Abstract
Toxoplasma gondii takes two different life cycle stages within intermediate hosts including humans. Tachyzoites proliferate during the acute stage, and they transform into cysts to establish a chronic infection preferentially in the brain. IFN-γ production by infiltrated CD4+ and CD8+ T cells is required for the prevention of cerebral tachyzoite growth. IFN-γ production by brain-resident cells, most likely microglia, plays a key first line defense role to facilitate both innate and T cell-mediated protective immunity to control the tachyzoite growth. IFN-γ produced by brain-resident cells activates cerebral expression of IFN-dependent effector molecules to suppress tachyzoite growth during the early stage of infection. Their IFN-γ production also induces an expression of CXCL9 and CXCL10 chemokines to recruit immune T cells into the brain, and upregulates cerebral expression of MHC class I and II molecules for antigen presentation to the recruited T cells to activate their IFN-γ production. CD8+ T cells also have the activity to remove T. gondii cysts from the brains of infected hosts. Of interest, the anti-cyst activity of CD8+ T cells does not require their IFN-γ but does require perforin. Notably, we discovered that CD8+ cytotoxic T cells penetrate in the cysts in a perforin-mediated manner, which induces morphological deterioration and destruction of the cysts and an accumulation of microglia and macrophages for their elimination. Thus, the immune system employs two distinct effector mechanisms mediated by IFN-γ or perforin depending on two different life cycle stages of a single pathogen, T. gondii, to control its cerebral infection.
Keywords: Toxoplasma, IFN-γ, perforin, tachyzoite, cysts, T cell invasion
Graphical Abstract

1. Introduction
Chronic infection with Toxoplasma gondii, an obligate intracellular protozoan parasite, is one of the most common parasitic infections in humans worldwide including developed countries. One third of human population is estimated to be infected with this parasite (1). Infection in humans occurs through an oral ingestion of the tissue cysts present in undercooked meat of infected animals or an ingestion of oocysts present in contaminated food such as water and fresh vegetables. During the acute stage of infection, tachyzoites proliferate within a various types of nucleated cells and can cause serious diseases such as lymphadenopathy, retinochoroiditis, and congenital infection of the fetuses (1). IFN-γ-mediated immune responses are required to restrict the tachyzoite proliferation (2–4). However, this protective immunity is unable to completely eradicate the tachyzoites, and the parasite converts into tissue cysts to establish a chronic infection. There are drugs effective to the tachyzoite stage of this parasite, but these drugs cannot prevent the establishment of chronic infection. In addition, there are currently no drugs effective against the cyst stage of the parasite.
Chronic infection with T. gondii can reactivate and cause life-threatening toxoplasmic encephalitis in immunocompromised patients such as those with AIDS, organ transplants, and neoplastic diseases (1). Even in immunocompetent individuals, there are increasing evidences indicating significant pathogenic effects of this chronic infection. Recent epidemiological studies reported increased incidence of brain cancers in individuals positive for IgG antibodies to this parasite (5, 6). In addition, higher mortality of brain cancer patients was noted in those infected with T. gondii than those uninfected with this parasite (7). Therefore, to improve public health, it is crucial to develop a method to prevent an infection with T. gondii.
To address this crucial public health issue, immunological interventions to activate the protective immunity capable of preventing T. gondii infection are an intriguing approach. For achieving this goal, it is essential to elucidate the mechanisms of the protective immunity against this parasite, especially in the brain where cysts are preferentially formed. Our recent studies revealed that the immune system utilizes two distinct effector mechanisms to control T. gondii depending on two different life cycle stages of the parasite. One is IFN-γ-mediated activation of cerebral cells to prevent the proliferation of tachyzoites (2–4). The other is perforin-mediated eradication of cysts by cytotoxic T cells (8, 9). Notably, in the IFN-γ-mediated protective immunity against tachyzoites, a production of this cytokine by brain-resident cells, in addition to T cells, was identified to be critical to promptly activate both innate and T cell-mediated immune responses to prevent the pathogen growth. In this review, we summarize recent advances in elucidating the mechanisms of the two different effector systems selected by the immune system to fight against two different life cycle stages of a single pathogen, T. gondii, in the brain.
2. IFN-γ-mediated protective immunity against the acute stage form, tachyzoites, of T. gondii
2.1. IFN-γ-mediated activation of host cells is required to control the proliferation of tachyzoites
We initially identified the requirement of IFN-γ for preventing tachyzoite proliferation during an acute acquired infection with T. gondii (2). Whereas both CD4+ and CD8+ T cells produce this cytokine against the infection (3, 4), CD8+ T cells play a major efferent limb in the resistance against the acute infection, and CD4+ T cells functions additively or synergistically with CD8+ T cells (3, 4). IFN-γ activates not only phagocytic cells but also non-phagocytic cells such as fibroblasts to inhibit tachyzoite growth within these cells. Multiple effector molecules are involved in this IFN-γ-mediated inhibition of tachyzoite growth. Production of nitric oxide (NO) from L-arginine by inducible NO synthase (NOS2) is important for the activity of murine microglia activated by IFN-γ against tachyzoites in vitro (10). Tachyzoites resides and proliferate within the parasitophorous vacuoles (PV) in infected cells. In IFN-γ-activated host cells, immunity-related GTPases (IRGs), such as Irgm3, and granulate binding proteins (GBPs), such as Gbp1 and Gbp2, accumulate onto the PV and destroy it to kill the parasite (11–13). The depletion of intracellular L-tryptophan pools by indoleamine-2, 3-dioxxygenase (IDO) is another important mechanism by which IFN-γ controls the intracellular tachyzoite replication (14).
A recent study using a murine model demonstrated an importance of Ly6C+ inflammatory monocytes in controlling cerebral tachyzoite proliferation (15). In vitro studies previously showed that NO production by NOS2 is important for macrophages activated by IFN-γ to restrict tachyzoite growth (16). Previous studies using bone marrow chimeric mice demonstrated that NOS2 in hematopoietic cells, but not by non-hematopoietic cells, are important for controlling T. gondii in the later stage of infection (17). Therefore, it is possible that Ly6C+ inflammatory monocytes prevent T. gondii tachyzoite proliferation in the brain through NO production by NOS2.
Mice deficient in GBPs are susceptible to T. gondii infection and die in the acute stage of infection (18). A recent study using Gbp1-knockout mice showed that Gbp1 is important to prevent mortality of the mice infected with a less virulent type II strain of the parasite in the later stage of infection, and their mortality is associated with inflammatory changes in their brains (12). Treatment of mice with 1-methyl-tryptophane (1-MT), an IDO inhibitor, during the later stage of T. gondii infection results in mortality of mice with increased numbers of cysts in the brain (14). Therefore, IDO is also most likely involved in controlling T. gondii proliferation in the brain.
2.2. IFN-γ production by brain-resident cells is required for the early stage innate defense by activating an expression of effector molecules to limit cerebral tachyzoite growth
In prevention of cerebral tachyzoite proliferation, both CD4+ and CD8+ T cells produce IFN-γ and contribute to the protective immunity (19, 20). Of interest, our study revealed that in addition to T cells, cells other than T or NK cells need to produce this cytokine to prevent reactivation of chronic cerebral T. gondii infection, which is initiated by rupture of the cysts followed by conversion of released bradyzoites to tachyzoites and proliferation of tachyzoites. Our recent study with bone marrow chimeric mice identified that the non-T, non-NK IFN-γ-producing cells required for the host defense are not hematopoietic innate immune cells but brain-resident cells (21). In this study, RAG1-knockout (RAG1−/−) and IFN-γ-knockout (IFN-γ−/−) mice were irradiated to eliminate hematopoietic cell populations, and then received bone marrow cells from RAG1−/− mice that lack T cells but have innate immune cells such as NK cells and macrophages capable of producing IFN-γ (namely RAG1−/−→RAG1−/− and RAG1−/−→IFN-γ−/−, respectively). Only difference in the brains of these two groups of bone marrow chimeras is the presence (RAG1−/−→RAG1−/−) and absence (RAG1−/−→IFN-γ−/−) of the capability to produce IFN-γ in brain-resident cells, which are irradiation-resistant, in the presence of hematopoietic innate immune cells capable of producing IFN-γ.
The RAG1−/−→RAG1−/− and RAG1−/−→IFN-γ−/− mice were infected and treated with sulfadiazine to control tachyzoite proliferation and establish cysts in their brains. Following an initiation of reactivation of the cerebral T. gondii infection by discontinuing sulfadiazine treatment, IFN-γ mRNA and proteins were detected only in the brains of RAG1−/−→RAG1−/− mice (21). Consistently, an expression of IFN-γ-dependent effector molecules, NOS2, IRGs, Gbp1, and IDO1 in the brain were upregulated only in the RAG1−/−→RAG1−/− mice (21). In agreement, cerebral tachyzoite growth was more efficiently suppressed in RAG1−/−→RAG1−/− than RAG1−/−→IFN-γ−/− mice. These results indicate that IFN-γ production by brain-resident cells is essential to activate IFN-γ-dependent protective innate immunity in the brain to limit tachyzoite growth following reactivation of cerebral T. gondii infection. In the absence of IFN-γ production by brain-resident cells, hematopoietic innate immune cells such as NK cells and macrophages capable of producing IFN-γ cannot activate this protective innate immune responses in the brain even though they are circulating systemically in the periphery.
Our previous studies demonstrated that CD11b+CD45low microglia and CD11b+CD45high blood-derived macrophages are major innate immune cell populations other than NK cells that express IFN-γ in the brain during both acute acquired infection and reactivation of chronic infection of T. gondii (22, 23). Consistently, when CD11b+CD45low microglia are purified from the brains of mice by sorting during reactivation of the infection and placed in a culture without any additional in vitro stimulation, microglia secrete large amounts of IFN-γ into the culture supernatants (22). In addition, a microglia cell line (EOC20) produces IFN-γ following stimulation with total lysate antigens of T. gondii tachyzoites in vitro (21). These results together suggest that uninfected microglia play a critical sentinel role in the brain to sense an occurrence of tachyzoite proliferation in the organ by detecting the parasite antigens released from infected cells and produce IFN-γ for promptly activating brain-resident cells around the pathogen growth to express effector molecules and inhibit tachyzoite proliferation. Lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, has been shown to activate microglial IFN-γ production in vitro (24, 25). Therefore, microglia appear to have the capability to play a key first line defense role in the brain to detect a proliferation of various microorganisms including protozoan parasites and bacteria, to facilitate cerebral protective innate immune responses.
2.3. IFN-γ production by brain-resident cells is pivotal to activate cerebral expression of CXCL9 and CXCL10 chemokines to recruit immune T cells into the brain for prevention of cerebral tachyzoite growth
Whereas IFN-γ-mediated innate immune responses are critical for limiting T. gondii tachyzoite proliferation in the brain, this innate immunity alone is not able to eliminate the cerebral growth of this parasite. T cells are ultimately required to shut off its growth, and the function of the T cells for this host defense is identified as their IFN-γ (19, 20, 26). The brain is isolated from the periphery by the blood-brain barrier. Therefore, in order to operate the critical host defense to shut off cerebral tachyzoite growth, immune T cells circulating in the periphery need to be recruited into the brain through the blood-brain barrier. Chemokines play crucial roles in mediating the migration of T cells into various organs. We identified that most of T cells that migrate into the brains of T. gondii-infected mice express CXCR3 chemokine receptor (27). There are three ligands, CXCL9, CXCL10, and CXCL11, for CXCR3. Our recent study identified that CXCL9 plays a pivotal role in recruiting both CD4+ and CD8+ immune T cells not only into the brain but also into the areas of tachyzoite growth within the brain to prevent the pathogen growth (27). Neutralization of CXCL9 by treatment with antibodies against this chemokine causes five and ten times fewer numbers of CD4+ and CD8+ recruited into the brains of T. gondii-infected mice during the early stage of reactivation of the infection (27). The reduced numbers of T cells recruited into the brain result in 9 times increase in tachyzoite burden in the anti-CXCL9 antibody-treated mice when compared to control antibody-treated animals (27). A study by others showed that CXCL10 is also involved in the T cell recruitment into the brains and controlling cerebral tachyzoite growth (28).
A comparison of chemokine expression in the brains of IFN-γ−/− and wild-type mice infected with T. gondii determined that cerebral expression of CXCL9 requires IFN-γ (29). The cerebral expression of CXCL10 is also dependent on this cytokine but low levels of this chemokine can be detected in the brains of IFN-γ−/− mice (29). Our recent study with RAG1−/−→RAG1−/− and RAG1−/−→IFN-γ−/− bone marrow chimeric mice uncovered that IFN-γ production by brain-resident cells is critical for activating cerebral CXCL9 expression in response to a reactivation of T. gondii infection (21). The CXCL10 expression was also identified to be largely dependent on IFN-γ produced by brain-resident cells (21). Consistently, the IFN-γ production by brain-resident cells was found to be crucial for recruiting both CD4+ and CD8+ T cells into the brain following reactivation of the infection (21).
2.4. IFN-γ production by brain-resident cells is required for upregulating cerebral expression of the MHC class I and II molecules to activate IFN-γ production of T cells recruited into the brain to prevent tachyzoite growth
After T cells migrate into the brain, they need to be activated by recognizing their target antigens presented by the MHC class I and II molecules. Expression of these MHC molecules is suppressed in brain cells in the regular condition. IFN-γ is known as a potent activator of MHC class I and II molecule expressions. The study using RAG1−/−→RAG1−/− and RAG1−/−→IFN-γ−/− bone marrow chimeric mice revealed that IFN-γ production by brain-resident cells is essential for upregulating cerebral expression of both MHC class I and II molecules in response to reactivation of T. gondii infection (21). In agreement, when immune T cells from infected wild-type mice were transferred into infected RAG1−/−→RAG1−/− and RAG1−/−→IFN-γ−/− mice, five times greater expression of IFN-γ was detected in the brains of the former than the latter (21). Consistently, the transfer of immune T cells inhibited cerebral tachyzoite growth much more efficiently in RAG1−/−→RAG1−/− than in RAG1−/−→IFN-γ−/− mice (21).
These evidences all together indicate that IFN-γ production by brain-resident cells, most likely microglia, is the key sentinel function of host defense in the brain to promptly activate IFN-γ-dependent protective innate immunity to limit tachyzoite growth and facilitate recruitment of immune T cells into the brain and activation of the recruited T cells to induce their IFN-γ production for preventing reactivation of cerebral T. gondii infection (see Figure 1). To our knowledge, this is the first evidence that sheds light on an importance of IFN-γ production by brain-resident cells in resistance against a cerebral infection in general. Since IFN-γ is important for resistance against cerebral infections with various microorganisms including bacteria and viruses (30, 31) in addition to T. gondii, it is quite possible that IFN-γ production by brain-resident cells plays crucial roles in orchestrating the protective immunity against not only T. gondii but also these other microorganisms in the brain.
Figure 1.
Schematic figure summarizing how IFN-γ-mediated protective immunity in the brain functions to prevent a proliferation of T. gondii tachyzoites.
3. Perforin-mediated protective immunity against the chronic stage form, cysts, of T. gondii
3.1. CD8+ T cells have a potent activity to remove the cysts from the brain of infected hosts
Since the majority of individuals infected with T. gondii remain positive for Toxoplasma IgG antibodies for long periods of time, probably for their life time, it was generally considered that the immune system cannot detect T. gondii cysts or unable to eliminate this chronic stage of the parasite. However, there was no solid evidence to support this general consideration. To address this largely overlooked but important point, we examined whether the immune system is able to detect and attack the cysts of T. gondii using a murine model. For this purpose, we performed an adoptive transfer of immune spleen cells obtained from wild-type mice chronically infected with T. gondii to infected, sulfadiazine-treated athymic nude mice deficient in T cells. The recipient animals had developed large numbers of T. gondii cysts when they received the transfer of immune spleen cells (8). The animals were kept under sulfadiazine treatment after the cell transfer, and 1 month later their cyst numbers in the brains were compared with those of the control group that had not received the immune spleen cells. The cyst numbers in the experimental groups with the cell transfer were found to be markedly fewer than those of the control group (8). T cells were identified to have the capability to reduce the cyst numbers. Notably, T cells were able to reduce numbers of the cysts in 1 week after their transfer into the recipients (8). Immunohistochemical studies visualized an accumulation of inflammatory cells around cysts only 2-3 days after a transfer of the immune T cells (8). Such histological changes were not detectable in the recipient of normal T cells from uninfected wild-type mice. Furthermore, CD8+ subset of immune T cells was identified to have a potent activity of reduce cyst numbers in the brain (8). These results provided the basis to depict the activity of CD8+ T cells to remove T. gondii cysts from the brains of infected hosts.
3.2. Anti-cyst activity of CD8+ T cells does not require their IFN-γ production but requires their perforin
The role of IFN-γ in the anti-T. gondii cyst activity of CD8+ T cells was examined by an adoptive transfer of CD8+ immune T cells from infected IFN-γ−/− and wild-type BALB/c mice to infected, sulfadiazine-treated SCID mice that lack T cells (8). Of interest, the T cells from either of these mice were equally efficient to markedly reduce numbers of T. gondii cysts in the brains of recipients (8), depicting a clear contrast to the effector mechanism of T cells required for preventing tachyzoite proliferation. Removal of the cysts through an IFN-γ-independent mechanism(s) was further supported by the evidence that NOS2, an important effector molecule in IFN-γ-mediated protective immunity against tachyzoites, is dispensable in the immune process to eliminate the tissue cysts (32). Importantly, a transfer of perforin-knockout (Prf1−/−) CD8+ immune T cells failed to reduce cerebral cyst burden of the recipient animals (8, 9). The Prf1−/− CD8+ immune T cells migrated into the brains of recipients and upregulated cerebral expression of IFN-γ and effector molecules (NOS2, Irgm3, Gbp1, and IDO1) required for preventing tachyzoite proliferation in the same manner as wild-type CD8+ immune T cells did (9), strongly suggesting that the lack of the capability of Prf1−/− T cells to remove T. gondii cysts is neither due to their failure of migration into the brain nor failure of controlling tachyzoite growth.
3.3. Antigen presentation by the H-2 Ld molecule and CD8+ T cells bearing T cell receptor (TCR) variable region β8.1, 8.2 (V β8.1, 8.2) play a major role in CD8+ T cell-mediated immune process to remove T. gondii cysts
In the process of determining T. gondii antigen(s) recognized by anti-cyst CD8+ cytotoxic T cells, the MHC class I molecule critical for activating these T cells was first identified. There is a genetic control in resistance against cerebral T. gondii infection in both mice (33, 34) and humans (35). In mice, animals with the H-2d haplotype are resistant, and only small numbers of cysts are detectable in their brains (33, 34). In contrast, mice with the H-2b or H-2k haplotypes are susceptible, and they form large numbers of cysts in their brain (33, 34). T. gondii cysts are formed and persist within infected cells (36). CD8+ T cells recognize their target antigens presented by the MHC class I molecules. The H-2Ld molecule was identified to mediate anti-cyst activity of CD8+ T cells in genetically resistant BALB/c mice (the H-2d haplotype) mice (37). A transfer of CD8+ immune T cells from infected BALB/c mice to infected BALB/c-H-2dm2 mice, which lack the H-2Ld molecule, failed to reduce cyst numbers in the brains of the recipient animals, whereas the same T cell transfer to infected SCID mice that have the H-2Ld molecule markedly reduced cyst numbers in the brains of the recipients (37).
T cells recognize their target antigens through the TCR composed of α and β chains expressed on their surface, and the specificity of the antigen recognition of each T cell is determined by the variable (V) region of each of the TCR α and β chains. To begin addressing a key T. gondii antigen(s) recognized by CD8+ T cells to initiate their anti-cyst activity, we first analyzed TCR Vβ usages of CD8+ T cells present in the brains of chronically infected BALB/c mice. CD8+ T cells bearing TCR Vβ8.1, 8.2 were identified to be most abundant in the brains of these animals (38). In contrast, in the brains of infected CBA/J mice (the H-2k haplotype, susceptible to cerebral T. gondii infection), this TCR Vβ chain was not most abundant among CD8+ T cells present in their brains (38). Therefore, CD8+ Vβ8.1, 8.2+ T cells appear to play an important role in the resistance of BALB/c mice against T. gondii cysts.
3.4. The N-terminal region of dense granule protein 6 (GRA6Nt) is a key T. gondii antigen that CD8+ T cells recognize to initiate the immune process to remove the cysts
To identify T. gondii antigen(s) recognized by the CD8+ Vβ8.1, 8.2+ T cells, T cell hybridomas were generated by using this population of CD8+ immune T cells purified from infected BALB/c mice (37). Of interest, the hybridomas did not respond to T. gondii epitopes previously reported to stimulate IFN-γ production of CD8+ T cells and/or a protective immunity against an acute acquired T. gondii infection through antigen presentation by the H-2Ld molecule (39, 40). In contrast, GRA6Nt was identified to potently induce a secretion of both perforin and granzyme B by the CD8+ Vβ8.1, 8.2+ T cell hybridoma (37). When BALB/c mice were immunized with bone marrow-derived dendritic cells pre-cultured with rGRA6Nt and their splenic CD8+ T cells were transferred to infected, sulfadiazine-treated SCID mice, significant reduction of cysts numbers were detected in their brains (37). The presence of GRA6Nt-specific cytotoxic T cells in the recipients was confirmed by a secretion of perforin and granzyme B in the culture supernatants following stimulation of spleen cells of the recipients with rGRA6Nt (37). Therefore, GRA6Nt is identified to be the first T. gondii antigen to activate CD8+ cytotoxic T cells capable of removing the cysts of the parasite in infected hosts.
3.5. CD8+ cytotoxic T cells penetrate into the cysts through a perforin-dependent manner
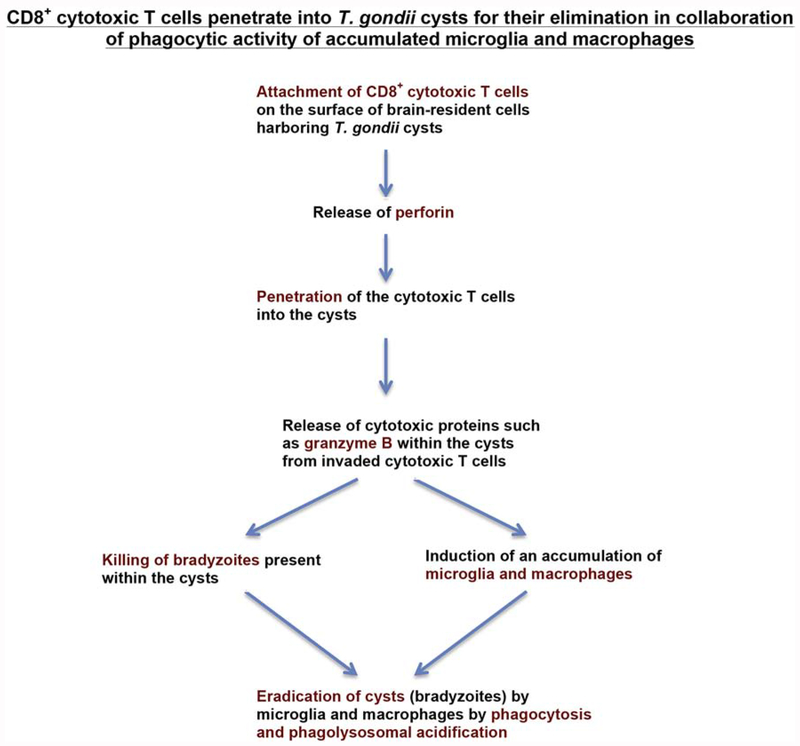
It is known that CD8+ cytotoxic T cells attach the surface of virus-infected cells and cancer cells to kill these cells by releasing perforin that forms pores in the cell membrane of the target cells and secrete cytotoxic proteins such as granzyme B into the target cells to induce death of the target cells. T. gondii cysts exist within infected host cells as mentioned earlier. To visualize the immune process in which CD8+ cytotoxic T cells remove T. gondii cysts, detailed immunohistochemical studies were performed. These studies detected numbers of cyst-holding cells attached by CD8+ T cells in the brains of nude or SCID mice at 2-3 days after receiving the T cells (9). Notably, numbers of cysts penetrated by CD8+ T cells were identified in the brains of the recipients (9). A transfer of Prf1−/− CD8+ immune T cells revealed that an attachment of the T cells on the surface of cyst-holding cells does not require perforin but their penetration into the cysts does require perforin of the T cells (9).
The invasion of CD8+ cytotoxic T cells was often detected in the initial stage (2-3 days after their transfer into recipient mice) of anti-cyst immune process (9). In addition, there were usually no other cells, or a few if any, detected on the surface of those cysts attached or invaded by CD8+ T cells (9), suggesting that CD8+ immune T cells are the first immune cell population that attacks the cysts. The cysts invaded by CD8+ T cells displayed morphological deterioration and destruction (9). Notably, large numbers of granular structures potently positive for granzyme B were detected within the cysts displaying morphological deterioration and destruction, and those granzyme B-positive structures were frequently associated with bradyzoites existing within these cysts (9). Granzyme B is a cytotoxic protein (serine protease) that CD8+ cytotoxic T cells secrete into the targets during their attack on the targets as mentioned earlier, suggesting that this molecule contributes to killing of the bradyzoites within the destroyed cysts penetrated by the CD8+ T cells. To our knowledge, an invasion of CD8+ cytotoxic T cells into a target of large mass, such as T. gondii cysts that can grow into the sizes of more than 50 μm, has not been reported before. Therefore, the analyses of the protective immunity against T. gondii cysts uncovered a previously unappreciated capability of CD8+ cytotoxic T cells to penetrate into a large target for its eradication.
3.6. CD8+ T cell-mediated destruction of cysts associates with an accumulation of microglia and macrophages for eradication of the cysts by phagolysosome acidification of the phagocytes
Immnohistological analyses also depicted an accumulation of large numbers of Iba1+ microglia and Ly6C+ inflammatory macrophages around and into morphological deteriorated or destroyed cysts during the anti-cyst immune process initiated by CD8+ cytotoxic T cells (9). Granzyme B in known to be involved in an induction of inflammation (41). Therefore, granzyme B could contribute, at least in part, to inducing an accumulation of these microglia and macrophages after CD8+ cytotoxic T cells invade into the cysts. In the areas of cyst destruction, most of bradyzoites were detected within the accumulated microglia and macrophages. Portions of the T. gondii-positive materials located within the microglia and macrophages did not display a clear morphology of the parasite, suggesting that they had been destroyed within these phagocytes. Therefore, the microglia and macrophages are most likely the scavenger cells that eliminate the bradyzoites once CD8+ immune T cells invaded into the cysts and displayed cytotoxic anti-cyst effector functions. Our study also showed that treatment of infected nude mice with chloroquine, an inhibitor of endolysosomal acidification, at least partially inhibited CD8+ T cell-mediated cyst removal following a transfer of these T cells (32). Therefore, microglia and macrophages phagocytose bradyzoites and eliminate them by phagolysosome acidification, once CD8+ T cells penetrate into the cysts and display their cytotoxic activities. Thus, the effector capability of invasive CD8+ cytotoxic T cells can be markedly amplified by large numbers of the accumulating phagocytes to eradicate the targets. Since one T. gondii cyst can contain hundreds to thousands of bradyzoites, the induction of an accumulation of large numbers of microglia and macrophages around the T cell-invaded cyst and sealing the target to avoid a leak of bradyzoites during their elimination appear to be an efficient mechanism for the host defense to fight against this parasite (see Figure 2).
Figure 2.
Schematic figure summarizing how perforin-mediated activity of CD8+ cytotoxic T cells eliminate T. gondii cysts from the brain of infected hosts
During the acute stage of T. gondii infection, tachyzoites invade into host cells and quickly proliferate within the cells. The proliferated tachyzoites then egress from the host cells and invade into new host cells located nearby to proliferate. Therefore, when T cells detect a tachyzoite-infected cell, there is a high probability that multiple cells around the detected infected cell are also infected with tachyzoites. In this situation, secreting a soluble mediator, IFN-γ, to activate the cells located around the infected cell to prevent the parasite growth is an effective strategy of the host immunity to control the tachyzoite proliferation. In contrast, the situation of the tissue cysts is different. The cysts are located one by one in a scattered manner, and it is unusual to detect multiple cysts nearby each other. In this situation, attacking the cyst-containing cells by CD8+ cytotoxic T cells through a direct cell-cell contact fashion, rather than secreting a soluble factor, could be an efficient strategy to destroy only the cyst-containing cells without inducing unnecessary activation and functional changes of cells located nearby. Therefore, the immune system seems to make compelling and effective choices on selecting effector mechanisms based on the characteristics of two different life cycle stages, tachyzoites and cysts, of a single microbial pathogen, T. gondii.
4. Conclusions
A little more than thirty years ago, a murine model of T. gondii infection contributed to uncovering the importance of IFN-γ for the protective immunity against an acute acquired infection with an intracellular microorganism. Since then, animal models of T. gondii infection have provided an excellent platform to investigate the molecular mechanisms of the IFN-γ-mediated protective immunity. Researchers worldwide have been elucidating numbers of effector mechanisms for an inhibition and killing of T. gondii tachyzoites within host cells activated by IFN-γ as shortly described in section 2.1. Recent analyses on the mechanisms of cerebral protective immunity against T. gondii tachyzoites revealed that IFN-γ plays key roles not only in activating effector cells to kill the pathogen but also in stimulating a production of mediators to recruit immune T cells from the periphery to the brain and to activate the recruited T cells for their IFN-γ production, which is required for ultimate prevention of cerebral growth of the pathogen. Notably, brain-resident cells are identified as the producer of this critical innate IFN-γ that facilitates both the protective innate and T cell immunity in the brain to prevent reactivation of cerebral T. gondii infection. Therefore, IFN-γ is not simply a powerful activator of effector cells to kill microbial pathogens but also a critical and potent mediator that organ-resident cells utilize to promote T cell-mediated protective immunity to prevent the pathogen growth in the organ. IFN-γ is important for resistance against cerebral infections with various microorganisms including bacteria and viruses (30, 31) in addition to T. gondii, as mentioned earlier. Therefore, IFN-γ production by brain-resident cells could probably play crucial roles in orchestrating the cerebral protective immunity against not only T. gondii but also these other microorganisms.
Notably, in contrast to the protective immunity to prevent a proliferation of tachyzoites of T. gondii, the immune system is uncovered to employ an effector mechanism independent from IFN-γ production by T cells to eliminate the chronic cyst stage of T. gondii. CD8+ T cells utilize perforin-dependent cytotoxic activity to eliminate the tissue cysts of the parasite. A striking finding is that CD8+ cytotoxic T cells not only attach the surface of cyst-containing cells but also directly penetrate into the cysts in a perforin-dependent manner. T cell-penetrated cysts display morphological deterioration and destruction associated with the presence of granular structures positive for granzyme B and an accumulation of microglia and macrophages that eradicate bradyzoites located within the cysts, at least in part, by phagocytosis and phagolysosome acidification. Therefore, the immune system utilizes two distinct effector mechanisms mediated by either IFN-γ or perforin depending on two different life cycle stage, the acute stage tachyzoites and the chronic stage cysts, of a single intracellular pathogen, T. gondii. In addition, to our knowledge, a penetration of CD8+ cytotoxic T cells into a target of large mass was not reported before in general. Therefore, T. gondii infection once again contributed to discovering a novel and important effector system of the protective immunity.
Since the penetration of CD8+ cytotoxic T cells into T. gondii cysts induces an accumulation of large numbers of phagocytes that can attack and eliminate the target, this collaboration of cytotoxic T cells and phagocytes is most likely a very powerful effector mechanism of the protective immunity. CD8+ cytotoxic T cells have been appreciated to be important for the protective immunity against various intracellular pathogens such as viruses and also against cancers. The presence of CD8+ T cells infiltrated into different types of solid cancers has been observed, and the presence of tumor-infiltrating T cells is an indicator of positive prognosis (42). It is possible that these tumor-infiltrating CD8+ T cells are, at least in part, aggressive penetrator CD8+ T cells that invade the tumor using their perforin-mediated activity as discovered in the protective immunity against T. gondii cysts. The invasion of the T cells into tumors could most likely induce an accumulation of large numbers of proinflammatory macrophages capable of attacking the cancer cells, as observed against T. gondii cysts (9). Therefore, effective activation of the aggressive and penetrating capability of CD8+ cytotoxic T cells appears to be an important and powerful approach to attack not only T. gondii cysts but also the other large targets, such as solid cancers, to induce their elimination.
Acknowledgements
The studies described in this review article were supported, in part, by NIH grants (AI095032, AI078756, AI134323, AI136821, and AI073576). The author appreciates contributions from individuals in my laboratory who participated in the studies and numbers of collaborators who provided valuable assistance in our studies described in this review article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The author declares that he has no conflict of interest.
References
- 1.Montoya JG, and Liesenfeld O. 2004 Toxoplasmosis. Lancet 363 (2014) 1965–1976. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Orellana MA, Schreiber RD, and Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240 (1988) 516–518. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Sa Q, Gehman M, and Ochiai E. Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain. Expert. Rev. Mol. Med 13 (2011) e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz M, Liesenfeld O, and Heimesaat MM. Immunology of Toxoplasma gondii. Immunol. Rev 240 (2011) 269–285. [DOI] [PubMed] [Google Scholar]
- 5.Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, and Misse D. Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol. Lett 8 (2012) 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong W, Liu GH, Meng QF, Dong W, Qin SY, Zhang FK, Zhang XY, Wang XY, Qian AD, and Zhu XQ. Toxoplasma gondii infection in cancer patients: prevalence, risk factors, genotypes and association with clinical diagnosis. Cancer Lett. 359 (2015) 307–313. [DOI] [PubMed] [Google Scholar]
- 7.Vittecoq M, Elguero E, Lafferty KD, Roche B, Brodeur J, Gauthier-Clerc M, Misse D, and Thomas F. Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect. Genet. Evol 12 (2012) 496–498. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, Xu B, Kudo T, and Perkins S. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am. J. Pathol 176 (2010) 1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiwari A, Hannah R, Lutshumba J, Ochiai E, Weiss LM, and Suzuki Y. Penetration of CD8(+) Cytotoxic T cells into large target, tissue cysts of Toxoplasma gondii, leads to its elimination. Am. J. Pathol 189 (2019) 1594–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao CC, Anderson WR, Hu S, Gekker G, Martella A, and Peterson PK. Activated microglia inhibit multiplication of Toxoplasma gondii via a nitric oxide mechanism. Clin. Immunol. Immunopathol 67 (1993) 178–183. [DOI] [PubMed] [Google Scholar]
- 11.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, and Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med 203 (2006) 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin H. W. t., Macmicking JD, and Sibley LD. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 9 (2013) e1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohshima J, Sasai M, Liu J, Yamashita K, Ma JS, Lee Y, Bando H, Howard JC, Ebisu S, Hayashi M, Takeda K, Standley DM, Frickel EM, and Yamamoto M. RabGDIalpha is a negative regulator of interferon-gamma-inducible GTPase-dependent cell-autonomous immunity to Toxoplasma gondii. Pro.c Natl. Acad. Sci. U S A 112 (2015) E4581–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divanovic S, Sawtell NM, Trompette A, Warning JI, Dias A, Cooper AM, Yap GS, Arditi M, Shimada K, Duhadaway JB, Prendergast GC, Basaraba RJ, Mellor AL, Munn DH, Aliberti J, and Karp CL. Opposing biological functions of tryptophan catabolizing enzymes during intracellular infection. J. Infect. Dis 205 (2012) 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas A, Bruder D, Wolf SA, Jeron A, Mack M, Heimesaat MM, and Dunay IR. Ly6Chigh monocytes control cerebral toxoplasmosis. J. Immunol 194 (2015) 3223–3235. [DOI] [PubMed] [Google Scholar]
- 16.Adams LB, Hibbs JB Jr., Taintor RR, and Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol 144 (1990) 2725–2729. [PubMed] [Google Scholar]
- 17.Yap GS, and Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med 189 (1999) 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, Huang DC, Soldati-Favre D, Horie K, Takeda J, and Takeda K. A cluster of interferon-gamma-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 37 (2012) 302–313. [DOI] [PubMed] [Google Scholar]
- 19.Gazzinelli R, Xu Y, Hieny S, Cheever A, and Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol 149 (1992) 175–180. [PubMed] [Google Scholar]
- 20.Wang X, Kang X,H, Kikuchi T, and Suzuki Y. Gamma interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect. Immun 72 (2004) 4432–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sa Q., Ochiai E, Tiwari A, Perkins S, Mullins J, Gehman M, Huckle W, Eyestone WH, Saunders TL, Shelton BJ, and Suzuki Y. Cutting Edge: IFN-gamma produced by brain-resident cells is crucial to Control cerebral infection with Toxoplasma gondii. J. Immunol 195 (2015) 796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, and Suzuki Y. Microglia produce IFN-gamma independently from T cells during acute toxoplasmosis in the brain. J. Interferon Cytokine Res 27 (2007) 599–605. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Claflin J, Wang X, Lengi A, and Kikuchi T. Microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int. J. Parasitol 35 (2005) 83–90. [DOI] [PubMed] [Google Scholar]
- 24.Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, and Bruce-Keller AJ. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J. Neuroimmunol 161: (2005) 123–136. [DOI] [PubMed] [Google Scholar]
- 25.De Simone R, Levi G, and Aloisi F. Interferon gamma gene expression in rat central nervous system glial cells. Cytokine 10 (1998) 418–422. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Conley FK, and Remington JS. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol 143 (1989) 2045–2050. [PubMed] [Google Scholar]
- 27.Ochiai E, Sa Q, Brogli M, Kudo T, Wang X, Dubey JP, and Suzuki Y. CXCL9 is important for recruiting immune T cells into the brain and inducing an accumulation of the T cells to the areas of tachyzoite proliferation to prevent reactivation of chronic cerebral infection with Toxoplasma gondii. Am. J. Pahol 185 (2015) 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, Liu AJ, and Hunter CA. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 486 (2012) 545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen X, Kudo T, Payne L, Wang X, Rodgers L, and Suzuki Y. Predominant interferon-gamma-mediated expression of CXCL9, CXCL10, and CCL5 proteins in the brain during chronic infection with Toxoplasma gondii in BALB/c mice resistant to development of toxoplasmic encephalitis. J Interferon Cytokine Res. 30 (2010) 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Broek MF, Muller U, Huang S, Zinkernagel RM, and Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev 148 (1995) 5–18. [DOI] [PubMed] [Google Scholar]
- 31.Chesler DA, A. D, and Reiss CS. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 13 (2002) 441–454. [DOI] [PubMed] [Google Scholar]
- 32.Sa Q, Tiwari A, Ochiai E, Mullins J, and Suzuki Y. Inducible nitric oxide synthase in innate immune cells is important for restricting cyst formation of Toxoplasma gondii in the brain but not required for the protective immune process to remove the cysts. Microbes Infect. 20 (2018) 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki Y, Joh K, Orellana MA, Conley FK, and Remington JS. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74 (1991) 732–739. [PMC free article] [PubMed] [Google Scholar]
- 34.Brown CR, and McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol 145 (1990) 3438–3441. [PubMed] [Google Scholar]
- 35.Suzuki Y, Wong SY, Grumet FC, Fessel J, Montoya JG, Zolopa AR, Portmore A, Schumacher-Perdreau F, Schrappe M, Koppen S, Ruf B, Brown BW, and Remington JS. Evidence for genetic regulation of susceptibility to toxoplasmic encephalitis in AIDS patients. J. Infect. Dis 173 (1996) 265–268. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson DJ, and Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol. Res 73 (1987) 483–491. [DOI] [PubMed] [Google Scholar]
- 37.Sa Q, Ochiai E, Tiwari A, Mullins J, Shastri N, Mercier C, Cesbron-Delauw MF, and Suzuki Y. Determination of a key antigen for immunological intervention to target the latent stage of Toxoplasma gondii. J. Immunol 198 (2017) 4425–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang H, Liesenfeld O, Remington JS, Claflin J, Wang X, and Suzuki Y. TCR V beta 8+ T cells prevent development of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. J. Immunol 170 (2003) 4254–4259. [DOI] [PubMed] [Google Scholar]
- 39.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, and Shastri N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol 9 (2008) 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frickel EM, Sahoo N, Hopp J, Gubbels MJ, Craver MP, Knoll LJ, Ploegh HL, and Grotenbreg GM. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J Infect Dis 198 (2008) 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wensink AC, Hack CE, and Bovenschen N. Granzymes regulate proinflammatory cytokine responses. J. Immunol 194 (2015) 491–497. [DOI] [PubMed] [Google Scholar]
- 42.Finn OJ, A Believer’s overview of cancer immunosurveillance and immunotherapy. J. Immunol 200 (2018) 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]