Abstract
Background
Human immunodeficiency virus (HIV) and hepatitis C virus (HCV) coinfection is associated with accelerated progression to cirrhosis, end-stage liver disease, and liver-associated death. It is fortunate that curative direct-acting antivirals for the treatment of HCV are widely available in the VA healthcare system. We attempted to identify, evaluate, and treat all HIV/HCV-coinfected persons at the Atlanta VA Healthcare System.
Methods
Human immunodeficiency virus/HCV-coinfected persons at Atlanta VA between 2015 and 2018 were identified using the HIV Atlanta Veterans Affairs Cohort Study and Hepatitis C VA Clinical Case Registry. Retrospective reviews of each electronic medical record were conducted by the hepatitis C clinical team for validation. The primary end point was achieving sustained virologic response.
Results
One hundred thirty-eight veterans with HIV and hepatitis C viremia were identified. One hundred twenty-five (90%) were evaluated for treatment and 113 (91%) were initiated on direct-acting antiviral therapy. Median age at initiation of treatment was 60 years and the majority were black race (90%). Genotype 1a was most common (70%) and 41% had compensated cirrhosis. One hundred eight completed treatment and 96% achieved sustained virologic response. Six veterans had virologic relapse; 4 had treatment-emergent resistance mutations in the NS5a gene. Mean CD4 was 580 cells/mm3 with HIV viral suppression in 82% of the cohort. In those not treated, unstable housing (25%), active substance use (31%), and psychiatric conditions (42%) were identified barriers to care.
Conclusions
Through a concerted, systematic effort, over 80% of HIV/hepatitis C persons in the Atlanta VA have been initiated on treatment for hepatitis C, 96% of which have been cured.
Keywords: care continuum, chronic hepatitis C virus, direct-acting antivirals, human immunodeficiency virus, veterans
This review describes the HCV care continuum for HIV/HCV-coinfected veterans treated in the Atlanta VA healthcare system between 2015 and 2018. One hundred twenty-five coinfected veterans were evaluated for treatment: 91% initiated HCV therapy, and 96% achieved sustained virologic response.
Hepatitis C virus (HCV) affects over 3 million persons in the United States and is one of the most common causes of end-stage liver disease (ESLD) [1]. Of the 1.2 million persons in the United States living with human immunodeficiency virus (HIV), 25% are coinfected with HCV [2]. There are significant consequences of HIV/HCV coinfection including lower likelihood of spontaneous clearance, faster progression to cirrhosis, greater incidence of decompensated cirrhosis, higher rates of hepatocellular carcinoma, and higher rates of liver-related and all-cause mortality [3–6]. With the advent of direct-acting antivirals (DAAs), these complications can be mitigated, underscoring the necessity of HCV eradication in this population.
Before 2014, the available therapy for HCV was injectable interferon (pegylated and nonpegylated) in combination with ribavirin. This regimen was poorly tolerated with (1) high rates of treatment limiting adverse events and (2) reported sustained virologic response (SVR) rates less than 50% in HCV-monoinfected persons [7, 8] and less than 25% in HIV/HCV-coinfected persons [9]. During the interferon era, it was reported that only half of the HCV-infected persons in the United States were tested and aware of their diagnosis, approximately one third had been referred for HCV care, and only 5% to 6% had been successfully treated [10].
Oral DAAs have allowed for improved ability to treat HCV, resulting in shorter treatment duration, minimal side effects, and significantly higher rates of SVR (>90%) in both monoinfected and coinfected persons [11–13]. With the availability of curative HCV therapy, treatment of HIV/HCV persons should be a priority. However, DAA treatment uptake has been limited by cost and associated insurance coverage restrictions [14].
The US Veterans Health Administration (VHA) is in a unique position to address challenges posed by chronic HCV infection and has now become the single largest provider of HCV care in the United States [15]. In 2001, the VHA implemented comprehensive screening guidelines inclusive of the Centers for Disease Control and Prevention screening recommendations and additional risk factors such as alcohol use, history of tattoos, multiple sexual partners, intranasal cocaine use, and service during the Vietnam era [16]. By 2013, more than 70% of veterans in the “baby boomer” birth cohort had been screened for HCV [17], and, when DAA therapy became widely available in 2016, the VHA announced a systematic and concerted effort to provide HCV treatment to all veterans with chronic HCV [18]. As of March 2019, 83% of veterans who started treatment for HCV have completed treatment and are cured [19].
Aligned with VHA efforts, we aimed to link HIV/HCV-coinfected veterans into care and initiate HCV treatment at the Atlanta VA. We describe the HCV care continuum that was provided to this cohort between 2015 and 2018.
MATERIALS AND METHODS
Study Population and Data Sources
We performed a retrospective cohort study of HCV care in HIV/HCV-coinfected adults (18 years and older) at the Atlanta VA Healthcare System (AVAHCS) between January 1, 2015 and October 31, 2018. Patient outreach, linkage, and treatment were performed through regular patient care but retrospectively analyzed as an institutional review board-approved research study.
Using the HIV Atlanta Veterans Affairs Cohort Study (HAVACS) and HCV VA Clinical Case Registries (HCV CCR), all veterans at the AVAHCS that were living with HIV and had either positive HCV serologic testing or ribonucleic acid (RNA)-based testing were identified.
Data Collection
Each electronic medical record was reviewed by an infectious disease physician or infectious disease-trained clinical pharmacist for validation of HCV and HIV status. The HCV clinical team was made up of a physician-led HCV team including a clinical pharmacy specialist, an advanced practice provider, and a registered nurse. The HCV clinical team educated the HIV staff and providers and additionally provided direct patient outreach to link patients into HCV care. Human immunodeficiency virus clinical providers alerted a designated HCV infectious disease physician to an HIV-positive veteran with HCV viremia. This triggered the process for the veteran to be seen and evaluated for treatment in hepatitis clinic. Pretreatment review of appropriateness of HCV treatment and potential drug-drug interaction with DAA therapy were assessed either through an electronic consult or during an in-person evaluation. Medications identified to have an interaction with DAA either were either safely discontinued, antiretroviral (ARV) medications were adjusted, or a different DAA regimen was chosen. Veterans who were not viremic, transferred to a different VA facility, lost to follow up, or died before the study were not included in the analysis.
Data elements of interest included age, sex, race, Charlson comorbidity index (CCI), active substance abuse, history of psychiatric condition, and unstable housing. Active substance use was defined as any use of cocaine, heroin, methamphetamines, or abuse of prescription opiates within 6 months of contact regarding HCV treatment and was ascertained via self-report and/or urine drug screens. Psychiatric condition was defined as any documented history of generalized anxiety disorder, posttraumatic stress disorder, major depression, bipolar disorder, or schizophrenia. Unstable housing was defined as lack of housing or requiring the assistance of the US Department of Housing and Urban Development-VA Supportive Housing (HUD-VASH) program within 3 months before or after initiating HCV treatment.
Through manual chart review, HIV- and HCV-specific factors were collected. These included CD4 lymphocyte count (cells/mm3), HIV RNA (copies/mL), ARV regimen, need for ART switch before initiation of DAA, HCV viral load, HCV genotype, prior HCV treatment experience, presence of cirrhosis (evidenced by fibrosis [FIB]4 score >3.25, aspartate aminotransferase-to-platelet ratio index [APRI] score >1, Fibroscan >12.5 kPa, or biopsy suggestive of cirrhosis), and DAA regimen. The resistance tests that were used included the HCV NS5A Resistance Genotype, the HCV NS5b drug resistance genotype, and the NS3 drug resistance genotype. Two veterans received treatment through the VA Choice Program (the VA Choice Program allowed veterans to see an HCV provider in the private sector and fill the DAA prescription at the VA pharmacy).
Study Definitions and Outcomes
The primary study objective was to identify HIV/HCV chronically coinfected veterans, link them into HCV care, and initiate HCV treatment to achieve an SVR. Chronic HCV was defined as having a detectable viral load at least 3 months before treatment initiation. If veterans were able to be contacted and presented to their scheduled HCV clinic appointment, they were considered to be linked into care. During the evaluation appointment, a pretreatment assessment was performed. This included the following: assessing their comorbidities; determining their fibrosis stage either through FIB4 score, APRI score, or Fibroscan; obtaining baseline laboratory tests including updated HCV viral load and HIV viral load if indicated; and checking for other hepatitis viruses. Veterans were then started on DAAs appropriate for their genotype, fibrosis stage, and renal function. If drug-drug interactions with DAA therapy was anticipated, veteran’s ARV regimen was adjusted before initial HCV treatment. Veterans were also assessed for baseline NS5a mutations before DAA therapy if they were treatment experienced, which assisted in determination of appropriate DAA regimen. Veterans were seen by an infectious disease physician, physician assistant, or infectious disease-trained clinical pharmacist monthly for additional medication and monitoring while on DAAs. Veterans were determined to have achieved SVR if their HCV RNA was undetectable (less than lower limit of quantification target or not detected) at least 12 weeks after completion of treatment. Relapse was defined as a detectable HCV viral load 12 weeks after completing DAA therapy. If a veteran experienced a relapse, resistance testing was performed to assess whether they developed a treatment-emergent mutation to their DAA therapy. Reinfection was defined as detectable HCV viremia after a veteran achieved SVR and with evidence of high-risk behavior and/or genotype switch.
Data Analysis
We tabulated the number of HIV/HCV veterans that were evaluated for treatment, seen in clinic, started on HCV therapy, and achieved SVR, which defined the HCV care continuum. We performed descriptive statistics on patient-level demographics, clinical characteristics, and treatment regimens. We compared those who were started on treatment to those who were not to identify factors associated with poor linkage into care. The χ 2 test was used for categorical variables, and Student’s t tests or Wilcoxon rank-sum test was used for continuous variables. Two-sided P values were used for all analyses, and P ≤ 0.05 was considered statistically significant. SAS version 9.4 (SAS Institute Inc.) was used for data management and statistical analysis.
RESULTS
Study Population
In this all male cohort, the median age was 60 years of age, 90% were of black race, and 70% were genotype 1a (Table 1). For clinical variables, cirrhosis was observed in 41% of veterans, median CCI was 3, and median absolute number of comorbidities was 6. In assessing psychiatric-related conditions, 46% had evidence of a psychiatric condition, and 16% had active substance abuse. The untreated group had more active substance use (31% vs 14%, P = .09) and unstable housing (25% vs 5%, P = .01) compared with treated group. Of the 12 veterans who were not treated, 3 (25%) died shortly after evaluation (2 of which were secondary to complications from ESLD), 5 (42%) had psychiatric conditions (3 of whom had active psychosis), 3 (25%) were consistently nonadherent to ARVs, 3 were unstably housed (25%), 1 decided to be treated outside of the VA, and 1 veteran was unable to be contacted for follow up.
Table 1.
Baseline Characteristics of HIV/HCV-Coinfected Veterans Evaluated for HCV Treatment at the Atlanta VA
| Variables | Entire Cohort, n (%) | Treated, n (%) | Untreated, n (%) | P Valuea |
|---|---|---|---|---|
| Total persons (n) | 125 | 113 | 12 | |
| Median age (IQR) | 60 (55–65) | 60 (55–64) | 62 (55–67) | 0.61 |
| Race | ||||
| White | 9 (7) | 8 (7) | 1 (8) | |
| Black | 113 (90) | 103 (91) | 10 (83) | |
| Unknown | 3 (2) | 2 (2) | 1 (8) | |
| Genotype | 0.86 | |||
| 1a | 88 (70) | 80 (71) | 8 (67) | |
| 1b | 32 (26) | 28 (25) | 4 (33) | |
| 1a/1b | 2 (2) | 2 (2) | 0 | |
| 2b | 2 (2) | 2 (2) | 0 | |
| 3a | 1 (1) | 1 (1) | 0 | |
| Cirrhosis | 48 (41) | 45(40) | 3 (25) | 0.38 |
| Decompensatedb | 4 (3) | 2 (2) | 2 (17) | 0.05 |
| Developed hepatocellular carcinoma | 3 (2) | 1 (1) | 2 (17) | 0.01 |
| CCI, median (IQR) | 3 (2–5) | 3 (2–5) | 4 (2–6) | 0.48 |
| Absolute number of comorbidities, median (IQR) | 6 (4–7) | 6 (4–7) | 5 (3–8) | 0.74 |
| Psychiatric condition | 57 (46) | 52 (46) | 5 (42) | 0.57 |
| Active substance use | 20 (16) | 16 (14) | 4 (31) | 0.09 |
| Unstable housing | 9 (6) | 6 (5) | 3 (25) | 0.01 |
Abbreviations: CCI, Charlson comorbidity index; HCV, hepatitis C virus, HIV, human immunodeficiency virus; IQR, interquartile range; VA, Veterans Affairs.
a P values compare those initiated on HCV treatment to those who were not.
bDecompensated cirrhosis was defined as presence of ascites, hepatic encephalopathy, history of bleeding esophageal varices, or hepatorenal syndrome.
Care Continuum and Virologic Response
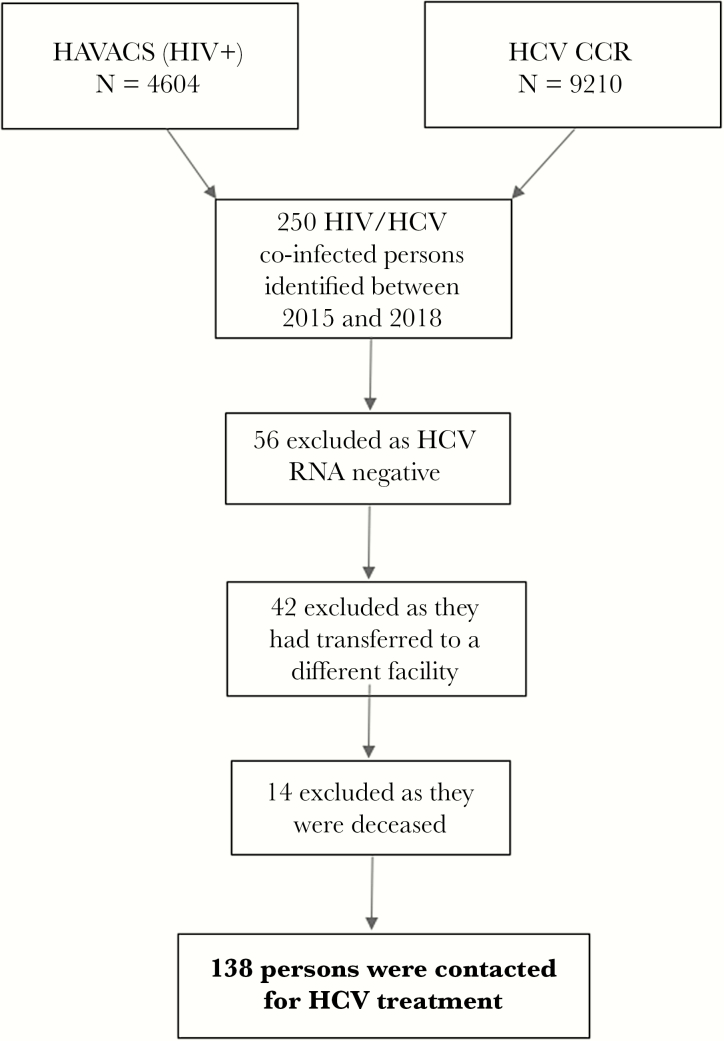
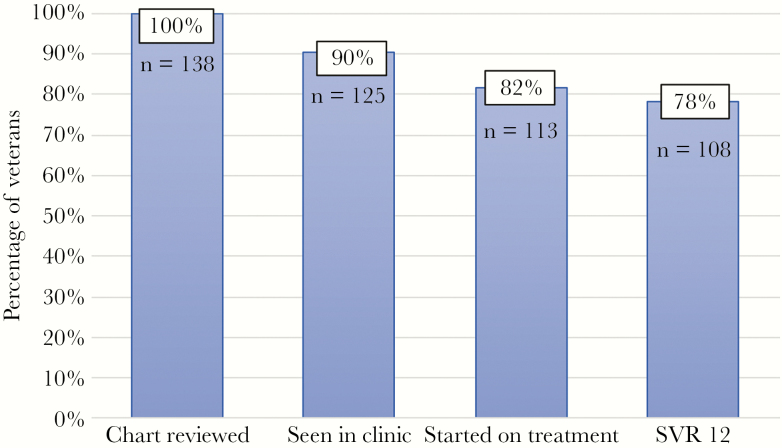
Two hundred fifty HIV/HCV-coinfected veterans were identified by the HAVACS and HCV CCR (Figure 1). Of those identified, 56 were not viremic, 14 died (2 of whom died from ESLD and 3 died from hepatocellular carcinoma), and 42 were transferred to a different VA facility. This resulted in 138 veterans who were contacted for HCV treatment. The HCV treatment care continuum is summarized in Figure 2. Of the 138 veterans, 13 (9%) could not be engaged in clinic follow up, whereas 125 (91%) presented for an initial evaluation in hepatitis clinic. These veterans were identified to be linked into HCV care between January 2015 and October 2018. Of the 125 persons, 113 (90%) were initiated on HCV treatment. At study end, 3 veterans remained on treatment and 2 veterans had completed treatment, although SVR data had not yet been obtained; thus, of the veterans who were started on treatment, 108 (96%) achieved SVR on either their first or second treatment attempt with documentation of clinical cure. During the study period, 78% of all HIV/HCV-coinfected veterans had achieved SVR. Of the 108 veterans who achieved SVR, there were 8 (7%) veterans who disrupted and discontinued therapy at either 4 or 6 weeks, all of whom were cured.
Figure 1.
Study inclusion criteria for human immunodeficiency virus (HIV)/hepatitis C virus (HCV)-coinfected veterans at the Atlanta VA Healthcare System. CCR, VA Clinical Case Registries; HAVACS, HIV Atlanta Veterans Affairs Cohort Study; RNA, ribonucleic acid.
Figure 2.
Hepatitis C virus (HCV) treatment care continuum model of human immunodeficiency virus/HCV-coinfected veterans at the Atlanta VA Healthcare System, January 2015–December 2018 (n = 138).
Antiretroviral Regimen
In general, the treated cohort had well controlled HIV, with a median CD4 of 580, and more than 80% of veterans were virally suppressed at initiation of therapy (Table 2). The most common ARV regimen at the time of HCV treatment start was an integrase-based regimen. Sixty-four percent of veterans were on an integrase-based regimen, 32% of whom required change to an integrase-based regimen before starting HCV treatment. Of those who required ARV regimen change, 69% were secondary to concerns of drug interactions with DAA therapy.
Table 2.
HIV Information for Coinfected Veterans Who Initiated HCV Treatment (n = 113)
| Variables | n (%) |
|---|---|
| Median CD4 count (IQR, cells/mm3) | 580 (379–765) |
| HIV viral load undetectable | 110 (82) |
| ARV regimen at time of HCV therapy | |
| Integrase-inhibitor-based | 79 (64) |
| NNRTI-based | 22 (17) |
| PI-based | 13 (10) |
| Other | 7 (6) |
| ARV regimen changed for HCV therapy | 36 (32) |
| ARV regimen change reason | |
| Medication interaction | 25 (69) |
| Optimization of regimen | 7 (19) |
| Other | 3 (8) |
Abbreviations: ARV, antiretroviral; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Hepatitis C Virus Treatment and Virologic Response
Table 3 encompasses the DAA regimens and clinical response. The most common DAA regimen used was ledipasvir/sofosbuvir (59%), followed by elbasvir/grazoprevir (10%) and sofosbuvir/velpatasvir (9%). There were 2 veterans who experienced adverse events leading to treatment discontinuation. One veteran experienced tongue ulcerations while on ledipasvir/sofosbuvir, which was discontinued. Once the tongue ulcerations resolved, he was started on glecaprevir/pibrentasvir, which was well tolerated, and he achieved SVR. Another veteran developed acute renal injury after approximately 6 weeks of sofosbuvir + daclatasvir, which resolved after discontinuation. This veteran also achieved clinical cure without having to be started on another treatment regimen.
Table 3.
HCV Treatment Regimen and Response (n = 113)
| HCV Treatment Regimen | n (%) |
|---|---|
| Ledipasvir/sofosbuvir | 73 (59) |
| Elbasvir/grazoprevir | 12 (10) |
| Sofosbuvir/velpatasvir | 11 (9) |
| Dasabuvir + ombitasvir/paritaprevir/ritonavir | 5 (4) |
| Simeprevir + sofosbuvir | 4 (3) |
| Daclatasvir + sofosbuvir | 3 (2) |
| Glecaprevir/pibrentasvir | 1 (1) |
| Unknown* | 1 (1) |
| DAA discontinued early although cured | 8 (7) |
| Relapsed | 6 (5) |
| Number with resistance | 4 (66) |
| Cured with retreatment | 5 (83) |
Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus.
*Patient was treated outside of Atlanta VA Healthcare System through the choice program.
In total, 6 veterans (5%) experienced a virologic relapse, only 1 of whom was treatment experienced. Four veterans had treatment-emergent resistance mutations in the NS5a gene and all were of black race. Five of the 6 veterans had cirrhosis and 4 of the 6 were on ledipasvir/sofosbuvir. One veteran had a 2-week drug interruption, which we believed contributed to developing resistance. One veteran’s genotype changed from 1a to 1b. Two veterans had Q30R mutations, 2 veterans had L31 mutations, and 2 veterans had Y93H mutations. Five of 6 veterans were cured after retreatment, and 1 veteran declined retreatment. Retreatment regimens included sofosbuvir/velpatasvir/voxilaprevir for 12 weeks (1 veteran), sofosbuvir velpatasvir + ribavirin for 24 weeks (1 veteran), and simeprevir + sofosbuvir + ribavirin for 24 weeks (3 veterans). Only 1 of the 6 veterans had a pretreatment CD4 count less than 200, although it improved to greater than 200 at the next check 3 months later. All veterans who experienced a clinical relapse were HIV virally suppressed. To date, only 1 veteran has been reinfected.
DISCUSSION
In this study, we successfully identified 100% of HIV/HCV-coinfected veterans at the AVAHCS, saw 91% in HCV clinic, initiated treatment in over 80%, and 78% of the total cohort achieved clinical cure by end of the study. The World Health Organization HCV elimination goals are to diagnose 90% of persons with HCV and treat everyone identified to have HCV viremia. In this study, we were close to achieving these goals [20].
Hepatitis C virus treatment in this coinfected population was well tolerated with limited adverse events; supporting the safety and tolerability of DAAs in this population. Our findings are similar to other published data of >90% cure rate in HIV/HCV-coinfected persons [11, 13, 21, 22], further corroborating that HIV/HCV patients can achieve similar cure rates as HCV-monoinfected persons. Although there is growing evidence that HCV regimens shorter than 12 weeks may lead to treatment failure in HIV/HCV-coinfected persons [23], 8 veterans discontinued treatment at 4 or 6 weeks and still achieved SVR.
Our cohort has a higher percentage of persons with cirrhosis than prior HIV/HCV studies [11, 24]. Cirrhosis was determined through noninvasive measures, thus there is a possibility that fibrosis stage was over estimated. Given the risk of complications and sampling error with biopsy, noninvasive measures of fibrosis have become established methods of diagnosing advanced fibrosis. Between 2000 and 2013, the prevalence of cirrhosis in the veteran population increased by approximately 2-fold, largely secondary to HCV-related cirrhosis [25], which could explain the prevalence of cirrhosis in this cohort. In addition, comorbid alcohol use disorder is prevalent in the veteran population and contributes to accelerated fibrosis progression [26].
In this study, there were 6 veterans who experienced a virologic relapse, and they were all of black race. A study that included over 1000 veterans in the greater Los Angeles area noted that African Americans had a 57% lower odds of achieving SVR [27]. In addition, Naggie et al [11] examined HCV treatment with ledipasvir/sofosbuvir in an HIV/HCV-coinfected population and found that black patients had lower response rates than other races. They also noted that the majority of failures were in persons taking efavirenz therapy, which was not seen in this study. Only 1 of the veterans who relapsed was on efavirenz before HCV treatment. Sixty-seven percent of the relapses were in patients who received ledipasvir/sofosbuvir as their first HCV therapy. Rossi et al [28] noted that of their 23 clinical failures, 65% were in persons who received ledipasvir/sofosbuvir, possibly suggesting that lower cure rates are achieved with ledipasvir/sofosbuvir in the HIV/HCV population. In contrast, in a large Spanish study, ledipasvir/sofosbuvir was not a factor associated with treatment failure [22]. Furthermore, 83% of our virologic relapse occurred in persons with cirrhosis. Several studies have described lower cure rates in persons with cirrhosis, indicating that advanced liver fibrosis may slightly decrease the effectiveness of DAA therapy [21, 22, 28].
The importance of prior knowledge of potential drug-drug interactions between ARVs and DAAs was underscored in this study. More than 30% of patients required a change in ARV regimen before starting HCV treatment with the majority of these changes secondary to potential drug-drug interaction with DAA therapy. This finding was also noted by Cope et al [29] where more than 60% of patients required a change in their ARV regimen before being started on DAA therapy. Most of the ARV changes were due to patients on older ARV regimens, and as more patients transition to newer INSTI-based regimens, there may be decreases in ARV-DAAs drug interaction.
It has been previously demonstrated that socioeconomic and psychiatric comorbidities serve as barriers to engaging in care [30, 31], which was also identified in our study [32]. In our cohort, reasons that veterans were not evaluated in clinic or started on HCV treatment included transportation issues, mental health concerns, active substance use, unstable housing, or comorbidities affecting life expectancy (ie, advanced metastatic cancer). Unstably housed veterans suffer from higher rates of chronic disease and comorbidities; often a combination of psychiatric and medical illnesses [33]. Unstable housing and homelessness have previously been shown to reduce the likelihood of initiating HCV treatment and achieving SVR [34, 35]. Although substance abuse was not statistically significant, our study highlights the role that substance abuse and unstable housing can play in preventing engagement into care. In addition, some veterans had medical conditions that limited their HCV treatment initiation. Those who were not started on treatment had a slighter higher CCI (4 vs 3).
A strength of this study is that the integrated VHA system enhanced our ability to identify and treat willing HIV/HCV-coinfected veterans. The pressures of obtaining insurance coverage before initiating treatment are not present, allowing for patients to be quickly initiated on therapy after being evaluated. Even when HIV and HCV clinics are colocated, Rizk et at [36] recently published that 70.5% were linked into care and 56.1% achieved SVR12. Falade-Nwulia [37] had similar findings in inner city Baltimore, Maryland where 72% were initiated on treatment and 62% achieved clinical cure. In both studies, there were high rates of public insurance, and Falade-Nwulia [37] noted that Medicaid was negatively associated with HCV treatment initiation. In addition, this study was multidisciplinary with the involvement of physicians, advanced practitioners, pharmacy, and nursing. Given that our study population was an exclusively male, majority black, veteran population from a single center, there may be decreased generalizability of our findings. In addition, we did not include the risk factor for HCV transmission. As previously mentioned, 1 patient has been reinfected, and people who inject drugs carry the highest risk for reinfection [38]. This highlights the need to identify these individuals and perform additional counseling on reinfection risk. Finally, the small sample size of our study may limit our ability to identify differences between HCV-treated and -untreated groups.
CONCLUSIONS
Treatment of HCV in HIV/HCV-coinfected persons is critical to decrease progression of fibrosis, development of decompensated cirrhosis, and liver associated-mortality. In this study, we achieved HCV virologic cure in approximately 80% of the Atlanta VA’s HIV/HCV-coinfected population, contributing to the growing knowledge that this population can perform similarly to monoinfected persons. With safe, effective, and widely available HCV DAA therapy, HCV elimination is attainable among HIV/HCV-coinfected persons.
Acknowledgments
Financial support. Our research activities are funded, in part, by grants from the National Institutes of Health/National Center for Advancing Translational Sciences (TL1TR002382, UL1TR002378).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. HHS.gov. Hepatitis C. 2019. Available at: https://www.hhs.gov/opa/reproductivehealth/fact-sheets/sexually-transmitted-diseases/hepatitis-c/index.html. Accessed 8 October 2019.
- 2. Centers for Disease Control and Prevention. Coinfection with HIV and Viral Hepatitis | Division of Viral Hepatitis | CDC. 2019. Available at: https://www.cdc.gov/hiv/pdf/library/factsheets/hiv-viral-hepatitis.pdf. Accessed 22 May 2019.
- 3. Pineda JA, Romero-Gómez M, Díaz-García F, et al. ; Grupo Andaluz para el Estudio de las Enfermedades Infecciosas; Grupo Andaluz para el Estudio del Hígado HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology 2005; 41:779–89. [DOI] [PubMed] [Google Scholar]
- 4. Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 2001; 32:492–7. [DOI] [PubMed] [Google Scholar]
- 5. Chen TY, Ding EL, Seage GR III, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis 2009; 49:1605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danta M, Semmo N, Fabris P, et al. Impact of HIV on host-virus interactions during early hepatitis C virus infection. J Infect Dis 2008; 197:1558–66. [DOI] [PubMed] [Google Scholar]
- 7. Butt AA, Yan P, Lo Re V III, Shaikh OS, Ross DB. Trends in treatment uptake and provider specialty for hepatitis C virus (HCV) infection in the Veterans Affairs Healthcare System: results from the electronically retrieved cohort of HCV-infected veterans (ERCHIVES). Clin Infect Dis 2019; 68:857–9. [DOI] [PubMed] [Google Scholar]
- 8. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–82. [DOI] [PubMed] [Google Scholar]
- 9. Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS 2006; 20:2361–9. [DOI] [PubMed] [Google Scholar]
- 10. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med 2013; 368:1859–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naggie S, Cooper C, Saag M, et al. ; ION-4 Investigators Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collins LF, Chan A, Zheng J, et al. Direct-acting antivirals improve access to care and cure for patients with HIV and chronic HCV infection. Open Forum Infect Dis 2018; 5:ofx264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishida H, Ishihara A, Tanaka S, et al. Favorable outcome with direct acting antiviral treatment in hepatitis C patients coinfected with human immunodeficiency virus. Hepatol Res 2019; 49:1076–82. [DOI] [PubMed] [Google Scholar]
- 14. AASLD/IDSA HCV Guidance Panel; Chung RT, Davis GL, Jensen DM, et al. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015; 62:932–54. [DOI] [PubMed] [Google Scholar]
- 15. Maier MM, Ross DB, Chartier M, et al. Cascade of care for hepatitis C virus infection within the US Veterans Health Administration. Am J Public Health 2016; 106:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cartwright EJ, Rentsch C, Rimland D. Hepatitis C virus screening practices and seropositivity among US veterans born during 1945 - 1965. BMC Res Notes 2014; 7:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Global Academy for Medical Education. Best Practices in Screening, Diagnosis, and Treatment Along the Veteran Health Administration’s Cascade of HCV Care.2017. Available at: https://www.globalacademycme.com/cme/emergency-medicine/addressing-unique-needs-military-veterans-chronic/best-practices-screening-diagnosis-and-treatment. Accessed 9 February 2019.
- 18. Graham J. VA extends new hepatitis C drugs to all veterans in its health system. JAMA 2016; 316:913–5. [DOI] [PubMed] [Google Scholar]
- 19. US Department of Veteran Affairs. Office of Public and Intergovernmental Affairs. VA on path to cure 100,000 Veterans of hepatitis C. Available at: https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5219. Accessed 16 August 2019.
- 20. Cooke GS, Andrieux-Meyer I, Applegate TL, et al. ; Lancet Gastroenterology & Hepatology Commissioners Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2019; 4:135–84. [DOI] [PubMed] [Google Scholar]
- 21. Boesecke C, Ingiliz P, Berger F, et al. ; GECCO Study Group Liver cirrhosis as a risk factor for direct-acting antiviral therapy failure in real-life hepatitis c virus/human immunodeficiency virus coinfection. Open Forum Infect Dis 2017; 4:ofx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berenguer J, Gil-Martin Á, Jarrin I, et al. All‐oral direct‐acting antiviral therapy against hepatitis C virus (HCV) in human immunodeficiency virus/HCV-coinfected subjects in real‐world practice: Madrid coinfection registry findings. Hepatology 2018; 68:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corma-Gómez A, Macías J, Merino Muñoz D, et al. Higher relapse rate among HIV/HCV-coinfected patients receiving sofosbuvir/ledipasvir for 8 weeks. J Infect 2019; 79:30–5. [DOI] [PubMed] [Google Scholar]
- 24. Kim HN, Nance RM, Williams-Nguyen JS, et al. ; Centers for AIDS Research Network of Integrated Clinical Systems Effectiveness of direct-acting antiviral therapy in patients with human immunodeficiency virus-hepatitis C virus coinfection in routine clinical care: a multicenter study. Open Forum Infect Dis 2019; 6:ofz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology 2015; 149:1471–82.e5; quiz e17–8. [DOI] [PubMed] [Google Scholar]
- 26. Teeters JB, Lancaster CL, Brown DG, Back SE. Substance use disorders in military veterans: prevalence and treatment challenges. Subst Abuse Rehabil 2017; 8:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benhammou JN, Dong TS, May FP, et al. Race affects SVR12 in a large and ethnically diverse hepatitis C‐infected patient population following treatment with direct‐acting antivirals: analysis of a single‐center Department of Veterans Affairs cohort. Pharmacol Res Perspect 2018; 6:e00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rossi C, Young J, Martel-Laferrière V, et al. Direct-acting antiviral treatment failure among hepatitis C and HIV-coinfected patients in clinical care. Open Forum Infect Dis 2019; 6:ofz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cope R, Pickering A, Glowa T, et al. Majority of HIV/HCV patients need to switch antiretroviral therapy to accommodate direct acting antivirals. AIDS Patient Care STDs 2015; 29:379–83. [DOI] [PubMed] [Google Scholar]
- 30. McGowan CE, Fried MW. Barriers to hepatitis C treatment. Liver Int 2012; 32(Suppl 1):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. el-Serag HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology 2002; 123:476–82. [DOI] [PubMed] [Google Scholar]
- 32. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis 2012; 9:E45. [PMC free article] [PubMed] [Google Scholar]
- 33. Goldstein G, Luther JF, Haas GL, et al. Factor structure and risk factors for the health status of homeless veterans. Psychiatr Q 2010; 81:311–23. [DOI] [PubMed] [Google Scholar]
- 34. Beiser ME, Smith K, Ingemi M, et al. Hepatitis C treatment outcomes among homeless-experienced individuals at a community health centre in Boston. Int J Drug Policy 2019; 72:129–37. [DOI] [PubMed] [Google Scholar]
- 35. Olafsson S, Tyrfingsson T, Runarsdottir V, et al. Treatment as prevention for hepatitis C (TraP Hep C) - a nationwide elimination programme in Iceland using direct-acting antiviral agents. J Intern Med 2018; 283:500–7. [DOI] [PubMed] [Google Scholar]
- 36. Rizk C, Miceli J, Shiferaw B, et al. Implementing a comprehensive hepatitis C virus (HCV) clinic within a human immunodeficiency virus clinic: a model of care for HCV microelimination. Open Forum Infect Dis 2019; 6:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Falade-Nwulia O, Sutcliffe CG, Mehta SH, et al. Hepatitis C elimination in people living with HIV is contingent on closing gaps in the HIV continuum. Open Forum Infect Dis 2019; 6:ofz426. doi: 10.1093/ofid/ofz426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossi C, Butt ZA, Wong S, et al. ; BC Hepatitis Testers Cohort Team Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol 2018; 69:1007–14. [DOI] [PubMed] [Google Scholar]