Abstract
Objective
The concept of enhanced recovery after surgery (ERAS) is relatively new to the neurosurgical field. The introduction of an ERAS protocol in lumbar fusion surgery has aimed to accelerate patient recovery from surgery by reducing in-hospital opioid consumption.
Methods
Patients with 1- or 2-level degenerative lumbar spine disease and who underwent ERAS transforaminal lumbar interbody fusion (TLIF) were retrospectively reviewed. Patients’ general demographic data, in-hospital opioid dosage (converted to morphine equivalents), and hospital stay were compared to those who underwent standard minimally-invasive (MIS)-TLIF.
Results
Twenty-four patients who received ERAS TLIF (the ERAS group) were compared to a series of 24 patients who received standard MIS-TLIF (the MIS group). The demographic data were similar. The operation time and blood loss significantly favored ERAS TLIF. The average daily opioid consumption was remarkably lower in the ERAS group than the MIS group. Average opioid dosage throughout the entire in-hospital period was also significantly reduced in the ERAS group compared to the MIS group. The average length of hospital stay was substantially shorter in the ERAS group (1.4 ± 1.13 days vs. 4.0±1.98 days, p <0.001).
Conclusion
The present study demonstrated a significant decline in the consumption of opioids and in the hospital length of stay for patients undergoing ERAS TLIF for 1- or 2-level degenerative lumbar spine disease.
Keywords: Enhanced recovery after surgery, Minimally invasive, Transforaminal lumbar interbody fusion, Awake fusion, Endoscopic discectomy and fusion, Expandable cage
INTRODUCTION
The development of minimally-invasive (MIS) techniques in spine surgery has accelerated in the last two decades. With the introduction of MIS transforaminal lumbar interbody fusion (MIS-TLIF), experienced spine surgeons are able to reduce surgical trauma while achieving long-term clinical and radiological outcomes comparable to open surgery [1-3]. MIS techniques have become popularized for degenerative lumbar spine pathology, offering numerous benefits: a smaller incision with less muscle trauma, reduced blood loss, shorter hospital stays, and earlier return to work [4-6]. Although postoperative pain remains the main reason for suboptimal mobility and dissatisfaction, advances in improving this aspect have been slow. Minimally-invasive surgery offers a relative advantage over open surgery, but ongoing improvements are still critical [5,7-9].
Our team previously reported initial results of an innovative MIS technique, the enhanced recovery after surgery (ERAS) TLIF. The centerpiece of our global ERAS spine program for lumbar interbody fusion is this technique, which combines novel technologies such as endoscopic decompression and fusion, conscious sedation without general anesthesia, expandable cage technology, a small-caliber percutaneous pedicle screw fixation system, multimodal pain control including long-acting local analgesia, and osteobiologics. Our initial results with the ERAS program demonstrated excellent clinical and radiologic outcomes for patients undergoing TLIF surgery [10,11]. Our ERAS program was designed specifically to address the general trend for minimizing pain and shortening the recuperation time after surgery. However, the effect of our ERAS program on acute postoperative pain and recovery time were unknown compared to standard MIS-TLIF using a microscope.
In this study, we examined opioid consumption and other inpatient metrics following an ERAS program at our institution. We aimed to assess postoperative pain control and initial recovery in a series of patients in the ERAS program, and compare their outcomes to patients who had undergone a standard MIS-TLIF by the same surgeon.
MATERIALS AND METHODS
1. ERAS TLIF (ERAS group)
The ERAS transforaminal lumbar interbody fusion (TLIF) program—as an advanced version of MIS-TLIF—was conceived at our institution in 2014. The initial consecutive series of patients in the ERAS TLIF program were treated by a single surgeon (MYW), and were enrolled in this retrospective study (ERAS group).
After Institutional Review Board (IRB) approval (University of Miami, Miller School of Medicine; IRB No. 20080954), a retrospective chart review was performed for patients’ demographic characteristics, indications for surgery, length of stay (LOS), estimated blood loss, fusion level, and distribution, operation time, perioperative complications, and readmission rate (within 30 days). All patients’ low back pain and disability levels before and after surgery were assessed with the Oswestry Disability Index (ODI). Postoperative ODI scores were assessed at 3, 6, and 12 months after surgery during outpatient clinic visits. The present study focused on the patients’ postoperative pain level during the acute in-patient period. Individual patient’s oral or intravenous opioid consumption during each day in the hospital was calculated and converted to morphine equivalent dosages (mg), followed by total opioid consumption during hospitalization. Nonsteroidal anti-inflammatory drugs were not administered for pain control.
ERAS TLIF employs several distinct techniques compared to the standard MIS-TLIF (Table 1) [10,12]. First, patients are placed in a prone position on a Jackson table. The patient is sedated using a continuous infusion of propofol, ketamine, and precedex with oxygen supplementation through a nasal cannula or nasal trumpet. Opioids are completely avoided to prevent respiratory depression. After cannulating Kambin triangle (Fig. 1), an 8-mm working channel with a rigid 30° angled endoscope is used to visualize and remove the intervertebral disc, replacing the conventional surgical microscope. With sequential dilation of this small tract, muscular dissection is completely eliminated. Subsequently, a mesh expandable cage (OptiMesh cage, Spineology, MN, USA) is utilized for interbody fusion. No other rigid or expandable cage system was utilized in the ERAS group in this study. Finally, Exparel (Pacira BioSciences, Inc. Parsippany, NJ. USA), a liposomal depoform bupivacaine, is injected alongthe entire planned pedicle screw tract. Aside from these unique aspects, ERAS TLIF shares other common elements with the standard MIS-TLIF performed at our institution, including the OptiMesh cage, standard percutaneous pedicle screw placement and rod fixation, and the off-label use recombinant human bone morphogenetic protein (BMP 2). The Viper (DePuy-Synthes Inc., Raynham, MA, USA) percutaneous screw system was used in all ERAS TLIF cases (Fig. 2). It must be emphasized that the OptiMesh cage and Exparel are also currently off-label for these techniques, according to the U.S. Food and Drug Administration.
Table 1.
Six elements of ERAS TLIF
| Element | Content |
|---|---|
| Intravenous sedation without general anesthesia | Patient is sedated using a continuous infusion of propofol, ketamine, and precedex with oxygen supplementation through nasal cannula or nasal trumpet. Opioids are completely avoided to prevent respiratory depression. |
| Endoscopic discectomy | Small-diameter (8 mm) working channel to minimize soft tissue trauma |
| Mesh expandable cage | Interbody fusion |
| Exparel | A liposomal depoform bupivacaine, is injected along the entire planned pedicle screw tract for local analgesia. |
| Recombinant human bone morphogenetic protein | To enhance bone fusion |
| Percutaneous pedicle screw | Posterior supplemental fixation |
ERAS, enhanced recovery after surgery; TLIF, transforaminal lumbar interbody fusion.
Fig. 1.
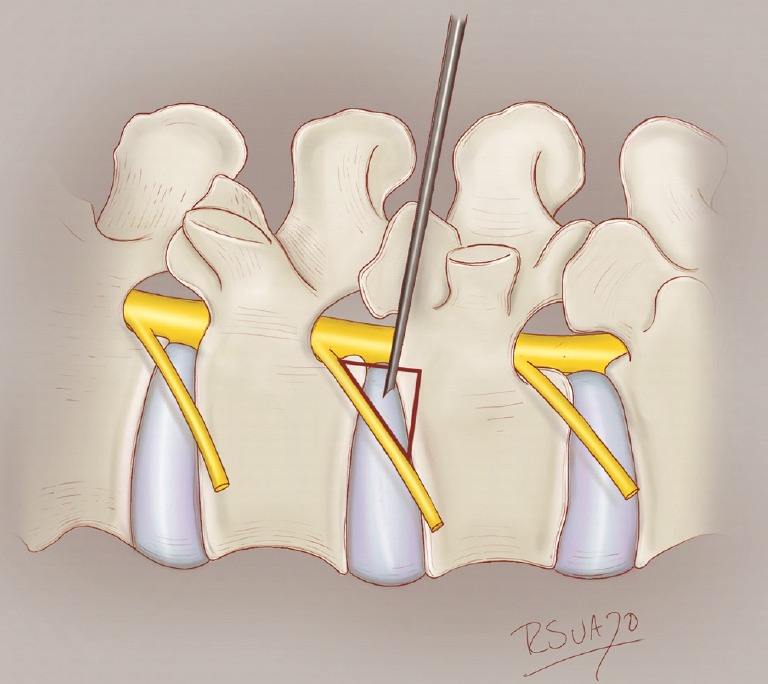
An illustration of Kambin triangle (red).
Fig. 2.
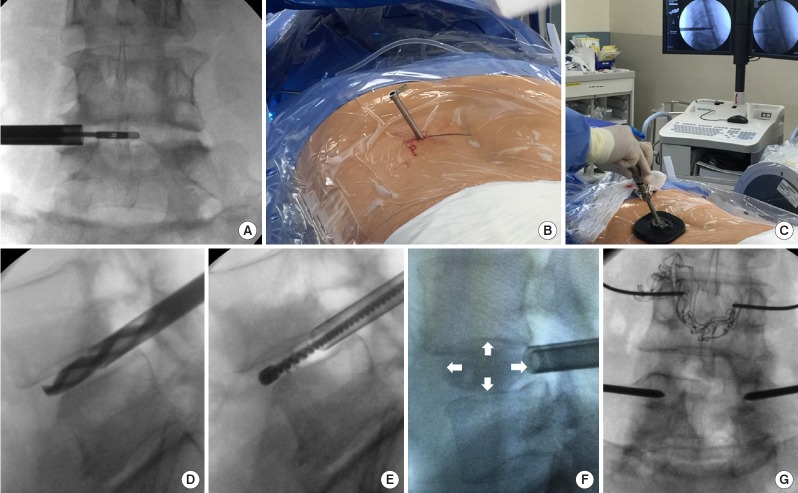
Schematic procedure of ERAS TLIF. Initially, endoscopic discectomy (A) is done via an 8-mm channel (B). Subsequently, the vertebral endplate is prepared through a small working channel (C) with specialized instruments such as drill (D) and electric brush (E), etc. No soft tissue or muscular dissection is needed. An OptiMesh expandable cage (F, white arrow) is inserted and filled with allograft through a small tube. Finally, the cannulation of Jamshidi needles and guide wire are performed for the placement of percutaneous pedicle screws (G). ERAS, enhanced recovery after surgery; TLIF, transforaminal lumbar interbody fusion.
2. Perioperative Pain Management and Nutrition Protocol in the ERAS Group
The ERAS protocol for TLIF leverages multimodal analgesia under monitored anesthesia care (MAC). At the time that these patients were enrolled, this was solely done with the injection of approximately 5–10 mL of 1:1 long-acting liposomal bupivacaine mixed with plain 0.25% Bupivacaine hydrochloride in the soft tissue tracts of the percutaneous pedicle screw tracts. Preand intraoperative doses of narcotic medications were not given in order to prevent respiratory suppression during MAC. The postoperative regimen consisted of standard and Pro re nata (PRN) opioid medications such as Percocet 5-325, Tramadol, and Dilaudid IV for breakthrough pain.
For ERAS preoperative nutrition management, patients were counseled on the negative effects of obesity and sarcopenia on surgical outcomes and were encouraged to lose weight but maintain or increase lean body mass with a high protein diet. They were also encouraged to have a carbohydrate load the night before surgery.
3. Standard MIS-TLIF (MIS Group)
A comparison series of patients at the same institution underwent standard MIS-TLIF by the same single surgeon, and were also reviewed for the present study (MIS group). All metrics in the MIS group were retrospectively reviewed and analyzed in comparison to the ERAS group, including in-patient narcotic consumption.
The standard MIS-TLIF procedure involved a small midline incision, microscope-assisted unilateral muscular dissection and facetectomy to achieve nerve root decompression, interbody fusion with a cage fixation device, and percutaneous pedicle screw placement. BMP-2 was routinely used in all standard MIS-TLIF procedures. The OptiMesh expandable cage or Luna 3D expandable cage system (Benvenue Medical Inc., Santa Clara, CA, USA) was used for interbody grafting, and the Viper percutaneous screw system was used in all MIS-TLIF cases.
The inclusion criteria were similar for each group. Indications for surgery included: (1) grade I and II spondylolisthesis (Fig. 3); (2) degenerative disc disease with spinal stenosis and nerve entrapment. The predominant symptoms in the present series were mainly radiculopathy, neurogenic claudication, and back pain. All patients in this series had demonstrable instability on preoperative images. Of note, patient size, Malampati score, and neck size were necessarily taken into account when considering prone conscious sedation for ERAS TLIFs; however, there were no firm exclusion criteria. Other comorbidities such as major cardiopulmonary disease and advanced age were actually not a contraindication for the ERAS program, since the use of conscious sedation eliminates the majority of the pathophysiologic derangements and risks incurred by general anesthesia. From an anatomical standpoint, we did not favor to operate at the L5–S1 level with ERAS TLIF due to iliac crest impediment to cannulation of Kambin triangle. Extremely wide hypertrophic facet joints are a relative contraindication, as these require a more lateral trajectory to Kambin triangle, which can place the dorsal root ganglion at risk of injury. We tended to offer ERAS TLIF to elderly patients and those with comorbidities since no general anesthesia was given. Patients’ psychological status were evaluated during clinical visits. Patients with anxiety issues were excluded from ERAS TLIF.
Fig. 3.
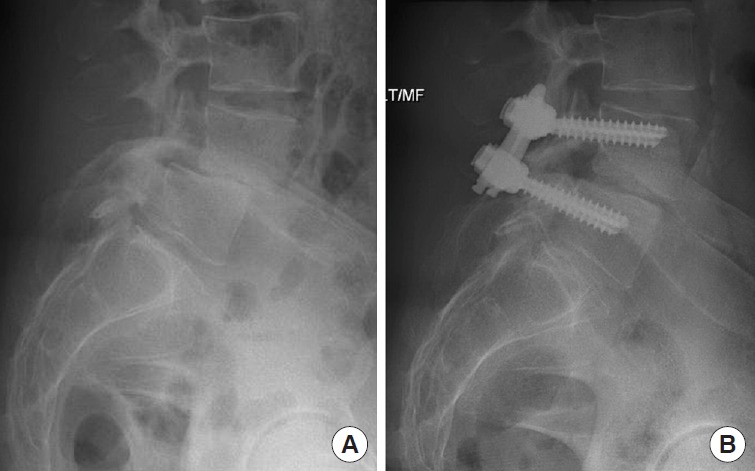
(A) A 61-year-old female underwent ERAS TLIF. A preoperative lumbar X-ray demonstrated grade II L4–5 spondylolisthesis. (B) A postoperative X-ray demonstrated rigid fixation and remarkable reduction of spondylolisthesis. ERAS, enhanced recovery after surgery; TLIF, transforaminal lumbar interbody fusion.
4. Statistical Analysis
An independent t-test was used for analysis of continuous variables. Pearson chi-square test was used for analysis of categorical variables. Statistical analysis was performed with the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was determined to be statistically significant.
RESULTS
1. Demographics
The first 24 patients treated in the ERAS program from 2014 to 2015 were enrolled to the ERAS group. A comparison series (MIS group) consisting of 24 patients treated with the standard MIS-TLIF from 2012 to 2015 were also retrospectively reviewed. Demographic data are shown in Table 2.
Table 2.
Demographic data
| Variable | ERAS TLIF (n = 24) | Standard MIS-TLIF (n = 24) | p-value |
|---|---|---|---|
| Age (yr) | 64.3 ± 11.59 | 60.1 ± 12.23 | 0.232 |
| Sex, male:female | 14:10 | 11:13 | 0.386 |
| Operation levels | 26 | 25 | 0.551 |
| 1-Level | 22 | 23 | |
| 2-Level | 2 | 1 | |
| Level distribution | 0.328 | ||
| L1–2 | 1 | 0 | |
| L2–3 | 1 | 1 | |
| L3–4 | 4 | 1 | |
| L4–5 | 18 | 19 | |
| L5–S1 | 1 | 4 | |
| Operation time (min) | 110.7 ± 21.23 | 154.8 ± 39.53 | < 0.001* |
| Estimated blood loss (mL) | 66.0 ± 37.24 | 121.4 ± 62.39 | 0.001* |
| Length of stay† (day) | 1.4 ± 1.13 | 4.0 ± 1.98 | < 0.001* |
| Preoperative ODI | 42.0 ± 14.67 | 46.6 ± 16.69 | 0.386 |
Values are presented as mean±standard deviation or number.
ERAS, enhanced recovery after surgery; TLIF, transforaminal lumbar interbody fusion; MIS, minimally-invasive; ODI, Oswestry Disability Index.
p <0.05, statistically significant.
Not including the day of surgery.
Patients in the ERAS group trended to be slightly older, but this difference was not significant (64.3±11.59 years vs. 60.1±12.23 years, p = 0.232). The gender distribution was similar between the 2 groups (p = 0.386). Twenty-six levels were treated in the ERAS group, compared to 25 levels in the MIS group. The majority of interbody fusions were performed at L4/5, which accounted for 72.5% of levels treated (37 out of 51 levels). Fusion level distributions did not differ significantly between groups (p = 0.328), but L5–S1 were primarily treated with standard MIS-TLIF due to aforementioned anatomical constraints. The average operation time was much more expeditious in the ERAS group than in the MIS group (skin to skin, 110.7±21.23 minutes vs. 154.8±39.53 minutes, p < 0.001). Also, the blood loss was significantly minimal in the ERAS group (p = 0.001) (Table 2). Postsurgical improvement according to the Oswestry Disability Index (ODI) demonstrated excellent clinical outcomes for the whole study cohort. Postoperative 12-month ODI scores were available for final evaluation, except that 1 patient was assessed at 6 months (preoperative vs. final follow-up: 43.8±15.63 vs. 14.4±9.17, p < 0.01).
2. In-Patient Opioid Consumption and Hospital Stay
The pain level before surgery was evaluated in both groups by using the ODI and it did not differ significantly (42.0±14.67 vs. 46.6±16.69, p = 0.386) (Table 2). All patients were provided with standard postoperative care, including adequate pain control until discharge. The majority of the patients in the ERAS group were discharged on postoperative day one (79%, n = 19). Hospital LOS ranged from 1–6 days in the ERAS group, compared to 2–9 days in the MIS group. The average hospital LOS was significantly shorter in the ERAS group (1.4±1.13 days vs. 4.0±1.98 days, p < 0.001) (Table 2).
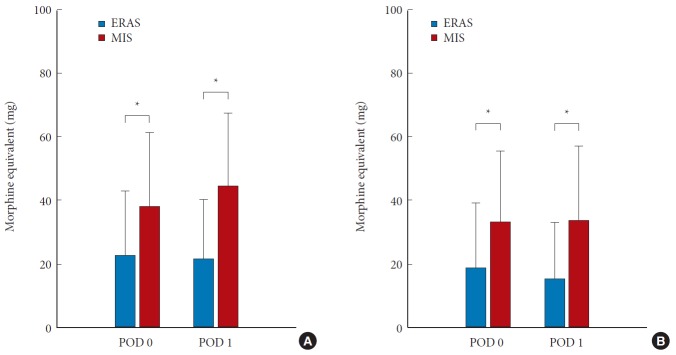
Acute pain control in the hospital was achieved with narcotic medications in both groups. The narcotic consumption in both groups was converted to morphine equivalent dosages for comparison (Table 3). Narcotic consumption at postoperative day 0 and day 1 is shown in Fig. 4. Narcotic consumption in the ERAS group was 22.8±20.20 mg and 21.6±18.72 mg (morphine equivalent) on postoperative days 0 and 1, respectively. In contrast, narcotic consumption in the MIS group was 38.1±23.27 mg and 44.3±23.10 mg on postoperative day 0 and day 1, respectively. These values differed significantly between the 2 groups (p = 0.019 and p = 0.001, respectively) (Fig. 4A). Patients in the ERAS group also received a significantly lower intravenous (IV) dose compared to the MIS group, on both postoperative day 0 and day 1 (p = 0.025 and p = 0.005, respectively) (Fig. 4B). Total opioid consumption throughout the entire hospital course was also significantly reduced in the ERAS group, both for total opioids (oral + IV) and IV opioids alone (Fig. 5).
Table 3.
Narcotic medication and its morphine equivalent dosage
| Variable | Morphine equivalent dosage (mg) |
|---|---|
| Morphine | 1 |
| Hydromorphine (1 mg) | 6.7 |
| Demerol (50 mg) | 1.7 |
| Percocet (5-mg oxycodone/325-mg acetaminophen) | 2.5 |
| Vicodin (5-mg hydrocodone/300-mg acetaminophen) | 1.7 |
| Tramadol (50 mg) | 2 |
Fig. 4.
Average narcotic consumption in both groups on postoperative day (POD) 0 and 1. (A) All narcotic consumption (oral + intravenous route). (B) Only intravenous narcotic consumption. ERAS, enhanced recovery after surgery; MIS, minimally-invasive. *p <0.05.
Fig. 5.
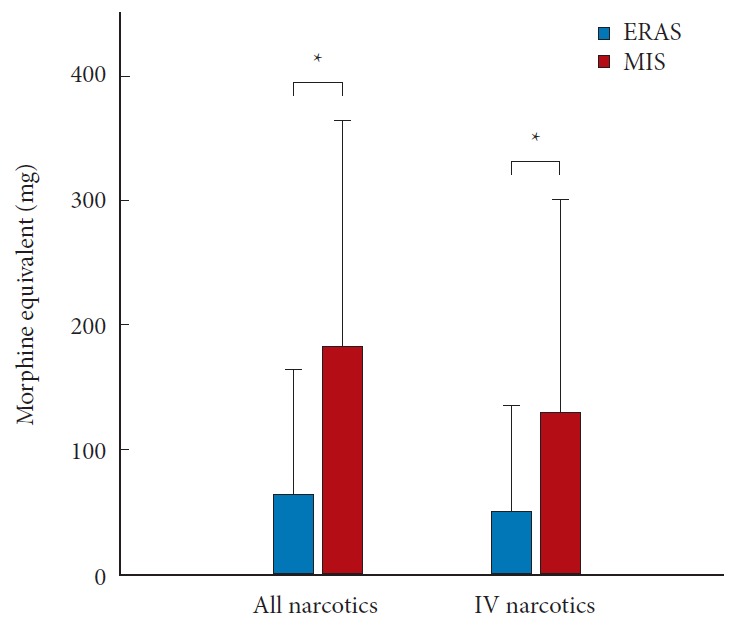
Average narcotic consumption throughout entire inpatient period in both groups. ERAS, enhanced recovery after surgery; MIS, minimally-invasive; IV, intravenous. *p <0.05.
3. Complication Profile
There were no intraoperative complications noted in this series. However, there was one patient in the ERAS group who developed an infection at the interbody space 2 months after the surgery and subsequently underwent anterior lumbar interbody fusion.
DISCUSSION
An ERAS program for lumbar interbody fusion has been active at our university since 2014. In the current atmosphere of healthcare expenditure reductions, decreasing reimbursements, and rising hospital costs, it is critical for spine surgery to evolve and adapt to this new culture of value-based care. This ERAS program is a marriage of technologies to enhance patient recovery, to accelerate discharge, and ultimately to reduce the cost of treatment [13].
In the present study, we successfully demonstrated that inhospital postoperative opioid consumption in the ERAS group was remarkably reduced compared to that in the standard MIS-TLIF group. Postoperative in-patient care following spine surgery was mainly for pain control. The patients in the ERAS group were discharged significantly earlier than those who underwent standard MIS-TLIF. Approximately 80% of these patients were discharged on postoperative day 1, while patients in the MIS-TLIF group were discharged on postoperative day 4 on average.
Acute postoperative pain has always been a major concern in spine surgery. Adequate postoperative pain management has been associated with early mobilization, better outcomes, and reduced adverse events in several surgical fields [14-17]. In-patient opioid consumption is often considered a surrogate measurement for assessing patients’ acute pain level [15,18]. The authors aimed to investigate acute postoperative pain levels by reviewing opioid consumption after ERAS TLIF, and comparing these data to standard MIS-TLIF patients. The results of our study suggested a lower postoperative pain level and shorter hospital stay in the ERAS group.
Although ERAS has become increasingly popular in many surgical subspecialties, the concept is relatively new in neurosurgery [19]. There is strong evidence from randomized-controlled trials and meta-analyses demonstrating the efficacy of ERAS in reducing morbidity, mortality, and hospital stay [20]. Generally, ERAS protocols consist of step-by-step programs advocating less invasive surgical techniques, pre- and postoperative reorganization, and multidisciplinary cooperation focused toward the ultimate goal of accelerated patient recovery and discharge.
Key elements prolonging postoperative recovery include increased opioid consumption, reduced appetite arising from the use of opioids, altered mental status, compensatory intravenous fluid, and immobilization [21]. These interrelated factors are often a function of postoperative pain levels. Therefore, a critical component of ERAS is to reduce surgery-related pain and to avoid the side effects of opioid analgesics [22]. These effects include respiratory failure, gut dysfunction, nausea/vomiting, and urinary retention [23]. There is strong evidence suggesting that prolonged hospital stays may be related to opioid analgesia [21]. The ERAS TLIF program described in our study adopted multimodal technology to minimize pain levels following lumbar fusion surgery, a procedure notorious for postoperative pain. The method included an exceedingly MIS technique and instruments, including endoscopic discectomy, expandable mesh cages, and percutaneous pedicle screws. Besides the technique aspect, regional long-acting analgesia (i.e., Exparel) and a significantly shorter operation time (mean operative time 110 minutes) were also demonstrated to be extremely helpful in reducing patients’ pain levels [24,25]. In return, our patients in the ERAS program required less opioid consumption, and achieved early mobility.
A fundamental outcome measurement in evaluating the ERAS program is the hospital LOS. There are several interventions affecting postoperative recovery, beyond analgesia alone. These include early mobilization, reduced intravenous fluid, early resumption of enteral feeding, and prevention of deep vein thrombosis [26-28]. These components are incorporated into ERAS protocols. However, there has been recent argument over whether the LOS truly reflects patients’ recovery. Functional recovery has been advocated as a more meaningful assessment. To date, little research has evaluated patients’ functional recovery as a primary outcome (as opposed to LOS), as it is difficult to assess and analyze [29]. Future ERAS studies in neurosurgery should pay more attention to patients’ function status when measuring outcomes.
Previous publications have demonstrated reduced pain levels after MIS-TLIF compared to open TLIF by assessing the visual analogue scales (VAS) or ODI scores [3,30]. The VAS and ODI scores are rather subjective measurements, and so may vary between patients. On the other hand, calculation of opioid consumption provides a quantitative evaluation of a given patient’s pain level during the acute in-patient period. Some studies in the literature have investigated postoperative opioid consumption. Isaacs et al. [31] reported a significant decrease in morphine equivalent consumption (average, 37.5 mg/day) in a series of MIS-TLIF patients compared to open TLIF. Cheng et al. [18] also demonstrated a remarkable reduction in opioid consumption for MIS-TLIF patients (average 66.5 mg/day around the clock, plus asneeded [PRN] dose) in contrast to open TLIF patients. The mean opioid consumption in the MIS-TLIF group was approximately 40 mg per day in our study, which resembled values in previous literature. Moreover, the average narcotic consumption in the ERAS group was nearly half of that in the MIS-TLIF group. Hospital LOS for MIS-TLIF patients ranged from 3.0 to 4.8 days in previous studies [3,18,30-33]. Average hospital LOS (4 days) in our MIS-TLIF group was comparable to these values. The mean hospital LOS in the ERAS group, however, was significantly shorter, averaging only 1.4 days without any early readmissions for inadequate pain control. This result corresponds with our previous publications [10,11].
The present study faced several limitations. First, the preoperative opioid consumption was not analyzed in this study. Since most of our patients received their pain medication from pain management or primary care physicians, the type of medication, dosage, and the prescription frequency were highly heterogeneous and therefore difficult to evaluate. More than 50% of the patients were prescribed with nonsteroid anti-inflammatory drugs rather than opioids for pain control before surgery. Some of the patients only took the medication as-needed and were not able to track the medication they had taken. All these factors rendered the evaluation of preoperative narcotic consumption to be unreliable and unrealistic. However, the ODI scale appeared to be similar between both groups before surgery, which provided a much more reliable measurement of the patients’ preoperative status in this study. As most patients (80%) in the ERAS group were discharged 1 day after surgery, only the data of narcotic consumption from postoperative day 0 and day 1 are available to be analyzed and compared to the standard MIS-TLIF group. Therefore, it would be underpowered to perform statistical analysis between both groups after postoperative day 1, although most patients in the MIS group were discharged 4 days after surgery. However, if we considered the total opioid consumption for each patient throughout the hospital stay, the average consumption in the ERAS group was significantly reduced. The number of patients in both groups was small, limiting generalizability. Further, this study focused on the acute postoperative period only. Comparison of long-term clinical and radiographic outcomes between the ERAS and standard MIS-TLIF groups were outside the scope of this study. We have demonstrated short-term efficacy, and excellent clinical and radiographic outcomes of our approach in a series of ERAS TLIF programs in 2 recent publications [10,11]. Although the general indications for surgery in both groups were similar—including: (1) grade I and II spondylolisthesis; (2) degenerative disc disease with spinal stenosis and nerve entrapment—all patients in this series had demonstrable instability on preoperative images. However, there could still be some selection bias in this retrospective study. The major difference in surgical techniques between the ERAS and MIS-TLIF groups was the utilization of a small-diameter endoscope instead of a microscope. It may have been that patients with severe stenosis that required direct decompression were more likely offered MIS-TLIF. Direct and complete decompression of bony structures and the ligamentum flavum are less likely to be done, yet indirect decompression can be achieved in ERAS TLIF. The lower operative time and blood loss in the ERAS group compared to the MIS-TLIF group may have resulted from the lack of facetectomy, hemilaminectomy, and ligamentum flavum removal. The patient’s age, psychological status, anatomical restriction, or comorbidity also need to be taken into consideration before performing ERAS TLIF. Future long-term and large-population results comparing the ERAS program to standard MIS-TLIF is currently underway. Ultimately, a large-scale randomized-controlled study is needed to validate the advantages of our ERAS program in spine surgery.
CONCLUSION
The data here demonstrate a significant decrease in the consumption of opioid medication and the hospital LOS for patients undergoing ERAS TLIF for 1- or 2-level degenerative lumbar spine disease. The immediate postoperative effects of pain reduction and accelerated recovery in the ERAS program for lumbar fusion surgery are dramatic, garnering outcomes superior to the standard MIS-TLIF.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Goldstein CL, Macwan K, Sundararajan K, et al. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. 2016;24:416–27. doi: 10.3171/2015.2.SPINE14973. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein CL, Phillips FM, Rampersaud YR. Comparative effectiveness and economic evaluations of open versus minimally invasive posterior or transforaminal lumbar interbody fusion: a systematic review. Spine (Phila Pa 1976) 2016;41 Suppl 8:S74–89. doi: 10.1097/BRS.0000000000001462. [DOI] [PubMed] [Google Scholar]
- 3.Lee KH, Yue WM, Yeo W, et al. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21:2265–70. doi: 10.1007/s00586-012-2281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker SL, Lerner J, McGirt MJ. Effect of minimally invasive technique on return to work and narcotic use following transforaminal lumbar inter-body fusion: a review. Prof Case Manag. 2012;17:229–35. doi: 10.1097/NCM.0b013e3182529c05. [DOI] [PubMed] [Google Scholar]
- 5.Adogwa O, Parker SL, Bydon A, et al. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24:479–84. doi: 10.1097/BSD.0b013e3182055cac. [DOI] [PubMed] [Google Scholar]
- 6.McGirt MJ, Parker SL, Lerner J, et al. Comparative analysis of perioperative surgical site infection after minimally invasive versus open posterior/transforaminal lumbar interbody fusion: analysis of hospital billing and discharge data from 5170 patients. J Neurosurg Spine. 2011;14:771–8. doi: 10.3171/2011.1.SPINE10571. [DOI] [PubMed] [Google Scholar]
- 7.Adogwa O, Elsamadicy AA, Han JL, et al. 30-Day readmission after spine surgery: an analysis of 1400 consecutive spine surgery patients. Spine (Phila Pa 1976) 2017;42:520–4. doi: 10.1097/BRS.0000000000001779. [DOI] [PubMed] [Google Scholar]
- 8.Gornitzky AL, Flynn JM, Muhly WT, et al. A rapid recovery pathway for adolescent idiopathic scoliosis that improves pain control and reduces time to inpatient recovery after posterior spinal fusion. Spine Deform. 2016;4:288–95. doi: 10.1016/j.jspd.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Vela J, Lobo-Escolar A, Joven-Aliaga E, et al. Perioperative and short-term advantages of mini-open approach for lumbar spinal fusion. Eur Spine J. 2009;18:1194–201. doi: 10.1007/s00586-009-1010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang MY, Grossman J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurg Focus. 2016;40:E13. doi: 10.3171/2015.11.FOCUS15435. [DOI] [PubMed] [Google Scholar]
- 11.Wang MY, Chang PY, Grossman J. Development of an enhanced recovery after surgery (ERAS) approach for lumbar spinal fusion. J Neurosurg Spine. 2017;26:411–8. doi: 10.3171/2016.9.SPINE16375. [DOI] [PubMed] [Google Scholar]
- 12.Chang HK, Kolcun JPG, Chang PY, et al. Enhanced recovery after SurgeryTM awake minimally-invasive transforaminal lumbar interbody fusion: 2-dimensional operative video. Oper Neurosurg (Hagerstown) 2019;16:519. doi: 10.1093/ons/opy187. [DOI] [PubMed] [Google Scholar]
- 13.Wang MY, Chang HK, Grossman J. Reduced acute care costs with the ERAS® minimally invasive transforaminal lumbar interbody fusion compared with conventional minimally invasive transforaminal lumbar interbody fusion. Neurosurgery. 2018;83:827–34. doi: 10.1093/neuros/nyx400. [DOI] [PubMed] [Google Scholar]
- 14.Devin CJ, McGirt MJ. Best evidence in multimodal pain management in spine surgery and means of assessing postoperative pain and functional outcomes. J Clin Neurosci. 2015;22:930–8. doi: 10.1016/j.jocn.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Essving P, Axelsson K, Kjellberg J, et al. Reduced hospital stay, morphine consumption, and pain intensity with local infiltration analgesia after unicompartmental knee arthroplasty. Acta Orthop. 2009;80:213–9. doi: 10.3109/17453670902930008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugely AJ, Martin CT, Gao Y, et al. Causes and risk factors for 30-day unplanned readmissions after lumbar spine surgery. Spine (Phila Pa 1976) 2014;39:761–8. doi: 10.1097/BRS.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 17.Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12–5. doi: 10.1016/j.arth.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Cheng JS, Park P, Le H, et al. Short-term and long-term outcomes of minimally invasive and open transforaminal lumbar interbody fusions: is there a difference? Neurosurg Focus. 2013;35:E6. doi: 10.3171/2013.5.FOCUS1377. [DOI] [PubMed] [Google Scholar]
- 19.Martín N, Valero R, Hurtado P, et al. Experience with “Fast track” postoperative care after deep brain stimulation surgery. Neurocirugia (Astur) 2016;27:263–8. doi: 10.1016/j.neucir.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Adamina M, Kehlet H, Tomlinson GA, et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149:830–40. doi: 10.1016/j.surg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 2013;37:259–84. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 22.Wu CL, Fleisher LA. Outcomes research in regional anesthesia and analgesia. Anesth Analg. 2000;91:1232–42. doi: 10.1097/00000539-200011000-00035. [DOI] [PubMed] [Google Scholar]
- 23.Oderda GM, Evans RS, Lloyd J, et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage. 2003;25:276–83. doi: 10.1016/s0885-3924(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Burke SM, Kryzanski JT, et al. The role of liposomal bupivacaine in reduction of postoperative pain after transforaminal lumbar interbody fusion: a clinical study. World Neurosurg. 2016;91:460–7. doi: 10.1016/j.wneu.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 25.Gu J, Guan F, Zhu L, et al. Risk factors of postoperative low back pain for lumbar spine disease. World Neurosurg. 2016;94:248–54. doi: 10.1016/j.wneu.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: enhanced recovery after surgery (ERAS) group recommendations. Arch Surg. 2009;144:961–9. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 27.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359:1812–8. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 28.Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev. 2006;(4):CD004080. doi: 10.1002/14651858.CD004080.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Coolsen MM, Wong-Lun-Hing EM, van Dam RM, et al. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB (Oxford) 2013;15:245–51. doi: 10.1111/j.1477-2574.2012.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng CW, Yue WM, Poh SY, et al. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 2009;34:1385–9. doi: 10.1097/BRS.0b013e3181a4e3be. [DOI] [PubMed] [Google Scholar]
- 31.Isaacs RE, Podichetty VK, Santiago P, et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3:98–105. doi: 10.3171/spi.2005.3.2.0098. [DOI] [PubMed] [Google Scholar]
- 32.Dhall SS, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine. 2008;9:560–5. doi: 10.3171/SPI.2008.9.08142. [DOI] [PubMed] [Google Scholar]
- 33.Guan J, Bisson EF, Dailey AT, et al. Comparison of clinical outcomes in the national neurosurgery quality and outcomes database for open versus minimally invasive transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 2016;41:E416–21. doi: 10.1097/BRS.0000000000001259. [DOI] [PubMed] [Google Scholar]