Abstract
Objective
To examine the effect of chronic demyelination in the optic nerve of patients with MS on progressive loss of retinal ganglion cell (RGC) axons.
Methods
Progressive retinal nerve fiber layer (RNFL) loss, as measured by optical coherence tomography, was longitudinally examined in 51 patients with MS with a history of unilateral optic neuritis (ON) and 25 normal controls. Patients were examined annually with a median of 4-year follow-up. Pairwise intereye comparison was performed between ON and fellow non-ON (NON) eyes of patients with MS using the linear mixed-effects model and survival analysis. The latency asymmetry of multifocal visual evoked potential (mfVEP) was used to determine the level of demyelination in the optic nerve.
Results
Although both ON and NON eyes demonstrate significantly faster loss of RGC axons compared with normal subjects, ON eyes with severe chronic demyelination show accelerated thinning in the RNFL in the temporal sector of the optic disc (temporal RNFL [tRNFL]) compared with fellow eyes (evidenced by both the linear mixed-effects model and survival analysis). Furthermore, progressive tRNFL thinning is associated with the degree of optic nerve demyelination and reflects the topography of pathology in the optic nerve. More rapid axonal loss in ON eyes is also functionally evidenced by mfVEP amplitude reduction, which correlates with the level of optic nerve demyelination.
Conclusions
Although the effect of demyelination on axonal survival has been demonstrated in experimental studies, our results provide first clinically meaningful evidence that chronic demyelination is associated with progressive axonal loss in human MS.
Axonal loss is now accepted as the major cause of irreversible neurologic disability in MS. Acute inflammatory demyelination is thought to be a principal cause of axonal transection and subsequent axonal degeneration.1 However, during the chronic phase of MS, even in the absence of acute inflammation, there is still considerable neuroaxonal loss evidenced by ongoing brain atrophy and worsening of clinical status.2,3 This suggests that there are other mechanisms involved in neurodegenerative changes in MS and emphasizes the shortcomings of the current disease-modifying therapies, which diminish the formation of new inflammatory lesions, but have little impact on axonal loss during the progressive stage of the disease.2
In addition, laboratory studies have indicated that loss of myelin can eventually lead to neuroaxonal damage.4 There is evidence that permanent demyelination may contribute to accelerated axonal degeneration by increasing vulnerability of axons to physiologic stress.5 Furthermore, lack of neurotrophic support from oligodendrocytes and disruption of normal axon-myelin interactions may also lead to degeneration of chronically demyelinated axons.4,6 Although numerous animal and in vitro studies have demonstrated negative effects of demyelination on axonal survival, the clinical significance of chronic demyelination as a precursor of axonal damage in humans has never been ascertained. Myelin pathology has also been identified in many neurodegenerative diseases other than MS7–9 and may play an important role in their pathogenesis. Moreover, transneuronal spread of degeneration along human neuronal projections could also be partially mediated by demyelination.9 Therefore, an understanding of whether chronic demyelination can cause neuroaxonal loss in humans is of paramount importance.
The aim of the current study was to investigate the effect of chronic demyelination on neuroaxonal loss in humans using the visual system as a model. The degree of demyelination following acute optic neuritis (ON) can be accurately estimated using the latency of visual evoked potentials (VEPs),10,11 and neuroaxonal loss of retinal ganglion cells (RGCs) can be monitored with a high level of precision using optical coherence tomography (OCT).12 Furthermore, using intrasubject comparison of 2 eyes in patients with a history of unilateral ON, intersubject variability is eliminated and the impact of other factors (such as age, sex, disease duration, treatment, and the effect of retrochiasmal lesions13) on the evaluation of an effect of chronic demyelination considerably reduced.
Methods
Participants
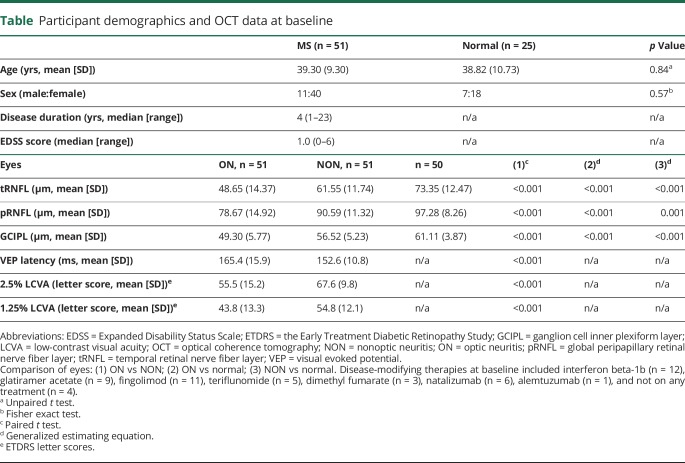
Fifty-one consecutive patients with relapsing-remitting MS with a history of unilateral ON (reported by patients and confirmed by treating neurologists) from 4 tertiary neuro-ophthalmology or neurology clinics in Sydney (Royal North Shore Hospital, Brain & Mind Centre, Inner West Neurology, and the Save Sight Institute) were recruited in this prospective cohort study. MS was diagnosed according to the 2010 revised McDonald criteria.14 Exclusion criteria included a history of other ocular, neurologic, or systemic diseases that could affect the results. Only patients who did not have ON 12 months before the study were included. The median post-ON duration in the study cohort was 4 years (range: 1–26 years). One patient had a relapse of ON during the follow-up, and only the data collected before the flare-up were included in analysis. Patients underwent regular ocular examinations (including visual acuity using the Early Treatment Diabetic Retinopathy Study low-contrast chart), OCT scans and multifocal visual evoked potential (mfVEP) recordings, and both eyes were analyzed. Patients with MS were examined annually and were followed up for a median of 4.0 years (range: 0.9–7.6 years) (n = 21, ≤2 years of follow-up; n = 12, 3–4 years of follow-up; n = 18, ≥5 years of follow-up). In addition, 25 healthy participants with similar sex and age distributions were also recruited as normal controls (median follow-up of 3.1 years [0.9–7.8]). The data analyzed in this study were collected between July 2010 and June 2018.
Standard protocol approvals, registrations, and patient consents
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Human Research Ethics Committee of the University of Sydney. Written consent was signed by all participants.
OCT scans
All participants underwent a peripapillary ring scan and a macular radial pattern scan using the Heidelberg Spectralis OCT and Eye Explorer software version 6.9.5.0 (Heidelberg Engineering, Germany) as described previously.15,16 In this study, the OCT data were reported according to the APOSTEL recommendations.17 The pupils of participants were not dilated, and the scans were performed by the same operator and device under room light conditions. The peripapillary ring scan (manual placement of the ring; diameter = 3.50 mm) was used to obtain retinal nerve fiber layer (RNFL) thickness measures. Both global and sectoral (including temporal, nasal, superior, and inferior) RNFL thicknesses were analyzed. The follow-up function was activated when scans were performed to ensure that the scans were obtained at the same locations as the baseline scans. All images were checked by 2 investigators (Y.Y. and A.K.), and those with poor quality and segmentation errors were excluded to ensure that all OCT images included in the analysis fulfilled the OSCAR-IB criteria.18 Baseline RNFL and ganglion cell inner plexiform layer (GCIPL) thicknesses are reported for all cases. Due to the fact that substantial thinning of the RNFL was present in ON eyes at baseline, relative change of RNFL was used for analysis, which has also been used by Talman et al.19 previously. Longitudinal analysis of relative loss of GCIPL was not able to be used for this study as the inner plexiform layer is relatively preserved and separating the 2 layers by segmentation is not reliable.15 A detailed explanation of GCIPL issues and schematic diagram is provided in supplementary materials (links.lww.com/NXI/A224).
Multifocal VEP recordings
The level and topography of demyelination in the visual pathways was assessed by the latency delay of mfVEP recorded using the Vision Search system (VisionSearch, Sydney, Australia) with standard stimulus conditions as described previously.16,20 In brief, 4 gold-disc electrodes (Grass, West Warwick, RI) were used for bipolar recording with 2 electrodes positioned 4 cm on either side of the inion, one electrode 2.5 cm above and another 4.5 cm below the inion in the midline. Electrical signals were recorded along 2 channels, measured as the difference between superior and inferior and between the left and right electrodes. All the VEP traces were checked by 2 experts (Y.Y. and A.K.) to ensure the quality of signal. Intereye asymmetry of mfVEP latency was used to determine the level of ON-related demyelination (postchiasmal/optic radiation lesions are assumed to have similar effects on latency delay in both eyes). For VEP analysis,16 the largest peak-to-trough amplitude within the interval of 70–210 ms was selected, and the second peak of the trace was used to determine the latency.
Statistics
Statistical analysis was performed using SPSS (version 24.0, IBM) and Graphpad Prism (version 7.0; Graphpad, La Jolla, CA). Baseline characteristics are summarized using descriptive statistics. Mean and CIs are reported for longitudinal continuous variables. Frequencies are reported for categorical data. Longitudinal OCT measures were analyzed in both eyes in the study subjects for intereye comparisons. Annual RNFL progression rates were estimated and compared by using linear mixed-effects models that included age, sex, and disease duration (for comparison between ON vs non-ON [NON] eyes) as covariates. The models have a multilevel structure with repeated measures nested within eye (left/right), nested within subjects. The frequency distribution of dependent variables was visually confirmed as normal, and no transformation was required. Boxplots showed that the assumption of equal variances of groups was valid. Subjects had varying numbers of repeated measures, and this missing data were handled by the mixed model restricted maximum likelihood estimation, without the need for imputation or deletion of cases. In addition, patients were separated into 2 groups for a further subanalysis based on the level of demyelination in the optic nerve (determined by asymmetry of mfVEP latency; <10 ms and ≥10 ms). Kaplan-Meier event rate curves and the log-rank (Mantel-Cox) test were also used to assess the cumulative risk ratio of progression and compared between ON and fellow NON eyes. The first time that more than 5% of RNFL thickness loss was found as compared to the baseline was defined as the end point in survival analysis.21 Five percent was selected as a statistically meaningful end point to determine progression based on previously reported intertest coefficient of variation of RNFL measures to be between 1% and 2%.22,23 Correlation between optic nerve demyelination (determined by mfVEP latency asymmetry) and asymmetry in the progression rate in temporal RNFL (tRNFL)/mfVEP amplitude between ON and NON eyes was examined using bivariate correlation and partial correlation controlling for age, sex, and disease duration. A p value of less than 0.05 was considered significant.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and from the corresponding author on reasonable request.
Results
Demographic data of all the participants are shown in the table. At baseline, both ON and NON eyes exhibited significant RGC neuronal and axonal loss as compared to age- and sex-matched control eyes, which is consistent with previous OCT studies in MS.24 Compared with NON eyes, eyes with a history of ON demonstrated significantly worse low-contrast visual acuity (LCVA) and thinner global and temporal RNFL. Furthermore, there was significant prolongation of mfVEP latency in ON eyes compared with NON eyes, which indicates a considerable degree of chronic ON-related demyelination (figure e-1, links.lww.com/NXI/A224).
Table.
Participant demographics and OCT data at baseline
Progressive RNFL thinning in ON and NON eyes
The rate of progressive RGC neuronal and axonal loss was examined in patients with a history of unilateral ON using intrasubject between-eye comparison. Because we have previously demonstrated that tRNFL is the most sensitive parameter to detect progressive loss of RGC fibers in MS,15 longitudinal loss of tRNFL was analyzed first. The linear mixed-effects model showed that age and disease duration at baseline were not associated with progressive tRNFL change. Although male subjects had overall thinner tRNFL (p = 0.02), sex did not have significant effects on the tRNFL progression rate. Both ON (−1.3% per year, 95% CI: −1.7% to −0.8%) and NON fellow (−1.0% per year, 95% CI: −1.3% to −0.7%) eyes displayed faster rates of tRNFL loss than normal controls (−0.6% per year, 95% CI: −1.1% to −0.2%; p < 0.001 vs ON and p = 0.008 vs NON). Although ON eyes demonstrated faster tRNFL thinning compared with fellow NON eyes, this difference was not statistically significant (mean difference = −0.3% [95% CI: −0.7 to 0.1]; p = 0.1) (figure e-2, links.lww.com/NXI/A224 for the trend of tRNFL thinning in µm in ON and NON eyes).
Similar analysis, applied to the global peripapillary retinal nerve fiber layer (pRNFL) measurement, also demonstrated a trend for reduction of RNFL in both ON and NON eyes. Relative pRNFL thinning was significantly faster in ON eyes (−0.5% per year, 95% CI: −0.8% to −0.3%) and NON eyes (−0.5% per year, 95% CI: −0.7% to −0.4%) compared with normal eyes (−0.2% per year, 95% CI: −0.4% to 0.0%, p = 0.04 vs ON and p = 0.01 vs NON). However, no difference in the rate of pRNFL thinning between ON and fellow NON eyes was detected (p = 0.63).
Survival analysis demonstrates faster thinning of tRNFL in ON eyes
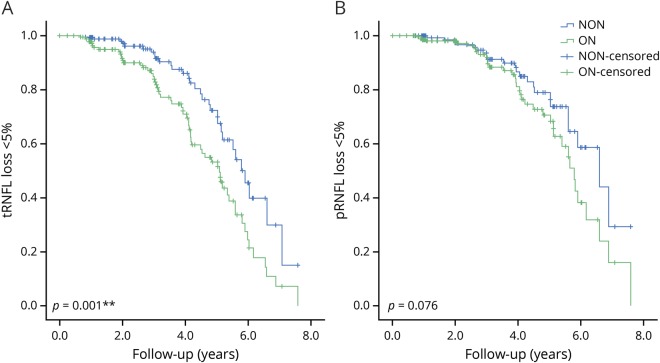
Although mean rates of progression were not significantly different between ON and NON eyes, time to reach an end point did show a difference. Because survival analysis has been shown to demonstrate high sensitivity in detecting subtle difference in RNFL thinning in patients with glaucoma,21 it was applied here to investigate RNFL progression in patients with MS with unilateral ON. More than 5% of RNFL thickness loss was defined as the end point. The analysis demonstrated significantly faster progressive tRNFL loss in ON eyes compared with their fellow NON eyes (p = 0.001, figure 1A). The difference between ON and NON eyes in pRNFL progression did not reach statistical significance (p = 0.08, figure 1B). To determine whether the above borderline change in pRNFL was mainly driven by tRNFL loss, RNFL changes in the other sectors were also evaluated. The subanalysis demonstrates no statistical difference in RNFL progression in the nasal (p = 0.65), superior (p = 0.95), and inferior (p = 0.14) disc areas between ON and NON eyes.
Figure 1. ON eyes showed a faster rate of RNFL loss in the temporal disc area.
Comparisons of progressive tRNFL (A) and pRNFL (B) thinning between ON and fellow NON eyes in patients with unilateral ON (n = 51) using Kaplan-Meier curves and the log-rank test. More than 5% of RNFL thickness loss as compared to the baseline was defined as the end point. NON = non-ON; ON = optic neuritis; pRNFL = global peripapillary retinal nerve fiber layer; RNFL = retinal nerve fiber layer; tRNFL = temporal RNFL.
Faster thinning of tRNFL in ON eyes is associated with the degree of optic nerve demyelination
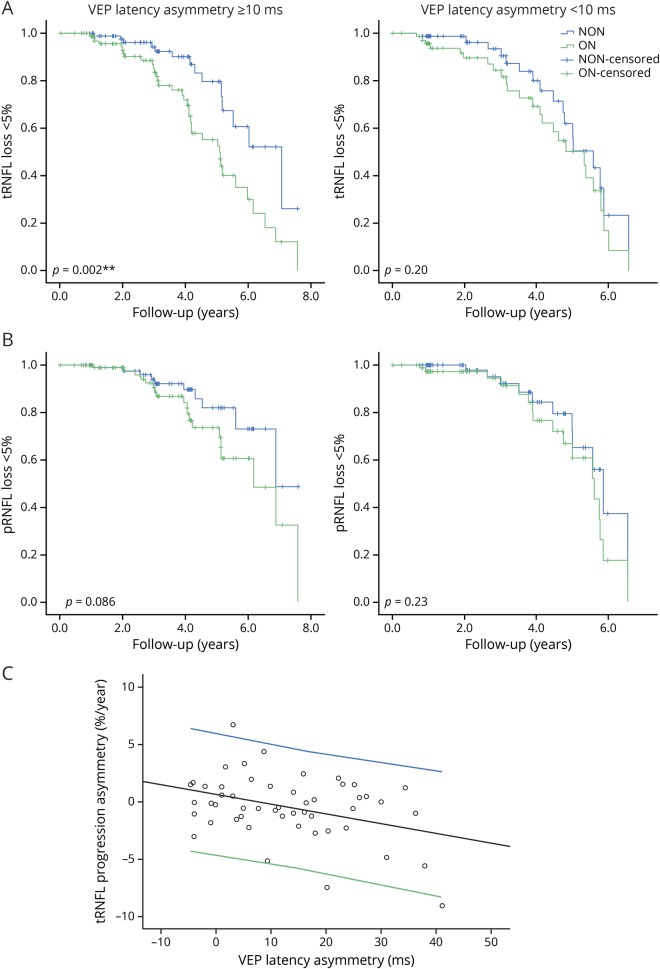
To investigate whether the tRNFL loss in ON eyes was associated with the degree of underlying chronic optic nerve demyelination, the patients were subdivided into 2 groups based on severity of optic nerve demyelination. Intereye mfVEP latency asymmetry was used to characterize the degree of demyelination caused by ON.10,11 There were 24 patients with no or mild ON-related chronic demyelination (latency asymmetry <10 ms, average 2.1 ± 4.6 ms) and 27 patients with moderate to severe optic nerve demyelination (latency asymmetry ≥10 ms, average 21.9 ± 8.8 ms). In the more severe demyelination group, progressive loss of tRNFL was significantly faster in the ON eyes than in the fellow eyes, evidenced by both the linear mixed-effects model (mean difference = −0.6% per year, 95% CI: −0.1% to −1.0%; p = 0.02) and survival analysis (p = 0.002). By contrast, no significant intereye difference was observed in the group with minimal degree of chronic demyelination (linear mixed-effects model: mean difference = −0.1% per year, 95% CI: −0.8% to 0.7%; p = 0.89; survival analysis: p = 0.20, figure 2A). Survival analysis of pRNFL in the severe latency delay group also showed a borderline difference in ON eyes in the progression rate compared with NON (p = 0.08) (figure 2B).
Figure 2. Faster tRNFL thinning in ON eyes is associated with the level of chronic demyelination in the optic nerve.
(A and B) Comparisons of progressive RNFL loss between ON and NON eyes in 2 patient groups with different levels of demyelination in the optic nerve determined by asymmetry of mfVEP latency (n = 27, latency asymmetry ≥10 ms; n = 24, latency asymmetry <10 ms). Faster tRNFL thinning was only present in the patients with more severe optic nerve demyelination (latency asymmetry ≥10 ms). There was also borderline difference (p = 0.086) in pRNFL progression between ON and fellow NON eyes in patients with advanced demyelination; by contrast, no difference was observed in the latency asymmetry <10 ms group, which also supports an association between accelerated RNFL loss in the temporal disc area and the level of optic nerve demyelination. (C) There was a significant correlation between asymmetry of the tRNFL progression rate (%/year) and optic nerve demyelination, as determined by intereye asymmetry of mfVEP latency between ON and fellow NON eyes (r = −0.38, p = 0.007), which remained significant after controlling for age, sex, and disease duration by partial correlation (p = −0.37, p = 0.01). NON = non-ON; ON = optic neuritis; pRNFL = peripapillary retinal nerve fiber layer; RNFL = retinal nerve fiber layer; tRNFL = temporal RNFL; VEP = visual evoked potential.
In addition, accelerated tRNFL thinning, was also significantly associated with the degree of ON-related optic nerve demyelination (r = −0.38, p = 0.007) (figure 2C). This result supports the assumption that a chronic deficit of myelin is associated with progressive axonal loss in MS.
Preferential loss of tRNFL in ON eyes reflects the topography of optic nerve demyelination
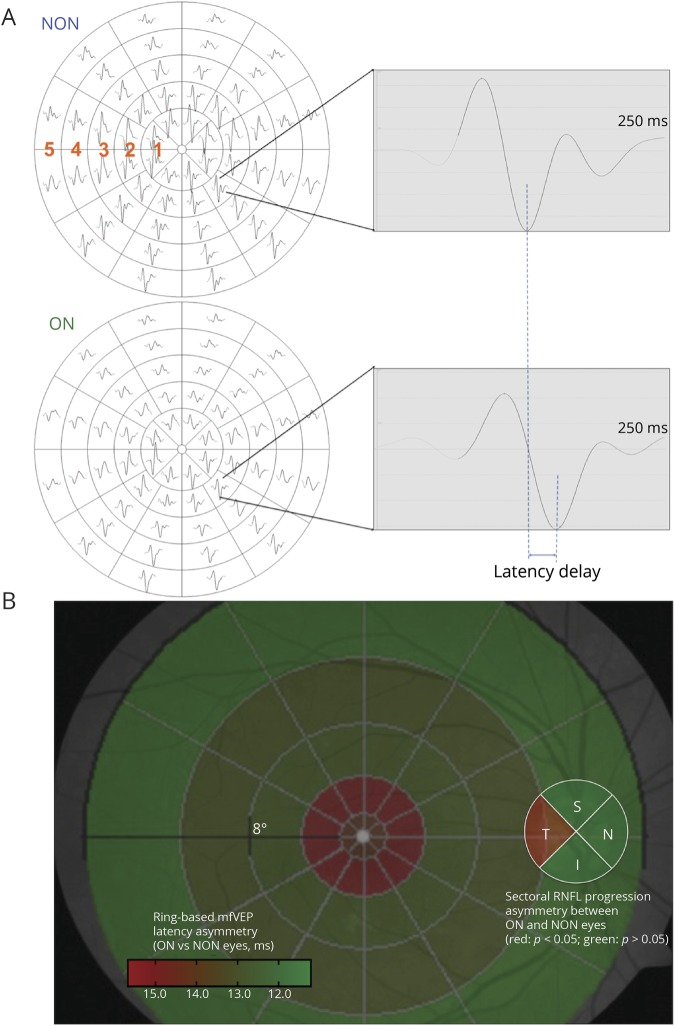
As described above, only tRNFL demonstrates accelerated thinning in ON eyes compared with fellow NON eyes. Because tRNFL consists of the RGC fibers projecting to the central part of the retina, it raises the question of whether those fibers are more susceptible to demyelination. Therefore, the degree of ON-related demyelination at different eccentricities of the retina was examined using ring-based analysis of asymmetry of the mfVEP latency between ON and fellow NON eyes (figure 3A).
Figure 3. Accelerated tRNFL thinning reflects the topography of optic nerve demyelination in ON eyes.
(A) Representative mfVEP traces from a patient with MS with a history of unilateral ON. Significant latency delay is observed in the ON eye. Asymmetry of the latency of mfVEP was calculated for each of the 5 eccentricity rings to determine the pattern of optic nerve demyelination. (B) Optic nerve fibers projecting to rings 1–2 showed a significantly higher level of demyelination than those projecting to rings 3–5 (p = 0.0005, 2-way ANOVA). The central retinal area topographically corresponds to the RNFL in the temporal sector of the optic disc (shown in red). This result could explain the observation of accelerated tRNFL thinning in ON eyes compared with fellow NON eyes. Compared with traditional pVEP, mfVEP used in this study represents a superior technique. Because of opposite orientation of upper and lower calcarine cortices, the dipoles generating responses from stimulation of the upper and lower hemifields are opposite and, therefore, producing oppositely oriented waveforms, which results in a cancelation effect.40 Therefore, pVEP actually represents the difference between signals from upper and lower calcarine cortices. In addition, the majority of the pVEP power is generated by 3–5 central degrees of the visual field, practically ignoring the paracentral and midperipheral fields. ANOVA = analysis of variance; NON = non-ON; ON = optic neuritis; pVEP = pattern VEP; RNFL = retinal nerve fiber layer; tRNFL = temporal RNFL.
The highest level of relative latency delay in the optic nerve fibers was found in the optic nerve fibers corresponding to the central 2 rings of the mfVEP stimulus, which represent the foveal to parafoveal region of the visual field (figure 3B). Optic nerve fibers projecting to rings 1–2 showed significantly higher level of latency delay than those projecting to rings 3–5 (p < 0.001, 2-way analysis of variance).
The fact that more severe latency delay is topographically related to the area demonstrating the most profound RNFL thinning supports the notion that accelerated axonal loss in MS is associated with chronic demyelination.
Faster axonal loss in ON eyes is also evidenced by VEP amplitude reduction
The amplitude of the mfVEP represents an objective functional measure of the visual pathway. While in acute ON reduction of the mfVEP amplitude is a result of conduction block caused by inflammation, in post-acute (chronic) stage ON, the mfVEP amplitude mainly reflects permanent axonal damage along the visual pathway.25–27 Therefore, the relationship between longitudinal change of mfVEP amplitude and degree of chronic optic nerve demyelination was investigated. Because of variability of mfVEP amplitude, only the subjects who had at least 3 follow-up visits were included (n = 28). Although 30 of 51 patients had at least 3 follow-up visits, in 2 patients, the VEP data for progression analysis were not available. Therefore, only 28 patients were included in the mfVEP substudy. Two patients had very low (nonrecordable) amplitude even at the baseline visit. Therefore, data from 26 patients were analyzed. The analysis demonstrated a significant difference in the rate of amplitude reduction between ON and fellow NON eyes. Annual reduction of mfVEP amplitude was 1.3% ± 0.7% faster in ON eyes compared with NON eyes (p = 0.007).
Both relative reduction of mfVEP amplitude in ON eyes (vs amplitude of the fellow NON eyes) and increase of intereye amplitude asymmetry correlated significantly with baseline latency delay (r = −0.50, p = 0.02, figure 4). In addition, the slope of mfVEP amplitude reduction also correlates with tRNFL thinning (r = 0.40, p = 0.045).
Figure 4. Correlation between baseline latency delay and asymmetry of the rate of mfVEP amplitude reduction (r = −0.47, p = 0.02).
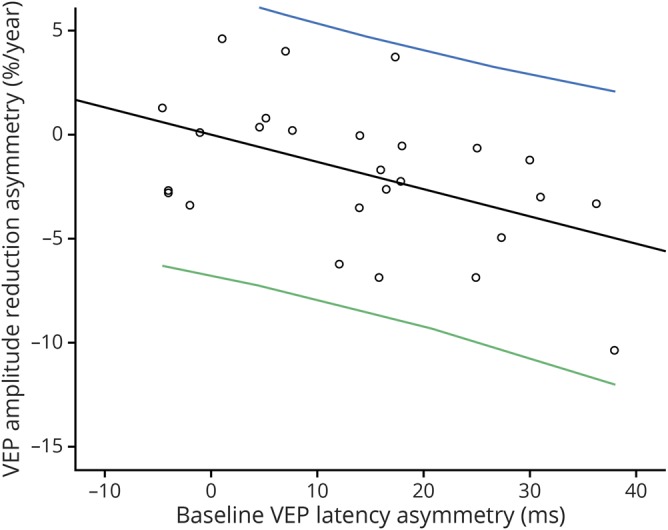
The correlation remained significant after adjusted for age, sex, and disease duration (r = −0.50, p = 0.02). VEP = visual evoked potential.
No evidence of new subclinical ON during the study
To exclude the potential effect of new subclinical ON, the longitudinal analysis of mfVEP latency was also performed in the same cohort of patients. There was no increase in the VEP latency in ON eyes detected. In fact, there was slight but significant shortening of the latency in ON eyes between the baseline and the last visit (1.6 ± 2.9 ms, p = 0.01, paired t test), whereas the latency of NON eyes remained stable (mean difference = 0.09 ± 3.2 ms, p = 0.8, paired t test), suggesting no active inflammatory demyelination during follow-up.
Discussion
Although chronic (not ON related) axonal and neuronal loss of RGC has been well documented in patients with MS, the underlying mechanisms remain elusive.24 Primary neurodegeneration and transsynaptically mediated damage have been suggested as potential causes of chronic axonal loss in MS.9,13,28–31 RGC and its axon are thought to be analogous to brain gray and white matter. Therefore, studying mechanisms of its damage in MS may provide crucial insights into pathogenesis of the disease. The current study examined the effect of chronic demyelination of the optic nerve on loss of RGC axons in patients with MS and provided the first direct in vivo evidence supporting contribution of permanent demyelination to progressive axonal loss in MS. This assumption is based on the fact that the faster rate of RNFL thinning in ON eyes is related to both severity and topography of demyelination in the optic nerve. Accelerated loss of axons was predominantly seen in tRNFL, which represents RGC axons subserving the macular area and are most severely demyelinated.
It should be noted that even in tRNFL, the preferential loss of axons in ON eyes was only apparent in cases with substantial demyelination. This emphasizes the fact that a clinical history of ON by itself is not sufficient to adequately estimate the presence of residual demyelination, which can vary significantly between patients. Therefore, a more direct measure must be used to assess the degree of chronic loss of myelin in the optic nerve. This together with the use (in some studies) of less sensitive time-domain OCT and absence of tRNFL sector analysis may partially explain the fact that previously reported longitudinal studies have not demonstrated a faster rate of axonal loss in ON eyes.19,32–35 In a 5-year study by Garcia-Martin et al.,32 no differences were detected between eyes with and without previous ON. Although VEP was recorded, its correlation with the rate of RNFL thinning was not analyzed. Furthermore, latency asymmetry of only 3 ms between ON and NON eyes, reported by the authors, indicates minimal degree of residual demyelination in the cohort of patients with ON. Although the study by Talman et al.19 also failed to show a difference in the relative rate of RNFL loss between ON and NON eyes, time since the episode of acute ON appeared to be a major determinant of RNFL thinning, suggesting that the duration of chronic demyelination may have some effects on axonal loss. A similar result was recently reported by Behbehani et al.33; however, only total RNFL thickness was measured, and no estimation of the degree of demyelination was performed.
One limitation of this study is a potential deleterious effect of subclinical ON on axonal integrity during the course of the follow-up. Although recurrence of ON is usually reported by patients and detected by worsening of VEP latency, VEP has been described to be highly sensitive even in subclinical cases.36 Longitudinal latency analysis in the current study did not reveal any evidence of subclinical inflammatory demyelination in the optic nerve. Conversely, slight latency shortening in ON eye was observed, which can be explained by preferential axonal loss of the most extensively demyelinated axons, leading to paradoxically improved nerve conduction. Furthermore, the potential effect of disease-modifying therapy was not examined due to the relatively small sample size for each medication group, which represents another potential limitation of the study. In addition, in this study, we were not able to identify any difference in LCVA deterioration between ON and NON eyes, which was possibly due to low sensitivity of the subjective test. Many ON eyes already had significant LCVA loss at baseline, which might also mask subtle further deterioration during follow-up. Although using VEP latency alone as the measurement of demyelination may by itself be a limiting factor, we are not familiar with any other techniques, which would allow to precisely measure degree of demyelination. In addition, compared with traditional pattern VEP, which has been used in MS remyelination trials with variable results, mfVEP used in the current study represents a superior technique (figure 3). Future MS clinical trials could consider using mfVEP to provide more accurate measure of remyelination. Although slightly more time consuming, the latency results of mfVEP are more meaningful and reliable, which results in significantly smaller sample size needed to demonstrate remyelination.37 Although we did not perform mfVEP in control subjects (which may be considered as another limitation), the conclusions were mainly drawn from intrasubject analysis between ON and NON in patients with MS.
To the best of our knowledge, this study provides the first clinical evidence supporting the hypothesis that chronic demyelination is associated with progressive axonal damage in MS. Although the result of the study indicates that the short-term (3–5 years) effect of chronic demyelination on axonal survival is small, it is clearly measurable, suggesting its potentially detrimental role in disease progression and functional deterioration during long course of the disease. The current study, therefore, strongly supports the need for development of new pro-remyelination therapies, a rapidly emerging research area in MS.38,39 In addition, given the fact that demyelination has also been observed as a pathologic component in other neurodegenerative disorders7,8 as well as being implicated in transneuronal degeneration,9 the revealed association between chronic demyelination and axonal loss may have broader implications for human neurodegenerative diseases.
Glossary
- GCIPL
ganglion cell and inner plexiform layer
- LCVA
low-contrast visual acuity
- NON
non-ON
- OCT
optical coherence tomography
- ON
optic neuritis
- pRNFL
global peripapillary retinal nerve fiber layer
- RGC
retinal ganglion cell
- RNFL
retinal nerve fiber layer
- tRNFL
temporal RNFL
- VEP
visual evoked potential
Appendix. Authors
Study funding
This study was funded by the National Multiple Sclerosis Society (NMSS), the National Health and Medical Research Council (NHMRC), the Ophthalmic Research Institute of Australia (ORIA), and the Sydney Medical School Foundation.
Disclosure
Disclosures available: Neurology.org/NN.
References
- 1.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–285. [DOI] [PubMed] [Google Scholar]
- 2.Beck ES, Reich DS. Brain atrophy in multiple sclerosis: how deep must we go? Ann Neurol 2018;83:208–209. [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Barnett MH, Yiannikas C, et al. . Lesion activity and chronic demyelination are the major determinants of brain atrophy in MS. Neurol Neuroimmunol Neuroinflamm 2019;6:e593 doi: 10.1212/NXI.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nave KA. Myelination and support of axonal integrity by glia. Nature 2010;468:244–252. [DOI] [PubMed] [Google Scholar]
- 5.Kornek B, Storch MK, Weissert R, et al. . Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol 2000;157:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci 2003;23:4967–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornari E, Maeder P, Meuli R, Ghika J, Knyazeva MG. Demyelination of superficial white matter in early Alzheimer's disease: a magnetization transfer imaging study. Neurobiol Aging 2012;33:428.e7–419. [DOI] [PubMed] [Google Scholar]
- 8.Dean DC III, Sojkova J, Hurley S, et al. . Alterations of myelin content in Parkinson's disease: a cross-sectional neuroimaging study. PLoS One 2016;11:e0163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You Y, Joseph C, Wang C, et al. . Demyelination precedes axonal loss in the transneuronal spread of human neurodegenerative disease. Brain 2019;142:426–442. [DOI] [PubMed] [Google Scholar]
- 10.You Y, Klistorner A, Thie J, Graham SL. Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Invest Ophthalmol Vis Sci 2011;52:6911–6918. [DOI] [PubMed] [Google Scholar]
- 11.van der Walt A, Kolbe S, Mitchell P, et al. . Parallel changes in structural and functional measures of optic nerve myelination after optic neuritis. PLoS One 2015;10:e0121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. . Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol 2016;15:574–584. [DOI] [PubMed] [Google Scholar]
- 13.Klistorner A, Graham EC, Yiannikas C, et al. . Progression of retinal ganglion cell loss in multiple sclerosis is associated with new lesions in the optic radiations. Eur J Neurol 2017;24:1392–1398. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham E, You Y, Yiannikas C, et al. . Progressive loss of retinal ganglion cells and axons in non-optic neuritis eyes in multiple sclerosis: a longitudinal optical coherence tomography study. Invest Ophthalmol Vis Sci 2016;57:2311–2317. [DOI] [PubMed] [Google Scholar]
- 16.Shen T, You Y, Arunachalam S, et al. . Differing structural and functional patterns of optic nerve damage in multiple sclerosis and neuromyelitis optica spectrum disorder. Ophthalmology 2019;126:445–453. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. . The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016;86:2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tewarie P, Balk L, Costello F, et al. . The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 2012;7:e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talman LS, Bisker ER, Sackel DJ, et al. . Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 2010;67:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triplett JD, Yiannikas C, Barnett MH, et al. . Pathophysiological basis of low contrast visual acuity loss in multiple sclerosis. Ann Clin Transl Neurol 2018;5:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen T, Gupta VK, Klistorner A, Chitranshi N, Graham SL, You Y. Sex-specific effect of BDNF Val66Met genotypes on the progression of open-angle glaucoma. Invest Ophthalmol Vis Sci 2019;60:1069–1075. [DOI] [PubMed] [Google Scholar]
- 22.Vizzeri G, Weinreb RN, Gonzalez-Garcia AO, et al. . Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br J Ophthalmol 2009;93:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadhwani M, Bali SJ, Satyapal R, et al. . Test-retest variability of retinal nerve fiber layer thickness and macular ganglion cell-inner plexiform layer thickness measurements using spectral-domain optical coherence tomography. J Glaucoma 2015;24:e109–e115. [DOI] [PubMed] [Google Scholar]
- 24.Petzold A, Balcer LJ, Calabresi PA, et al. . Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2017;16:797–812. [DOI] [PubMed] [Google Scholar]
- 25.You Y, Thie J, Klistorner A, Gupta VK, Graham SL. Normalization of visual evoked potentials using underlying electroencephalogram levels improves amplitude reproducibility in rats. Invest Ophthalmol Vis Sci 2012;53:1473–1478. [DOI] [PubMed] [Google Scholar]
- 26.You Y, Klistorner A, Thie J, Gupta VK, Graham SL. Axonal loss in a rat model of optic neuritis is closely correlated with visual evoked potential amplitudes using electroencephalogram based scaling. Invest Ophthalmol Vis Sci 2012;53:3662. [DOI] [PubMed] [Google Scholar]
- 27.Klistorner A, Arvind H, Garrick R, Graham SL, Paine M, Yiannikas C. Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Invest Ophthalmol Vis Sci 2010;51:2770–2777. [DOI] [PubMed] [Google Scholar]
- 28.Petzold A, de Boer JF, Schippling S, et al. . Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2010;9:921–932. [DOI] [PubMed] [Google Scholar]
- 29.Klistorner A, Sriram P, Vootakuru N, et al. . Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology 2014;82:2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 2010;133:1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You Y, Graham EC, Shen T, et al. . Progressive inner nuclear layer dysfunction in non-optic neuritis eyes in MS. Neurol Neuroimmunol Neuroinflamm 2018;5:e427 doi: 10.1212/NXI.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Martin E, Ara JR, Martin J, et al. . Retinal and optic nerve degeneration in patients with multiple sclerosis followed up for 5 years. Ophthalmology 2017;124:688–696. [DOI] [PubMed] [Google Scholar]
- 33.Behbehani R, Adnan H, Al-Hassan AA, Al-Salahat A, Alroughani R. Predictors of retinal atrophy in multiple sclerosis: a longitudinal study using spectral domain optical coherence tomography with segmentation analysis. Mult Scler Relat Disord 2018;21:56–62. [DOI] [PubMed] [Google Scholar]
- 34.Zhostkova MA, Davydovskaya MV, Boyko AN, Akopyan VS. The three-year follow-up study of retinal changes in patients with multiple sclerosis [in Russian]. Zh Nevrol Psikhiatr Im S S Korsakova 2016;116:35–41. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Martin E, Pueyo V, Almarcegui C, et al. . Risk factors for progressive axonal degeneration of the retinal nerve fibre layer in multiple sclerosis patients. Br J Ophthalmol 2011;95:1577–1582. [DOI] [PubMed] [Google Scholar]
- 36.Yang EB, Hood DC, Rodarte C, Zhang X, Odel JG, Behrens MM. Improvement in conduction velocity after optic neuritis measured with the multifocal VEP. Invest Ophthalmol Vis Sci 2007;48:692–698. [DOI] [PubMed] [Google Scholar]
- 37.Klistorner A, Chai Y, Leocani L, et al. . Assessment of opicinumab in acute optic neuritis using multifocal visual evoked potential. CNS Drugs 2018;32:1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You Y, Gupta V. The extracellular matrix and remyelination strategies in multiple sclerosis. eNeuro 2018;5:e0435–0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plemel JR, Liu WQ, Yong VW. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov 2017;16:617–634. [DOI] [PubMed] [Google Scholar]
- 40.Klistorner AI, Graham SL, Grigg JR, Billson FA. Multifocal topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci 1998;39:937–950. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and from the corresponding author on reasonable request.