Abstract
Kinesin is a molecular motor that moves along microtubules. Kinesin family member 9 (KIF9) is evolutionarily conserved and expressed strongly in mouse testis. In the unicellular flagellate Chlamydomonas, KLP1 (ortholog of KIF9) is localized to the central pair microtubules of the axoneme and regulates flagellar motility. In contrast, the function of KIF9 remains unclear in mammals. Here, we mutated KIF9 in mice using the CRISPR/Cas9 system. Kif9 mutated mice exhibit impaired sperm motility and subfertility. Further analysis reveals that the flagella lacking KIF9 showed an asymmetric waveform pattern, which leads to a circular motion of spermatozoa. In spermatozoa that lack the central pair protein HYDIN, KIF9 was not detected by immunofluorescence and immunoblot analysis. These results suggest that KIF9 is associated with the central pair microtubules and regulates flagellar motility in mice.
Keywords: fertilization, male fertility, sperm motility
Abbreviations
- ASH
ASPM‐SPD2‐Hydin
- ES
embryonic stem
- IVF
in vitro fertilization
- Kif9
kinesin family member 9
- KO
knockout
- LD
large deletion
- MEF
mouse embryonic fibroblast
- TEM
transmission electron microscopy
- VAP
average path velocity
- VCL
curvilinear velocity
- VSL
straight line velocity
- WT
wild type
- ZP
zona pellucida
1. INTRODUCTION
Spermatozoa are highly specialized cells that are composed of two parts, head, and flagellum. The head contains the nucleus where the paternal genetic information is stored and an acrosome, an exocytotic vesicle that surrounds the nucleus. The flagellum is a motile, thread‐like appendage that can be divided into three parts, midpiece, principal piece, and end piece.1, 2 The midpiece contains a mitochondrial sheath that plays roles in energy production, whereas the principal piece contains a fibrous sheath that provides elastic rigidity and a scaffold for glycolytic and signaling molecules. These accessory structures are not localized in the end piece.1, 2 Any defects in the formation or function of these structures could lead to male sterility.
The central component of the flagellum is the axoneme, a “9+2” structure that consists of a central pair of two singlet microtubules surrounded by nine outer microtubule doublets.3 In addition to microtubules, there are several macromolecular complexes that compose the axoneme such as outer and inner dynein arms that slide doublet microtubules and radial spokes that are localized between the central pair and doublet microtubules. Molecular components of these structures have been extensively studied in the unicellular flagellate Chlamydomonas.3 Many proteins identified in Chlamydomonas are conserved in mammals including mice and humans; however, their functions and association with infertility in mammals remain to be understood.
Kinesin is a motor protein that moves along microtubules, usually in an anterograde manner. Forty‐five kinesins with varying functions have been found in humans,4 which compose the kinesin superfamily of proteins (KIFs). Kif9 is evolutionarily conserved and its function has been studied in unicellular organisms. In Chlamydomonas, KLP1 (ortholog of KIF9) is localized in the central pair of the axoneme.5, 6 Knocking down KLP1 leads to a reduction in swimming velocity, suggesting that KLP1 is involved in flagellar motility.6 Supporting this idea, knockdown of KIF9A (ortholog of Kif9) in Trypanosoma brucei leads to impaired motility without visible structural abnormalities of their flagella.7
In addition to these studies with unicellular organisms, Northern blot analysis using mouse tissues showed that Kif9 is expressed strongly in the testis,8 suggesting that KIF9 is involved in regulating sperm motility. In this study, we confirmed that KIF9 is localized to the mouse sperm flagella. Further, we mutated Kif9 in mice using the CRISPR/Cas9 system and analyzed its function in male fertility and sperm motility.
2. MATERIALS AND METHODS
2.1. Animals
All animal experiments were approved by the Animal Care and Use Committee of the Research Institute for Microbial Diseases, Osaka University. Mice were purchased from CLEA Japan (Tokyo, Japan) or Japan SLC (Shizuoka, Japan).
2.2. RT‐PCR
Mouse cDNA was prepared from various tissues of adult ICR mice or testes from 1‐ to 5‐week‐old males with SuperScript III First‐Strand Synthesis System (Thermo Fisher Scientific, MA, USA) using an oligo (dT) primer. RT‐PCR was performed using 10 ng of cDNA with the following forward and reverse primers: 5′‐AGAAGGACACTCGGAGAGGG‐3′ and 5′‐CGCGGTGCTTGTAATTCTCC‐3′ for Kif9, 5′‐AAGTGTGACGTTGACATCCG‐3′, and 5′‐GATCCACATCTGCTGGAAGG‐3′ for Actb. The amplification conditions were 1 minute at 94°C, followed by 35 cycles of 94°C for 30 seconds, 65°C for 30 seconds, and 72°C for 30 seconds, with a final 1‐minute extension at 72°C.
2.3. In silico data analysis
Single cell transcriptome data in the mouse testis that was published previously9 was obtained. Kif9 expression in those cells was analyzed using Loupe Cell Browser 3.3.1 (10× Genomics, CA, USA).
2.4. Immunofluorescence
Spermatozoa collected from the cauda epididymis were diluted in PBS, spotted onto slides, air‐dried, fixed with 4% paraformaldehyde for 10 minutes, and washed in PBS for 5 minutes. The slides were blocked with 5% BSA and 10% goat serum in PBS for 1 hour at room temperature. The slides were then incubated with rabbit anti‐KIF9 antibody (1:50, #HPA022033, Atlas Antibodies, Bromma, Sweden) overnight at 4°C and washed with PBS three times for 10 minutes each. After incubation with Alexa Fluor 488 or Alexa Fluor 546‐conjugated secondary antibody (1:200, #A11070 or #A11071, Thermo Fisher Scientific) at room temperature for 2 hours, the slides were washed with PBS three times for 10 minutes each. The slides were then incubated with Hoechst 33342 (2 µg/mL) (Thermo Fisher Scientific) for 15 minutes and washed with PBS three times for 10 minutes each. Slides were observed with an Olympus BX‐53 microscope (Tokyo, Japan).
2.5. Sperm protein fractionation
Sperm protein fractionation was performed as described previously.10, 11 Spermatozoa obtained from the cauda epididymis were suspended in 1% Triton X‐100 lysis buffer (50 mM NaCl, 20 mM Tris‐HCl, pH 7.5, protease inhibitor mixture) and incubated for 2 hours at 4°C. The sample was centrifuged at 15 000 g for 10 minutes to separate the Triton‐soluble fraction and the Triton‐resistant fraction. The pellet (Triton‐resistant fraction) was resuspended in 1% SDS lysis buffer (75 mM NaCl, 24 mM EDTA, pH 6.0) and incubated for 1 hour at room temperature. The sample was centrifuged at 15 000 g for 10 minutes to separate the SDS‐soluble fraction and SDS‐resistant fraction. The pellet (SDS‐resistant fraction) was resuspended in sample buffer (66 mM Tris‐HCl, 2% SDS, 10% glycerol and 0.005% Bromophenol Blue), boiled for 5 minutes, and centrifuged at 15 000 g for 10 minutes.
2.6. Immunoblot analysis
Immunoblot analysis was performed as described previously.12 Samples were subjected to SDS‐PAGE followed by western blotting. After blocking with 10% skim milk, blots were incubated with primary antibodies overnight at 4°C and then incubated with secondary antibodies conjugated to horseradish peroxidase (1:10,000, #805‐035‐180, #111‐036‐045, #115‐036‐062, or #112‐035‐167, Jackson ImmunoResearch, PA, USA) for 2 hours at room temperature. Antibodies used: goat anti‐KIF9 1:100 (#SC99958, Santa Cruz Biotechnology, CA, USA); rabbit anti‐ACTB 1:1000 (#PM053, Medical & Biological Laboratories, Aichi, Japan); goat anti‐BASIGIN 1:500 (#SC9757, Santa Cruz Biotechnology), mouse anti‐acetylated tubulin 1:1000 (#T7451, Sigma‐Aldrich, MO, USA); mouse anti‐AKAP4 1:5000 (#611564, BD Biosciences, CA, USA); mouse anti‐phosphotyrosine 1:1000 (#05‐321, Merck Millipore, MA, USA); rabbit anti‐RSPH9 1:200 (#HPA031703, Atlas Antibodies); rat anti‐PA 1:1000 (#012‐25863, FUJIFILM Wako Pure Chemical, Osaka, Japan); and rabbit anti‐FLAG 1:1000 (#PM020, Medical & Biological Laboratories). Immunoreactive proteins were detected by an ECL western blotting detection kit (GE Healthcare, Little Chalfont, UK).
2.7. gRNA design
gRNAs with fewer off‐target sites were found using the online source CRISPRdirect.13 The gRNA sequence for an indel mutation was 5′‐TCATGAGCAAAGTCATCAGT‐3′ (exon 2) and target sequences for a large deletion were 5′‐TAAAATGGGTACTAGGAAAA‐3′ (exon 2) and 5′‐AGCAGCTCTAGTCTGTTCTA‐3′ (exon 21).
2.8. Generation of Kif9 mutant mice (indel)
Superovulated B6D2F1 females were mated with B6D2F1 males and fertilized eggs were collected. Circular pX330 plasmids14, 15 were injected into one of the pronuclei at 5 ng/µL. The injected zygotes were cultured in KSOM medium16 for one day. Two‐cell embryos were then transferred into the oviduct of pseudo‐pregnant ICR mice. Obtained pups were genotyped by PCR and Sanger sequencing.
2.9. Generation of Kif9 mutant mice (large deletion)
Kif9 large deletion mice were generated using ES cells as described previously.17 Briefly, the EGR‐G01 ES cells (1 × 103‐4)18 were cultured on mouse embryonic fibroblasts (MEF) in a 6‐well plate and transfected with pX330 targeting exon 2 (1.0 µg) and PX459 targeting exon 21 (1.0 µg) using Lipofectamine LTX & PLUS (Thermo Fisher Scientific). After 14‐18 hours, the cells were selected with puromycin (0.1 µg/mL) for 48 hours, passaged, cultured for 5‐6 more days, picked, and transferred onto MEF cells in 96‐well plates. After 48‐72 hours of culture, each ES cell clone was genotyped. The mutant ES cell clones with normal karyotypes were injected into 8‐cell ICR embryos and the blastocysts were transplanted into the uteri of pseudo‐pregnant ICR females. Obtained chimeric mice were mated with B6D2F1 females to obtain the next generation through germline transmission.
2.10. Genotyping
Genotyping was performed with PCR. For the indel mutation, “primer a” (5′‐CACAAAGCAGCTGAAAGACAGG‐3′) and “primer b” (5′‐CTCCACCATTCGGATGGAGG‐3′) were used for PCR and the PCR product was digested with StuI. For large the deletion, “primer a” and “primer b” were used for the WT allele and “primer a” and “primer c” (5′‐TTCTGTGAAGAGGAGCAAGG‐3′) were used for the large deletion allele.
2.11. Mating tests
Sexually matured male mice were individually caged with two 8‐week‐old B6D2F1 female mice for 2 months and plugs were checked every morning. The number of pups was counted on the day of birth.
2.12. Histological analysis of testis
PAS staining of testis sections was performed as previously described.19 The sections were observed with an Olympus BX‐53 microscope.
2.13. In vitro fertilization (IVF)
IVF was performed as described previously.20 Briefly, spermatozoa collected from cauda epididymis were incubated in TYH medium21 for 2 hours at 37°C under 5% CO2. Eggs collected from superovulated females were treated with 330 µg/mL of hyaluronidase (Sigma‐Aldrich) for 10 minutes to remove the cumulus cells (cumulus‐free eggs) or with 1 mg/mL of collagenase (Sigma‐Aldrich) for 10 minutes to remove the zona pellucida (ZP) (zona‐free eggs). The incubated spermatozoa were added to a drop of the TYH medium containing intact, cumulus‐free, or zona‐free eggs at a final density of 2 × 105 spermatozoa/mL. When IVF was performed using intact or cumulus‐free eggs, two‐cell embryos were counted the next day. When IVF was performed using zona‐free eggs, the pronuclear formation was observed 6 hours after insemination. For the ZP binding assay, cumulus‐free eggs were incubated with spermatozoa at a density of 2 × 105 spermatozoa/mL and eggs were observed under an Olympus IX‐73 microscope.
2.14. Isolation of sperm proteins for tyrosine phosphorylation
Spermatozoa collected from the cauda epididymis were incubated in TYH medium for 10 minutes or 2 hours. Spermatozoa were then collected in PBS and centrifugated at 2000 g for 2 minutes at room temperature. The collected spermatozoa were resuspended in sample buffer, boiled for 5 minutes, and centrifuged at 15 000 g for 10 minutes. Immunoblot analysis was performed as described above using 5% BSA instead of 10% skim milk for blocking.
2.15. Sperm motility analysis
Sperm motility was analyzed as described previously.22 Spermatozoa obtained from cauda epididymis were incubated in the TYH medium. Sperm motility was analyzed using the CEROS sperm analysis system (Version 12.3; Hamilton Thorne Biosciences, MA, USA). Analysis settings were as described previously.23 For tracing sperm waveforms, spermatozoa were observed with an Olympus BX‐53 microscope equipped with a high‐speed camera (HAS‐L1, Ditect, Tokyo, Japan). The motility was videotaped at 200 frames per second or 50 frames per second. Obtained images were analyzed for waveforms using the sperm motion analyzing software (BohBohsoft, Tokyo, Japan).24
2.16. Transmission electron microscopy (TEM)
Cauda epididymis samples were prepared for TEM analysis as described previously.25 Sections were examined using a JEM‐1400 plus electron microscope (JEOL, Tokyo, Japan) at 80 kV with a CCD Veleta 2K × 2X camera (Olympus).
2.17. Generation of Hydin KO chimeric mice
Hydin KO ES cells that were established previously26 were injected into 8‐cell ICR embryos. Obtained blastocysts were transplanted into the uteri of pseudo‐pregnant ICR females.
2.18. Generation of KIF9 and HYDIN recombinant proteins
Kif9 was amplified from mouse testis cDNA, digested with BamHI and EcoRV, and ligated into the FLAG‐tagged (C‐terminus) pCAG vector that contains the CAG promoter and a rabbit globin poly (A) signal.27 Primers that were used to amplify the cDNA were 5′‐AAGGATCCGCCGCCATGGGTACTAGGAAAAAGGTTCAAGC‐3′ and 5′‐AAGATATCTTTTCTGTGTGACTGTTGGAGG‐3′. Hydin was also amplified from mouse testis cDNA, digested with EcoRV and NheI, and ligated into the PA‐tagged (C‐terminus) pCAG vectors. Primers used were 5′‐AAGATATCGCCGCCATGACCCTGAAGATCAAATGTGTGG‐3′ and 5′‐AAGCTAGCGCTGGTTTCCTGCTTTTCCTCC‐3′ for Hydin #1 (1‐408), 5′‐AAGATATCGCCGCCATGATCCTTGAAGACAGCG‐3′ and 5′‐AAGCTAGCCCCACAGGGGGAGGGGCTGGAGAGCAGC‐3′ for Hydin #2 (409‐800), and 5′‐AAGATATCGCCGCCATGGTCATCTCCCCCCACAGCACTGTGAGC‐3′ and 5′‐AAGCTAGCCACCTCAAAGCTGAGGTTGG‐3′ for Hydin #3 (801‐1218).
2.19. Co‐immunoprecipitation
Plasmids were transiently transfected into HEK293T cells and cultured for 24 hours. Immunoprecipitation using harvested cells was performed as previously described.19 FLAG M2 antibody (#F1804, Sigma‐Aldrich) was used for immunoprecipitation.
2.20. Statistical analysis
Statistical analyses were performed using Student’s t test. Differences were considered significant at P < .05 (*) or highly significant at P < .01 (**) and P < .001 (***). Error bars are standard deviation.
3. RESULTS
3.1. KIF9 is a testis‐enriched protein localized to sperm flagellum
RT‐PCR analysis using mouse tissues confirmed that Kif9 is expressed strongly in the testis with weak expression found in the brain, thymus, lung, and heart (Figure 1A). Western blotting analysis confirmed that KIF9 is expressed strongly in the testis (Figure 1A). Further, RT‐PCR analysis using mouse postnatal testes revealed that Kif9 starts to express from two weeks, which corresponds to the production of primary spermatocytes. KIF9 protein was detected from three weeks when round spermatids begin to appear (Figure 1B). We confirmed the expression of Kif9 in spermatocytes and spermatids using an in silico approach by examining an expression database (Supplemental Figure S1). These results suggest that KIF9 may play roles in spermatogenesis and/or fertilization.
Figure 1.
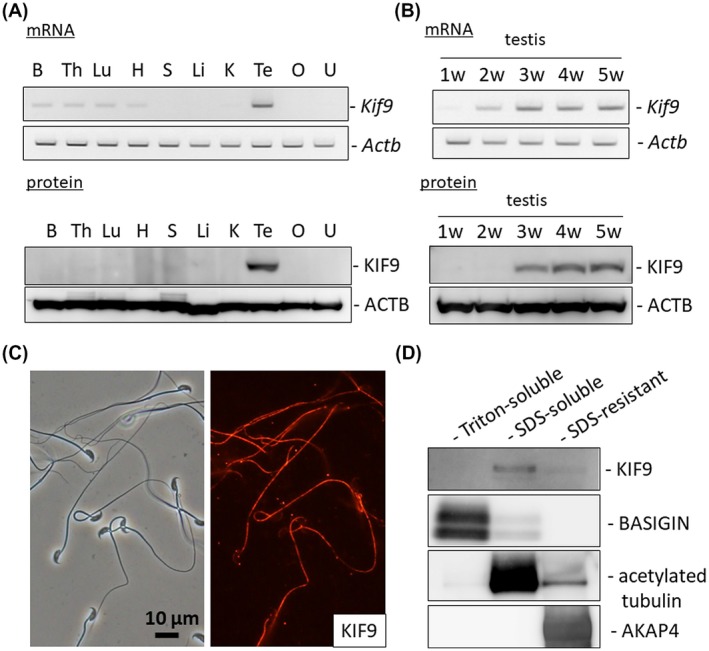
KIF9 is testis‐enriched and localized to mouse flagella. A, Upper, RT‐PCR of Kif9 using RNAs obtained from various tissues of ICR mice. Actb as control. Lower, immunoblot analysis of KIF9 using proteins obtained from various tissues of ICR mice. ACTB as control. B: brain, Th: thymus, Lu: lung, H: heart, S: spleen, Li: liver, K: kidney, Te: testis, O: ovary, and U: uterus. B, Upper, RT‐PCR of Kif9 using RNAs obtained from various postnatal testes of ICR mice. Actb as control. Lower, immunoblot analysis of KIF9 using proteins obtained from various postnatal testes of ICR mice. ACTB as control. C, Localization of KIF9 in spermatozoa. KIF9 is detected in the flagellum. D, Fractionation of mouse spermatozoa. KIF9 was found in the SDS‐soluble fraction. BASIGIN, acetylated tubulin, and AKAP4 were used as makers for the Triton‐soluble, SDS‐soluble, and SDS‐resistant fractions, respectively
Immunofluorescence analysis indicated that KIF9 was localized to the flagellum (Figure 1C). To further analyze KIF9 localization in the flagellum, we fractionated sperm proteins into a Triton X‐100 soluble fraction that contains transmembrane and cytosolic proteins, an SDS‐soluble fraction that contains axonemal proteins, and an SDS‐resistant fraction that contains proteins localized in the accessory structures such as outer dense fibers and fibrous sheath.10, 11 KIF9 was found in the SDS‐soluble fraction (Figure 1D), suggesting that KIF9 is localized in the axoneme, which is consistent with the studies done in Chlamydomonas.5, 6
3.2. Kif9‐mutated male mice are subfertile and exhibit partially impaired zona pellucida (ZP) penetration
To analyze the function of KIF9 in the spermatozoa, we generated Kif9‐mutant mice using the CRISPR/Cas9 system. We injected a pX330 plasmid expressing Cas9 and a gRNA that targets exon 2 (Figure 2A)14 into the pronuclei of fertilized oocytes and obtained Kif9‐mutant mice that possessed a 16 bp deletion (Figure 2B). Because this deletion disrupts the StuI restriction enzyme site, genotyping can be done by digesting the PCR product with the StuI enzyme (Figure 2C). The 16 bp deletion resulted in a frameshift mutation (P15L) with a premature stop codon introduced three amino acids later (Figure 2D). Obtained Kif9−16/−16 mice did not exhibit overt abnormalities including hydrocephalus that is often observed when the motility of ependymal cilia is impaired.28, 29 We confirmed that KIF9 was depleted in Kif9−16/−16 male testis and spermatozoa with Western blotting (Figure 2E) and in the null spermatozoa with immunofluorescence (Supplemental Figure S2A). We then analyzed the testis sections of Kif9−16/−16 mice (Supplemental Figure S2B). Although there is a study showing that KIF9 regulates matrix degradation by macrophage podosomes,30 no abnormal structures were observed in Kif9−16/−16 testis including spermatogenesis.
Figure 2.
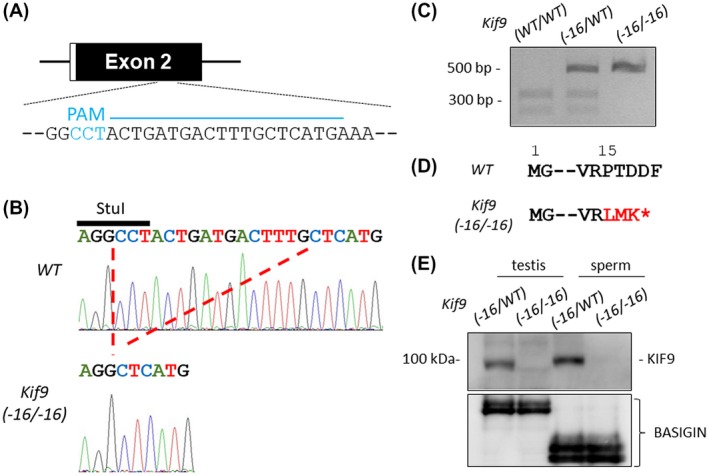
Generation of Kif9‐mutant mice. A, CRISPR/Cas9 targeting scheme. gRNA was designed within exon 2 that contains the start codon. Cyan characters indicate PAM (protospacer adjacent motif) sequence. B, Wave pattern sequence of Kif9. In mutants, 16 bp nucleotides were deleted. C, Genotyping Kif9−16/−16 mice by StuI digestion. D, The 16 bp deletion caused a P15L mutation resulting in a premature stop codon introduced three amino acids later. E, Protein expression of KIF9 in testis and cauda epididymal spermatozoa. BASIGIN as a loading control
Next, to examine fertility, Kif9−16/−16 male mice were mated with wild‐type females for two months and found that homozygous male mice were subfertile (Figure 3A). Further, fewer numbers of eggs were fertilized when we performed in vitro fertilization (IVF) using the spermatozoa from Kif9−16/−16 mice (Figure 3B). Lower fertilization rates in IVF could not be rescued by removing cumulus cells (Figure 3C); however, eggs were fertilized when the ZP was removed (Figure 3D), indicating that ZP penetration is partially impaired in Kif9−16/−16 mice. Although several KO mouse lines exhibit impaired ZP binding,31 spermatozoa from Kif9−16/−16 mice could bind to the ZP (Supplemental Figure S3A). We also analyzed the phosphorylation status of tyrosine residues, a hallmark of the capacitation process32; however, no differences were observed between Kif9‐16/WT and Kif9−16/−16 mice (Supplemental Figure S3B).
Figure 3.
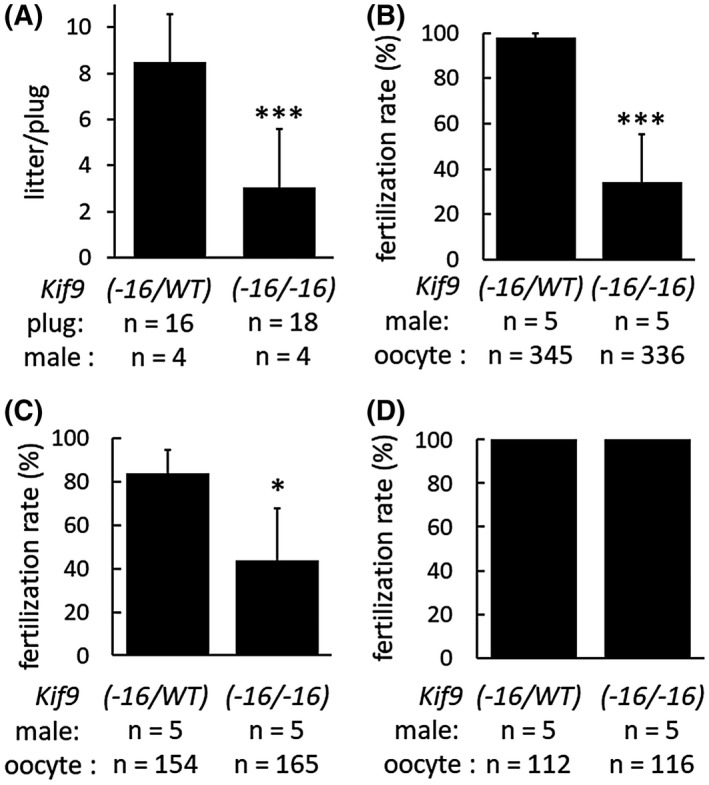
In vivo and in vitro fertility of Kif9−16/−16 male mice. A, Number of litters born per plug detected. n = 4 males each for Kif9‐16/WT and Kif9−16/−16 mice. B, IVF with cumulus‐intact oocytes. n = 5 males each for Kif9‐16/WT and Kif9−16/−16 mice. C, IVF with cumulus‐free oocytes. n = 5 males each for Kif9‐16/WT and Kif9−16/−16 mice. D, IVF with zona pellucida‐free oocytes. n = 5 males each for Kif9‐16/WT and Kif9−16/−16 mice
3.3. Kif9−16/−16 mice exhibit impaired sperm motility
Localization of KIF9 to the flagellum and partially impaired ZP penetration observed in Kif9−16/−16 mice suggest that KIF9 may play roles in regulating flagellar motility. Therefore, we analyzed sperm motility using a computer‐assisted sperm analysis system. In contrast to the control spermatozoa that move linearly, the trajectory of the moving spermatozoa was circular in Kif9−16/−16 mice (Figure 4A and Supplemental Movies S1, S2), although percentages of motile spermatozoa were comparable between Kif9‐16/WT and Kif9−16/−16 mice (Figure 4B). Consistent with this observation, velocity parameters such as average path velocity (VAP), straight line velocity (VSL), and curvilinear velocity (VCL) were lower in the Kif9−16/−16 mice than those of Kif9‐16/WT mice (Figure 4C), indicating that sperm motility is impaired in Kif9−16/−16 mice. To further analyze sperm motility defects, we traced the flagellar waveform (Figure 4D). Flagella of the control spermatozoa could bend to both sides (pro‐hook and anti‐hook)33; however, the majority of spermatozoa from Kif9−16/−16 mice could bend only to the side of the hook (pro‐hook) (the number of pro‐hook stall = 105, the number of anti‐hook stall = 30, the number of spermatozoa without stall = 18 out of 153 spermatozoa examined, number of males = 3), which may cause the circular motion of spermatozoa. These results indicate that KIF9 is important in regulating the flagellar waveform pattern.
Figure 4.
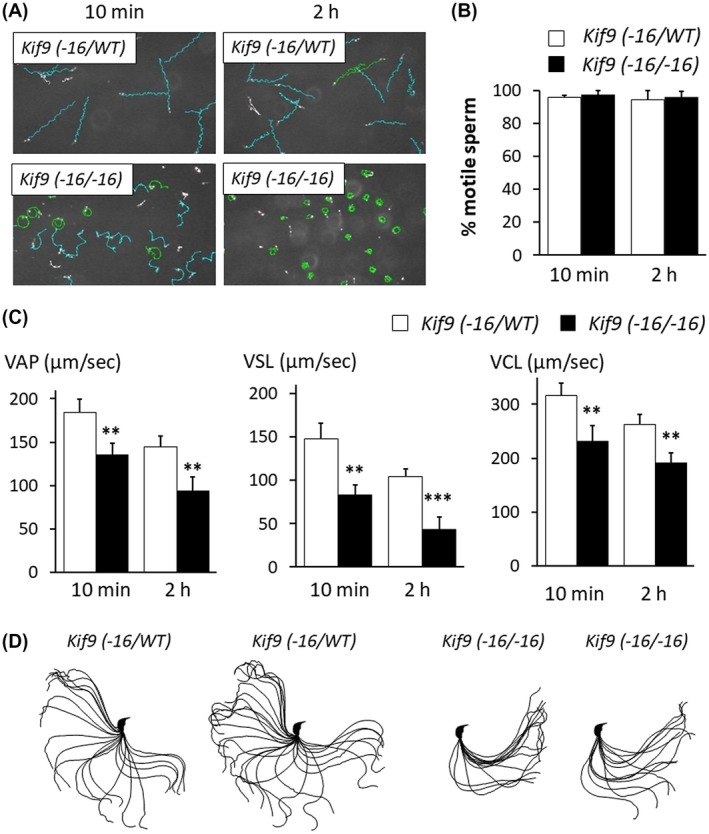
Sperm motility is impaired in Kif9−16/−16 mice. A, Sperm motility tracing performed by a computer‐assisted sperm analysis system after 10 minutes and 2 hours incubation. Cyan tracks were defined as spermatozoa with progressive motility (VSL/VAP ≧ 0.5 and VAP ≧ 50 µm/sec). B, Percentage of motile sperm. n = 4 males each for Kif9‐16/WT and Kif9−16/−16 mice. C, VAP (average path velocity), VSL (straight line velocity), and VCL (curvilinear velocity) were analyzed. n = 4 males each for Kif9−16/WT and Kif9−16/−16 mice. D, Flagellar waveforms were analyzed 2 hours after incubation. The motility was videotaped at 200 frames per second. Single frames throughout one beating cycle were superimposed
3.4. Generation and phenotypic analysis of Kif9 “large deletion” mice
Because antibodies used to analyze KIF9 depletion (Figure 2E and Supplemental Figure S2A) recognize the N‐terminus region, there is a possibility that a truncated protein is still produced from a different methionine in Kif9−16/−16 mice. To eliminate the possibility a truncated protein of KIF9 is causing the phenotype observed in Kif9−16/−16 mice, we designed two gRNAs to excise the entire Kif9 gene, one near the start codon that is different from gRNA used for the 16 bp deletion and another one near the stop codon (Supplemental Figure S4A). In the large deletion (LD) mutant mice, 41 902 bp was deleted and the LD was verified by PCR (Supplemental Figure S4B). Kif9LD/LD mice did not exhibit overt abnormalities including hydrocephalus, which is consistent with Kif9−16/−16 mice. The depletion of KIF9 in the testis and spermatozoa of Kif9LD/LD mice was confirmed with Western blotting (Supplemental Figure S4C). Kif9LD/LD male mice were subfertile (Supplemental Figure S4D) and exhibit impaired sperm motility (Supplemental Figure S4E), as observed in Kif9−16/−16 mice. These results indicate that male subfertility and impaired sperm motility are attributed to the deletion of KIF9.
3.5. KIF9 is associated with the axoneme central pair protein HYDIN
Because the deletion of axonemal proteins often leads to the disruption of axonemal structures,12, 34, 35, 36 we observed spermatozoa using transmission electron microscopy. No abnormalities were observed in both the midpiece (Figure 5A and Supplemental Figure S5) and principal piece (Figure 5B) of Kif9−16/−16 mice, indicating that impaired sperm motility is not caused by obvious structural defects of the axoneme.
Figure 5.
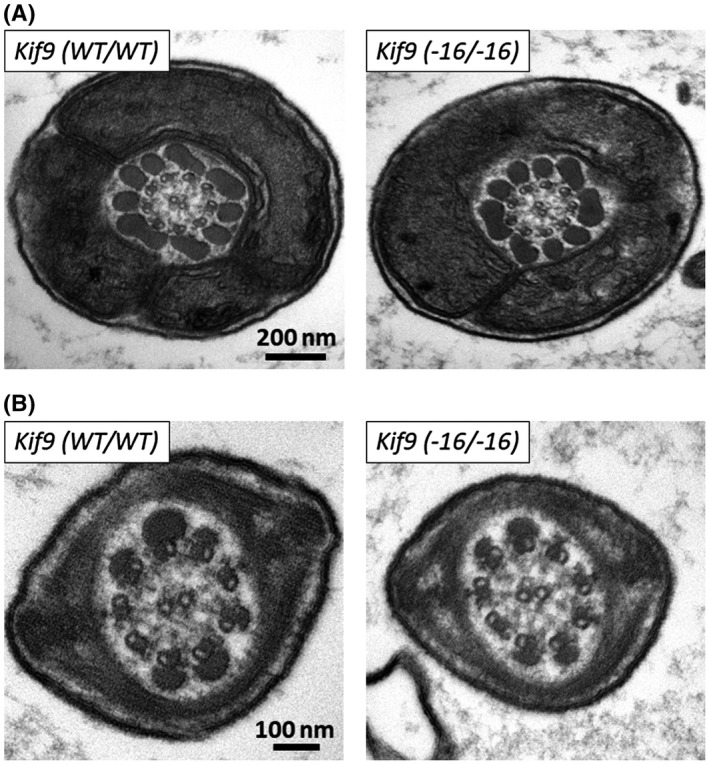
No obvious ultrastructural abnormalities were observed in the flagella of Kif9−16/−16 mice. The midpiece (A) and principal piece (B) of spermatozoa within the cauda epididymis were observed with transmission electron microscopy
In Chlamydomonas, KLP1 (Chlamydomonas ortholog of KIF9) is localized to the central pair of the axoneme and is associated with HYDIN, another central pair protein.37 In Chlamydomonas, HYDIN knockdown leads to a strong reduction in the amount of KLP1,37 suggesting an interaction between HYDIN and KLP1. In mice, HYDIN is localized to the central pair as well28; however, immunoprecipitation analysis of the KIF9‐HYDIN association could not be performed because it is difficult to solubilize KIF9 with mild lysis buffers (Figure 1D). Further, the lack of anti‐HYDIN antibodies makes it difficult to analyze HYDIN localization in the Kif9 mutant mice. Therefore, we analyzed the localization of KIF9 in Hydin KO spermatozoa. Hydin KO causes hydrocephalus and lethality before sexual maturation, which hampers the analysis of the mature spermatozoa.28 Recently, we knocked out Hydin in fluorescently tagged ES cells using the CRISPR/Cas9 system.26 By making chimeric mice with these ES cells, we were able to analyze spermatozoa derived from Hydin KO ES cells and we found that HYDIN is essential for flagellum formation.26 To analyze if KIF9 is associated with HYDIN in mice, we analyzed KIF9 localization using these chimeric mice. Consistent with a previous study,26 Hydin KO spermatozoa exhibit short tails (Figure 6A). When we performed immunofluorescence with KIF9 antibody, no signals were detected in Hydin KO spermatozoa (Figure 6A). Further, when we performed Western blotting using the spermatozoa from the cauda epididymis in which the contribution of Hydin KO ES cells was high, no KIF9 bands were observed, although signals of acetylated tubulin and RSPH9, a protein localized in the radial spoke, were detected (Figure 6B). The disappearance of KIF9 in Hydin KO spermatozoa suggests that KIF9 may be associated with HYDIN and is localized to the central pair of the axoneme in mice.
Figure 6.
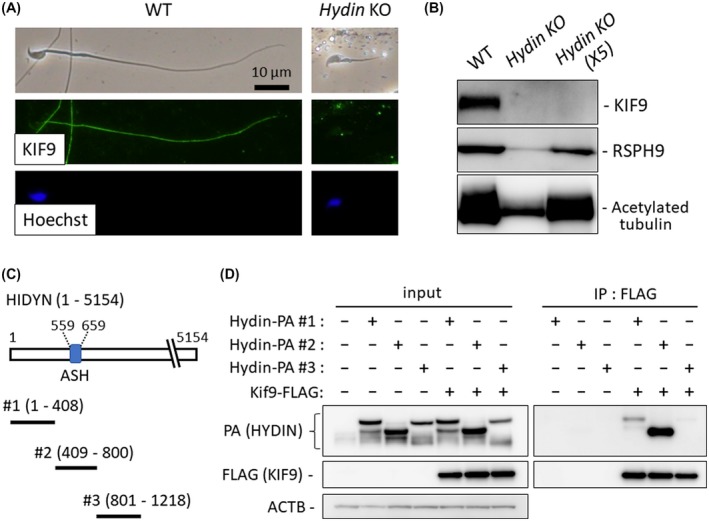
KIF9 disappeared in Hydin KO spermatozoa. A, Hydin KO spermatozoa obtained from the epididymis of Hydin KO chimeric mice were stained for KIF9. B, Western blotting analysis with the spermatozoa obtained from Hydin KO chimeric cauda epididymis. KIF9 was not detected even when five times the amount of protein was loaded (Hydin KO X5). In contrast, acetylated tubulin, indicating the presence of flagellum microtubules, and RSPH9, indicating the presence of radial spokes of the axoneme, were detected. C, Regions near the ASH domain of HYDIN were cloned for co‐immunoprecipitation analysis. D, Hydin‐PA #1, Hydin‐PA #2, or Hydin‐PA #3 were co‐expressed with Kif9‐FLAG in HEK293T cells and immunoprecipitation with FLAG M2 antibody was performed. ACTB as control
To further analyze KIF9‐HYDIN interaction, we expressed FLAG‐tagged KIF9 and PA‐tagged HYDIN in HEK293T cells. Because HYDIN contains 5154 amino acids, which makes it difficult to clone the whole Hydin sequence, we focused on the ASPM‐SPD2‐Hydin (ASH) domain. The ASH domain is found in cilia‐ or centrosome‐associated proteins and is shown to interact with a different kinesin, KIF13B.38 Immunoprecipitation analysis revealed that KIF9 bound to the region containing the ASH domain (HYDIN #2) and weakly bound to the N‐terminus region of HYDIN (#1), but not to region #3 (Figure 6C,D). These results suggest that KIF9 could bind to the N‐terminus region of HYDIN that contains the ASH domain.
4. DISCUSSION
In this study, we revealed that KIF9 is localized to the mouse flagellum. Further, KIF9 was detected in the SDS soluble fraction, suggesting that KIF9 is associated with the axoneme. Because KIF9 disappeared in Hydin KO spermatozoa and interaction of KIF9 and HYDIN was confirmed with co‐immunoprecipitation assay, it is likely that KIF9 is localized to the central pair of the axoneme, consistent with Chlamydomonas.5, 6
By mutating Kif9 in mice, we revealed that KIF9 is important for the progressive motility of spermatozoa and normal male fertility. Kif9 mutant mice were not completely infertile likely because there are variations in the motility of individual spermatozoa and the spermatozoa with good motility could fertilize oocytes. Detailed analysis of flagellar motility of Kif9 mutant mice showed that waveform patterns are asymmetric, indicating that switching of microtubule sliding is impaired. In Chlamydomonas, HYDIN is localized to the C2 microtubule of the central pair and is thought to be essential for the switch in bending direction by regulating dynein arm activity.37 By interacting with HYDIN through the ASH domain, KIF9 may also be involved in the switching. KIF9 possesses a motor domain in the C‐terminus and a previous study suggests that KIF9 possesses motor activity.30 It remains to be determined if the motor activity of KIF9 is involved in the directional switch in bending.
KIF9 belongs to the kinesin 9 family that contains another kinesin, KIF6.4 Northern blot analysis showed that Kif6 is expressed in mouse testis8; however, Kif6 mutant mice exhibited hydrocephalus leading to postnatal lethality,39 which makes it difficult to analyze KIF6 function in mature spermatozoa. The milder phenotype of Kif9 mutant mice compared to Hydin KO mice, such as subfertility, no abnormalities in axonemal ultrastructures, or no overt hydrocephalus, may be due to the compensation by KIF6. It is also possible that KIF9 plays more specific roles in regulating sperm flagella, rather than regulating ciliary motility that exhibits different waveform patterns from flagella.
There are studies showing that kinesins play roles in spermiogenesis through intraflagellar transport or intramanchette transport.40, 41, 42 Although we cannot exclude the possibility that KIF9 is involved in these transports, which is important for regulating sperm motility, we could not observe any abnormalities in ultrastructures with transmission electron microscopy. Other KIFs may be involved in these transports during spermiogenesis. It is noteworthy to mention that we also mutated Kif2b that is expressed strongly in the testis; however, the mutant male mice exhibited normal fertility.43
In summary, we reveal that Kif9‐mutant male mice exhibit impaired sperm motility and male subfertility. Because Kif9 is conserved in humans, revealing how KIF9 regulates flagellar motility may lead to better treatment for individuals with asthenozoospermia.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
H. Miyata and M. Ikawa designed the research; H. Miyata, K. Shimada, A. Morohoshi, S. Oura, T. Matsumura, Z. Xu, and Y. Oyama performed the research; H. Miyata, K. Shimada, A. Morohoshi, S. Oura, T. Matsumura, Z. Xu, Y. Oyama, and M. Ikawa analyzed the data; H. Miyata and M. Ikawa wrote the paper.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank Eri Hosoyamada and Mariko Tamura for technical assistance and Dr. Julio M. Castaneda for critical reading of the manuscript. This research was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI grants (JP17H04987 to H.M., JP17K17852 to K.S., JP19J12450 to A.M., JP19J21619 to S.O., and JP25112007, JP17H01394, JP19H05750 to M.I.); Takeda Science Foundation grant to H.M.; and the Japan Agency for Medical Research and Development (AMED) grant (JP19gm5010001 to M.I.); and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01HD087157 and R01HD088412 to M.I.); and the Bill & Melinda Gates Foundation (Grand Challenges Explorations grant OPP1160866 to M.I.).
Miyata H, Shimada K, Morohoshi A, et al. Testis‐enriched kinesin KIF9 is important for progressive motility in mouse spermatozoa. The FASEB Journal. 2020;34:5389–5400. 10.1096/fj.201902755R
Contributor Information
Haruhiko Miyata, Email: hmiya003@biken.osaka-u.ac.jp.
Masahito Ikawa, Email: ikawa@biken.osaka-u.ac.jp.
REFERENCES
- 1. Toshimori K, Eddy EM. The spermatozoon In: Knobil E, Neil JD, eds. Physiology of reproduction. 4th ed London: Academic Press; 2014:99‐148. [Google Scholar]
- 2. Eddy EM, Toshimori K, O'Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103‐115. [DOI] [PubMed] [Google Scholar]
- 3. Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod. 2011;17:524‐538. [DOI] [PubMed] [Google Scholar]
- 4. Hirokawa N, Tanaka Y. Kinesin superfamily proteins (KIFs): various functions and their relevance for important phenomena in life and diseases. Exp Cell Res. 2015;334:16‐25. [DOI] [PubMed] [Google Scholar]
- 5. Bernstein M, Beech PL, Katz SG, Rosenbaum JL. A new kinesin‐like protein (Klp1) localized to a single microtubule of the chlamydomonas‐flagellum. J Cell Biol. 1994;125:1313‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yokoyama R, O'Toole E, Ghosh S, Mitchell DR. Regulation of flagellar dynein activity by a central pair kinesin. Proc Natl Acad Sci USA. 2004;101:17398‐17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demonchy R, Blisnick T, Deprez C, et al. Kinesin 9 family members perform separate functions in the trypanosome flagellum. J Cell Biol. 2009;187:615‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakagawa T, Tanaka Y, Matsuoka E, et al. Identification and classification of 16 new kinesin superfamily (KIF) proteins in mouse genome (vol 94, pg 9654, 1997). Proc Natl Acad Sci USA. 1999;94:4214‐4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hermann BP, Cheng K, Singh A, et al. The mammalian spermatogenesis single‐cell transcriptome, from spermatogonial stem cells to spermatids. Cell Rep. 2018;25(1650‐1667):e1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao WL, Gerton GL, Moss SB. Proteomic profiling of accessory structures from the mouse sperm flagellum. Mol Cell Proteomics. 2006;5:801‐810. [DOI] [PubMed] [Google Scholar]
- 11. Castaneda JM, Hua R, Miyata H, et al. TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proc Natl Acad Sci USA. 2017;114:E5370‐E5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbasi F, Miyata H, Shimada K, et al. RSPH6A is required for sperm flagellum formation and male fertility in mice. J Cell Sci. 2018;131:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naito Y, Hino K, Bono H, Ui‐Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off‐target sites. Bioinformatics. 2015;31:1120‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep. 2013;3:3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho YG, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in Ksom ‐ augmentation by amino‐acids and analysis of gene‐expression. Mol Reprod Dev. 1995;41:232‐238. [DOI] [PubMed] [Google Scholar]
- 17. Oji A, Noda T, Fujihara Y, et al. CRISPR/Cas9 mediated genome editing in ES cells and its application for chimeric analysis in mice. Sci Rep. 2016;6:31666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujihara Y, Kaseda K, Inoue N, Ikawa M, Okabe M. Production of mouse pups from germline transmission‐failed knockout chimeras. Transgenic Res. 2013;22:195‐200. [DOI] [PubMed] [Google Scholar]
- 19. Morohoshi A, Miyata H, Shimada K, et al. Nexin‐Dynein regulatory complex component DRC7 but not FBXL13 is required for sperm flagellum formation and male fertility in mice. PLoS Genet. 2020;16:e1008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gyobu S, Miyata H, Ikawa M, et al. A role of TMEM16E carrying a scrambling domain in sperm motility. Mol Cell Biol. 2016;36:645‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muro Y, Hasuwa H, Isotani A, et al. Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization. Biol Reprod. 2016;94:1-7. [DOI] [PubMed] [Google Scholar]
- 22. Miyata H, Satouh Y, Mashiko D, et al. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science. 2015;350:442‐445. [DOI] [PubMed] [Google Scholar]
- 23. Goodson SG, Zhang ZJ, Tsuruta JK, Wang W, O'Brien DA. Classification of mouse sperm motility patterns using an automated multiclass support vector machines model. Biol Reprod. 2011;84:1207‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baba SA, Mogami Y. An approach to digital image‐analysis of bending shapes of eukaryotic flagella and cilia. Cell Motil Cytoskel. 1985;5:475‐489. [Google Scholar]
- 25. Shimada K, Kato H, Miyata H, Ikawa M. Glycerol kinase 2 is essential for proper arrangement of crescent‐like mitochondria to form the mitochondrial sheath during mouse spermatogenesis. J Reprod Dev. 2019;65:155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oura S, Miyata H, Noda T, et al. Chimeric analysis with newly established EGFP/DsRed2‐tagged ES cells identify HYDIN as essential for spermiogenesis in mice. Exp Anim. 2019;68:25‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niwa H, Yamamura K, Miyazaki J. Efficient selection for high‐expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193‐199. [DOI] [PubMed] [Google Scholar]
- 28. Lechtreck KF, Delmotte P, Robinson ML, Sandersoll MJ, Witman GB. Mutations in Hydin impair ciliary motility in mice. J Cell Biol. 2008;180:633‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sasaki K, Shiba K, Nakamura A, et al. Calaxin is required for cilia‐driven determination of vertebrate laterality. Commun Biol. 2019;2:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cornfine S, Himmel M, Kopp P, et al. The kinesin KIF9 and reggie/flotillin proteins regulate matrix degradation by macrophage podosomes. Mol Biol Cell. 2011;22:202‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujihara Y, Miyata H, Ikawa M. Factors controlling sperm migration through the oviduct revealed by gene‐modified mouse models. Exp Anim. 2018;67:91‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visconti PE, Bailey JL, Moore GD, Pan D, Olds‐Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129‐1137. [DOI] [PubMed] [Google Scholar]
- 33. Ishijima S, Baba SA, Mohri H, Suarez SS. Quantitative analysis of flagellar movement in hyperactivated and Acrosome‐reacted golden hamster spermatozoa. Mol Reprod Dev. 2002;61:376‐384. [DOI] [PubMed] [Google Scholar]
- 34. Sapiro R, Kostetskii I, Olds‐Clarke P, Gerton GL, Radice GL, Strauss JF. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm‐associated antigen 6. Mol Cell Biol. 2002;22:6298‐6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sironen A, Kotaja N, Mulhern H, et al. Loss of SPEF2 Function in mice results in spermatogenesis defects and primary ciliary dyskinesia. Biol Reprod. 2011;85:690‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McKenzie CW, Craige B, Kroeger TV, et al. CFAP54 is required for proper ciliary motility and assembly of the central pair apparatus in mice. Mol Biol Cell. 2015;26:3140‐3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lechtreck KF, Witman GB. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J Cell Biol. 2007;176:473‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schou KB, Mogensen JB, Morthorst SK, et al. KIF13B establishes a CAV1‐enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling. Nat Commun. 2017;8:14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Konjikusic MJ, Yeetong P, Boswell CW, et al. Mutations in Kinesin family member 6 reveal specific role in ependymal cell ciliogenesis and human neurological development. Plos Genet. 2018;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller MG, Mulholland DJ, Vogl AW. Rat testis motor proteins associated with spermatid translocation (dynein) and spermatid flagella (kinesin‐II). Biol Reprod. 1999;60:1047‐1056. [DOI] [PubMed] [Google Scholar]
- 41. Lehti MS, Kotaja N, Sironen A. KIF3A is essential for sperm tail formation and manchette function. Mol Cell Endocrinol. 2013;377:44‐55. [DOI] [PubMed] [Google Scholar]
- 42. Kierszenbaum AL. Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Dev. 2002;63:1‐4. [DOI] [PubMed] [Google Scholar]
- 43. Miyata H, Castaneda JM, Fujihara Y, et al. Genome engineering uncovers 54 evolutionarily conserved and testis‐enriched genes that are not required for male fertility in mice. Proc Natl Acad Sci USA. 2016;113:7704‐7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


