Abstract
Background
The major risk of kidney biopsy is severe bleeding. Numerous risk factors for bleeding after biopsy have been reported, but findings have been inconsistent.
Methods
We retrospectively reviewed medical records of adult patients enrolled in a native kidney biopsy cohort study to identify major bleeding events (red blood cell [RBC] transfusions, invasive procedures, kidney loss, or death). We used logistic and linear regression models to identify characteristics associated with postbiopsy RBC transfusions and decline in hemoglobin within a week after the procedure.
Results
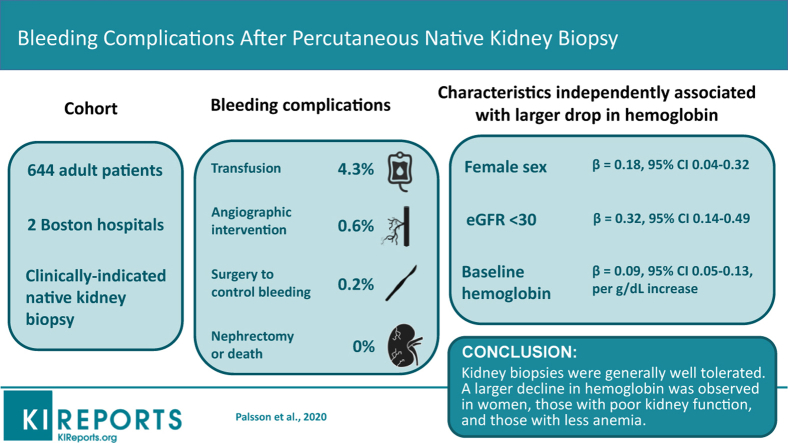
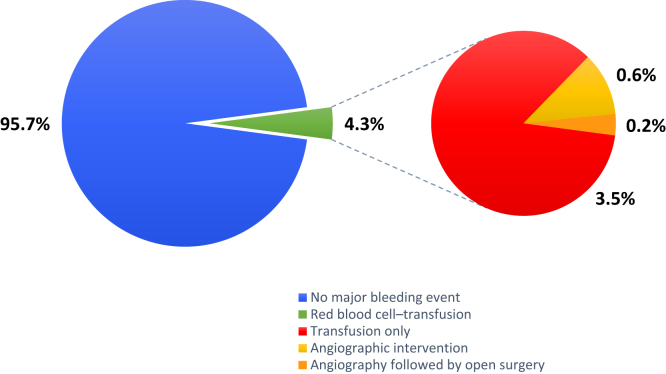
Major bleeding events occurred in 28 of 644 (4.3%) patients (28 required an RBC transfusion, 4 underwent angiographic intervention, and 1 had open surgery to control bleeding). No patient lost a kidney or died because of the biopsy. Postbiopsy RBC transfusion risk was driven by the baseline hemoglobin level (odds ratio [OR] 13.6; 95% confidence interval [CI] 5.4–34.1 for hemoglobin <10 vs. ≥10 g/dl). After adjusting for hemoglobin, no other patient characteristics were independently associated with RBC transfusions. Female sex (β = 0.18; 95% CI: 0.04–0.32), estimated glomerular filtration rate (eGFR) <30 ml/min per 1.73 m2 (β = 0.32; 95% CI: 0.14–0.49), and baseline hemoglobin (β = 0.09; 95% CI: 0.05–0.13, per g/dl increase) were independently associated with a larger drop in hemoglobin. Histopathologic lesions were not independently associated with major bleeding after biopsy.
Conclusion
Biopsies were generally well tolerated. Baseline hemoglobin was the dominant risk factor for RBC transfusions, but female sex and eGFR <30 ml/min per 1.73 m2 were also associated with a larger decline in hemoglobin after the procedure.
Keywords: biopsy, bleeding, blood transfusions, kidney diseases, risk factors
Graphical abstract
The kidney biopsy is a critical diagnostic and prognostic tool for evaluation of patients with kidney disease.1 Its results can guide management and provide information on response to treatment.2 Due to the high blood flow through the kidneys, which receive approximately 20% of the cardiac output,3 the major risks of kidney biopsies are related to bleeding. Bleeding complications can range from self-limited hematuria and asymptomatic perinephric hematomas of minimal clinical significance to life-threatening hemorrhage, resulting in hemodynamic instability, kidney loss, and even death. In current practice, kidney biopsies are most commonly obtained percutaneously using real-time ultrasound guidance and automatic spring-loaded biopsy devices, which have been shown to decrease the risk of the procedure.4, 5, 6, 7 The frequency of biopsy complications after percutaneous native kidney biopsies, however, varies across studies, due to differences in patient populations and procedural techniques, as well as variability in complication definitions and the intensity of post-procedural monitoring. Different studies have collectively identified numerous risk factors for bleeding complications following kidney biopsy, but findings have been inconsistent.8 Associations of bleeding risk with specific forms of kidney disease or histologic lesions have been reported sporadically, but generally lack validation. We sought to further define the risk of major bleeding complications after percutaneous native kidney biopsy and its associated clinical and pathologic risk factors using a cohort of patients who underwent biopsy at 2 academic hospitals in the northeast United States.
Methods
Study Design and Population
We retrospectively examined medical records of patients enrolled in the Boston Kidney Biopsy Cohort to identify major biopsy-associated bleeding complications and gather data on covariates that had not been collected as part of the original prospective design. Details on the Boston Kidney Biopsy Cohort have been described previously.9 For the current analysis, we used data from adult (≥18 years old) patients who underwent clinically indicated percutaneous native kidney biopsy at 2 of the participating academic hospitals in Boston, Massachusetts, between September 2006 and June 2018. We included only the first biopsy in the analysis if patients had more than 1 performed. Procedures were performed either by a nephrologist or interventional radiologist with use of imaging guidance (either ultrasound or computed tomography) and spring-loaded biopsy devices. Imaging was regularly performed at the end of biopsy procedures to evaluate for hematoma development, but subsequent imaging was performed only as clinically indicated. Data were stored using REDCap electronic data capture tools hosted at Partners Healthcare.10 The study was approved by the Partners Human Research Committee (Institutional Review Board #2007P000003) and is in accordance with the principles of the Declaration of Helsinki.
Outcomes and Covariates
Data on biopsy-associated bleeding events were collected by review of patient records. Outcomes of interest included postbiopsy hematomas, RBC transfusions, angiographic or open surgical interventions to control bleeding, kidney loss, and death. Decline in hemoglobin was also examined by recording the closest hemoglobin level available up to 30 days before the procedure and the lowest hemoglobin within 7 days after biopsy.
Covariates examined included age, sex, race, baseline laboratory tests (eGFR, prothrombin time, activated partial thromboplastin time, and proteinuria), diagnosis of hypertension or diabetes mellitus, biopsy indication, and the setting in which the procedure took place (i.e., urgently in the inpatient setting vs. following outpatient evaluation). Systolic blood pressure was also examined, using the measurement most proximal to the time of the biopsy. Histopathologic lesions of interest, which included interstitial fibrosis and tubular atrophy, global glomerulosclerosis, acute tubular injury, and arterial and arteriolar sclerosis, were semiquantitatively scored. Clinicopathologic diagnoses were adjudicated by reviewing biopsy reports alongside patient medical records.
Statistical Analysis
We summarized continuous variables with means and SDs if normally distributed and with medians and interquartile ranges if not normally distributed and performed group comparisons using t tests or Wilcoxon rank sum tests, respectively. We summarized count data as percentages and compared them between groups using χ2 or Fisher’s exact tests. We used Spearman correlation coefficients to determine the association between continuous variables.
In our primary analysis, we examined the association of patient characteristics, selected a priori by review of the literature and based on adequate availability in the medical records, with the need for RBC transfusion after biopsy. We first examined these associations using univariable logistic regression models and then analyzed them after adjusting for prebiopsy hemoglobin levels, which we found to be the most strongly associated characteristic. As bleeding risk did not change linearly with the laboratory studies in our analysis, they were dichotomized at thresholds determined by examining the distribution of the data among those who did versus did not receive an RBC transfusion.
In a post hoc analysis, we explored the associations of the same patient characteristics with decline in hemoglobin using linear regression models. Each characteristic of interest was initially examined in a univariable model and all were subsequently entered together into a multivariable model to identify variables independently associated with hemoglobin decline. We also used an automated stepwise selection procedure as a secondary analysis to verify the characteristics most strongly associated with postbiopsy hemoglobin decline.
Regression analyses were initially conducted using only cases with complete data (>90%). In sensitivity analyses, the same models were examined after multiple imputation under multivariate normal distribution for missing data. In a separate sensitivity analysis, an automated stepwise selection procedure was used in a logistic regression model to identify which of the clinical characteristics of interest were most predictive of postbiopsy blood transfusion. Statistical tests were 2-sided, and we considered P < 0.05 as the threshold for significance. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The study cohort consisted of 644 individuals. The median age was 53 years, 52% were female, and 65% were white. The most commonly reported indications for biopsy, which were not mutually exclusive, were proteinuria (54.5%), worsening eGFR (35.4%), and hematuria (25.2%). Computed tomography guidance used to perform 9.9% of biopsies, whereas the remainder were guided by ultrasound. Before biopsy, the median (interquartile range) hemoglobin was 11.6 g/dl (10.1–13.3 g/dl), serum creatinine was 1.57 mg/dl (0.98–2.48 mg/dl), eGFR was 44.9 ml/min per 1.73 m2 (23.4–81.6 ml/min per 1.73 m2), platelet count was 251 K/μl (201–315 K/μl), and international normalized ratio (INR) was 1.0 (1.0–1.1) (Table 1).
Table 1.
Characteristics of study cohort
| Characteristic | Transfusion required postbiopsy (n = 28) | No transfusion required postbiopsy (n = 616) | P value | Missing (%) |
|---|---|---|---|---|
| Site | 0.76 | 0 | ||
| Hospital 1 (%) | 4.2 | 95.8 | ||
| Hospital 2 (%) | 5.1 | 94.9 | ||
| Age, yr | 48.5 (35.1–64.8) | 53.3 (37.2–66.3) | 0.39 | 0 |
| Female (%) | 57.1 | 51.3 | 0.57 | 0 |
| Race (%) | 0.81 | 0 | ||
| White | 60.7 | 65.1 | ||
| Black | 21.4 | 18.5 | ||
| Other | 17.9 | 16.4 | ||
| DM (%) | 32.1 | 17.9 | 0.08 | 0 |
| HTN (%) | 50.0 | 49.0 | 0.92 | 0 |
| SBP, mm Hg | 132 (117–144) | 130 (118–142) | 0.74 | 4.2 |
| ACE inhibitor or ARB (%) | 32.1 | 43.3 | 0.33 | 0 |
| Serum Cr, mg/dl | 2.88 (1.57–5.27) | 1.54 (0.98–2.41) | <0.01 | 0.1 |
| Serum Cr >2 mg/dl (%) | 67.9 | 34.4 | <0.01 | |
| eGFR, ml/min per 1.73 m2 | 21.3 (10.8–43.3) | 45.6 (24.9–82.0) | <0.01 | 0.1 |
| eGFR >60 ml/min per 1.73 m2 (%) | 21.4 | 37.2 | <0.01 | 0.1 |
| 30 < eGFR ≤ 60 ml/min per 1.73 m2 (%) | 14.3 | 31.2 | ||
| eGFR ≤ 30 ml/min per 1.73 m2 (%) | 64.3 | 31.6 | ||
| Proteinuria, g/g Cr | 1.30 (0.66–3.94) | 1.47 (0.34–3.89) | 0.48 | 1.6 |
| Proteinuria > 3.5 g/g Cr (%) | 26.9% | 28.6% | 0.85 | |
| INR | 1.1 (1.0–1.1) | 1.0 (1.0–1.1) | 0.02 | 2.3 |
| INR ≥1.1 (%) | 66.7 | 34.4 | <0.01 | |
| aPTT | 30.5 (27.6–33.6) | 29.2 (26.7–31.8) | 0.21 | 7.6 |
| Platelet count, K/μl | 232.5 (170–343.5) | 251 (201–313) | 0.62 | 2.3 |
| Platelet count <150 K/μl (%) | 17.9 | 8.8 | 0.17 | |
| Hemoglobin, g/dl | 8.7 (8.1–9.7) | 11.7 (10.2–13.4) | <0.01 | 2.4 |
| Hemoglobin <10 g/dl (%) | 78.6 | 21.3 | <0.01 | |
| Reason for biopsy (%)a | 0 | |||
| Proteinuria | 42.9 | 55.0 | 0.24 | |
| Hematuria | 28.6 | 25.0 | 0.66 | |
| Worsening eGFR | 64.3 | 34.1 | <0.01 | |
| CT-guided instead of US-guided biopsy (%) | 3.6 | 10.2 | 0.34 | 0 |
| Urgent inpatient biopsy (%) | 57.1 | 17.5 | <0.01 | 0 |
ACE, angiotensin-converting enzyme; aPTT, activated partial thromboplastin time; ARB, angiotensin II receptor blockers; Cr, creatinine; CT, computed tomography; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; INR, international normalized ratio; SBP, systolic blood pressure; US, ultrasound.
Reasons for biopsy are not mutually exclusive.
The median decline in hemoglobin was 0.7 g/dl (0.3–1.2 g/dl) or 6.2% (2.7%–10.6%); 39 patients had a decline in hemoglobin >2 g/dl and 11 patients had a decline >3 g/dl. Higher baseline hemoglobin was weakly positively correlated with the absolute decline in hemoglobin after biopsy (r = 0.09, P = 0.03). We found baseline hemoglobin to be negatively correlated with eGFR (r = −0.50, P < 0.01), INR (r = −0.34, P < 0.01), and age (r = −0.10, P = 0.01). Median (interquartile range) hemoglobin was lower among women than men (11.2 g/dl [9.8–12.7 g/dl] vs. 12.2 g/dl [10.2–14.0 g/dl], respectively, P < 0.01), among patients with versus without diabetes (10.5 g/dl [9.1–12.1 g/dl] vs. 11.9 g/dl [10.2–13.6 g/dl], respectively, P < 0.01), among patients with worsening eGFR as an indication for biopsy compared with those with other indications (10.5 g/dl [9.2–12.2 g/dl] vs. 12.2 g/dl [10.7–13.7 g/dl], respectively, P < 0.01), and among patients undergoing urgent inpatient biopsies compared with those whose biopsies were arranged after outpatient evaluation (9.5 g/dl [8.7–10.4 g/dl] vs. 12.2 g/dl [10.7–13.6 g/dl], respectively, P < 0.01).
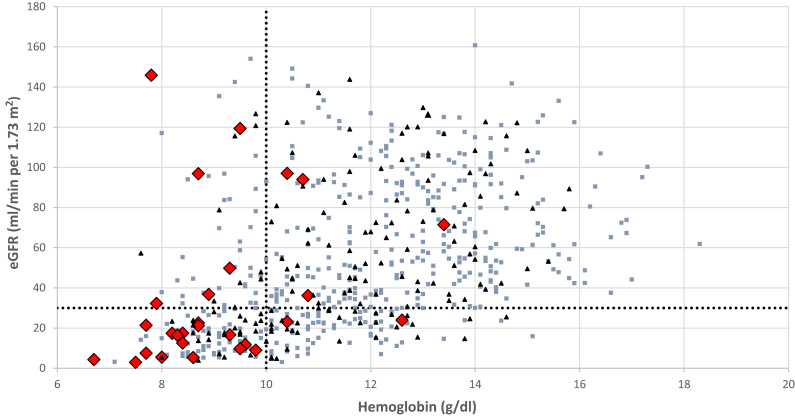
Hematomas were identified after biopsy in 29.0% of cases, usually on immediate postprocedure imaging (26.7%). Twenty-eight patients (4.3%) required RBC transfusion postbiopsy. We found neither a significant difference in transfusion rates between the 2 hospitals (4.2% vs. 5.1%, P = 0.76) nor a temporal trend during the period of observation. Patients requiring transfusion typically had lower baseline hemoglobin and eGFR before the procedure, with 22 of 28 transfused patients having a prebiopsy hemoglobin level <10 g/dl and 18 of 28 having an eGFR <30 ml/min per 1.73 m2 (Figure 1). Of the transfused patients, 4 (0.6% of the total cohort) underwent angiography due to severe bleeding and 1 (0.2%) subsequently required surgical intervention where a bleeding vessel was ligated. No patient lost a kidney or died because of the biopsy (Figure 2).
Figure 1.
Distribution of baseline hemoglobin and estimated glomerular filtration rate (eGFR) among patients in the cohort. Red diamonds represent individuals who received a red blood cell transfusion after biopsy, black triangles represent those who were not transfused but developed a hematoma postbiopsy, and blue boxes represent those in which neither of these events occurred. The vertical dotted line marks the hemoglobin level of 10 g/dl and the horizontal dotted line marks the eGFR level of 30 ml/min per 1.73 m2.
Figure 2.
Of 644 patients included in the analysis, 28 (4.3%) required a red blood cell transfusion after undergoing percutaneous native kidney biopsy; 4 (0.6%) required angiographic intervention and 1 (0.2%) subsequently underwent open surgery to control bleeding. No patients lost a kidney or died because of the procedure.
Transfusion was required in 14.5% (95% CI: 9.4%–21.4%) of patients with prebiopsy hemoglobin <10 g/dl compared with 1.2% (95% CI: 0.5%–2.8%) of patients with prebiopsy hemoglobin ≥10 g/dl. Transfusion was required in 8.5% (95% CI: 5.1%–13.1%) of patients with an eGFR <30 ml/min per 1.73 m2 and 2.3% (95% CI: 1.1%–4.2%) of patients with eGFR ≥30 ml/min per 1.73 m2. Table 2 shows the association of patient characteristics of interest with the need for blood transfusion after biopsy. In univariable models, blood transfusions were significantly associated with baseline hemoglobin <10 g/dl (OR: 13.6; 95% CI: 5.4–34.1), eGFR <30 ml/min per 1.73 m2 (OR: 3.9; 95% CI: 1.8–8.6), INR ≥1.1 (OR: 3.8; 95% CI: 1.7–8.7), worsening renal function as the indication for biopsy (OR: 3.5; 95% CI: 1.6–7.7) and urgent inpatient biopsies (OR: 6.3; 95% CI: 2.9–13.6). After adjusting for baseline hemoglobin (<10 vs. ≥10 g/dl), however, none of the other patient characteristics remained significantly associated with postbiopsy transfusions, although urgent inpatient biopsies remained nominally associated (OR: 2.13; 95% CI: 0.89–5.11; P = 0.09). In line with this, when the same patient characteristics were entered together in a logistic regression model and an automated stepwise selection procedure was utilized to choose predictors, prebiopsy hemoglobin remained the sole independent variable in the final model.
Table 2.
Association of clinical characteristics with need for transfusion after biopsy, before and after adjustment for the prebiopsy hemoglobin level (<10 vs. ≥10 g/dl)
| Characteristic | Univariable models |
Models adjusted for prebiopsy hemoglobin <10 vs. ≥10 g/dl |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, per 10-yr increase | 0.91 (0.73–1.13) | 0.39 | 0.92 (0.73–1.15) | 0.45 |
| Female sex | 1.27 (0.59–2.72) | 0.55 | 0.98 (0.44–2.18) | 0.96 |
| Diabetes | 2.18 (0.96–4.95) | 0.06 | 1.49 (0.63–3.52) | 0.37 |
| Systolic blood pressure, per 10 mm Hg increase | 1.06 (0.86–1.30) | 0.59 | 1.05 (0.86–1.29) | 0.63 |
| eGFR <30 ml/min per 1.73 m2 | 3.89 (1.76–8.58) | <0.01 | 1.55 (0.65–3.70) | 0.33 |
| Platelet count <150 K/μl | 2.25 (0.82–6.16) | 0.11 | 1.53 (0.53–4.43) | 0.43 |
| INR ≥1.1 | 3.82 (1.69–8.65) | <0.01 | 1.89 (0.79–4.54) | 0.16 |
| Worsening eGFR as indication for biopsy | 3.48 (1.58–7.67) | <0.01 | 1.82 (0.79–4.22) | 0.16 |
| Urgent inpatient procedure | 6.27 (2.88–13.64) | <0.01 | 2.13 (0.89–5.11) | 0.09 |
| Hemoglobin <10 g/dl | 13.55 (5.38–34.12) | <0.01 | N/A | N/A |
CI, confidence interval; eGFR, estimated glomerular filtration rate; INR, international normalized ratio; N/A, not applicable; OR, odds ratio.
Hematomas visualized at the end of biopsy procedures were nominally associated with the need for subsequent RBC transfusion both before (OR: 2.14; 95% CI: 0.99–4.62; P = 0.05) and after (OR: 2.00; 95% CI: 0.89–4.49; P = 0.09) adjustment for baseline hemoglobin. When viewed as a predictive test in this setting, the sensitivity and specificity of hematomas were 42.9% and 74.0%, respectively. The positive predictive value of hematomas was 7.0%, whereas the negative predictive value was 96.6%.
Table 3 shows results of the post hoc analysis, where we explored the association of the same patient characteristics with decline in hemoglobin within 7 days after biopsy. In univariable models, the factors significantly associated with decline in hemoglobin were eGFR <30 ml/min per 1.73 m2 (β = 0.16; 95% CI: 0.03–0.30) and baseline hemoglobin (β = 0.03; 95% CI: 0.00–0.06 per g/dl increase). When all the variables of interest were incorporated in a multivariable model, female sex (β = 0.18; 95% CI: 0.04–0.32), eGFR <30 ml/min per 1.73 m2 (β = 0.32; 95% CI: 0.14–0.49) and baseline hemoglobin (β = 0.09; 95% CI: 0.05–0.13 per g/dl increase) were independently associated with the hemoglobin decline. The same 3 variables remained in the final model when an automated stepwise selection procedure was used. When the analysis was repeated after multiple imputation for missing data or after additional adjustment for the center at which the biopsy took place, the results did not meaningfully change (data not shown).
Table 3.
Association of clinical characteristics with absolute decline in hemoglobin within 7 days after biopsy, before and after multivariable adjustment
| Characteristic | Univariable models |
Multivariable modela |
Model using stepwise selection procedureb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β coefficient | 95% CI | P value | β coefficient | 95% CI | P value | β coefficient | 95% CI | P value | |
| Age, per 10-yr increase | 0.02 | −0.02 to 0.05 | 0.44 | 0.00 | −0.04 to 0.04 | 0.89 | – | – | – |
| Female sex | 0.07 | −0.06 to 0.20 | 0.29 | 0.18 | 0.04 to 0.32 | 0.01 | 0.17 | 0.04 to 0.31 | 0.01 |
| Diabetes | 0.09 | −0.08 to 0.26 | 0.28 | 0.10 | −0.08 to 0.27 | 0.29 | – | – | – |
| Systolic blood pressure, per 10 mm Hg increase | −0.02 | −0.05 to 0.02 | 0.31 | −0.03 | −0.04 to 0.04 | 0.89 | – | – | – |
| eGFR <30 ml/min per 1.73 m2 | 0.16 | 0.03 to 0.30 | 0.02 | 0.32 | 0.14 to 0.49 | <0.01 | 0.36 | 0.20 to 0.51 | <0.01 |
| Platelet count <150 K/μl | −0.05 | −0.27 to 0.17 | 0.66 | 0.00 | −0.22 to 0.22 | 0.97 | – | – | – |
| INR ≥1.1 | 0.00 | −0.13 to 0.14 | 0.96 | 0.03 | −0.12 to 0.19 | 0.70 | – | – | – |
| Worsening eGFR as indication for biopsy | 0.06 | −0.07 to 0.19 | 0.37 | 0.07 | −0.08 to 0.22 | 0.35 | – | – | – |
| Urgent inpatient biopsy | 0.02 | −0.14 to 0.18 | 0.81 | 0.01 | −0.18 to 0.19 | 0.94 | – | – | – |
| Baseline Hgb, per 1 g/dl increase | 0.03 | 0.00 to 0.06 | 0.03 | 0.09 | 0.05 to 0.13 | <0.01 | 0.08 | 0.04 to 0.11 | <0.01 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; Hgb, hemoglobin; INR, international normalized ratio.
All clinical characteristics of interest, shown in the table, were included in the model.
All clinical characteristics of interest, shown in the table, were included in the initial model and an automated stepwise selection procedure was then used to choose predictors.
Table 4 shows associations of histopathologic lesions with postbiopsy RBC transfusion. Although acute tubular injury was significantly associated with transfusion in a univariable model, it was no longer independently associated with this outcome after adjustment for prebiopsy hemoglobin (<10 vs. ≥10 g/dl). Interstitial fibrosis and tubular atrophy, global glomerulosclerosis, arterial sclerosis, and arteriolar sclerosis were not significantly associated with transfusions in any of the corresponding univariable or multivariable models. We did not find significant correlation between the ordinally scored histopathologic lesions and postbiopsy hemoglobin decline. Consistent with these findings, when adjusting for baseline hemoglobin levels, we found no associations between major clinicopathologic diagnostic categories and the need for transfusion after biopsy (Table 5).
Table 4.
Association of histopathologic lesions with need for transfusion after biopsy
| Characteristic | Univariable models |
Models adjusted for prebiopsy hemoglobin <10 vs.10 g/dl |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| IFTA (>25%) | 0.98 (0.45–2.12) | 0.95 | 0.59 (0.26–1.35) | 0.21 |
| Global glomerulosclerosis (>25%) | 1.01 (0.46–2.23) | 0.97 | 0.82 (0.36–1.87) | 0.63 |
| Arterial sclerosis (moderate or severe vs. none or mild) | 1.22 (0.55–2.68) | 0.62 | 1.16 (0.51–2.64) | 0.73 |
| Arteriolar sclerosis (moderate or severe vs. none or mild) | 1.11 (0.52–2.38) | 0.78 | 0.90 (0.41–2.00) | 0.80 |
| Acute tubular injury (moderate or severe vs. none or mild) | 3.76 (1.66–8.50) | <0.01 | 1.46 (0.61–3.51) | 0.40 |
CI, confidence interval; IFTA, interstitial fibrosis and tubular atrophy; OR, odds ratio.
Table 5.
Association of clinicopathologic diagnostic categories with need for transfusion after biopsy, before and after adjustment for the prebiopsy hemoglobin level (<10 vs. ≥10 g/dl)
| Diagnostic category | Events/total | Univariable models |
Models adjusted for prebiopsy hemoglobin <10 vs. ≥10 g/dl |
||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Proliferative GN | 9/198 | Ref | Ref | Ref | Ref |
| Nonproliferative GN | 5/113 | 0.97 (0.32–3.00) | 0.95 | 1.43 (0.44–4.66) | 0.95 |
| Paraprotein-associated renal disease | 0/49 | No events | N/A | No events | N/A |
| Diabetic nephropathy | 3/55 | 1.21 (0.32–4.64) | 0.94 | 0.86 (0.21–3.46) | 0.97 |
| Vascular disease | 2/70 | 0.62 (0.13–2.93) | 0.96 | 0.80 (0.16–3.99) | 0.97 |
| Tubulointerstitial disease | 6/58 | 2.42 (0.83–7.12) | 0.91 | 1.23 (0.40–3.78) | 0.95 |
| Advanced chronic changes | 2/56 | 0.78 (0.16–3.71) | 0.96 | 1.08 (0.21–5.50) | 0.96 |
| Other | 1/40 | 0.54 (0.07–4.37) | 0.97 | 1.11 (0.13–9.91) | 0.96 |
CI, confidence interval; GN, glomerulonephritis; N/A; not applicable; OR, odds ratio.
Five biopsies were nondiagnostic and not included in the table.
Discussion
Severe bleeding complications were relatively rare (4.3%) in our cohort of patients undergoing percutaneous native kidney biopsy. Higher transfusion rates were seen particularly among those with baseline hemoglobin levels <10 g/dl or eGFR <30 ml/min per 1.73 m2. These findings may aid clinicians when discussing the risk of kidney biopsy with individual patients and obtaining their informed consent for the procedure. The rates of severe complications were consistent across the 2 hospitals included in our analysis, and fell within ranges previously reported in the literature, supporting the validity and generalizability of our findings.11, 12, 13, 14, 15, 16, 17 Even though perinephric hematomas were frequently visualized on imaging after biopsy and the median drop in hemoglobin was 0.7 g/dl, patients seldom required blood transfusions or invasive interventions to control bleeding. No patients lost a kidney or died because of the procedure.
Although several patient characteristics were associated with the need for postbiopsy blood transfusion in unadjusted analyses, we found that the risk of transfusion was first and foremost driven by the hemoglobin level before the procedure. Indeed, other patient characteristics associated with transfusion postbiopsy were correlated with baseline hemoglobin, and after adjusting only for hemoglobin, they did not remain independently associated with the need for transfusion. Patients with higher baseline levels of hemoglobin notably tended to lose slightly more blood after biopsy in absolute terms (i.e., g/dl) but were still less likely to require transfusion. Although it has been suggested that anemia may itself hamper hemostasis,18 our results in this regard mimic those of Whittier et al.,19 who reported in a large prospective study that anemia is a predictor of receiving a blood transfusion after biopsy, but not of bleeding.
Hematomas were commonly visualized at the end of biopsy procedures. Most were self-limited and inconsequential, as evidenced by their positive predictive value of only 7% for RBC transfusions. Although we found that their negative predictive value was high at 96.6%, this was primarily a result of the low rate of blood transfusions. Sensitivity and specificity of postprocedure hematomas for bleeding requiring transfusion were notably both modest.
Previous studies of the risk of bleeding complications after kidney biopsy have commonly focused on blood transfusions as we did in our primary analysis, because this allows severe bleeding events to be broadly captured, whether in the form of internal hematomas or hematuria, and because transfusions are both clinically relevant and can be readily adjudicated. Given how infrequently transfusions are required after kidney biopsy and the relatively modest size of most biopsy cohorts, however, studies designed in this manner tend not to be well powered to identify risk factors for excessive bleeding. It is likely that the literature on this subject, which contains multiple such observational studies, each with a low number of events, may thus be susceptible to publication bias. A meta-analysis by Corapi et al.17 summarized findings of 32 studies involving 9456 native kidney biopsies where erythrocyte transfusions were examined as the outcome, and identified significantly higher rates of transfusions after biopsy in women, in patients with a serum creatinine >2 mg/dl, in the setting of acute kidney injury, and with use of larger-gauge biopsy needles. Significant heterogeneity between studies was noted and the possibility of publication bias in this field was acknowledged, although funnel plots to examine this for these individual risk factors for RBC transfusion after biopsy were not shown.
Given the above limitations of blood transfusions as a study outcome, we performed a post hoc analysis with maximum decline in hemoglobin within a week after biopsy as the outcome, to identify characteristics associated with excessive bleeding after kidney biopsy in a more sensitive manner. Interestingly, in this analysis we found female sex and eGFR <30 ml/min per 1.73 m2 to be independent predictors of bleeding after multivariable adjustment, similar to the aforementioned meta-analysis. Although it is possible that the observed hemoglobin nadir would have been lower in patients who received an RBC transfusion if this intervention had not taken place, this potential bias applied only to a small minority of the cohort. We believe that this method, although not without limitations, can thus be a useful adjunct in studies exploring bleeding risk after kidney biopsy.
Patients with advanced kidney disease are well known to be at increased risk of bleeding. The pathophysiology of uremic bleeding is incompletely understood, but abnormal platelet function plays a major role.20,21 Decreased kidney function, both in the setting of chronic kidney disease and acute kidney injury, has accordingly been identified as a risk factor for bleeding after kidney biopsy.16,2,2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Our data lend further support to an association between kidney function and bleeding risk after biopsy. Although desmopressin may decrease the risk of bleeding when administered to patients with decreased kidney function before invasive procedures,26, 27, 28 we were unable to evaluate its effect in our cohort because of missing data. Female sex has also repeatedly been identified as a risk factor for bleeding after kidney biopsy.15, 16, 17,29 It has been suggested previously that this association might be due to confounding by the lower eGFR among women at a given serum creatinine compared with men, as older studies have commonly adjusted for renal function using serum creatinine rather than eGFR. Interestingly, however, our analysis again demonstrates increased risk among women despite adjustment for eGFR in our regression models. It is possible that women are at increased risk of bleeding due to the smaller size of their kidneys compared with men’s, which might increase the chance of inadvertent injury to deeper structures during the biopsy procedure.
Biopsies obtained in urgent settings among hospitalized patients have previously been associated with increased risk of major bleeding events,30 and were nominally associated with postbiopsy blood transfusions in our analysis. Although it might be expected that acutely ill hospitalized patients were at higher risk of bleeding because of other associated risk factors, such as recent exposure to thromboprophylactic agents, we did not find them to have a larger drop in hemoglobin, suggesting rather that the threshold to transfuse patients in this setting after a given drop in their blood counts might be lower.
Uncontrolled hypertension and coagulopathy have long been viewed as major risk factors for bleeding after kidney biopsy.15,16,29,31, 32, 33, 34, 35, 36 Patients in our cohort with severe hypertension, thrombocytopenia, or INR elevation were therefore not biopsied without earlier intervention to address such abnormalities. For example, only 13 patients in our cohort had a platelet count <100 K/μl, and although 3 of them received a transfusion after biopsy, our study was underpowered to appropriately examine the association of such severe thrombocytopenia with bleeding events. Hence, even though systolic blood pressure, thrombocytopenia, and INR were not independent predictors of major bleeding complications in our study, these results must be interpreted with caution and should not extrapolated to patients with more abnormal values.
Many forms of kidney disease have been associated with increased risk of bleeding, including acute tubular injury, amyloidosis, cast nephropathy, and hypertensive renal disease.29,35,37,38 However, such findings have not been consistent across studies. For example, a study by Soares et al.39 attempted to verify the higher bleeding risk of patients with amyloidosis using a cohort of 101 patients with and 188 patients without amyloidosis but found no evidence of heightened risk. We also found no increase in risk of bleeding among patients with paraprotein-associated kidney disease, and none of the patients in our cohort with amyloidosis had a major bleeding event postbiopsy. Although acute tubular injury was associated with higher risk of transfusion after biopsy in a univariable model, it was no longer significant after covariate adjustment. We similarly found no independently significant associations of other histopathologic lesions or major categories of kidney disease with bleeding.
Our study has several limitations. Because of its retrospective observational design, residual confounding is likely to be present. Certain covariates of interest, such as biopsy needle gauge and the number of passes made during the procedure, could not be included in this analysis because of missing data.40 Our cohort derived from 2 high-volume teaching hospitals in the same city in the United States and results may not be generalizable to smaller centers or those in different geographic locations. Importantly, although our cohort is large, major bleeding events are quite rare and our power to detect statistically significant risk factors is thus limited.
In summary, we found that the risk of major bleeding complications after imaging guided percutaneous native kidney biopsy at 2 Boston academic hospitals was 4.3%. Anemia was the dominant risk factor for RBC transfusion after biopsy. Patient characteristics independently associated with a larger drop in hemoglobin after biopsy, albeit with modest effect sizes, included female sex, eGFR <30 ml/min per 1.73 m2, and higher baseline hemoglobin. Exploratory analyses of changes in hemoglobin after biopsy may serve a complementary role to analyses of severe bleeding events and help further define populations at risk for excessive bleeding.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank all members of the Waikar Lab for their work on the Boston Kidney Biopsy Cohort.
This study was supported by NIH R01DK093574 (SSW). RP was supported by an ASN Ben J. Lipps Research Fellowship Grant.
References
- 1.Luciano R.L., Moeckel G.W. Update on the native kidney biopsy: core curriculum 2019. Am J Kidney Dis. 2019;73:404–415. doi: 10.1053/j.ajkd.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Richards N.T., Darby S., Howie A.J. Knowledge of renal histology alters patient management in over 40% of cases. Nephrol Dial Transplant. 1994;9:1255–1259. [PubMed] [Google Scholar]
- 3.Botti R.E., Razzak M.A., MacIntyre W.J., Pritchard W.H. The relationship of renal blood flow to cardiac output in normal individuals as determined by concomitant radioisotopic measurements. Cardiovasc Res. 1968;2:243–246. doi: 10.1093/cvr/2.3.243. [DOI] [PubMed] [Google Scholar]
- 4.Doyle A.J., Gregory M.C., Terreros D.A. Percutaneous native renal biopsy: comparison of a 1.2-mm spring-driven system with a traditional 2-mm hand-driven system. Am J Kidney Dis. 1994;23:498–503. doi: 10.1016/s0272-6386(12)80370-2. [DOI] [PubMed] [Google Scholar]
- 5.Riehl J., Maigatter S., Kierdorf H. Percutaneous renal biopsy: comparison of manual and automated puncture techniques with native and transplanted kidneys. Nephrol Dial Transplant. 1994;9:1568–1574. [PubMed] [Google Scholar]
- 6.Birnholz J.C., Kasinath B.S., Corwin H.L. An improved technique for ultrasound guided percutaneous renal biopsy. Kidney Int. 1985;27:80–82. doi: 10.1038/ki.1985.13. [DOI] [PubMed] [Google Scholar]
- 7.Burstein D.M., Schwartz M.M., Korbet S.M. Percutaneous renal biopsy with the use of real-time ultrasound. Am J Nephrol. 1991;11:195–200. doi: 10.1159/000168303. [DOI] [PubMed] [Google Scholar]
- 8.Whittier W.L. Complications of the percutaneous kidney biopsy. Adv Chronic Kidney Dis. 2012;19:179–187. doi: 10.1053/j.ackd.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava A., Palsson R., Kaze A.D. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol. 2018;29:2213–2224. doi: 10.1681/ASN.2017121260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittier W.L., Gashti C., Saltzberg S., Korbet S. Comparison of native and transplant kidney biopsies: diagnostic yield and complications. Clin Kidney J. 2018;11:616–622. doi: 10.1093/ckj/sfy051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabatabai S., Sperati C.J., Atta M.G. Predictors of complication after percutaneous ultrasound-guided kidney biopsy in HIV-infected individuals: possible role of hepatitis C and HIV co-infection. Clin J Am Soc Nephrol. 2009;4:1766–1773. doi: 10.2215/CJN.03880609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldo B., Korbet S.M., Freimanis M.G., Lewis E.J. The value of post-biopsy ultrasound in predicting complications after percutaneous renal biopsy of native kidneys. Nephrol Dial Transplant. 2009;24:2433–2439. doi: 10.1093/ndt/gfp073. [DOI] [PubMed] [Google Scholar]
- 14.Mackinnon B., Fraser E., Simpson K. Is it necessary to stop antiplatelet agents before a native renal biopsy? Nephrol Dial Transplant. 2008;23:3566–3570. doi: 10.1093/ndt/gfn282. [DOI] [PubMed] [Google Scholar]
- 15.Manno C., Strippoli G.F.M., Arnesano L. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int. 2004;66:1570–1577. doi: 10.1111/j.1523-1755.2004.00922.x. [DOI] [PubMed] [Google Scholar]
- 16.Moledina D.G., Luciano R.L., Kukova L. Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol. 2018;13:1633–1640. doi: 10.2215/CJN.04910418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corapi K.M., Chen J.L.T., Balk E.M., Gordon C.E. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60:62–73. doi: 10.1053/j.ajkd.2012.02.330. [DOI] [PubMed] [Google Scholar]
- 18.Livio M., Gotti E., Marchesi D. Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet. 1982;2:1013–1015. doi: 10.1016/s0140-6736(82)90050-2. [DOI] [PubMed] [Google Scholar]
- 19.Whittier W.L., Sayeed K., Korbet S.M. Clinical factors influencing the decision to transfuse after percutaneous native kidney biopsy. Clin Kidney J. 2016;9:102–107. doi: 10.1093/ckj/sfv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohal A.S., Gangji A.S., Crowther M.A., Treleaven D. Uremic bleeding: pathophysiology and clinical risk factors. Thromb Res. 2006;118:417–422. doi: 10.1016/j.thromres.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Molino D., De Lucia D., Gaspare De Santo N. Coagulation disorders in uremia. Semin Nephrol. 2006;26:46–51. doi: 10.1016/j.semnephrol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Korbet S.M., Volpini K.C., Whittier W.L. Percutaneous renal biopsy of native kidneys: a single-center experience of 1,055 biopsies. Am J Nephrol. 2014;39:153–162. doi: 10.1159/000358334. [DOI] [PubMed] [Google Scholar]
- 23.Tøndel C., Vikse B.E., Bostad L., Svarstad E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin J Am Soc Nephrol. 2012;7:1591–1597. doi: 10.2215/CJN.02150212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simard-Meilleur M.-C., Troyanov S., Roy L. Risk factors and timing of native kidney biopsy complications. Nephron Extra. 2014;4:42–49. doi: 10.1159/000360087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waikar S.S., McMahon G.M. Expanding the role for kidney biopsies in acute kidney injury. Semin Nephrol. 2018;38:12–20. doi: 10.1016/j.semnephrol.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannucci P.M., Remuzzi G., Pusineri F. Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308:8–12. doi: 10.1056/NEJM198301063080102. [DOI] [PubMed] [Google Scholar]
- 27.Hedges S.J., Dehoney S.B., Hooper J.S. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. 2007;3:138–153. doi: 10.1038/ncpneph0421. [DOI] [PubMed] [Google Scholar]
- 28.Manno C., Bonifati C., Torres D.D. Desmopressin acetate in percutaneous ultrasound-guided kidney biopsy: a randomized controlled trial. Am J Kidney Dis. 2011;57:850–855. doi: 10.1053/j.ajkd.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Korbet S.M., Gashti C.N., Evans J.K., Whittier W.L. Risk of percutaneous renal biopsy of native kidneys in the evaluation of acute kidney injury. Clin Kidney J. 2018;11:610–615. doi: 10.1093/ckj/sfy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lees J.S., McQuarrie E.P., Mordi N. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin Kidney J. 2017;10:573–577. doi: 10.1093/ckj/sfx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa E., Nomura S., Hamaguchi T. Ultrasonography as a predictor of overt bleeding after renal biopsy. Clin Exp Nephrol. 2009;13:325–331. doi: 10.1007/s10157-009-0165-7. [DOI] [PubMed] [Google Scholar]
- 32.Shidham G.B., Siddiqi N., Beres J.A. Clinical risk factors associated with bleeding after native kidney biopsy. Nephrology (Carlton) 2005;10:305–310. doi: 10.1111/j.1440-1797.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 33.Nass K., O’Neill W.C. Bedside renal biopsy: ultrasound guidance by the nephrologist. Am J Kidney Dis. 1999;34:955–959. doi: 10.1016/S0272-6386(99)70058-2. [DOI] [PubMed] [Google Scholar]
- 34.Chen T.K., Estrella M.M., Fine D.M. Predictors of kidney biopsy complication among patients with systemic lupus erythematosus. Lupus. 2012;21:848–854. doi: 10.1177/0961203312439334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eiro M., Katoh T., Watanabe T. Risk factors for bleeding complications in percutaneous renal biopsy. Clin Exp Nephrol. 2005;9:40–45. doi: 10.1007/s10157-004-0326-7. [DOI] [PubMed] [Google Scholar]
- 36.Kriegshauser J.S., Patel M.D., Young S.W. Risk of bleeding after native renal biopsy as a function of preprocedural systolic and diastolic blood pressure. J Vasc Interv Radiol. 2015;26:206–212. doi: 10.1016/j.jvir.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Parrish A.E. Complications of percutaneous renal biopsy: a review of 37 years’ experience. Clin Nephrol. 1992;38:135–141. [PubMed] [Google Scholar]
- 38.Stratta P., Canavese C., Marengo M. Risk management of renal biopsy: 1387 cases over 30 years in a single centre. Eur J Clin Invest. 2007;37:954–963. doi: 10.1111/j.1365-2362.2007.01885.x. [DOI] [PubMed] [Google Scholar]
- 39.Soares S.M., Fervenza F.C., Lager D.J. Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: single-center experience in 101 patients. Am J Kidney Dis. 2008;52:1079–1083. doi: 10.1053/j.ajkd.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Cui S., Heller H.T., Waikar S.S., McMahon G.M. Needle size and the risk of kidney biopsy bleeding complications. Kidney Int Rep. 2016;1:324–326. doi: 10.1016/j.ekir.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]