu Y., Aquino C. J., Cowan D. J., Anderson D. L., Ambroso J. L., Bishop M. J., Boros E. E., Chen L., Cunningham A., Dobbins R. L., Feldman P. L., Harston L. T., Kaldor I. W., Klein R., Liang X., et al. (2013) Discovery of a highly potent, nonabsorbable apical sodium-dependent bile acid transporter inhibitor (GSK2330672) for treatment of type 2 diabetes. J. Med. Chem. 56, 5094–5114 10.1021/jm400459m [DOI] [PubMed] [Google Scholar]
- 5. Tiessen R. G., Kennedy C. A., Keller B. T., Levin N., Acevedo L., Gedulin B., van Vliet A. A., Dorenbaum A., and Palmer M. (2018) Safety, tolerability and pharmacodynamics of apical sodium-dependent bile acid transporter inhibition with volixibat in healthy adults and patients with type 2 diabetes mellitus: A randomised placebo-controlled trial. BMC Gastroenterol. 18, 3 10.1186/s12876-017-0736-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rao A., Kosters A., Mells J. E., Zhang W., Setchell K. D. R., Amanso A. M., Wynn G. M., Xu T., Keller B. T., Yin H., Banton S., Jones D. P., Wu H., Dawson P. A., and Karpen S. J. (2016) Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 8, 357ra122 10.1126/scitranslmed.aaf4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xia X., Roundtree M., Merikhi A., Lu X., Shentu S., and Lesage G. (2004) Degradation of the apical sodium-dependent bile acid transporter by the ubiquitin-proteasome pathway in cholangiocytes. J. Biol. Chem. 279, 44931–44937 10.1074/jbc.M400969200 [DOI] [PubMed] [Google Scholar]
- 8. Miyata M., Yamakawa H., Hayashi K., Kuribayashi H., Yamazoe Y., and Yoshinari K. (2013) Ileal apical sodium-dependent bile acid transporter protein levels are down-regulated through ubiquitin-dependent protein degradation induced by bile acids. Eur. J. Pharmacol. 714, 507–514 10.1016/j.ejphar.2013.06.036 [DOI] [PubMed] [Google Scholar]
- 9. Sun A.-Q., Salkar R., Sachchidanand Xu S., Zeng L., Zhou M.-M., and Suchy F. J. (2003) A 14-amino acid sequence with a β-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J. Biol. Chem. 278, 4000–4009 10.1074/jbc.M207163200 [DOI] [PubMed] [Google Scholar]
- 10. Muthusamy S., Malhotra P., Hosameddin M., Dudeja A. K., Borthakur S., Saksena S., Gill R. K., Dudeja P. K., and Alrefai W. A. (2015) N-glycosylation is essential for ileal ASBT function and protection against proteases. Am. J. Physiol. Cell Physiol. 308, C964–C971 10.1152/ajpcell.00023.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarwar Z., Annaba F., Dwivedi A., Saksena S., Gill R. K., and Alrefai W. A. (2009) Modulation of ileal apical Na+-dependent bile acid transporter ASBT by protein kinase C. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G532–G538 10.1152/ajpgi.00052.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Annaba F., Sarwar Z., Gill R. K., Ghosh A., Saksena S., Borthakur A., Hecht G. A., Dudeja P. K., and Alrefai W. A. (2012) Enteropathogenic Escherichia coli inhibits ileal sodium-dependent bile acid transporter ASBT. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1216–G1222 10.1152/ajpgi.00017.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chamberlain L. H., and Shipston M. J. (2015) The physiology of protein S-acylation. Physiol. Rev. 95, 341–376 10.1152/physrev.00032.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korycka J., Łach A., Heger E., Bogusławska D. M., Wolny M., Toporkiewicz M., Augoff K., Korzeniewski J., and Sikorski A. F. (2012) Human DHHC proteins: A spotlight on the hidden player of palmitoylation. Eur. J. Cell Biol. 91, 107–117 10.1016/j.ejcb.2011.09.013 [DOI] [PubMed] [Google Scholar]
- 15. Tabaczar S., Czogalla A., Podkalicka J., Biernatowska A., and Sikorski A. F. (2017) Protein palmitoylation: Palmitoyltransferases and their specificity. Exp. Biol. Med. (Maywood). 242, 1150–1157 10.1177/1535370217707732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemonidis K., Salaun C., Kouskou M., Diez-Ardanuy C., Chamberlain L. H., and Greaves J. (2017) Substrate selectivity in the zDHHC family of S-acyltransferases. Biochem. Soc. Trans. 45, 751–758 10.1042/BST20160309 [DOI] [PubMed] [Google Scholar]
- 17. Banerjee A., Ray A., Chang C., and Swaan P. W. (2005) Site-directed mutagenesis and use of bile acid-MTS conjugates to probe the role of cysteines in the human apical sodium-dependent bile acid transporter (SLC10A2). Biochemistry. 44, 8908–8917 10.1021/bi050553s [DOI] [PubMed] [Google Scholar]
- 18. Chothe P. P., Czuba L. C., Moore R. H., and Swaan P. W. (2018) Human bile acid transporter ASBT (SLC10A2) forms functional non-covalent homodimers and higher order oligomers. Biochim. Biophys. Acta 1860, 645–653 10.1016/j.bbamem.2017.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang H., Zhang X., Chen X., Aramsangtienchai P., Tong Z., and Lin H. (2018) Protein lipidation: Occurrence, mechanisms, biological functions, and enabling technologies. Chem. Rev. 118, 919–988 10.1021/acs.chemrev.6b00750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mueller G. M., Maarouf A. B., Kinlough C. L., Sheng N., Kashlan O. B., Okumura S., Luthy S., Kleyman T. R., and Hughey R. P. (2010) Cys palmitoylation of the β subunit modulates gating of the epithelial sodium channel. J. Biol. Chem. 285, 30453–30462 10.1074/jbc.M110.151845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mukherjee A., Mueller G. M., Kinlough C. L., Sheng N., Wang Z., Mustafa S. A., Kashlan O. B., Kleyman T. R., and Hughey R. P. (2014) Cysteine palmitoylation of the γ subunit has a dominant role in modulating activity of the epithelial sodium channel. J. Biol. Chem. 289, 14351–14359 10.1074/jbc.M113.526020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McClure M., DeLucas L. J., Wilson L., Ray M., Rowe S. M., Wu X., Dai Q., Hong J. S., Sorscher E. J., Kappes J. C., and Barnes S. (2012) Purification of CFTR for mass spectrometry analysis: Identification of palmitoylation and other post-translational modifications. Protein Eng. Des. Sel. 25, 7–14 10.1093/protein/gzr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McClure M. L., Wen H., Fortenberry J., Hong J. S., and Sorscher E. J. (2014) S-palmitoylation regulates biogenesis of core glycosylated wild-type and F508del CFTR in a post-ER compartment. Biochem. J. 459, 417–425 10.1042/BJ20131037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foster J. D., and Vaughan R. A. (2011) Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation. J. Biol. Chem. 286, 5175–5186 10.1074/jbc.M110.187872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moritz A. E., Rastedt D. E., Stanislowski D. J., Shetty M., Smith M. A., Vaughan R. A., and Foster J. D. (2015) Reciprocal phosphorylation and palmitoylation control dopamine transporter kinetics. J. Biol. Chem. 290, 29095–29105 10.1074/jbc.M115.667055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Werno M. W., and Chamberlain L. H. (2015) S-acylation of the insulin-responsive aminopeptidase (IRAP): Quantitative analysis and identification of modified cysteines. Sci. Rep. 5, 12413 10.1038/srep12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forrester M. T., Hess D. T., Thompson J. W., Hultman R., Moseley M. A., Stamler J. S., and Casey P. J. (2011) Site-specific analysis of protein S-acylation by resin-assisted capture. J. Lipid Res. 52, 393–398 10.1194/jlr.D011106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jang D., Kwon H., Jeong K., Lee J., and Pak Y. (2015) Essential role of flotillin-1 palmitoylation in the intracellular localization and signaling function of IGF-1 receptor. J. Cell Sci. 128, 2179–2190 10.1242/jcs.169409 [DOI] [PubMed] [Google Scholar]
- 29. Annaba F., Sarwar Z., Kumar P., Saksena S., Turner J. R., Dudeja P. K., Gill R. K., and Alrefai W. A. (2008) Modulation of ileal bile acid transporter (ASBT) activity by depletion of plasma membrane cholesterol: Association with lipid rafts. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G489–G497 10.1152/ajpgi.00237.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Annaba F., Ma K., Kumar P., Dudeja A. K., Kineman R. D., Shneider B. L., Saksena S., Gill R. K., and Alrefai W. A. (2010) Ileal apical Na+-dependent bile acid transporter ASBT is upregulated in rats with diabetes mellitus induced by low doses of streptozotocin. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G898–G906 10.1152/ajpgi.00139.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaebler A., Milan R., Straub L., Hoelper D., Kuerschner L., and Thiele C. (2013) Alkyne lipids as substrates for click chemistry-based in vitro enzymatic assays. J. Lipid Res. 54, 2282–2290 10.1194/jlr.D038653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yap M. C., Kostiuk M. A., Martin D. D. O., Perinpanayagam M. A., Hak P. G., Siddam A., Majjigapu J. R., Rajaiah G., Keller B. O., Prescher J. A., Wu P., Bertozzi C. R., Falck J. R., and Berthiaume L. G. (2010) Rapid and selective detection of fatty acylated proteins using omega-alkynyl-fatty acids and click chemistry. J. Lipid Res. 51, 1566–1580 10.1194/jlr.D002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jump D. B. (2002) The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 277, 8755–8758 10.1074/jbc.R100062200 [DOI] [PubMed] [Google Scholar]
- 34. Liang X., Nazarian A., Erdjument-Bromage H., Bornmann W., Tempst P., and Resh M. D. (2001) Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J. Biol. Chem. 276, 30987–30994 10.1074/jbc.M104018200 [DOI] [PubMed] [Google Scholar]
- 35. Casey W. M., Gibson K. J., and Parks L. W. (1994) Covalent attachment of palmitoleic acid (C16:1 delta 9) to proteins in Saccharomyces cerevisiae. Evidence for a third class of acylated proteins. J. Biol. Chem. 269, 2082–2085 [PubMed] [Google Scholar]
- 36. Shipston M. J. (2014) Ion channel regulation by protein S-acylation. J. Gen. Physiol. 143, 659–678 10.1085/jgp.201411176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chavda B., Arnott J. A., and Planey S. L. (2014) Targeting protein palmitoylation: Selective inhibitors and implications in disease. Expert Opin. Drug Discov. 9, 1005–1019 10.1517/17460441.2014.933802 [DOI] [PubMed] [Google Scholar]
- 38. Zhou F., Xue Y., Yao X., and Xu Y. (2006) CSS-Palm: Palmitoylation site prediction with a clustering and scoring strategy (CSS). Bioinformatics 22, 894–896 10.1093/bioinformatics/btl013 [DOI] [PubMed] [Google Scholar]
- 39. Ren J., Wen L., Gao X., Jin C., Xue Y., and Yao X. (2008) CSS-Palm 2.0: An updated software for palmitoylation sites prediction. Protein Eng. Des. Sel. 21, 639–644 10.1093/protein/gzn039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu L.-L., Wan S.-B., Niu S., Shi X.-H., Li H.-P., Cai Y.-D., and Chou K.-C. (2011) Prediction and analysis of protein palmitoylation sites. Biochimie 93, 489–496 10.1016/j.biochi.2010.10.022 [DOI] [PubMed] [Google Scholar]
- 41. Xie Y., Zheng Y., Li H., Luo X., He Z., Cao S., Shi Y., Zhao Q., Xue Y., Zuo Z., and Ren J. (2016) GPS-lipid: A robust tool for the prediction of multiple lipid modification sites. Sci. Rep. 6, 28249 10.1038/srep28249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muszbek L., Haramura G., Cluette-Brown J. E., Van Cott E. M., and Laposata M. (1999) The pool of fatty acids covalently bound to platelet proteins by thioester linkages can be altered by exogenously supplied fatty acids. Lipids 34 (suppl.) S331–S337 10.1007/bf02562334 [DOI] [PubMed] [Google Scholar]
- 43. Greaves J., Munro K. R., Davidson S. C., Riviere M., Wojno J., Smith T. K., Tomkinson N. C. O., and Chamberlain L. H. (2017) Molecular basis of fatty acid selectivity in the zDHHC family of S-acyltransferases revealed by click chemistry. Proc. Natl. Acad. Sci. U.S.A. 114, E1365–E1374 10.1073/pnas.1612254114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou B., An M., Freeman M. R., and Yang W. (2014) Technologies and challenges in proteomic analysis of protein S-acylation. J. Proteomics Bioinform. 7, 256–263 10.4172/jpb.1000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hussainzada N., Banerjee A., and Swaan P. W. (2006) Transmembrane domain VII of the human apical sodium-dependent bile acid transporter ASBT (SLC10A2) lines the substrate translocation pathway. Mol. Pharmacol. 70, 1565–1574 10.1124/mol.106.028647 [DOI] [PubMed] [Google Scholar]
- 46. Moore R. H., Chothe P., and Swaan P. W. (2013) Transmembrane domain V plays a stabilizing role in the function of human bile acid transporter SLC10A2. Biochemistry 52, 5117–5124 10.1021/bi400028q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ren W., Jhala U. S., and Du K. (2013) Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte 2, 17–28 10.4161/adip.22117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sanders S. S., Martin D. D. O., Butland S. L., Lavallée-Adam M., Calzolari D., Kay C., Yates J. R. 3rd, and Hayden M. R. (2015) Curation of the mammalian palmitoylome indicates a pivotal role for palmitoylation in diseases and disorders of the nervous system and cancers. PLoS Comput. Biol. 11, e1004405 10.1371/journal.pcbi.1004405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thorne R. F., Ralston K. J., de Bock C. E., Mhaidat N. M., Zhang X. D., Boyd A. W., and Burns G. F. (2010) Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum. Biochim. Biophys. Acta 1803, 1298–1307 10.1016/j.bbamcr.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 50. Zhao L., Zhang C., Luo X., Wang P., Zhou W., Zhong S., Xie Y., Jiang Y., Yang P., Tang R., Pan Q., Hall A. R., Luong T. V., Fan J., Varghese Z., Moorhead J. F., Pinzani M., Chen Y., and Ruan X. Z. (2018) CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J. Hepatol. 69, 705–717 10.1016/j.jhep.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 51. Spinelli M., Fusco S., and Grassi C. (2018) Nutrient-dependent changes of protein palmitoylation: Impact on nuclear enzymes and regulation of gene expression. Int. J. Mol. Sci. 19, 3820 10.3390/ijms19123820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu Z., and Zhong L. (2015) New insights into the posttranslational regulation of human cytosolic thioredoxin by S-palmitoylation. Biochem. Biophys. Res. Commun. 460, 949–956 10.1016/j.bbrc.2015.03.132 [DOI] [PubMed] [Google Scholar]
- 53. Fritz K. S., Green M. F., Petersen D. R., and Hirschey M. D. (2013) Ethanol metabolism modifies hepatic protein acylation in mice. PLoS One 8, e75868 10.1371/journal.pone.0075868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spinelli M., Fusco S., Mainardi M., Scala F., Natale F., Lapenta R., Mattera A., Rinaudo M., Li Puma D. D., Ripoli C., Grassi A., D'Ascenzo M., and Grassi C. (2017) Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 8, 2009 10.1038/s41467-017-02221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gill R. K., Saksena S., Alrefai W. A., Sarwar Z., Goldstein J. L., Carroll R. E., Ramaswamy K., and Dudeja P. K. (2005) Expression and membrane localization of MCT isoforms along the length of the human intestine. Am. J. Physiol. Cell Physiol. 289, C846–C852 10.1152/ajpcell.00112.2005 [DOI] [PubMed] [Google Scholar]
- 56. Dudeja P. K., Baldwin M. L., Harig J. M., Cragoe E. J. Jr., Ramaswamy K., and Brasitus T. A. (1994) Mechanisms of Na+ transport in human distal colonic apical membrane vesicles. Biochim. Biophys. Acta 1193, 67–76 10.1016/0005-2736(94)90334-4 [DOI] [PubMed] [Google Scholar]
- 57. Harig J. M., Dudeja P. K., Knaup S. M., Shoshara J., Ramaswamy K., and Brasitus T. A. (1990) Apical plasma membrane vesicles formed from organ donor colon demonstrate Na+ and H+ conductances and Na+/H+ exchange. Biochem. Biophys. Res. Commun. 167, 438–443 10.1016/0006-291X(90)92042-X [DOI] [PubMed] [Google Scholar]
- 58. Hong V., Steinmetz N. F., Manchester M., and Finn M. G. (2010) Labeling live cells by copper-catalyzed alkyne–azide click chemistry. Bioconjugate Chem. 21, 1912–1916 10.1021/bc100272z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 60. Ticho A. L., Lee H., Gill R. K., Dudeja P. K., Saksena S., Lee D., and Alrefai W. A. (2018) A novel bioluminescence-based method to investigate uptake of bile acids in living cells. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G529–G537 10.1152/ajpgi.00133.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]