Abstract
Objective
To investigate the diagnostic yield of commercial immunodots to detect onconeural antibodies associated with paraneoplastic neurologic syndromes (PNSs), we analyzed the proportion of confirmed positive results using alternative techniques.
Methods
Sera (n = 5,300) of patients with suspected PNS were tested by PNS+2 blot (Ravo Diagnostika; January 2016–May 2017) or EUROLINE PNS 12 Ag (Euroimmun; July 2017–November 2018). Positive samples were further explored by in-house indirect immunofluorescence and a third in-house technique (Western blot or cell-based assay) using recombinant protein. Those found negative by these 2 techniques were considered as nonconfirmed. We analyzed the relationship between band intensity and final confirmation. Clinical data were collected for all confirmed results and nonconfirmed EUROLINE immunodots.
Results
PNS+2 blot was positive in 128/1,658 (7.7%) sera and confirmed in 47/128 (36.7%). EUROLINE was positive in 186/3,626 (5.1%) and confirmed in 56/186 (30.1%). Confirmation was highly variable among the antibodies tested, from 7.2% (PNS+2 blot) and 5.8% (EUROLINE) for anti-Yo to 88.2% (PNS+2 blot) and 65.0% (EUROLINE) for anti-Hu. None of the 27 weak positive sera by EUROLINE was confirmed. Band intensity in confirmed cases was variable among the antibodies from strong positive for all anti-Yo (n = 3) and anti-Hu (n = 11) to positive (n = 19) or strong positive (n = 9) for anti-SOX1. Among patients with a nonconfirmed EUROLINE result and available clinical information, all had an alternative diagnosis, and only 6.7% had cancer.
Conclusions
Immunodots may be useful for PNS screening, but a threshold should be established for each antibody, and clinical information and confirmation by other techniques are essential.
Classification of evidence
The study provides Class IV evidence that immunodot assays for onconeural antibodies accurately identify patients with paraneoplastic neurologic syndromes.
Paraneoplastic neurologic syndromes (PNSs) are rare but now well-characterized immune-mediated neurologic diseases triggered by cancer and diagnosed by the presence of circulating autoantibodies.1 Among them, autoantibodies directed against intracellular neural antigens (also known as onconeural antibodies) are strongly associated with the presence of an underlying cancer, and its detection is a cornerstone of PNS diagnosis.
Indirect immunofluorescence (IIF) on rat brain slices is the preferred screening test for identification of onconeural antibodies, but the result should be confirmed by a second technique, either Western blot or for some cases such as anti-delta/notch-like epidermal growth factor–related receptor (anti-Tr/DNER) by cell-based assays (CBAs).2,3 These techniques have been developed mainly in research laboratories and are not available for routine analysis. However, 2 commercial immunodot assays are currently marketed: PNS+2 blot (Ravo Diagnostika, Freiburg, Germany) and EUROLINE PNS 12 Ag (Euroimmun, Lübeck, Germany). These immunodot assays present the advantage to be easily and quickly performed as they are fully automated; they also screen several antibodies at the same time. However, very little is known about the reliability of these immunodot assays, as only a few published studies have analyzed the sensitivity for the detection of anti-CV2/CRMP5 (collapsin response-mediator protein-5) antibodies,4 and the sensitivity and specificity for anti-Ma2 antibodies,5 and anti-SOX1 antibodies.6 In our laboratory, we use commercial immunodot assays as the first step of biological PNS diagnosis for all onconeural antibodies. Herein, we studied the diagnostic yield of 2 commercial immunodots by investigating the proportion of positive results confirmed by alternative techniques, taking also into account the clinical information when it was available.
Methods
This study is a single-center retrospective analysis of samples (sera) from patients with suspicion of PNS that were analyzed at the French Reference Center on Paraneoplastic Neurological Syndromes (Lyon, France). First, sera were screened by commercial immunodot assays, using PNS+2 blot (Ravo Diagnostika), from January 2016 to May 2017, and EUROLINE PNS 12 Ag (Euroimmun), from July 2017 to November 2018. Only the sera that were found positive by the immunodot assay for at least one of the onconeural antibodies were further analyzed by 2 in-house techniques: IIF followed by a technique using recombinant protein, either a Western blot for anti-CV2/CRMP5 and anti-amphiphysin antibodies or a CBA for the other antibodies. When a positive immunodot result was also found positive using the 2 different confirmatory techniques (IIF and Western blot/CBA), the case was considered as confirmed. When both IIF and the third technique were negative, the immunodot result was considered as nonconfirmed. All confirmed cases were included in the database of the French Reference Center, along with clinical information. For the current study, we also collected clinical data (including clinical phenotype, cancer association, and final diagnosis) for patients whose serum was tested using the EUROLINE PNS 12 Ag (Euroimmun) but were nonconfirmed; these data were not available for patients whose serum was tested using the PNS+2 blot (Ravo Diagnostika). When a tumor was detected, it was considered coincidental or truly associated with the result obtained by the immunodot according to previous reports.1 As anti-SOX1 and anti-Zic4 are antibodies that are mostly cancer related, without clear evidence of specificity for a given PNS, they were not included in the clinical comparison.7
Commercial immunodot assays
The PNS+2 blot (Ravo Diagnostika) includes 9 different antigens for PNS: Yo, Hu, Ri CV2/CRMP5, Ma2, SOX1, amphiphysin, Ma1, and GAD65 (glutamic acid decarboxylase 65). Serum samples were analyzed following the manufacturer's instructions. Immunodots were performed on the EUROBlotMaster system (Euroimmun), and bands were scanned and analyzed using the BLOTrix4 Cubos software (BioSciTec, Frankfurt, Germany), giving an arbitrary unit of intensity. There was no manufacturer recommendation for interpretation of band intensity.
EUROLINE PNS 12 Ag (Euroimmun) includes 12 different antigens for PNS: Yo, Hu, Ri, CV2/CRMP5, Ma2, SOX1, amphiphysin, Zic4, Tr/DNER, GAD65, recoverin, and titin. Serum samples were analyzed following the manufacturer's instructions. Immunodots were performed on the EUROBlotOne system (Euroimmun), and bands were scanned and analyzed using EUROLineScan (Euroimmun), giving an arbitrary unit of intensity. According to the manufacturer's instructions, samples were considered negative when presenting intensity between 0 and 7, weak positive between 8 and 14, positive between 15 and 70 (15–35, +; 36–70, ++), and strong positive equal or above 71.
Anti-GAD65 antibodies were not included in the present study as they are usually detected in non-PNS.8,9 Moreover, the commercial immunodots do not establish a precise titer that is important to differentiate the neurologic syndromes from other non-neurologic diseases that may be also positive for anti-GAD65 antibodies.8 Anti-titin autoantibodies, which are associated with myasthenia gravis with malignant thymoma,10 and anti-recoverin autoantibodies, which are associated with paraneoplastic retinopathy,11 were not considered in this study as they are not confirmed in our laboratory.
To investigate whether band intensity is indicative of true positivity, the scan values of positive EUROLINE PNS 12 Ag immunodot (Euroimmun) were compared with the confirmation tests. When the band intensity value was lacking, sera were excluded from the analysis. As there is no manufacturer recommendation for interpretation of band intensity, we did not perform the same analysis for PNS+2 blot (Ravo Diagnostika).
Indirect immunofluorescence
The first confirmatory test performed was IIF on rat brain sections. For this, rat brain was taken, cut in half, and immediately frozen in isopentane at −50°C for 2 minutes. The frozen brain was cut into 12-µm-thick sagittal sections. Brain sections were blocked in phosphate-buffered saline (PBS) containing 3% bovine serum albumin and 3% normal goat serum for 1 hour. Patient serum was then incubated overnight at room temperature (dilution 1/100). Slides were washed 3 times in PBS and incubated with secondary antibody (goat anti-human coupled to Invitrogen Alexa 488; Thermo Fisher Scientific, Waltham, MA) for 1 hour at room temperature. After 3 washes, slides were mounted in Mowiol medium (Sigma-Aldrich, Saint-Louis, MO) and read using a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany).
Cell-based assay
CBAs were performed using human embryonic kidney (HEK) cells transfected with the plasmid of interest. HEK 293T cells were cultured on 12-mm glass coverslips in Dulbecco modified Eagle's medium, supplemented with 10% fetal calf serum and penicillin/streptomycin. Cells were incubated at 37°C in an atmosphere containing 5% CO2. Cells were transiently transfected with cDNAs coding for the recombinant protein of interest fused with green fluorescent protein tag using the kit Lipofectamine LTX Reagent (Invitrogen 15338-100, Thermo Fisher Scientific). Twenty-four hours after transfection, coverslips were washed and fixed 15 minutes with 4% paraformaldehyde and washed again twice. Coverslips were incubated 1 hour 30 minutes at room temperature with patient serum, and after 3 PBS washes, a goat anti-human coupled to Invitrogen Alexa 555 (Thermo Fisher Scientific) secondary antibody was incubated for 1 hour at room temperature. Coverslips were mounted in Mowiol (Sigma-Aldrich) medium and read using a Zeiss Axiophot microscope (Zeiss).
Western blot
Briefly, HEK cells were plated in a 100-mm-diameter dish and transfected with a plasmid coding for the target antigen to be confirmed as described above. At 36–48 hours post-transfection, cells were washed with cold PBS, and proteins were extracted in lysis buffer (50 mM Tris-HCl [tris-aminomethane hydrochloride] pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40 [nonyl phenoxypolyethoxylethanol], 0.1% sodium dodecyl sulfate [SDS], protease inhibitors, and Benzonase) for 30 minutes at 4°C. The extract was cleared by centrifugation (10,000g, 20 minutes), denatured in Laemmli buffer (62.5 mM Tris-HCl, 2% SDS, 10% glycerol, 100 mM DTT, 0.01% bromophenol blue), subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) separation, and then transferred on a polyvinylidene difluoride membrane using a semi-dry Western blot apparatus. Serum (diluted 1/100) was incubated overnight with the membrane. Antibody fixation was visualized using peroxidase goat anti-human IgG (Jackson ImmunoResearch, Cambridgeshire, United Kingdom) and chromogenic substrate (Fast 3,3′ Diaminobenzidine tablet, Sigma-Aldrich). A sample was positive if it bound to the recombinant protein on the membrane. A positive and a negative control were included in each experiment.
Patient consent
Observational studies conducted in France using data obtained from a retrospective study without any additional therapeutic or monitoring procedure do not require informed consent, and this was the case herein as onconeural antibodies were analyzed as part of routine diagnostic investigation. Patient records and information were anonymized before analysis. The Institutional Review Board of the University Claude Bernard Lyon 1 and Hospices Civils de Lyon approved the study.
Data availability
All data reported are available in the article and in the supplementary materials.
Results
Confirmation of positive commercial immunodots
From January 2016 to November 2018, 5,300 sera were analyzed using PNS+2 blot (Ravo Diagnostika) or EUROLINE PNS 12 Ag (Euroimmun) for suspected PNS in our laboratory; a positive result was obtained in 330/5,300 (6.2%) sera.
Between January 2016 and May 2017, we analyzed 1,660 sera using PNS+2 blot (Ravo Diagnostika), and 130 (7.8%) were positive for at least 1 antibody; 20/130 (15.3%) sera were positive for 2 or more antibodies, and there were therefore a total of 152 positive results. After excluding 3 cases of anti-GAD65 antibody, 2 of them as isolated positivity, 128/1,658 (7.7%) sera were positive, corresponding to a total of 149 antibody positive results. Using in-house techniques, 47/128 (36.7%) sera positive according to PNS+2 blot (Ravo Diagnostika) were confirmed. The total number of confirmed positive results was 52/149 (34.9%). The proportion of confirmation varied among the antibodies included in the immunodot, from 0.0% for anti-amphiphysin (0/5) and anti-Ma1 (0/6), 7.2% for anti-Yo (4/55), 50% for anti-Ri (2/4), 55.5% for anti-CV2/CRMP5 (5/9), to 88.2% for anti-Hu (15/17; table 1).
Table 1.
Positive results for each antibody using PNS+2 blot (Ravo Diagnostika) and EUROLINE PNS 12 Ag (Euroimmun) and confirmation using in-house techniques
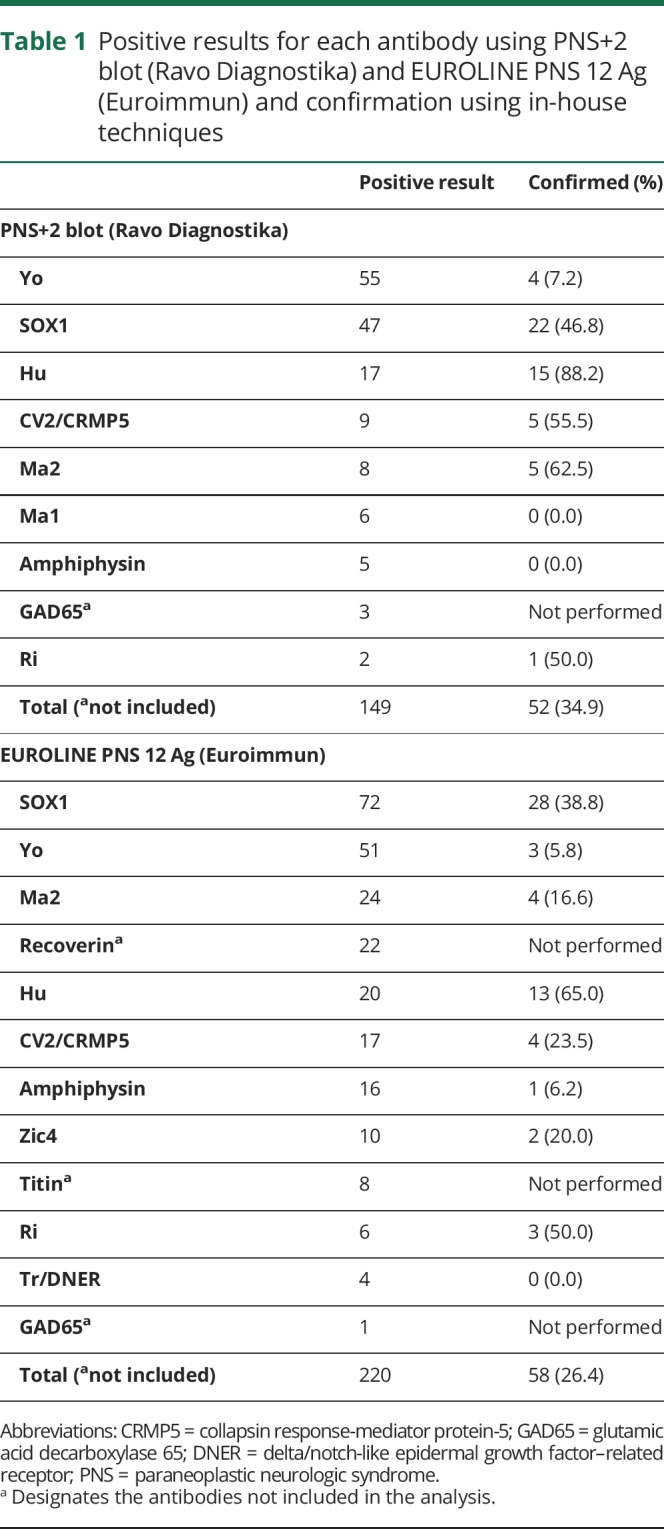
Between July 2017 and November 2018, we analyzed 3,640 sera using EUROLINE PNS 12 Ag (Euroimmun), and 200 (5.4%) were positive for at least 1 antibody; 43/200 (21.5%) sera were positive for 2 or more antibodies, and there were a total of 251 positive results. After excluding anti-recoverin (n = 14 as isolated positivity, and n = 22 in sera also positive for another antibody), anti-titin (n = 8, all of them also positive for other antibodies), and anti-GAD65 (n = 1, also positive for anti-Hu); 186/3,626 (5.1%) sera were positive, and antibody positivity reached 220. Using in-house techniques, 56/186 (30.1%) sera positive according to EUROLINE PNS 12 Ag (Euroimmun) were confirmed (figure 1). The total number of confirmed antibody positive results was 58/220 (26.4%). The proportion of confirmation varied among the antibodies included in the immunodot, from 0.0% (0/4) for anti-Tr/DNER, and 5.8% (3/51) for anti-Yo, to 65.0% (13/20) for anti-Hu results (table 1).
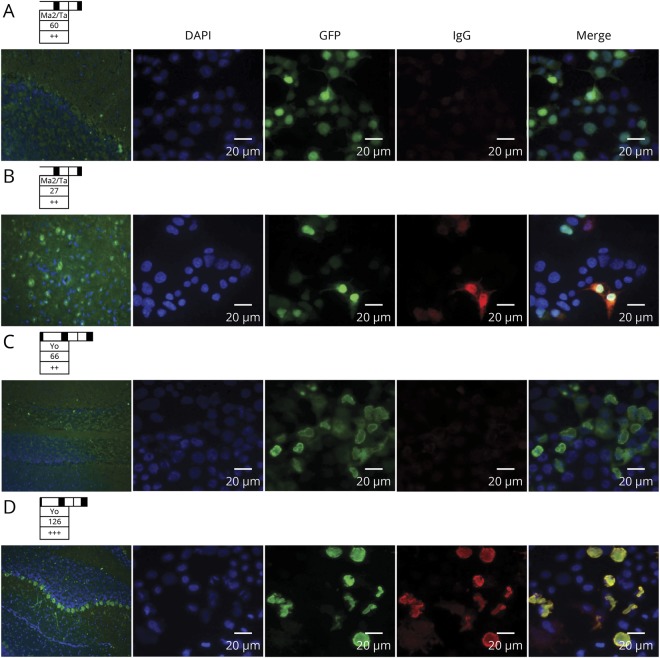
Figure 1. Alternative techniques used for confirmation of commercial immunodots.
EUROLINE PNS 12 Ag (Euroimmun) positive for anti-Ma2 (A), and anti-Yo (C), not confirmed by indirect immunofluorescence (IIF) on the rat cerebellum (left), and cell-based assay (CBA, right). A confirmed case for anti-Ma2 (B) and anti-Yo (D) is also provided for comparison. Immunodot band and scan value on the top of each case. For IIF, nuclei are stained with DAPI and anti-human IgG in green. For CBA, nuclei are also stained with DAPI, the protein of interest (Ma2 or Yo) in green as transfection control (GFP), and human IgG in red; a merged image is shown at the end of each row. GFP = green fluorescent protein.
In summary, over the total period of analysis, 103/314 (32.8%) sera and 110/369 (29.8%) antibody positive results obtained by both commercial immunodots were confirmed by the 2 in-house techniques, which always showed concordant results between them (figure 2).
Figure 2. Diagnostic algorithm followed in our laboratory to confirm positive immunodots.
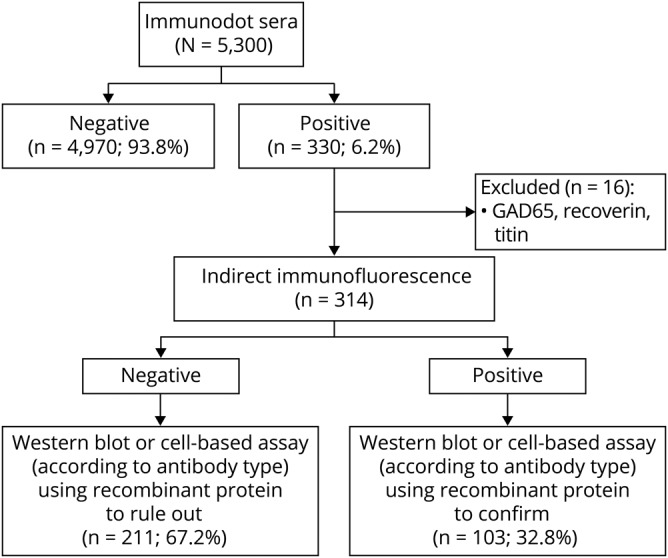
All positive sera by immunodots (except those positive for anti-glutamic acid decarboxylase [GAD65], anti-recoverin, and anti-titin, which were excluded from the current study) were analyzed using indirect immunofluorescence (IIF) and a technique using recombinant protein, either a Western blot for anti-amphiphysin and anti-CV2/CRMP5 (collapsin response-mediator protein-5) antibodies or a cell-based assay for the other antibodies. The results from the 2 confirmatory tests were always concordant. When IIF and the third technique were positive and consistent with the immunodot, the result was considered as confirmed. When IIF and the third technique were both negative, the immunodot result was considered as nonconfirmed.
Clinical data
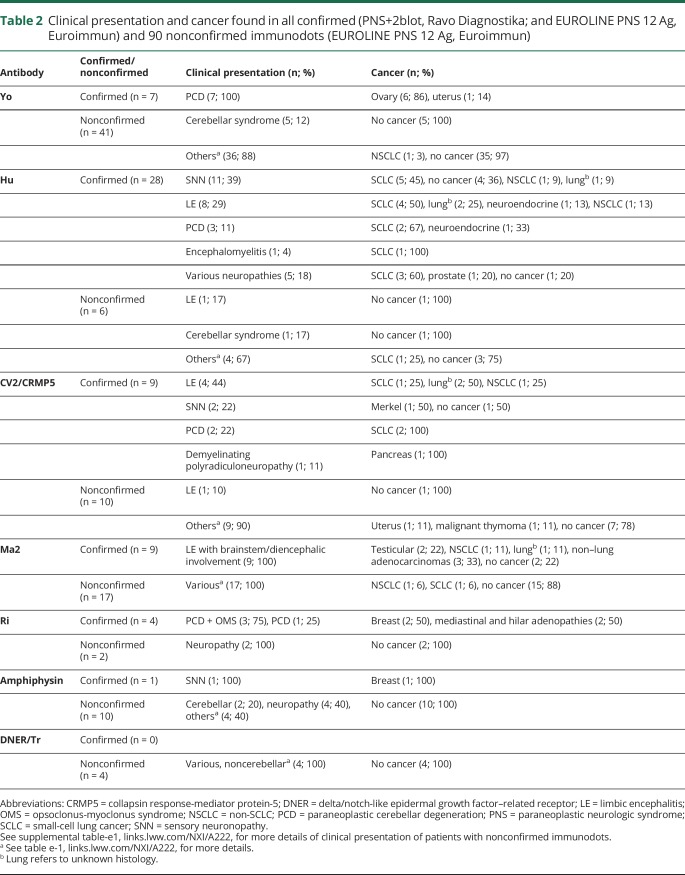
We collected the clinical data of all (n = 58) confirmed immunodots (Ravo Diagnostika and Euroimmun) and for 90/110 (81.8%) nonconfirmed EUROLINE PNS 12 Ag (Euroimmun) for classical onconeural antibodies (table 2). All patients with confirmed immunodots (58/58, 100%) presented clinical symptoms classically described with the identified antibody, and 50/58 (86.2%) presented also a concomitant cancer known to be associated with the particular antibody. In 8/58 (13.8%) patients with confirmed immunodots, a cancer was not yet diagnosed at last follow-up; these included 4 patients with anti-Hu and typical sensory neuronopathy, 1 anti-Hu patient with severe axonal polyneuropathy, 1 patient with anti-CV2/CRMP5 and sensory neuronopathy, and 2 patients with anti-Ma2 and limbic encephalitis with brainstem/diencephalic involvement. All patients with confirmed immunodots fulfilled the current diagnostic criteria for PNS published in 2004.1
Table 2.
Clinical presentation and cancer found in all confirmed (PNS+2blot, Ravo Diagnostika; and EUROLINE PNS 12 Ag, Euroimmun) and 90 nonconfirmed immunodots (EUROLINE PNS 12 Ag, Euroimmun)
Conversely, the clinical presentation of most (76/90; 84.4%) of the nonconfirmed immunodots was incompatible with the antibody identified by immunodots. For nonconfirmed anti-Yo antibodies, the demographic features were also atypical with a male predominance (26/41; 63.4%). A cancer was detected in only 6/76 patients (7.9%) with nonconfirmed immunodots, and the presenting clinical symptoms were not classically described in association with such antibodies (table 2). In 2 patients (2/6, 33.3%), the tumor was considered coincidental. It was a lung adenocarcinoma with anti-Yo in a patient with a final diagnosis of stroke and a uterine cancer with anti-CV2/CRMP5 antibodies in a patient with typical amyotrophic lateral sclerosis. In the other cases (4/6, 66.7%), a tumor usually described with the antibodies suspected by immunodots was present, but the neurologic features were attributed to an alternative diagnosis unrelated to a possible PNS (small-cell lung cancer [SCLC] with anti-Hu and diagnosis of leukoencephalopathy after radiotherapy, n = 1; SCLC with anti-Ma2 and only attention deficit, n = 1; lung adenocarcinoma with anti-Ma2 but with proved meningeal carcinomatosis, n = 1; malignant thymoma with anti-CV2/CRMP5 in a patient with Morvan syndrome and also anti–contactin-associated protein-like 2 [CASPR2] antibodies in the serum, n = 1). None of the 14/90 (15.6%) nonconfirmed immunodots with compatible clinical picture had a cancer (table 2). All patients with nonconfirmed immunodot had an alternative diagnosis different than PNS (table e-1, links.lww.com/NXI/A222).
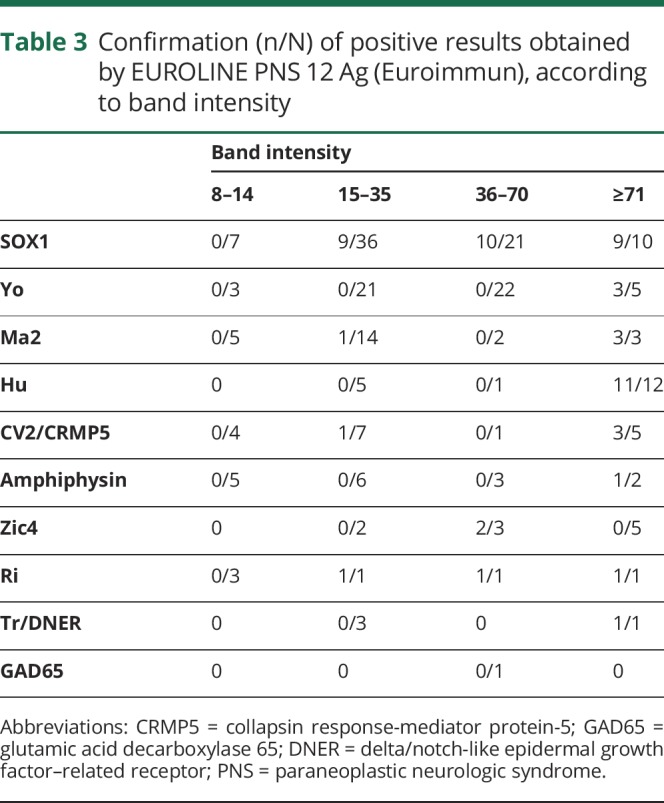
Immunodot intensity stratification
Band intensity was not available for 2 anti-Hu antibodies detected by EUROLINE PNS 12 Ag (Euroimmun), so they were excluded from the analysis. Irrespective of the antigen, among the 27 positive sera with intensity between 8 and 14 (weak positivity), none was confirmed (table 3). However, the relationship between band intensity and final confirmation was not uniform among the onconeural antibodies tested. While all confirmed sera for anti-Yo (n = 3) and anti-Hu (n = 11) were strong positive (above 70) according to EUROLINE PNS 12 Ag (Euroimmun); 9/28 confirmed SOX1 antibodies were within the lower part of positive range (15–35).
Table 3.
Confirmation (n/N) of positive results obtained by EUROLINE PNS 12 Ag (Euroimmun), according to band intensity
Discussion
Commercial immunodots provide an easy and rapid detection of onconeural antibodies in routine laboratories, which is one of the main steps in PNS diagnosis.1 However, no independent and large studies have been performed concerning their diagnostic yield. In the present study, we found that positive immunodots were only confirmed by alternative techniques in just under a third of the cases (positive predictive value). Nevertheless, the proportion of confirmation was highly variable among the antibodies tested using both PNS+2 blot (Ravo Diagnostika) or EUROLINE PNS 12 Ag (Euroimmun). Whereas most anti-Hu positivity was confirmed, anti-SOX1 showed more modest results, and anti-Yo was almost never confirmed. Other onconeural antibodies also had low rates of confirmation, such as anti-Ri, but they were uncommon in the current study, limiting the interpretation of these data.
We also analyzed whether the band intensity obtained using EUROLINE PNS 12 Ag (Euroimmun) was related to the final confirmation using other techniques. Of interest, among the 27 positive sera with intensity between 8 and 14 (weak positive), none was confirmed irrespective of the antibody, demonstrating frequent nonconfirmed results among immunodots with low band intensity. At the opposite end of the band intensity range, we found that for some onconeural antibodies (such as anti-Yo or anti-Hu), only those with the highest levels of intensity were confirmed, indicating therefore a different threshold from the one proposed by the manufactures. However, this was not the case for other onconeural antibodies, such as anti-SOX1 or anti-Ma2, in which an optimal threshold dividing the confirmed from the nonconfirmed seems more difficult to establish. Thus, more studies are needed to determine the particular thresholds for each antibody to achieve a higher specificity.
The reason why the confirmation of positive immunodots was generally infrequent and, in some cases, highly dependent on band intensity is likely to be related to the nature of the antigens. Commercial immunodots use recombinant proteins that may not present the conformational structure of the natural protein in vivo. Epitopes may therefore be modified leading to recognition by unspecific antibodies that can be present in patients with other autoimmune or inflammatory diseases, as it has been already reported.12 In addition, the proteins used as targets for the detection of onconeural antibodies may not be the true antigens. For instance, cerebellar degeneration protein 2 (CDR2) was the first antigen described for anti-Yo antibodies in paraneoplastic cerebellar degeneration associated with breast and ovary cancer in women.13 However, later, it was reported that they also recognized the CDR2 paralog, CDR2L (CDR2-like).14 Recently, it has been shown that anti-Yo antibodies only bind endogenous CDR2L and not CDR2.15 We also found that more than a half of the patients with anti-Yo nonconfirmed immunodots were men, reinforcing the hypothesis that Yo proteins used in commercial immunodots (CDR2) do not represent the natural Yo antigen.
We described herein that the majority of patients with nonconfirmed immunodots lacked suitable clinical presentations to suspect a PNS. Cancer was rarely present and sometimes corresponded to types not typically associated with the antibody identified by immunodot, such as lung adenocarcinoma with anti-Yo or uterine cancer with anti-CV2/CRMP5. Taken together, these findings underline the importance of taking into account all clinical features when considering a positive immunodot. Thereby, interpretation of immunodots should be dependent on sex, clinical phenotype, and subtype of tumor, which must be in accordance with previously published cases. Ideally, opinion of an expert center should be sought in cases of antibody positivity that do not match with clinical presentation, and confirmation by alternative diagnostic methods should be proposed.
The present study is limited by the fact that negative immunodots were not further analyzed, and we did not have access to clinical information concerning these patients. As we cannot determine false and true negatives, we are not able to provide the exact sensitivity and specificity of the commercial kits. Nevertheless, specificity is likely to be very high (probably nearly 95%), as a large number of true negatives are expected due to the low prevalence of PNS. Another fact that should be mentioned is that we also identified 4 patients with nonconfirmed immunodots for onconeural antibodies who, although with an alternative diagnosis, had a cancer usually associated with the antibodies found, raising the possibility of a true positivity. Indeed, positive immunodots with negative immunofluorescence have been described in a few patients with idiopathic inflammatory myositis.16
In conclusion, commercial immunodots assays may be useful to screen patients with PNS suspicion, but clinical information and confirmatory tests at reference centers are needed to minimize misdiagnosis.
Acknowledgment
The authors thank NeuroBioTec Hospices Civils de Lyon BRC (France, AC-2013-1867, NFS96-900) for banking sera and CSF samples. They thank Philip Robinson for help in manuscript preparation (Direction de la Recherche Clinique, Hospices Civils de Lyon).
Glossary
- CBA
cell-based assay
- IIF
indirect immunofluorescence
- HEK
human embryonic kidney
- PBS
phosphate-buffered saline
- PNS
paraneoplastic neurologic syndrome
- SCLC
small-cell lung cancer
- SDS
sodium dodecyl sulfate
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
This study is supported by research grants from ANR (ANR-14-CE15-0001-MECANO) and FRM (Fondation pour la recherche médicale) DQ20170336751. This work has been developed within the BETPSY project, which is supported by a public grant overseen by the French National Research Agency (ANR), as part of the second “Investissements d´Avenir” program (reference ANR-18-RHUS-0012).
Disclosure
The authors have no conflicts of interest to disclose. Go to Neurology.org/NN for full disclosures.
References
- 1.Graus F. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Höftberger R, Dalmau J, Graus F. Clinical neuropathology practice guide 5-2012: updated guideline for the diagnosis of antineuronal antibodies. Clin Neuropathol 2012;31:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricken G, Schwaiger C, De Simoni D, et al. . Detection methods for autoantibodies in suspected autoimmune encephalitis. Front Neurol 2018;9:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabater L, Saiz A, Dalmau J, Graus F. Pitfalls in the detection of CV2 (CRMP5) antibodies. J Neuroimmunol 2016;290:80–83. [DOI] [PubMed] [Google Scholar]
- 5.Johannis W, Renno JH, Wielckens K, Voltz R. Ma2 antibodies: an evaluation of commercially available detection methods. Clin Lab 2011;57:321–326. [PubMed] [Google Scholar]
- 6.Ruiz-García R, Martínez-Hernández E, García-Ormaechea M, et al. . Caveats and pitfalls of SOX1 autoantibody testing with a commercial line blot assay in paraneoplastic neurological investigations. Front Immunol 2019;10:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol 2010;257:509–517. [DOI] [PubMed] [Google Scholar]
- 8.Saiz A, Blanco Y, Sabater L, et al. . Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain 2008;131:2553–2563. [DOI] [PubMed] [Google Scholar]
- 9.Ariño H, Höftberger R, Gresa-Arribas N, et al. . Paraneoplastic neurological syndromes and glutamic acid decarboxylase antibodies. JAMA Neurol 2015;72:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto AM, Gajdos P, Eymard B, et al. . Anti-titin antibodies in myasthenia gravis: tight association with thymoma and heterogeneity of nonthymoma patients. Arch Neurol 2001;58:885–890. [DOI] [PubMed] [Google Scholar]
- 11.Thirkill CE, Tait RC, Tyler NK, Roth AM, Keltner JL. The cancer-associated retinopathy antigen is a recoverin-like protein. Invest Ophthalmol Vis Sci 1992;33:2768–2772. [PubMed] [Google Scholar]
- 12.Infantino M, Tampoia M, Fabris M, et al. . Combining immunofluorescence with immunoblot assay improves the specificity of autoantibody testing for myositis. Rheumatology (Oxford) 2019;58:1239–1244. [DOI] [PubMed] [Google Scholar]
- 13.Totland C, Aarskog NK, Eichler TW, et al. . CDR2 antigen and Yo antibodies. Cancer Immunol Immunother 2011;60:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichler TW, Totland C, Haugen M, et al. . CDR2L antibodies: a new player in paraneoplastic cerebellar degeneration. PLoS One 2013;8:e66002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kråkenes T, Herdlevær I, Raspotnig M, Haugen M, Schubert M, Vedeler CA. CDR2L is the major Yo antibody target in paraneoplastic cerebellar degeneration. Ann Neurol 2019;86:316–321. [DOI] [PubMed] [Google Scholar]
- 16.Picard C, Vincent T, Lega JC, et al. . Heterogeneous clinical spectrum of anti-SRP myositis and importance of the methods of detection of anti-SRP autoantibodies: a multicentric study. Immunol Res 2016;64:677–686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported are available in the article and in the supplementary materials.