Autoimmune hemolytic anemia (AIHA) has been reported after treatment with an anti-CD52 monoclonal antibody (alemtuzumab) in 7 MS cases.1 All underwent positive direct Coombs test, i.e., antibodies to red blood cells (RBCs); however, no autoantibody (Ab) specificity was identified.1 Aquaporin 1 (AQP1), expressed in RBCs2 and human astrocytes,3,4 has been linked with autoimmunity: in some AIHA cases (Abs to Colton group antigens located on AQP1)2 and in some patients with CNS demyelinating disorders.3,4 Therefore, AQP1-Abs deserve investigation as the possible linking cause of the concurrent presence of the 2 disorders. Here, we present a patient characterized with MS, however, with extensive longitudinal transverse myelitis (LETM), who developed AIHA in parallel with brain demyelinating relapse, 1 year after alemtuzumab infusion; interestingly, a high AQP1-Ab titer was detected, which dropped in parallel with patient recovery.
Case report
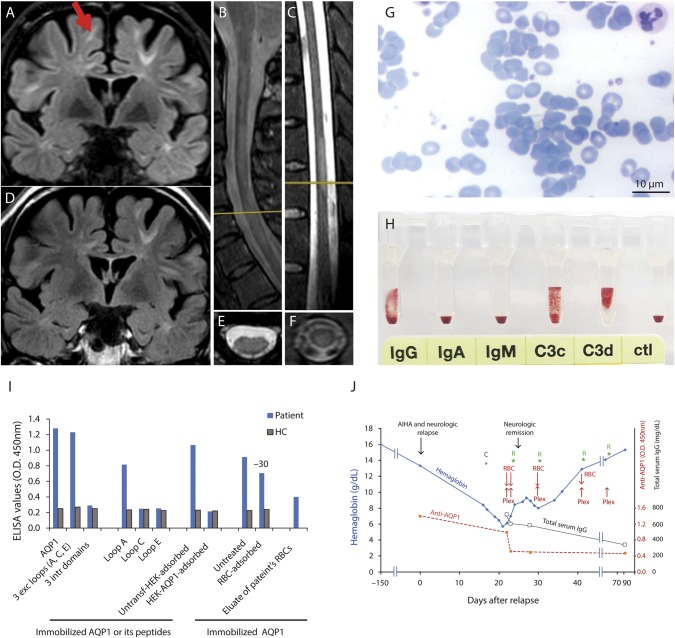
The patient's history started 16 years ago (15-year-old; Caucasian) with optic neuritis, followed by tingling of lower extremities and diplopia. Brain lesions fulfilled the 2017 McDonald MS criteria. The patient partially responded to IFNβ-1α (treated for 3 years), responded well to natalizumab (discontinued after 3 years on JC virus positivity), followed by fingolimod with good response, which was subsequently discontinued because of sustained B-cell lymphopenia. After B-cell recovery, the patient received a 5-day alemtuzumab course (EDSS: 3,5) with good response. A year later, the patient simultaneously developed demyelinating relapse (MRI, figure, A–F) with severe left lower limb weakness (EDSS: 6) and hemolytic anemia (figure, G, H, J). At admission, the patient had a hematocrit value of 19.0%, hemoglobin concentration of 7.8g/dL, reticulocyte value of 3.7%, LDH level of 303U/L, total bilirubin concentration of 3.3 mg/dL, undetectable serum haptoglobin, hemoglobinuria, RBC agglutination on peripheral blood smear (figure, G), and positive direct Coombs test (figure, H). A moderate titer (1:8) of cold agglutinin was detected, and thus, mixed-type AIHA was diagnosed. No autoantibody specificity was identified because the patient's RBC eluate (i.e., containing RBC-bound IgG released by low pH treatment) reacted with all RBCs tested (panel-pan-reactive). The indirect Coombs test was also positive, without identification of Ab-specificity (panel-pan-reactive). The Eginition Hospital IRB approved this study.
Figure. Clinical, radiologic, and laboratory findings of the patient with concurrent AIHA and demyelinating relapse after alemtuzumab infusion.
(A, B, C, E, F) Brain, cervical, and thoracic MRI findings 1 month after AIHA onset and demyelinating relapse; (A) shows periventricular and juxtacortical brain lesions (the red arrow shows a new lesion not present in D); (B, C, E, and F) show transverse myelitis in the cervical (with LETM) (B and E) and thoracic (C and F) cord; T2 spinal cord lesion load was identical with that at the time of alemtuzumab initiation. (D) MRI 6 months before the AIHA onset. (G) Large RBC agglutinates detected on the peripheral blood smear film. (H) Direct Coombs test using DiaMed gel test ID microtyping system (DiaMed GmbH); positive for IgG (2+) and complement fragments: C3d (4+) and C3c (2+). (I) Serum (from patient and a healthy control, HC) binding on ELISA wells with immobilized AQP1, peptide mixture corresponding to the AQP1 extracellular and intracellular loops, or the individual AQP1 extracellular loops. “RBC-adsorbed” and “HEK-AQP1-adsorbed” denote serum preincubated with healthy RBCs (group O) or HEK293 cells transfected with human AQP1, respectively, tested for binding on immobilized AQP1 (1 μL serum was preincubated with 75,000 to 300,000 cells in total 100 μL volume; 300,000 AQP1-transfected cells resulted in complete immunoadsorption). The last bar shows the binding of patient's RBC eluate to AQP1. The values are averages of 3 experiments. (J) hemoglobin, AQP1-Ab, and total IgG level fluctuations and therapies of the patient. Hemoglobin levels started to decrease gradually and dropped down to 5.7 g/dL. High AQP1-Ab levels (red dashed line) were detected at the onset of demyelinating relapse and AIHA and remained quite high up to the maximum drop of hemoglobin. Subsequently, AQP1-Ab levels decreased, remaining at very low values during the recovery and remission phase of both diseases (up to at least 91 days after AIHA onset and demyelinating relapse). The black line with empty square symbols represents the total serum IgG levels. Blood samples at day 22 and days 23 were collected before the plasmapheresis and RBC transfusion of the day. The steep AQP1-Ab drop at day 23, despite only small decrease of total IgG, could be attributed to Ab immunoadsorption on the transfused RBCs. Overall, patient started corticosteroid treatment resulting in neurologic recovery within 8 days, received 4 courses of rituximab, 5 plasmapheresis sessions, and transfusions of 4 RBC units. C: Start steroid treatment; Plex: plasmapheresis; R: rituximab treatment; RBC: RBC transfusion. LETM = longitudinal transverse myelitis.
The simultaneous demyelinating relapse and AIHA development prompted us to search for a common pathogenic factor. High AQP1 levels in RBCs2 and astrocytes3,4 and occasional AQP1-Ab presence in AIHA2 and in demyelinating diseases3,4 led us to search for AQP1-Abs. We used ELISA with yeast-expressed human AQP1 and synthetic peptides corresponding to AQP1 segments.3 AQP1-ELISA detected high IgG-Ab binding to AQP1 and to its extracellular loop-A during the acute phase (figure, I and J). As previously reported,3 the patient's AQP1-Abs were of the complement-activating IgG1 isoform. Because a reliable direct cell-based-assay (CBA) for AQP1-Abs has not been developed yet, probably because of low AQP1 expression,3,5 Ab binding on the extracellular side of cell-embedded AQP1 was confirmed by an indirect CBA: serum was preincubated with AQP1-expressing HEK293 cells followed by ELISA on the treated serum3; the serum was totally AQP1-Ab depleted under these conditions (figure, I).
Interestingly, in similar immunoadsorption experiments with healthy RBCs (ABO-group O), 30 ± 2% of the patient's AQP1-Abs were adsorbed (figure, I). The AQP1-Abs were also detected in the eluate of the patient's RBCs (figure, I), confirming in vivo binding.
The patient recovered (EDSS 3.5) after a combination therapy with corticosteroids, rituximab, plasmapheresis, and RBC transfusions, with a parallel dramatic drop of the AQP1-Ab titer (figure, J). Ten months later, after rituximab treatment, there was no clinical or MRI activity.
Discussion
In conclusion, our patient simultaneously developed AIHA and demyelinating relapse 1 year after alemtuzumab infusion, with a high AQP1-Ab titer, which dropped in parallel with patient recovery. The high AQP1-Ab titer and in vivo Ab-binding on RBCs during AIHA, as well as the drop of Ab-titer during recovery, suggest that a pathogenic role of these Abs is possible. The patient’s Abs bound on AQP1 loop-A, which contains Colton group antigens (Coa, Cob), were previously linked with hemolytic reactions.2 The concurrent presence of demyelinating relapse may also be related to AQP1-Abs; indeed, the role of AQP-Abs in demyelination was recently supported by our observation of AQP1 loss and IgG deposition in a tumefactive demyelinating brain lesion of a patient with AQP1-Ab+ neuromyelitis optica (NMO).4 Our present patient's brain lesions fulfilled the MS criteria (figure, A and D), seronegative for AQP4-/MOG-Abs, however, with LETM (figure, B and E; a typical NMO spectrum disorder finding6), suggesting an MS/NMO overlap syndrome.3 Moreover, disease exacerbation after alemtuzumab infusion has been linked with NMOsd, suggesting a possible explanation of our case. It would be interesting to test for AQP1-Abs in other reported MS cases with AIHA development after alemtuzumab infusion. One case also had nephropathy,7 i.e., affecting an additional AQP1-rich organ,2 responding to steroids and IVIG. Our data suggest that AQP1-Abs could be a triggering factor of alemtuzumab-induced AIHA and demyelinating relapse and justify further studies on this hypothesis.
Appendix. Authors
Study funding
The study was supported by a grant from the Hellenic Academy of Neuroimmunology (HELANI) to JST and KK.
Disclosure
Dr. Tzartos has shares in the research and diagnostic laboratory of Tzartos NeuroDiagnostics, and he is coinventor in a patent application that relates to the detection of antibodies to aquaporin 1. He has also received travel grants from Genzyme, Genesis Pharma, Teva, and Novartis and advisory boards from Genesis Pharma and Novartis. Dr. Valsami reports no disclosures. Dr. Tzanetakos is a subinvestigator in the prospective, multicenter, observational, postauthorization safety study (PASS) to evaluate the long-term safety profile of LEMTRADA (alemtuzumab) treatment in patients with relapsing forms of MS (PASS—OBS13434). He has also received travel grants from Genzyme, Genesis Pharma, Teva, and Novartis and advisory boards from Genesis Pharma, Novartis, and Roche. Dr. Stergiou is coinventor in a patent application that relates to the detection of antibodies to aquaporin 1. Dr. Dandoulaki reports no disclosures. Dr. Barbarousi reports no disclosures. Dr. Psimenou has received research grants from Hospira, Novartis, and Astellas. Dr. Velonakis reports no disclosures. Dr. Stefanis, in recent years, has received the following grants: MULTISYN European Program (EU, FP7-HEALTH.2013.1.2-1, number 602646), PPMI (supported by the Michael J. Fox Foundation), IMPRIND-IMI2 Number 116060 (EU, H2020), SANTE 2017, SANTE 2019 Research Grants in Biomedical Sciences, “Transferring autonomous and nonautonomous cell degeneration 3D models between EU and USA for development of effective therapies for neurodegenerative diseases (ND) - CROSS NEUROD” (H2020-EU 1.3.3., Grant Number778003), and “CMA as a Means to Counteract alpha-Synuclein Pathology in Non-Human Primates” grants by the Michael J. Fox Foundation (collaborator). He has served on an advisory board for AbbVie and has received honoraria from AbbVie and Sanofi. Dr. Kilindireas has received research grants from Genesis Pharma and Teva and consultation fees, advisory boards, and honoraria from Genzyme, Genesis Pharma, Teva and Novartis and is a coinventor in a patent application that relates to the detection of antibodies to aquaporin 1. He is one of the primary investigators in the prospective, multicenter, observational, postauthorization safety study (PASS) to evaluate the long-term safety profile of LEMTRADA (alemtuzumab) treatment in patients with relapsing forms of MS PASS—OBS13434. Go to Neurology.org/NN for full disclosures.
References
- 1.Desai PA, Romere CM, Nguyen L, Saksena A, Abdullah SJ, Diaz AE. Severe Coombs positive autoimmune hemolytic anemia after Alemtuzumab Infusion for relapsing remitting multiple sclerosis. What can we learn? Blood 2018;132(suppl 1):2331.30487130 [Google Scholar]
- 2.Halverson GR, Peyrard T. A review of the Colton blood group system. Immunohematology 2010;26:22–26. [PubMed] [Google Scholar]
- 3.Tzartos JS, Stergiou C, Kilidireas K, Zisimopoulou P, Thomaidis T, Tzartos SJ. Aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders. PLoS One 2013;8:e74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Türkoğlu R, Lassmann H, Aker FV, Tzartos J, Tzartos S, Tüzün E. Recurrent tumefactive demyelinating lesions: a pathological study. Clin Neuropathol 2017;36:195–198. [DOI] [PubMed] [Google Scholar]
- 5.Schanda K, Waters P, Holzer H, et al. . Antibodies to aquaporin-1 are not present in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm 2015;2:e160. doi:10.1212/NXI.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Banwell B, Bennett JL, et al. . International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.di Ioia M, Farina D, di Tommaso V, et al. . Simultaneous early-onset severe autoimmune hemolytic anemia and albuminuria during alemtuzumab treatment for multiple sclerosis. Mult Scler 2018;24:813–815. [DOI] [PubMed] [Google Scholar]