Abstract
Migraine is a burdensome disease with an especially high prevalence in women between the age of 15 and 49 years. Non-pharmacological, non-invasive therapeutic methods to control symptoms are increasingly in demand to complement a multimodal intervention approach in migraine. Thirty-seven subjects (age: 25.0 ± 4.1 years; 36 females) diagnosed with high-frequency episodic migraine who presented at least one active myofascial trigger point (mTrP) in the trapezius muscles and at least one latent mTrP in the deltoid muscles bilaterally prospectively underwent six sessions of repetitive peripheral magnetic stimulation (rPMS) over two weeks. Patients were randomly assigned to receive rPMS applied to the mTrPs of the trapezius (n = 19) or deltoid muscles (n = 18). Whereas the trapezius muscle is supposed to be part of the trigemino-cervical complex (TCC) and, thus, involved in the pathophysiology of migraine, the deltoid muscle was not expected to interfere with the TCC and was therefore chosen as a control stimulation site. The headache calendar of the German Migraine and Headache Society (DMKG) as well as the Migraine Disability Assessment (MIDAS) questionnaire were used to evaluate stimulation-related effects. Frequency of headache days decreased significantly in both the trapezius and the deltoid group after six sessions of rPMS (trapezius group: p = 0.005; deltoid group: p = 0.003). The MIDAS score decreased significantly from 29 to 13 points (p = 0.0004) in the trapezius and from 31 to 15 points (p = 0.002) in the deltoid group. Thus, rPMS applied to mTrPs of neck and shoulder muscles offers a promising approach to alleviate headache frequency and symptom burden. Future clinical trials are needed to examine more profoundly these effects, preferably using a sham-controlled setting.
Subject terms: Headache, Migraine, Paediatric neurological disorders
Introduction
According to the Global Burden of Disease Study more than one billion people were suffering from migraine in 2016, making migraine one of the most prevalent neurological disorders worldwide1. Especially women between the age of 15 and 49 years are concerned – in this group migraine is also the first cause of disability worldwide1–4. Further, migraineurs show a considerably reduced health-related quality of life (QoL) and considerable loss in work productivity5–7. The high impact of migraine on QoL is reflected by percentages as high as 85% of those affected who feel helpless, depressed or not understood, with 83% reporting sleeping difficulties and 55% being afraid of the next migraine attack8. However, this frequent and debilitating headache disorder is still under-diagnosed and often inadequately treated9.
Despite the high global prevalence of migraine and the considerable worsening of QoL among affected subjects, the complex pathophysiology of migraine is not completely understood10,11. Over the recent years, the neck and shoulder region of migraineurs is more and more focused on in the field of migraine research. Specifically, neck pain may play an important role as a trigger or premonitory symptom or part of migraine attacks, with muscular pain and generalized hyperalgesia in the neck and shoulder region being more frequent in subjects with migraine than in healthy controls12–16. The interrelation of neck pain and migraine might be explained by the concept of the trigemino-cervical complex (TCC), which suggests that peripheral sensitization of trigemino-cervical neurons may influence central nociception by a potential convergence of cervical and dural nociceptive afferents in the caudal nuclei of the trigeminal nerves in the brain stem17–19. Myofascial trigger points (mTrPs) in migraineurs could be regarded key components related to muscular pain and hyperalgesia as well as headache symptoms, and mTrPs have shown to respond positively to local treatment and, thus, could be promising sites for targeted intervention20–25. Hence, mTrPs in the neck and shoulder region seem to be a gateway to enter and influence the TCC with the aim of achieving symptom alleviation in migraineurs20.
Central modulation in migraineurs can be reached by different invasive and non-invasive techniques26–29. Promising examples include transcranial magnetic stimulation (TMS)30–33, transcranial direct current stimulation (tDCS)34–36, supra-orbital nerve stimulation (SONS)37–39, and vagus nerve stimulation (VNS)40–43. In addition, repetitive peripheral magnetic stimulation (rPMS) has been introduced recently as a novel method to stimulate the upper trapezius muscles in subjects with migraine, with its application being feasible, well-tolerated, and mostly free of any adverse effects among migraineurs44. Moreover, migraine frequency decreased substantially according to three-month follow-up after rPMS to trapezius muscles as assessed by the Migraine Disability Assessment (MIDAS) questionnaire44. Of note, the local pressure pain threshold (PPT) of the trapezius muscles was steadily increasing during the course of the six sessions of rPMS applied in this previous study, indicating local muscular effects besides alleviation of headache symptoms44. In another trial rPMS was administered either to the trapezius muscle (considered part of the TCC) or the deltoid muscle (considered not to be part of the TCC) to evaluate local effects and differences depending on the muscle stimulated45. Despite using the same stimulation protocol for both the trapezius and deltoid muscles, the trapezius muscles showed considerably higher PPTs than the deltoid muscles according to post-interventional examination, which was found regardless of the stimulated muscle (rPMS to the trapezius but also the deltoid muscles influenced the PPTs in trapezius muscles)45.
These previous results imply that rPMS could alleviate muscular sensitivity at the stimulated area as well as headache symptoms within the context of the TCC, making rPMS a promising neuromodulatory technique44. To date, rPMS offers an auspicious non-invasive neuromodulatory approach that can directly intervene at peripheral muscular structures in the neck and shoulder area whilst potentially using the TCC as a gateway to modulate central nociception simultaneously. However, a closer look at the clinical outcome following rPMS applied to muscles inside and outside of the TCC in migraineurs is still needed to evaluate in detail the efficacy and specificity of this approach within the concept of the TCC.
Against this background, the aim of this study is to systematically investigate potential central effects of rPMS applied to either the trapezius or the deltoid muscles. We hypothesize a stronger alleviation of migraine symptoms when rPMS is applied to the trapezius muscles when compared to the deltoid muscles.
Results
Demographics and baseline characteristics
Table 1 gives an overview about demographics and baseline characteristics of the 37 participants included in this study. Thirty-six of them were female, one was male. The mean age was 25.0 ± 4.1 years (range: 19–35 years). The included subjects were randomly assigned to the trapezius group (n = 19) or the deltoid group (n = 18). No significant differences were found between subjects of the trapezius group compared to subjects of the deltoid group regarding demographics or items of the headache diary of the German Migraine andHeadache Society (DMKG) or the MIDAS questionnaire (p > 0.05). No dropouts were registered.
Table 1.
Demographics and baseline characteristics according to the headache diary of the German Migraine and Headache Society (DMKG) and Migraine Disability Assessment (MIDAS) questionnaire.
| Trapezius group N = 19 | Deltoid group N = 18 | p* | |
|---|---|---|---|
| Median (range) or % (N) | |||
| Subject characteristics | |||
| Age (in years) | 25 (19–35) | 24.5 (19–32) | 0.702 |
| Female sex | 100.0 (19) | 94.4 (17) | 0.978 |
| Headache diary of the DMKG (assessed daily over the course of 90 days before and after intervention) | |||
| Number of days with headache | 23 (17–37) | 20 (15–40) | 0.057 |
| Cumulative headache duration (hours) | 194 (78–429) | 121 (60–482) | 0.448 |
| Duration per headache attack (hours) | 6.8 (4.0–14.8) | 6.1 (3.3–19.3) | 0.988 |
| Average headache intensity (according to VAS) | 5.3 (3.5–6.9) | 5.2 (3.9–6.5) | 0.727 |
| Vomiting (incidences per 90 days) | 0 (0–4) | 0 (0–9) | 0.521 |
| Nausea (incidences per 90 days) | 7 (0–25) | 5 (0–16) | 0.344 |
| Medication (intake per 90 days) | 12 (0–29) | 11 (3–27) | 0.927 |
| MIDAS questionnaire (assessed for the 90 days before and after intervention) | |||
| Missing school/work (days) | 1 (0–5) | 1 (0–12) | 0.405 |
| Productivity at school/work reduced by half (days) | 10 (2–20) | 7.5 (3–23) | 0.247 |
| Could not do household work (days) | 5 (0–11) | 4.5 (0–18) | 0.903 |
| Household work productivity reduced by half (days) | 5 (0–15) | 6 (0–14) | 0.843 |
| Missing family, social, or leisure activities (days) | 3 (0–10) | 4.5 (0–17) | 0.375 |
*Wilcoxon signed-rank test or Chi-squared test.
Pre- vs. post-interventional results according to the DMKG headache diary and the MIDAS questionnaire
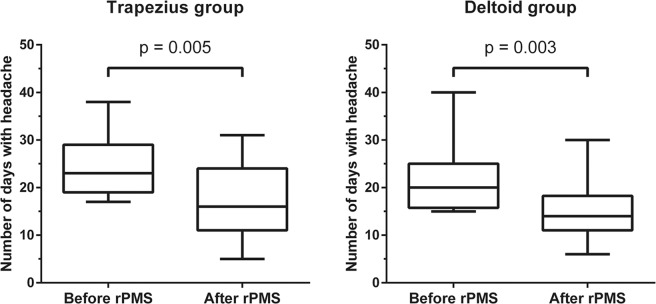
Table 2 gives an overview about the pre- and post-interventional status according to the DMKG headache diary and the MIDAS questionnaire for both groups. The headache frequency per 90 days significantly decreased in the trapezius group from 23 to 16 days (p = 0.005, relative reduction −34.8%) and in the deltoid group from 20 to 14 days (p = 0.003, relative reduction −32.5%; Fig. 1). Regarding the cumulative duration of headache attacks there was a tendency towards declining values (trapezius group: p = 0.068, relative reduction −23.2%; deltoid group: p = 0.076, relative reduction −37.2%).
Table 2.
Pre- versus post-interventional results according to the headache diary of the German Migraine and Headache Society (DMKG) and Migraine Disability Assessment (MIDAS) questionnaire.
| Trapezius group N = 19 | p* | Deltoid group N = 18 | p* | |||
|---|---|---|---|---|---|---|
| Pre-stimulation | Post-stimulation | Pre-stimulation | Post-stimulation | |||
| Median (range) | Median (range) | |||||
| Headache diary of the DMKG (assessed daily over the course of 90 days before and after intervention) | ||||||
| Number of days with headache | 23 (17–37) | 16 (5–31) | 0.005 | 20 (15–40) | 14 (6–30) | 0.003 |
| Cumulative headache duration (hours) | 194 (78–429) | 146.5 (40–336) | 0.068 | 121 (60–482) | 97.5 (19–420) | 0.076 |
| Duration per headache attack (hours) | 6.8 (4.0–14.8) | 7.8 (3.6–16.9) | 0.606 | 6.1 (3.3–19.3) | 6.9 (2.8–17.5) | 0.704 |
| Average headache intensity (according to VAS) | 5.3 (3.5–6.9) | 5.9 (4.3–7.9) | 0.161 | 5.2 (3.9–6.5) | 5.3 (3.3–6.7) | 0.584 |
| Vomiting (incidences per 90 days) | 0 (0–4) | 0 (0–3) | 0.523 | 0 (0–9) | 0 (0–2) | 0.819 |
| Nausea (incidences per 90 days) | 7 (0–25) | 4 (0–29) | 0.138 | 5 (0–16) | 3.5 (0–17) | 0.666 |
| Medication (intake per 90 days) | 12 (0–29) | 9 (0–27) | 0.254 | 11 (3–27) | 9 (2–17) | 0.302 |
| MIDAS questionnaire (assessed for the 90 days before and after intervention) | ||||||
| Missing school/work (days) | 1 (0–5) | 1 (0–5) | 0.914 | 1 (0–12) | 1 (0–6) | 0.630 |
| Productivity at school/work reduced by half (days) | 10 (2–20) | 4 (0–10) | 0.001 | 7.5 (3–23) | 4 (0–12) | 0.005 |
| Could not do household work (days) | 5 (0–11) | 2 (0–15) | 0.095 | 4.5 (0–18) | 2 (0–12) | 0.160 |
| Household work productivity reduced by half (days) | 5 (0–15) | 2 (0–7) | 0.002 | 6 (0–14) | 3 (0–11) | 0.077 |
| Missing family, social, or leisure activities (days) | 3 (0–10) | 2 (0–10) | 0.324 | 4.5 (0–17) | 2.5 (0–12) | 0.245 |
*Wilcoxon signed-rank test. P-values printed in bold are statistically significant after correction for multiple testing using the Benjamini-Hochberg procedure with a false discovery rate (FDR) of 10%.
Figure 1.
Number of days with headache. The box plots depict the number of days with headache according to evaluation by the headache diary of the German Migraine and Headache Society (DMKG), which was carried out before and after the two-week interval of repetitive peripheral magnetic stimulation (rPMS). Median values with 25% and 75% percentiles and minimum and maximum whiskers are shown separately for the trapezius group and deltoid group. There was a statistically significant difference between the pre and post-interventional assessments in both groups (trapezius group: p = 0.005, deltoid group: p = 0.003).
The MIDAS score decreased from 29 to 13 points (p = 0.0004) in the trapezius group and from 31 to 15 points in the deltoid group (p = 0.002). In both groups, the median MIDAS score changed from “severe impairment” to “moderate impairment” (trapezius group: p = 0.0005; deltoid group: p = 0.009). Overall, the MIDAS score improved significantly after rPMS (p = 0.001), considering all four MIDAS subgroups with the 37 study participants. After intervention, the productivity at school/work showed to be less affected by headache events (trapezius group: p = 0.001, relative reduction −40.0%; deltoid group: p = 0.005, relative reduction −53.3%) than prior to intervention. Also, productivity in household improved significantly after rPMS in the trapezius group (p = 0.002, relative reduction −60.0%), but not in the deltoid group.
Table 3 depicts the intervention effects for each variable and compares the effects between the trapezius und deltoid group, indicating no statistically significant differences (p > 0.05). Furthermore, we conducted a sensitivity analysis excluding the single man without any relevant changes regarding the results (Supplementary Table 1).
Table 3.
Differences between pre- and post-interventional results according to the headache diary of the German Migraine and Headache Society (DMKG) and Migraine Disability Assessment (MIDAS) questionnaire.
| Trapezius group N = 19 | Deltoid group N = 18 | p* | |
|---|---|---|---|
| Median (range) | |||
| Headache diary of the DMKG (assessed daily over the course of 90 days before and after intervention) | |||
| Frequency in 3 months | 8 (−9–23) | 6.5 (−6–12) | 0.647 |
| Cumulative duration | 45 (−69–228) | 45.05 (−13–138) | 0.486 |
| Average duration | −0.1 (−6.6–4.8) | 0.4 (−2.1–7.3) | 0.214 |
| Average intensity | −0.2 (−3.0–0.6) | 0.1 (−1.1–2.4) | 0.070 |
| Vomiting | 0 (0–2) | 0 (−2–7) | 0.207 |
| Nausea | 4 (−15–11) | 0 (−6–9) | 0.092 |
| Frequency of use of medication | 2 (−8–11) | 2.5 (−12–11) | 0.689 |
| MIDAS questionnaire (assessed for the 90 days before and after intervention) | |||
| Missing school/work | 0 (−5–4) | 0 (−3–7) | 0.533 |
| Productivity at school/work reduced by half | 4 (−1–18) | 4 (−1–11) | 0.541 |
| Could not do household work | 1 (−5–9) | 2 (−8–14) | 0.583 |
| Household work productivity reduced by half | 3 (−3–14) | 3 (−11–12) | 0.938 |
| Missing family, social, or leisure activities | 1 (−4–4) | 1.5 (−5–11) | 0.427 |
*Wilcoxon signed-rank test.
Discussion
This study evaluated the central effects of rPMS applied to trapezius or deltoid muscles in young adults suffering from high-frequency episodic migraine with a focus on possible differences in stimulation effects between subjects stimulated on either of these muscles. Our main findings were that days suffering from headache substantially decreased in both groups, whereas headache intensity and duration per attack did not significantly change when comparing the pre- to the post-interventional status (Tables 2 and 3; Fig. 1). Moreover, the MIDAS score, which is a measurement for the impairment in daily life due to migraine, considerably improved in both groups, with the productivity at school/work being less constrained in both groups and productivity at household being less impaired in the trapezius group after rPMS (Tables 2 and 3).
In general, alterations in neck and shoulder muscles like mTrPs as well as musculoskeletal dysfunction are supposed to play an essential role in the pathophysiology of migraine20,46,47. According to the concept of the TCC, peripheral sensitization and central convergence of cervical and meningeal nociceptive afferents in the brain stem could explain the important correlation of neck pain and migraine17–19. To date, rPMS seems to be a promising non-pharmacological, non-invasive approach that allows modulation of peripheral as well as central migraine-related symptoms via peripheral inflow, which is most likely taking effect on the basis of the TCC44. In a pilot study, rPMS was applied to the trapezius muscles of young migraineurs, leading to a decrease of migraine attacks and intensity of headache44. Furthermore, in another study local effects of rPMS on the trapezius muscles, supposed to be part of the TCC, and deltoid muscles, supposed not to be part of the TCC, were described by pre- and post-interventional measurements of the local PPT45. In detail, depending on the examined muscles the increase of PPTs differed significantly (subjects with stimulation of trapezius muscles: p = 0.021; subjects with stimulation of deltoid muscles: p = 0.080)45. Despite these promising first results focusing on the peripheral part of the TCC, further insights into rPMS and its central effects for intervention in migraine are lacking. Comparing rPMS to the latest investigations of other available neuromodulative techniques for intervention in migraine, the reduction of migraine attacks and days suffering from headache are generally in a comparable range33,36,39,43. In the ESPOUSE study, single-pulse TMS was applied to the occiput by the study participants twice a day during three months as a prophylactic treatment and also as an acute intervention during any migraine attack occurring in this period of time33. Application of single-pulse TMS led to an average reduction of 2.8 days with headache per month33. In comparison, tDCS applied to the area corresponding to the M1 in the dominant hemisphere ten times during three to five weeks led to a decrease of 3.0 days with headache per month36. Furthermore, SONS was applied to the center of the forehead by participants once a day over three months, being capable of reducing headache frequency by 2.8 days per month39. Participants deploying VNS three times a day for twelve weeks experienced a decline of 2.3 days with headache per month43. In the present study, rPMS applied to the trapezius muscle was able to decrease headache frequency by 2.7 days per month and by 2.2 days per month when applied to the deltoid muscle, respectively. It has to be noticed that rPMS, in comparison to other methods, is usually well tolerated, painless, and non-invasive44. Changes in the MIDAS score were only examined in a recent TMS study with a reduction of 3 points according to post-interventional evaluation48. In comparison, rPMS led to a clearer decrease depending on the stimulated muscle, which probably points to a considerably higher improvement in the QoL. In this context, the side effects of rPMS tend to be less severe than those reported for VNS and SONS and, consequently, might result in higher acceptance of rPMS and better satisfaction39,43,45.
Moreover, rPMS offers the possibility of simultaneously improving local hyperalgesia in neck and shoulder muscles of migraineurs by increasing the PPT of mTrPs in the trapezius muscles by direct or indirect stimulation45. Thus, on the one hand, rPMS has a substantial positive effect on musculature44,45. On the other hand, the peripheral modulation of the TCC via stimulation of the trapezius muscle could lead to a central modulation of nociceptive afferents in the brain stem17,44,45. This means that rPMS, although delivered peripherally at the muscle level, is able to influence central mechanisms that play a role in migraine pathophysiology and, hence, could improve migraine symptoms and frequency. Of note, a comparable effect occurs when rPMS is applied to the deltoid muscle in migraineurs. As the deltoid muscle is not expected to be part of the TCC, we initially hypothesized a less pronounced effect on migraine-related symptoms in the deltoid group – supported by the results of the previous publication, suggesting a more intense peripheral effect of rPMS on the trapezius muscles when compared to the deltoid muscles45. The positive central effect after stimulation of the deltoid muscle might be explained by the uplifting movement of the shoulder, which is provoked by rPMS on the deltoid muscle and which might indirectly active the trapezius muscle45. This would mean that stimulation of muscles outside the TCC that are, however, linked to the trapezius muscle might potentially allow an interaction with the central elements of the TCC in the brain stem.
Regarding the impact of rPMS among migraineurs, we have to acknowledge the important role placebo effects may play as they could generally influence the participants’ outcome considerably regarding treatment effects. In principal, the more complex a treatment is, the bigger potential placebo effects seem to be49,50. Especially procedures delivered by medical devices seem to entail more distinct placebo effects than oral pharmacological treatments49. Positive response expectancies might also intensify the placebo effect in analgesia51. Considering the placebo effect in migraine, there is a mean recovery rate of 40.7% in control groups according to an analysis of systematic reviews of pharmacological and non-pharmacological treatments in migraine52. In total, we cannot estimate the influence of a potential placebo effect as long as randomized controlled studies on rPMS are mostly lacking. Thus, a sham-controlled study is needed to explore the extent of potential placebo effects. A sham-controlled study setup could be feasible by using a dedicated sham coil that is surrounded by an isolating shell to interrupt the electric field induced by the coil, thus avoiding real stimulation as suggested for TMS applications, for instance53. This would mean that during its use the device’s typical noise is audible without the electric field passing the plastic tube and the musculature is, however, not stimulated53. Moreover, a closer look at the deltoid muscle is needed to explain the influence and effect of its stimulation regarding central effects of rPMS. In this context, recent literature on electrical stimulation of skin afferents by a wearable device applied between the bellies of the deltoid and triceps muscles supports our observations54,55. On behalf of this device acute migraine attacks were effectively controlled, but preventive data have not become available so far. The concept of conditioned pain modulation could be the basis for those positive effects in acute migraine treatment as well as for our observations in the deltoid group54. Further, assessing the effects of a novel method like rPMS on advanced migraine markers evaluated by emerging technologies, i.e. the expression of specific neurosteroid patterns or facial electronic thermography, may track progress in the understanding of distinct neuromodulatory mechanisms56–60.
Due to the small sample size in each group and the inclusion of young patients suffering from high-frequency episodic migraine, the results are not to be generalized to other groups of migraine patients. Moreover, the female predominance of this cohort has to be considered. However, females usually outnumber male patients in migraine trials, including studies of the other neuromodulatory approaches61–63. On the one hand, this fact corresponds to the overall higher prevalence of migraine in women in epidemiological studies4,64,65. On the other hand, this ratio tends to be even more pronounced in treatment trials – an observation not extensively studied so far.
In conclusion, this study examined central effects of rPMS when applied to mTrPs of the trapezius muscles, considered part of the TCC, and of the deltoid muscles not being supposed to be part of the TCC in young adults with high-frequency episodic migraine. After six sessions of rPMS, suffering from headache decreased substantially in both the trapezius and the deltoid group. Consequently, rPMS offers a promising tool to intervene at muscular structures in migraineurs with both central, but also peripheral effects. Further clinical studies are needed to examine more profoundly the impact of a possible placebo effect, preferably using a sham-controlled setting.
Materials and Methods
Ethics and study enrollment
The institutional review boards of both universities of Munich (TUM and LMU) approved the study protocol. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all enrolled subjects. The study was registered with the German Clinical Trials Register (clinical trial registration number: DRKS00019870, 15/11/2019).
The following criteria needed to be met for inclusion: (1) age between 18 and 35 years, (2) migraine (according to the German version of the headache questionnaire modified according to the International Classification of Headache Disorders [ICHD], 3rd edition66–68), (3) a frequency of 15 to 44 days of headache during the 90 days prior to the first rPMS intervention (verified by the headache diary of the DMKG), (4) at least one active mTrP in one of the upper trapezius muscles (identified by a physiotherapist specialized in manual palpation of mTrPs), (5) no metallic implants (e.g. pacemaker, cochlear implants), and (6) written informed consent. The following criteria were defined as exclusion criteria: (1) any neurological illnesses except for migraine, (2) intake of any medication for migraine prophylaxis, (3) any changes in hormonal contraception during rPMS or 90 days before and after rPMS, and (4) pregnancy.
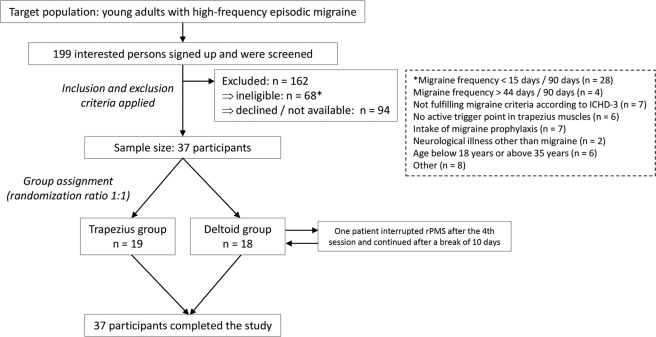
Recruitment of participants was achieved via announcements in the hospitals and local libraries of the two universities of Munich. Overall, 37 subjects (mean age: 25.0 ± 4.1 years, age range: 19–35 years, 36 females) who fulfilled the inclusion criteria were enrolled (Fig. 2). Sample size estimation for the present trial was based on a previous pilot study evaluating the feasibility of rPMS to the trapezius muscles in migraineurs, reporting an average reduction of headache frequency of 33% (SD = 33)44. To achieve a similar effect considering statistical power of 90% and an alpha error of 5%, a sample size of 18 subjects per group would be needed. The cohort considered in the present trial has been assessed in an earlier publication for other objectives, focusing on the methodological setup presentation of rPMS and evaluation of local muscular effects of rPMS45.
Figure 2.
Study design and enrollment. This flow chart provides an overview of the study design, its inclusion and exclusion criteria, and group assignments. Overall, 199 subjects were screened, with a final sample size of 37 participants undergoing repetitive peripheral magnetic stimulation (rPMS) after consideration of the study’s inclusion and exclusion criteria. No dropouts were registered.
Study design and setup
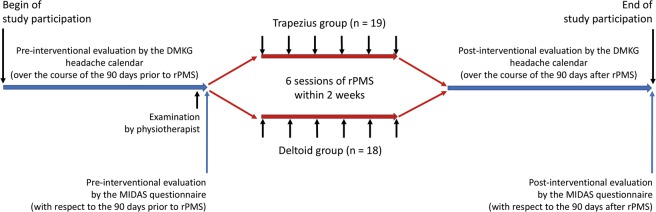
We chose a monocentric, prospective, randomized study design to systematically investigate the mid-term effects of rPMS on migraine when applied to skeletal musculature (Figs. 1 and 2). The enrollment phase was between August 2016 and April 2018. The pre- and post-interventional evaluation periods lasted for 90 days each and surrounded a two-week rPMS intervention phase; thus, the complete study participation covered almost seven months (Fig. 3).
Figure 3.
Timeline of study participation. This flow chart depicts the steps of the study in a chronological order, consisting of pre- and post-interventional assessments using the headache diary of the German Migraine and Headache Society (DMKG) and Migraine Disability Assessment (MIDAS) questionnaire. These assessments were grouped around a two-week interval of repetitive peripheral magnetic stimulation (rPMS) that was subdivided into six single sessions. Stimulation by rPMS was applied to either myofascial trigger points (mTrPs) of the trapezius muscles (trapezius group) or deltoid muscles (deltoid group). Determination of the presence and location of mTrPs was done by a physiotherapist.
The 37 enrolled subjects were randomized into two groups with a randomization ratio of 1:1. One group was supposed to receive rPMS bilaterally on the trapezius muscles (trapezius group: n = 19), the other group on the deltoid muscles (deltoid group: n = 18). Block randomization was achieved by drawing notes labeled with one of the group assignments from a sealed envelope, which was performed by a person other than the investigator conducting rPMS. The envelope contained the same number of notes for the trapezius group and deltoid group (n = 18 each). Consideration of an additional subject in the trapezius group (n = 19) was due to initial loss of a subject during post-interventional evaluation; however, this subject was reachable again later and provided completed evaluation.
In the course of the study, each subject underwent six sessions of rPMS on the designated muscles during two consecutive weeks in regular intervals (e.g., Monday/Wednesday/Friday or Tuesday/Thursday/Saturday). The right- and left-sided trapezius or deltoid muscles, depending on group assignment, were consecutively stimulated in each session, with the starting side of the first session being randomized as well (left side to be stimulated first: n = 18; right side to be stimulated first: n = 19).
Evaluation of migraine
For this study we applied the German version of the headache questionnaire modified according to the ICHD (3rd edition)66–68, the headache diary of the DMKG69, and the MIDAS questionnaire70,71 (Fig. 3).
Initially, all subjects had to fill in the German version of the headache questionnaire modified according to the ICHD (3rd edition) to verify migraine diagnosis by the following items: localization, duration and quality of pain, nausea, photophobia, phonophobia, and the influence of physical activity on the intensity of pain. A minimum of the mentioned criteria had to be fulfilled to receive a migraine-positive result67,68. Moreover, the presence of aura symptoms and an association with tension-type-headache (TTH) were recorded as well. The sensitivity and specificity of the German version of the headache questionnaire is 73% and 96% for the diagnosis of migraine, 85% and 98% for the diagnosis of TTH, and 62% and 97% for the diagnosis of a combination of both headache disorders67. Furthermore, the questionnaire was confirmed and revalidated to be used in epidemiological studies in order to assess the prevalence of different headache disorders68.
To monitor the headache frequency and characteristics the 90 days before the first rPMS session, the headache calendar of the DMKG needed to be filled in on a daily basis. With the help of the headache calendar, subjects recorded numerous items of each headache attack like date, trigger mechanisms (stress, relaxation, disturbance of sleep-awake rhythm, menstruation etc.), intensity, duration, quality, localization, forerunning symptoms (scintillating scotoma, paresthesia, aphasia etc.), concomitant symptoms like nausea, vomiting, photophobia, phonophobia or osmophobia, drug intake, dosage form, and pain relief. Subsequently, they were advised to continue filling in the headache diary during the period of stimulation sessions and also during the course of the 90 days after the last rPMS intervention. A basic diagnostic headache diary, such as the DMKG headache calendar, is a well-accepted tool that can facilitate a considerably higher diagnosis rate for subjects who filled in such a calendar for one month before consulting a specialist (complete diagnosis rate: 97.7%) when compared to subjects without any documentation of headache attacks (complete diagnosis rate: 87.7%)69. Moreover, a headache diary is useful to increase understanding of primary headaches and to strengthen awareness for triggers and medication intake69.
Besides, subjects were instructed to fill in the MIDAS questionnaire to estimate the impairment by headache events in different aspects of daily life before and after the two-week interval of rPMS application. Therefore, they had to estimate the number of days of incapacity for work and housekeeping, reduced capacity for work and housekeeping as well as absence in social activities due to headache symptoms during the 90 days before and after the interval of rPMS, respectively. The MIDAS questionnaire had to be completed prior to the first rPMS intervention and again after the 90 days of completion of the headache calendar after the last rPMS intervention. The MIDAS questionnaire has shown high internal consistency and reliability and correlates well with physicians’ clinical judgements of pain, disability, and need for medical care70,71. Correlation of the MIDAS score to the physicians’ assessment for “need of medical care” with r = 0.69 supports the suitability of the MIDAS questionnaire in clinical practice70.
Determination of myofascial trigger points
To identify mTrPs in trapezius or deltoid muscles, a certified physiotherapist qualified for mTrP palpation examined all participants by manual examinations few days before the first stimulation session (Fig. 3). The three standard criteria defining active mTrPs were carefully checked during examination by the physiotherapist: (1) a palpable taut band with local hypersensitivity, (2) a referred pain at the typical localization of the subject’s headache must be provoked by palpation of the mTrP, (3) a spontaneous evasive movement called “jump sign” as reaction to palpation of the mTrP47,72–74. However, a latent mTrP does not show any referred pain recognized as the typical headache during palpation, but fulfills the following two criteria of (1) a taut band with a sensitive spot, and (2) the so-called “jump sign”75.
The subjects needed to show either two active mTrPs in the trapezius muscles, e.g. one active mTrP in each of them, or, alternatively, one active and one latent mTrP in the trapezius muscles. Concerning the deltoid muscles, one latent mTrP needed to be identified by the physiotherapist bilaterally. In case that more than one active or latent mTrP could be identified in one muscle, the point which was most responsive in terms of painful sensation due to manual palpation was chosen by the physiotherapist, the other points were not further considered in the study. Overall, our aim was to identify four mTrPs in each subject, one mTrP within each side of the trapezius and deltoid muscles, respectively.
The four defined mTrPs were documented by marking the chosen points with a waterproof pen. The distances between the seventh cervical vertebra and the acromion were taken as well as photos to guarantee thorough documentation of mTrP locations in each subject.
Repetitive peripheral magnetic stimulation
During a two-week intervention period a total of six sessions of rPMS were applied to mTrPs of the trapezius or deltoid muscles, depending on group assignment, with the starting side of rPMS being alternated from session to session (Fig. 3). For stimulation, a Nexstim eXimia NBS system with a figure-of-eight stimulation coil was used (version 4.3; Nexstim Plc., Helsinki, Finland).
At the beginning of the first rPMS session, the intensity of rPMS was defined individually on the muscles to be stimulated and was kept for both sides for the following sessions. Stimulation was initiated with an intensity of 15% of the maximum output and gradually increased by steps of 5%. The participant was advised to evaluate the sensation caused by rPMS on a visual analogue scale (VAS) from 0 (maximum comfort) to 10 (maximum discomfort and pain). We chose the highest intensity still being rated lower than 5 points on this scale for stimulation of both sides. Then, for application of a standardized stimulation protocol during each session, we fixed the stimulation coil with direct skin contact above the mTrPs of the trapezius or deltoid muscles and ensured a constant and stable position of the coil in each session (Fig. 4)44,45. During each visit the left and right mTrPs of the trapezius muscles (trapezius group) or the left and right mTrPs of the deltoid muscles (deltoid group) were stimulated for 15 minutes per side. Stimulation of each side consisted of 20 bursts with a total of 6,000 stimuli and a 20-Hz frequency. A single burst was composed of 300 stimuli taking 15 seconds, followed by a relaxation time of 30 seconds. Besides, there was a break of approximately two minutes between stimulation to each side, allowing the operator to change the coil position for stimulation of the contralateral side.
Figure 4.
Setup of stimulation. This figure depicts the setup of stimulation by repetitive peripheral magnetic stimulation (rPMS). During pulse application to either myofascial trigger points (mTrPs) in the trapezius or deltoid muscles, the subject sat on a comfortable chair with armrests, headrest, and footplate in a relaxing position. After careful positioning of the stimulation coil over the individually defined mTrPs, a static coil holder was used to fix the correct position. Written informed consent was obtained from the subject of this figure to use this photo for publication.
Data analysis and statistics
All statistical data analyses were performed using R software (version 3.1.0; The R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism (version 6.04; GraphPad Software Inc., La Jolla, CA, USA).
For demographics, scores of the DMKG headache calendar, and MIDAS questionnaire, descriptive statistics including mean, standard deviation (SD), median, and ranges or absolute and relative frequencies were calculated. Furthermore, the overall MIDAS score (0–5 points: none to minimal impairment, 6–10 points: mild impairment, 11–20 points: moderate impairment, and >20 points: severe impairment) was calculated based on the results of the questionnaire. This was performed separately for the trapezius group and deltoid group and separately for the pre- and post-interventional assessments, respectively. To compare demographic data and scores between subjects assigned to the trapezius or deltoid group or between pre- and post-interventional status, we used Chi-squared tests or Wilcoxon signed-rank tests. For continuous variables, non-parametric tests were performed as normal distributions could not be assumed (based on Shapiro-Wilk tests and graphical examinations). A sensitivity analysis including only female patients was performed in addition, thus excluding the single male subject included in this study (results shown in Supplementary Table 1). Correction for multiple testing was performed using the Benjamini-Hochberg procedure with a false discovery rate (FDR) of 10%76. The level of statistical significance was set at p < 0.05.
Supplementary information
Author contributions
T.R., N.S., F.H., F.T.F., and M.L. were involved in data acquisition and data handling. T.R., N.S., F.H., L.A., M.B., and M.L. performed data analyses including statistics. H.K. and B.K. were responsible for determination and documentation of trigger points by manual palpation. T.R., N.S., F.H., L.A., M.B., and M.L. performed literature research and drafted the submitted version of the manuscript. N.S., F.H., S.K., and M.L. were responsible for the study design. F.H., S.K., and M.L. supervised the study. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
N.S. received honoraria from Nexstim Plc (Helsinki, Finland). S.K. is consultant for Nexstim Plc (Helsinki, Finland). T.R., F.H., L.A., F.T.F., B.K., H.K., M.B., and M.L. have nothing to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tabea Renner, Nico Sollmann, Michaela V. Bonfert and Mirjam N. Landgraf.
Supplementary information
is available for this paper at 10.1038/s41598-020-62701-9.
References
- 1.Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology17, 954-976, 10.1016/s1474-4422(18)30322-3 (2018). [DOI] [PMC free article] [PubMed]
- 2.Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: will health politicians now take notice? The journal of headache and pain. 2018;19:17. doi: 10.1186/s10194-018-0846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet390, 1211–1259, 10.1016/s0140-6736(17)32154-2 (2017). [DOI] [PMC free article] [PubMed]
- 4.Lipton RB, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 5.Vo P, Fang J, Bilitou A, Laflamme AK, Gupta S. Patients’ perspective on the burden of migraine in Europe: a cross-sectional analysis of survey data in France, Germany, Italy, Spain, and the United Kingdom. The journal of headache and pain. 2018;19:82. doi: 10.1186/s10194-018-0907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buse DC, et al. Life With Migraine: Effects on Relationships, Career, and Finances From the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59:1286–1299. doi: 10.1111/head.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonardi M, Raggi A. A narrative review on the burden of migraine: when the burden is the impact on people’s life. The journal of headache and pain. 2019;20:41. doi: 10.1186/s10194-019-0993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martelletti P, et al. My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. The journal of headache and pain. 2018;19:115. doi: 10.1186/s10194-018-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsarava Z, Mania M, Lampl C, Herberhold J, Steiner TJ. Poor medical care for people with migraine in Europe - evidence from the Eurolight study. The journal of headache and pain. 2018;19:10. doi: 10.1186/s10194-018-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charles A. The pathophysiology of migraine: implications for clinical management. The Lancet. Neurology. 2018;17:174–182. doi: 10.1016/s1474-4422(17)30435-0. [DOI] [PubMed] [Google Scholar]
- 11.Diener HC, Slomke MA, Limmroth V. [Headache and migraine] Der Nervenarzt. 2007;78(Suppl 1):7–13. doi: 10.1007/s00115-007-2332-y. [DOI] [PubMed] [Google Scholar]
- 12.Blaschek A, et al. Self-reported muscle pain in adolescents with migraine and tension-type headache. Cephalalgia: an international journal of headache. 2012;32:241–249. doi: 10.1177/0333102411434808. [DOI] [PubMed] [Google Scholar]
- 13.Blaschek A, et al. Self-reported neck pain is associated with migraine but not with tension-type headache in adolescents. Cephalalgia: an international journal of headache. 2014;34:895–903. doi: 10.1177/0333102414523338. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-de-las-Penas C, et al. Generalized neck-shoulder hyperalgesia in chronic tension-type headache and unilateral migraine assessed by pressure pain sensitivity topographical maps of the trapezius muscle. Cephalalgia: an international journal of headache. 2010;30:77–86. doi: 10.1111/j.1468-2982.2009.01901.x. [DOI] [PubMed] [Google Scholar]
- 15.Ozer, G. & Benlier, N. Neck pain: is it part of a migraine attack or a trigger before a migraine attack? Acta neurologica Belgica, 10.1007/s13760-018-1030-9 (2018). [DOI] [PubMed]
- 16.Lampl C, Rudolph M, Deligianni CI, Mitsikostas DD. Neck pain in episodic migraine: premonitory symptom or part of the attack? The journal of headache and pain. 2015;16:566. doi: 10.1186/s10194-015-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartsch T, Goadsby PJ. The trigeminocervical complex and migraine: current concepts and synthesis. Current pain and headache reports. 2003;7:371–376. doi: 10.1007/s11916-003-0036-y. [DOI] [PubMed] [Google Scholar]
- 18.Piovesan EJ, et al. Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia: an international journal of headache. 2001;21:107–109. doi: 10.1046/j.1468-2982.2001.00166.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnston MM, Jordan SE, Charles AC. Pain referral patterns of the C1 to C3 nerves: implications for headache disorders. Annals of neurology. 2013;74:145–148. doi: 10.1002/ana.23869. [DOI] [PubMed] [Google Scholar]
- 20.Giamberardino MA, et al. Contribution of myofascial trigger points to migraine symptoms. The journal of pain: official journal of the American Pain Society. 2007;8:869–878. doi: 10.1016/j.jpain.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Calandre EP, Hidalgo J, Garcia-Leiva JM, Rico-Villademoros F. Trigger point evaluation in migraine patients: an indication of peripheral sensitization linked to migraine predisposition? European journal of neurology. 2006;13:244–249. doi: 10.1111/j.1468-1331.2006.01181.x. [DOI] [PubMed] [Google Scholar]
- 22.Gandolfi M, et al. Does myofascial and trigger point treatment reduce pain and analgesic intake in patients undergoing onabotulinumtoxinA injection due to chronic intractable migraine? European journal of physical and rehabilitation medicine. 2018;54:1–12. doi: 10.23736/s1973-9087.17.04568-3. [DOI] [PubMed] [Google Scholar]
- 23.Castien R, De Hertogh W. A Neuroscience Perspective of Physical Treatment of Headache and Neck Pain. Frontiers in neurology. 2019;10:276. doi: 10.3389/fneur.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palacios-Cena M, et al. The Number of Active But Not Latent Trigger Points Associated with Widespread Pressure Pain Hypersensitivity in Women with Episodic Migraines. Pain medicine (Malden, Mass.) 2017;18:2485–2491. doi: 10.1093/pm/pnx130. [DOI] [PubMed] [Google Scholar]
- 25.Ge HY, Fernandez-de-Las-Penas C, Yue SW. Myofascial trigger points: spontaneous electrical activity and its consequences for pain induction and propagation. Chin. Med. 2011;6:13. doi: 10.1186/1749-8546-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puledda F, Goadsby PJ. An Update on Non-Pharmacological Neuromodulation for the Acute and Preventive Treatment of Migraine. Headache. 2017;57:685–691. doi: 10.1111/head.13069. [DOI] [PubMed] [Google Scholar]
- 27.Reuter, U., McClure, C., Liebler, E. & Pozo-Rosich, P. Non-invasive neuromodulation for migraine and cluster headache: a systematic review of clinical trials. Journal of neurology, neurosurgery, and psychiatry, 10.1136/jnnp-2018-320113 (2019). [DOI] [PMC free article] [PubMed]
- 28.Schoenen J, Roberta B, Magis D, Coppola G. Noninvasive neurostimulation methods for migraine therapy: The available evidence. Cephalalgia: an international journal of headache. 2016;36:1170–1180. doi: 10.1177/0333102416636022. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann, J. & May, A. Neuromodulation for the treatment of primary headache syndromes. Expert review of neurotherapeutics, 1-8, 10.1080/14737175.2019.1585243 (2019). [DOI] [PubMed]
- 30.Clarke BM, Upton AR, Kamath MV, Al-Harbi T, Castellanos CM. Transcranial magnetic stimulation for migraine: clinical effects. The journal of headache and pain. 2006;7:341–346. doi: 10.1007/s10194-006-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipton RB, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. The Lancet. Neurology. 2010;9:373–380. doi: 10.1016/s1474-4422(10)70054-5. [DOI] [PubMed] [Google Scholar]
- 32.Brighina F, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. Journal of the neurological sciences. 2004;227:67–71. doi: 10.1016/j.jns.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Starling AJ, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study) Cephalalgia: an international journal of headache. 2018;38:1038–1048. doi: 10.1177/0333102418762525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antal A, Kriener N, Lang N, Boros K, Paulus W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia: an international journal of headache. 2011;31:820–828. doi: 10.1177/0333102411399349. [DOI] [PubMed] [Google Scholar]
- 35.Rocha S, et al. Transcranial direct current stimulation in the prophylactic treatment of migraine based on interictal visual cortex excitability abnormalities: A pilot randomized controlled trial. Journal of the neurological sciences. 2015;349:33–39. doi: 10.1016/j.jns.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Przeklasa-Muszynska A, Kocot-Kepska M, Dobrogowski J, Wiatr M, Mika J. Transcranial direct current stimulation (tDCS) and its influence on analgesics effectiveness in patients suffering from migraine headache. Pharmacol Rep. 2017;69:714–721. doi: 10.1016/j.pharep.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Magis D, Sava S, d’Elia TS, Baschi R, Schoenen J. Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly(R) device in headache treatment: a survey of 2,313 headache sufferers in the general population. The journal of headache and pain. 2013;14:95. doi: 10.1186/1129-2377-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenen J, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697–704. doi: 10.1212/WNL.0b013e3182825055. [DOI] [PubMed] [Google Scholar]
- 39.Ordas, C. M. et al. Transcutaneous Supraorbital Stimulation as a Preventive Treatment for Chronic Migraine: A Prospective, Open-Label Study. Pain medicine (Malden, Mass.), 10.1093/pm/pnz119 (2019). [DOI] [PubMed]
- 40.Goadsby PJ, Grosberg BM, Mauskop A, Cady R, Simmons KA. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia: an international journal of headache. 2014;34:986–993. doi: 10.1177/0333102414524494. [DOI] [PubMed] [Google Scholar]
- 41.Barbanti P, et al. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. The journal of headache and pain. 2015;16:61. doi: 10.1186/s10194-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silberstein SD, et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology. 2016;87:529–538. doi: 10.1212/wnl.0000000000002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diener, H. C. et al. Non-invasive vagus nerve stimulation (nVNS) for the preventive treatment of episodic migraine: The multicentre, double-blind, randomised, sham-controlled PREMIUM trial. Cephalalgia: an international journal of headache, 333102419876920, 10.1177/0333102419876920 (2019). [DOI] [PMC free article] [PubMed]
- 44.Sollmann N, et al. Magnetic stimulation of the upper trapezius muscles in patients with migraine - A pilot study. Eur J Paediatr Neurol. 2016;20:888–897. doi: 10.1016/j.ejpn.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Renner, T. et al. Repetitive Peripheral Magnetic Stimulation (rPMS) in Subjects With Migraine—Setup Presentation and Effects on Skeletal Musculature. Frontiers in neurology10, 10.3389/fneur.2019.00738 (2019). [DOI] [PMC free article] [PubMed]
- 46.Luedtke K, Starke W, May A. Musculoskeletal dysfunction in migraine patients. Cephalalgia: an international journal of headache. 2018;38:865–875. doi: 10.1177/0333102417716934. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-de-Las-Penas C. Myofascial Head Pain. Current pain and headache reports. 2015;19:28. doi: 10.1007/s11916-015-0503-2. [DOI] [PubMed] [Google Scholar]
- 48.Conforto AB, et al. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia: an international journal of headache. 2014;34:464–472. doi: 10.1177/0333102413515340. [DOI] [PubMed] [Google Scholar]
- 49.Kaptchuk TJ, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. Bmj. 2006;332:391–397. doi: 10.1136/bmj.38726.603310.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786–792. doi: 10.1016/s0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 51.Pollo A, et al. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/s0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 52.de Groot FM, et al. Headache: the placebo effects in the control groups in randomized clinical trials; an analysis of systematic reviews. J Manipulative Physiol Ther. 2011;34:297–305. doi: 10.1016/j.jmpt.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Duecker F, Sack AT. Rethinking the role of sham TMS. Frontiers in psychology. 2015;6:210. doi: 10.3389/fpsyg.2015.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rapoport AM, et al. Remote electrical neuromodulation (REN) in the acute treatment of migraine: a comparison with usual care and acute migraine medications. The journal of headache and pain. 2019;20:83. doi: 10.1186/s10194-019-1033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yarnitsky D, et al. Remote Electrical Neuromodulation (REN) Relieves Acute Migraine: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Headache. 2019;59:1240–1252. doi: 10.1111/head.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersen HH, Duroux M, Gazerani P. Serum MicroRNA Signatures in Migraineurs During Attacks and in Pain-Free Periods. Mol Neurobiol. 2016;53:1494–1500. doi: 10.1007/s12035-015-9106-5. [DOI] [PubMed] [Google Scholar]
- 57.Tafuri E, et al. MicroRNA profiling in migraine without aura: pilot study. Ann Med. 2015;47:468–473. doi: 10.3109/07853890.2015.1071871. [DOI] [PubMed] [Google Scholar]
- 58.Koverech A, et al. Migraine and cluster headache show impaired neurosteroids patterns. The journal of headache and pain. 2019;20:61. doi: 10.1186/s10194-019-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pavlidis I, et al. Dynamic Quantification of Migrainous Thermal Facial Patterns - A Pilot Study. IEEE J Biomed Health Inform. 2019;23:1225–1233. doi: 10.1109/jbhi.2018.2855670. [DOI] [PubMed] [Google Scholar]
- 60.Gratt BM, Pullinger A, Sickles EA, Lee JJ. Electronic thermography of normal facial structures: a pilot study. Oral Surg Oral Med Oral Pathol. 1989;68:346–351. doi: 10.1016/0030-4220(89)90222-3. [DOI] [PubMed] [Google Scholar]
- 61.Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial Magnetic and Direct Current Stimulation (TMS/tDCS) for the Treatment of Headache: A Systematic Review. Headache. 2019;59:339–357. doi: 10.1111/head.13479. [DOI] [PubMed] [Google Scholar]
- 62.Tao H, et al. Effectiveness of transcutaneous electrical nerve stimulation for the treatment of migraine: a meta-analysis of randomized controlled trials. The journal of headache and pain. 2018;19:42. doi: 10.1186/s10194-018-0868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robbins NM, Bernat JL. Minority Representation in Migraine Treatment Trials. Headache. 2017;57:525–533. doi: 10.1111/head.13018. [DOI] [PubMed] [Google Scholar]
- 64.Straube A, Andreou A. Primary headaches during lifespan. The journal of headache and pain. 2019;20:35. doi: 10.1186/s10194-019-0985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. Journal of the neurological sciences. 2017;372:307–315. doi: 10.1016/j.jns.2016.11.071. [DOI] [PubMed] [Google Scholar]
- 66.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia: an international journal of headache33, 629-808, 10.1177/0333102413485658 (2013). [DOI] [PubMed]
- 67.Fritsche G, et al. Validation of a german language questionnaire for screening for migraine, tension-type headache, and trigeminal autonomic cephalgias. Headache. 2007;47:546–551. doi: 10.1111/j.1526-4610.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 68.Yoon MS, et al. Population-based validation of a German-language self-administered headache questionnaire. Cephalalgia: an international journal of headache. 2008;28:605–608. doi: 10.1111/j.1468-2982.2008.01560.x. [DOI] [PubMed] [Google Scholar]
- 69.Jensen R, et al. A basic diagnostic headache diary (BDHD) is well accepted and useful in the diagnosis of headache. a multicentre European and Latin American study. Cephalalgia: an international journal of headache. 2011;31:1549–1560. doi: 10.1177/0333102411424212. [DOI] [PubMed] [Google Scholar]
- 70.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56:S20–28. doi: 10.1212/WNL.56.suppl_1.S20. [DOI] [PubMed] [Google Scholar]
- 71.Lipton RB, Stewart WF, Sawyer J, Edmeads JG. Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2001;41:854–861. doi: 10.1111/j.1526-4610.2001.01156.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhuang X, Tan S, Huang Q. Understanding of myofascial trigger points. Chinese medical journal. 2014;127:4271–4277. [PubMed] [Google Scholar]
- 73.Fernandez-de-las-Penas C, Cuadrado ML, Arendt-Nielsen L, Simons DG, Pareja JA. Myofascial trigger points and sensitization: an updated pain model for tension-type headache. Cephalalgia: an international journal of headache. 2007;27:383–393. doi: 10.1111/j.1468-2982.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 74.Landgraf MN, et al. Alterations in the trapezius muscle in young patients with migraine–a pilot case series with MRI. Eur J Paediatr Neurol. 2015;19:372–376. doi: 10.1016/j.ejpn.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez-de-Las-Penas C, Dommerholt J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain medicine (Malden, Mass.) 2018;19:142–150. doi: 10.1093/pm/pnx207. [DOI] [PubMed] [Google Scholar]
- 76.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.