Abstract
Introduction
Monoclonal Ig deposition disease (MIDD) frequently leads to kidney failure, and a large proportion of these patients would greatly benefit from kidney transplantation. However, data on kidney transplantation outcomes in MIDD are limited.
Methods
This was a retrospective analysis of long-term renal outcomes of 23 patients with MIDD, including 6 patients who underwent kidney transplantation.
Results
The 1-, 5-, and 10-year overall survival (OS) from diagnosis were 95%, 78%, and 65%, respectively. Approximately half of the patients (n = 12) progressed to end-stage renal disease (ESRD) with a median time from diagnosis to ESRD of 3.4 years. The 1-, 5-, and 10-year renal survival from diagnosis were 77%, 48%, and 29% respectively. Renal response was observed only in 5 patients (22%), all of them after achieving hematologic complete response. Median OS from diagnosis was significantly better for those who underwent kidney transplantation versus those who remained on dialysis (19.8 years vs. 8.3 years, P = 0.016). Among patients who underwent kidney transplantation, the shortest survival from MIDD diagnosis was 13.7 years and the longest was 27.8 years. Of the 3 patients with kidney transplants who died, the time from the first kidney transplantation to death was 7.4, 18.8, and 20.4 years. Graft loss due to disease recurrence occurred at 4 months and 3.8 years after kidney transplantation in 2 patients who either were not treated or did not respond to treatment.
Conclusion
As treatments for MIDD have dramatically improved, more patients are achieving sustained hematologic responses with longer patient and graft survival after kidney transplantation.
Keywords: kidney transplantation, MIDD, monoclonal Ig deposition disease
Monoclonal Ig deposition disease (MIDD) is a rare type of monoclonal gammopathy that is characterized by a nonamyloid deposition of light chains or heavy chains in various organs, with light chain deposition disease (LCDD) occurring more frequently than heavy chain deposition disease. Rarely there is deposition of both light and heavy chains. MIDD usually occurs in the setting of a plasma cell dyscrasia or a lymphoproliferative disorder, and the kidneys are affected in almost all cases.1 Other organ involvement including the heart, liver, lungs, and nerves is much less frequent, but has been increasingly recognized as part of this disease.2, 3, 4 Similar to AL amyloidosis, kidney involvement in MIDD usually manifests as albuminuria >0.5 g in 24 hours and/or decreased kidney function, leading to a high incidence of progression to end-stage renal disease (ESRD) without successful treatment of the underlying hematologic disease.5,6 Until 2 decades ago, the options for treatment of MIDD were very limited, and often with poor hematologic response, with a mean overall survival (OS) of 4 to 7.5 years.7, 8, 9 Recent advances in therapies for plasma cell dyscrasias have led to longer disease-free survival due to sustained hematologic responses, as well as a dramatic improvement in OS to approximately 14 years.4,10,11 Median time from diagnosis to ESRD has been 2.7 to 9 years, depending on the level of kidney dysfunction at presentation.10
In comparison to AL amyloidosis, both OS and kidney prognosis are better in MIDD because of the lower incidence of extrarenal organ involvement.10
Historically, patients with AL amyloidosis were not referred for kidney transplantation because of concerns about disease recurrence leading to shorter OS and early graft loss. Given the similarities between AL amyloidosis and MIDD, the same has been true for patients with MIDD. In 1 study from the 1990s, Leung et al. evaluated 7 patients with MIDD who underwent kidney transplantation. The median time from diagnosis to kidney transplantation in this cohort was 6.1 years (range, 4 months−12.8 years), and 5 of the 7 patients had disease recurrence with OS of 12 years (range, 3.9−19.3 years). After disease recurrence, median graft survival was only 10.9 months. Median graft survival for all 7 patients, including those who died with a functioning allograft, was 3.1 years.12 We have recently shown that a selected group of AL amyloidosis patients who underwent kidney transplantation had good outcomes with median OS from transplantation of 10.5 years (range, 1−20.4 years) and median graft survival of 8.3 years (range, 4 months−20.4 years), which were comparable to those in other high-risk populations.13 Patients with hematologic complete response (CR) or very good partial response (VGPR) before kidney transplantation had significantly better patient survival than patients with partial hematologic response (PR) or no hematologic response (NR).
Given the high incidence of kidney involvement in MIDD and the frequently late diagnosis, many patients still progress to ESRD and would greatly benefit from kidney transplantation. However, data on kidney transplantation outcomes in MIDD are limited.10, 11, 12,14 In this study, we report the outcomes of 23 patients with MIDD and renal involvement.
Methods
A total of 23 patients with MIDD were included in this analysis. All patients were followed up at Boston University Medical Center and/or the Amyloidosis Center at Boston University School of Medicine between January 1989 and December 2018. Of these 23 patients, 6 patients underwent a total of 7 kidney transplantations after advancing to ESRD between the years 1996 to 2017. Data were prospectively collected during follow-up years and analyzed retrospectively. All patients had authorized the use of their medical records for research, and the study was approved by the Boston University Institutional Review Board in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act guidelines. Clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul. All patients had renal involvement as evidenced by biopsy-proven monoclonal Ig deposition in their renal parenchyma and were showing typical signs of renal involvement with urine protein (albumin) excretion of >0.5 g/d and/or a decreased estimated glomerular filtration rate (eGFR). The eGFR was estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation for patients who were <70 years of age and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Creatinine Equation for patients >70 years of age.15 Histological findings were noted from biopsy reports. As there are no established criteria for renal response in MIDD, organ response was defined using criteria used for AL amyloidosis.16
Statistical analysis
Values are specified as counts and percentages for categorical variables and as median with range for continuous variables, unless otherwise specified. Survival analyses were performed using the Kaplan–Meier method, and between-group comparisons were performed using the log-rank test. All statistical analyses were performed using R software version 3.5.1 (R Core Team [2018]. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org).
Results
Clinical Features at Diagnosis
All 23 patients were diagnosed with MIDD by kidney biopsy at a median age of 51.7 years (range, 32.6−71.2 years). A total of 19 patients (83%) were male, and all patients were of white ethnicity (2 were Hispanic). At the time of diagnosis, median creatinine was 3 mg/dl (range, 0.8−12 mg/dl), median 24-hour urine protein excretion was 3 g (range, 0−22 g), and median eGFR was 22 ml/min per 1.73 m2 (range, 4−91 ml/min per 1.73 m2). In all, 22 patients had LCDD: 5 patients (22%) with lambda chain clonality and 17 (74%) with kappa chain clonality. One patient had heavy chain deposition disease with IgG3 and no LC restriction. At the time of diagnosis, 1 patient had concurrent multiple myeloma (MM) (lambda isotype), 1 patient had both chronic lymphocytic leukemia and a history of testicular cancer, and 3 additional patients had a past history of solid organ malignancies (prostate, bladder: in remission during the study period; colon: diagnosed and treated after the diagnosis of MIDD was established). Four patients had clear evidence of cardiac involvement (Table 1).
Table 1.
Clinical characteristics
| Clinical parameter | |
|---|---|
| Median age at diagnosis, yr (range) | 51.7 (32.6–71.2) |
| Sex, n (%) | |
| Male | 19 (83) |
| Female | 4 (17) |
| Type of lesion, n (%) | |
| Light chain deposition disease | 22 (96) |
| Heavy chain deposition disease | 1 (4) |
| Light chain clonality, n (%) | |
| Kappa | 17 (74) |
| Lambda | 5 (22) |
| Renal presentation | |
| Median creatinine (mg/dl), (range) | 3 (0.8–12) |
| Median proteinuria (g/24 h), (range) | 3 (0–22) |
| Chronic kidney disease stage, n (%) | |
| I–II | 2 (9) |
| III | 4 (17) |
| IV | 12 (52) |
| V and/or end-stage renal disease | 5 (22) |
| Comorbidities, n | |
| Other hematologic diagnoses, n | |
| Monoclonal gammopathy of unknown significance | 2 |
| Chronic lymphocytic lymphoma | 1 |
| Multiple myeloma | 1 |
| Solid malignancies, n (%) | 4 (13) |
| Diabetes, n (%) | 4 (13) |
| Hypertension, n (%) | 11 (57) |
| Cardiac involvement, n (%) | 4 (17) |
During the median follow up of 8.1 years (range, 0.4−25.6 years), 3 patients were lost to follow-up. Two patients (8%) were on dialysis at the time of diagnosis, and 10 more patients (43%) progressed to ESRD after a median time of 3.4 years (range, 0−9.1 years) from diagnosis.
Treatment Outcomes of MIDD Patients
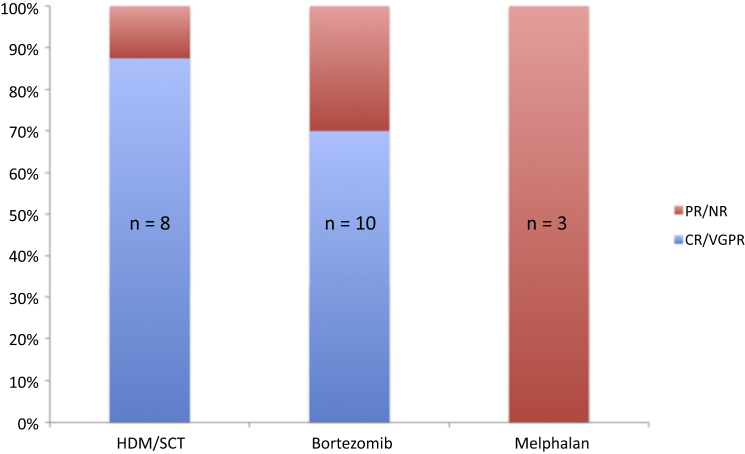
The 1-, 5-, and 10-year renal survival from diagnosis were 77%, 48%, and 29%, respectively. Six of the 12 patients who reached ESRD underwent kidney transplantation (1 patient had 2 kidney transplants). At the end of the study period, 9 of the 23 patients (39%) were deceased. The 1-, 5-, and 10-year OS from diagnosis were 95%, 78%, and 65%, respectively. Following the diagnosis of MIDD, 8 patients (35%) received first-line treatment with high-dose melphalan/stem cell transplantation (HDM/SCT), 10 (43%) received bortezomib, 3 (13%) received oral melphalan, and 1 patient received rituximab and bendamustine for chronic lymphocytic leukemia−related LCDD. One more patient had isolated renal MIDD with no systemic evidence of plasma cell dyscrasia and did not receive any clonal targeted therapy. This patient progressed to ESRD 8.5 months after diagnosis and died after 7 years on hemodialysis while awaiting deceased donor kidney transplantation. Of the 18 patients who received first-line treatment with HDM/SCT or bortezomib, 14 (78%) achieved VGPR or better (Figure 1). Of the 8 patients who underwent HDM/SCT, as first or second line treatment, 1 required acute initiation of dialysis because of severe sepsis and acute tubular injury post-treatment and died shortly thereafter.
Figure 1.
Status of hematologic response according to initial hematologic treatment. CR, complete response; HDM/SCT, high-dose melphalan/stem cell transplantation; NR, no response; PR, partial response; VGPR, very good partial response.
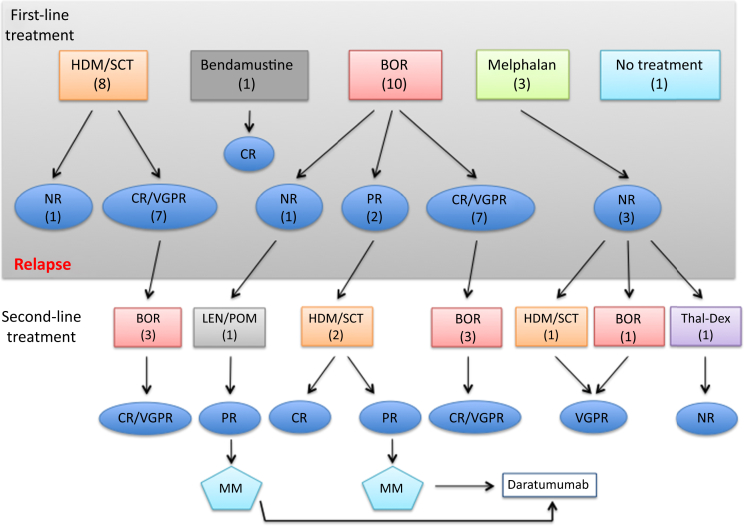
Of the 22 patients who received treatment, median time to achieving hematologic response after initial treatment was 0.5 year (range, 0.2−2.5 years). Eleven patients achieved CR, 4 achieved VGPR, 2 had a PR, and 5 patients did not respond or were not treated at all (NR). Altogether, 12 patients required subsequent hematologic treatment because of a lack of response to first-line therapy or disease recurrence. Three patients received second-line treatment with HDM/SCT, and 7 were treated with bortezomib-based therapy (Figure 2). Of those who did not respond to first-line treatment, 1 patient died within 6 months of HDM/SCT because of progression of his disease, and another patient who was treated with bortezomib followed by lenalidomide and then pomalidomide progressed to multiple myeloma and was started on daratumumab. This patient is still alive and remains on dialysis. None of the 3 patients who had initial treatment with oral melphalan had a hematologic response. One received second-line treatment with HDM/SCT, and another received bortezomib, both achieving VGPR. The third patient was treated with thalidomide−dexamethasone with no response and died 13.7 years after diagnosis.
Figure 2.
Hematologic response status after first- and second-line treatment. BOR, bortezomib; CR, complete response; HDM/SCT, high-dose melphalan/stem cell transplantation; LEN, lenalidomide; MM, multiple myeloma; NR, no response; POM, pomalidomide; PR, partial response; VGPR, very good partial response.
When comparing the outcomes of patients according to type of hematologic treatment, there was a trend toward better median OS from diagnosis in patients who received HDM/SCT versus all other treatments including bortezomib (19.8 years vs. 8.25 years); however, the difference was not statistically significant (P = 0.25). When combining both bortezomib and SCT/HDM versus other treatments, the trend was similar (19.8 years vs. 13.7 years, P = 0.83).
Progression-free survival from diagnosis was 8.3 years (range, 0.4−16.8 years), and was not different between patients who received first-line treatment with HDM/SCT versus all other treatments. The same is true for those who achieved a favorable hematologic response (CR+VGPR) versus those who did not (PR+NR) (data not shown). Median OS from diagnosis as well as from dialysis initiation according to initial hematologic response (CR+VGPR vs. PR+NR) was not statistically different between groups (from diagnosis 27.7 vs. 13.7 years, P = 0.35; and from dialysis 20.8 vs. 14 years, P = 0.55)
Renal response was achieved in only 5 patients (22%), with stabilization or improvement of renal function and a decrease of >30% in proteinuria16 after a median time of 1 year (range, 0.9−1.2 years) from diagnosis and 11 months (range, 7.9−12 years) from treatment initiation. At last follow-up, these patients had a median creatinine of ≤1.9 mg/dl (eGFR ≥34 ml/min per 1.73 m2). Of note, all of these patients were in a CR. Renal organ response was not achieved in patients who did not achieve a CR.
Kidney Transplantation Outcomes
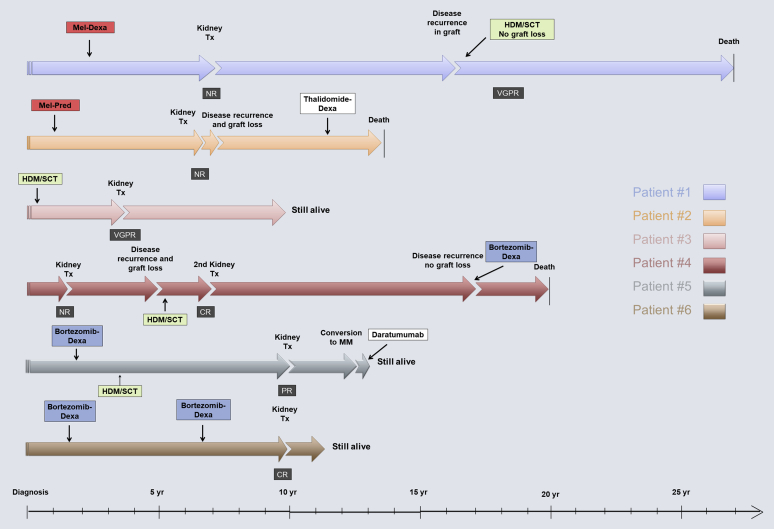
Six patients (26%) with MIDD underwent a total of 7 kidney transplantations over the course of 21 years (1996−2017). Graft loss because of disease recurrence occurred in only 2 patients at 4 months and 3.8 years post-transplantation. Two more grafts were lost because of patient death (11 and 13 years after renal transplantation), and 3 grafts were still functioning at the end of the study. Median age at kidney transplantation was 50 years (range, 38.7−58.4 years). Four of the 7 transplants were from deceased donors. Before kidney transplantation, 2 patients (patients 1 and 2) received only oral melphalan-based therapy. Patient 1 did not respond to treatment with oral melphalan and progressed to ESRD. He received a living related kidney transplant and had disease recurrence in the graft after 8.9 years. He was then treated with HDM/SCT, achieving a VGPR that halted disease progression, and survived 11 more years after re-treatment. Patient 2 did not respond to treatment and experienced graft loss within 4 months after transplantation because of disease recurrence, was then treated with thalidomide, and died 7.5 years after kidney transplantation while on dialysis. Patients 3 and 4 received HDM/SCT. Patient 3 achieved a VGPR and is still alive with a functioning graft at 6 years after kidney transplantation. Patient 4 received his first kidney transplant with no prior hematologic treatment and lost his graft after 3.8 years because of disease recurrence. He was then treated with HDM/SCT, achieving a CR, then received a second kidney transplant and survived 13 more years but needed re-treatment with cyclophosphamide, bortezomib, and dexamethasone 9.5 years after kidney transplantation. He eventually died of metastatic renal cell carcinoma of his native kidneys, but with a transplanted kidney that was still functioning before his death. Patients 5 and 6 received bortezomib-based therapies before kidney transplantation. Patient 5 received HDM/SCT, achieving a PR. He subsequently progressed to multiple myeloma and is currently undergoing treatment with daratumumab. Patient 6 achieved a CR after treatment with bortezomib−dexamethasone and subsequently received a repeat cycle of bortezomib−dexamethasone because of worsening proteinuria and kidney function, achieving a CR. He underwent transplantation while in CR and is still alive with a functioning graft at last follow-up.
Among patients who underwent kidney transplantation, the shortest survival from MIDD diagnosis was 13.7 and the longest was 27.8 years. Death occurred in 3 patients at 7.4, 18.8, and 20.4 years from first kidney transplantation. Three patients remain alive with functioning grafts 1.7, 2.8, and 6.5 years after kidney transplantation (Figure 3).
Figure 3.
Disease course over time for transplantation patients. CR, complete response; Dexa, dexamethasone; HDM/SCT, high-dose melphalan/stem cell transplantation; Mel, melphalan; MM, multiple myeloma; NR, no response; PR, partial response; Pred, prednisone; Tx, transplantation; VGPR, very good partial response.
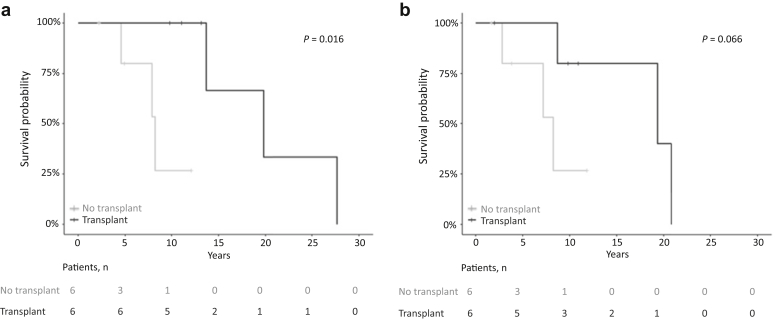
Median OS from diagnosis was significantly better for those who underwent kidney transplantation versus those who stayed on dialysis (19.8 years vs. 8.3 years, P = 0.016) (Figure 4a). Median OS from dialysis initiation was also better in the kidney transplant group; however, the difference was not statistically significant (19.3 years vs. 8.25 years, P = 0.06) (Figure 4b).
Figure 4.
(a) Overall survival from diagnosis of patients with end-stage renal disease (ESRD): kidney transplantation versus no kidney transplantation. (b) Overall survival from dialysis initiation: kidney transplantation versus no kidney transplantation.
Discussion
This study reports the outcomes of 23 patients with MIDD who were followed up at the Boston University Amyloidosis Center over 3 decades. During the past 15 to 20 years, the treatments for plasma cell dyscrasias, including MIDD have evolved, and the use of HDM/SCT17, 18, 19 and proteasome inhibitor−based therapies as first-line treatments has resulted in improved patient and kidney survival, especially in patients who achieve a favorable response to treatment (CR/VGPR).4,20 Before these newer therapies, 5-year patient survival in MIDD was 70% and 5-year kidney survival was only 37%.5 More recent data demonstrated a higher 5-year renal survival rate of 57%.11 In another recent study from the UK, median OS from diagnosis was 14 years and kidney survival was 5.4 years.10 Similarly, we observed a median OS of 13.7 years and improved 1-, 5-, and 10-year patient survival rates (95%, 78%, and 65%, respectively). Despite improvement in patient survival, kidney survival is still lagging behind because of delayed diagnosis or failure of initial treatment, and many patients require dialysis initiation (12 of 23 patients, 52%). In a recent retrospective study from France, 166 patients with MIDD demonstrated a much better renal survival in patients with eGFR >30 ml/min per 1.73 m2 at diagnosis compared with those who had eGFR <30 ml/min per 1.73 m2 (17.4 years vs. 7.3 years, respectively).4 In our center, patients with MIDD had median eGFR of 22 ml/min at diagnosis, and a median renal survival of 3.4 years with 1-, 5-, and 10-year survival of 72%, 36%, and 21% respectively. This is partly due to the fact that diagnosis of MIDD is often delayed,11 which allows for increased deposition of light or heavy chains in the renal parenchyma over time, ultimately leading to significant fibrosis of the kidneys and therefore diminished organ response. As there are no established criteria for renal response in MIDD, we considered renal responses based on established and validated criteria developed for AL amyloidosis as has been previously reported.11 In our cohort, most of the patients had advanced chronic kidney disease at presentation, and as a result we observed a low incidence of renal organ response (22%). Notably, renal organ response was demonstrated only in patients who achieved CR after treatment and had better renal parameters at presentation (serum creatinine ≤3.1 mg/dl or eGFR ≥21). The 5 patients with renal response had a creatinine of 3.1, 2.9, 2.3, 1.3, and 0.8 mg/dl at diagnosis. Similarly, in the study by Sayed et al., there was improvement in GFR among the patients who achieved a hematologic CR/VGPR at 6 months after treatment compared with GFR loss in patients who achieved only PR or NR.10 A recent study from France also showed better renal survival in patients achieving a favorable hematologic response (CR/VGPR).4
We did observe a trend toward better survival from diagnosis or dialysis initiation in patients who received HDM/SCT versus all other treatments including bortezomib; however, the difference was not statistically significant, likely because of the small number of patients. The same is true for HDM/SCT or bortezomib versus all other treatments and for achieving CR/VGPR versus PR/NR. The benefit of HDM/SCT and bortezomib was more clearly demonstrated in a study by Kourelis et al., in which patients treated with either of these 2 modalities were more likely to achieve CR/VGPR and to have better renal survival.11
Reports of kidney transplantation outcomes in MIDD patients are very scarce because of the rarity of this disease and the hesitation of physicians to refer these patients for kidney transplantation (Table 2).4,10, 11, 12 In a study of 7 patients from Mayo Clinic published in 2004, the authors concluded that kidney transplantation should not be performed in MIDD patients, given the short graft survival in their cohort.12 The patients in this study were diagnosed before the routine use of HDM/SCT in MIDD and newer plasma cell−directed agents that led to the improved outcomes that we have been observing in the last 2 decades. Indeed, a later study from 2016 published by the same group reported outcomes of 88 patients with MIDD. In all, 61% of patients received HDM/SCT or proteasome inhibitor−based therapies, and those patients were more likely to achieve CR/VGPR. Nine patients in this cohort underwent kidney transplantation, 7 with CR after hematologic treatment (5 treated with HDM/SCT, 2 with proteasome inhibitors), and only 1 of them had hematologic and renal progression 9 years after kidney transplantation. Graft survival was not reported; however, the remaining 6 patients had hematologic progression at various points, were initiated on plasma cell−directed therapy with good hematologic response, and had no reported renal progression at last follow-up (median time of last follow-up not indicated).11 Not surprisingly, the 2 patients who lost their grafts because of disease recurrence had no response to chemotherapy or no treatment before their kidney transplantation. In our cohort of 6 patients who underwent 7 renal transplantations, the shortest graft survivals were 4 months and 3.8 years; yet, again, both were in patients who did not respond to treatment or were not treated before kidney transplantation (patients 2 and 4, respectively). In another cohort of 53 patients with LCDD, 7 patients underwent kidney transplantation with 3 graft losses: 2 because of LCDD recurrence in the graft after 1.6 and 1.9 years (no salvage therapy was given) and 1 because of rejection secondary to noncompliance with immunosuppressive medications.10 In our cohort, no graft was lost because of rejection. Joly et al. reported 14 patients who underwent kidney transplantation after achieving hematologic response to treatment. In this cohort, MIDD recurrence in the allograft was documented in 4 patients after a median time of 38 months (range, 24.5−42 months). All patients received chemotherapy, and only 1 graft was lost after 5 years.4 In our cohort, the OS of patients who progressed to ESRD and underwent kidney transplantation was significantly better than that in patients with ESRD who did not have kidney transplantation (Figure 4), likely partially related to selection bias.
Table 2.
Renal transplantation outcomes in various MIDD cohorts
| Summary of data on renal transplantation | Mayo Clinic 200412 | UK cohort 201510 | Mayo Clinic 201611 | French cohort 20194 | BU cohort 2020 |
|---|---|---|---|---|---|
| Number of kidney transplant patients | 7 | 7 | 9 | 23 | 6 patients, 7 grafts |
| Study period | 1972–1999 | 2002–2015 | 1992–2014 | 1981–2015 | 1996–2017 |
| Type of hematologic treatment (no. of patients) | Mel-Pred (3) No treatment (4) |
ASCT (4 ) No treatment (3) |
PI (2) ASCT (5) Unknown chemotherapy (1) No treatment (1) |
N/A | Mel-Pred (2) ASCT (2) PI (2) |
| Hematologic response at time of kidney transplant (number of patients) | N/A | CR (3) Not treated (4) |
CR (7) Unknown (2) |
CR/VGPR (14) Not treated, diagnosed after MIDD was found in the graft (9) |
CR (2) VGPR (1) PR (1) NR (3) |
| Number of patients with renal recurrence | 5 | 2 | 3 | 4 of 14 previously treated 9 of 9 previously not diagnosed/treated |
4 (1 PR, 3 NR) |
| Time from kidney transplant to disease recurrence | 33.3 mo (range, 3–45 mo) | 1.6 and 1.9 yr | 2.8, 9 yr | 38 mo (range, 32.5–42 mo) in previously treated patients 32 mo (range, 23–42 mo) in previously untreated patients |
3.2 yr (range, 4 mo–3.8 yr) |
| Median overall survival | From kidney transplant: 6.1 yr (range, 4 mo–12.8 yr) | N/A | N/A | N/A | From diagnosis: 19.8 yr (range, 13.7–27.7 yr) |
| Graft survival | 37.3 mo | 3 grafts lost: 2 due to disease recurrence and 1 due to rejection 4 functioning grafts (0.8–9.7 yr from kidney transplantation) |
2 grafts lost to disease recurrence 6 patients re-treated for hematologic relapse, have functioning graft |
1 graft lost due to disease recurrence (5 yr post-transplantation) 4 grafts lost after MIDD diagnosis after kidney transplantation at 46 mo (range, 40–52.5 mo) 3 grafts loss due to death 16 functioning grafts after median follow-up of 89 mo (range, 35–163 mo) |
4 grafts lost: 2 due to disease recurrence (4 mo and 3.8 yr after transplantation) and 2 due to death (13 and 20.4 yr after transplantation) 3 functioning grafts (1.7–6.5 yr from kidney transplant) |
ASCT, autologous stem cell transplant; CR, complete response; Dexa, dexamethasone; Dx, diagnosis; Mel-Pred, melphalan-prednisone; MIDD, monoclonal Ig deposition disease; N/A, not applicable; NR, no response; PI, proteasome inhibitor, PR, partial response; VGPR, very good partial response.
Of note, LC are cleared by the kidneys, and, as a result, when advanced chronic kidney disease or ESRD is present, the levels increase because of decreased renal clearance. Consequently, hematologic response to treatment cannot be accurately evaluated, as that status is based partly on serum free LC levels. In these cases, elevated free LC levels might lead to bias toward a worse hematologic response.
Recently, newer methods for evaluation of hematologic response such as flow cytometry and next-generation gene sequencing used primarily in multiple myeloma have enabled detection of malignant clones that are undetectable by standard methods.21,22 These clones are defined as minimal residual disease, and their presence in patients with multiple myeloma and AL amyloidosis was associated with inferior disease-free and overall survival.21,22 When used in patients with AL amyloidosis, next-generation flow cytometry revealed that 45% to 60% of those previously classified as CR were in fact minimal residual disease positive, and in those cases continued amyloid deposition in the kidney might still be possible, leading to worsening renal parameters. Using these newer methods for diagnosis of minimal residual disease in MIDD has not been done routinely, and their implications are currently unknown in this disease; however, having a more accurate way of assessing the presence of monoclonal LC production could potentially aid in better selection of patients for successful kidney transplantation or for early treatment.
The retrospective nature of this study as well as the relatively small number of patients are limitations of this study. The small number of patients likely made it difficult to show statistical significance in multiple analyses. In addition, there was no racial diversity in our cohort, perhaps representing underdiagnosis of MIDD in individuals of other races or ethnicities. The choice of hematologic treatment and kidney transplantation were physician and patient dependent and likely contributed to selection bias. Despite these shortcomings, our data along with recently published data from the Mayo Clinic, the United Kingdom, and France4,10,11 support the notion that kidney transplantation can have good outcomes in selected patients with MIDD who respond to hematologic treatment (Table 2).
In summary, our study as well as other recently published studies show that recent improvements in treatments for plasma cell dyscrasias have allowed more patients to achieve a CR or VGPR and to have sustained hematologic response. These robust hematologic responses have led to longer patient and graft survival after kidney transplantation. Clearly, more data regarding the outcomes of MIDD patients are needed; however, in concordance with our recent conclusions regarding AL amyloidosis patients,13 we recommend that patients with MIDD who reach ESRD and have a favorable response to hematologic treatments, such as a CR or VGPR, be considered for kidney transplantation.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported in parts by funds provided by the McCaleb Award from the Amyloidosis Center, Boston University School of Medicine, Alan and Sandra Gerry Amyloid Research Laboratory (AA-K and AH) and National Institutes of Health grant 1KO8DK090143 (AH).
References
- 1.Pozzi C., D'Amico M., Fogazzi G.B. Light chain deposition disease with renal involvement: clinical characteristics and prognostic factors. Am J Kidney Dis. 2003;42:1154–1163. doi: 10.1053/j.ajkd.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Ronco P., Plaisier E., Mougenot B. Immunoglobulin light (heavy)-chain deposition disease: from molecular medicine to pathophysiology-driven therapy. Clin J Am Soc Nephrol. 2006;1:1342–1350. doi: 10.2215/CJN.01730506. [DOI] [PubMed] [Google Scholar]
- 3.Pozzi C., Fogazzi G.B., Banfi G. Renal disease and patient survival in light chain deposition disease. Clin Nephrol. 1995;43:281–287. [PubMed] [Google Scholar]
- 4.Joly F., Cohen C., Javaugue V. Randall-type monoclonal immunoglobulin deposition disease: novel insights from a nationwide cohort study. Blood. 2019;133:576–587. doi: 10.1182/blood-2018-09-872028. [DOI] [PubMed] [Google Scholar]
- 5.Heilman R.L., Velosa J.A., Holley K.E. Long-term follow-up and response to chemotherapy in patients with light-chain deposition disease. Am J Kidney Dis. 1992;20:34–41. doi: 10.1016/s0272-6386(12)80314-3. [DOI] [PubMed] [Google Scholar]
- 6.Adam Z., Krejci M., Pour L. [Complete remission of nephrotic syndrome and improvement of renal function in a patient with light chain deposition disease following high dose chemotherapy with transplantation of autologous haematopoietic stem cells. A case study and review of literature] Vnitr Lek. 2009;55:1089–1096. [PubMed] [Google Scholar]
- 7.Montseny J.J., Kleinknecht D., Meyrier A. Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol Dial Transplant. 1998;13:1438–1445. doi: 10.1093/ndt/13.6.1438. [DOI] [PubMed] [Google Scholar]
- 8.Nasr S.H., Valeri A.M., Cornell L.D. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7:231–239. doi: 10.2215/CJN.08640811. [DOI] [PubMed] [Google Scholar]
- 9.Mohan M., Buros A., Mathur P. Clinical characteristics and prognostic factors in multiple myeloma patients with light chain deposition disease. Am J Hematol. 2017;92:739–745. doi: 10.1002/ajh.24756. [DOI] [PubMed] [Google Scholar]
- 10.Sayed R.H., Wechalekar A.D., Gilbertson J.A. Natural history and outcome of light chain deposition disease. Blood. 2015;126:2805–2810. doi: 10.1182/blood-2015-07-658872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kourelis T.V., Nasr S.H., Dispenzieri A. Outcomes of patients with renal monoclonal immunoglobulin deposition disease. Am J Hematol. 2016;91:1123–1128. doi: 10.1002/ajh.24528. [DOI] [PubMed] [Google Scholar]
- 12.Leung N., Lager D.J., Gertz M.A. Long-term outcome of renal transplantation in light-chain deposition disease. Am J Kidney Dis. 2004;43:147–153. doi: 10.1053/j.ajkd.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Angel-Korman A., Stern L., Sarosiek S. Long-term outcome of kidney transplantation in AL amyloidosis. Kidney Int. 2019;95:405–411. doi: 10.1016/j.kint.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Short A.K., O'Donoghue D.J., Riad H.N. Recurrence of light chain nephropathy in a renal allograft. A case report and review of the literature. Am J Nephrol. 2001;21:237–240. doi: 10.1159/000046254. [DOI] [PubMed] [Google Scholar]
- 15.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Palladini G., Hegenbart U., Milani P. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–2332. doi: 10.1182/blood-2014-04-570010. [DOI] [PubMed] [Google Scholar]
- 17.Weichman K., Dember L.M., Prokaeva T. Clinical and molecular characteristics of patients with non-amyloid light chain deposition disorders, and outcome following treatment with high-dose melphalan and autologous stem cell transplantation. Bone Marrow Transplant. 2006;38:339–343. doi: 10.1038/sj.bmt.1705447. [DOI] [PubMed] [Google Scholar]
- 18.Salant D.J., Sanchorawala V., D'Agati V.D. A case of atypical light chain deposition disease--diagnosis and treatment. Clin J Am Soc Nephrol. 2007;2:858–867. doi: 10.2215/CJN.00970207. [DOI] [PubMed] [Google Scholar]
- 19.Seldin D.C., Andrea N., Berenbaum I. High-dose melphalan and autologous stem cell transplantation for AL amyloidosis: recent trends in treatment-related mortality and 1-year survival at a single institution. Amyloid. 2011;18(suppl 1):127–129. doi: 10.3109/13506129.2011.574354047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batalini F., Econimo L., Quillen K. High-dose melphalan and stem cell transplantation in patients on dialysis due to immunoglobulin light-chain amyloidosis and monoclonal immunoglobulin deposition disease. Biol Blood Marrow Transplant. 2018;24:127–132. doi: 10.1016/j.bbmt.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Lopez J., Lahuerta J.J., Pepin F. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073–3079. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores-Montero J., Sanoja-Flores L., Paiva B. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:2094–2103. doi: 10.1038/leu.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]