MS is a chronic inflammatory disease of the CNS involving T cell and B cell responses. Recently, several studies have described modifications of specific bacterium abundances of gut microbiota in patients with remitting–relapsing MS compared with healthy individuals (see for review1). This was often associated with an increase in Akkermansia muciniphila bacteria. In experimental autoimmune encephalomyelitis, transfer of gut microbiota from patients with MS to mice induced proinflammatory responses and exacerbation of the disease, whereas microbiota from healthy volunteers (HVs) were less inflammatory.2,3 Because bacteria in the gut modulate immune responses, we assessed the antibody production against A muciniphila in patients with MS. In CSF, levels of anti-A muciniphila immunoglobulin G (IgG) were increased in patients with MS compared with controls, whereas no difference was found for levels of IgG against Escherichia coli, Fusobacterium necrophorum, Acinetobacter baumannii, Prevotella melaninogenica, and Bacteroides fragilis.
Methods
Patients with relapsing–remitting MS (RRMS, n = 62) were enrolled in the neurology department of CHU de Nantes. Sex and age-matched patients (HVs, n = 41), patients with noninflammatory neurologic disease (e.g., suffering from sudden headaches or idiopathic intracranial hypertension) (noninflammatory neurologic disease [NIND], n = 23), and patients with inflammatory neurologic disease (peripheral neuropathy, brain lymphoma) (inflammatory neurological disease [IND], n = 10) were used for comparison. Informed written consent was obtained from all the patients before any study-related procedure was performed. Patients had not received any disease-modifying drugs before the sampling.
IgG concentration was measured with an immunonephelometric assay performed using a Beckman Immage Analyzer (Beckman Coulter). To detect antibody against bacteria, ELISA tests were performed using serum and CSF samples. Briefly, bacteria lysates from A muciniphila, F necrophorum, A baumannii, P melaninogenica, and E coli were coated on plates (Nunc) at 1 µg/mL of proteins in phosphate buffer saline (PBS). Bovine serum albumin (Sigma Aldrich) at 1% in PBS was used for blocking. Patient samples were incubated for 2 hours at 37°C in PBS (dilutions 1/100 for serum, 1/10 for CSF) and 1% bovine serum albumin. Antihuman IgG antibodies coupled with horseradish peroxidase (Bethyl Laboratories) at 1/5,000, 1 hour at 37°C, were used. The reaction with the substrate (3,3',5,5'-Tetramethylbenzidine, BD Biosciences) was stopped with sulfuric acid (0.18M, Sigma Aldrich). Plates were read at 450 nm using a Spark 10M multimode microplate reader (Tecan). The mean values of optical density were compared using the Mann-Whitney test and analysis of variance with Dunn's multiple comparisons test for more than 2 groups. Aberrant values for biological variables were determined by a Dixon test and excluded.
Results
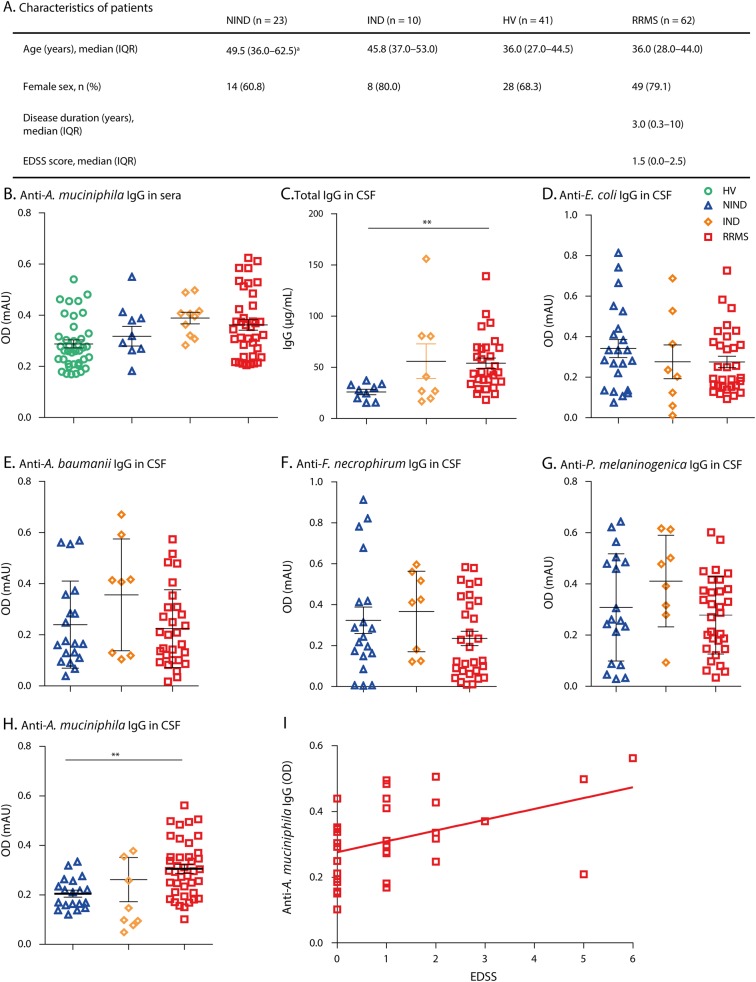
The levels of anti-A muciniphila IgG were measured by using ELISA in sera and appeared equivalent in patients with RRMS, NIND, IND, and HV (figure, B). The IgG levels of anti-E. coli, F. necrophorum, A baumannii, and P melaninogenica were also similar in the tested groups (data not shown). As expected and previously described, the total IgG concentrations were increased in CSF from patients with RRMS compared with those of NIND (p < 0.01, figure, C). There was no difference in the IgG levels against E coli, F necrophorum, A baumannii, and P melaninogenica in CSF from the 3 groups (figure, D–G). We were able to reveal an increased IgG reaction against A muciniphila in patients with RRMS compared with those with NIND and IND (p < 0.005, figure, H). There was no correlation between the total IgG concentration and levels of anti-A muciniphila IgG in CSF (data not shown). Strikingly, the levels of anti-A muciniphila correlated with Expanded Disability Status Scale score in RRMS (rho = 0.36, p = 0.027, figure, I).
Figure. Increased levels of Anti-Akkermansia IgG in RRMS CSF and correlation with the EDSS score.
(A) Clinical characteristics of patients are listed in Table A. Patients with RRMS; patients with noninflammatory neurological disease of the CNS (NIND), patients with IND, and HVs; EDSS, median ± IQR, ap < 0.01 NIND vs HV, and RRMS. (B) Levels of anti-Akkermansia muciniphila IgG in sera from patients with RRMS, NIND, IND, and HV. (C) Total IgG concentrations in CSF from patients with RRMS, NIND, and IND. (D) Levels of anti-Escherichia coli IgG in CSF from patients with RRMS, NIND, and IND. (E) Levels of anti-Acinetobacter baumannii IgG in CSF. (F) Levels of anti-Fusobacterium necrophorum IgG in CSF. (G) Levels of anti-Prevotella melaninogenica IgG in CSF. (H) Levels of anti-A muciniphila IgG in CSF. Mean ± standard error. **p < 0.01. (I) Correlation between anti-A muciniphila IgG in CSF from patients with RRMS and EDSS score (Spearman's rank correlation rho = 0.36, p = 0.027). EDSS = Expanded Disability Status Scale; HV = healthy volunteer; IND = inflammatory neurological disease; IQR = interquartile range; NIND = noninflammatory neurological disease; RRMS = relapsing–remitting MS.
Discussion
This is the first study to display that the levels of IgG against A muciniphila are increased in CSF of patients with RRMS compared with control samples. Interestingly, anti-E Coli IgG levels in CSF were equivalent in patients with RRMS and controls, revealing a specific response against a particular bacterium. An increase in A muciniphila, Fusobacterium, and Acinetobacter bacteria and a decrease in Prevotella have been shown in the gut microbiota of patients with RRMS. However, we found that, although the CSF samples also exhibited an increase in anti-A muciniphila IgG levels, no such modification was detected in the blood. This CSF-localized specific antibody signature suggests an intrathecal secretion of specific B cells retained in the CNS compartment, which may have migrated from the gut. B cells were indeed found while draining cervical lymph nodes of patients with MS.4 These B cells were able to populate the CNS in this inflammatory context. Moreover, in a mouse model, plasma cells specific for commensal bacteria were diminished in the gut and circulate to the CNS, producing in situ immunoglobulins.5 The local role of anti-A muciniphila IgG in MS pathology remains yet to be elucidated. Antibodies could be also elicited or act through cross-reactivity/molecular mimicry to myelin components as was described recently for T cell responses to some microbiota-derived peptides.6 Thus, along with previous lines of evidence for gut microbiota modifications in MS, our present findings highlight a CNS-specific antibacterial antibody response in patients with RRMS.
Appendix. Authors
Study funding
This study was supported by the Région Pays de La Loire, ARSEP (Association for research on multiple sclerosis), and Antares foundations. This work has been carried out thanks to the support of the LabEx IGO project (n° ANR-11-LABX-0016-01) funded by the «Investissements d'Avenir » French Government program, managed by the French National Research Agency (ANR).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Freedman SN, Shahi SK, Mangalam AK. The “gut feeling”: breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics 2018;15:109–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berer K, Gerdes LA, Cekanaviciute E, et al. . Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A 2017;114:10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cekanaviciute E, Yoo BB, Runia TF, et al. . Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A 2017;114:10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern JN, Yaari G, Vander Heiden JA, et al. . B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med 2014;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rojas OL, Probstel AK, Porfilio EA, et al. . Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 2019;176:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Planas R, Santos R, Tomas-Ojer P, et al. . GDP-l-fucose synthase is a CD4(+) T cell-specific autoantigen in DRB3*02:02 patients with multiple sclerosis. Sci Transl Med 2018;10:1–15. [DOI] [PubMed] [Google Scholar]