Abstract
Background
Older patients with non-small cell lung cancer (NSCLC) are often not prescribed standard therapy. It is important to know which older patients would be candidates for aggressive therapy based on their prognosis, and to develop a model that can help determine prognosis.
Methods
Data on older patients (≥70 years) enrolled on 38 NCI cooperative group trials of advanced NSCLC from 1991 to 2011 were analyzed. Multivariable Cox PH model was built with a stepwise selection. We derived a prognostic score using the estimated Cox PH regression coefficient. We then calculated the area under receiver operating characteristic (ROC) curve of survival in the testing set.
Results
The final analysis included 1467 patients, who were randomly divided into a training (n=963) and a testing set (n=504). The prognostic risk score was calculated as: 3 (if male) + 3 (if PS=1) + 8 (if PS=2) + 11 (if initial stage=IV) + 4 (if weight loss). Patients were classified into two prognostic groups: good (0–8) and poor (≥9). The median survival in the two groups in the testing set were 13.15 (95% CI, 10.82–15.91) and 8.52 months (95% CI, 7.5–9.63), respectively. The model had area under the 1-year and 2-year ROCs (0.6 and 0.65, respectively) that were higher than existing models.
Conclusions
Male gender, poor performance status, distant metastases and recent weight loss predict for poor overall survival (OS) in older patients with advanced NSCLC. This study proposes a simple prognostic model for older adults with advanced NSCLC.
Keywords: Prognostic models, Older patients, Survival, Non-small cell lung cancer, Geriatric oncology
Introduction
Lung cancer is the second-most common malignancy and the leading cause of cancer-related death in the United States, estimated to account for 222,500 new cases and 155,780 deaths in 2017 (1). According to recent available estimates, 60% of patients with lung cancer are 65 years of age or older at diagnosis making this a significant burden in older patients (2). Despite recent advances in the understanding of the biology of lung cancer, patients with advanced lung cancer usually die of their disease. Median survival of patients with advanced lung cancer is currently estimated to be about thirteenmonths with the possibility of some improvement once the currently ongoing immunotherapy trials mature (3).
However, the majority of evidence used in treatment of older patients with non-small cell lung cancer (NSCLC) is based on data from younger patients because of the lack of good quality evidence specific to them (4, 5). Older patients are often not prescribed standard therapy despite evidence that older patients with good performance status respond to chemotherapy just as well as young patients (6–8). One concern is that older patients do not tolerate chemotherapy well and have an increased incidence of adverse events (9). Due to multiple competing causes of death, older patients often do not demonstrate a benefit in overall survival even though they may have similar progression survival benefit as younger patients in the same trial (10).
Given these, it is important to know which older patient would actually be candidates for aggressive therapy based on their prognosis. The currently available models can help determine clinical prognosis, but were not developed specifically for older patients (11, 12). The Mandrekar analysis included 1053 patients with advanced NSCLC from nine North Central Cancer Treatment Group trials (11). They found that performance status, body mass index (BMI), disease stage, hemoglobin level and white blood cell (WBC) count were significant prognostic factors for overall survival. The Blanchon study evaluated the four year mortality of 4669 patients with NSCLC (12). The final prognostic model included age, sex, performance status (reduced activity, active >50%, inactive >50% and total incapacity), histological type and stage. Only 34% of patients in the Blanchon study were >70 years of age (12) and 46% of patients in the Mandrekar study were 65 years or older (11).
While comprehensive geriatric assessment (CGA) is emerging to be a promising tool to predict this, it still needs validation in prospective randomized clinical trials (13, 14). Moreover, the CGA may not always be possible to administer in a busy clinical practice. Hence, it is important to develop a simple prognostic model that can help clinicians determine individual prognosis and thereby determine the best treatment option. This analysis evaluates older patients with newly diagnosed advanced non-small cell lung cancer enrolled onto National Cancer Institute (NCI) Cooperative group trials
Patients and Methods
This study was approved by Duke University Institutional Review Board. We identified 38 treatment trials of advanced non-small cell lung cancer from 1990 to 2011 conducted by NCI-sponsored cooperative groups (CALGB, ECOG, NCCTG, RTOG, SWOG) (Supplementary Table S1). The final analysis cohort consisted of enrolled 1467 patients ≥70 years of age, with advanced non-small cell lung cancer. This age was chosen on the basis of the median age at diagnosis for lung cancer (15). The primary endpoint overall survival (OS) was defined as the time between random assignment or registration and death resulting from any cause. Progression-free survival was defined as the time interval between random assignment or registration and progression or death, whichever came first. Survival endpoints were considered right-censored if patients were alive or lost-to-follow-up at the time of last follow-up.
Statistical analysis
We randomly split our cohort into a training set (two thirds) and a testing set. The baseline characteristics of the training and testing sets were compared using chi-square tests. Multivariable Cox proportional hazards (PH) model was built with a backward stepwise procedure with alpha=0.05 as inclusion criterion. Predictors used were age, sex, race, performance status, initial stage, and weight loss in the past 3/6 months. A weight loss of ≥5% in the preceding 3 months or ≥10% in the preceding 6 months were considered as weight loss for this analysis.
For ease of use, we defined a prognostic score that is calculated using the estimated Cox PH regression coefficient divided by log(2) and multiplied by ten rounded to the nearest integer. To assess the performance of our prognostic model, we calculated the c-index for survival times and the area under receiver operating characteristic (ROC) curve of one and two year survival in the testing set. The 95% confidence intervals for these statistics were obtained using bootstrapping. Our prognostic model was compared to PS only model and two existing models, Blanchon et al. (B model) and Mandrekar et al. (M model).
The overall survival by the two risk groups were reported in forms of median and its 95% confidence interval (CI) and plotted using Kaplan-Meier method. Two-sided tests with a P-value < .05 were considered statistically significant. SAS and R software were used for data analysis.
Results
We reviewed records of 2823 patients with advanced non-small cell lung cancer, aged ≥70 years, who were enrolled in 71 NCI-sponsored cooperative group clinical trials between 1990 and 2011. After excluding patients who had missing information, the final analysis included 1467 patients. The CONSORT diagram is shown in Figure 1. These patients were randomly divided into a training set (n=963) and a testing set (n=504). The two cohorts were similar in all characteristics, except performance status (PS) and weight loss. The testing set had a higher proportion of patients with PS 1 (66.3% vs. 56.8%) and a higher proportion of patients who had not lost weight (72.6% vs. 67.4%) (Table 1).
Figure 1 –
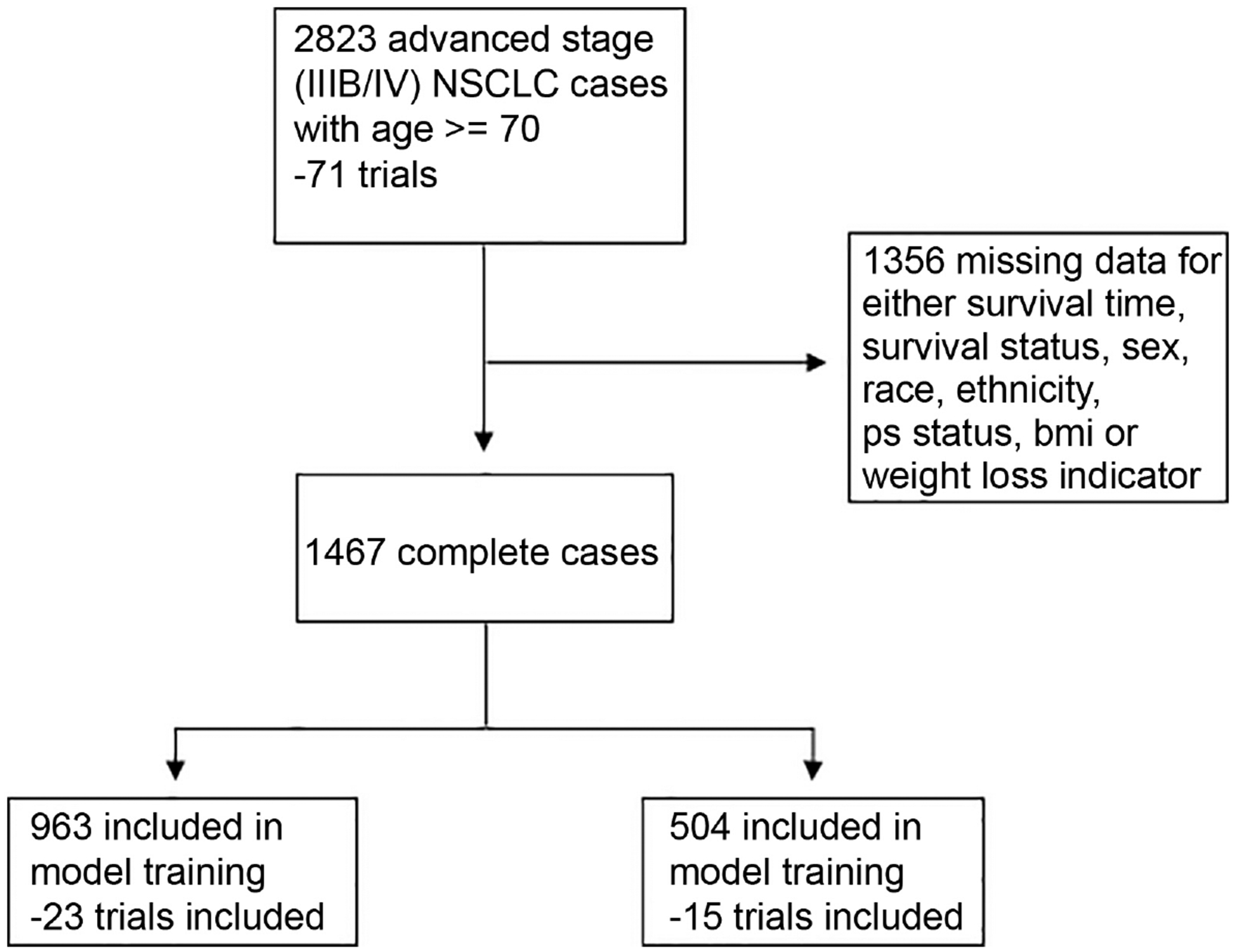
Consort Diagram
Table 1.
Baseline characteristics of enrolled older patients with advanced NSCLC in training and testing cohorts.
| Training (N = 963) | Testing (N = 504) | ||
|---|---|---|---|
| N (%) | N (%) | P | |
| Age group | 0.410 | ||
| 70–74 | 583 (60.54) | 317 (62.90) | |
| 75+ | 380 (39.46) | 187 (37.10) | |
| Sex | 0.836 | ||
| Male | 322 (33.44) | 172 (34.13) | |
| Female | 641 (66.56) | 332 (65.87) | |
| Race group | 0.422 | ||
| White | 881 (91.48) | 467 (92.66) | |
| Black | 67 (6.96) | 27 (5.36) | |
| Others | 15 (1.56) | 10 (1.98) | |
| Ethnicity | 0.979 | ||
| Non-Hispanic | 948 (98.44) | 497 (98.61) | |
| Hispanic | 15 (1.56) | 7 (1.39) | |
| Performance Status | <0.001 | ||
| 0 | 336 (34.89) | 153 (30.36) | |
| 1 | 547 (56.80) | 334 (66.27) | |
| 2 | 80 (8.31) | 17 (3.37) | |
| Initial stage | 0.331 | ||
| IIIB | 441 (45.79) | 245 (48.61) | |
| IV | 522 (54.21) | 259 (51.39) | |
| Body Mass Index | 0.803 | ||
| Healthy | 390 (40.50) | 214 (42.46) | |
| Underweight | 52 (5.40) | 23 (4.56) | |
| Overweight | 370 (38.42) | 186 (36.90) | |
| Obese | 151 (15.68) | 81 (16.07) | |
| 5/10% weight loss in the past 3/6 months | 0.046 | ||
| No | 649 (67.39) | 366 (72.62) | |
| Yes | 314(32.61) | 138 (27.38) |
On multivariable analysis, performance status, initial stage (IIIB vs. IV) and weight loss immediately prior to diagnosis were predictive of overall survival and were assigned a weighted score (Table 2). The prognostic risk score was calculated using the following formula: 5 (if PS=1) + 11 (if PS=2) + 4 (if initial stage=‘IV’) + 5 (if weight loss=‘Yes’). Based on these variables, patients were classified into two groups: good prognosis (0–8 points) and poor prognosis (≥9 points).
Table 2.
Multivariate Cox’s regression analysis for overall survival of enrolled older patients with advanced NSCLC in training cohort.
| Regression coefficient | P | Hazard ratio (95% CI) | Point score | |
|---|---|---|---|---|
| Performance status | ||||
| 0 | 0 | 1 | 0 | |
| 1 | 0.331 | <0.001 | 1.39(1.21,1.61) | 5 |
| 2–3 | 0.735 | <0.001 | 2.08 (1.61,2.69) | 11 |
| Initial stage | ||||
| IIIB | 0 | 1 | 0 | |
| IV | 0.273 | <0.001 | 1.31 (1.15,1.50) | 4 |
| 5/10% weight loss in the past 3/6 months | ||||
| No | 0 | 1 | 0 | |
| Yes | 0.355 | <0.001 | 1.43 (1.24,1.64) | 5 |
The median OS in the two groups in the training set were as follows: good prognosis – 11.77 months (95% CI, 10.92, 13.70 months) and poor prognosis – 6.21 (95% CI, 5.59, 7.50 months) (Table 3, Figure 2a). These findings were validated in the testing set where the findings were similar: good prognosis – 13.15 months (95% CI, 10.82, 15.91 months) and poor prognosis – 8.52 (95% CI, 7.50, 9.63 months) (Table 3, Figure 2b). The area under the 1-year and 2-year ROC and the c-index observed in the training and testing cohorts are shown in Table 4.
Table 3.
Median OS of two risk groups of the developed model in training and testing cohorts.
| Risk strata | N (%) | Number of event | Median OS in months (95% C.I.) |
|---|---|---|---|
| Training cohort | |||
| 1 (0–8 points) | 452 (46.94) | 412 | 11.77 (10.92,13.70) |
| 2 (9+ points) | 511 (53.06) | 497 | 6.21 (5.59, 7.50) |
| Testing cohort | |||
| 1 (0–5 points) | 254 (50.40) | 236 | 13.15(10.82,15.91) |
| 2 (9+ points) | 250 (49.60) | 248 | 8.52 (7.50, 9.63) |
OS – Overall Survival.
CI – Confidence Interval.
Figure 2 –
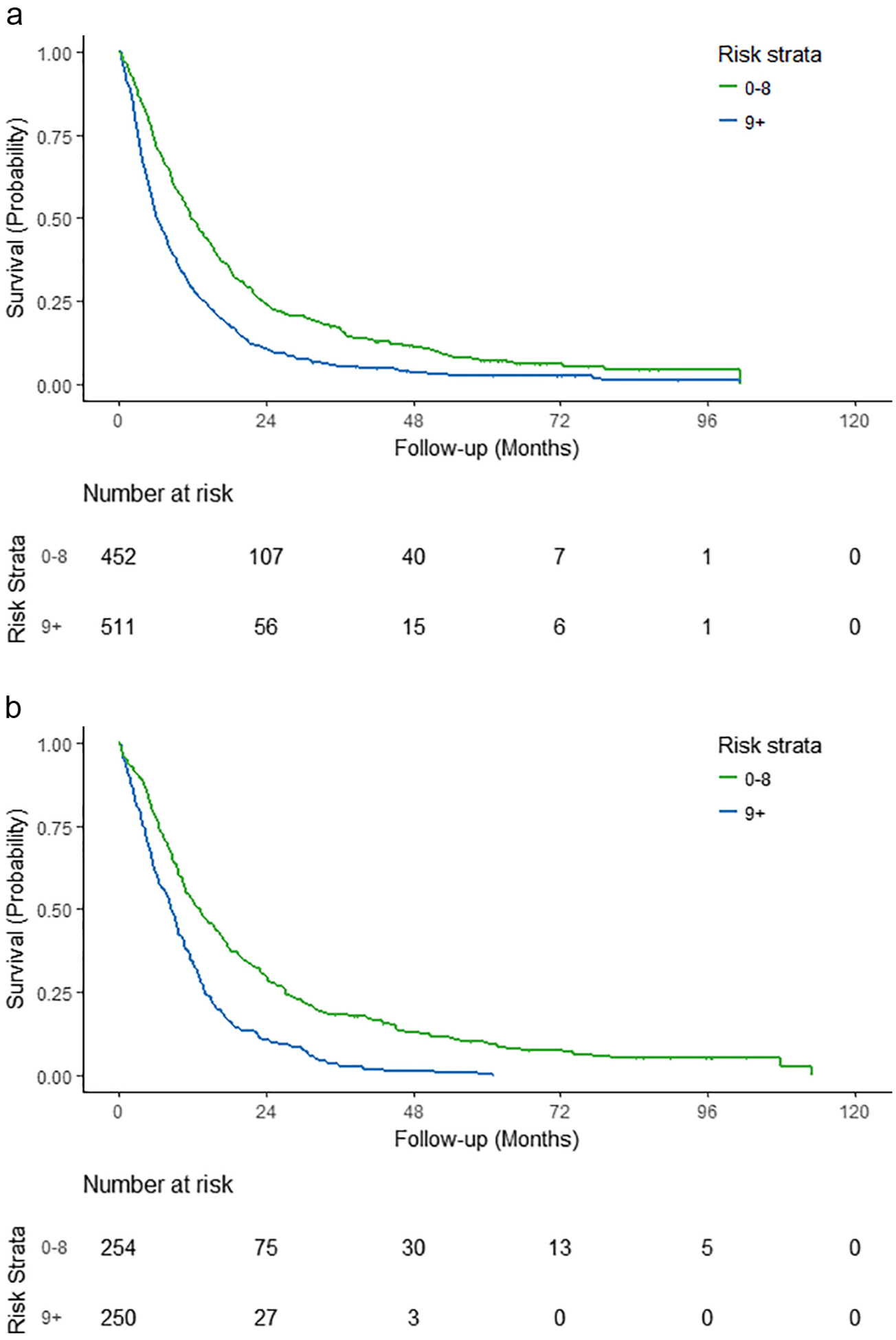
Overall Survival based on risk stratification among older patients with advanced non-small cell lung cancer (a) – training set; (b) – testing set
Table 4.
Area under 1-, 2- year ROC and C-index in training and testing cohorts.
| Training cohort (N = 963) | Testing cohort (N = 504) | |
|---|---|---|
| 1-year ROC (95%C.I.) | 0.61 (0.58, 0.64) | 0.60 (0.55, 0.64) |
| 2-year ROC (95% C.I.) | 0.62 (0.58, 0.66) | 0.65 (0.59, 0.70) |
| C-index (95% C.I.) | 0.57 (0.56, 0.59) | 0.57 (0.55, 0.60) |
ROC – Receiver Operating Characteristics.
CI – Confidence Interval.
The present model was then compared to the currently existing prognostic models for advanced non-small cell lung cancer, namely the Blanchon and Mandrekar models (Supplemental Tables S2 and S3). Despite a lower number of variables in the present model and the simplicity of estimation, the present model had a better area under the 1-year and 2-year ROC than both models; the c-index was better than the Mandrekar model and similar to the Blanchon model (0.59 vs. 0.56 and 0.59 respectively) (Supplementary Table S4).
Discussion
This study proposes a simple prognostic model for older adults with advanced NSCLC based on basic clinical characteristics that are part of the evaluation process for every patient with NSCLC, such as performance status, stage and weight loss immediately prior to diagnosis. The key advantage of this model over existing models is that it is developed exclusively from an older patient cohort, unlike the existing models that included patients of all ages. Thus, our model overcomes some of the drawbacks of existing models on the applicability to older patients.
There have been a number of studies that have studied the significance of different patient and tumor factors in determining prognosis of NSCLC patients. In a review summarizing the literature on this subject, Brundage et al reviewed 887 articles evaluating 169 tumor- or host-related prognostic factors (16). The median number of patients enrolled per study was 120 and the median number of 4 factors were identified as being significant in a multivariate analysis. They concluded that these studies were underpowered and heterogeneous in their conclusions.
Two models were subsequently developed to predict outcomes in NSCLC. The Mandrekar model included data from over 1000 patients to develop an equation that provided overall survival estimates at different time points following the diagnosis (11). The variables included in this model were performance status, body mass index, hemoglobin level and WBC counts prior to treatment. The Blanchon model included approximately 4500 patients of all ages and all stages of NSCLC to develop a score that predicted for 4 year mortality (12). The final score was calculated based on age, sex, performance status at diagnosis, histological type and stage. The patients were classified into six prognostic subgroups. In contrast to these two models, the main advantage of the present model is its simplicity. This model developed from almost 1500 patients, includes performance status, stage and weight loss to divide older patients into two prognostic subgroups.
The inclusion of these variables itself is not surprising, as multiple studies have shown that these factors are individual prognostic markers in lung cancer. In an analysis of the international staging database of the International Association for the Study of Lung Cancer, Sculier et al analyzed records of 12,428 patients (17). Similar to the findings of the present analysis, they found that male gender was associated with an increased risk of death, after adjusting for clinical stage, age, PS, and histology (HR - 1.17, 95% CI, 1.11, 1.23; p<0.001).
Performance status is a well-recognized independent prognostic marker in lung cancer. In the aforementioned analysis, patients with ECOG PS 1, 2 and 3–4 did significantly worse than those with PS 0 (17). The corresponding hazard ratios and 95% CI when compared to PS 0 were, PS 1 – 1.38; 1.32, 1.44; PS 2 – 2.09; 1.95, 2.23; PS 3–4 – 3.48; 3.17, 3.83 (p-values < 0.0001). In a similar analysis of 6462 patients with stage IV NSCLC, Kawaguchi et al observed a significant worsening of median survival with worsening performance status (18). Patients with PS 0 had a median survival of 11.7 months as compared to 8.3, 4.4, 2.8 and 2.1 months for patients with PS 1, 2, 3 and 4 respectively (p<0.0001).
The present study did not stage patients according to the AJCC 7th Edition, as the majority of the trials included in the analysis were completed prior to the use of this system. Stage IIIB in the present analysis includes patients who would be considered as having M1a disease (stage IV) in the AJCC 7th Edition TNM classification. Nevertheless, we found a significantly increased risk of death in patients with distant metastases (Stage IV in the previous AJCC classification; Stage IV - M1b in the 7th Edition). This further highlights the prognostic significance of intrathoracic as opposed extra-thoracic metastatic disease.
Geriatric assessment of all older patients with cancer prior to treatment, has been recommended as a way to detect hitherto hidden health problems even in those with a good performance status (19). A recent study analyzed the prognostic value of the Geriatric 8 (G8) assessment in olderpatients with lung cancer. In this analysis of 142 patients (84 with NSCLC), the potentially frail patients had a significantly greater risk of 1-year mortality (hazard ratio, 4.08; 95% confidence interval 1.67–9.99; P = 0.02). The only other significant variable was a higher disease stage. While geriatric assessment may provide a useful approach to identifying older patients with lung cancer at risk for early mortality, it is time consuming and hence its incorporation in routine clinical care has been limited.
The main strengths of this study are the large sample size and its development using data only from older individuals. Despite the simplicity of the model, its performance characteristics are similar to the presently available models, namely the Blanchon and Mandrekar models. Also since the variables in the present model are routinely captured as part of clinical care, it does not increase the burden on the oncologist or clinic staff. Hence this can be used routinely to determine prognosis of older patients with advanced NSCLC, especially if a comprehensive geriatric assessment is not feasible. Furthermore, a simpler model can be more robust and therefore more useful as compared to a complex model, in which additional predictors are included but have low added value in prediction accuracy.
The limitations of this study include that it comprised of only those patients, who were enrolled in NCI cooperative group clinical trials. Previous studies have indicated that patients approached for clinical trial participation may have certain specific characteristics and may not necessarily represent the population at large (20). However, given the diverse nature of enrollment in cooperative group trials, we feel that our findings are valuable to the practicing oncologist. In addition, this model would help the development of practical clinical trials for older adults with advanced NSCLC.
Secondly, most of the trials included in this analysis were conducted before current knowledge regarding specific driver mutations and immunotherapeutic agents were available and hence that information is lacking. Lastly, the clinical trials included in this analysis did not have a component of geriatric assessment. Nonetheless, we believe that this information will be valuable as the same parameters will be relevant to those patients as well.
In conclusion, we have developed a simple, pragmatic, prognostic model using performance status, distant metastases and weight loss immediately prior to diagnosis predict for overall survival in older patients with advanced non-small cell lung cancer.
Supplementary Material
Funding Source:
Supported by National Institutes of Health Grant No. R21-AG042894 (T.E.S., E.E.V., H.P., X.W.), Health and Medical Research Fund No. 15162491 (H.P.), and National Cancer Institute Grant No. P01-CA142538 (X.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None of the authors has any conflicts of interest with the contents of this manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015;1(1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socinski M, Creelan B, Horn L, Reck M, Paz-Ares L, Steins M, et al. NSCLC, metastaticCheckMate 026: A phase 3 trial of nivolumab vs investigator’s choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1)−positive NSCLC. Annals of Oncology. 2016;27(suppl_6):LBA7_PR–LBA_PR. [Google Scholar]
- 4.Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr., Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–9. [DOI] [PubMed] [Google Scholar]
- 6.Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol. 2001;19(4):1064–70. [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Venditti LN, Neumann PJ, Gelber RD, Weinstein MC, Potosky AL, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117(5):1239–46. [DOI] [PubMed] [Google Scholar]
- 8.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2191–7. [DOI] [PubMed] [Google Scholar]
- 9.Chrischilles EA, Pendergast JF, Kahn KL, Wallace RB, Moga DC, Harrington DP, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(4):620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramalingam SS, Dahlberg SE, Langer CJ, Gray R, Belani CP, Brahmer JR, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26(1):60–5. [DOI] [PubMed] [Google Scholar]
- 11.Mandrekar SJ, Schild SE, Hillman SL, Allen KL, Marks RS, Mailliard JA, et al. A prognostic model for advanced stage nonsmall cell lung cancer: pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2006;107(4):781–92. [DOI] [PubMed] [Google Scholar]
- 12.Blanchon F, Grivaux M, Asselain B, Lebas FX, Orlando JP, Piquet J, et al. 4-year mortality in patients with non-small-cell lung cancer: development and validation of a prognostic index. Lancet Oncol. 2006;7(10):829–36. [DOI] [PubMed] [Google Scholar]
- 13.Schulkes KJ, Hamaker ME, van den Bos F, van Elden LJ. Relevance of a Geriatric Assessment for Elderly Patients With Lung Cancer-A Systematic Review. Clin Lung Cancer. 2016;17(5):341–9 e3. [DOI] [PubMed] [Google Scholar]
- 14.Schulkes KJ, Souwer ET, Hamaker ME, Codrington H, van der Sar-van der Brugge S, Lammers JJ, et al. The Effect of A Geriatric Assessment on Treatment Decisions for Patients with Lung Cancer. Lung. 2017;195(2):225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang HH, Wang X, Stinchcombe TE, Wong ML, Cheng P, Ganti AK, et al. Enrollment Trends and Disparity Among Patients With Lung Cancer in National Clinical Trials, 1990 to 2012. J Clin Oncol. 2016;34(33):3992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122(3):1037–57. [DOI] [PubMed] [Google Scholar]
- 17.Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P, International Staging C, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol. 2008;3(5):457–66. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5(5):620–30. [DOI] [PubMed] [Google Scholar]
- 19.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kehl KL, Arora NK, Schrag D, Ayanian JZ, Clauser SB, Klabunde CN, et al. Discussions about clinical trials among patients with newly diagnosed lung and colorectal cancer. J Natl Cancer Inst. 2014;106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


