Abstract
Background
The infant microbiome contributes to health status across the lifespan, but environmental factors affecting microbial communities are poorly understood, particularly when toxic and essential elements interact.
Objective
We aimed to identify the associations between a spectrum of other early-postnatal nutrient or toxic elemental exposures measured and the infant gut microbiome.
Methods
Our analysis included 179 six-week-old infants from the New Hampshire Birth Cohort Study. Eleven elements were measured in infant toenail clippings. The gut microbiome was assessed using 16S rRNA V4-V5 hypervariable region targeted sequencing. Multivariable zero-inflated logistic normal regression (MZILN) was used to model the association between element concentrations and taxon relative abundance. To explore interactive and nonlinear associations between the exposures and specific taxa we employed Bayesian Kernel Machine Regression (BKMR). Effect modification by delivery mode, feeding mode, peripartum antibiotic exposure, and infant sex was assessed with stratified models.
Results
We found a negative association between arsenic and microbial diversity in the full population that was accentuated among infants exposed to peripartum antibiotics. Arsenic, cadmium, copper, iron, lead, manganese, nickel, selenium, tin, and zinc were each associated with differences in at least one taxon in the full study population, with most of the related taxa belonging to the Bacteroides and Lactobacillales. In stratified analyses, mercury, in addition to the other elements, was associated with specific taxa. Bifidobacterium, which associated negatively with zinc in MZILN and BKMR models, had a quadratic association with arsenic concentrations. These associations varied with the concentration of the other element.
Conclusions
Early postnatal toxic and nutrient elemental exposures are associated with differences in the infant microbiome. Further research is needed to clarify the whether these alterations are a biomarker of exposure or if they have implications for child and lifelong health.
Keywords: Infant gut microbiome, 16S rRNA gene, Metals/metalloids, Elemental nutrients, Mixtures, Bayesian kernel machine regression
1. INTRODUCTION
For nearly a decade there have been calls to examine the interaction between environmental toxicants and the gut microbiome (Betts, 2011; Feingold et al., 2010), but most of the current research has been conducted in animal models and, with few exceptions (Gaulke et al., 2018; Guo et al., 2014; Yu et al., 2016), has explored the effects of individual chemicals (Chi et al., 2018, 2016; Rosenfeld, 2017). The literature is particularly sparse regarding studies of human infants. The neonatal gut microbiome is unstable until a balanced community is established (Lozupone et al., 2012; Palmer et al., 2007), and during this period may be especially sensitive to modifying factors such as environmental chemicals. Infants are exposed to an onslaught of exposures simultaneously, including metals and metalloids, collectively referred to as “metals” (Rauh et al., 2010; Stiemsma and Michels, 2018). Some exposures, including essential nutrients from breastmilk, are vital for infant growth and development, but others are harmful toxicants (Oskarsson et al., 1998; Rauh et al., 2010). Although some nascent work has examined how these elements interact to impact child health (Henn et al., 2014; Valeri et al., 2017), mixture methods have not yet been applied to the microbiome.
Many epidemiological and experimental studies have explored the detrimental effects of infant exposure to metals on growth (Sabra et al., 2017; Vrijheid et al., 2016), neurodevelopment (Rodríguez-Barranco et al., 2013; Vahter, 2008; Vrijheid et al., 2016), and gastrointestinal symptoms (Farzan et al., 2016; Rahman Anisur et al., 2011; Rivera-Dominguez and Castano, 2019). However, the mechanisms by which metals affect child health are not fully understood (Jaishankar et al., 2014), and the microbiome remains an underexplored target of these elements. As the most critical barrier organ, the intestines house the majority of the microbes in and on the human body (The Human Microbiome Project Consortium et al., 2012); toxicants directly impact microbes and are often metabolized by them. Our previous work found associations between six-week-old urinary arsenic and the gut microbiome (Hoen et al., 2018). Another recent paper described associations between organic compounds in breastmilk and the infant gut microbiome one month post-partum (Iszatt et al., 2019). However, these works did not consider the myriad of elements to which the gut is exposed concomitantly, especially at the taxon-level.
This study expands on our earlier findings of an association between arsenic and the infant gut microbiome by examining exposure to eleven trace elements concurrently in the neonatal period of 0–6 weeks of age. In addition to controlling for confounding by the other metals/nutrients, which are common co-exposures, we implemented novel statistical methods to allow for potential nonlinear and interactive associations between essential and toxic elements and the microbiome.
2. MATERIALS AND METHODS
2.1. Study cohort
Pregnant women (approximately 24–28 weeks gestation) ages 18–45 were recruited from New Hampshire prenatal clinics, as previously described (Gilbert-Diamond et al., 2011). Inclusion criteria for the original cohort (designed to examine the effects of drinking-water arsenic on infant health) included using a private, unregulated well at their residence since last menstrual period and no plans to move. Participants provided written and informed consent. All study protocols were reviewed and approved by the Center for the Protection of Human Subjects at Dartmouth.
2.2. Toenail and stool sample collection
At approximately two weeks postpartum, participants were sent materials to collect and return a clipping of their infant’s toenails. Infant toenails grow roughly 0.1 mm/day (Goullé et al., 2009) and are on average 3.2–5.7 mm in length at birth (Davis et al., 2014), and thus clippings reflect exposures approximately six to seven weeks prior, including the relevant window during which the naïve gut is colonized by bacteria. These peripartum exposures primarily result from transplacental transfer (Chen et al., 2014). Infant stools were collected as previously described (Madan et al., 2016). At a regularly scheduled postpartum visit (approximately six weeks postpartum), mothers were asked to bring in the infant toenail clipping sample and to collect an infant stool sample in a provided diaper and store it in their home freezer until they were able to return it to the study site (within 24 hours). Stool was thawed at 4°C so that it could be aliquoted into cryovial tubes containing RNAlater and homogenized before storing at −80°C.
2.3. Trace element/metal/metalloid analysis in infant toenails
Trace elements were measured in infant toenails as previously described (Punshon et al., 2016). Briefly, samples were digested with a low-pressure microwave method and subjected to inductively coupled plasma mass spectrometry (ICP-MS) to measure the concentration of sixteen metal/metalloid compounds (aluminum, vanadium, chromium, manganese, iron, nickel, copper, zinc, arsenic, selenium, molybdenum, cadmium, tin, antimony, mercury, and lead). Of these, we a priori identified manganese, iron, nickel, copper, zinc, arsenic, selenium, cadmium, tin, mercury, and lead as exposures of interest because toenail concentrations of these compounds are considered reproducible biomarkers of exposure (Garland et al., 1993), including in infant samples (Appleton et al., 2017; Davis et al., 2014; Maccani et al., 2015; Punshon et al., 2016). Mean recovery of standard reference material [National Institute for Environmental Sciences (Japan) Certified Reference Material No. 13, hair] was 92% (± 7%) and the average coefficient of variation between duplicates was 5% (all ≤ 7%). Any variability of precision in measurement is independent of outcome assessment and therefore likely biases any associations toward the null by introducing noise. Machine-reported concentrations below the limit of detection (LOD) were used (Whitcomb and Schisterman, 2008). Undetectable concentrations were imputed with the analyte-specific LOD/sqrt(2). For comparability, concentrations were log-transformed and z-scaled, and exposures were treated continuously.
2.4. Microbiome analysis in stool samples
As previously described (Madan et al., 2016), DNA was extracted from infant stool samples with the Zymo Fecal DNA extraction kit (Cat# D6010, Zymo Research, Irvine, CA), using the manufacturer’s instructions. For each extraction, 400ul RNAlater stool slurry (50–100mg of stool) was used to isolate DNA. Extractions were run in batches of multiple samples, including a composite RNAlater stool positive control and RNAlater negative control. Lysis was perform using 750ul Lysis Buffer in ZR BashingBead™ Lysis Tubes (0.5 mm), mixed and then shaken on a Disruptor Genie for 6 minutes. Eluted DNA was quantified on a Qubit™ fluorometer using the Qubit™ dsDNA BR Assay. Average coefficient of variation of DNA yields (ng/ul) for composite RNAlater stool positive controls was 28%. No DNA was ever detectable in negative control elutions. The V4-V5 hypervariable region of the 16S rRNA gene (Degnan and Ochman, 2012) was sequenced on an Illumina MiSeq at the Marine Biological Laboratory in Woods Hole, Massachusetts. Representative negative controls were submitted for Illumina v4v5 16S sequencing, but no reads were obtained. Details on quality control measures were described previously (Madan et al., 2016). Amplicon sequence variants (ASVs) were inferred using the DADA2 pipeline (Callahan et al., 2016) and taxonomies were assigned using the Greengenes classifier and reference dataset (DeSantis et al., 2006).
2.5. Statistical analysis
Our analysis was restricted to term (≥ 37 weeks gestational age) infants who had stool samples collected at approximately six weeks postpartum, whose toenail clippings were collected no more than 10 weeks after stool collection, and who had complete covariate data (Figure S1, n =179). Covariates were selected a priori or if they had previously been identified as predictors of the six-week microbiome in New Hampshire Birth Cohort Study (NHBCS) analyses (Coker et al., 2019; Hoen et al., 2018; Lundgren et al., 2018; Madan et al., 2016; Singh et al., 2019). These included feeding method, delivery mode, peripartum antibiotic exposure, infant sex, maternal fruit and dairy intake during pregnancy, and parity. Based on prior work, feeding method, delivery mode, peripartum antibiotic exposure, and infant sex were considered potential effect modifiers. Thus, analyses stratified on these factors were conducted. Statistical significance was defined as p < 0.05. All analyses were conducted in R 3.5.2 (R Core Team, Vienna, Austria).
Alpha (within-subject) diversity was calculated with the Shannon (1949) and Simpson (1949) Indices, and, because the exposures and outcome relations appeared linear, we used linear regression to model each metal/metalloid with the alpha diversity indices, adjusted for all other metal/metalloids and covariates. Beta diversity (community structure) was assessed using generalized UniFrac distances (Chen et al., 2012). Using the adonis2 function in the “vegan” package with 10,000 permutations, we tested the statistical significance of the contribution of each exposure to community structure while adjusting for all metal/metalloid exposures and covariates (considered significant if p < 0.05). Alpha and beta diversity supplemental analyses considered each exposure individually (not adjusting for other exposures) for comparison to mutually adjusted models. To identify ASV-specific associations each of the elements, we employed Multivariate zero-inflated logistic normal (MZILN) models (Li et al., 2018). MZILN statistically models zero-inflated and compositional relative abundance data using both discrete and continuous components to allow for zero-valued relative abundances (with a random taxon selected as the reference) as the outcome and uses ordinary least squares with a minimax concave penalty for estimation based on log-likelihood function. Given the predictive nature of this model, multiple corrections testing is neither appropriate nor necessary because we do not interpret significance or p-values. All exposures and relative abundance outcomes were included concurrently in the models, which were run 100 times; we used a threshold of having a non-zero estimate in at least 90 runs to ensure robustness of results. This method was shown to be insensitive to the reference taxon selected (Li et al., 2018), but in sensitivity analyses we ran these models with each taxon serving as the reference. MZILN, like all available methods for microbiome data, assumes a linear relationship between each exposure and outcome, and cannot account for correlations among exposures.
Finally, because we hypothesized that some elements may have nonlinear and/or interactive effects, we utilized Bayesian kernel machine regression (BKMR) with variable selection to flexibly model the association between the metals/metalloids and the top ten most abundant taxa and each metric of alpha diversity in the unstratified population (Bobb et al., 2015). BKMR exploits the (dis)similarity of subjects’ exposure profiles in a kernel machine to estimate the exposure-response function, from which one can examine cross-sections of this high-dimensional surface by setting all but one or two of the predictors to a fixed value. To better approximate a normal distribution of the outcome (taxon relative abundance), a pseudo count of 0.5 was added to zero-count data before calculating relative abundance and log transformation (Chen and Li, 2013). Using a Gaussian kernel with 30,000 iterations, we explored the nonlinear dose-response curve for each individual exposure when all other exposures were held at their median. Elements selected for inclusion in more than 50% of the iterations [posterior inclusion probability (PIP) > 0.5] were deemed to be significant contributors to the variability in the outcome. To examine whether any two elements interacted, we visually compared the dose-response curve for a given exposure when one other exposure was fixed at the 10th, 50th, or 90th percentile and all other exposures were fixed at the 50th percentile. After visually assessing each BKMR fitted univariate and bivariate exposure-response function, we confirmed and quantified our findings by applying the most appropriate traditional regression method.
3. RESULTS
3.1. Study participant characteristics
Of 1305 pregnant women enrolled in our study since stool collection began, we obtained a stool sample from 661 infants at approximately six weeks of age and performed 16S sequencing on 391 of these (Figure S1). We further obtained trace element data, including toxic metals/metalloids and nutrient elements, measured in toenail clippings at six weeks on 222 of these infants and complete exposure, outcome, and covariate data on 179. Mothers of infants included in our analysis were 60% parous and 93% were married (Table 1). Approximately half of infants were exposed to peripartum antibiotics and a quarter were born via Caesarean delivery (Table 1). Participants included in the analysis were more likely to have any higher education compared to those not included in the analysis (43% compared to 33%). Many element concentrations were positively correlated with one another (positive Spearman’s correlation coefficient range: 0.003, 0.87; Figure S2). Mercury was not correlated with other elements except for selenium, with which it had a weak positive correlation (Spearman’s correlation coefficient = 0.29). Exposure distributions were similar for individuals included in the analysis and those not included except for nickel and zinc, both of which had higher means in our analytical cohort (Table 1, Table S1).
Table 1.
Characteristics of New Hampshire Birth Cohort Study subjects [N (%) or Mean (± SD)] with infant toenail metal concentrations after stool collection began compared to those included in analyses
| Characteristic | Subjects with Toenail Metals (n = 458) | Included in Analysis (n = 179) | p* |
|---|---|---|---|
| Maternal Characteristics | |||
| Parity | 0.92 | ||
| Nulliparous | 182 (39.7) | 71 (39.7) | |
| Parous | 272 (59.3) | 108 (60.3) | |
| Education | < 0.05 | ||
| College Graduate or Less | 287 (62.6) | 102 (57.3) | |
| Any Higher Education | 153 (33.4) | 77 (42.7) | |
| Relationship Status | 0.59 | ||
| Married | 406 (88.6) | 167 (93.3) | |
| Never Married or Separated | 34 (7.4) | 12 (6.7) | |
| Fruit Intake (servings/day) | 2.4 (± 1.4) | 2.6 (± 1.5) | 0.10 |
| Dairy Intake (servings/day) | 3.7 (± 1.9) | 3.7 (± 1.9) | 0.46 |
| Delivery Characteristics | |||
| Delivery Mode | 0.46 | ||
| Vaginal | 331 (72.3) | 133 (74.3) | |
| Caesarean | 127 (27.7) | 46 (25.7) | |
| Peripartum Antibiotic Exposure | 0.56 | ||
| Any | 208 (45.4) | 87 (48.6) | |
| None | 236 (51.5) | 92 (51.4) | |
| Gestational Age (weeks) | 39.1 (± 1.6) | 39.4 (± 1.1) | 0.02 |
| Birth Weight (g) | 3470 (± 524) | 3527 (± 452) | 0.05 |
| Infant Characteristics | |||
| Feeding Mode | 0.68 | ||
| Exclusively Breast Fed | 238 (52) | 111 (62.0) | |
| Formula Fed or Mixed Fed | 153 (33.4) | 68 (38.0) | |
| Infant Sex | 0.34 | ||
| Male | 240 (52.4) | 99 (55.3) | |
| Female | 218 (47.6) | 80 (44.7) | |
| Toenail Metal at Six Weeks (μg/g) | |||
| Arsenic | 0.24 (± 0.78) | 0.25 (± 0.56) | 0.65 |
| Cadmium | 0.12 (± 0.33) | 0.14 (± 0.41) | 0.69 |
| Copper | 18.7 (± 44.9) | 23.3 (± 63.4) | 0.23 |
| Iron | 464.5 (± 2524.8) | 709.1 (± 3824.6) | 0.29 |
| Lead | 1.8 (± 4.5) | 2.2 (± 6.1) | 0.71 |
| Manganese | 7.4 (± 29.5) | 10.3 (± 41.5) | 0.35 |
| Mercury | 0.25 (± 0.75) | 0.21 (± 0.46) | 0.66 |
| Nickel | 918.9 (± 7636) | 1922.1 (± 12094) | 0.04 |
| Selenium | 2.2 (± 4) | 2.5 (± 4.6) | 0.09 |
| Tin | 1.7 (± 4.5) | 1.8 (± 4.9) | 0.45 |
| Zinc | 973.3 (± 4947.3) | 1376.3 (± 6051.1) | 0.03 |
| Microbial Diversity at Six Weeks | |||
| Shannon Index (unitless) | - | 1.73 (± 0.56) | |
| Simpson Index (unitless) | - | 0.69 (± 0.17) |
Comparing those included in analysis to those not included
3.2. Within-subject (alpha) diversity and community structure (beta diversity)
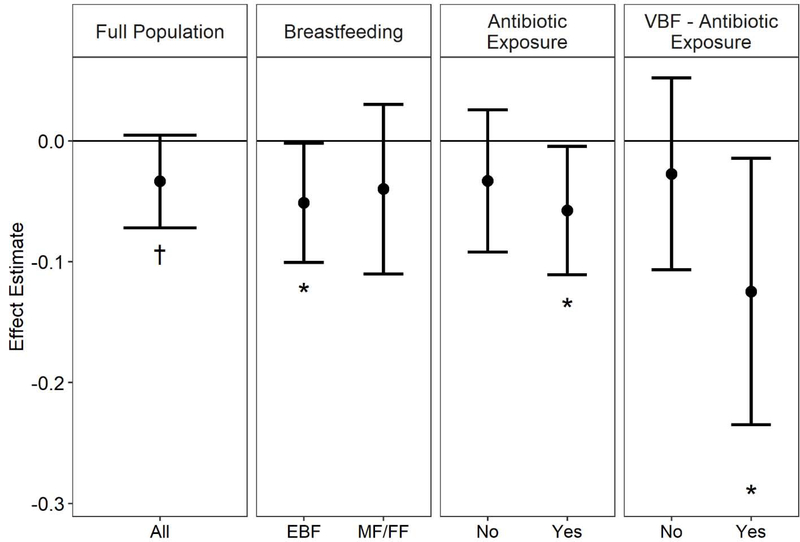
Infant toenail arsenic was marginally negatively associated with the Simpson Index, a measure of within-subject diversity [β = −0.03 per standard deviation increase in log arsenic concentration, 95% CI (−0.07, 0); Figure 1, Table S2]. This association was stronger and more statistically significant (smaller p-value) among infants who were exclusively breast fed [β = −0.05 per standard deviation increase in log arsenic concentration, 95% CI (−0.1, 0)], exposed to peripartum antibiotics [β = −0.06 per standard deviation increase in log arsenic concentration, 95% CI (−0.11, 0)], or vaginally delivered, exclusively breastfed and exposed to peripartum antibiotics [β = −0.12 per standard deviation increase in log arsenic concentration, 95% CI (−0.24, −0.01)]. Estimates were mostly similar in single-exposure models, but the negative association with arsenic among vaginally delivered, exclusively breastfed infants was not as apparent [β = −0.06 per standard deviation increase in log arsenic concentration, 95% CI (−0.14, 0.02); Table S3]. Although no association between arsenic and the Shannon Index was observed in the unstratified population, we again found stronger associations among infants exposed to peripartum antibiotics, particularly those who were delivered vaginally and exclusively breastfed (Table S4). Additionally, a negative association was observed between mercury and the Shannon Index among infants fed any formula [mixed fed or exclusively formula fed, β = −0.15 per standard deviation increase in log mercury concentration, 95% CI (−0.27, −0.02)]. For the most part, estimates from single-exposure models were encompassed in the confidence intervals from multi-exposure models (Table S5). BKMR indicated that no element contributed to either metric of alpha diversity (PIPs < 0.5; Table S6). No significant associations were found between nutrients/metals and beta diversity, measured with generalized UniFrac distances in the unstratified population in either multi- or single-exposure analyses (Table S7–8). Cadmium was a marginal contributor to community structure among infants who were not exclusively breastfed in multi- but not in single-exposure models.
Figure 1.
Association between log-transformed, z-centered infant toenail arsenic exposure and Simpson Index within-subject diversity in the full population and stratified by relevant covariates, adjusting for all other nutrient/metal/metalloid exposures and covariates concurrently. EBF: exclusively breast fed. MF/FF: mixed fed/formula fed. VBF: vaginally delivered and exclusively breast fed.
3.3. Specific-taxon relative abundance
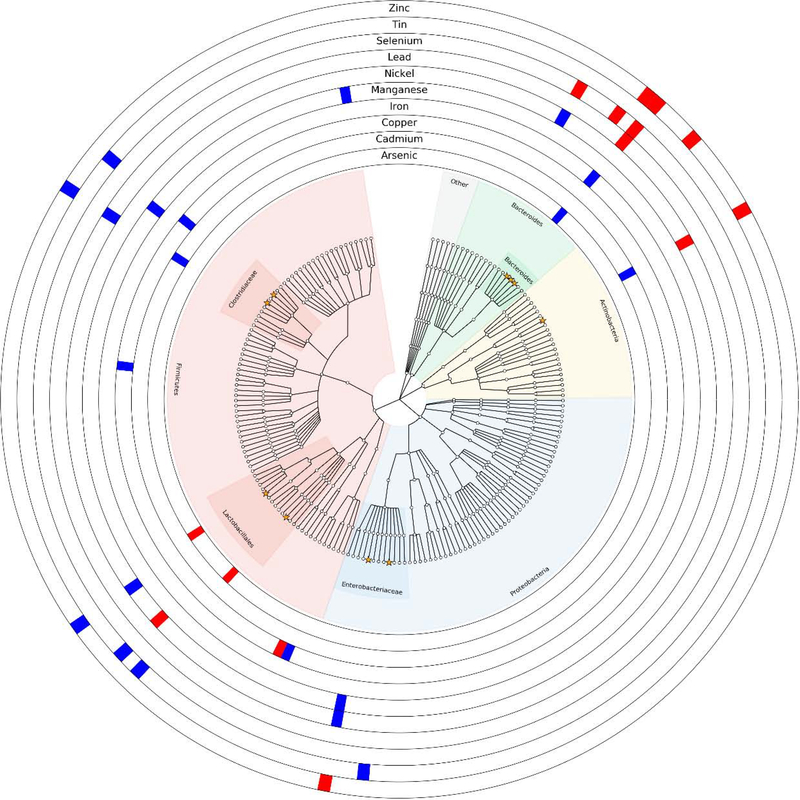
Associations with the relative abundance of at least one ASV were detected for several elements MZILN models (Figure 2). The strongest associations were detected with the relative abundance of an ASV identified as Escherichia coli, with both negative (zinc) and positive (nickel and manganese) associations. A strong negative association was also detected between zinc and the relative abundance of an ASV annotated as Bacteroides fragilis. The most abundant infant gut ASV, Bifidobacterium, decreased with increasing zinc concentrations and increased with increasing cadmium concentrations. Some of these associations were consistent in stratified analyses while others were not (Tables S9–19). In general, the relative abundance of taxa in the gut microbiome of male infants was more often associated with trace element/metal exposures female infants. Additionally, the microbiomes of infants born vaginally were more associated with exposures than those born via Caesarean. A sensitivity analysis with each taxon as the reference produced similar results (data not shown).
Figure 2.
Cladogram of amplicon sequence variants statistically significantly associated either positively (blue) or negatively (red) with infant toenail metal/metalloid exposures in the full population (n = 179). Orange stars indicate the ten most abundant taxa on average in the population. Models adjust for all element/metal/metalloid exposures and covariates concurrently. No statistically significant associations were found with mercury, and thus it does not appear in the figure, but was included as a covariate in all models.
3.4. Nonlinearity and interactions
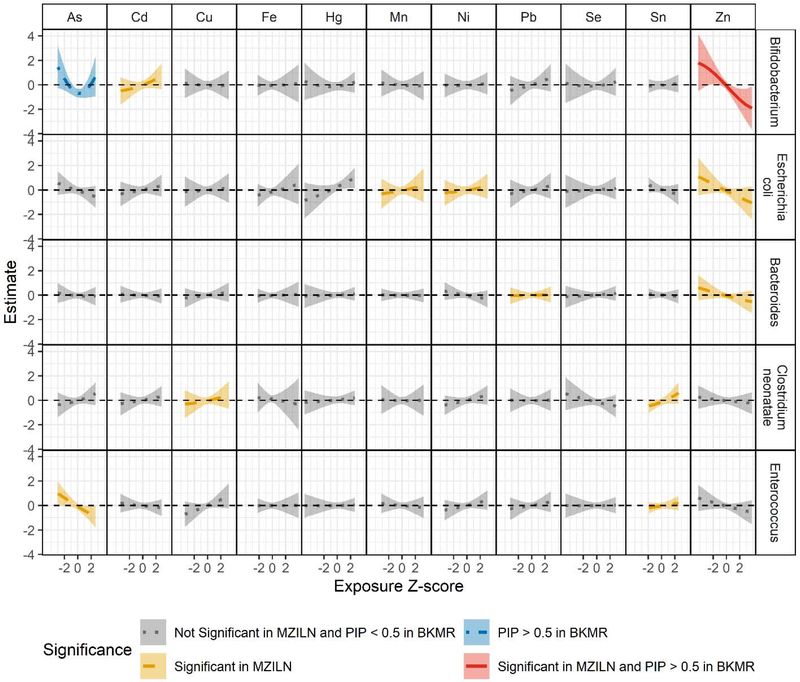
We next explored potential exposure interactions using BKMR. PIPs for associations identified using MZILN models ranged from 0.024 (lead and a Bacteroides ASV) to 0.632 (zinc and Bifidobacterium; Table S20). Based on the PIPs, elements were significant contributors (i.e., PIP > 0.5) to the relative abundance of Bifidobacterium (arsenic and zinc). For those associations detected with MZILN where the PIP was < 0.5, BKMR dose-response curves showed similar patterns to those described by MZILN (i.e., in the same direction, Figure S3).
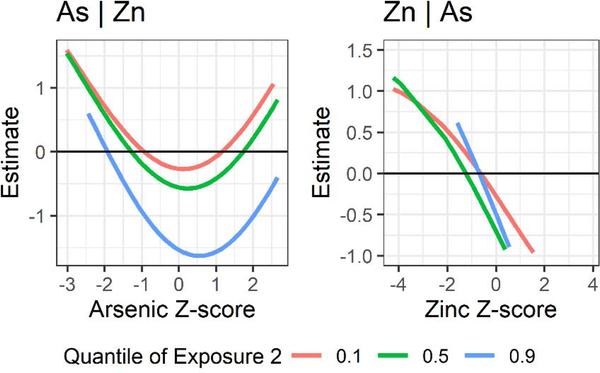
Dose-response curves from BKMR suggested a negative linear association between zinc and Bifidobacterium, which was also positively associated with cadmium in MZILN models (Figure 3). The dose-response curves for cadmium from BKMR reflected this positive association, but less conspicuously (PIP = 0.223). Although we found no association between arsenic and Bifidobacterium with MZILN, we detected a nonlinear (quadratic) association using BKMR (PIP = 0.578). Furthermore, in BKMR bivariate exposure plots, the association between arsenic and Bifidobacterium differed by concentration of zinc, with the reciprocal plot suggesting a similar interaction (Figure 4). Specifically, arsenic was more strongly associated with Bifidobacterium relative abundance at high concentrations of zinc (90th percentile), whereas the reverse was also true (i.e., zinc was more strongly associated with Bifidobacterium relative abundance when arsenic was in the 50th or 90th percentile compared to the 10th percentile). To confirm these associations, we fit linear regression models including all elements and covariates as well as a quadratic term for arsenic and an interaction term between zinc and arsenic. Despite the limited power of this model, the main effect of zinc was significant [βZn = −0.52, 95% CI (−0.91, −0.13)], as was the quadratic arsenic term [βAs2 = 0.43, 95% CI (0.18, 0.68)]. Neither the main effect of arsenic nor the interaction between arsenic and zinc were significant [βAs = −0.11, 95% CI (−0.49, 0.28); βAs*Zn = −0.21, 95% CI (−0.47, 0.04)], although the direction of the interaction suggested a synergistic relationship. No other nutrient/toxic exposures or their interactions were significant in their associations with Bifidobacterium (Figure S4).
Figure 3.
Bayesian Kernel Machine Regression (BKMR) dose-response curves for each exposure when all other exposures are fixed at the median, adjusting for covariates in the full population (n = 179) for taxa with associations with at least two exposures in either multivariate zero-inflated logistic normal (MZILN) or BKMR models. Color/line type indicate exposures that were found to be significantly associated with the given amplicon sequence variant in MZILN models, to have a posterior inclusion probability (PIP) > 0.5 in BKMR, both, or neither.
Figure 4.
Bivariate plots of the interaction between arsenic and zinc in their associations with Bifidobacterium in the unstratified population (n = 179) when all other exposures are fixed at the median, adjusting for covariates. For example, the first panel shows the association between arsenic and Bifidobacterium when zinc is fixed at the 10th, 50th, or 90th percentile and all other elements are fixed at their medians.
4. DISCUSSION
In our novel study of infant multiple exposures to eleven trace elements, which include environmental toxicants found in our food and water sources, we found that arsenic was associated with decreased microbial alpha diversity, particularly in exclusively breastfed infants or those exposed to peripartum antibiotics. None of the exposures we investigated appeared to alter beta diversity, entire bacterial community structures. We identified associations with most nutrient and metal elements and the relative abundance of at least one taxon. We also found differences in the microbiome associated with toxicant exposures by delivery mode, breast feeding status, infant sex, and peripartum antibiotic exposure, although the small sample size in these subpopulations may lead to spurious results. In addition, we identified a nonlinear (quadratic) association between arsenic and Bifidobacterium and a synergistic interaction between arsenic and zinc in their associations with Bifidobacterium using BKMR.
We found evidence that arsenic was associated with decreased diversity as measured by the Simpson Index. Our finding that the association between arsenic concentration and Simpson Index was more pronounced among infants exposed to peripartum antibiotics (primarily penicillin) suggests that these exposures may act synergistically on alpha diversity. Interestingly, certain forms of arsenic were previously used as antimicrobials, and have recently been proposed as a novel broad-spectrum antibiotic (Nadar et al., 2019). We also found that the association between arsenic and Simpson Index was more significant among infants who were exclusively breastfed, although the estimate among mixed fed/exclusively formula fed infants was not appreciably different. Because of the limited number of exclusively formula fed infants in our population, we cannot determine whether infants with any breastfeeding experience a different effect of arsenic than those with no breastfeeding. Although urinary arsenic of formula or mixed fed infants is significantly higher than that of exclusively breastfed infants in this population (Hoen et al., 2018), their toenail arsenic concentrations at six weeks were similar. This is likely due to the time period of exposure represented by infant toenails trace element testing (i.e., infant peripartum exposure which can include late fetal exposure) versus infant urine element testing, which captures metal exposure in the past few days.
In addition to arsenic, copper (Grass et al., 2011) and mercury (Fildes, 1940) have been identified and utilized as antimicrobials, and nickel, lead, and tin have been shown to have some weak antimicrobial properties (Yasuyuki et al., 2010). We found that mercury was associated with decreased within-subject diversity, but only among infants who were not exclusively breast fed. While a recent meta-analysis found that not exclusively breast feeding was associated with increased alpha diversity in infants under six months of age, this was not true in our six-week-old population and mercury concentration was also not related to breastfeeding status (Ho et al., 2018). It is possible that exclusive breastfeeding confers some protection against mercury, or, conversely, that formula supplementation imparts susceptibility. However, without in vitro modeling any hypothesized mechanisms remain speculative. Notably, Bridges et al. did not report changes to within-subject diversity with increasing exposure to mercury in a mouse model (2018). We did not find any associations between copper and within-subject diversity. Copper concentrations in our study were comparable to other child/infant populations (Karatela et al., 2018; Wilhelm et al., 1994), and our null findings with within-subject diversity reflect similar results in a study of dietary copper intake in rats (Song et al., 2018).
No individual elements were associated with changes in bacterial community structure (beta diversity) in the infant gut using generalized UniFrac distances. In our previous report of associations between urinary arsenic concentrations and infant gut microbial diversity, we described a significant correlation, particularly among formula-fed male infants (Hoen et al., 2018). This discrepancy may relate to the different exposure metric used (urinary vs. toenail), which could indicate an importance of timing of exposure, or the inclusion of the wide array of exposure elements as covariates. Inorganic arsenic is the majority of toenail arsenic, whereas organic forms such as metabolic products of inorganic arsenic dominate urinary arsenic (Button et al., 2009; Signes-Pastor et al., 2017). Since the gut microbiome is known to play a role in arsenic metabolism (i.e., methylation), our findings could reflect an effect of the microbiome on arsenic rather than the reverse (Van de Wiele et al., 2010). Future metagenomic studies examining the abundance of arsenic methylation genes or studies in populations with acute rather than chronic exposure may elucidate the bidirectional association between arsenic and the gut microbiome.
We identified at least one ASV associated with ten of the examined elements in the cohort. Bacteria utilize certain metals in vital biological processes and thus their growth could be inhibited by insufficient supply of those metals (Chandrangsu et al., 2017); others that can transport or detoxify metals may increase in abundance in the presence of those metals. Some bacteria have been shown to accumulate toxic metals (Gadd, 1990), which can ultimately cause cell death (Yasuyuki et al., 2010). In our stratified analyses, we found more associations between metals and the relative abundance of ASVs among vaginally delivered infants than those delivered via Caesarean section. While this could be a result of greater statistical power in the vaginally delivered population due to a larger sample size or chance, it may also reflect changes in the microbiome of the pregnant woman related to her metal exposure, which is correlated with infant exposure (Arbuckle et al., 2016). Because vertical transfer is disrupted by Caesarean surgery, these associations may be better preserved in the vaginally delivered subset (Dominguez-Bello et al., 2010). It may also indicate that the taxa transferred vaginally are more likely to be affected by nutrient or toxic exposures. Further research on the microbiome during pregnancy, particularly related to environmental exposures and seeding of the infant gut, is needed to investigate this possibility.
Applying BKMR allowed us to detect nonlinear and interactive associations between infant toenail element concentrations and Bifidobacterium, as well as affirming its strong negative linear association with zinc. On average, Bifidobacterium is the most abundant taxon in the infant gut and its increased abundance in the six-week infant has been associated with vaginal delivery and exclusive breastfeeding (Madan et al., 2016). The bacterium is recognized as a probiotic capable of decreasing incidence of viral diarrhea and with multiple immunologic benefits (López et al., 2010; Saavedra, 2000). Similarly, zinc has been used in over-the-counter medications to boost immune function (McElroy and Miller, 2002); zinc deficiency, for which up to 17% of the world’s population is at risk (Wessells and Brown, 2012), has been associated with impaired immunity (Wintergerst et al., 2007). However, in populations with adequate intake, zinc supplementation was associated with increased nausea and diarrhea (Science et al., 2012). It is possible that decreased Bifidobacterium abundance serves as a mechanism by which zinc exposure influences gastrointestinal symptoms. The association between zinc and Bifidobacterium may be different (i.e., positive) in a zinc-deficient population such that across a broad spectrum of zinc exposure there is a nonlinear, negative quadratic effect. In fact, in a mouse model of zinc deficiency researchers found that Bifidobacterium was positively correlated with plasma zinc concentrations (Gaulke et al., 2018). Future analyses in populations with a broad range of zinc exposure (i.e., including zinc-deficient individuals) are warranted to examine this hypothesis.
With BKMR we were able to detect a nonlinear association between arsenic and Bifidobacterium that could not be detected with traditional statistical methods for microbiome data, highlighting a gap in the current microbiome literature. The observed nonmonotonic relationship, where arsenic is associated with lower relative abundance of Bifidobacterium at moderate concentrations, may indicate desensitization at higher concentrations, a negative feedback mechanism, or dose-dependent metabolism (Lagarde et al., 2015). We found that this association was strongest when zinc concentrations were in their 90th percentile, indicating a potential synergism between zinc and arsenic in their associations with Bifidobacterium. Gaulke et al. examined co-exposure to zinc deficiency and arsenic exposure and found interactions with community structure and individual taxa (2018). However, these results are not directly comparable to the interaction between arsenic and zinc observed in our analysis because animal models are imperfect representations of the human microbiome, especially in this case where the mice were adults and exclusively female. More in vitro and in vivo research is needed to determine how zinc and arsenic may affect the growth of Bifidobacterium, and how they may interact in doing so.
Our study, while examining the association between environmental factors and the infant gut microbiome using novel interaction methods, is not without its limitations. Our sample size, particularly in certain sub-populations (e.g., Caesarean deliveries), provides limited power. However, our findings suggest important associations that should be confirmed in larger cohorts. Additionally, the cohort inclusion criterion of private well use may cause recruitment individuals with unique elemental exposure profiles that do not reflect those of the general population. Stool samples were thawed during aliquoting, which may result in deterioration of some bacterial DNA, but that degradation should be independent of exposure and therefore not result in bias even if some taxa are underrepresented. Because there have been few studies on environmental exposures and the microbiome, it is unclear what window of nutrient/metal/metalloid exposure is most influential in modifying the infant gut microbiome. Elemental exposures are relatively stable over time, and thus, infant toenails collected around six weeks postpartum are likely reflective of late prenatal and early postnatal exposures. The small sample weight of infant toenails may lead to nondifferential misclassification of exposure. It is possible that the infant microbiome could affect the metabolism and excretion/storage of metal/metalloids.
Our analysis, like all microbiome studies, is also limited by the novel but imperfect methods available for obtaining meaningful estimates from high-dimensional and complex data. While MZILN accounts for the zero-inflated nature of microbiome data and allows for the consideration of many exposures, it identifies significant associations by shrinking estimates toward zero, thereby biasing all estimates toward the null. Similarly, because BKMR is not designed to handle zero-inflated, autocorrelated microbiome data, it is not a practical or appropriate first approach in this type of study. Rather, as utilized here, it can be used to tease apart complex interactions between multiple exposures with a few taxa of interest. Here, we elected to examine some of the most abundant taxa with BKMR to minimize the effect of zero-count data, but in doing so we have missed other novel associations. Further, imperfect strategies for modeling co-exposure to multiple elements in an epidemiologic setting make it possible that our estimates are subject to co-exposure amplification bias (Weisskopf et al., 2018). Our results highlight the need for statistical methods that can handle the structure of microbiome data, but also can reveal nonlinear and interactive associations.
Despite its limitations, our study also has many strengths. First, it is among the earliest epidemiologic studies with a large sample size to consider the association between early-life exposure to nutrients/metals/metalloids and the gut microbiome, and the first to consider the potential confounding by co-exposure to multiple elements. In addition, we explored the interactive effects of metals that were associated with the same ASV by applying BKMR, a novel approach in the microbiome literature. This study also applies cutting-edge statistical techniques to handle the complex structure of microbial sequencing data. For example, by using generalized UniFrac distances we did not inflate the importance of abundant taxa as weighted UniFracs do, but still reduced the noise of less abundant taxa which can drive findings with unweighted UniFracs. Furthermore, the NHBCS, a longitudinal pregnancy cohort, captures the exposures and outcomes of this study at multiple time points, which will allow us to explore whether these associations persist in later infancy and have relevance to childhood health outcomes.
5. CONCLUSIONS
In summary, this study found novel associations of common metal and metalloid exposures with the gut microbiome diversity and specific taxa in the infant gut in the neonatal period. Our findings suggest that environmental exposures, including metals, could be important drivers of infant gut microbial development. Future studies are needed to evaluate the impact of elements during pregnancy and on the vertical transmission of the microbiome to offspring. In addition, pre- and perinatal metal exposure has been associated with many diverse health effects, and it remains to be seen whether the alterations observed in the infant gut microbiome are a biomarker of exposure or represent a biological mechanism by which metals ultimately affect human health.
Supplementary Material
HIGHLIGHTS.
Aim was to examine mixture of nutrient and toxic elements and the infant microbiome
Arsenic and antibiotics were synergistically associated with lower diversity
Found associations with Bifidobacterium and zinc (negative) and arsenic (quadratic)
Acknowledgments
FUNDING
This work is part of the specific aims of NIEHS P01ES022832, NIEHS P20ES018175, US EPA RD-83544201, and RD-83459901. Biorepository services were provided by NIGMS P20GM104416. The original cohort was funded by NIEHS P42ES007373. The funding agencies were not involved in the study design or collection, analysis, and interpretation of data, or writing of the manuscript. We appreciate the participation of all New Hampshire Birth Cohort Study families and the support of the cohort study staff without whom this research would not be possible.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
DATA REFERENCES
The 16S rRNA gene sequencing data used in this study are available through the National Center for Biotechnology Information (NCBI) Sequence Read Archive: http://ncbi.nlm.nih.gov/sra under accession number PRJNA296814.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appleton AA, Jackson BP, Karagas M, Marsit CJ, 2017. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics 12, 607–615. 10.1080/15592294.2017.1320637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE, Liang CL, Morisset A-S, Fisher M, Weiler H, Cirtiu CM, Legrand M, Davis K, Ettinger AS, Fraser WD, 2016. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 163, 270–282. 10.1016/j.chemosphere.2016.08.023 [DOI] [PubMed] [Google Scholar]
- Betts KS, 2011. A Study in Balance: How Microbiomes Are Changing the Shape of Environmental Health. Environ. Health Perspect 119, a340–a346. 10.1289/ehp.119-a340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA, 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16, 493–508. 10.1093/biostatistics/kxu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges KN, Zhang Y, Curran TE, Magnuson JT, Venables BJ, Durrer KE, Allen MS, Roberts AP, 2018. Alterations to the Intestinal Microbiome and Metabolome of Pimephales promelas and Mus musculus Following Exposure to Dietary Methylmercury. Environ. Sci. Technol 52, 8774–8784. 10.1021/acs.est.8b01150 [DOI] [PubMed] [Google Scholar]
- Button M, Jenkin GRT, Harrington CF, Watts MJ, 2009. Human toenails as a biomarker of exposure to elevated environmental arsenic. J. Environ. Monit 11, 610 10.1039/b817097e [DOI] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP, 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrangsu P, Rensing C, Helmann JD, 2017. Metal Homeostasis and Resistance in Bacteria. Nat. Rev. Microbiol 15, 338–350. 10.1038/nrmicro.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Bittinger K, Charlson ES, Hoffmann C, Lewis J, Wu GD, Collman RG, Bushman FD, Li H, 2012. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics 28, 2106–2113. 10.1093/bioinformatics/bts342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li H, 2013. Variable selection for sparse Dirichlet-multinomial regression with an application to microbiome data analysis. Ann. Appl. Stat 7, 418–442. 10.1214/12-AOAS592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, Ji Y, Hong X, Caruso D, Bartell T, Gong Y, Strickland P, Navas-Acien A, Guallar E, Wang X, 2014. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo. Sci. Environ. Epidemiol 24, 537–544. 10.1038/jes.2014.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Bian X, Gao B, Ru H, Tu P, Lu K, 2016. Sex-Specific Effects of Arsenic Exposure on the Trajectory and Function of the Gut Microbiome. Chem. Res. Toxicol 29, 949–951. 10.1021/acs.chemrestox.6b00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Gao B, Tu P, Liu C-W, Xue J, Lai Y, Ru H, Lu K, 2018. Individual susceptibility to arsenic-induced diseases: the role of host genetics, nutritional status and the gut microbiome. Mamm. Genome Off. J. Int. Mamm. Genome Soc 29, 63–79. 10.1007/s00335-018-9736-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker MO, Hoen AG, Dade E, Lundgren S, Li Z, Wong AD, Zens MS, Palys TJ, Morrison HG, Sogin ML, Baker ER, Karagas MR, Madan JC, 2019. Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study. BJOG Int. J. Obstet. Gynaecol 10.1111/1471-0528.15799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Li Z, Gilbert-Diamond D, Mackenzie TA, Cottingham KL, Jackson BP, Lee JS, Baker ER, Marsit CJ, Karagas MR, 2014. Infant toenails as a biomarker of in utero arsenic exposure. J. Expo. Sci. Environ. Epidemiol 24, 467–473. 10.1038/jes.2014.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan PH, Ochman H, 2012. Illumina-based analysis of microbial community diversity. ISME J. 6, 183–194. 10.1038/ismej.2011.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL, 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol 72, 5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R, 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A 107, 11971–11975. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Li Z, Korrick SA, Spiegelman D, Enelow R, Nadeau K, Baker E, Karagas MR, 2016. Infant Infections and Respiratory Symptoms in Relation to in Utero Arsenic Exposure in a U.S. Cohort. Environ. Health Perspect 124, 840–847. 10.1289/ehp.1409282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold BJ, Vegosen L, Davis M, Leibler J, Peterson A, Silbergeld EK, 2010. A Niche for Infectious Disease in Environmental Health: Rethinking the Toxicological Paradigm. Environ. Health Perspect 118, 1165–1172. 10.1289/ehp.0901866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fildes P, 1940. The Mechanism of the Anti-bacterial Action of Mercury. Br. J. Exp. Pathol 21, 67–73. [Google Scholar]
- Gadd GM, 1990. Heavy metal accumulation by bacteria and other microorganisms. Experientia 46, 834–840. 10.1007/BF01935534 [DOI] [Google Scholar]
- Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, Willett WC, Hunter DJ, 1993. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol. Prev. Biomark 2, 493–497. [PubMed] [Google Scholar]
- Gaulke CA, Rolshoven J, Wong CP, Hudson LG, Ho E, Sharpton TJ, 2018. Marginal Zinc Deficiency and Environmentally Relevant Concentrations of Arsenic Elicit Combined Effects on the Gut Microbiome. mSphere 3 10.1128/mSphere.00521-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, Baker ER, Jackson BP, Folt CL, Karagas MR, 2011. Rice consumption contributes to arsenic exposure in US women. Proc. Natl. Acad. Sci. U. S. A 108, 20656–20660. 10.1073/pnas.1109127108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goullé JP, Saussereau E, Mahieu L, Bouige D, Groenwont S, Guerbet M, Lacroix C, 2009. Application of Inductively Coupled Plasma Mass Spectrometry Multielement Analysis in Fingernail and Toenail as a Biomarker of Metal Exposure. J. Anal. Toxicol 33, 92–98. 10.1093/jat/33.2.92 [DOI] [PubMed] [Google Scholar]
- Grass G, Rensing C, Solioz M, 2011. Metallic Copper as an Antimicrobial Surface. Appl Env. Microbiol 77, 1541–1547. 10.1128/AEM.02766-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Liu S, Wang Z, Zhang X, Li M, Wu B, 2014. Metagenomic profiles and antibiotic resistance genes in gut microbiota of mice exposed to arsenic and iron. Chemosphere 112, 1–8. 10.1016/j.chemosphere.2014.03.068 [DOI] [PubMed] [Google Scholar]
- Henn BC, Coull BA, Wright RO, 2014. Chemical Mixtures and Children’s Health. Curr. Opin. Pediatr 26, 223–229. 10.1097/MOP.0000000000000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho NT, Li F, Lee-Sarwar KA, Tun HM, Brown BP, Pannaraj PS, Bender JM, Azad MB, Thompson AL, Weiss ST, Azcarate-Peril MA, Litonjua AA, Kozyrskyj AL, Jaspan HB, Aldrovandi GM, Kuhn L, 2018. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat. Commun 9 10.1038/s41467-018-06473-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen AG, Madan JC, Li Z, Coker M, Lundgren SN, Morrison HG, Palys T, Jackson BP, Sogin ML, Cottingham KL, Karagas MR, 2018. Sex-specific associations of infants’ gut microbiome with arsenic exposure in a US population. Sci. Rep 8, 12627 10.1038/s41598-018-30581-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iszatt N, Janssen S, Lenters V, Dahl C, Stigum H, Knight R, Mandal S, Peddada S, González A, Midtvedt T, Eggesbø M, 2019. Environmental toxicants in breast milk of Norwegian mothers and gut bacteria composition and metabolites in their infants at 1 month. Microbiome 7, 34 10.1186/s40168-019-0645-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN, 2014. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol 7, 60–72. 10.2478/intox-2014-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatela S, Ward NI, Zeng IS, Paterson J, 2018. Status and interrelationship of toenail elements in Pacific children. J. Trace Elem. Med. Biol 46, 10–16. 10.1016/j.jtemb.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C, 2015. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ. Health 14 10.1186/1476-069X-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lee K, Karagas MR, Madan JC, Hoen AG, O’Malley AJ, Li H, 2018. Conditional Regression Based on a Multivariate Zero-Inflated Logistic-Normal Model for Microbiome Relative Abundance Data. Stat. Biosci 10, 587–608. 10.1007/s12561-018-9219-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López P, Gueimonde M, Margolles A, Suárez A, 2010. Distinct Bifidobacterium strains drive different immune responses in vitro. Int. J. Food Microbiol 138, 157–165. 10.1016/j.ijfoodmicro.2009.12.023 [DOI] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R, 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230. 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren SN, Madan JC, Emond JA, Morrison HG, Christensen BC, Karagas MR, Hoen AG, 2018. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 6, 109 10.1186/s40168-018-0490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani JZJ, Koestler DC, Lester B, Houseman EA, Armstrong DA, Kelsey KT, Marsit CJ, 2015. Placental DNA Methylation Related to Both Infant Toenail Mercury and Adverse Neurobehavioral Outcomes. Environ. Health Perspect 123, 723–729. 10.1289/ehp.1408561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan JC, Hoen AG, Lundgren SN, Farzan SF, Cottingham KL, Morrison HG, Sogin ML, Li H, Moore JH, Karagas MR, 2016. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 170, 212–219. 10.1001/jamapediatrics.2015.3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy BH, Miller SP, 2002. Effectiveness of Zinc Gluconate Glycine Lozenges (Cold-Eeze) Against the Common Cold in School-Aged Subjects: A Retrospective Chart Review. Am. J. Ther 9, 472. [DOI] [PubMed] [Google Scholar]
- Nadar VS, Chen J, Dheeman DS, Galván AE, Yoshinaga-Sakurai K, Kandavelu P, Sankaran B, Kuramata M, Ishikawa S, Rosen BP, Yoshinaga M, 2019. Arsinothricin, an arsenic-containing non-proteinogenic amino acid analog of glutamate, is a broad-spectrum antibiotic. Commun. Biol 2, 131 10.1038/s42003-019-0365-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson A, Hallén IP, Sundberg J, Grawé KP, 1998. Risk assessment in relation to neonatal metal exposure†. The Analyst 123, 19–23. 10.1039/a705136k [DOI] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO, 2007. Development of the Human Infant Intestinal Microbiota. PLOS Biol. 5, e177 10.1371/journal.pbio.0050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punshon T, Li Z, Marsit CJ, Jackson BP, Baker ER, Karagas MR, 2016. Placental Metal Concentrations in Relation to Maternal and Infant Toenails in a U.S. Cohort. Environ. Sci. Technol 50, 1587–1594. 10.1021/acs.est.5b05316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisur Rahman, Marie Vahter, Ekström Eva-Charlotte Persson Lars-Åke, 2011. Arsenic Exposure in Pregnancy Increases the Risk of Lower Respiratory Tract Infection and Diarrhea during Infancy in Bangladesh. Environ. Health Perspect 119, 719–724. 10.1289/ehp.1002265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Horton MK, Miller RL, Whyatt RM, Perera F, 2010. Neonatology and the Environment: Impact of Early Exposure to Airborne Environmental Toxicants on Infant and Child Neurodevelopment. NeoReviews 11, 363–369. 10.1542/neo.11-7-e363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Dominguez G, Castano G, 2019. Gastroenteritis, Pediatric, in: StatPearls. StatPearls Publishing, Treasure Island (FL). [Google Scholar]
- Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, Rojas-García A, 2013. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Sci. Total Environ 454–455, 562–577. 10.1016/j.scitotenv.2013.03.047 [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, 2017. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front. Cell. Infect. Microbiol 7, 396 10.3389/fcimb.2017.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J, 2000. Probiotics and infectious diarrhea. Am. J. Gastroenterol 95, S16–S18. 10.1016/S0002-9270(99)00811-4 [DOI] [PubMed] [Google Scholar]
- Sabra S, Malmqvist E, Saborit A, Gratacós E, Roig MDG, 2017. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PLOS ONE 12, e0185645 10.1371/journal.pone.0185645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Science M, Johnstone J, Roth DE, Guyatt G, Loeb M, 2012. Zinc for the treatment of the common cold: a systematic review and meta-analysis of randomized controlled trials. CMAJ Can. Med. Assoc. J. J. Assoc. Medicale Can 184, E551–561. 10.1503/cmaj.111990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C, Weaver W, 1949. The Mathematical Theory of Communication. The University of Illinois Press, Urbana. [Google Scholar]
- Signes-Pastor AJ, Carey M, Vioque J, Navarrete-Muñoz EM, Rodríguez-Dehli C, Tardón A, Begoña-Zubero M, Santa-Marina L, Vrijheid M, Casas M, Llop S, Gonzalez-Palacios S, Meharg AA, 2017. Urinary Arsenic Speciation in Children and Pregnant Women from Spain. Expo. Health 9, 105–111. 10.1007/s12403-016-0225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, 1949. Measurement of Diversity. Nature 163, 688 10.1038/163688a0 [DOI] [Google Scholar]
- Singh SB, Madan J, Coker M, Hoen A, Baker ER, Karagas MR, Mueller NT, 2019. Does birth mode modify associations of maternal pre-pregnancy BMI and gestational weight gain with the infant gut microbiome? Int. J. Obes 1 10.1038/s41366-018-0273-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Li X, Zhang X, Shi H, Vos MB, Wei X, Wang Y, Gao H, Rouchka EC, Yin X, Zhou Z, Prough RA, Cave MC, McClain CJ, 2018. Dietary copper-fructose interactions alter gut microbial activity in male rats. Am. J. Physiol. - Gastrointest. Liver Physiol 314, G119–G130. 10.1152/ajpgi.00378.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiemsma LT, Michels KB, 2018. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 141, e20172437 10.1542/peds.2017-2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Human Microbiome Project Consortium, Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, Giglio MG, Hallsworth-Pepin K, Lobos EA, Madupu R, Magrini V, Martin JC, Mitreva M, Muzny DM, Sodergren EJ, Versalovic J, Wollam AM, Worley KC, Wortman JR, Young SK, Zeng Q, Aagaard KM, Abolude OO, Allen-Vercoe E, Alm EJ, Alvarado L, Andersen GL, Anderson S, Appelbaum E, Arachchi HM, Armitage G, Arze CA, Ayvaz T, Baker CC, Begg L, Belachew T, Bhonagiri V, Bihan M, Blaser MJ, Bloom T, Bonazzi V, Paul Brooks J, Buck GA, Buhay CJ, Busam DA, Campbell JL, Canon SR, Cantarel BL, Chain PSG, Chen I-MA, Chen L, Chhibba S, Chu K, Ciulla DM, Clemente JC, Clifton SW, Conlan S, Crabtree J, Cutting MA, Davidovics NJ, Davis CC, DeSantis TZ, Deal C, Delehaunty KD, Dewhirst FE, Deych E, Ding Y, Dooling DJ, Dugan SP, Michael Dunne W, Scott Durkin A, Edgar RC, Erlich RL, Farmer CN, Farrell RM, Faust K, Feldgarden M, Felix VM, Fisher S, Fodor AA, Forney LJ, Foster L, Di Francesco V, Friedman J, Friedrich DC, Fronick CC, Fulton LL, Gao H, Garcia N, Giannoukos G, Giblin C, Giovanni MY, Goldberg JM, Goll J, Gonzalez A, Griggs A, Gujja S, Kinder Haake S, Haas BJ, Hamilton HA, Harris EL, Hepburn TA, Herter B, Hoffmann DE, Holder ME, Howarth C, Huang KH, Huse SM, Izard J, Jansson JK, Jiang H, Jordan C, Joshi V, Katancik JA, Keitel WA, Kelley ST, Kells C, King NB, Knights D, Kong HH, Koren O, Koren S, Kota KC, Kovar CL, Kyrpides NC, La Rosa PS, Lee SL, Lemon KP, Lennon N, Lewis CM, Lewis L, Ley RE, Li K, Liolios K, Liu B, Liu Y, Lo C-C, Lozupone CA, Dwayne Lunsford R, Madden T, Mahurkar AA, Mannon PJ, Mardis ER, Markowitz VM, Mavromatis K, McCorrison JM, McDonald D, McEwen J, McGuire AL, McInnes P, Mehta T, Mihindukulasuriya KA, Miller JR, Minx PJ, Newsham I, Nusbaum C, O’Laughlin M, Orvis J, Pagani I, Palaniappan K, Patel SM, Pearson M, Peterson J, Podar M, Pohl C, Pollard KS, Pop M, Priest ME, Proctor LM, Qin X, Raes J, Ravel J, Reid JG, Rho M, Rhodes R, Riehle KP, Rivera MC, Rodriguez-Mueller B, Rogers Y-H, Ross MC, Russ C, Sanka RK, Sankar P, Fah Sathirapongsasuti J, Schloss JA, Schloss PD, Schmidt TM, Scholz M, Schriml L, Schubert AM, Segata N, Segre JA, Shannon WD, Sharp RR, Sharpton TJ, Shenoy N, Sheth NU, Simone GA, Singh I, Smillie CS, Sobel JD, Sommer DD, Spicer P, Sutton GG, Sykes SM, Tabbaa DG, Thiagarajan M, Tomlinson CM, Torralba M, Treangen TJ, Truty RM, Vishnivetskaya TA, Walker J, Wang L, Wang Z, Ward DV, Warren W, Watson MA, Wellington C, Wetterstrand KA, White JR, Wilczek-Boney K, Wu Y, Wylie KM, Wylie T, Yandava C, Ye L, Ye Y, Yooseph S, Youmans BP, Zhang L, Zhou Y, Zhu Y, Zoloth L, Zucker JD, Birren BW, Gibbs RA, Highlander SK, Methé BA, Nelson KE, Petrosino JF, Weinstock GM, Wilson RK, White O, 2012. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, 2008. Health Effects of Early Life Exposure to Arsenic. Basic Clin. Pharmacol. Toxicol 102, 204–211. 10.1111/j.1742-7843.2007.00168.x [DOI] [PubMed] [Google Scholar]
- Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, Kile ML, Quamruzzaman Q, Afroz S, Golam M, Amarasiriwardena C, Bellinger DC, Christiani DC, Coull BA, Wright RO, 2017. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ. Health Perspect 125, 067015 10.1289/EHP614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wiele T, Gallawa CM, Kubachk KM, Creed JT, Basta N, Dayton EA, Whitacre S, Laing GD, Bradham K, 2010. Arsenic Metabolism by Human Gut Microbiota upon in Vitro Digestion of Contaminated Soils. Environ. Health Perspect 118, 1004–1009. 10.1289/ehp.0901794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M, 2016. Environmental pollutants and child health—A review of recent concerns. Int. J. Hyg. Environ. Health 219, 331–342. 10.1016/j.ijheh.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Seals RM, Webster TF, 2018. Bias Amplification in Epidemiologic Analysis of Exposure to Mixtures. Environ. Health Perspect 126, 047003 10.1289/EHP2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells KR, Brown KH, 2012. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLOS ONE 7, e50568 10.1371/journal.pone.0050568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb BW, Schisterman EF, 2008. Assays with lower detection limits: implications for epidemiological investigations. Paediatr. Perinat. Epidemiol 22, 597–602. 10.1111/j.1365-3016.2008.00969.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Lombeck I, Ohnesorge FK, 1994. Cadmium, copper, lead and zinc concentrations in hair and toenails of young children and family members: a follow-up study. Sci. Total Environ 141, 275–280. 10.1016/0048-9697(94)90034-5 [DOI] [PubMed] [Google Scholar]
- Wintergerst ES, Maggini S, Hornig DH, 2007. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab 51, 301–323. 10.1159/000107673 [DOI] [PubMed] [Google Scholar]
- Yasuyuki M, Kunihiro K, Kurissery S, Kanavillil N, Sato Y, Kikuchi Y, 2010. Antibacterial properties of nine pure metals: a laboratory study using Staphylococcus aureus and Escherichia coli. Biofouling 26, 851–858. 10.1080/08927014.2010.527000 [DOI] [PubMed] [Google Scholar]
- Yu H, Wu B, Zhang X-X, Liu S, Yu J, Cheng S, Ren H-Q, Ye L, 2016. Arsenic Metabolism and Toxicity Influenced by Ferric Iron in Simulated Gastrointestinal Tract and the Roles of Gut Microbiota. Environ. Sci. Technol 50, 7189–7197. 10.1021/acs.est.6b01533 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.