Abstract
Prolongation of the QT interval can predispose patients to fatal arrhythmias such as torsade de pointes. While arrhythmia can occur spontaneously in patients with a genetic predisposition, drugs such as ondansetron and droperidol, which are frequently used in the perioperative period, have been implicated in the prolongation of the QT interval. As the list of medications that cause QT prolongation grows, anesthesia providers and perioperative nurses must be up to date on the QT interval. The article reviews the physiology and measurement of the QT interval, the risk factors of QT prolongation, the mechanism of drug-induced QT prolongation, and perioperative considerations for patient care.
Keywords: QT prolongation, torsade de pointes, perioperative care, drugs
Prolongation of the QT interval may lead to fatal ventricular arrhythmias such as torsade de pointes, which is associated with cardiac arrest.1 In the perioperative setting, the incidence of cardiac arrest due to torsade de pointes is relatively low;2 however, its occurrence is devastating to the patient, family, and health care providers. Risks factors of torsade de pointes include underlying coronary artery disease, congenital QTc prolongation, electrolyte imbalance, advanced age, and hepatorenal disease, among others.2,3 Patients with these risk factors frequently present for surgery, and the risk of torsade de pointes increases with the number of risk factors. The risk of torsade de pointes is higher in the perioperative setting because of the combination of preexisting risk factors, electrolyte shifts due to fluid replacement, and the use of multiple medications that can induce QT prolongation.
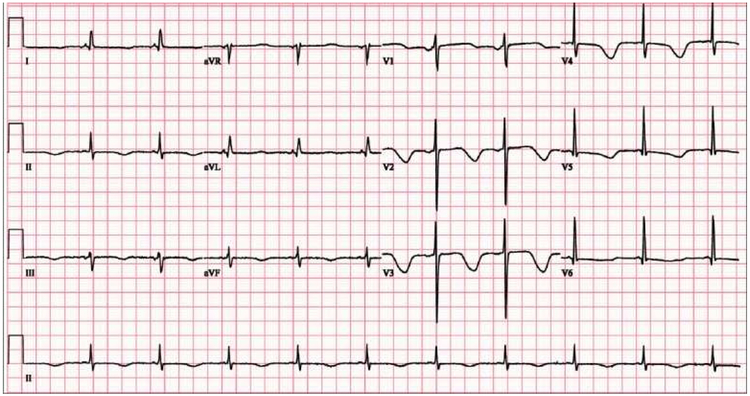
Drug-induced prolongation of the QT interval has been cited as the most common cause of withdrawal or restriction of the usage of marketed drugs.4,5 However, many medications that are associated with the prolongation of the QT interval are still in clinical use. Figure 1 shows the electrocardiograph (ECG) of a patient who develop a prolonged QTc interval “due to a combination of clarithromycin use, hypomagnesemia, and hypocalcemia.”6 Given that a patient’s ECG is continuously monitored in the perioperative setting, it is possible to detect changes in the QT interval and prevent impending fatal arrhythmias.7 Under contract with the Food and Drug Administration (FDA), CredibleMEds is a non-profit 501c3 organization that maintains an up-to-date list of medications that have a risk of ventricular arrhythmias and QT prolongation.8 As the list of drugs that induce QT prolongation grows, and more research emerges about risk factors of QT prolongation, anesthesia providers and perioperative nurses must be knowledgeable about QT prolongation to prevent potentially fatal arrhythmias.
Figure 1.
ECG shows sinus rhythm with deep T wave inversion and prolongation of the QTc interval.
Reproduced with permission. Ambhore A, Teo SG, Omar AR, Poh KK. Importance of QT interval in clinical practice. Singapore Medical Journal. 2014; 55(12):607-612
The purpose of this review is to educate anesthesia providers and perioperative nurses about the risk, ECG monitoring, and prevention of drug-induced QT prolongation. To achieve these goals, we first discuss the physiology and measurement of the QT interval. Second, we describe the genetic and non-genetic risk factors of QT prolongation. Third, we examine the list and mechanism of action of commonly used drugs that induced QT interval prolongation. Finally, we discuss preoperative, intraoperative, and postoperative considerations for patients with known risk factors of QT prolongation.
What is the QT interval?
The electrical activity of the heart is generated by the back-and-forth movement of ions across the cell membrane of cardiac cells. The movement of ions, which is regulated by the autonomic nervous system, results in four interrelated characteristics: chronotropy (automatic generation of rhythm, sometimes referred to as heart rate), dromotropy (impulse conduction), bathmotropy (excitation of cardiac myocytes), and ionotropy (contraction of myocardium, sometimes referred to as contractility).9 Clinically, this movement of ions and the resultant electrical activity is recorded by placing electrodes on the surface of the body, known as an electrocardiogram (ECG).
Appropriate lead placement is essential to generate a reliable ECG tracing that reflects the current electrical activity of the heart. Clinically, the ECG is frequently generated with three (3) electrodes, five (5) electrodes, or twelve (12) electrodes.10 The 12-lead ECG is recognized as the gold standard for accurate diagnosis and treatment of cardiac arrhythmias and dysrhythmias and should be used for the accurate measurement of the QT interval. 10,11 However, it may not be clinically feasible to continuously monitor all patients in the perioperative setting with 12 leads. Thus, these authors strongly advocate the routine use of the five-lead (rather than the 3-lead) monitor during the perioperative period. In the 5-lead monitor, four electrodes are placed around the heart on the torso corresponding to the right arm (RA), right foot (RF), left arm (LA) and left foot (LF). The fifth electrode (also known as precordial or V lead) may be adjusted to various V1 to V6 locations, depending on the monitoring needs.11 However, in adults, placement on the V1 location (fourth intercostal space and right sternal border) is recommended for the monitoring of ventricular arrhythmias.11,12
A complete evaluation of the ECG should include the measurement and interpretation of the QT interval because of the risk of life-threatening arrhythmias associated with changes in the QT interval.10 The QT interval is the time from the beginning of the QRS complex to the end of the T-wave (Figure 2). This period represents atrial repolarization, ventricular depolarization, and ventricular repolarization. Atrial repolarization usually occurs during ventricular depolarization, which is recorded as the QRS complex. Ventricular depolarization is initiated when voltage-gated fast sodium channels open, causing a rapid influx of sodium ions (Na+) into the cardiac myocytes along a concentration gradient. During the rapid depolarization, slow voltage-gated calcium channels also open, allowing the slow influx of Ca2+ ions. This rapid depolarization is often described as Phase 0 of the ventricular action potential. Phase 1 is characterized by the closure of the fast sodium channels, which terminate the influx of Na+ ions, and the transient efflux of potassium ions (K+). During Phase 2 (the Plateau phase), slow Ca2+ continues to enter the cell to offset the decrease in polarization caused by the efflux of K+. The ventricles finally repolarize (Phase 3) when the efflux of K+ exceeds the influx of Ca2+. Following the ventricular repolarization, the cells enter the resting membrane potential phase (Phase 4) during which the energy (via the Na+/K+ ATPase pump) is used to re-establish the resting membrane potential.9 The essential role played by sodium, potassium, and calcium channels is evident by the fact that mutations that affect these channels affect the duration of ventricular depolarization and repolarization (QT-interval).
Figure 2.
Measurement of the QT interval
The rate of ventricular depolarization and repolarization determines the heart rate. Thus, an accurate measurement of the QT interval must adjust the variability of the heart rate. This adjusted or corrected heart rate is known as the corrected QT interval (QTc).10 The formulae that are used for calculating the QTc include the Bazetts formula, the Hodges correction, the Fridericia exponential correction, and the Framingham method.10,13 Of these methods, the Fridericia and Framingham QTc correction methods have been shown to more accurately predict the 30-day and 1-year mortality in patients with QTc prolongation.13
Where: QT = QT interval (ms); RR = interval between two successive R-waves (ms); QTc = Corrected QT interval (ms)
QTc values between 350 and 450 ms for males 360 and 460 ms for females is considered normal.10 According to the American Heart Association (AHA), recommendations for the Standardization and Interpretation of the Electrocardiogram, a QTc ≥450 ms (males) and ≥460 ms (females) “be considered a prolonged QT interval.”14
Risk factors for QT prolongation
Delayed cardiac repolarization (measured as a prolonged QTc interval) can be the result of genetic (usually congenital) or non-genetic (acquired) factors. Irrespective of their classification (genetic versus non-genetic), all factors that prolong the QTc interval can lead to ventricular arrhythmias and sudden cardiac arrest.3 While acquired (drug-induced) LQTS is the primary focus of this paper, it is essential to recognize that patients with congenital LQTS have a higher risk for ventricular arrhythmias, or sudden cardiac arrest, if exposed to a drug that prolongs the QT interval.1,15 Thus, a good knowledge of these risk factors may prevent cardiovascular deaths in a perioperative setting.
Genetic risk factors of QTc prolongation
Contemporary research has shown that genetic differences play an important role in the structure and function of ion channels and ventricular myocardium. At the molecular level, proteins such as sodium ion channels, potassium ion channels, and myocardium are coded by genes. As such genetic mutations may result in structural and functional alterations in the resultant protein. About 1 in 2000 individuals are born with long QT syndrome (LQTS), a potentially life-threatening cardiac arrhythmia that predisposes them to delayed repolarization of the ventricular myocardium.16 LQTS is inherited primarily as an autosomal dominant trait, and to a lesser extent as an autosomal recessive trait. In addition, up to 10 percent of LQTS results from spontaneous de novo germline mutations.16,17 Genetic variants in at least 17 genes that code for various proteins, such as ion channels, have been identified in patients with LQTS. About three-quarters of congenital LQTS is associated with three (3) genes that code for rapidly (IKr) and slowly (IKs) activating rectifier potassium and sodium channels: KCNQ1, KCNH2, and SCN5A.16,18 These potassium and sodium channels play a crucial role in ventricular depolarization and repolarization.
Potassium channels are a superfamily of voltage-gated six transmembrane channel made up of a voltage-sensitive domain and a pore domain.19 The KCNQ1 gene is located on chromosome 11 (11p15.5) and codes for the α subunit of the IKs potassium channel (KvLQT1, Kv7.1). Genetic variation of the KCNQ1 gene has been associated with variability in the functions of the slow K+ pore seen in LQTS type I.16,17,19 Similarly, the human Ether-à-go-go-Related (hERG) gene is located on chromosome 7 (7q35-36) and encodes the IKr potassium channel (hERG).16,18 Variations in the hERG gene (is also known as KCNH2 or Kv11) has been associated with the alterations in cardiac repolarization seen in LQTS type 2.19,20 Overall, loss of function mutation in the KCNQ1 and KCNH2 genes have been associated with a decrease in potassium conduction that results in the prolongation of the action potential.18
Like potassium channels, sodium channels are a superfamily of transmembrane voltage sensing and pore-forming channels. The SCN5A gene encodes the pore-forming α subunit, Nav1.5, that regulates sodium conduction during atrial, ventricular, and Purkinje fiber depolarization.21 On the other hand, a gain of function mutation in the SCN5A gene (that codes for) increases sodium conduction across the myocardium.16,21 Both decrease potassium conduction and increase sodium (and calcium to some extent) conduction, resulting in the prolongation of the QT interval on the ECG. Inheritance of these genetic mutations alone is not diagnostic of LQTS. A patient must have one of the LQTS mutant genes, show a QTc ≥ 500 ms, and possess a Schwartz score of ≥ 3.5 without an additional cause for QT prolongation before he or she can be diagnosed with clinical LQTS.22 Schwartz and Ackerman22 provide an excellent review of a clinical approach to diagnose and treat a patient suspected of having congenital LQTS.22
Non-genetic risk factors
In 2010, Drew and colleagues published a comprehensive discussion on the risk factors and strategies for preventing drug-induced LQTS and torsade de pointes in hospitalized patients.1 Their paper raised awareness about patient-specific and non-patient-specific factors that increase the risk for LQTS. More recently, Vandael and colleagues published a systematic review that identified over 20 non-genetic risk factors of QTc prolongation.23 These factors include the patients’ demographic factors, smoking, electrolyte imbalances, cardiovascular disease, co-comorbidities, and drugs.1,23 Many studies have shown that advanced age and the female sex are associated with an increased risk of QT prolongation and torsade de pointes.1,23-25 A recent study of 'healthy' smokers versus non-smokers found that smoking a single cigarette prolongs QT dispersion significantly, which is a strong predictor of ventricular arrhythmias.26 Vandael and colleagues also reported strong evidence that smoking, being over the age of 65 years, as well as the female gender, are associated with a higher risk of QTc prolongation.23
Given that an optimal electrochemical balance is required for normal cardiac function, electrolyte imbalances can increase the patient's risk of QTc prolongation. There is solid evidence linking hypokalemia (serum K+ levels less than 3.5 mmol/L) to QTc prolongation and torsade de pointes.23,24 The common causes of hypokalemia include the administration of diuretics (e.g., furosemide), high levels of circulating epinephrine (sustained sympathetic nervous system activation), and the activation of the renin-angiotensin-aldosterone pathways. Studies in animal models suggest that hypokalemia-induced QT prolongation results from a decrease in the K+ electrochemical gradient across the cardiac myocytes, reduction in the efflux of potassium through the Ikr channels, and delayed depolarization.27 Diuretic-induced reduction in the serum potassium level is often associated with a reduction in the magnesium level. Hypomagnesemia (serum Mg2+ less than 1.7mg/dL) slows the activity of the Na+-K+ ATPase pump, with resultant exacerbation of the hypokalemic prolongation of the QTc interval. Clinically, hypomagnesemia is frequently associated with torsade de pointes.23,24,27 Other causes of QTc prolongation and torsade de pointes include hypocalcemia, hypothermia, extreme bradycardia, apheresis/blood transfusion, carbon monoxide, hyperparathyroidism, and hypothyroidism.3,7,8,23
Besides the diuretic-induced hypokalemia, many medications administered during the perioperative period have been associated with QTc prolongation. The Arizona Center for Education and Research (AZCERT) maintains an updated list of all medications known to have the risk of QT interval prolongation and torsade de pointes.8 Clinicians are advised to check this site frequently for the updates on the list. Table 1 summarizes the use and alternatives of some of the medications that are frequently used in the perioperative setting. The blockade of the voltage-gated potassium channel plays an important role in the pathopharmacology of most drug-induced QTc prolongations. 7 Some of these medications, such as opioids, anesthetics, and anti-emetics, deserve additional discussion because of their central role in perioperative care.
Table 1.
Perioperative medications with the known risk of QTc prolongation and suggested alternatives
| Drug (Brand Name) | Classification | Perioperative Use | Suggested Alternatives |
|---|---|---|---|
| Albuterol& | Bronchodilator | Asthma, bronchoconstriction, and bronchospasm | Ipratropium, Lidocaine, Fluticasone, Budesonide, Beclomethasone Ketamine# |
| Azithromycin*& | Antibiotic | Bacterial infection | Doxycycline |
| Chlorpromazine*& | Antiemetic | PONV | Dexamethasone |
| Ciprofloxacin*& | Antibiotic | Bacterial infection | Doxycycline |
| Cocaine*& | Local anesthetic | Topical anesthetic | Lidocaine |
| Dexmedetomidine &# | α2 adrenergic agonist | Sedation, anxiety, pain, and shivering | Midazolam, Clonidine |
| Droperidol*& | Anti-emetic | PONV | Dexamethasone |
| Ephedrine& | Vasoconstrictor | Hypotension | Vasopressin or fluids |
| Epinephrine& | Vasoconstrictor | Hypotension | Vasopressin or fluids |
| Erythromycin*& | Antibiotic | Bacterial infection | Doxycycline |
| Famotidine&# | H2-receptor antagonist | Gastroesophageal reflux disease (GERD) | Ranitidine |
| Furosemide&# | Loop diuretic | Hypertension and fluid overload | Aldactone |
| Haloperidol*& | Anti-emetic | PONV | Dexamethasone |
| Hydrochlorothiazide&# | Diuretic | Hypertension and fluid overload | Aldactone |
| Levofloxacin*& | Antibiotic | Bacterial infection | Doxycycline |
| Methadone*& | Opioid agonist | Pain and opioid use disorder | Fentanyl, Remifentanil, Alfentanil |
| Metoclopramide &# | Anti-emetic and Prokinetic | PONV and GERD | Dexamethasone |
| Nicardipine# | Calcium channel blocker | Hypertension | Esmolol |
| Ondansetron*& | Anti-emetic | PONV | Dexamethasone |
| Oxytocin &# | Oxytonic | Uterine atony and stimulate uterine contraction | Carboprost, Methylergonovine |
| Phenylephrine& | α1 adrenergic agonist | Hypotension | Vasopressin or fluids |
| Propofol*& | Intravenous anesthetic | Induction and maintenance of general anesthesia | Midazolam, Fentanyl, Remifentanil, Morphine, Etomidate# Ketamine# |
| Sevoflurane *& | Volatile anesthetic | Induction and maintenance of general anesthesia | Isoflurane |
| Succinylcholine | Neuromuscular blocking agent | Muscle relaxation | Rocuronium, Vecuronium, Cisatricurium |
| Terbutaline& | Bronchodilator | Asthma | Ipratropium bromide, Lidocaine |
= drugs with known risks of TdP;
= drugs to avoid in patients with congenital LQTS;
= drugs with a possible risk of TdP (exercise caution). PONV = post-operative nausea and vomiting, GERD = gastro-esophageal reflux disease
Opioids are used to treat acute and chronic pain. Most opioids exert their analgesic effect in the central nervous system via the mu, kappa, and delta receptors. These opioid receptors are also located in other organs, such as the heart, where the binding of the opioid results in a decrease in cardiac contractility.28 Most opioids do not affect cardiac conduction; in fact, a high dose opioid technique is recommended for the induction of anesthesia in patients with cardiac diseases. However, a 2018 systematic review of the effects of opioids on the QT interval found that some opioids carry a risk of QTc prolongation and torsade de pointes.29 Notably, methadone (a synthetic mu receptor agonist) appears to have the highest risk of QT prolongation and the induction of arrhythmias. Even low doses of methadone increase the risk of QT prolongation, and the risk increases with the dose.29 In addition, buprenorphine (a partial mu agonist, kappa receptor antagonist, and delta receptor agonist) has been shown to increase the risk of QTc prolongation in a dose-related manner.28-30 Other non-opioid analgesics that are known to increase the QT interval include Ketorolac and Tramadol. Ketorolac (Toradol) is a non-steroidal anti-inflammatory drug that inhibits the cyclooxygenase enzyme and also prolongs cardiac repolarization30 while Tramadol is a weak synthetic mu receptor agonist that can cause serotonin syndrome and associated cardiac arrhythmias.29
Intravenous and volatile anesthetics are used to induce a state of unconsciousness for patients undergoing various painful procedures. It is known that most volatile (inhalational) and intravenous anesthetics have myocardial depressant properties. All volatile anesthetics (sevoflurane, isoflurane, and desflurane) that are commonly used in the United States have the potential to increase the QTc interval.31,32 Both animal and human studies have shown that sevoflurane, isoflurane, and desflurane inhibit the Iks channel in a dose-dependent manner.32 Inhibition of the Iks has been implicated in drug-induced torsade de pointes. Among the intravenous anesthetics, Propofol has been shown to inhibit the Iks (IhERG) channel and induce torsade de pointes.33 A retrospective study of over 680,000 patients reported that Propofol administration was associated with a torsade de pointes incidence rate of 1.93 per million.34 However, a 2014 review noted that many studies had not found any significant association between Propofol, QTc interval, and torsade de pointes. In fact, some investigators have suggested that Propofol may shorten the QT interval and reverse the inhibitory effects of volatiles on the K+ channels. 32
During the perioperative period, antiemetics are frequently administered to prevent and treat postoperative nausea and vomiting. The US Food and Drug Administration (FDA) has a black box with and drug safety warning on droperidol and ondansetron, respectively, because of QT interval prolongation risk.35,36 The updated communication on ondansetron warns against the use of the 32mg single dose.35 Ondansetron, a 5-hydroxytryptamine receptor antagonist, is one of the most frequently used medications for antiemetic prophylaxis. Many studies have shown that a single dose of ondansetron can increase the QTc interval, especially when used in patients with underlying cardiac disease.37,38 In 2017, Lee and colleagues reported 2 cases of patients who developed torsade de pointes following the administration of several doses of ondansetron.39 One of the patients became unresponsive and pulseless, requiring chest compressions after 36mg of intravenous ondansetron, while the other had a slight increase in troponin level with non-ischemic changes on ECG.39 A retrospective chart review of patients who underwent various surgical procedures did not find any statistically significant increase in QTc interval, following the perioperative administration of ondansetron or dolasetron.36 Similarly, a randomized, double-blind control trial of patients undergoing non-cardiac surgery did not find any significant increase in ventricular repolarization, following the perioperative administration of the standard doses of ondansetron (4mg) and droperidol (2.5mg)40 These findings and others have called to question the FDA’s warning on droperidol and ondansetron’s risk for QTc prolongation and torsade de pointes.36,40,41 However, current clinical guidelines recommend the avoidance of droperidol and ondansetron in patients with cardiac disease and congenital LQTS.8 Other antiemetic medications that have been reported to increase QTc interval and the risk of torsade de pointes are haloperidol and metoclopramide.30 Both haloperidol and metoclopramide are dopamine receptor antagonists. A recent meta-analysis of randomized controlled trials found that there are no significant differences between haloperidol and 5-hydroxytryptamine receptor antagonists with regard to antiemetic effectiveness and incidence of QTc prolongation.42
Considerations for perioperative care
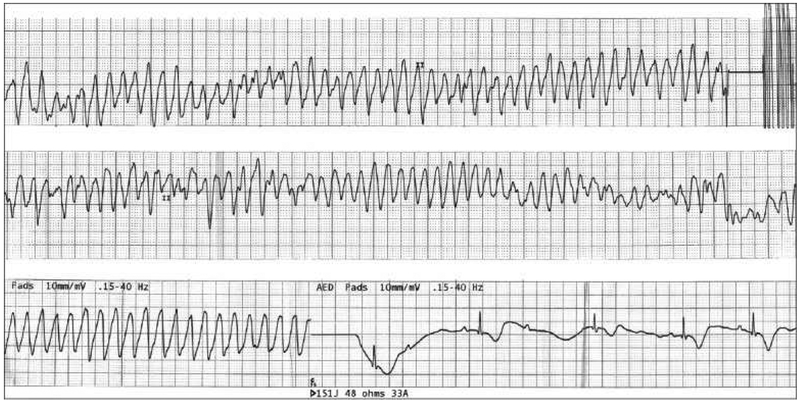
Patients with LQTS have a significant risk for morbidity and mortality during the perioperative period because of abnormal ventricular repolarization, and perioperative health providers (especially Registered Nurses, Certified Registered Nurse Anesthetists, and Anesthesiologists) must be aware of medications that can increase this risk. There are no consensus guidelines for patients with LQTS. However, careful considerations may prevent fatal ventricular arrhythmias and potential morbidity and mortality. Figure 3 shows the ECG of a patient who developed torsades the pointes and successively degenerated into ventricular fibrillation, which was cardioverted with 150 joules of direct current.6
Figure 3.
ECG of a patient who developed torsade’s de pointes and successively degenerated into ventricular fibrillation, and was cardioverted with 150 joules of direct current
Reproduced with permission. Ambhore A, Teo SG, Omar AR, Poh KK. Importance of QT interval in clinical practice. Singapore Medical Journal. 2014; 55(12):607-612
Preoperative considerations
Patients with congenital LQTS or cardiac disease or those taking medications that can prolong the QTc interval require careful preoperative evaluation. Ideally, medications that can prolong the QT interval or increase the risk of torsade de pointes should be avoided. However, given the long list of medications that present this risk, complete avoidance may be nearly impossible.
Preoperative assessment should include a thorough assessment of the patient’s cardiac history. For patients with a history of congenital LQTS, a baseline 12-lead ECG should be obtained. If the QTc > 500ms the cause of the QT prolongation should be investigated further. Such an investigation may require consultation with a cardiologist. For patients taking antiarrhythmic medications and undergoing major surgical procedures that are associated with significant blood loss and fluid shifts, a cardiologist should be consulted for preoperative optimization.
Preoperative laboratory tests, especially serum electrolytes, should be assessed to obtain baseline values. Any electrolyte imbalances should be corrected preoperatively. Hypomagnesemia, hypokalemia, and hypocalcemia are associated with QTc prolongation and should be corrected immediately. For patients with LQTS, it has been suggested that preoperative serum potassium and magnesium levels should be >4.5 mmol/L and >2.0mg/dL, respectively.15,43 Given that smoking has been associated with an increase in arrhythmogenicity, the patient should be counseled to stop smoking prior to the surgery.26 Besides the medications discussed in this paper, many other drugs (such as antipsychotics and antiarrhythmics) can increase the risk of QTc prolongation and torsade de pointes. Hence, caution must be exercised when administering any medications that can prolong the QTc interval or induce arrhythmias.
Intraoperative Considerations
Most patients undergoing surgery under general anesthesia and monitored anesthesia care will most likely receive at least one medication that has been reported to increase the QTc interval. As a result, intraoperative care should include continuous monitoring by means of an ECG. The printing of a baseline strip before the induction of general anesthesia is a good clinical practice that may provide a quick point-of-care reference.
Stress and anxiety increase the risk of arrhythmias, especially in patients with LQTS.44 Midazolam is a safe anxiolytic.8 The induction of anesthesia should occur in a calm, quiet environment. Propofol, etomidate, ketamine, and thiopental may induce QTc prolongation.33,45,46 The technique of using a high dose of opioid (e.g., Fentanyl or Morphine) may be a safer alternative to propofol. Hypotension should be treated with balanced solutions, such as lactated ringers, and the use of phenylephrine, ephedrine, and epinephrine should be avoided in high-risk patients. Intraoperative blood loss and fluid replacement therapy may increase the electrolyte imbalances, such as hypocalcemia, hypokalemia, hypomagnesemia, and hypernatremia.23,24,27,47 The close monitoring of electrolytes and the prompt replacement of any deficits are recommended.15,43
Another intraoperative consideration is the avoidance of drug-drug interactions that increase the risk of QTc prolongation.48 As depicted in Table 1, sevoflurane, which is frequently used for the maintenance of anesthesia, should be avoided in patients with LQTS.8,31 Similarly, succinylcholine (a commonly used depolarizing neuromuscular blockade) should be avoided because it can prolong the QT interval. Despite the need to ensure adequate pain management in the perioperative period, methadone, dexmedetomidine, and levo-alpha-acetylmethadol should be avoided in high-risk patients.29 Unfortunately, no studies have examined the effect of polypharmacy on the risk of QTc prolongation or torsade de pointes, especially in high-risk patients. However, it would be good clinical judgment to assume that the administration of multiple drugs that increase the QTc interval would increase the probability that a patient would develop a potentially fatal ventricular arrhythmia, such as torsade de pointes or ventricular fibrillation.
Postoperative Considerations
During the postoperative period, the nurse and providers should continue to monitor the patient’s ECG closely. The QT interval should be assessed frequently until fully normalized. A 12 lead ECG should be obtained if the patient develops an arrhythmia or if the QTc interval is ≥ 500ms.3 In addition, cardiology services should be consulted for patients who QTc prolongation does not resolve spontaneously, or who develop any tachyarrhythmia or sustained QTc prolongation into the postoperative period. Electrolyte imbalances should be identified and corrected promptly. If a patient with congenital LQTS or cardiac disease develop PONV, caution must be exercised when administering ondansetron,36,39 droperidol,40 haloperidol, and metoclopramide.30 If any of these medications are administered, the patient must be closely monitored through ECG, and escalating doses must be avoided because the risk of QTc prolongation is dose-dependent. Non-opioid analgesics (e.g., acetaminophen) and opioids (e.g., fentanyl) that do not increase the risk of QTc prolongation should be used for postoperative pain control. For patients with opioid use disorder who require methadone maintenance therapy, close monitoring of the ECG and caution must be exercised. Postoperative discharge should be delayed until resolution of symptoms and normalization of QTc interval. In addition, discharge teaching should include education about QTc prolongation, and signs and symptoms of arrhythmias, such as chest pain, palpitations, dizziness, shortness of breath, lightheadedness, presyncopal or syncopal episodes, and/or any unusual feeling.3 The patient should be instructed to contact their primary care provider or consult a health care provider should any of these symptoms occur. Overall, critical thinking and good clinical judgment are essential for the management of patients with drug-induced QTc prolongation.
Conclusion
Drug-induced prolongation of the QTc interval increases a patient’s risk for perioperative morbidity and mortality, yet many perioperative health care providers may not be aware of this risk. The key recommendation is to avoid drugs that may increase the QTc interval, especially in patients with congenital LQTS and cardiac disease. Volatile and intravenous anesthetics, opioids, and antiemetic medications are among the medications that must be used with caution. Knowing that perioperative fluid shifts and associated electrolyte imbalance can exacerbate QTc prolongation, prompt correction of electrolyte imbalances is essential for the prevention of potentially lethal arrhythmias, such as torsade de pointes, during the perioperative period. Medications that may cause QT prolongation are common in the perioperative period, and these drugs should be identified by the nurses and anesthesia provider to prevent a potentially life-threatening ventricular dysrhythmia. Finally, patients’ identified to have LGTS should be educated about signs and symptoms of arrhythmias and instructed to contact a health care provider immediately, when these signs and symptoms occur.
Acknowledgement:
We are immensely grateful to Lisa Jean, CRNA for her comments on an earlier version of the manuscript.
Financial Support: Edwin Aroke is supported by an Administrative Supplement Grant from the National Institute on Minority Research and Health Disparities/National Institutes of Health.
Footnotes
Conflict of interest: The authors have declared no conflicts of interest for this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: A scientific statement from the american heart association and the american college of cardiology foundation endorsed by the american association of critical-care nurses and the international society for computerized electrocardiology. J Am Coll Cardiol. 2010;55(9):934–947. doi: 10.1016/j.jacc.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston J, Pal S, Nagele P. Perioperative torsade de pointes: A systematic review of published case reports. Anesth Analg. 2013;117(3):559–564. doi: 10.1213/ANE.0b013e318290c380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinkley KE, Lee Page R, Lien H, et al. QT interval prolongation and the risk of torsades de pointes: Essentials for clinicians. Curr Med Res Opin. 2013;29(12):1719–1726. doi: 10.1185/03007995.2013.840568 [DOI] [PubMed] [Google Scholar]
- 4.van Noord C, Eijgelsheim M, Stricker BHC. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70(1):16–23. doi: 10.1111/j.1365-2125.2010.03660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013–1022. doi: 10.1056/nejmra032426 [DOI] [PubMed] [Google Scholar]
- 6.Ambhore A, Teo SG, Bin Omar AR, Poh KK. Importance of QT interval in clinical practice. Singapore Med J. 2014;55(12):607–611; quiz 612. doi: 10.11622/smedj.2014172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639–1650. doi: 10.1016/j.jacc.2015.12.063 [DOI] [PubMed] [Google Scholar]
- 8.QT drug list. AZCERT, Inc; 2019. https://www.crediblemeds.org/index.php/?cID=328. Accessed February 19 2019. [Google Scholar]
- 9.Heerdt PM, Crystal GJ. Cardiovascular physiology: Cellular and molecular regulation In: Hemmings HC, Egan TD, eds. Pharmacology and physiology for anesthesia: Foundations and clinical application. Philadelphia, PA, USA: Elsevier Saunders; 2013. [Google Scholar]
- 10.Postema PG, Wilde AA. The measurement of the QT interval. Curr Cardiol Rev. 2014;10(3):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis J Ecg monitoring leads and special leads. Indian Pacing Electrophysiol J. 2016;16(3):92–95. doi: 10.1016/j.ipej.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: A scientific statement from the american heart association. Circulation. 2017;136(19):e273–e344. doi:doi: 10.1161/CIR.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 13.Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc. 2016;5(6):e003264. doi: 10.1161/JAHA.116.003264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rautaharju PM, Surawicz B, Gettes LS. Aha/accf/hrs recommendations for the standardization and interpretation of the electrocardiogram. Circulation. 2009;119(10):e241–e250. doi:doi: 10.1161/CIRCULATIONAHA.108.191096 [DOI] [PubMed] [Google Scholar]
- 15.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 aha/acc/hrs guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2018;72(14):e91–e220. doi: 10.1016/j.jacc.2017.10.054 [DOI] [PubMed] [Google Scholar]
- 16.Tester DJ, Ackerman MJ. Genetics of long QT syndrome. Methodist DeBakey Cardiovasc J. 2014;10(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz Peter J, Crotti L, Insolia R. Long-QT syndrome. Circ Arrhythm Electrophysiol. 2012;5(4):868–877. doi: 10.1161/CIRCEP.111.962019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano Y, Shimizu W. Genetics of long-QT syndrome. J Hum Genet. 2015;61:51. doi: 10.1038/jhg.2015.74 [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Sampson KJ, Kass RS. Cardiac delayed rectifier potassium channels in health and disease. Card Electrophysiol Clin. 2016;8(2):307–322. doi: 10.1016/j.ccep.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin AS, Wilde AA. Genetic control of potassium channels. Card Electrophysiol Clin. 2016;8(2):285–306. doi: 10.1016/j.ccep.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Wilde AAM, Amin AS. Clinical spectrum of scn5a mutations: Long QT syndrome, brugada syndrome, and cardiomyopathy. JACC Clin Electrophysiol. 2018;4(5):569–579. doi: 10.1016/j.jacep.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz PJ, Ackerman MJ. The long QT syndrome: A transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34(40):3109–3116. doi: 10.1093/eurheartj/eht089 [DOI] [PubMed] [Google Scholar]
- 23.Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: Systematic review of the evidence. Int J Clin Pharm. 2017;39(1):16–25. doi: 10.1007/s11096-016-0414-2 [DOI] [PubMed] [Google Scholar]
- 24.Heemskerk CPM, Pereboom M, van Stralen K, et al. Risk factors for QTc interval prolongation. Eur J Clin Pharmacol. 2018;74(2):183–191. doi: 10.1007/s00228-017-2381-5 [DOI] [PubMed] [Google Scholar]
- 25.Castro VM, Clements CC, Murphy SN, et al. Qt interval and antidepressant use: A cross sectional study of electronic health records. BMJ. 2013;346:f288–f288. doi: 10.1136/bmj.f288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbarzadeh MA, Yazdani S, Ghaidari ME, et al. Acute effects of smoking on QT dispersion in healthy males. ARYA Atheroscler. 2014;10(2):89–93. [PMC free article] [PubMed] [Google Scholar]
- 27.Osadchii OE. Mechanisms of hypokalemia-induced ventricular arrhythmogenicity. Fund Clin Pharmacol. 2010;24(5):547–559. doi: 10.1111/j.1472-8206.2010.00835.x [DOI] [PubMed] [Google Scholar]
- 28.Chen A, Ashburn MA. Cardiac effects of opioid therapy. Pain Med. 2015;16(suppl 1):S27–S31. doi: 10.1111/pme.12915 [DOI] [PubMed] [Google Scholar]
- 29.Behzadi M, Joukar S, Beik A. Opioids and cardiac arrhythmia: A literature review. Med Princ Pract. 2018;27(5):401–414. doi: 10.1159/000492616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klivinyi C, Bornemann-Cimenti H. Pain medication and long QT syndrome. Korean J Pain. 2018;31(1):3–9. doi: 10.3344/kjp.2018.31.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huneke R, Fassl J, Rossaint R, Luckhoff A. Effects of volatile anesthetics on cardiac ion channels. Acta Anaesthesiolog Scand. 2004;48(5):547–561. doi: 10.1111/j.0001-5172.2004.00391.x [DOI] [PubMed] [Google Scholar]
- 32.Staikou C, Stamelos M, Stavroulakis E. Impact of anaesthetic drugs and adjuvants on ecg markers of torsadogenicity. Br J Anaesth. 2014;112(2):217–230. doi: 10.1093/bja/aet412 [DOI] [PubMed] [Google Scholar]
- 33.Han S-N, Jing Y, Yang L-L, Zhang Z, Zhang L-R. Propofol inhibits herg k+ channels and enhances the inhibition effects on its mutations in hek293 cells. Eur J Pharmacol. 2016;791:168–178. doi: 10.1016/j.ejphar.2016.08.028 [DOI] [PubMed] [Google Scholar]
- 34.Abrich VA, Ramakrishna H, Mehta A, Mookadam F, Srivathsan K. The possible role of propofol in drug-induced torsades de pointes: A real-world single-center analysis. Int J Cardiol. 2017. doi: 10.1016/j.ijcard.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration. Fda drug safety communication: New information regarding QT prolongation with ondansetron (zofran). In: Administration USFaD, ed2017. [Google Scholar]
- 36.Obal D, Yang D, Sessler DI. Perioperative doses of ondansetron or dolasetron do not lengthen the QT interval. Mayo Clin Proceed. 2014;89(1):69–80. doi: 10.1016/j.mayocp.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 37.Li K, Vo K, Lee BK, Addo N, Coralic Z. Effect of a single dose of i.V. Ondansetron on QTc interval in emergency department patients. Am J Health Syst Pharm. 2018;75(5):276–282. doi: 10.2146/ajhp161070 [DOI] [PubMed] [Google Scholar]
- 38.Hafermann MJ, Namdar R, Seibold GE, Page RL 2nd. Effect of intravenous ondansetron on QT interval prolongation in patients with cardiovascular disease and additional risk factors for torsades: A prospective, observational study. Drug Healthc Patient Saf. 2011;3:53–58. doi: 10.2147/DHPS.S25623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee DY, Trinh T, Roy SK. Torsades de pointes after ondansetron infusion in 2 patients. Tex Heart I J. 2017;44(5):366–369. doi: 10.14503/THIJ-16-6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agamez Medina GL, Gonzalez-Arevalo A, Gomez-Arnau JI, et al. Effects of droperidol and ondansetron on dispersion of ventricular repolarization: A randomized double-blind clinical study in anesthetized adult patients. Rev Esp Anestesiol Reanim. 2015;62(9):495–501. doi: 10.1016/j.redar.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 41.Tracz K, Owczuk R. Small doses of droperidol do not present relevant torsadogenic actions: A double-blind, ondansetron-controlled study. Br J Clin Pharmacol. 2015;79(4):669–676. doi: 10.1111/bcp.12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh PM, Borle A, Makkar JK, Trikha A, Fish D, Sinha A. Haloperidol versus 5-ht3 receptor antagonists for postoperative vomiting and QTc prolongation: A noninferiority meta-analysis and trial sequential analysis of randomized controlled trials. J Clin Pharmacol. 2018;58(2):131–143. doi: 10.1002/jcph.999 [DOI] [PubMed] [Google Scholar]
- 43.Sorajja D, Munger TM, Shen WK. Optimal antiarrhythmic drug therapy for electrical storm. J Biomed Res. 2015;29(1):20–34. doi: 10.7555/jbr.29.20140147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrassy G, Szabo A, Ferencz G, Trummer Z, Simon E, Tahy A. Mental stress may induce QT-interval prolongation and T-wave notching. Ann Noninvas Electrocardiol. 2007;12(3):251–259. doi: 10.1111/j.1542-474X.2007.00169.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oji M, Terao Y, Toyoda T, et al. Differential effects of propofol and sevoflurane on QT interval during anesthetic induction. J Clin Monit Comput. 2013;27(3):243–248. doi: 10.1007/s10877-012-9420-7 [DOI] [PubMed] [Google Scholar]
- 46.Kim DH, Kweon TD, Nam SB, Han DW, Cho WY, Lee JS. Effects of target concentration infusion of propofol and tracheal intubation on QTc interval. Anaesthesia. 2008;63(10):1061–1064. doi: 10.1111/j.1365-2044.2008.05564.x [DOI] [PubMed] [Google Scholar]
- 47.Ho KM, Leonard AD. Concentration-dependent effect of hypocalcaemia on mortality of patients with critical bleeding requiring massive transfusion: A cohort study. Anaesth Intens Care. 2011;39(1):46–54. doi: 10.1177/0310057x1103900107 [DOI] [PubMed] [Google Scholar]
- 48.Li EC, Esterly JS, Pohl S, Scott SD, McBride BF. Drug-induced QT-interval prolongation: Considerations for clinicians. Pharmacotherapy. 2010;30(7):684–701. doi: 10.1592/phco.30.7.684 [DOI] [PubMed] [Google Scholar]