Abstract
Background
Opioid dependence, misuse, and abuse in the United States continue to rise. Prior studies indicate an important risk factor for persistent opioid use includes elective surgical procedures, though the probability following thoracic procedures remains unknown. We analyzed the incidence and factors associated with new persistent opioid use after lung resection
Methods
We evaluated data from opioid-naïve cancer patients undergoing lung resection between 2010 and 2014 using insurance claims from the Truven Health MarketScan Databases. New persistent opioid usage was defined as continued opioid prescription fills between 90 and 180 days following surgery. Variables with p<0.10 by univariate analysis were included in a multivariable logistic regression performed for risk adjustment. Multivariable results were each reported with odds ratio (OR) and confidence interval (CI)
Results
3,026 patients (44.8% male; 55.2% female) were identified as opioid-naïve undergoing lung resection. Mean age was 64 ± 11 years and mean postoperative length of stay (LOS) was 5.2±3.3 days. 6.5% underwent neoadjuvant therapy, while 21.7% underwent adjuvant therapy. Among opioid-naïve patients, 14% continued to fill opioid prescriptions following lung resection. Multivariable analysis showed that age ≤ 64 (OR 1.28 [CI 1.03–1.59], p=0.028), male sex (1.40 [1.13–1.73], p=0.002), postoperative LOS (1.32 [1.05–1.65], p=0.016), thoracotomy (1.58 [1.24–2.02], p<0.001), and adjuvant therapy (2.19 [1.75–2.75], p<0.001) were independent risk factors for persistent opioid usage
Conclusions
The greatest risk factors for persistent opioid use (14%) following lung resection were adjuvant therapy and thoracotomy. Future studies should focus on reducing excess prescribing, perioperative patient education, and safe opioid disposal.
Keywords: Lung cancer surgery, Pain, Chemotherapy, Radiation therapy, Surgery, complications, Thoracoscopy/VATS, Thoracotomy
INTRODUCTION
Opioid abuse and opioid-related deaths in the United States have continued to rise, with an increase in drug overdoses from 52,400 in 2015 to 63,600 in 2016, highlighting a national crisis that last year accounted for more deaths than breast cancer [1].
Surgeons play a unique role in the opioid crisis by oftentimes prescribing opioids to previously opioid-naïve patients. This prescription initially serves as an appropriate therapeutic measure, though may be the sentinel opioid prescription leading to persistent usage. Rates of persistent opioid usage beyond 90 days following surgery have been previously reported between 3% and 8% in analyses of minor and major elective operations [2–4], and over 10% in early-stage cancer patients undergoing curative surgery [5].
While previous analyses have characterized all surgical patients, little is known about patients undergoing lung resection for cancer. This cohort of patients carries established risk factors for increased opioid usage due to their cancer diagnosis, potential for chemoradiation, and morbid operations, whether through a thoracotomy or minimally-invasive thoracoscopic approach.
We undertook a retrospective cohort study of opioid-naïve, privately insured patients undergoing resection for lung cancer in the United States. We aimed to define the incidence of new persistent opioid usage among previously opioid-naïve patients and explore risk factors associated with new persistent opioid usage in this population. We hypothesized that the rate of new persistent opioid users would equal established rates for surgical cancer patients. We further evaluated risk factors such as surgical approach, in addition to previously reported risk factors.
PATIENTS AND METHODS
Patient Data Source
We identified eligible patients through insurance claims from the Truven Health Marketscan Research Databases [6]. This database encompasses over 100 health plans in the United States. The University of Michigan Institutional Review Board granted this study “exempt” status.
Opioid Prescription Definitions
As with prior work [4,5], opioid prescriptions were attributed to surgery if filled between 30 days prior to surgery and 14 days after discharge. Patients were defined as opioid-naïve if no opioid prescriptions were filled between 12 months and 31 days before surgery, consistent with prior analyses [2,4,5]. This 30-day window accounts for opioids prescribed immediately prior to surgery, intended for use by the patient postoperatively.
Patient Population
We included lung cancer patients age ≥ 18 years old who were opioid-naïve, underwent curative-intent lung resection between January 1, 2010 and June 30, 2014, and filled an opioid prescription attributed to the operation. Patients had continuous insurance enrollment for at least one year before and after surgery. Exclusion criteria consisted of patients with hospital admissions > 30 days, underwent a subsequent procedure within 180 days, discharged to home hospice care, and those who died during the index hospitalization.
As described previously [5], data on age, sex, household income based on metropolitan statistical area code, and insurance type were included. Patients who underwent neoadjuvant chemotherapy and/or radiation during the 12 months before surgery and patients who underwent adjuvant chemotherapy and/or radiation during the 180 days after surgery were included. Comorbidities were quantified using the Elixhauser comorbidity score [7]. Elixhauser software assigns variables identifying comorbidities in using ICD-9 diagnosis coding, from which an index score is created that describes the comorbidity burden and may be useful for multivariate analyses of inpatient procedures [8]. The Agency of Healthcare Research and Quality (AHRQ) Clinical Classification System was used to identify psychiatric diagnoses, including mood, personality, and substance use disorders. Variables included were age (≤64 years old vs >64), sex, income (≤$70,000 vs >$70,000), history of anxiety, depression, or substance abuse, neoadjuvant therapy, procedure type (thoracotomy versus video-assisted thoracoscopic surgery [VATS]), occurrence of a postoperative complication, postoperative length of stay (≤5 days vs > 5 days), and adjuvant therapy. The AHRQ classification system collapses ICD-9 diagnosis and procedure codes into clinically meaningful categories useful for presenting descriptive statistics, including categories from the Clinical Classifications Software for Mental Health and Substance Abuse.
Thoracotomy-related resections included CPT procedure codes 32440, 32442, 32445, 32480, 32482, 32484, 32486, 32488, and 32503–32505, while VATS resections were captured in codes 32663, 32666, and 32669–32671. Eighty-one patients were coded for both thoracotomy and VATS in the database. These patients were treated as VATS cases converted to thoracotomy and were thus reported as thoracotomy for the purposes of this analysis. Sixty-one patients did not carry a code for thoracotomy or VATS and therefore were excluded from the regression analysis.
Outcome
The primary outcome was new persistent opioid usage, defined as opioid-naïve patients who filled an opioid prescription attributed to surgery and filled at least one additional opioid prescription between 91 and 180 days after surgery. The choice of 91 days repeats a standard used in prior studies [2,4,5,9], after which recovery from surgery is expected.
Statistical Analyses
Statistical analyses were conducted using SPSS, version 25 (IBM Corp). Univariate analysis was performed using chi-square testing. Variables found to have p<0.10 were selected for inclusion in a multivariable model. Multivariable logistic regression analysis was then performed for risk adjustment. Multivariable results were reported with odds ratio (OR) and 95% confidence interval (CI) for each. P-values were deemed significant if less than 0.05. Model fit was evaluated using the Hosmer-Lemeshow test.
RESULTS
A total of 3,026 opioid-naïve patients (44.8% male; 55.2% female) underwent lung resection. Mean age was 64 ± 11 years and 67% (2026/3026) of patients had an annual income of ≤$70,000. Anxiety, depression, and substance abuse were reported amongst 7.5% (227/3026), 5.7% (173/3026), and 17.1% (518/3026) of patients, respectively. 6.5% (196/3026) of patients underwent neoadjuvant, while 21.7% (657/3026) underwent adjuvant therapy following surgery. Thoracotomy was performed in 56.9% (1722/3026) of patients, VATS was performed in 41.1% (1243/3026), and surgical approach was unknown for 2.0% (61/3026). Mean postoperative length of stay (LOS) was 5.2 ± 3.3 days (Table 1).
Table 1.
Patient characteristics.
| Variable | Total n = 3,026 |
|---|---|
| Male sex | 1356 (44.8%) |
| Mean + SD age, years | 64 + 11 |
| Income ≤ $70,000 | 2026 (67.0%) |
| Anxiety | 227 (7.5%) |
| Depression | 173 (5.7%) |
| Prior substance abuse | 518 (17.1%) |
| Neoadjuvant chemotherapy | 184 (6.1%) |
| Neoadjuvant radiation | 44 (1.5%) |
| Adjuvant chemotherapy | 603 (19.9%) |
| Adjuvant radiation | 164 (5.4%) |
| Surgical Approach | |
| Thoracotomy | 1722 (56.9%) |
| VATS | 1243 (41.1%) |
| Unknown | 61 (2.0%) |
| Mean postoperative length of stay + SD, days | 5.2 + 3.3 |
| Median OME perioperative prescribed | 315 (IQR 225–500) |
IQR, interquartile range; OME, oral morphine equivalent; SD, standard deviation; VATS, video-assisted thoracoscopic surgery.
Overall 14% (424/3,026) of opioid-naïve patients continued to fill opioid prescriptions 90–180 days after lung resection. Among those who underwent thoracotomy, 17.1% (294/1722) persistently used opioids, as compared to 9.4% (117/1243) of VATS patients (p<0.001). Among patients undergoing adjuvant therapy, 23.9% (157/657) became persistent opioid users as compared to 11.4% (267/2369) of patients who did not undergo adjuvant therapy (p<0.001). Additionally, univariate analysis indicated patients with persistent opioid usage were more likely to be younger, male, earn an annual income of ≤$70,000, have a history of substance abuse, have a post-operative complication, and have a LOS longer than 5 days (Table 2).
Table 2.
Univariate analysis of patient characteristics and new persistent opioid usage.
| Variable | No Persistent Use, Total n = 2602 | Persistent Opioid Use, Total n = 424 | P-value |
|---|---|---|---|
| Age ≤ 64 years* | 1345 (51.7%) | 245 (57.8%) | 0.021 |
| Male sex* | 1130 (43.4%) | 226 (53.3%) | <0.001 |
| Income ≤ $70,000* | 1715 (65.9%) | 311 (73.3%) | 0.003 |
| Anxiety | 198 (7.6%) | 29 (7.8%) | 0.620 |
| Depression | 147 (5.6%) | 26 (6.1%) | 0.653 |
| Substance Abuse* | 423 (16.3%) | 95 (22.4%) | 0.003 |
| Neoadjuvant Therapy | 164 (6.3%) | 32 (7.5%) | 0.338 |
| Surgical Approach | |||
| Thoracotomy* | 1428 (54.8%) | 294 (69.3%) | |
| VATS | 1126 (43.2%) | 117 (27.6%) | <0.001 |
| Unknown | 48 (1.8%) | 13 (3.1%) | |
| Postoperative Length of Stay > 5 days* | 852 (32.7%) | 188 (44.3%) | <0.001 |
| Adjuvant Therapy* | 500 (19.2%) | 157 (37.0%) | <0.001 |
VATS, video-assisted thoracoscopic surgery. All variables marked with
were significant on univariate analysis and included in multivariable binary regression model.
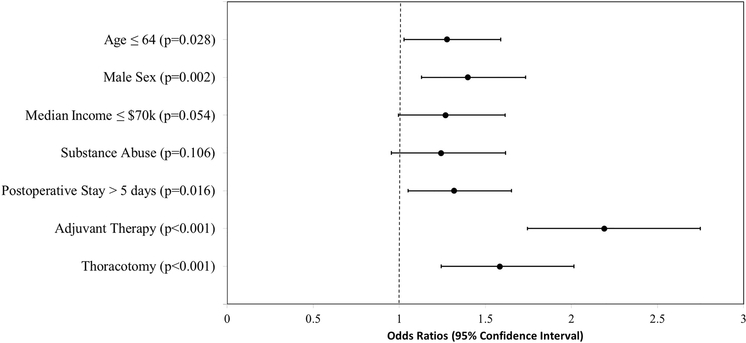
Each significant variable from univariate analysis was included in a multivariable logistic regression model. Independent patient risk factors for persistent opioid usage from multivariable analysis were age ≤ 64 (OR 1.30 [CI 1.05 to 1.62]), male sex (1.39 [1.12 to 1.72]), postoperative LOS > 5 days (1.30 [1.04 to 1.63]), adjuvant therapy (2.19 [1.74 to 2.75]), and thoracotomy (1.58 [1.24 to 2.01]) [Figure].
Figure.
Multivariable regression analysis of new persistent opioid usage after lung resection.
COMMENT
Patients undergoing resection for lung cancer had a 14% rate of new persistent opioid usage among previously opioid-naïve cancer patients. Notably, 17.1% of patients undergoing thoracotomy for lung resection developed new persistent opioid usage versus 9.4% of patients undergoing VATS. Multivariable analysis showed that resection via thoracotomy conferred a greater than 50% increased risk of new persistent opioid usage (OR 1.58 [95% CI 1.24 to 2.02]) compared to a VATS approach. Additional independent risk factors for new persistent usage included age ≤ 64, male sex, postoperative LOS > 5 days, and adjuvant therapy.
Multiple prior studies have utilized our definitions of opioid-naïve and new persistent opioid usage. Clarke et al. [2] found a new persistent opioid usage rate of 3.1% among a cohort of patients ≥ 66 years old having major elective cardiac, thoracic, intra-abdominal, and pelvic procedures. The authors compared open pelvic procedures such as prostatectomies to thoracic procedures and found that both open and minimally-invasive thoracic procedures were associated with significantly higher risks of persistent opioid usage (OR 2.58 [CI 2.03 to 3.28] and OR 1.95 [CI 1.36 to 2.78], respectively). They found rates of 8.5% and 6.3% of prolonged opioid use for open and minimally-invasive intrathoracic procedures, respectively, as compared to our findings of 17.1% and 9.4%, respectively. However, the patients in Clarke et al’s analysis were all ≥ 66 years old, whereas our analysis included all patients over 18 years old and identified age of less than 65 to be an independent risk factor for persistent opioid usage.
Additionally, Clarke’s analysis includes all intrathoracic elective procedures, as opposed to our study’s population, consisting entirely of lung cancer patients. It is clear from prior work [5] that cancer diagnoses confer additional risk of persistent opioid usage, as compared to non-cancer patients undergoing elective operations [4]. A wide-ranging analysis of curative-intent cancer patients by Lee et al. [5] concluded a rate of more than 10% new persistent opioid usage, substantially higher than prior studies and comparable to our figure of 14% for lung resection patients. A longer term follow-up of Clarke’s analysis to report ongoing opioid therapy at 180, 270, and 365 days after surgery found that while only 0.4% of the patients continued receiving opioid prescriptions one year after surgery, the highest risk of persistent opioid use occurred after open (1.7% [CI 1.2% to 2.3%]) and minimally invasive (1.3% [CI 0.6% to 2.5%]) lung resection procedures [3].
Lung resection patients confer pain management challenges due to both disease process and surgical approach. Cancer patients confer a higher risk of persistent opioid usage, which is enhanced by adjuvant therapy, found to be the only variable significantly associated with new persistent opioid usage across all cancer procedures [5]. This is consistent with our analysis identifying adjuvant therapy as the strongest risk factor for new persistent opioid usage after lung resection (OR 2.19 [CI 1.75 to 2.75]). Definitive chemoradiation has demonstrated a 33% rate of new persistent opioid usage [14], and this may be due to the agents themselves and associated neurotoxicity [10–13]. Adjuvant therapy may also present a psychological, emotional, and physical challenge when compounded with recovery from the index surgical operation. We did not find neoadjuvant therapy to be a risk factor for persistent usage (p=0.338), suggesting the effects of adjuvant chemotherapy on opioid usage may be additive or synergistic with surgical morbidity.
Much of the pain-generating morbidity from lung resection is from the incision(s) made during the operation. The two most common approaches which are compared in this analysis are a traditional thoracotomy versus a VATS approach. While existing data detail a variety of elective and curative-intent surgery on non-cancer and cancer patients of varying morbidity, thoracic lung resection through a thoracotomy is associated with significant pain-related morbidity. Prior studies have shown that 40–80% of patients may develop post-thoracotomy pain syndrome, with significant disruption to daily living [16,17] and increased use of pain medication [17,18]. It has additionally been shown that post-thoracotomy pain is often neuropathic and does not respond well to opioids [18–20]. These figures put into perspective why over 17% of lung resection patients undergoing thoracotomy in our analysis may still be using opioids 90–180 days following surgery, well beyond the period during which acute pain should have resolved. In our cohort we only found a 1.6% rate of documented post-thoracotomy pain syndrome, though this may be underestimated since we excluded patients who died, went to home hospice, or had a hospital stay beyond 30 days, and not all cases may have been captured through insurance claims. A large systematic review investigating the incidence of severe chronic pain at 3 and 6 months after thoracotomy found rates of approximately 50%, and these reported rates have remained largely unchanged since the 1990s [15], suggesting that despite an array of pain management strategies, postsurgical thoracotomy pain remains a challenge. While our data captured a 17.1% rate of persistent opioid usage in patients following thoracotomy, we may not have captured additional patients with chronic pain managed through non-opioid therapies.
With these concerns in mind, VATS has popularized as an alternate approach. Multiple observational studies have demonstrated benefit of VATS over thoracotomy in postoperative recovery, lung function, and perioperative morbidity [21–24], but multicenter, prospective, randomized evidence is sparse. Regarding postoperative pain specifically, two randomized controlled trials in Europe found that VATS was associated with less postoperative pain and better quality of life as measured by multiple metrics [25–26]. Our data support these findings, as undergoing thoracotomy rather than VATS conferred a greater than 50% risk of persistent opioid usage on multivariable analysis of our cohort.
In efforts to decrease opioid usage, adjunct pain medications may assist in managing postoperative pain. Adjuncts such as gabapentin may prove especially useful in treating neuropathic pain, associated with post-thoracotomy pain syndrome and adjuvant chemotherapy [27–29]. Additionally, scheduled acetaminophen may be used in conjunction with an opioid. Intraoperatively, injectable medications may be used to help manage postoperative pain. Extrapleural infusion for intercostal nerve blockade was found to be at least as effective as an epidural and significantly better than opioid treatment alone [30]. More recently, liposomal bupivacaine, providing an intercostal nerve blockade for 72 to 96 hours as compared to the 6- to 8-hour blockade provided by standard bupivacaine, has been proposed as an alternative to thoracic epidurals, local anesthetic blockades, and intercostal nerve catheters with patient-controlled analgesia (PCA) [31]. One report concluded liposomal bupivacaine (versus epidural) had lower mean pain scores on postoperative days 1 and 3, less pulmonary complications, and a nearly 2-day shorter length of hospital stay, though no difference in opioid utilization [32]. An analysis of orthopedic patients undergoing total knee arthroplasty found significantly reduced opioid usage and need for PCA in the first 24 hours after surgery, but higher pain scores at each of 48, 72, and 96 hours postoperatively [33]. One randomized study of non-pharmacologic management of postoperative pain found back massage to result in short-term decrease in pain intensity, unpleasantness, and anxiety as compared to a control group, though opioid consumption did not differ between groups [34]. The increased use of non-opioid medications and non-pharmacologic interventions is an area which requires further investigation in thoracic patients.
Postoperative iatrogenic opioid dependence following lung resection occurs as often as other common and significant postoperative complications, such as atrial fibrillation [35]. Multiple well-powered studies have shown that surgical patients are using substantially less opioids than prescribed [36–37]. While some evidence-based guidelines have been established to reduce excess prescribing [38], nationwide guidelines have not been determined. A recent study of laparoscopic cholecystectomy patients found the median amount of opioid prescribed was reduced from 250 milligrams (mg) to 75 mg (p<0.01) after implementing guidelines based on the 66th percentile of the number being taken, reducing prescription size by 63% without affecting refill requests or pain scores [39].
Acute inpatient postoperative pain as a predictor of chronic postoperative pain following discharge could not be examined with these administrative data but warrants further investigation for pain management optimization. Additionally, multidisciplinary care centered around patient education is paramount to reducing persistent and excessive opioid usage. In addition to controlling overprescribing, care-givers must educate patients about the risks of opioids, alternatives and adjuncts to opioids, pain expectations following surgery, and instructions for proper disposal of unused pills. These efforts have begun in Michigan with active participation from clinicians in collaboration with payers [40].
Our study has several limitations. First, our population was limited to adults with enrollment in employer-based insurance and did not adjust for race and ethnicity, though we did adjust for sex, income, and insurer. Second, while new persistent opioid usage fits a very specific definition, our follow up is limited to 180 days after surgery. Third, insurance claims for filled opioid prescriptions is not specific to any one operation, though excluding patients with subsequent operations within 180 days helps to minimize this limitation. While our exclusion criteria may relate to a higher risk of opioid usage, each of the criteria represent exceptional clinical scenarios that could affect data reliability if included. Fourth, these administrative data include prescription pain medication only and thus did not include information about over-the-counter preoperative, intraoperative, and postoperative adjuncts used for pain management. Finally, we were unable to control potential confounding by lung cancer stage.
In conclusion, we demonstrate 14% of opioid-naïve lung cancer patients undergoing surgery persistently used opioids postoperatively. This cohort of patients carries the highest reported rate of new persistent opioid usage following surgery, with patients undergoing an thoracotomy (OR 1.58 [CI 1.24 to 2.02]) and receiving adjuvant therapy (OR 2.19 [CI 1.75 to 2.75]) representing the two groups at highest risk of persistent opioid usage. This iatrogenic complication which matches or exceeds the prevalence of atrial fibrillation necessitates further studies to elucidate risk factors associated with persistent opioid usage, prioritizing and establishing standard protocols for patient education and safe disposal of excess medications, and development and implementation of evidence-based opioid prescribing guidelines.
Supplementary Material
REFERENCES
- 1.Hedegaard H, Warner M, Minino AM. Drug overdose deaths in the United States, 1999–2016. NCHS data brief, no 294, Hyattsville, MD: National Center for Health Statistics, 2017. [Google Scholar]
- 2.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014. February;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soneji N, Clarke HA, Ko DT, et al. Risks of Developing Persistent Opioid Use After Major Surgery. JAMA Surg 2016. November;151(11):1083–1084. [DOI] [PubMed] [Google Scholar]
- 4.Brummett CM, Waljee JF, Goesling J, et al. : New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JSJ, Hu HM, Edelman AL, et al. New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol 2017;35(36):4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen L: The Truven Health MarketScan Databases for life sciences researchers. Ann Arbor, Michigan, Truven Health Analytics, 2017. [Google Scholar]
- 7.van Walraven C, Austin PC, Jennings A, et al. : A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 47:626–633, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Identifying Increased Risk of Readmission and In-Hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Exit Disclaimer Medical Care 2017. July; 55(7):698–705. [DOI] [PubMed] [Google Scholar]
- 9.Alam A, Gomes T, Zheng H, et al. : Long-term analgesic use after low-risk surgery: A retrospective cohort study. Arch Intern Med 172:425–430, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Jung BF, Herrmann D, Griggs J, et al. : Neuropathic pain associated with non-surgical treatment of breast cancer. Pain 118:10–14, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Land SR, Kopec JA, Cecchini RS, et al. : Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 25:2205–2211, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Mols F, Beijers T, Lemmens V, et al. : Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: Results from the population-based PROFILES registry. J Clin Oncol 31:2699–2707, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Whelan TJ, Levine M, Julian J, et al. : The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer 88:2260–2266, 2000. [PubMed] [Google Scholar]
- 14.Kwon JH, Hui D, Chisholm G, et al. : Predictors of long-term opioid treatment among patients who receive chemoradiation for head and neck cancer. Oncologist 18:768–774, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayman EO, Brennan TJ: Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: Meta-analysis. J Pain 15:887–897, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Tiippana E, Nilsson E, Kalso E. Post-thoracotomy pain after thoracic epidural analgesia: a prospective follow-up study. Acta Anaesthesiol Scand 2003;47:433–8. [DOI] [PubMed] [Google Scholar]
- 17.Wildgaard K, Ravn J, Nikolajsen L, Jakobsen E, Jensen TS, Kehlet H. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand 2011;55:60–8. [DOI] [PubMed] [Google Scholar]
- 18.Steegers MAH, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OHG. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 2008;9:955–61. [DOI] [PubMed] [Google Scholar]
- 19.Maguire MF, Ravenscroft A, Beggs D, Duffy JP. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg 2006;29:800–5. [DOI] [PubMed] [Google Scholar]
- 20.Duale C, Guastella V, Morand D, Cardot JM, Aublet-Cuvelier B, Mulliez A, et al. Characteristics of the neuropathy induced by thoracotomy: a 4-month follow-up study with psychophysical examination. Clin J Pain 2011;27:471–80. [DOI] [PubMed] [Google Scholar]
- 21.Pages PB, Delpy JP, Orsini B, et al. Propensity Score Analysis Comparing Videothoracoscopic Lobectomy with Thoracotomy: a French Nationwide Study. Ann Thora Surg 2016;101(4):1370–8. [DOI] [PubMed] [Google Scholar]
- 22.Zhao J, Zhao Y, Qiu T, et al. Quality of life and survival after II stage nonsmall cell carcinoma surgery: Video-assisted thoracic surgery versus thoracotomy lobectomy. Indian J Cancer 2015;52 Suppl 2:e130–3. [DOI] [PubMed] [Google Scholar]
- 23.Agostini P, Lugg ST, Adams K, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy. Interact Cardiovasc Thorac Surg 2017;24(6):931–937. [DOI] [PubMed] [Google Scholar]
- 24.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86(6):2008–16; discussion 2016–8. [DOI] [PubMed] [Google Scholar]
- 25.Palade E, Guenter J, Kirschbaum A, Wiesemann S, Passlick B. Postoperative pain in the acute phase after surgery: VATS lobectomy vs. open lung resection – results of a prospective randomized trial. Zentralbl Chir 2014;139 s1l 1:S59–66. [DOI] [PubMed] [Google Scholar]
- 26.Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomized controlled trial. Lancet Oncol 2016;17(6):836–844. [DOI] [PubMed] [Google Scholar]
- 27.Bostick GP, Toth C, Carr EC, et al. : Physical functioning and opioid use in patients with neuropathic pain. Pain Med 2015;16:1361–1368. [DOI] [PubMed] [Google Scholar]
- 28.Gaskell H, Derry S, Stannard C, et al. : Oxycodone for neuropathic pain in adults. Cochrane Database Syst Rev 2016;7:CD010692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kehlet H, Jensen TS, Woolf CJ: Persistent postsurgical pain: Risk factors and prevention. Lancet 2006;367:1618–1625. [DOI] [PubMed] [Google Scholar]
- 30.Detterbeck FC. Efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg 2005. October;80(4):1550–9. [DOI] [PubMed] [Google Scholar]
- 31.Rice DC, Cata JP, Mena GE, et al. Posterior Intercostal Nerve Block with Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. Ann Thorac Surg 2015;99(6):1953–60. [DOI] [PubMed] [Google Scholar]
- 32.Khalil KG, Boutrous ML, Irani AD, et al. Operative Intercostal Nerve Blocks With Long-Acting Bupivacaine Liposome for Pain Control After Thoracotomy. Ann Thorac Surg 2015;100(6):2013–8. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of Liposomal Bupivacaine Infiltration on the Management of Total Knee Arthroplasty. JAMA Surg 2017;152(1):90–95. [DOI] [PubMed] [Google Scholar]
- 34.Mitchinson AR, Kim HM, Rosenberg JM, et al. Acute postoperative pain management using massage as an adjuvant therapy: a randomized trial. Arch Surg 2007. December;142(12):1158–67. [DOI] [PubMed] [Google Scholar]
- 35.Ziarnik E and Grogan EL. Postlobectomy Early Complications. Thorac Surg Clin 2015;25(3):355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feinberg AE, Chesney TR, Srikandarajah S, Acuna SA, McLeod RS. Opioid Use After Discharge in Postoperative Patients: A Systematic Review. Ann Surg 2017; 10.1097/SLA.0000000000002591 [Epub ahead of print on Dec 5, 2017]. [DOI] [PubMed] [Google Scholar]
- 37.Hill MV, McMahon ML, Stucke RS, Barth RJ Jr. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg 2017;265(4):709–714. [DOI] [PubMed] [Google Scholar]
- 38.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ Jr. An Educational Intervention Decreases Opioid Prescribing After General Surgical Operations. Ann Surg 2017. 10.1097/SLA.0000000000002198 [Epub ahead of print on Mar 6, 2017]. [DOI] [PubMed] [Google Scholar]
- 39.Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in Opioid Prescribing Through Evidence-Based Prescribing Guidelines. JAMA Surg 2017; 10.1001/jamasurg.2017.4436 [Epub ahead of print on Dec 6, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic Opioid Dependence in the United States: Are Surgeons the Gatekeepers? Ann Surg 2017;265(4):728–730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.