Abstract
S-Nitrosation is a key posttranslational modification in regulating proteins in both normal physiology and diverse human diseases. To identify novel therapies for human diseases linked to oxidative and nitrosative stress, understanding how cells control S-nitrosation specificity could be critical. Among the enzymes known to control S-nitrosation of proteins, thioredoxin 1 (Trx1), a conserved disulfide reductase, transnitrosates and denitrosates distinct sets of target proteins. To recognize the function of Trx1 in both normal and dysfunctional cells, S-nitrosation targets of Trx1 in different cells need to be identified. However, S-nitrosation is usually too labile to be detected directly by mass spectrometry (MS). Here we present two optimized MS techniques to identify S-nitrosated Trx1 and its transnitrosation targets, using both direct and indirect MS methods.
Keywords: S-nitrosation, Thioredoxin, Transnitrosation, Mass spectrometry
1. Introduction
S-Nitrosation, the covalent addition of the nitric oxide (NO) moiety onto cysteine thiols, is an important posttranslational modification (PTM) for regulating protein functions [1, 2]. This PTM is dynamic, reversible, and site-specific [3, 4]. The studies on the mechanisms that control the specificity of S-nitrosation draw broad interest. Thioredoxin 1 (Trx1) is a key regulator of S-nitrosation. It is a conserved antioxidant protein that is well known not only for its disulfide reductase activity but also for its transnitrosation and denitrosation activities [3, 5]. What distinguishes Trx1 from other modulators of S-nitrosation is its capacity to reversibly regulate this PTM on distinct target proteins, depending on the redox status of its key cysteines [5–9]. To identify S-nitrosation targets of Trx1 in different cells, we have developed several mass spectrometry (MS) methods that we share here.
It is problematic to identify and quantify S-nitrosation, due to its chemical instability [4, 10–14]. For example, several methods have been developed to detect S-nitrosated proteins separated by gel electrophoresis; they involve either a direct Western blotting detection with an anti-S-nitroso-cysteine (SNO-Cys) antibody [15] or an indirect Western blotting detection using a biotin-switch technique (BST) [12, 16]. To detect chemically labile SNO-Cys, Western blotting requires the proteins to be separated by inefficient nonreducing SDS-PAGE, under reduced ambient light, and with shortened binding time of the primary antibodies, compromising both detection sensitivities and specificities. To overcome such limitations, BST has been developed and widely used for detecting S-nitrosated proteins and peptides. The reaction conditions for BST are less onerous than the direct approach; yet, BST is sometimes confounded by false positive signals due to imperfect chemical reaction specificity. Thus, the inclusions of both positive and negative controls are necessary to draw conclusions from BST analyses [17]. Overall, Western blotting methods can only identify S-nitrosated proteins, but not SNO-Cys sites.
Only optimized MS methods can effectively analyze S-nitrosated peptides, and identify SNO-Cys sites. For example, SNO-Cys is rarely observed in the spectra obtained from Matrix-Assisted Laser Desorption Ionization Time-of-Flight (MALDITOF) mass spectrometers, because the laser energies used for peptide protonation could easily deduct NO from the precursor ions during ionization [18]. Under “softer” electrospray ionization (ESI) conditions, S-nitrosated peptides can be observed as a +29 atomic mass unit (amu) peptide ion (with an NO replacing a H) over the unmodified peptide ions for each SNO-Cys [5, 19, 20]. However, the ESI condition needs to be optimized for each type of instrument to avoid NO loss in ESI. In this chapter, we will first describe an ESI method on a Quadrupole-Time-of-Flight (QTOF)-MS to directly detect SNO-Cys sites in a peptide derived from human caspase 3 and in a recombinant human Trx1. Specifically, we will describe how to (1) adjust the buffer compositions and the pH in order to minimize the solution-phase chemical denitrosation, and (2) fine-tune both cone and collision energy voltages for optimal ionization of both S-nitrosated peptides and proteins. This direct approach is specific, but not highly sensitive or quantitative. To overcome these deficiencies and analyze small amounts of S-nitrosated proteins from biological samples, we will also describe a BST approach using the isotope-coded affinity tag (ICAT) reagents to quantify Trx1-induced S-nitrosation in target peptides [21, 22].
2. Materials
2.1. Reagents and Solutions
BCA Protein Assay Kit (Pierce, Rockford, IL, USA).
Half M EDTA, pH 8.0 (Cellgro, Mediatech Inc., Herndon, VA, USA).
One hundred mM ammonium bicarbonate (NH4HCO3), pH 8.0.
N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide (biotin-HPDP) (Thermo Fisher Scientific, Rockford, IL, USA).
pH Test strips (EMD Millipore, Billerica, MA, USA).
Protease inhibitor cocktail (Sigma, St. Louis, MO, USA).
Sequencing-grade trypsin (Promega, Madison, WI, USA).
S-Nitrosoglutathione (GSNO) stock solution: 10 mM in HPLC-grade water, freshly made.
Fifty mM Tris(2-carboxyethyl) phosphine (TCEP) solution, adjusted to pH 8.5 with 100 mM of NaOH; one M TECP diluted 20 times in HPLC-grade water.
Human caspase 3 (Casp3) peptide (163-CRGTELDCGIETD-175) (AnaSpec, San Jose, CA, USA) (see Note 1).
Recombinant human thioredoxin1 (Trx1) (Sigma, St. Louis, MO, USA).
Anti-biotin antibody (Vector Laboratories, Burlingame, CA, USA).
Enhanced chemiluminescence substrate kit (PerkinElmer, Waltham, MA, USA).
Cleavable ICAT Reagent Kit (AB Sciex, Framingham, MA, USA), containing: (a) ICAT Light reagent (ICAT-L) and ICAT Heavy reagent (ICAT-H); (b) Cleaving reagent A, containing concentrated trifluoroacetic acid (TFA), and Cleaving reagent B, contains a scavenger that reduces the side reactions during the cleaving reactions; (c) Cation-exchange and affinity buffers and avidin cartridges.
Cell line: HeLa cells.
Culture medium: Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO, USA).
Fetal bovine serum (FBS, Atlanta Biologicals, Flowery Branch, GA, USA).
2.2. Buffers
Eight M Urea in HPLC grade water (see Note 2).
Affinity loading buffer: 2× PBS, pH 7.2.
Cell lysis buffer (LB): 50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA and 0.1 mM neocuproine, and supplemented with a protease inhibitor cocktail (see Note 3).
Blocking buffer: LB supplemented with 2.5% SDS and 20 mM methyl methanethiosulfonate (MMTS).
Biotinylation buffer: LB supplemented with 1% SDS, 1.2 μg/μL ICAT-L or ICAT-H reagent and 10 mM sodium ascorbate.
Elute buffer: 30% acetonitrile (ACN) and 0.4% TFA.
LC/MS infusion buffer: 50% ACN and 0.1% formic acid (FA).
LC/MS/MS solvent A: 2% ACN and 0.1% FA in HPLC-grade water.
LC/MS/MS solvent B: 95% ACN and 0.1% FA in HPLC-grade water.
SDS-PAGE loading buffer: 100 mM Tris, pH 6.8, 2% SDS, 15% glycerol and 0.01% Bromophenol Blue.
S-nitrosation buffer (NB): 10% ACN, 1 mM EDTA, and 0.1 mM neocuproine.
Wash buffer 1: 2× PBS, pH 7.2.
Wash buffer 2: 50 mM NH4HCO3 and 20% methanol.
2.3. Instruments and Software
Dinoex Ultimate 3000 RSLC nano LC system (Thermo Electron, Rockford, IL, USA).
LTQ-Orbitrap Velos tandem MS system (Orbitrap MS) with a nano-ESI source (Thermo Fisher Scientific, Rockford, IL, USA).
QTOF-MS system equipped with a nano-ESI source (Waters Corporation, Milford, MA, USA).
Proteome Discoverer software (Thermo Fisher Scientific, version 1.4.12).
MassLynx software (Waters, Corporation, Milford, MA, USA).
pH Meter (AB15 Basic, Thermo Fisher Scientific).
PepClean C18 spin columns (Pierce, Rockford, IL, USA).
PepMap 100 C18 LC column (75 μm × 150 mm, 3 μm, 100 Å, Dionex, Sunnyvale, CA, USA).
Vacufuge concentrator (Eppendorf North America, Hauppauge, NY, USA).
3. Methods
3.1. Direct Detection of S-Nitrosation Sites by QTOF-MS
3.3.1. S-Nitrosation of the Casp3 Peptide
Reduction of the disulfide bond: Reduce 25 μg of the Casp3 peptide using 25 μL of 50 mM TCEP and incubate the solution at 37 °C for 60 min (see Note 4).
Peptide desalting: Desalt the reduced peptide using a PepClean C18 spin column based on the manufacture protocol. Elute the peptide with 20 μL of 70% ACN for three times and concentrate the solution to ~25 μL with a Vacufuge.
Peptide S-nitrosation: After desalting, suspend an aliquot of the peptide (1 nmol) in 50 μL of NB. S-Nitrosate select cysteines in the Casp3 peptide with either 10 nmol of GSNO or 1 nmol of S-nitrosated Trx1 (SNO-Trx1, see Subheading 3.1.2 below). Incubate the reaction solutions at 37 °C for 30 min in the dark (see Note 5).
3.1.1. S-Nitrosation of the Recombinant Human Trx1 Protein
S-Nitrosation of Trx1: Mix 25 μg of oxidized Trx1 (commercial Trx1 is usually heavily oxidized with disulfides) with a 25-fold molar excess of GSNO in 50 μL of NB at 37 °C for 30 min in the dark (see Note 6).
Acetone precipitation: Mix the SNO-Trx1 with 200 μL of cold 100% acetone in a volume ratio of 1:4 and keep the mixture at −20 °C for 1 h. Centrifuge the resulting solution at 5000 × g for 8 min. Wash the pellet using 200 μL of ice cold 80% acetone and centrifuge the solution at 5000 × g for 8 min, repeat four times.
Suspend the SNO-Trx1 protein pellet with 30 μL of NB (see Note 7). Use 10 μL of the SNO-Trx1 solution for direct detection by QTOF MS. Use the rest of the SNO-Trx1 solution for trypsin digestion and identification of the SNO-Cys sites in Trx1.
Trypsin digestion of SNO-Trx1: Mix 20 μL of SNO-Trx1 with 20 μL of 100 mM NH4HCO3 (pH 8.0). Add trypsin to the SNO-Trx1 solution at a trypsin: Trx1 molar ratio of 1:5. Incubate the digestion solution at 37 °C overnight in the dark.
Desalt the resulting peptides via a PepClean C18 spin column prior to LC/MS/MS analysis on the QTOF-MS (see Note 8).
3.1.2. Direct Detection of S-Nitrosation of Casp3 Peptide and SNO-Trx1 on QTOF-MS
QTOF-MS instrument setting: Set the ESI capillary voltage at 3 kV and the MS scan range from m/z 400 to 1900 amu. Acquire MS spectra in the positive ion mode by direct infusion. Alter the cone and collision energy voltages to locate the maximal ion intensities of the S-nitrosated Casp3 peptide (SNO-Casp3p) in the MS spectra (see Note 9).
Optimization of the cone voltage: Set cone voltages from 15 to 30 V stepwise with a 5-V interval. Measure the ion intensity of SNO-Casp3p in the MS1 spectra. Select the cone voltage giving the highest ion intensity to the SNO-Casp3p in the MS1 spectra (see Note 10).
Optimization of the collision energy voltage: Set the collision energy voltages from 0 to 10 V with a 2-V interval. Measure the ion intensities of SNO-Casp3p in the MS1 spectra. Choose the collision energy voltage producing the highest SNOCasp3p ion intensity in the MS1 spectra (see Note 11).
MS1 and MS2 acquisition: Acquire the MS1 spectra of SNO-Casp3p, SNO-Trx1 tryptic peptides, and SNO-Trx1 protein by a direct infusion analysis for 2 min each, using the optimized cone and collision voltages based on the results from steps 2 and 3. To acquire MS2 spectra of SNO-Casp3p, set the collision energy to 37 V for the selected SNO-peptide precursor ions (MS2 collision energy may vary in other instruments). The collision energy of 37V likely would knock off the NO group from the SNO-peptide and provide b- and y- fragments ion series in MS2 spectra for peptide identification.
Data analysis: Interpret the MS1 and MS2 spectra of S-nitrosated peptides manually based on the m/z values of the precursor and the fragment ions. Deconvolute the MS1 spectra of SNO-Trx1 using the MaxEnt1 module in the MassLynx software (see Note 12).
3.2. Indirect Detection of Targets of the Trx1 Transnitrosation Using BST with ICAT
3.2.1. Cell Lysate Preparation
Cell culture: Grow HeLa cells at 37 °C in a DMEM media containing 10% FBS in atmosphere, supplemented with 5% CO2.
Cell harvest: Harvest 1 × 107 cells via centrifugation at 500 × g for 5 min and wash the cell pellets with PBS.
Protein extraction: Lyse cells in 1 mL LB.
Protein concentration assay: After the removal of cell debris from the lysate via centrifugation at 10,000 × g for 10 min, measure the protein concentrations using the BCA Protein Assay Kit and adjust the protein solution to 1 μg/μL with the LB.
3.2.2. Indirect SNO-Trx1 Transnitrosation of the Cellular Proteins in Vitro
Freshly prepare 100 μg of SNO-Trx1 (see steps 1 and 2 in Subheading 3.1.2).
SNO-Trx1 transnitrosation of target proteins: Mix 100 μg in Vitro of either SNO-Trx1 or un-nitrosated Trx1 as a control with 1 mg of HeLa proteins in 1 mL of LB at 37 °C for 30 min in the dark. After the transnitrosation reaction, process the resulting proteins by the BST procedures described below, under Subheading 3.2.3 (see Notes 13 and 14).
3.2.3. BST Analysis of SNO-Proteins
Following step 2 under Subheading 3.2.2, denature S-nitrosated proteins by 2.5 % SDS (100 μg/sample) and block the free thiols by MMTS in 100 μL of the Blocking buffer, with frequent mixing via a vortex, at 50 °C for 30 min in the dark.
Remove the excess MMTS by cold acetone precipitation of the proteins (see step 2 under Subheading 3.1.2).
Reconstitute the protein pellets in 100 μL of the biotinylation buffer at room temperature (RT) in the dark (see Note 15, an important BST negative control). The newly exposed free ascorbate-reduced thiols are labeled with either ICAT-L for the control proteins that were treated by Trx1 or ICAT-H for the SNO-Trx1 transnitrosation target proteins (see Note 16).
Following the ICAT labeling reactions, remove the excess ICAT reagents from each reaction mixture by ice-cold acetone precipitation (see step 2 under Subheading 3.1.2) and dissolve the resulting protein pellets in 8 M urea.
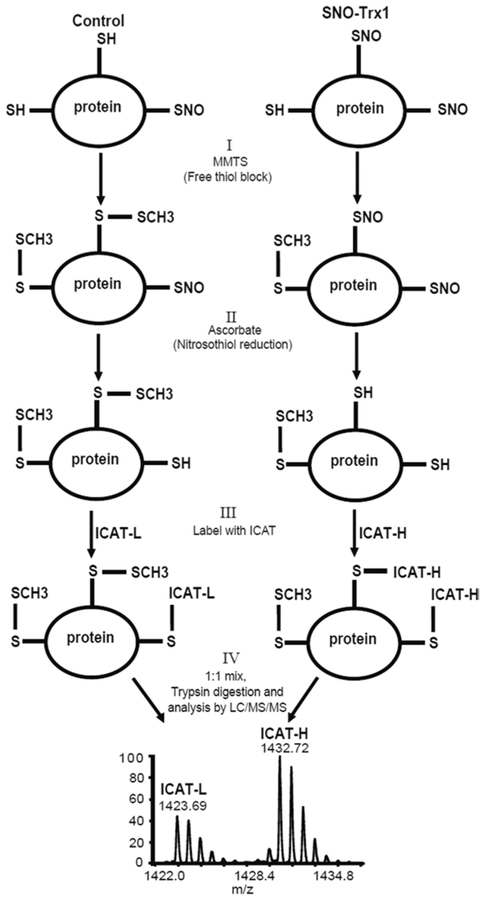
The labeling efficiency (Fig. 1) can be confirmed by Western blotting using anti-biotin antibody (see Note 17).
Fig. 1.
ICAT-based quantitative identification of SNO-Trx1 target proteins. Following incubation of cellular proteins without (Trx1 control) or with SNO-Trx1, protein SNO-Cys sites are labeled with the ICAT reagents and detected by BST: (I) free thiols are blocked with MMTS. (II) S-Nitrosated residues are reduced by ascorbate. (III) Nascent free thiols are labeled by the ICAT reagents. In this example, SNO-Trx1 treated sample is labeled with ICAT-H, while untreated sample is labeled with ICAT-L. (IV) Labeled proteins are combined, proteolytically digested, and their SNO-Cys sites identified and quantified by LC/MS/MS analysis on an Orbitrap MS. Modified from Wu et al. [22] with permission
3.2.4. In-Solution Digestion of ICAT-Labeled Proteins for MS Analyses
For each pairwise comparison, mix 100 μg each of the corresponding ICAT-H and ICAT-L labeled proteins in total 200 μL of 8 M urea and dilute the mixture protein solution with 900 μL of 50 mM NH4HCO3 (pH 8.3).
Digest the resulting protein mixture (200 μg in total) with trypsin at a 25:1 protein–trypsin ratio (w/w), at 37 °C overnight.
3.2.5. Enrich the Biotinylated Peptides
Add 500–1000 μL of the affinity loading buffer to the tryptic peptide solution, and adjust the pH to 7.0 with 100 mM HCl (see Notes 18 and 19).
Slowly inject (~1 drop/s) the peptide solution onto the avidin cartridge and collect all the flow-through. Inject 500 μL of the affinity loading buffer onto the cartridge.
Inject 1 mL of the wash buffer 1 followed by 1 mL of the wash buffer 2 and 1 mL of distilled water to clean the cartridge and remove nonspecifically bound peptides and discard the eluent (see Note 20).
Slowly inject (~1 drop/s) 750 μL of the elute buffer onto the cartridge and collect the eluate.
Evaporate the eluted solution to dryness in a Vacufuge.
Add to the sample tube 90 μL of the freshly prepared Cleaving Reagent, vortex and incubate the mixture for 2 h at 37 °C and dry the solution in a Vacufuge concentrator (see Note 21).
Desalt the cleaved peptides using a C18 spin column and reconstitute in 5 μL of the LC/MS/MS solvent A for analysis (see Subheading 3.2.6).
3.2.6. Identify ICAT-Labeled Peptides and Their Modification Sites by Orbitrap MS
Separate the ICAT-labeled peptides on a Dionex UltiMate 3000 reversed phase liquid chromatography system, using a PepMap 100 C18 column with an 85-min gradient (1–50% Solvent B) at a flow rate of 250 nL/min [21, 22].
Analyze the LC-resolved peptides using an LTQ-Orbitrap Velos tandem MS system with a nano-ESI source [21, 22].
Set the ESI spray voltage to 2.15 kV and the capillary temperature at 275 °C.
Acquire the full scan MS spectra (from m/z 300–2000) in the Orbitrap MS at a resolution of 60,000 (at m/z 400), with the lock mass option enabled.
Acquire the MS/MS spectra in a data-dependent mode. Sequentially isolate and fragment the ten most intense peptide ions, with charge states of 2–4, using the collision-induced dissociation with a normalized collision energy of 30%.
Set the precursor ion-selection abundance threshold at 3000 counts for the MS/MS analysis.
Submit the resulting raw spectral files to the Proteome Discoverer to identify and quantify ICAT-linked peptide pairs (see Subheading 3.2.7) (Fig. 1).
3.2.7. Quantitative ICAT Analysis of SNO-Trx1 Transnitrosation of Target Peptides
Search the MS/MS spectra (Fig. 2) against a SwissProt human database using the Mascot search engine (V.2.4.1) via the Protein Discoverer (PD) platform.
Set the search parameters: trypsin digestion with up to two missed cleavages; precursor mass tolerance at 10 ppm and fragment mass tolerance at 0.5 Da; methionine oxidation, Cysteine MMTS, ICAT-H and ICAT-L modifications as variable modifications. Also search the spectra against a decoy database containing all the reversed protein sequences to estimate the false discovery rate (FDR).
Search the raw spectrum files for peptide identification using Spectrum Selector, Mascot and Events Detector. In the Spectrum Selector, set the minimum and maximum precursor masses as 350 Da and 10,000 Da, respectively.
Filter the .msf files from PD and compile them into a list of nonredundant proteins with Scaffold (v. 4.2.1, Proteome Software, Portland, OR).
Accept the protein and peptide identifications with FDRs of no more than 1.0%, based on both the Protein Prophet and the Peptide Prophet algorithms.
Once an ICAT-L or ICAT-H labeled peptide is identified, use the extracted ion chromatograms for the corresponding ICAT-L and ICAT-H pairs to calculate the relative abundance ratios between the ICAT-H- and ICAT-L-labeled peptides, using PD.
For peptide quantification, set the mass precision at 2 ppm for event detection. Use the factory preset ICAT quantification method as the Precursor Ions Quantifier. Use the areas of the extracted ion chromatograms of ICAT-labeled peptides for calculating the ratios of ICAT-H to ICAT-L.
Repeat the biological experiments at least three times to calculate the standard deviations among the ICAT ratios and estimate the analytical variations (see Note 22).
Fig. 2.
Identification and quantification of a Trx1 target SNO-peptide in GAPDH. A MS spectrum of GAPDH 235-VPTANVSVVDLTC*R-248 (C*: ICAT-label site. ICAT light labeled: M2+, m/z 850.96; ICAT heavy labeled: M2+, m/z 855.45. An increased ICAT-H peak over ICAT-L represents SNO-Trx1 transnitrosation of Cys247 in GAPDH. Modified from Wu et al. [22], with permission
4. Notes
Cys163 and Cys170 of the Casp3p readily form a disulfide bond within an ambient environment. These residues need to be reduced prior to S-nitrosation of Cys163.
All buffers should be prepared using HPLC grade water, to minimize background noise in LC/MS.
All ESI sample buffers should be prepared containing 1 mM EDTA, and 0.1 mM neocuproine, pH 6.8, to protect the SNO-Cys in the peptides and proteins from artificial denitro sation. The pH of all buffers is adjusted using 100 mM HCl or NaOH.
S-Nitrosation experiments should ideally be performed in the dark or low room lights to minimize chemical denitrosation.
The S-nitrosated peptide in NB needs to be diluted at least ten times in the LC/MS infusion buffer before direct infusion analysis. GSNO S-nitrosated Casp3p is used to optimize the MS instrument parameters. S-nitrosated Casp3p obtained from SNO-Trx1 treatment is used to identify the specific transnitrosation target site(s) by TOF-MS. The SNO-Casp3p can be identified by a mass of 1440.63 amu, and the SNO-Trx1(His-tag modified) can be identified by a mass of 13795.80 Da. Trx1 does not have to be removed prior to analyzing SNO-Casp3p.
Human Trx1 contains five cysteines. The oxidized Trx1 has one known disulfide bond between Cys32 and Cys35, and three free thiols at Cys62, Cys69 and Cys73. We previously found that only Trx1 with Cys32–Cys35 oxidized can be nitrosated, whereas Trx1 with reduced Cys32 and Cys35 thiols could not be S-nitrosated and transnitrosated target proteins [5].
Due to the labile nature of S-nitrosation, it is important to keep the S-nitrosated peptides or proteins in NB containing both EDTA and neocuproine, to minimize copper-mediated denitrosation during the sample handlings. These two reagents are chelating agents to sequester metal ions that are detrimental to the preservation of the SNO-Cys on S-nitrosated peptides and proteins.
The direct detection by MS should be performed right after the S-nitrosation treatments and tryptic digestions, and ideally should be performed in the dark or under reduced light, to minimize denitrosation.
S-NO bond is fragile and can be denitrosated both during sample preparation and MS analysis. Therefore, the parameters of a MS instrument need to be fine-tuned to both ionize the peptide and retain the S-nitrosation on the peptides or proteins. In Subheading 3.1 is an example of how to optimize the parameters on a QTOF-MS to directly analyze S-nitrosated peptides and proteins in MS1 spectra. For other types of MS instruments, the parameters and optimization procedures may vary. Direct detection of SNO-Cys is simple, quick and accurate for mapping SNO-Cys to identify S-nitrosation in peptides and proteins. Since S-nitrosation is unstable, the S-nitrosated sample solutions should not be stored long-term for delayed detection.
In general, most ESI MS ion intensities increase when the cone voltages increase from 15 to 30 V. However, due to the fragility of the S-NO bonds in SNO-Cys residues, the ion intensities of S-nitrosated peptides tend to decrease after the cone voltages exceed 20 V.
The MS1 ion intensities of S-nitrosated peptides are also sensitive to the collision energy voltages. Using several different S-nitrosated peptides, we found that the MS1 ion intensities of the S-nitrosated peptides increased when the voltage increased from 0 to 4 V. However, the S-nitrosated peptide ion intensities would decrease when the collision energy voltage were above 4 V [19].
Our previous study indicated that SNO-Trx1 could transnitrosate Casp3p more effectively than GSNO [5]. For the analysis of SNO-Trx1 transnitrosation of Casp3p, the analytical procedure is the same as for the analysis of SNO-Casp3p obtained from GSNO S-nitrosation.
The procedure of indirect detection of SNO-Cys by using BST is complex, less specific than the direct methods, and may produce false signals. However, the BST-labeled samples are stable and can be stored for delayed MS detection.
GSNO treatment of cellular proteins can be performed as a positive control. Negative controls, such as unnitrosated Trx1 or buffer treatment of the cellular proteins need to be included to distinguish SNO-Trx1 targets from other unrelated SNO-proteins, which would not have increased ICAT-H to -L ratios.
Ascorbate is a weak reductant that is believed to specifically reduce SNO-peptides but not disulfide bonds or other common oxidative PTMs of the thiols. Since that reaction may not be 100% selective, the inclusion of the proper controls to ensure specific detection of Trx1-regulated SNO-protein signals is essential. Thus, a negative control reaction should be included; in which ascorbate is omitted, so no SNO-Cys-derived free thiol would be available for subsequent biotinylation and detection by either Western blotting or MS [17].
The method for biotinylation using the ICAT reagents is based on Paige et al. [13] and Wu et al. [21, 22] with some modifications.
Western blotting confirmation of biotinylation: To confirm efficient ICAT labeling of the S-nitrosated proteins (SNO-proteins) following BST, one can use Western blotting to visualize the SNO-proteins from both control and SNO-Trx1-treated samples. Solubilize 15 μg of the protein pellets in a SDS-PAGE loading buffer and separate the proteins by SDS-PAGE. Transfer the resolved proteins onto a nitrocellulose membrane (0.45 μm; Bio-Rad, Benicia, CA, USA), and block the nonspecific antibody binding sites on the membrane with 5% milk and 0.1% Tween 20. Probe the membrane with an anti-biotin antibody (1:3000 dilution) (Vector Laboratories, Burlingame, CA, USA) and visualize the ICAT-L or H-labeled proteins with an enhanced chemiluminescence substrate (PerkinElmer, Waltham, MA, USA).
Mixtures of the ICAT-H and ICAT-L-labeled peptides are enriched by a biotin affinity chromatography procedure, using an avidin column provided in the cleavable ICAT kit modified from the manufacturer’s protocol as described in Wu et al. [21, 22].
The peptide mixture can be cleaned using an ICAT Cation Exchange Buffer Pack prior to avidin enrichment, if the samples contain excessive salts.
More extensive washes in step 3 in Subheading 3.2.5 can reduce the nonspecific binding of nonbiotinylated peptides and may enable the identification of more biotinylated peptides and the modified sites in LC/MS/MS.
The ICAT cleavable reagent is composed of an acid cleavage site, in which the biotin portion of the label and part of the linker can be cleaved by TFA. The cleavage can reduce the peptide mass and improve the overall peptide fragmentation efficiency in the tandem mass analyzers. Scavengers (thioanisole, EDT, anisole, phenol) can be used to reduce side reactions during the cleaving reaction.
Statistical analysis can be performed using a two-tailed unpaired Student’s t-test. Consider the ICAT fraction differences as significant when the P-values are less than 0.05 and ICAT ratios of >1.22 or <0.78 with a 95% confidence interval, based on the analytical variations of the ICAT samples estimated from prior studies [21]. When making inferences from proteomics data, it is important to know that different LC/MS/MS systems may produce different analytical variations, and thus different ratio cutoffs for determining whether an ICAT ratio represents a meaningful change.
Acknowledgments
This chapter is produced with a grant support from the NIH-National Institute of General Medical Sciences (R01GM112415, to A.B. and H.L.), and the Orbitrap MS described here was purchased with a grant from the NIH-National Institute of Neurological Disorders and Stroke (P30NS046593).
References
- 1.Foster MW, Hess DT, Stamler JS (2009) Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15(9):391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannick JB (2007) Regulation of apoptosis by protein S-nitrosylation. Amino Acids 32(4):523–526 [DOI] [PubMed] [Google Scholar]
- 3.Benhar M, Forrester MT, Hess DT, Stamler JS (2008) Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320(5879):1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao C, Guo H, Wei J, Mi Z, Wai PY, Kuo PC (2005) Identification of S-nitrosylated proteins in endotoxin-stimulated RAW264.7 murine macrophages. Nitric Oxide 12(2):121–126 [DOI] [PubMed] [Google Scholar]
- 5.Wu C, Liu T, Chen W, Oka S, Fu C, Jain MR, Parrott AM, Baykal AT, Sadoshima J, Li H (2010) Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol Cell Proteomics 9(10):2262–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S (2002) Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol 4(10):743–749 [DOI] [PubMed] [Google Scholar]
- 7.Haendeler J, Weiland U, Zeiher AM, Dimmeler S (1997) Effects of redox-related congeners of NO on apoptosis and caspase-3 activity. Nitric Oxide 1(4):282–293 [DOI] [PubMed] [Google Scholar]
- 8.Hashemy SI, Holmgren A (2008) Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J Biol Chem 283(32):21890–21898 [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Parrott AM, Fu C, Liu T, Marino SM, Gladyshev VN, Jain MR, Baykal AT, Li Q, Oka S, Sadoshima J, Beuve A, Simmons WJ, Li H (2011) Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxid Redox Signal 15(9):2565–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greco TM, Hodara R, Parastatidis I, Heijnen HF, Dennehy MK, Liebler DC, Ischiropoulos H (2006) Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proc Natl Acad Sci USA 103(19):7420–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao G, Derakhshan B, Shi L, Campagne F, Gross SS (2006) SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci USA 103(4):1012–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH (2001) Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3(2):193–197 [DOI] [PubMed] [Google Scholar]
- 13.Paige JS, Xu G, Stancevic B, Jaffrey SR (2008) Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem Biol 15(12):1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torta F, Usuelli V, Malgaroli A, Bachi A (2008) Proteomic analysis of protein S-nitrosylation. Proteomics 8(21):4484–4494 [DOI] [PubMed] [Google Scholar]
- 15.Gow AJ, Davis CW, Munson D, Ischiropoulos H (2004) Immunohistochemical detection of S-nitrosylated proteins. Methods Mol Biol 279:167–172 [DOI] [PubMed] [Google Scholar]
- 16.Jaffrey SR, Snyder SH (2001) The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001(86):PL1. [DOI] [PubMed] [Google Scholar]
- 17.Huang B, Chen C (2006) An ascorbate-dependent artifact that interferes with the interpretation of the biotin switch assay. Free Radic Biol Med 41(4):562–567 [DOI] [PubMed] [Google Scholar]
- 18.Knipp M, Braun O, Gehrig PM, Sack R, Vasák M (2003) Zn(II)-free dimethylargininase-1 (DDAH-1) is inhibited upon specific Cys-S-nitrosylation. J Biol Chem 278(5):3410–3416 [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Liu T, Wu C, Li H (2008) A strategy for direct identification of protein S-nitrosylation sites by quadrupole time-of-flight mass spectrometry. J Am Soc Mass Spectrom 19(9):1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SJ, Lee JR, Kim YH, Park YS, Park SI, Park HS, Kim KP (2007) Investigation of tyrosine nitration and nitrosylation of angiotensin II and bovine serum albumin with electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 21(17):2797–2804 [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Parrott AM, Liu T, Beuve A, Li H (2013) Functional proteomics approaches for the identification of transnitrosylase and denitrosylase targets. Methods 62(2):151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Parrott AM, Liu T, Jain MR, Yang Y, Sadoshima J, Li H (2011) Distinction of thioredoxin transnitrosylation and denitrosylation target proteins by the ICAT quantitative approach. J Proteome 74(11):2498–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]