Abstract
Evidence suggests that the CXXC type zinc finger (ZF-CXXC) protein 5 (CXXC5) is a critical regulator/integrator of various signaling pathways that include the estrogen (E2)-estrogen receptor α (ERα). Due to its ZF-CXXC domain, CXXC5 is considered to be a member of the ZF-CXXC family, which binds to unmethylated CpG dinucleotides of DNA and through enzymatic activities for DNA methylation and/or chromatin modifications generates a chromatin state critical for gene expressions. Structural/functional features of CXXC5 remain largely unknown. CXXC5, suggested as transcription and/or epigenetic factor, participates in cellular proliferation, differentiation, and death. To explore the role of CXXC5 in E2-ERα mediated cellular events, we verified by generating a recombinant protein that CXXC5 is indeed an unmethylated CpG binder. We uncovered that CXXC5, although lacks a transcription activation/repression function, participates in E2-driven cellular proliferation by modulating the expression of distinct and mutual genes also regulated by E2. Furthermore, we found that the overexpression of CXXC5, which correlates with mRNA and protein levels of ERα, associates with poor prognosis in ER-positive breast cancer patients. Thus, CXXC5 as an unmethylated CpG binder contributes to E2-mediated gene expressions that result in the regulation of cellular proliferation and may contribute to ER-positive breast cancer progression.
Subject terms: Cell signalling, Breast cancer
Introduction
Estrogen hormones, particularly 17β-estradiol (E2), are involved in the physiological and pathophysiological regulation of many organ and tissue functions, including breast tissue1. The effects of E2 in breast tissue are mediated mainly by estrogen receptor α (ERα) as a transcription factor1. Because of the critical role of the E2-ERα signaling in physiology and pathophysiology of breast tissue, identifying key genes regulated by E2-ERα to alter cellular phenotype could provide new prognostic tools and/or therapeutic targets for breast cancer1. We previously and others recently2 reported that CXXC5 is an E2-ERα responsive gene3,4. Due to a highly conserved zinc-finger CXXC domain (ZF-CXXC), CXXC5 is considered to be a member of the functionally distinct ZF-CXXC family encompassing 12 proteins5–7. The ZF-CXXC domain is characterized by two conserved cysteine-rich motifs (CXXCXXC; wherein X indicates other residues) that associate with two Zn++ ions forming zinc-finger structures. The ZF-CXXC family proteins through the ZF-CXXC domain recognize and bind to unmethylated CpG dinucleotides with varying affinities and, in a sequence context, specificities7 to regulate gene expressions5,6.
Expressed in cells of different tissues in response to morphogenic retinoic acid8, multifunctional cytokine family member transforming growth factor-β9 and bone morphogenetic protein 410,11 as well as the Wnt family of secreted glycolipoprotein Wnt3a12–14, CXXC5 is suggested to participate as transcription factor, co-regulator and/or epigenetic factor in a wide variety of cellular functions. These include the modulation of signal transduction, DNA damage response, cellular energy metabolism, proliferation, differentiation, angiogenesis and cell death8–10,12,15–19. In vivo experimental models further indicate that CXXC5 contributes to osteoblast differentiation, growth plate senescence, cutaneous wound healing, hair loss, and antiviral responses as well as kidney and heart development2,12,13,20–24. In accordance with the importance of CXXC5 in cellular events, de-regulated expressions of CXXC5 appear to correlate with the development, and resistance to therapies, of various pathologies including cardiovascular disease, diminished ovarian reserve (DOR), Blepharophimosis Ptosis Epicantus Inversus Syndrome (BPES), Acute Myeloid Leukemia (AML), prostate and breast cancer8,25–30.
Although we showed that the E2-ERα signaling augments the expression of CXXC5 and the synthesis of the encoded protein3,4, the role of CXXC5 in cellular events mediated by E2-ERα is unclear. To address this issue, we here initially verified by generating a full-length recombinant protein that CXXC5 is indeed an unmethylated CpG binding protein. In assessing intracellular functions in ERα-synthesizing and E2-responsive MCF7 cells derived from a breast adenocarcinoma, we found that CXXC5, although lacks a transcription activation or repression function, modifies gene expressions independently from and in collaboration with the E2-ERα signaling. This results in the modulation of E2-mediated cellular proliferation.
Results
CXXC5 is an unmethylated CpG binding protein
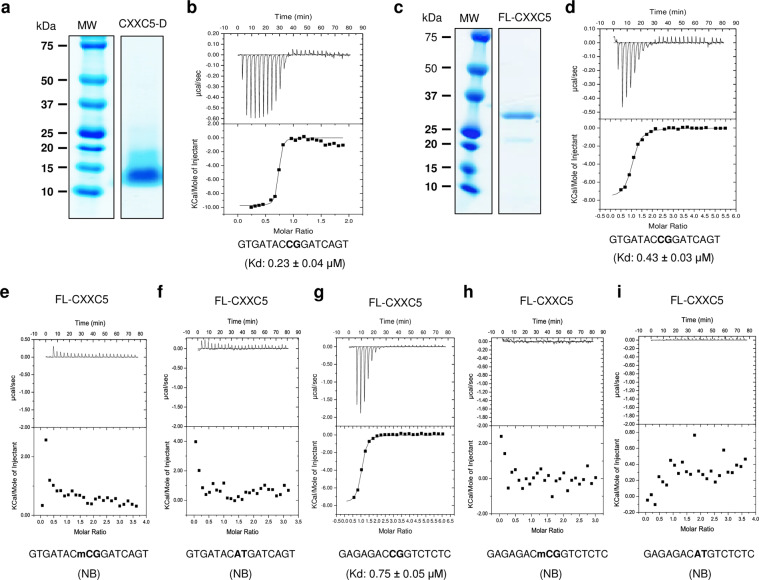
CXXC5 encodes a 322 amino-acid long protein with a molecular mass of 33 kDa. Due to its ZF-CXXC domain, CXXC5 is considered to be a member of the ZF-CXXC family5–7. The CXXC domain specifically binds to an unmethylated CpG dinucleotide containing DNA. Consistent with this, recent structural studies clearly indicated that the CXXC domain of CXXC5, as the other members of the ZF-CXXC family, also preferentially binds to unmethylated CpG dinucleotides7.
Despite the structured CXXC domain located at the carboxyl-terminus of CXXC5 (250–322), our in silico analyses suggest that the amino-terminus of the protein is highly disordered (Supplementary Fig. S1) and lacks, as indicated previously5–7, any known structural motif. Since intramolecular interactions among structural regions are also critical for the functional features of a protein31, we wanted to assess whether or not CXXC5 as the full-length protein binds to unmethylated CpG dinucleotide containing DNA as well. To examine this issue, we expressed and purified, to a near homogeneity, the recombinant the CXXC domain of CXXC5 (CXXC-D) and the full-length CXXC5 protein (FL-CXXC5) using a bacterial expression system (Fig. 1a,c). To assess the DNA binding ability of the recombinant FL-CXXC5 in comparison with that of CXXC-D, we used isothermal titration calorimetry (ITC), which monitors heat changes caused by macromolecular, including DNA and protein, interactions. For the assay, 10 µM recombinant protein and 300 µM DNA were mixed in and subjected to ITC. The binding isotherms fitted to a one-site binding mode revealed that CXXC-D (Fig. 1b), as shown previously7, and FL-CXXC5 (Fig. 1d) binds effectively to a centrally located unmethylated CG dinucleotide bearing DNA (5′-GTGATACCGGATCAGT-3′). Moreover, this binding was abolished when we used a DNA fragment containing identical sequences but with the methylated-CG (mCG) dinucleotide (5′- GTGATACmCGGATCAGT-3′) (Fig. 1e). The binding of FL-CXXC5 to the unmethylated CG containing DNA fragment is specific; because, a DNA fragment containing the central AT (5′-GTGATACATGATCAGT-3′), exhibited no binding (Fig. 1f). Furthermore, FL-CXXC5 also binds with similar affinities to DNA fragment with different sequences (5′- GAGAGACCGGTCTCTC-3′) that surround the central CG (Fig. 1g) but not mCG (Fig. 1h) or AT (Fig. 1i) dinucleotide. These results suggest that FL-CXXC5 as its CXXC-D binds specifically to unmethylated CpG dinucleotide containing DNA.
Figure 1.
Purification and interaction with DNA of the recombinant full-length CXXC5 (FL-CXXC5) and the CXXC domain (CXXC-D) proteins. CXXC-D (a) and FL-CXXC5 (c), expressed in bacteria and purified sequentially with ion exchange, heparin, and size-exclusion chromatographies, were loaded onto an SDS-PAGE gel (4–20% gradient) and stained with InstantBlue coomassie dye. “MW” indicates molecular masses in kDa. 10 µM CXXC-D (b) or FL-CXXC5 (d) was subjected to isothermal titration calorimetry (ITC) using a 300 µM double-stranded DNA fragment that bears a central unmethylated CG dinucleotide (5′-GTGATACCGGATCAGT-3′). (e,f) The binding of FL-CXXC5 was also assessed with ITC using a double-stranded DNA fragment with the central mCG or AT nucleotides embedded into the same surrounding DNA sequence. To ensure that sequences surrounding the central nucleotides have no effect on the ability of FL-CXXC5 to bind to DNA, a double-stranded DNA fragment with a distinct surrounding sequence (5′-GAGAGACxxGTCTCTC-3′) bearing the central CG (G), mCG (h) or AT (i) nucleotides was subjected to ITC. Results are the mean ± S.E. of two experiments. NB denotes no detectable binding.
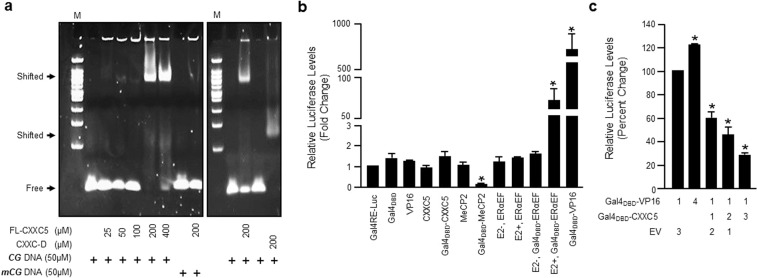
To further confirm these results, we also tested the ability of FL-CXXC5 and CXXC-D to bind specifically to an unmethylated CpG bearing DNA fragment with the central CG, but not mCG, sequence using electrophoretic mobility shift assay (EMSA) at 1:0.5, 1:1, 1:2, 1:4 and 1:8 molar ratio of DNA (50 μM) to FL-CXXC5 or 1:4 CXXC-D (Fig. 2a). EMSA, confirming ITC results, indicates that FL-CXXC5 as CXXC-D is an unmethylated CpG dinucleotide binder.
Figure 2.
Electrophoretic mobility shift (EMSA) and reporter assay. (a) To assess the ability of the recombinant FL-CXXC5 or its CXXC domain (CXXC-D), 50 μM DNA with the central unmethylated (CG) or methylated (mCG) CpG dinucleotides was mixed with FL-CXXC5 at 1:0.5, 1:1, 1:2, 1:4 and 1:8 molar ratios or with CCCX-D at 1:4 molar ratios. Samples were run onto 5% native TBE gel, stained and visualized with UV spectrometry. “M” represents the molecular marker. “Free” denotes unbound DNA; “Shifted” indicates DNA bound protein. A representative experiment performed with two independent times is shown. (b) To assess the intrinsic transcription regulatory function of FL-CXXC5, pGal4-RE Luciferase Reporter vector (pGal4RE-Luc, 125 ng) containing tandem Gal4 response elements (Gal4-RE) juxtaposed to a simple TATA box promoter that drives the expression of the firefly Luciferase enzyme cDNA together with an expression vector (75 ng) bearing Gal4DBD, VP16, FL-CXXC5, WT-MeCP2 or ERα-EF domain cDNA or Gal4DBD-VP16, Gal4DBD-CXXC5, Gal4DBD-MeCP2 or Gal4DBD-ERαEF cDNA transfected into MCF7 cells. MCF7 cells grown in 10% CD-FBS for 48 h were transfected with expression vector bearing ERαEF cDNA or Gal4DBD-ERαEF and were treated without (%0.01 ethanol) or with 10−8 M E2 for 24 h. Transfection efficiency was monitored with the co-expression of a reporter plasmid bearing the Renilla Luciferase enzyme cDNA (0.5 ng). Results indicating relative firefly/Renilla luciferase activity determined using a dual luciferase assay kit are the mean ± S.E. of three independent experiments performed in duplicate. The asterisk denotes significant differences compared to responses observed with Gal4-RE, which is set to 1. (c) Cells were also transfected with pGal4RE-Luc together with the expression vector bearing none (empty vector, EV), Gal4DBD-VP16 and/or Gal4DBD-CXXC5 cDNA. In transfections, we used a total of 300 ng expression vector (1 denotes 75 ng), for which appropriate amounts of the parent expression vector (EV) were supplemented to equalize the total plasmid DNA amount. Results presented as relative firefly/Renilla luciferase levels indicate percent change and are the mean ± S.E. of three independent experiments performed in duplicate. The asterisk denotes significant differences from responses observed with Gal4DBD-VP16, which was set to 100%.
CXXC5 lacks a transcription activation/repression function
Studies suggest that CXXC5 is a critical regulator/integrator of various signaling networks4,10–12,17. Functional studies also suggest that CXXC5 in a cell- and tissue-type dependent manner can act as a transcription activator10,14,16 or repressor32 to modulate gene expressions. These findings raise the possibility that CXXC5 has an intrinsic transcription regulatory function. To preliminary address this issue, we used a mammalian one-hybrid approach, a derivative of the mammalian two-hybrid system33, which exploits the modular nature of eukaryotic transcription factors that possess functionally distinct regions: DNA binding (DBD) and transcription activation domains. The binding of Gal4DBD, as the DNA binding module of a chimeric protein generated with genetic fusion, to Gal4 response elements (Gal4RE) preceding a simple TATA box promoter positions the protein to the promoter to drive the expression of the reporter enzyme: firefly luciferase from a Gal4RE-Luc reporter vector, as we described previously34. This approach, thereby, circumvents the need for the presence of transcription factor-specific responsive elements to drive reporter gene expressions in comparative analysis. We genetically fused FL-CXXC5 to the carboxyl-terminus of Gal4DBD to generate the chimeric Gal4DBD-CXXC5 protein. We also used a chimeric transcription activator, Gal4DBD-ERαEF that bears the carboxyl-terminally located E/F domain of ERα34, which possesses a ligand (17β-estradiol, E2)-dependent transcription activation function. In addition, we utilized the Gal4DBD-VP16 fusion protein that bears the potent VP16 transcription activation domain. MeCP2 (methyl-CpG binding protein 2) was shown to act as a potent transcription repressor when fused to Gal4DBD35. Based on this finding, we also genetically fused MeCP2 to the Gal4DBD to generate the Gal4DBD-MeCP2 chimeric protein. The proteins were then synthesized by transient transfections in E2-responsive MCF7 cells together with the Gal4RE-Luc reporter vector (Fig. 2B). If a protein has a transcription regulatory function, an augmentation/repression of reporter enzyme activity by a chimeric protein compared with levels induced by each protein alone is expected to occur. Results revealed that Gal4DBD-VP16 and Gal4DBD-ERαE/F, the latter which only in the presence of 10–8 M E2, effectively increased the reporter enzyme activity compared to that induced by Gal4DBD alone. As shown previously35, Gal4DBD-MeCP2 robustly repressed the reporter enzyme levels. VP16, MeCP2 or ERαE/F, the latter which in the absence or presence of E2, alone have no effect on the enzyme activity. These results indicate that the positioning of a transcription regulator to a promoter independent of its DNA binding ability exposes the intrinsic transcription regulatory potential of the protein. In contrast, CXXC5 alone or as the Gal4DBD fusion protein did not affect the levels of the reporter enzyme activity. To ensure that the fusion between Gal4DBD and CXXC5 did not alter the ability of the chimeric protein to interact with promoter elements, we also carried out a promoter competition assay in MCF7 cells (Fig. 2c). Since Gal4DBD-VP16 potently enhances the reporter enzyme activity from the Gal4RE-promoter, a reduction in the extent of the reporter enzyme activity induced by Gal4DBD-VP16 through the competitive binding of Gal4DBD-CXXC5 to Gal4REs would indicate the ability of Gal4DBD-CXXC5 to bind to DNA. Indeed, the increasing concentrations of the expression vector bearing Gal4DBD-CXXC5, but not CXXC5 alone (data not shown), cDNA effectively decreased the reporter enzyme activity induced by Gal4DBD-VP16. Thus, these results indicate that CXXC5 lacks a transcription activation or repression function and suggest that CXXC5 may act as a nucleation factor for the establishment of a transcription state.
The reduction of the intracellular CXXC5 level affects cell proliferation induced by E2
Our previous studies indicated that CXXC5 is an E2-ERα responsive gene3,4. To assess the functional significance of CXXC5 through an augmented or a repressed level of CXXC5 synthesis in cell models derived from breast, or other tissues, adenocarcinomas, we used stable transfections with expression vectors bearing the CXXC5 cDNA or shRNA, the latter which was designed according to our previous study4. Unfortunately, however, the chronic over-expression or the repression of CXXC5 synthesis led to cell death independent of cell type or tissue-of-origin. This necessitated the use of an siRNA approach to examine the role of CXXC5 in MCF7 cells.
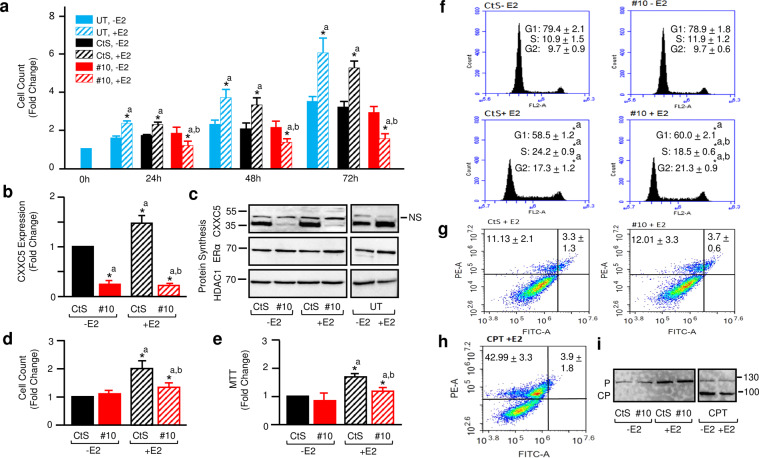
We, therefore, carried out studies with siRNAs to assess CXXC5 functions in MCF7 cells treated without or with E2. In our previous study4, we had evaluated four different siRNAs that target CXXC5 transcripts (FlexitubeGene Solution, Qiagen siRNA #2, 7, 9 and 10) as well as a scrambled siRNA control (CtS) to alter levels of CXXC5 transcript and protein. Of the siRNAs, siRNA#10 and siRNA#2 (for siRNA sequences: Supplementary Table S1) effectively repressed CXXC5 transcript levels and protein synthesis in MCF7 cells in comparison with CtS, which had no effect on CXXC5 transcript or protein levels compared to untransfected (UT) cells4. Based on these findings, we assessed the effect of siRNA#10 initially on cellular growth. MCF7 cells grown in 10% CD-FBS containing medium for 48 h to reduce endogenous E2 levels, were transiently transfected without or with CtS or siRNA#10 in the absence (-E2; ethanol as vehicle control, 0.01%) or the presence of E2 (E2; 10–8 M) up to 72 hours (Fig. 3a). We found by cell counting that E2 treatment of UT cells, as expected, increased the cellular proliferation at every time point tested. CtS did not adversely affect cell number compared to that of UT cells in the absence or presence of E2. On the other hand, siRNA#10 effectively prevented specifically E2-mediated cellular growth at time points tested. Similar results were also observed with siRNA#2 (Supplementary Fig. S2a).
Figure 3.
Effects of siRNAs specific to CXXC5 on cellular growth. MCF7 cells grown in 10% CD-FBS containing medium for 48 h were transiently transfected without (UT) or with 10 nM CtS or siRNA#10 in the absence (0.01% ethanol) or the presence of 10−8 M E2 up to 72 h. (a) Cells were subjected to cell counting using a hemocytometer. Results, as the mean ± S.E. of three independent determinations, depict fold change in cellular growth compared with those observed with cells at time 0 in the absence of E2, which is set to 1. In (a–f) the asterisk with a superscript “a” indicates significant difference from the corresponding ethanol-treated group (-E2); whereas superscript “b” depicts a significant difference from CtS transfected cells in the presence of E2. (b) The cDNA library generated from total RNA (50 ng) isolated from transfected cells for 48 h was subjected to qPCR using a primer set specific to CXXC5. (c) Nuclear extracts from untransfected (UT) or transfected cells were subjected to WB using an antibody for CXXC5, ERα or HDAC1. NS denotes non-specific protein; Molecular mass in KDa is indicated. Transfected cells were subjected to cell counting (d) and MTT assay (e). In (b,d,e), results depicting the mean ± S.E. of three independent determinations indicate fold change in mRNA levels (b) or cell numbers (d,e) compared with CtS in the absence of E2, which is set to 1. Transfected MCF7 cells were subjected to flow cytometry (f) with results depicting the percent of cells in the G1, G2 and S phases, or to Annexin V assay followed by flow cytometry (g). In (f,g), results are the mean ± SEM of three independent experiments. (h & i) Untransfected (UT) or transfected cells were also subjected to the cell death inducer camptothecin (2 nM) for 24 h without (−E2) or with E2 (E2). Cells were then subjected to Annexin V assay (h) with results as the mean ± S.E. of three independent experiments depicting percent change in apoptotic (upper right quadrant) and death (upper left quadrant) cells, or to WB (i) using a PARP1-specific antibody. P and CP denote the uncut and cut PARP1, respectively.
Based on these initial results, MCF7 cells maintained in 10% CD-FBS for 48 h were transiently transfected with CtS or siRNA#10 in the absence or presence of 10–8 M E2 for 48 h. E2, as we showed previously4, augmented the expression and protein synthesis of CXXC5 in cells transfected with CtS. siRNA#10, on the other hand, effectively reduced CXXC5 at both transcript (Fig. 3b) and protein (Fig. 3c) levels in the absence or presence of E2. siRNA#10 did not adversely affect the protein level of ERα, nor that of HDAC1 used as a loading control, that could account for cellular events mediated by E2 in transfected cells (Fig. 3c).
Confirming our initial results (Fig. 3a), we also observed that siRNA#10 reduced cellular proliferation only in the presence of E2 assessed by cell counting (Fig. 3d) or MTT assay (Fig. 3e). The E2-induced proliferation of MCF7 cells is mediated by ERα, as ICI (Imperial Chemical Industries 182,780), an ER antagonist, effectively prevented the growth of the cells transfected with CtS or siRNA#10 (Supplementary Fig. 2b). siRNA#10 also repressed the E2-mediated growth of ERα-synthesizing T47D cells derived from a breast ductal carcinoma (Supplementary Fig. S2c). These findings indicate that CXXC5 is involved in the cellular proliferation mediated by the E2-ERα signaling.
The reduction of the intracellular CXXC5 level alters cell cycle progression
To examine whether or not the reduction of E2-mediated cellular proliferation by siRNA#10 involves alterations in cell cycle phases, we transiently transfected MCF7 cells, grown in 10% CD-FBS containing medium for 48 h, with CtS or siRNA#10 in the absence (-E2) or the presence of E2 (E2; 10−8 M) for 48 h (Fig. 3f). E2 increased the cell population in the S and G2 phases with a concomitant decrease in the G1 phase in cells transfected with CtS. siRNA#10 reduced the E2-mediated augmentation of the cell population in the S phase and increased the population in the G2 phase without an effect on the cell number in the G1 phase. These results suggest that CXXC5 contributes to events in the E2-driven S phase and in the G2/G1 transition.
To assess whether or not the repression of cellular proliferation by siRNA#10 includes also cell death, MCF7 cells grown in 10% CD-FBS containing medium for 48 h were transiently transfected with CtS or siRNA#10 in the absence or presence of E2 (E2; 10−8 M) for 48 h. Cells were then subjected to an Annexin V assay followed by cytometry as well as WB for the cleavage of poly (ADP-ribose) polymerase-1 (PARP1) as a marker for cell death. Results revealed that siRNA#10 in the absence or presence of E2 (shown in the presence of E2) had no effect on cellular death compared to cells transfected with CtS nor did it affect the cleavage of PARP1, the level of which was increased in the presence of E2 (Fig. 3g,i). In contrast, camptothecin (CPT), a DNA topoisomerase inhibitor, effectively increased death cell populations (shown in the presence of E2) (Fig. 3h) and cleaved PARP1 (CP) in the absence or presence of E2 (Fig. 3i). These results collectively suggest that CXXC5 as an unmethylated CpG dinucleotide binder contributes to cellular proliferation mediated by E2-ERα signaling through events occurring primarily in the S phase of the cell cycle.
CXXC5 alone and together with E2-ERα is involved in the regulation of gene expressions
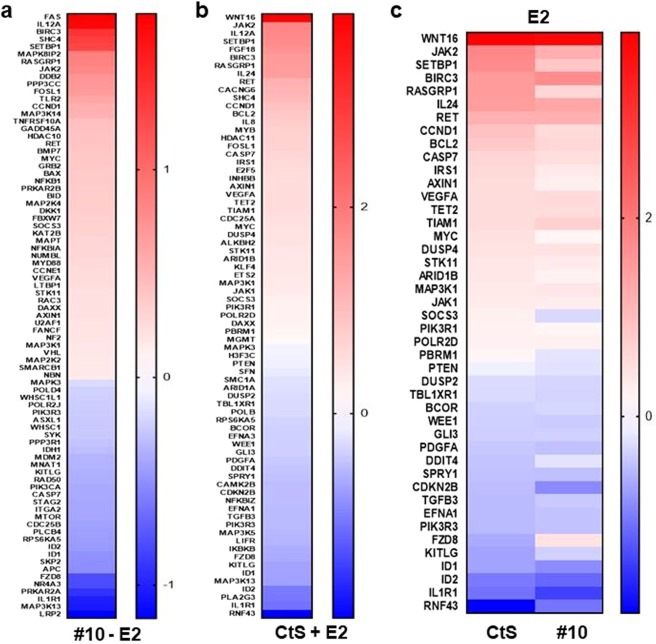
The contribution of CXXC5 to the E2-mediated cellular proliferation together with our observation that CXXC5 is an unmethylated CpG binder raises the possibility that CXXC5 alone or together with E2-ERα alter gene expressions. To test this prediction, we carried out a multiplex gene expression analysis using the PanCancer Pathway Panel (nCounter, Nanostring) that contains 770 genes from cancer-associated canonical pathways involved in cell cycle regulation, apoptosis, DNA damage control, transcriptional regulation, and chromatin modification. This approach provides direct measurements of cellular levels of mRNA transcripts36,37. MCF7 cells grown in 10% CD-FBS containing medium for 48 h were transiently transfected with CtS or siRNA#10 in the absence of E2. Forty-eight hours after transfection, cells were treated without (-E2, 0.01% ethanol) or with 10−8 M E2 (+E2) for 3 h, which is a time period critical for the assessment of changes in the expression of primary (protein synthesis independent) E2 target genes38,39. This treatment regimen generated four groups: Group 1) CtS and -E2; 2) #10 and -E2; 3) CtS and +E2; 4) #10 and +E2. Total RNA from each group was processed for and subjected to the Panel profiler. Following background correction, we assessed gene expressions mediated by CXXC5 in the absence of E2, Group 2 by normalizing to Group 1 (Fig. 4a & Table S2). Results revealed that CXXC5 in the absence of E2 modulates the expression of a number of genes including APC, BAX, BID, CCNE1, BMP7, DKK1, GADD45A, GRB2, HDAC10, ITGA2, KAT2B, MTOR, NF2, NFKB1, NFKBIA, POLD4, POLR2J, RAD50, SKP2 and SMARCB1, whose encoded proteins are involved in various intracellular events ranging from signal transduction to chromatin modeling. We also normalized Group 3 to Group 1 to reveal genes differentially regulated by E2 when CXXC5 is present (Fig. 4b & Table S2, Column 2). This group of genes includes AXIN1, BCL2, CCND1, CDKN2B, DUSP4, ETS2, ID1, ID2, IL24, IRS1, MAP3K5, MGMT, MYC, NFKBIZ, PDGFA, PTEN, TET2, and TGFB3, which were shown to be regulated by E23,39–50. The normalization of Group 4 to Group 2, on the other hand, indicates genes mediated in response to E2 in the absence of CXXC5 (Table S2), which, in addition to E2-regulated genes, also include the appearance of genes encoding the components of Interleukin (IL1RAP), Growth Factors (ERBB2, LTBP1, TGFB2, VEGFC), Wnt (FZD7, WNT7B), Notch (NOTCH3, JAG2) and Hedgehog (PTCH1) signaling pathways. To further reveal the impact of CXXC5 on gene expressions in response to E2, we then compared E2-mediated gene expressions observed in the presence (Group3/Group1) and the absence (Group4/Group2) of CXXC5. Results revealed that CXXC5 participates in the expression of a set of E2-responsive genes antagonistically or additively (Fig. 4c). CXXC5, for example, antagonistically modulated the expression of FZD8, PBRM1, SOC3 in the presence of E2. CXXC5, on the other hand, additively modulated (augmented or attenuated) the expression of AXIN1, CCND1, CDKN2B, IL24, IRS1, MYC, PTEN, RET, RINF43 or TGFB in response to E2. These results collectively indicate that the integrated effects of CXXC5 and E2-signaling define the state and the level of target gene transcriptions and suggest that CXXC5 is a critical regulatory component for the fine-tuning of E2-mediated gene expressions, thereby of cellular proliferation.
Figure 4.
Analysis of Gene Expression with the nCounter PanCancer Pathway Panel. MCF7 cells grown in 12-well tissue culture plates (9 × 104 cells/well) in 10% CD-FBS medium for 48 h were transiently transfected using 10 nM of CtS or siRNA#10, generating four treatment groups: Group (1) CtS and −E2; (2) #10 and −E2; (3) CtS and +E2; (4) #10 and +E2. Forty-eight hours after transfection, cells were treated without (ethanol, 0.01%) as vehicle control or with 10−8 M of E2 for 3 hours. Total RNA (50 ng) was subjected to the nCounter PanCancer Pathway Panel gene expression analysis with nCounter Digital Analyzer. Quality control, data normalization and differential expression analyses were carried out using Nanostring nSolver 3.0 Analysis software together with its Advanced Analysis plug-in. Each treatment is depicted as heat maps with increasing (red) and decreasing levels (blue) and are the mean of three independent experiments in the log2 scale. (a) To assess differentially expressed genes mediated by CXXC5 in the absence of E2 (#10 −E2), nanostring results obtained from cells transfected with siRNA#10 in the absence of E2 (Group 2) was normalized to those from cells transfected with CtS in the absence of E2 (Group 1). (b) The effects of E2 on gene expression (CtS and +E2) were assessed by normalizing nanostring results obtained from cells transfected with CtS in the presence of E2 (Group 3) to those from cells transfected with CtS in the absence of E2 (Group 1). (c) To reveal the impact of CXXC5 on gene expressions in response to E2, we compared differentially expressed genes observed in the presence (CtS and +E2) and the absence (#10 and +E2) of CXXC5, the latter which was obtained by normalizing nanostring results obtained from cells transfected with siRNA#10 in the presence of E2 (Group 4) to those from cells transfected with siRNA#10 in the absence of E2 (Group 2).
ERα expression is correlated with the expression of CXXC5, whose overexpression is associated with poor prognosis in ERα-positive (ER+) breast cancer
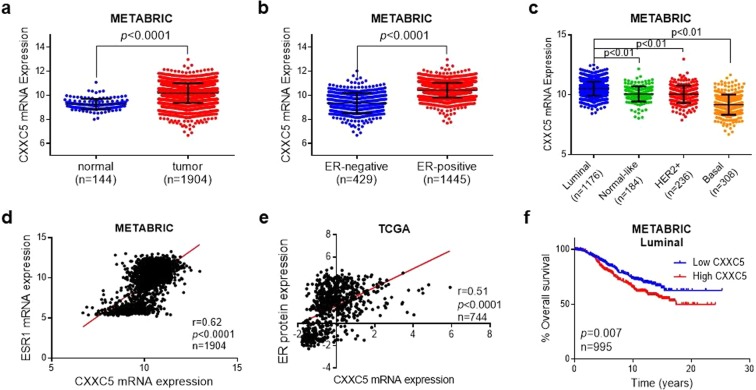
Since CXXC5 is an E2-ERα responsive gene and the CXXC5 protein appears to be critical for E2-mediated cellular events in MCF7 cells modeling ER-positive breast cancer, we wanted to assess whether there is a correlation between the CXXC5 and ESR1 (ERα) expressions and whether the CXXC5 expression has a prognostic value in ER+ breast cancer patients using two large, publically available breast cancer patient datasets, METABRIC (Molecular Taxonomy of Breast Cancer International Consortium) and TCGA (The Cancer Genome Atlas). We found that the mean CXXC5 expression is significantly higher in breast cancer than that observed with normal breast tissue (Fig. 5a). Previous studies have indicated that the overexpression of CXXC5 is associated with poor prognosis in ERα-positive (ER+) breast cancer25,51. Consistent with this, we found that the mean of CXXC5 expression is higher in ERα+ luminal breast tumors compared to that observed with normal-like and ERα-negative breast cancer subtypes, including HER2+ and Basal-like tumors (Fig. 5b,c). Moreover, mRNA levels of CXXC5 positively correlate with both mRNA and protein levels of ERα in breast cancer patients (Fig. 5d,e). Importantly, higher levels of the mean CXXC5 expression predict a poor overall survival only in luminal patients while the expression levels of CXXC5 are not prognostic in HER2+ or Basal-like subtypes (Fig. 5f, Supplementary Fig. S5), further supporting its clinical relevance to ER+ breast cancer. These results suggest that there is a correlation between CXXC5 and ESR1 expressions in ER+ breast cancer and that a deregulated expression of CXXC5 could contribute to the initiation and/or progression of ERα+ breast cancer.
Figure 5.
Clinicopathological relevance of CXXC5 expressions in breast cancer patients. (a) CXXC5 expression in breast cancer (BC) as compared to normal breast tissue from METABRIC. (b) CXXC5 expression in ER-negative versus ER-positive breast cancer patients from METABRIC. (c) CXXC5 expression in different molecular subtypes of tumors of breast cancer patients from METABRIC. (d) Pearson correlation analysis between mRNA expressions of CXXC5 and ESR1 (ERα) in patients from METABRIC. (e) Pearson correlation analysis between mRNA expression of CXXC5 and ERα protein level in breast cancer patients from TCGA. (f) Kaplan Meier survival analysis of basal breast cancer patients based on CXXC5 expression separated from the median.
Discussion
In assessing the functions of CXXC5 in vitro and in cellula we show here that CXXC5 is an unmethylated CpG dinucleotide binding protein and contributes to gene expressions and, consequently, to the modulation of cellular proliferation driven by the E2-ERα signaling.
In vertebrates, DNA methylation by DNA methyltransferase enzymes (DNMTs) is one of the mechanisms of gene silencing and it mostly occurs in CpG dinucleotides of the genome. Methylation of cytosine residues results in the recruitment of methyl-CpG-binding proteins (MBPs) that act as transcription repressors52. Although the majority of CpGs in mammalian genomic DNA is methylated53, about 70% of human gene promoters are associated with unmethylated DNA sequences called CpG islands (CGIs)54–56. CGIs are rich in C and G nucleotides and are defined by a high density of CpG dinucleotides. CGIs are often found in the promoter regions of genes that have characteristic transcription-associated chromatin organizations and are mostly hypomethylated. Recent structural studies indicated that the ZF-CXXC domains of the ZF-CXXC family proteins recognize and bind to unmodified CpG dinucleotides with varying affinities and specifies allowing to be grouped in distinct classes7. For example, MLL2 (Mixed-lineage leukemia 1 & 2); KDM2A and KDM2B (Lysine (K)-specific Demethylase 2A & 2B); FBXL19 (F-box and leucine rich repeat protein 19); MBD1 (Methyl-CpG-binding domain protein 1) as the Class II ZF-CXXC family proteins specifically recognize unmethylated CpG-containing DNA. The ZF-CXXC domains of TET1 and TET3 (Tet methylcytosine Dioxygenase 1 & 3), CXXC4 (CXXC-type zinc finger protein 4), CXXC5 bind to cytosine containing DNA with a preference for CpG over CpH (H any nucleotide other than G). On the other hand, CFP (CXXC finger protein 1) as the only member of Class I binds to CpGpGp sequence with high affinity. Whereas, the CXXC domain of DNMT1 (DNA (cytosine-5)-methyltransferase 1) as the Class IV protein displays a weak or no binding to DNA7. Although the CXXC domain of CXXC5 interacts with CpH as well as methylated CpG containing DNA, the binding affinity of the CXXC domain to these sequences is considerably lower (10–40 fold) than those observed with sequences containing the unmethylated CpG dinucleotide7. On the other hand, we observed here that the full-length CXXC5 binds specifically to unmethylated CpG dinucleotide containing DNA. As suggested for various proteins31, it is likely that intramolecular allosteric interactions between the disorder amino-terminus and the carboxyl-terminally located CXXC domain, or the presence of an affinity modulatory sub-region within the amino-terminus, provide selectivity to apoCXXC5 to interact primarily with unmethylated CpG dinucleotides. Dynamic conformational fluctuations in CXXC5 mediated by protein-protein interactions and/or post-translational modifications in a cellular environment could also be critical for the protein to recognize and bind to a permutation of unmodified or modified cytosine-centered DNA sequences in a signal- and cell type-specific manner.
Our results also indicate that the E2-responsive gene product CXXC5 is a critical contributor to the expression of genes mediated by the E2-ERα signaling. The expression of the primary E2 responsive genes by E2-ERα encompasses proteins involved in the metabolism of nucleic acid/proteins as well as transcription factors, membrane signaling cascade and receptor proteins. These proteins, in turn, participate in the regulation of late gene expressions responsible for the modulation of DNA replication, recombination, and repair, cell cycle, and division, consequently in the initiation of E2-mediated cellular proliferation38,57–59. Although limited in the gene number, our multiplex gene expression analysis using the PanCancer Pathway Panel revealed that the suppression of CXXC5 levels in cells without an active E2 signaling, i.e. cells transfected with siRNA#10 in the absence of E2, results in the regulation of a number of genes, including CCND1, CCNE1, CDC25B, MYC critical for cellular proliferation. However, the modulatory effect of CXXC5 on gene expressions was not reflected in the proliferation, as we observed no change in cellular growth compared to cells transfected with CtS in the absence of E2. On the other hand, E2 treatment of cells transfected with CtS effectively increased cellular proliferation through the expression of a set of genes including CDC25A, MYB, and WEE1 specifically regulated by E2 as well as CCND1 and MYC regulated by both E2 and CXXC5. In contrast, the diminished CXXC5 levels in cells resulted in an effective attenuation of E2-driven cellular proliferation. In addition to genes regulated independently as well as commonly by E2 and CXXC5 critical for cellular proliferation, the appearance of a set of genes modulated by E2 as a result of the diminished synthesis of CXXC5 that includes CDK6, which encodes a cyclin-dependent kinase critical for the initiation and transition of cell cycle phases and ATR, which encodes a serine/threonine-protein kinase involved in checkpoint control, could underlie the repression of E2-driven cellular proliferation. This implies that CXXC5 contributes to the regulation of not only known but also latent E2 responsive gene expressions critical for cellular proliferation.
Although it is clear that CXXC5 is involved in gene expressions, the underlying mechanism by which CXXC5 mediates transcription remains conjectural. Our findings that CXXC5 lacks a transcription regulatory function but is an unmethylated CpG dinucleotide binder suggest that upon binding to DNA, CXXC5 could act as a nucleation factor to establish a transcription state permissive or restrictive for transcription by preventing DNA methylation and/or recruiting epigenetic and/or transcription factors. In keeping with this prediction, our ongoing studies suggest that CXXC5 indeed interacts with various transcription factors as well as DNA and histone modifiers. This is also consistent with findings that the genetic ablation of CXXC5 in a mouse model results in epigenetic alterations, including decreased levels of promoter CpG methylation as well as histone modifications, at the Interferon regulatory factor 7 gene (IRF7) locus in plasmacytoid dendritic cells24. Likewise, the loss of CXXC5 was recently shown to alter the methylation state of embryonic stem cells contributing to altered gene expressions19. Furthermore, CXXC5 was reported to inhibit the expression of the CD40 ligand gene (CD40L) by interacting with histone-lysine N-methyltransferase SUV39H1 at the gene promoter60. Extending these, our observations based on a publicly available methylation profiling data set generated by the use of genome tiling arrays carried out with DNA from MCF7 cells in the absence or the presence of E2 (GSE132513) suggest that the methylation state of some of the estrogen-responsive genes and their expressions could correlate with the presence or absence of CXXC5 (Supplementary Fig. S5). We observed that MGMT, DAXX, CACNG6, HDAC11 and SHC4 expressions, which are upregulated by E2 only in the presence of CXXC5 but remain unchanged when CXXC5 is knockdown (Fig. 4, and Supplementary Table 2), tend to be hypomethylated upon E2 stimulation in MCF-7 cells. Of these, the hypomethylated regions of CACNG6 or SHC4 are located at the promoter. On the other hand, the expression of NFKBIZ, IKBKB, CAKM2B, MAP3K13, RPS6KA5 or POLB which is downregulated by E2 only in the presence of CXXC5 but remains unaltered when CXXC5 is knockdown (Fig. 4, and Supplementary Table 2), is likely to be hypermethylated at the promoter regions. While its importance remains to be explored, we also observed in vitro that CXXC5 binds to hemimethylated CG containing DNA fragment (Supplementary Fig. S6). This raises an intriguing possibility that CXXC5 could also be involved in the maintenance of replication, as suggested for DNMTs61,62 as well as monoallelic expression patterns of imprinted genes, which are lost in human cancer63.
Interestingly, some latent E2-responsive genes encode protein components of signal transduction pathways mediated by the cytokine, growth factor and frizzled family of proteins, which were previously shown to drive the expression of CXXC59–14. This suggests that the integrated effects of various signaling pathways at both basal- and induced-state determine the magnitude and direction of the CXXC5 expression. Since cell type-specific gene expression programs are controlled by the repertoire of lineage-specific transcription regulators responding to distinct stimuli, altered levels of the CXXC5 expression would also lead to diverse cellular responses ranging from proliferation to differentiation and death, as reported previously8–10,12,15–19,32, in a cell- and tissue-specific manner, contributing to the physiology and pathophysiology of various tissue functions.
We, as others25,51, find that high levels of CXXC5 expression predict poor overall survival, particularly in ERα-overexpressing luminal breast cancer patients. This is consistent with the prediction that the overexpression of CXXC5 represents an independent molecular marker for the unfavorable prognosis in ER+ breast tumors25,51. Moreover, we find here that the CXXC5 expression also correlates with the mRNA and protein levels of ERα. Since CXXC5 is an E2-ERα responsive gene, CXXC5 could indeed participate in the initiation and progression of breast cancer driven by a de-regulated E2-ERα signaling. This, in turn, implies that in addition to being a prognostic factor, CXXC5 could be an important therapeutic target for the disease as well.
Methods
Cell culture and biochemicals
The growth and maintenance of E2 responsive and ERα-synthesizing MCF7 and T47D cells were described previously4,64. In all experiments, media were changed every third day when appropriate.
Restriction and DNA modifying enzymes were obtained from New England Bio-Labs (Beverly, MA, USA) or Thermo-Fischer Sci. (Waltham, MA, USA). 17β-estradiol (E2) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The complete antagonist of estrogen receptor, Imperial Chemical Industries 182780, (ICI) was obtained from Tocris Biosciences (Ellisville, IL, USA). The antibody for Poly(ADP-ribose) polymerase 1 (PARP1; 9542) was obtained from Cell Signaling Technology (Beverly, MA, USA). The antibodies for CXXC5 (ab106533) and for HDAC1 (ab19845) were purchased from Abcam Inc. (Cambridge, MA, USA). Secondary antibodies conjugated with horseradish peroxidase were obtained from Santa Cruz Biotech (Santa Cruz, CA, USA). siRNAs (FlexiTube siRNA) for CXXC5 were purchased from Qiagen Inc. (Düsseldorf, Germany). Camptothecin (13637) was purchased from Cell Signaling Technology.
Cloning of cDNA for the full-length CXXC5 or the Zinc Finger CXXC domain into the pET28a-MHL vector for bacterial expression
The expression vector bearing the human CXXC5 cDNA was described previously4. The sequences encoding the full-length CXXC5 (322 aa) or the CXXC DNA binding domain (250–309 aa) generated by PCR using the CXXC5 cDNA as the DNA template were inserted into the pET28a-MHL vector (GenBank accession EF456735) with appropriate restriction enzyme sites. The vector contains sequences encoding the 6xHis tag at the 5′ end of the inserted cDNA followed by sequences for the cleavage with Tobacco Etch Virus (TEV) protease for the initial protein purification. TEV protease was produced at the Structural Genomics Consortium (University of Toronto, Toronto, CA). The vector also contains the kanamycin resistance gene for antibiotic selection.
Protein expression and purification
The recombinant full-length CXXC5 or the CXXC domain protein was overexpressed in Escherichia coli BL21(DE3)-V2R-pRARE2 strain (Structural Genomics Consortium; Toronto, CA) in terrific broth (Sigma-Aldrich, MO, USA) supplemented with 0.8% glycerol, 50 µg/ml kanamycin, 17 µg/ml chloramphenicol, 50 µM ZnCl2 and 800 µl antifoam-204 (Sigma-Aldrich) in a 2-liter flask and grown at 37 °C with shaking at 200 rpm. When OD600 reached 1.6, the temperature was reduced to 16 °C and 250 µM IPTG was added into the culture to induce protein synthesis. Cultures were aerated overnight at 16 °C with shaking at 200 rpm. Cells were collected by centrifugation at 4 °C, lysed in a lysis buffer (50 mM Tris pH 8.0, 500 mM NaCl, 1 mM PMSF, 3 mM β-mercaptoethanol, 50 µM ZnCl2 and benzonase) and homogenized using an Ultra-Turrax T8 homogenizer at a maximal setting for 60 seconds. The slurry was then sonicated briefly and was centrifuged at 15,000 rpm for 1 h at 4 °C to clear lysate. The cleared supernatant subsequently mixed with 10 ml of equilibrated His-Select resin (Sigma-Aldrich) and incubated for 30 min at 4 °C with continuous mixing. The mixture was then loaded onto a column and washed with 300 ml wash buffer (50 mM Tris pH: 8.0, 2 M NaCl, 13 mM imidazole pH: 8.0, 3 mM β-mercaptoethanol, 50 µM ZnCl2). Proteins were eluted from the resin by 20 ml of elution buffer (50 mM Tris pH: 8.0, 300 mM NaCl, 250 mM imidazole pH: 8.0, and 1 mM TCEP). To remove the high concentration of imidazole from the protein mixture, the mixture was dialyzed against 50 mM Tris pH 8.0, 150 mM NaCl and 1 mM TCEP at 4 °C overnight. During the dialysis procedure, TEV enzyme (1 µg TEV for 10 µg recombinant protein) was added directly into the protein mixture to remove the 6xHis tag from proteins. Protein samples were subsequently subjected to a HiTrap SP Fast Flow (GE Healthcare, Sigma-Aldrich) ion-exchange chromatography using buffers with gradient salt concentrations (Buffer A: 50 mM Tris pH 8.0, 20 mM NaCl, 1 mM TCEP, Buffer B: 50 mM Tris pH 8.0, 1 M NaCl; 1 mM TCEP). Fractions corresponding to protein peaks were loaded on SDS-PAGE (4–20% gradient) and stained by InstantBlue coomassie dye (Expedeon, San Diego, CA, USA) to ensure the protein purification procedure. Protein fractions were then collected and subjected to a Superdex 200 (10/300) gel filtration (size exclusion) column (GE Healthcare Bio-Sci., Pittsburgh, PA, USA) followed by a HiTrap Heparin HP (GE Healthcare) column for further purification. Eluted proteins in an elution buffer (20 mM Tris pH 7.5, 150 mM NaCl and 1 mM TCEP) were subjected to SDS-PAGE (4–20% gradient) and stained by the InstantBlue coomassie dye to ensure the purification procedure. The protein fractions that contained the full-length CXXC5 or the CXXC domain protein were collected and concentrated using 10K-MW (for the FL-CXXC5) and 3K-MW (for the CXXC-D) cut-off concentrators (Thermo-Fisher) for functional assays.
Isothermal titration calorimetry (ITC) binding assay
The abilities of the purified proteins to interact with DNA were assesses with ITC. Oligonucleotides containing a central CpG dinucleotide without or with methyl-cytosine residue or an AT dinucleotide as single-stranded DNA were purchased from Integrated DNA Technologies (IDT). Each pair of single-strand DNAs, dissolved in buffer 20 mM Tris pH 7.5 and 150 mM NaCl, was mixed in a 1:1 molar ratio, heated to 95 °C for 5 min and cooled to room temperature to obtain a double-stranded DNA. The full-length CXXC5 or the CXXC domain protein at the concentration of 10 µM and of 300 µM DNA were used in the ITC experiment using a VP-ITC (Malvern Panalytical, Malvern, UK) at 25 °C. A total of 25 injections was performed with 180-sec intervals using the reference power of 15 μcal/sec. Binding isotherms were plotted and analyzed using Origin 7.0 software (MicroCal Inc.). ITC measurements were fit to a one-site binding model.
Electrophoretic mobility shift assay (EMSA)
We also assessed the abilities of the recombinant FL-CXXC5 or the CXXC-D protein to bind to DNA with EMSA. EMSA was carried out as described previously4,65. As detailed for ITC experiments, the double-stranded DNA without or with methyl-cytosine residue, 50 µM, was mixed with the full-length CXXC5 or the CXXC domain protein in increasing molar ratios and incubated for 30 min on ice. The samples were then loaded onto 5% TBE ready gels (Bio-Rad Laboratories, CA, USA) in 0.5X TBE running buffer. The gel was stained by the SybrGold dye (Thermo-Fisher) for visualization.
Transfections
For siRNA transfection, cells (in 96-well tissue culture plates for cellular growth; in 12-well tissue culture plates for RNA extraction or 6-well tissue culture plates for WB and phenotypic assays) were cultured in charcoal-dextran treated fetal bovine serum-containing (CD-FBS) phenol red-free medium for 48 h to reduce/ablate steroid hormone levels. Cells then were transiently transfected with the HiPerfect transfection reagent (Qiagen) using 10 nM of a scrambled siRNA (AllStar, CtS) or of an siRNA specific for CXXC5 (FlexiTube GeneSolution, Qiagen) and treated in the absence (ethanol, 0.01%) or the presence of 10−8 M E2 and/or 10−6 M ICI, which is a complete ER antagonist, as we described previously4 for the duration of an experiment defined in the result section. The amount of siRNA we selected was based on our initial studies, with which we observed that 10 nM was the maximal amount of the control siRNA (CtS) without inducing phenotypic changes in transfected MCF7 cells up to 96 h.
Luciferase assay
To assess whether or not CXXC5 has a transcription activation or repression activity, we used luciferase assays. pGal4-RE Luciferase Reporter vector (pGal4-RE-Luc) contains a tandem Gal4 response elements (Gal4-RE) juxtaposed to a simple TATA box promoter that drives the expression of the Firefly Luciferase enzyme cDNA as the reporter enzyme (Promega Corp., Madison, WI, USA), as we described previously34. The expression vector (pM) bearing sequences encoding the GAL4 DNA Binding Domain, DBD, (Gal4DBD, amino acids 1–147), and a nuclear localization signal was used as the template to generate fusion proteins. The sequences encoding the FL-CXXC5, full-length MeCP2 (amino acids 1–486) or the multifunctional carboxyl-terminus of ERα-EF domain containing the ligand-dependent transaction activity (amino acids 301–595) were inserted into the 3′ end of the sequences encoding the GAL4DBD with appropriate restriction enzyme sites. We also used an expression vector bearing only the FL-CXXC5, FL-MeCP2 or ERαEF cDNA as control. Expression vectors (75 ng/transfection) were transfected into MCF7 cells together with the pGal4-RE-Luc reporter plasmid (125 ng/transfection) as described previously4. Transfection efficiency was monitored with the co-expression of a reporter plasmid that drives the expression of the Renilla Luciferase enzyme cDNA (0.5 ng). In transfections using the expression vector bearing ERαEF or Gal4DBD-ERαEF cDNA, cells were treated without (ethanol, 0.01%) or with 10−8 M E2. Twenty-four hours after transfections, cellular extracts were assayed for the change in luciferase enzyme activities using a Dual-Luciferase Assay kit (Promega Corp.), as described previously4.
PCR and RT-qPCR
Isolated total RNA from cells was used for the cDNA synthesis (The RevertAid First Strand cDNA Synthesis Kit, Thermo-Fisher). The SYBR Green Mastermix (BioRad, Hercules, CA, USA) and gene-specific primers (Supplementary Table S1) were used for RT-qPCR reactions on BioRad Connect Real-Time PCR, as we described previously4. For the normalization of results, we used the expression of RPLP0 (60 S acidic ribosomal protein P0), a reference gene for the normalization of E2-regulated genes in breast carcinomas66. The relative quantitation of gene expressions was assessed with the comparative 2-ΔΔCT method67. During the RT-qPCR experiments, MIQE Guidelines were followed68.
Western blotting (WB)
WB was performed as described previously4. In brief, cells grown in six-well tissue culture plates in medium supplemented with 10% CD-FBS to reduce the endogenous E2 concentration for 48 h were transfected with an siRNA specific for CXXC5 or a non-target control siRNA (AllStar, CtS) in the absence (EtOH, 0.01%) or the presence of E2 (10−8 M) for 48 h. At the termination, cells were collected and the nuclear content was isolated using NE-PER protein extraction kit (Thermo Fisher). Protein concentration was measured with Bradford Protein Assay (Bio-Rad). Nuclear extracts (50 μg) were then subjected to 12% SDS-PAGE. Proteins were probed with an antibody specific to CXXC5 (ab106533, Abcam) or PARP1 (9542; Cell Signaling Tech.) followed by a secondary antibody conjugated with horseradish peroxidase (Advansta Inc., San Jose, CA, USA). The HDAC1 antibody (Abcam, ab19845) was used for monitoring the levels of HDAC1, which was used as the loading control. Proteins were visualized with ECL (Advansta) and images were captured with ChemiDoc Imaging System (Bio-Rad). PageRuler Prestained Protein Ladder (Thermo-Fisher) was used as a molecular weight marker.
Cellular proliferation
To assess the effects of CXXC5 synthesis on cellular growth, cells were plated in 96-well culture plates in phenol red-free medium containing 10% CD-FBS for 48 h. Cells were then transiently transfected with siRNAs as described previously4 in the absence (ethanol, 0.01%) or the presence of E2 (10−8 M) and/or ICI (10−6 M). 48 h after transfection, cells were subjected to counting using a hemocytometer or 3-(4,5-Dimethylthiazol-2-yl)−2,5 diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich) as described previously3.
Cell cycle analysis
To assess the effects of reduced CXXC5 protein levels on cell cycle phases, cells, 16 ×104/well of a six-well tissue culture plate, were grown in phenol red-free medium containing 10% CD-FBS for 48 h. Cells were then transfected without or with siRNAs in the absence (ethanol, 0.01%) or the presence of 10−8 M E2 and grown for additional 48 h. Cells were collected with trypsinization, washed with PBS and pelleted. Cells were then processed for cell cycle distribution as described previously3,65. In brief, pelleted cells were gently re-suspended in 100 µl of 2%CD-FBS containing PBS, fixed and permeabilized with ice-cold 70% ethanol overnight. Cells were subsequently incubated with 200 µl of PBS containing propidium iodide (20 µg/ml; Sigma-Aldrich), 200 µg/ml RNase A (Thermo-Fisher) and 0.1% (v/v) Triton X-100 (AppliChem, Germany) for 30 min. Cell cycle analyses were then carried out with flow cytometry (BD Accuri C6 Cytometer; BD Biosciences, San Jose, CA, USA).
Annexin V assay
To assess whether reduced CXXC5 protein levels cause cell death in the absence or presence of E2, MCF7 cells, 16 × 104 cells/well of a six-well tissue culture plate, were grown in phenol red-free medium containing 10% CD-FBS for 48 h for. Cells were then transfected without or with siRNAs in the absence (ethanol, 0.01%) or the presence of 10−8 M E2 and grown for additional 48 h. Cells were subsequently subjected to Annexin V staining, as we described previously3,65, according to the manufacturer’s instructions (BioLegend, San Diego, CA, USA). In brief, cells were harvested and washed twice with cold 1X PBS and then re-suspended in 1X binding buffer. APC Annexin V (5 µl) and 7-Amino-Actinomycin (7-ADD) (5 µl) were added to 100 µl of cell suspension containing 105 cells. The cell suspension was gently mixed and incubated for 15 min at room temperature in the dark followed by the addition of 400 µl of 1X binding buffer and subjection to flow cytometry (BD Accuri C6 cytometer, BD Biosciences). Using the experimental approach described here for Annexin V assay, we also carried out WB for the cleavage of Poly(ADP-ribose) Polymerase (PARP) as an indication of apoptosis, with a PARP1-specific antibody (9542, Cell Signaling Tech.) that also detects the cleaved PARP. We also used camptothecin (CPT), a DNA topoisomerase inhibitor, as a control for the induction of cell death. Cells maintained for 48 h in the absence or presence of E2 were subjected to 2 nM CPT for 24 h. Cells were then collected and subjected to Annexin V staining or WB analysis for PARP1 cleavage using the PARP1 antibody.
Analysis of Gene Expression with nCounter PanCancer Pathway Panel gene expression analysis and RT-qPCR
MCF7 cells in 12-well tissue culture plates (9 ×104 cells/well) were cultured in phenol red-free medium containing 10% CD-FBS medium for 48 h. Cells were then transiently transfected with the HiPerfect transfection reagent (Qiagen) using 10 nM of an siRNA. Forty-eight hours after transfection, cells were treated without (ethanol, 0.01%) as vehicle control or with 10−8 M of E2 for 3 hours. For RNA extraction, Quick-RNA MiniPrep (Zymo Research, Irvine, CA, USA) was used as instructed by the manufacturer. The total RNA sample was measured using BioDrop μLITE (Cambridge, United Kingdom). 50 ng RNA/sample was used for processing the nCounter PanCancer Pathway Panel gene expression analysis (NanoString Technologies, Inc. Redwood City, CA, USA). This panel contains 730 different probes together with 40 internal control probes complementary to the corresponding target mRNAs. The fluorescence barcodes specific for a gene are attached to each probe, enabling digital counting of each transcript. Sample preparation was carried out with nCounter PrepStation and microscopy scanning of the barcode signals was carried out with nCounter Digital Analyzer (Nanostring) as instructed by the manufacturer. Quality control, data normalization and differential expression analyses were done using Nanostring nSolver 3.0 Analysis software together with its Advanced Analysis plug-in.
For RT-qPCR, total RNA isolated from MCF7 cells, treated as described for PanCancer Pathway Panel, was used for cDNA synthesis (The RevertAid First Strand cDNA Synthesis Kit, Thermo-Fisher). Based on the results from PanCancer Pathway Panel gene expression analysis, eight genes were selected for the verification of the results observed with PanCancer Pathway Panel with RT-qPCR using gene-specific primers (Supplementary Table S1). RT-qPCR products were detected with SYBR Green on BioRad Connect Real-Time PCR. We used the expression of RPLP0 for qPCR normalizations.
Metadata analysis for the clinical relevance of CXXC5 in breast cancer patients
mRNA expressions of CXXC5 and ERα in patients from METABRIC69 and TCGA (https://www.cancer.gov/tcga) databases were downloaded from cBioPortal70,71. Protein expression of ERα was downloaded from TCPA database72. Survival curves were generated based on median separation and the significance of the difference among groups was determined using the Log-rank test. Comparisons between the two groups were made by a two-tailed t-test. Pearson correlation coefficient was calculated to demonstrate the association between gene or protein expressions of CXXC5 and ERα. Box-plots depict median number and the 25th to 75th quartiles. “ns” denotes “not significant”.
Methylation profiling
A publically available methylation profiling dataset of MCF-7 cells in response to E2 (GSE132513) was used to analyze the differential methylation of E2-regulated genes that are affected by CXXC5 knockdown. To this end, normalized methylation scores were downloaded from the GEO database, and the ratio of the methylation scores in “E2 stimulated” cells to “E2-deprived” cells were taken. A fold change cut-off of 1.2 was used to determine differentially methylated genes73,74. A heatmap of the methylation scores of E2-regulated genes whose expression depends on CXXC5 modulation was drawn using Morpheus software.
Statistical analysis
Results, with biological replicates as indicated for each experiment, were presented as the mean ± standard error (S.E.) The statistical analyses were performed using one-way ANOVA with Tukey test for post-hoc analysis or two-tailed unpaired t-test with a confidence interval, minimum, of 95%.
Supplementary information
Acknowledgements
This work was supported by grants from TUBITAK-KBAG 114Z243 (MM) and METU-BAP-01-08-2016-001 (MM). Gamze Ayaz was supported by graduate study fellowships from TUBITAK 2214/A. We express our gratitude to Dr. Jinrong Min, Structural Genomics Consortium & Department of Physiology, University of Toronto, Toronto, Ontario, Canada, for his generous permission, and inputs, to utilize his laboratory resources to carry out recombinant protein expressions and characterizations. We also thank Dr. Serap Beldar, Structural Genomics Consortium, University of Toronto, Toronto, Canada, for the help in ITC data analyses and Öykü Deniz Demiralay, METU Biological Sciences, Ankara, Turkey, for the help in WB analyses. We thank Dr. Kunio Miyake, University of Yamanashi, Japan, for providing an expression vector bearing the full-length MeCP2 cDNA. We thank METABRIC Consortium for providing access to breast cancer patient data. We thank members of the Muyan laboratory for stimulating discussions, contributions and critical readings of the manuscript.
Author contributions
G.A., P.Y. and M.M. designed the study. G.A., N.R., P.Y. and G.K. performed the experiments. G.A. performed recombinant protein expression and characterization; N.R., D.C.K. and R.Ç.A. conducted PanCancer Pathway Panel gene expression analysis and Annexin V assays; Ö.S., P.Y. and Ö.Ş. conducted metadata analysis and methylation profiling; G.A., N.R., P.Y., G.K. and M.M. wrote the manuscript. All contributors reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/16/2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Supplementary information
is available for this paper at 10.1038/s41598-020-62912-0.
References
- 1.Ayaz G, et al. Dynamic transcriptional events mediated by estrogen receptor alpha. Front. Biosci. 2019;24:4716. doi: 10.2741/4716. [DOI] [PubMed] [Google Scholar]
- 2.Choi S, et al. CXXC5 mediates growth plate senescence and is a target for enhancement of longitudinal bone growth. Life Sci. Alliance. 2019;2:e201800254. doi: 10.26508/lsa.201800254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nott SL, et al. Genomic Responses from the Estrogen-responsive Element-dependent Signaling Pathway Mediated by Estrogen Receptor α Are Required to Elicit Cellular Alterations. J. Biol. Chem. 2009;284:15277–15288. doi: 10.1074/jbc.M900365200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaşar P, Ayaz G, Muyan M. Estradiol-Estrogen Receptor α Mediates the Expression of the CXXC5 Gene through the Estrogen Response Element-Dependent Signaling Pathway. Sci. Rep. 2016;6:37808. doi: 10.1038/srep37808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long HK, Blackledge NP, Klose RJ. ZF-CxxC domain-containing proteins, CpG islands and the chromatin connection. Biochem. Soc. Trans. 2013;41:727–740. doi: 10.1042/BST20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong X, Tu S, Wang J, Luo S, Yan X. CXXC5: A novel regulator and coordinator of TGF-β, BMP and Wnt signaling. J. Cell. Mol. Med. 2019;23:740–749. doi: 10.1111/jcmm.14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, et al. DNA Sequence Recognition of Human CXXC Domains and Their Structural Determinants. Structure. 2018;26:85–95.e3. doi: 10.1016/j.str.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Pendino F, et al. Functional involvement of RINF, retinoid-inducible nuclear factor (CXXC5), in normal and tumoral human myelopoiesis. Blood. 2009;113:3172–3181. doi: 10.1182/blood-2008-07-170035. [DOI] [PubMed] [Google Scholar]
- 9.Yan X, et al. CXXC5 suppresses hepatocellular carcinoma by promoting TGF-β-induced cell cycle arrest and apoptosis. J. Mol. Cell Biol. 2018;10:48–59. doi: 10.1093/jmcb/mjx042. [DOI] [PubMed] [Google Scholar]
- 10.Kim H-Y, et al. CXXC5 is a transcriptional activator of Flk-1 and mediates bone morphogenic protein-induced endothelial cell differentiation and vessel formation. FASEB J. 2014;28:615–626. doi: 10.1096/fj.13-236216. [DOI] [PubMed] [Google Scholar]
- 11.Andersson T, et al. CXXC5 Is a Novel BMP4-regulated Modulator of Wnt Signaling in Neural Stem Cells. J. Biol. Chem. 2009;284:3672–3681. doi: 10.1074/jbc.M808119200. [DOI] [PubMed] [Google Scholar]
- 12.Kim H-Y, et al. CXXC5 is a negative-feedback regulator of the Wnt/β-catenin pathway involved in osteoblast differentiation. Cell Death Differ. 2015;22:912–920. doi: 10.1038/cdd.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S-H, et al. The Dishevelled-binding protein CXXC5 negatively regulates cutaneous wound healing. J. Exp. Med. 2015;212:1061–1080. doi: 10.1084/jem.20141601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M-Y, et al. CXXC5 plays a role as a transcription activator for myelin genes on oligodendrocyte differentiation. Glia. 2016;64:350–362. doi: 10.1002/glia.22932. [DOI] [PubMed] [Google Scholar]
- 15.Marshall PA, et al. Discovery of novel vitamin D receptor interacting proteins that modulate 1,25-dihydroxyvitamin D3 signaling. J. Steroid Biochem. Mol. Biol. 2012;132:147–159. doi: 10.1016/j.jsbmb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, et al. CXXC5 regulates differentiation of C2C12 myoblasts into myocytes. J. Muscle Res. Cell Motil. 2014;35:259–265. doi: 10.1007/s10974-014-9400-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, et al. The CXXC finger 5 protein is required for DNA damage-induced p53 activation. Sci. China Ser. C Life Sci. 2009;52:528–538. doi: 10.1007/s11427-009-0083-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, et al. CXXC5 Associates with Smads to Mediate TNF-α Induced Apoptosis. Curr. Mol. Med. 2013;13:1385–1396. doi: 10.2174/15665240113139990069. [DOI] [PubMed] [Google Scholar]
- 19.Ravichandran M, et al. Rinf Regulates Pluripotency Network Genes and Tet Enzymes in Embryonic Stem Cells. Cell Rep. 2019;28:1993–2003.e5. doi: 10.1016/j.celrep.2019.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, et al. Small molecule inhibitors of the Dishevelled‐CXXC5 interaction are new drug candidates for bone anabolic osteoporosis therapy. EMBO Mol. Med. 2016;8:375–387. doi: 10.15252/emmm.201505714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S-H, et al. Targeting of CXXC5 by a Competing Peptide Stimulates Hair Regrowth and Wound-Induced Hair Neogenesis. J. Invest. Dermatol. 2017;137:2260–2269. doi: 10.1016/j.jid.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, et al. A Novel Wilms Tumor 1 (WT1) Target Gene Negatively Regulates the WNT Signaling Pathway. J. Biol. Chem. 2010;285:14585–14593. doi: 10.1074/jbc.M109.094334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng X, et al. CXXC5 is required for cardiac looping relating to TGFβ signaling pathway in zebrafish. Int. J. Cardiol. 2016;214:246–253. doi: 10.1016/j.ijcard.2016.03.201. [DOI] [PubMed] [Google Scholar]
- 24.Ma S, et al. Epigenetic regulator CXXC5 recruits DNA demethylase Tet2 to regulate TLR7/9-elicited IFN response in pDCs. J. Exp. Med. 2017;214:1471–1491. doi: 10.1084/jem.20161149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knappskog S, et al. RINF (CXXC5) is overexpressed in solid tumors and is an unfavorable prognostic factor in breast cancer†. Ann. Oncol. 2011;22:2208–2215. doi: 10.1093/annonc/mdq737. [DOI] [PubMed] [Google Scholar]
- 26.May-Panloup P, et al. Molecular characterization of corona radiata cells from patients with diminished ovarian reserve using microarray and microfluidic-based gene expression profiling. Hum. Reprod. 2012;27:829–843. doi: 10.1093/humrep/der431. [DOI] [PubMed] [Google Scholar]
- 27.L’Hôte D, et al. Discovery of novel protein partners of the transcription factor FOXL2 provides insights into its physiopathological roles. Hum. Mol. Genet. 2012;21:3264–3274. doi: 10.1093/hmg/dds170. [DOI] [PubMed] [Google Scholar]
- 28.Treppendahl MB, Möllgård L, Hellström-Lindberg E, Cloos P, Grønbaek K. Downregulation but lack of promoter hypermethylation or somatic mutations of the potential tumor suppressor CXXC5 in MDS and AML with deletion 5q. Eur. J. Haematol. 2013;90:259–260. doi: 10.1111/ejh.12045. [DOI] [PubMed] [Google Scholar]
- 29.Centritto F, et al. Cellular and molecular determinants of all‐ trans retinoic acid sensitivity in breast cancer: Luminal phenotype and RAR α expression. EMBO Mol. Med. 2015;7:950–972. doi: 10.15252/emmm.201404670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benedetti I, De Marzo AM, Geliebter J, Reyes N. CXXC5 expression in prostate cancer: implications for cancer progression. Int. J. Exp. Pathol. 2017;98:234–243. doi: 10.1111/iep.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forman-Kay JD, Mittag T. From Sequence and Forces to Structure, Function, and Evolution of Intrinsically Disordered Proteins. Structure. 2013;21:1492–1499. doi: 10.1016/j.str.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aras S, et al. Oxygen-dependent expression of cytochrome c oxidase subunit 4-2 gene expression is mediated by transcription factors RBPJ, CXXC5 and CHCHD2. Nucleic Acids Res. 2013;41:2255–2266. doi: 10.1093/nar/gks1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo Y, Batalao A, Zhou H, Zhu L. Mammalian Two-Hybrid System: A Complementary Approach to the Yeast Two-Hybrid System. Biotechniques. 1997;22:350–352. doi: 10.2144/97222pf02. [DOI] [PubMed] [Google Scholar]
- 34.Yi P, Bhagat S, Hilf R, Bambara RA, Muyan M. Differences in the Abilities of Estrogen Receptors to Integrate Activation Functions Are Critical for Subtype-Specific Transcriptional Responses. Mol. Endocrinol. 2002;16:1810–1827. doi: 10.1210/me.2001-0323. [DOI] [PubMed] [Google Scholar]
- 35.Nan X, Campoy FJ, Bird A. MeCP2 Is a Transcriptional Repressor with Abundant Binding Sites in Genomic Chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- 36.Wang, H. et al. NanoStringDiff: a novel statistical method for differential expression analysis based on NanoString nCounter data. Nucleic Acids Res. gkw677 10.1093/nar/gkw677 (2016). [DOI] [PMC free article] [PubMed]
- 37.Kahraman, D. C., Kahraman, T. & Atalay, R. C. Differential alteration of IL-8 in liver cancer stem cell enrichment in response to PI3K/Akt/mTOR inhibitors and sorafenib. In Experimental and Molecular Therapeutics 3937–3937 (American Association for Cancer Research, 10.1158/1538-7445.AM2018-3937 2018).
- 38.Bourdeau V, et al. Mechanisms of primary and secondary estrogen target gene regulation in breast cancer cells. Nucleic Acids Res. 2007;36:76–93. doi: 10.1093/nar/gkm945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frasor J, et al. Profiling of Estrogen Up- and Down-Regulated Gene Expression in Human Breast Cancer Cells: Insights into Gene Networks and Pathways Underlying Estrogenic Control of Proliferation and Cell Phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 40.Patterson AR, et al. Sustained Reprogramming of the Estrogen Response After Chronic Exposure to Endocrine Disruptors. Mol. Endocrinol. 2015;29:384–395. doi: 10.1210/me.2014-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pechenino AS, Frick KM. The effects of acute 17β-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol. Learn. Mem. 2009;91:315–322. doi: 10.1016/j.nlm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of Estrogen Receptor β on Gene Networks Regulated by Estrogen Receptor α in Breast Cancer Cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 43.Mauro L, et al. Estradiol Increases IRS-1 Gene Expression and Insulin Signaling in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2001;288:685–689. doi: 10.1006/bbrc.2001.5815. [DOI] [PubMed] [Google Scholar]
- 44.Paranjpe A, et al. New insights into estrogenic regulation of O6-methylguanine DNA-methyltransferase (MGMT) in human breast cancer cells: Co-degradation of ER-α and MGMT proteins by fulvestrant or O6-benzylguanine indicates fresh avenues for therapy. J. Biomed. Res. 2016;30:393–410. doi: 10.7555/JBR.30.20160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shanker G, Sorci-Thomas M, Adams MR. Estrogen modulates the inducible expression of platelet-derived growth factor mrna by monocyte/macrophages. Life Sci. 1995;56:499–507. doi: 10.1016/0024-3205(94)00479-c. [DOI] [PubMed] [Google Scholar]
- 46.Andruska ND, et al. Estrogen receptor α inhibitor activates the unfolded protein response, blocks protein synthesis, and induces tumor regression. Proc. Natl. Acad. Sci. 2015;112:4737–4742. doi: 10.1073/pnas.1403685112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chimge N-O, et al. RUNX1 prevents oestrogen-mediated AXIN1 suppression and β-catenin activation in ER-positive breast cancer. Nat. Commun. 2016;7:10751. doi: 10.1038/ncomms10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Donnell AJM, Macleod KG, Burns DJ, Smyth JF, Langdon SP. Estrogen receptor-α mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr. Relat. Cancer. 2005;12:851–866. doi: 10.1677/erc.1.01039. [DOI] [PubMed] [Google Scholar]
- 49.Sengupta K, Banerjee S, Saxena N, Banerjee SK. Estradiol-induced vascular endothelial growth factor-A expression in breast tumor cells is biphasic and regulated by estrogen receptor-alpha dependent pathway. Int. J. Oncol. 2003;22:609–14. [PubMed] [Google Scholar]
- 50.McInnes KJ, Brown KA, Hunger NI, Simpson ER. Regulation of LKB1 expression by sex hormones in adipocytes. Int. J. Obes. 2012;36:982–985. doi: 10.1038/ijo.2011.172. [DOI] [PubMed] [Google Scholar]
- 51.Fang, L., Wang, Y., Gao, Y. & Chen, X. Overexpression of CXXC5 is a strong poor prognostic factor in ER+ breast cancer. Oncol. Lett. 10.3892/ol.2018.8647 (2018). [DOI] [PMC free article] [PubMed]
- 52.Defossez, P.-A. & Stancheva, I. Biological Functions of Methyl-CpG-Binding Proteins. In 377–398, 10.1016/B978-0-12-387685-0.00012-3 (2011). [DOI] [PubMed]
- 53.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 54.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. 2006;103:1412–1417. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blackledge NP, Klose R. CpG island chromatin. Epigenetics. 2011;6:147–152. doi: 10.4161/epi.6.2.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hah N, et al. A Rapid, Extensive, and Transient Transcriptional Response to Estrogen Signaling in Breast Cancer Cells. Cell. 2011;145:622–634. doi: 10.1016/j.cell.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jagannathan V, Robinson-Rechavi M. Meta-analysis of estrogen response in MCF-7 distinguishes early target genes involved in signaling and cell proliferation from later target genes involved in cell cycle and DNA repair. BMC Syst. Biol. 2011;5:138. doi: 10.1186/1752-0509-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cicatiello L, et al. Estrogen Receptor α Controls a Gene Network in Luminal-Like Breast Cancer Cells Comprising Multiple Transcription Factors and MicroRNAs. Am. J. Pathol. 2010;176:2113–2130. doi: 10.2353/ajpath.2010.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuchiya Y, et al. ThPOK represses CXXC5, which induces methylation of histone H3 lysine 9 in Cd40lg promoter by association with SUV39H1: implications in repression of CD40L expression in CD8 + cytotoxic T cells. J. Leukoc. Biol. 2016;100:327–338. doi: 10.1189/jlb.1A0915-396RR. [DOI] [PubMed] [Google Scholar]
- 61.Bashtrykov P, et al. Specificity of Dnmt1 for Methylation of Hemimethylated CpG Sites Resides in Its Catalytic Domain. Chem. Biol. 2012;19:572–578. doi: 10.1016/j.chembiol.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Xu C, Corces VG. Nascent DNA methylome mapping reveals inheritance of hemimethylation at CTCF/cohesin sites. Science. 2018;359:1166–1170. doi: 10.1126/science.aan5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jelinic P, Shaw P. Loss of imprinting and cancer. J. Pathol. 2007;211:261–268. doi: 10.1002/path.2116. [DOI] [PubMed] [Google Scholar]
- 64.Huang J, et al. Targeting estrogen responsive elements (EREs): design of potent transactivators for ERE-containing genes. Mol. Cell. Endocrinol. 2004;218:65–78. doi: 10.1016/j.mce.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Muyan M, et al. Modulation of Estrogen Response Element-Driven Gene Expressions and Cellular Proliferation with Polar Directions by Designer Transcription Regulators. PLoS One. 2015;10:e0136423. doi: 10.1371/journal.pone.0136423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyng MB, Lænkholm A-V, Pallisgaard N, Ditzel HJ. Identification of genes for normalization of real-time RT-PCR data in breast carcinomas. BMC Cancer. 2008;8:20. doi: 10.1186/1471-2407-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 68.Bustin SA, et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 69.Curtis C, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao J, et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013;6:pl1–pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cerami E, et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, et al. TCPA: a resource for cancer functional proteomics data. Nat. Methods. 2013;10:1046–1047. doi: 10.1038/nmeth.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trolle C, et al. Widespread DNA hypomethylation and differential gene expression in Turner syndrome. Sci Rep. 2016;6:34220. doi: 10.1038/srep34220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laufer BI, et al. Long-lasting alterations to DNA methylation and ncRNAs could underlie the effects of fetal alcohol exposure in mice. Dis Model Mech. 2013;6:977–992. doi: 10.1242/dmm.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.