Abstract
Cardiovascular disorders are the most common cause of mortality in autosomal dominant polycystic kidney disease (ADPKD). This review considers recent clinical and basic science studies that address the contributing factors of cardiovascular dysfunction in ADPKD. In particular, attention is placed on how dysfunction of the polycystin proteins located in the cardiovascular system contributes to extrarenal manifestations of ADPKD.
Keywords: cardiovascular dysfunction, calcium signaling, heart failure, polycystic kidney disease
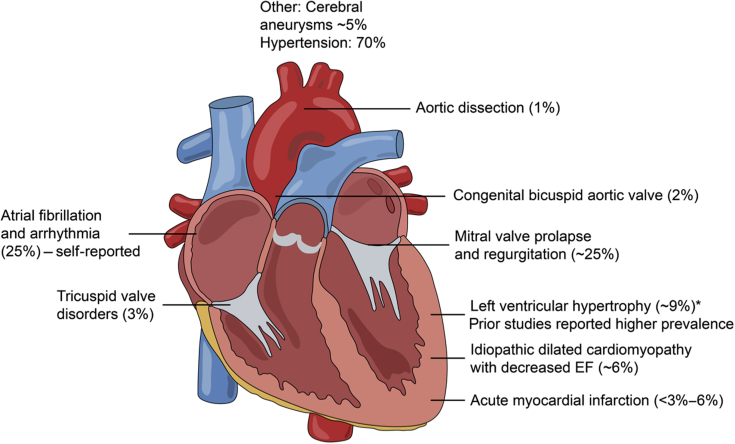
ADPKD is the most common hereditary cause of end-stage renal disease, and it is typified by renal cysts that can begin early in life, although clinical complications typically manifest in the third or fourth decade of life.1 Mutations in at least 2 genes, PKD1 and PKD2, result in reduced level and function of their respective proteins, polycystin 1 (PC1) and polycystin 2 (PC2), and the clinical manifestations of this disease. ADPKD is a systemic disease with extrarenal manifestations involving the cardiovascular system, including hypertension, intracranial aneurysms, left ventricular hypertrophy, arrhythmias, and dilated cardiomyopathy (Figure 1).2, 3, 4, 5 Cardiovascular complications are the most common cause of death in ADPKD, and whether defects in the polycystins play a primary role or are secondary to the consequences of hypertension and progressive kidney disease is still unclear.
Figure 1.
Spectrum of cardiovascular disorders reported in autosomal dominant polycystic kidney disease. Note that prevalence is based on more recent clinical studies (i.e., the past decade, in which blood pressure control has been administered). Please refer to the text for references. EF, ejection fraction.
The high incidence of cardiovascular complications may be secondary due to early-onset hypertension in ADPKD that occurs at a mean age of 27 years, typically prior to loss of kidney function. The relationship between ADPKD and the onset of hypertension is complex. As renal cysts enlarge, they compress the renal vasculature, cause localized ischemia, and activate the intrarenal renin–angiotensin–aldosterone system and the renal sympathetic nervous system.4,6,7 Moreover, as discussed further later in this review, the polycystin proteins are found in the vasculature, and both endothelial dysfunction and alterations in smooth muscle cell function have been observed in human and animal studies. Regardless of the sequelae of events that lead to hypertension, blood pressure levels in most hypertensive ADPKD patients are not malignant or accelerated, and they typically require only 1 or 2 antihypertensive agents for adequate blood pressure control. However, with chronic pressure overload, loss of diurnal decline in blood pressure and slowly increasing blood pressure in advanced kidney failure contribute to the increased frequency of cardiovascular events seen in ADPKD and are well-described key factors in the development of congestive heart failure (HF).8 In addition to the contribution of renal hypertension, and of particular importance, the polycystin proteins are found in all cells of the vasculature and heart, including vascular smooth muscle cells, endothelial cells, cardiomyocytes, and fibroblasts. Dysregulation of polycystin function in these cells may in fact be responsible for a primary cardiovascular defect in ADPKD. This review considers the characteristics of cardiovascular dysfunction in ADPKD, and the functional role of the polycystin proteins in cardiac tissues. In doing so, this review addresses the role of the polycystin proteins themselves in the cardiovascular pathophysiology of ADPKD, and how partial, or complete, loss of these proteins may contribute to the clinical cardiovascular manifestations in this disorder.
Clinical Studies of HF in ADPKD
HF is a collective term describing abnormal function of the chambers of the heart. Modern definitions of HF are evolving, referencing the ejection fraction (EF), that is, the ability of the left ventricle to pump blood by comparing the filling (diastolic phase) and ejected (systolic phase) volume. Since 2013, the American College of Cardiology guidelines have subdivided HF into 2 categories9, 10, 11: HF with reduced (≤40%) EF, also known as systolic HF, and HF with preserved (≥50%) EF, or diastolic HF. Additionally, the 2016 European Society of Cardiology HF guidelines12 introduced the term “heart failure with mid-range ejection fraction” to refer to patients with HF and a mildly reduced EF of 40%–49%.
To date, the vast majority of clinical studies in ADPKD have primarily evaluated structure only or left ventricular mass index (LVMI), which is a structural measure of cardiac mass. When LVMI is chronically elevated, this condition can create a predisposition to HF. However, LVMI is not a direct measure of cardiac function, and patients can have elevated LVMI without HF. In the setting of a single LVMI measurement, cardiac remodeling is complex, with different types of remodeling being associated with altered pump function (e.g., concentric hypertrophy with HF with preserved EF to a dilated cardiomyopathy or HF with reduced EF).
A registry of a cohort of 116 ADPKD patients (mean age: 41 years) had a 41% prevalence of left ventricular hypertrophy, and ∼23% subjects had it without hypertension, using echocardiographic methods.13 In a study of 31 ADPKD patients (mean age: ∼35–40 years), evaluating both systolic and diastolic function, hypertensive patients with ADPKD had a significantly greater LVMI, which is not surprising. Intriguingly, right ventricular function, as assessed by myocardial performance index, was significantly higher in both ADPKD patients and normotensives,14 and in ADPKD patients with well-preserved renal function.
More-current studies, initiated decades after the start of use of renin–angiotensin–aldosterone system inhibitors as the primary therapy for hypertension in ADPKD, show a different pattern of LVMI. The HALT-PKD study (NCT00283686) studied 558 hypertensive ADPKD patients and measured LVMI by magnetic resonance imaging. At baseline, the prevalence of left ventricular hypertrophy was only 4%.15 However there was a significant reduction in LVMI in those randomized to rigorous blood pressure control as compared to those in the moderate blood pressure control group. Potential contributors to the lower LVMI measurements in this large hypertensive cohort include an earlier diagnosis of hypertension, more rigorous control of blood pressure, use of renin–angiotensin–aldosterone system inhibitors in more than 80% prior to enrollment, and different imaging techniques.2,15 In confirmation of this, the HALT trial results are in good agreement with a self-report study of 419 subjects that noted that over 80% of the respondents had hypertension, and only 9% reported having an enlarged (or hypertrophic) heart.3 Included in this report was a self-report of intracranial aneurysms, which, consistent with prior reports, occurred in approximately 5%. Intriguingly, as elaborated on below, 25% of the respondents self-reported arrhythmias, a cardiovascular abnormality that has been less well-studied in ADPKD.
As mentioned above, the definition of HF, and the techniques to assess the condition, have undergone significant changes in the past decade. Thus, more recent publications have reported measures of EF by echocardiographic analysis in ADPKD patients. For example, an analysis of 667 ADPKD patients from the Mayo Clinic database with existing echocardiographic data found that 6% had idiopathic dilated cardiomyopathy with a mean EF of 25%, consistent with an idiopathic dilated cardiomyopathy phenotype.16 In contrast, 2.5% of the 667 ADPKD patients studied had hypertrophic obstructive cardiomyopathy and a reported EF of 70%. A 2019 single-site study from Maryland of 126 ADPKD patients (78% with hypertension) noted that regardless of the co-diagnosis of hypertension, left ventricular hypertrophy, as defined by LVMI (that is, left ventricular mass assessed with echocardiography, and indexed to body mass) was the same, at ∼21%.17 Echocardiography with speckle-tracking can also be used to detect subtle and early changes in cardiac mechanics. A 2019 substudy of the ALADIN-trial (testing the effectiveness of somatostatin treatment) at a single site with 34 ADPKD and matched controls found that those with ADPKD had significantly stiffer hearts, as assessed by decreased relaxation times and diminished torsional movement, both parameters that can precede and contribute to HF.18,19 Although a small cohort of patients were analyzed, this study is particularly interesting, as the ADPKD cohort did not have decreased EF (average EF of 63%) but exhibited some diastolic dysfunction (where the refilling pressure was decreased, as assessed by Doppler measures of E/e’, that is, the velocity of mitral valve inflow compared to the mitral annulus velocity) compared to matched controls.
It is curious to note that although acute myocardial infarction (MI) is the most common cause of HF in the general population, MI in the ADPKD population has not been previously associated with ADPKD. However, recent studies from 2 different Asian ADPKD populations suggest that the prevalence of MI in the ADPKD population is doubled compared to that in non-ADPKD subjects.20,21 Coronary artery disease differs in severity and distribution in different ethnic populations, and therefore, these findings may or may not apply to other populations.
On the whole, it has been consistently reported that cardiac dysfunction of both a systolic and diastolic nature is present in ADPKD, often early, prior to loss of kidney function and also in normotensive individuals (Table 13,13, 14, 15,16, 17, 18, 19, 20, 21). Comprehensive and consistent echocardiographic or magnetic resonance imaging measurements utilizing the new guidelines and definitions for HF are needed to obtain an accurate current estimate of the prevalence of HF with preserved ejection fraction and HF with reduced ejection fraction in the broader ADPKD population.
Table 1.
Summary of clinical cardiac findings and studies discussed in the review
| Reference | Main finding | Sample size (analyzed ADPKD cohort) | Key patient characteristics | Method |
|---|---|---|---|---|
| Chapman et al.13 1997 | 41% LVH 23% LVH without hypertension 16% in healthy controls |
116 | Mean age: 41 yr; Male: 47%; Age-adjusted creatine: 2.0 |
Echocardiography |
| Oflaz et al.14 2005 | Hypertensive patients with ADPKD had higher LVMI compared to controls; LVMI was higher in normotensive ADPKD subjects, although not significant |
31 | Age: 35–40 yr Male: 33% |
Echocardiography Myocardial performance index |
| HALT-PKD15 NCT00283686 |
4% LVMI | 558 | Males: 51% GFR: >60 |
Magnetic resonance imaging |
| Helal et al.3 2012 | 9.5% cardiac enlargement 25% arrhythmia 6% MI |
419 | Age: 53 yr Male: 47% Hypertension: 80% ESRD: 32% |
Self-reporting |
| Chebib16 2017 | 6% IDCM with EF of 25%; 2.5% had hypertrophic obstructive CM, with EF of 70% |
58 | Male: 48%–58% (depending on stratification) GFR: 52–55 |
Retrospective of co-existing ADPKD and cardiomyopathy from 1984 to 2015 |
| Spinelli et al.18,19 2018, 2019 (Aladin-Trial) |
No difference in LVM; Stiffer hearts— decreased relaxation times and tortional movement; dystolic dysfunction (e/e’ ratios) |
34 | Mean age: 35.8 yr Male: 38% Hypertension: 65% GFR: 82 |
Echo with speckle tracking |
| Sung et al.20 2017 | MI 3%, compared with 1% in non-ADPKD group; Arrhythmia: 19% |
2062 | Median age: 47 yr Male: 48.5% Hypertension: 81% |
Retrospective (1997–2008) from Taiwan national health insurance research database |
| Yang et al.21 2018 | 75% with ST segment elevation MI (compared with 59% for controls) 25% with non-ST segment elevation MI (compared with 41% controls) 11.5% sudden cardiac death (compared with 4.6% controls) |
52 | Age: 18–75 yr Hypertension: 79% |
Retrospective of 13 years at Peking University for AMI patients with ADPKD |
| Chen et al.17 2019 | 21% LVH; same between hypertensive and nonhypertensive | 126 | Hypertension: 78% eGFR: 63 Mean age: 46 yr Male: 40% |
Echocardiography |
ADPKD, autosomal dominant polycystic kidney disease; AMI, acute myocardial infarction; CM, cardiomyopathy; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; IDCM, idiopathic dilated cardiomyopathy; LVH, left ventricular hypertrophy; LVM, left ventricular mass; LVMI, left ventricular mass index; MI, myocardial infarction.
Valvular Disorders
The filling of the left ventricle by oxygenated blood from the left atria is provided by proper function of the mitral valve. The mitral valves of the heart are derived from the collagen and connective tissue. Dysfunction of the mitral valve can also contribute to rhythm disorders, or arrhythmias (see section Functional Effects of the Polycystin Proteins on Excitation–Contraction Coupling). A study from 1988 of 163 ADPKD patients reported that 26% had mitral valve prolapse, and 31% mitral valve incompetence, or regurgitation.22 A later study from 2001 in 73 patients with PKD1 mutations found a similar incidence of mitral valve prolapse (26%). Similar incidence rates of mitral valve prolapse were also found in a longitudinal study.23 The finding that unaffected family members had lower prevalence of mitral valve prolapse (14%) is suggestive that mutations in the PKD genes (but not nonfamilial or modifying familial causes) are responsible for the higher prevalence in the ADPKD patients.24 It should be noted that the tricuspid valve, which fills the right ventricle, is not generally affected in ADPKD. Intriguingly, ADPKD children, either with few renal cysts or genetically identified, also had a substantially higher incidence (12%) of mitral valve prolapse compared to unaffected siblings (3%).25 However, a smaller study of 19 affected and 20 unaffected young ADPKD patients did not detect any mitral valve or left ventricular abnormalities.26 The results of these studies suggest that the mitral valve abnormalities may arise in a subset of ADPKD patients independent of renal cyst development. The mechanism by which these valvular disorders arise, and whether they are related to the cardiomyocyte abnormalities, is unclear. It has been hypothesized that prolapse is due to changes in the extracellular matrix, but it is not yet clear if the mitral valve prolapse seen is due to collagen-fibronectin buildup or an actual vascular disorder. As the papillary muscles composed of cardiomyocytes act to contract the mitral valve, and the polycystins found in the cardiomyocytes can contribute here (as discussed below), it is possible that a papillary muscle phenotype may be responsible. Basic mechanistic experiments aimed at elucidating the progression of valvular disease have not been conducted, and the combination of ADPKD mouse genetic lines and newer technologies to carefully measure mitral valve function may provide additional insight.
Overview of Polycystin Proteins—Structure, Localization, and Cellular Function
It has been consistently noted that the clinical characteristics of ADPKD due to mutations to polycstin 1 and 2 manifest as different phenotypes. Thus, PKD1 mutations typically give rise to an earlier clinical onset, whereas the progression of disease in a PKD2 patient is typified by later and milder onset of symptoms.27 Part of the phenotypic difference is due to the different structures and functions of the 2 proteins. In spite of the similar names and a shared polycystin motif, there are striking structural differences between these 2 membrane proteins. PC1 is an 11 transmembrane protein, has structural characteristics reminiscent of a G-coupled protein receptor, and is found typically on the plasma membrane and primary cilia.28 In contrast, PC2 is typified by 6 transmembrane spans (these 6 transmembrane spans have some homology to the last 6 transmembrane spans of PC1) and can assemble as a tetramer29, 30, 31 where it can form a calcium-dependent cation-selective channel.29, 30, 31, 32, 33, 34 Although there is some sequence homology between the final 6 transmembrane spans of PC1 and PC2, there is no clear evidence that PC1 also acts as a bona fide channel by itself. However, there are varying reports of its conductance of calcium directly, and whether its channel activity is dependent on other modifying proteins.32,35,36 PC2 is also a member of the transient receptor potential family of channel proteins, along with polycystin 2-L1 and polycystin 2-L2 protein. Recent nomenclature assignment has designated PC2 as TRPP1; for the purposes of this review, which discusses both PC1 and PC2, the polycystin names have been utilized. As mentioned above, PC1 and PC2 can independently localize to different regions of the cell. However, the 2 proteins do interact through regions on the cytosolic C-terminal domains and/or transmembrane interaction sites, as evidenced by a cryo-electron microscopy structure of a PC1/PC2 complex that was largely lacking the cytoplasmic domains of PC1 and PC2.28 Functionally, the polycystin proteins can interact either in the primary cilium, or between the plasma membrane and endoplasmic reticulum. Renal cysts manifest when the common signaling pathway between these 2 proteins is disrupted. When either or both of these proteins are lost, there is, typically, an increase in intracellular cyclic adenosine monophosphate signaling, and a decrease in intracellular calcium signaling, which either, or together, can cause cellular proliferation and increased apoptosis.1
Congenital Abnormalities and Cardiac Development
The localization of the polycystin proteins to the primary cilia, and their modulatory role in calcium signaling, may in part explain why loss or mutations to these proteins cause congestive HF.37 Numerous animal model studies have demonstrated a functional role of the polycystins in left–right axis development, and in particular, a functional role in the sinus node.38,39 Although women with ADPKD have been noted to have more fetal complications,40 including congenital heart defects, large genetic screening studies have linked several previously idiopathic congenital heart disorders to polycystin 1 and polycystin 2 mutations.37 A key rationale for the role of polycystin 2 in the heart’s development is its function in the nodal cilia. In cardiomyocytes, these primary cilia are present in the embryo, but they become vestigial in the adult.41 Moreover, as discussed below, primarily cilia are present on fibroblasts.42 In rodents, as in humans, complete knockouts of the polycystins are developmentally lethal, due to the lack of full organ development. Zebrafish studies have been useful in the study of the proteins, as additional copies of polycystin enable the fish to survive, albeit with edema and a dextrocardia phenotype.43 It is unclear what functional signal the polycystins give within the primary cilia. Several studies indicate that there is a calcium signal from the cilia that enables left–right axis development, although this has not been uniformly found.39,44,45 Regardless, the central role that PC2 plays in the nodal cilia in organ development is not disputed. Once left–right symmetry has been established, and the chambers of the heart have been developed, the function of the polycystins in the developed heart appear to be more varied. As discussed below, the polycystin proteins are present in multiple cell types, which can all contribute to the confluence of cardiovascular dysfunction observed in ADPKD.
Functional Effects of the Polycystin Proteins on Excitation–Contraction Coupling
A substantial number of basic science studies have demonstrated that loss of polycystin proteins modify intracellular calcium levels, either directly or indirectly. The maintenance and determination of calcium signaling is exquisitely controlled, as calcium is a ubiquitous second messenger, where fluctuations can result in diverse cellular functions ranging from transcription factor initiation, to cellular contraction, to cell death. In nonmuscle cells, the intracellular calcium signals are largely governed by the inositol tris-phosphate receptor. The release of calcium from this receptor is on the order of seconds; in contrast, the calcium signal required for excitation–contraction coupling for cardiomyocytes or skeletal muscle is on the order of milliseconds. The difference in speed is, in part, due to the unique structural arrangement of the cardiomyocytes, and the types of proteins that regulate the excitation–contraction coupling. The contraction cycle of the ventricular cardiomyocyte starts with an action potential that triggers calcium entry from the L-type calcium channel and subsequent calcium release from the sarcoplasmic reticulum, controlled by the ryanodine receptor (RyR). The released calcium interacts with the contractile apparatus to elicit the contraction. Perturbations to these proteins would thus render any of these processes affected, and if left unchecked, would result in calcium contractility dysfunction, and, over time, HF (Figure 2). Early studies indicated that the PC2 can bind and interact with the RyR. In planar lipid bilayer electrophysiological experiments, where an artificial membrane was created and the channel activity of RyR assessed, PC2 was found to decrease RyR channel activity, and thus sarcoplasmic reticulum calcium release.46 This would, therefore, increase the amount of calcium that would interact with the contractile apparatus, resulting in a larger contraction. The excess calcium would be expected to be a contributing factor for HF, although as discussed below, there is additional complexity.
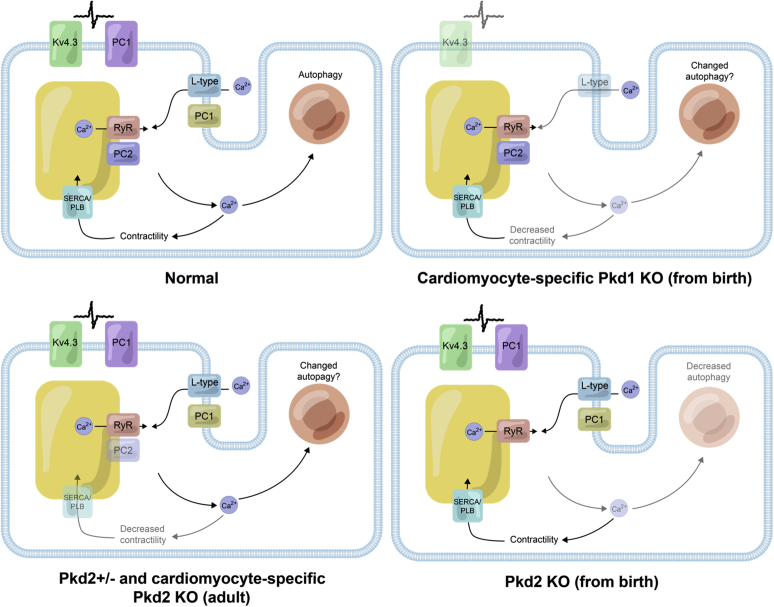
Figure 2.
Current understanding of the location and functional roles of polycystin (PC)1 and PC2 in cardiomyocytes. Normal: PC1 appears to be on the plasma membrane in cardiomyocytes and has been shown to interact with the potassium (Kv) channel 4.3 and regulate the expression of the L-type calcium channel (VDCC). PC2 has been shown to interact with the intracellular release channel ryanodine receptor (RyR). Arrows point to the movement of calcium in a cardiomyocyte contributing to contractility and autophagy. It is unclear if PC1 and PC2 interact directly with each other in cardiomyocytes, as demonstrated in the renal epithelial cells. PC1 knockout (KO) models: KO of PC1 from birth leads to decreased L-type VDCC; decreased Kv4.3 expression, reduced action potential duration, decreased calcium release, and decreased contractility. Autophagy in cardiomyocytes has not been addressed yet in the Pkd1 KO mouse. PC2 models: Heterozygous and adult induced PC2 KO causes increased released calcium and, paradoxically, decreased contractility due to desensitization of the myofilament to calcium. The calcium uptake mechanism into the sarcoplasmic reticulum via SERCA and phospholamban is less active. From-birth KO Pkd2: From-birth KO of cardiac PC2 causes decreased released calcium and decreased autophagy. SERCA, sarco/endoplasmic reticulum Ca2+-ATPase.
Subsequent studies have demonstrated that the PC2 haploinsufficient mouse (which does not have renal cysts) has larger stimulated calcium transients, yet diminished contracture, indicating decreased calcium sensitivity of the myofilament,47 and a decrease in EF was seen with aging.48 Similar loss of calcium regulation was also found in zebrafish knockout models of PC2.43 The calcium and contraction deficiency was further exacerbated by the addition of catecholamines or by pressure overload (trans aortic artery constriction, a rodent surgical procedure where a ligature constricts the arterial blood flow, thus generating a pressure-overload situation and eventual HF).43,47, 48, 49 Collectively, these studies point to roles of the polycystins in decoupling the calcium–contractile relationship that exists within cardiomyocytes, and this decoupling is further accentuated by pressure overload. It is expected that the decoupled calcium–contractile relationship would be contributory to loss of systolic function, but the exact mechanism by which loss of PC2 achieves the decoupling is not clear.
Loss of PC1 does not appear to cause the same cardiac phenotype as PC2 deficiency. Studies of the haploinsufficient PKD1 mouse revealed extensive diastolic dysfunction.50 However, to better understand if PC1 impacted cardiomyocytes function directly (rather than through systemic effects), one has to utilize cardiac-specific knockout models. Intriguingly, studies on cardiomyocyte-specific knockout of PC1 from birth showed reduction in the density of the L-type calcium channel.51 The functional regulation of the L-type calcium channel by PC1 was governed by an interaction with the trafficking subunit of the L-type calcium channel. Overall, these cardiomyocyte-specific PC1 knockout mice, compared to control mice, had increased wall thickness and decreased fractional shortening.51 These murine findings are similar to those from the human clinical studies discussed earlier, in which left ventricular hypertrophy was found in ∼41% of young ADPKD patients,13 but they are less consistent with the HALT-PKD study, which did not find a significantly higher prevalence of ventricular hypertrophy.2,15 This discrepancy may result from the fact that the mouse model had PKD1 knocked out in both alleles from birth, whereas humans are born with heterozygous mutations.
The authors of the cardiomyocyte-specific PC1 knockout mice study also reported that regulation of the L-type calcium channel density was osmotically dependent.51 Thus, hypo-osmotic stress caused upregulation of the L-type calcium channel only when PC1 was present. A role for PC1 in osmotic or pressure sensing would be highly relevant in the setting of HF, as many ADPKD patients have hypertension, which would induce pressure overload on the heart. However, due to the differential cardiac baseline characteristics in the from-birth cardiomyocyte PC1 knockout mice (i.e., increased wall thickness and decreased fractional shortening), there are limitations to interpreting the results when a pressure overload state is additionally imposed.51 Thus, to avoid the additional confounding factors, models in which PC1 is temporally knocked out once the mouse reaches maturity (rather than from birth) will help to clarify the role of cardiomyocyte-localized PC1 in HF.
More recently, cardiomyocyte-localized PC1 has also been found to co-assemble with multiple voltage-dependent potassium channels, Kv, and loss of polycystin protein reduced the action potential duration.52 These data may in part explain why 25% of ADPKD patients have self-reported arrhythmias3 and demonstrate conduction abnormalities such as atrial fibrillation.53 However, the role of the polycystins in the atria or the conduction system has not been systematically examined. As arrhythmogenic and atrial fibrillation events can increase the risk of developing cardiomyopathies and HF,54 additional insight into the timing and likelihood of inciting event(s) would be beneficial in the development of treatment stratagems.
Noncontractile Role of Polycystins in Cardiomyocytes
Although the present discussion emphasizes a direct role of the polycystin proteins in regulating cardiomyocyte contractile activity, increasing evidence indicates that these proteins are responsible for additional roles (Table 242,47,50, 51, 52,55, 56, 57, 58, 59). Of note, a function for autophagy has been reported.55 Autophagy is essential for the heart, as the cardiomyocytes, unlike the renal epithelial cells, do not undergo regular cell proliferation; thus, autophagy is a means by which the cell can remove the buildup of deleterious protein components. Impaired removal of this cellular debris from cardiomyocytes impairs sarcomeric function and the ability of the cardiomyocytes to contract, and ultimately results in a stiffer and less compliant heart. These recent findings may in part explain the decreased torsional compliance of ADPKD patients’ hearts.18
Table 2.
Summary of the main clinical findings, and the potential mechanisms, based on animal studies
| Main clinical finding | Potential mechanisms (using animal models) | References |
|---|---|---|
| Cardiac hypertrophy | Ciliary role of polycystin 1 in fibroblasts and fibrosis | 42 |
| Cardiac failure | Alteration in calcium signaling leading to dysfunctional contractility (e.g., via the L-type calcium channel or ryanodine receptor) | 47, 50, 51, 52, 55 |
| Change in autophagy | ||
| Arrhythmias | Change in potassium channel Kv4.3 expression | 52 |
| Hypertension | Endothelial dysfunction, loss of nitric oxide production Note, however, that SMC knockout of polycystin 2 is hypotensive |
56, 57, 58, 59 |
Noncardiomyocyte Cardiac Cells
Cardiomyocytes are the key cells of the heart that drive cardiac function, owing to their ability to contract in a coordinated manner. However, in a normal, healthy heart, fibroblast cells are believed to be the dominant population.60 The cardiac fibroblast population starts to expand in HF, via either infiltration or the conversion of cardiomyocytes in cardiomyofibroblasts. The replacement of functionally contracting cells with fibroblasts occurs later in HF and can be viewed as a point of no return for the heart. Intriguingly, a recent study points to a particular role for PC1 in the primary cilia of fibroblasts and indicates that the absence of PC1 from cardiac fibroblasts results in increased hypertrophy, although there was a reduction in scar size after myocardial infarction.42 Surprisingly, though, the degree of fibrosis in the areas surrounding the myocardial infarction in mice with or without fibroblast PC1 remained the same. Thus, there appears to be a novel signaling mechanism in cardiac fibroblasts, in which PC1 in the cilia does contribute to cardiac remodeling. In addition, signaling pathways in the heart appear to be different with regard to the way PC1 and PC2 contribute to cell proliferation and changes in the extracellular matrix in the kidney.
Vascular Function of PC1 and PC2
Given the early onset and frequency of hypertension in ADPKD patients, it is expected that the polycystin proteins contribute to vascular function. However, results from murine studies have not always paralleled the clinical findings of hypertension and endothelial dysfunction. Early studies on aorta-derived vascular smooth muscle cells (VSMCs) indicated that loss of PC1 and PC2 resulted in a decrease in calcium signaling,61 although the downstream consequences of this signaling loss were not elucidated. Yet in studies from germline heterozygous mice (without renal cysts), the loss of PC2 does not result in cardiovascular abnormalities, or changes in blood pressure, even if mice are aged, or if female mice have the additional vascular stress of pregnancy.62 These studies may suggest that the remaining intact allele and secondary protective mechanisms provide sufficient means of ensuring intact regulation of blood pressure and cardiac function. Confusing results have also been reported from the vascular smooth muscle. Knockout of PC1 from VSMCs resulted in no changes in blood pressure but did result in a loss of the myogenic response in the mesenteric artery.56 The loss in pressure sensing was found to be a combination of both polycystins acting together with filamin A. Conversely, knockout of PC2 from VSMCs resulted in hypotension.57 This hypotension was attributed to the ability of PC2 to conduct potassium into the VSMCs, where a change in potassium level may contribute to repolarization.57 It is not clear if loss of PC2 actually alters the basal membrane potential, as mesenteric resistance vessels from this knockout mouse still show a partial myogenic response. Thus, it appears that PC1 and PC2 may function in separate pathways in VSMCs.
Regarding endothelial function of the polycystins, a number of clinical studies have indicated endothelial dysfunction, and the endothelial dysfunction may precede any blood pressure changes. For example, in a study of 41 ADPKD patients of patients with GFR >60 ml/min and normal LVMI, it was found that there was no significantly different ambulatory blood pressures among the ADPKD patients, even in those stratified by systolic blood pressure shown to be subject to a nocturnal fall of more than 10%.8 Endothelial dysfunction, as assessed by brachial artery measurements, demonstrated that ADPKD patients, who did not have a dip in nocturnal systolic pressure, had significantly lower dilatory responses. This would potentially have a longer-term impact, as endothelial dysfunction is a precursor to chronic cardiovascular disease.
Clinical data suggest that there is decreased release of the intrinsic vasodilator nitric oxide,63 and an increase in asymmetric dimethylarginine, an endogenous competitor with L-arginine in the production of nitric oxide.64 Endothelial nitric oxide synthase was also elevated from serum samples.65 These studies are in agreement with other reports of increased levels of oxidative stress, including patients with preserved renal function.66,67 Collectively, these changes would make both the resistance vasculature and the conduit arteries stiffer and more prone to hypertension. Oxidative stress affects the conduit arteries by decreasing the coronary artery flow, and it can induce vascular remodeling, resulting in a thickening of the carotid intima media, which has been observed in normotensive ADPKD patients with well-preserved renal function. In rat models of autosomal recessive polycystic kidney disease (studied using the PCK rat, where there is a skipped exon in the Pkhd1 gene), there was an increase in oxidative stress and a decrease in L-arginine levels, and aortic endothelial cells isolated from these rats displayed increased proliferation and apoptosis, which would contribute to vascular remodeling68 and increased vascular stiffness. In vascular studies of the Lewis rat model of nephronophthisis, another recessive form of PKD (in which the cilliary protein Nek8 is disrupted69), an overall reduction in nephrons by ∼25% occurs.58 The adult Lewis rat has calcification of the aorta, elevated renal sympathetic nerve activity, and elevated mean arterial pressure.70 But at earlier time points, these rats also have a loss of remodeling of the systemic vasculature at 6 weeks, which then proceeds to a compromised endothelial-dependent vasorelaxation and a reduction in nitric oxide synthase activity in systemic vascular beds, including the mesenteric artery.59 Overall, it appears that rat endothelial vascular studies, even though they are conducted on autosomal recessive PKD rodent models, are broadly consistent with the clinical ADPKD studies. However, as no basic science studies have determined the phenotype when PC1 or PC2 is selectively removed from the endothelium, it is yet to be conclusively determined if there are differential roles for these proteins or if an endothelial phenotype exists in the absence of a renal contribution.
Relationship of the Heart to Renal Function
As mentioned above, activation of the renin–angiotensin–aldosteronesystem, and ensuing hypertension,contributes to the development of HF. However, what has also been intriguing is the observation of an upregulation of the polycystin proteins, in particular PC2, in HF models in the non-PKD mouse models.49 Although these studies are yet to be confirmed in a patient population, HF models of pressure overload or elevated catecholaminergic stimulus where cardiac knockout or haplosufficiency of PC1 and PC2 is present result in a lower than expected elevation in natriuretic peptides.14,51 Functionally, the natriuretic peptides are made in the atria and ventricles of the heart in response to stress. The natriuretic peptides can then enhance renal function by promoting salt wasting, or natriuresis. These animal studies may suggest that the loss of the polycystin proteins impairs the pressure-sensing role of the heart and results in a lower natriuresis in the setting of HF.
Future Perspectives
A multitude of functions for the polycystin proteins in the heart contribute to the cardiovascular phenotype in ADPKD. The increasing number of genetic and systematic mechanistic studies using murine and other models is providing valuable, although sometimes contradictory, insight into how ADPKD patients manifest their cardiovascular symptoms. Moreover, future studies that focus on genetic screening studies combined with mutation analysis may provide additional insight and screening methods for identifying ADPKD patients that are at risk for cardiovascular disease, even with normal blood pressure. Finally, the incorporation of diastolic and systolic functional assessments, in addition to structural measurements that utilize recent definitions for HF, will help determine the risk level that ADPKD patients have for cardiovascular events.
Disclosure
ABC acknowledges research/consultant support from Otsuka, Reata, and Sanofi. The other author declared no competing interests.
Acknowledgments
IYK acknowledges the financial support of National Institutes of Health (NIH) grant R00DK101585 and the Loyola University Chicago Cardiovascular Research Institute. ABC acknowledges NIH support with grant P30DK106912.
Contributor Information
Ivana Y. Kuo, Email: ikuo@luc.edu.
Arlene B. Chapman, Email: achapman1@bsd.uchicago.edu.
References
- 1.Torres V.E., Harris P.C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol. 2014;25:18–32. doi: 10.1681/ASN.2013040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam A., Perrone R.D. Left ventricular hypertrophy in ADPKD: changing demographics. Curr Hypertens Rev. 2013;9:27–31. doi: 10.2174/1573402111309010005. [DOI] [PubMed] [Google Scholar]
- 3.Helal I., Reed B., Mettler P. Prevalence of cardiovascular events in patients with autosomal dominant polycystic kidney disease. Am J Nephrol. 2012;36:362–370. doi: 10.1159/000343281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ecder T., Schrier R.W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5:221–228. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ecder T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr Hypertens Rev. 2013;9:2–11. doi: 10.2174/1573402111309010002. [DOI] [PubMed] [Google Scholar]
- 6.Rahbari-Oskoui F., Williams O., Chapman A. Mechanisms and management of hypertension in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2014;29:2194–2201. doi: 10.1093/ndt/gft513. [DOI] [PubMed] [Google Scholar]
- 7.Chapman A.B., Stepniakowski K., Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:153–163. doi: 10.1053/j.ackd.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turgut F., Oflaz H., Namli S. Ambulatory blood pressure and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease. Ren Fail. 2007;29:979–984. doi: 10.1080/08860220701641728. [DOI] [PubMed] [Google Scholar]
- 9.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 11.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 13.Chapman A.B., Johnson A.M., Rainguet S. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8:12921297. doi: 10.1681/ASN.V881292. [DOI] [PubMed] [Google Scholar]
- 14.Oflaz H., Alisir S., Buyukaydin B. Biventricular diastolic dysfunction in patients with autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:2244–2249. doi: 10.1111/j.1523-1755.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 15.Perrone R.D., Abebe K.Z., Schrier R.W. Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:2508–2515. doi: 10.2215/CJN.04610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chebib F.T., Hogan M.C., El-Zoghby Z.M. Autosomal dominant polycystic kidney patients may be predisposed to various cardiomyopathies. Kidney Int Rep. 2017;2:913–923. doi: 10.1016/j.ekir.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H., Watnick T., Hong S.N. Left ventricular hypertrophy in a contemporary cohort of autosomal dominant polycystic kidney disease patients. BMC Nephrol. 2019;20:386. doi: 10.1186/s12882-019-1555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinelli L., Pisani A., Giugliano G. Left ventricular dysfunction in ADPKD and effects of octreotide-LAR: A cross-sectional and longitudinal substudy of the ALADIN trial. Int J Cardiol. 2019;275:145–151. doi: 10.1016/j.ijcard.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 19.Spinelli L., Pisani A., Giugliano G. Data on the assessment of LV mechanics by speckle tracking echocardiography in ADPKD patients. Data Brief. 2018;21:2075–2081. doi: 10.1016/j.dib.2018.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung P.H., Chiang H.J., Yang Y.H. An association between autosomal-dominant polycystic kidney disease and the risk of acute myocardial infarction in Asian population—results of a nationwide study. Oncotarget. 2017;8:19365–19375. doi: 10.18632/oncotarget.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang B., Wang Q., Wang R., Xu T. Clinical manifestation, management and prognosis of acute myocardial infarction in autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2018;43:1806–1812. doi: 10.1159/000495638. [DOI] [PubMed] [Google Scholar]
- 22.Hossack K.F., Leddy C.L., Johnson A.M. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med. 1988;319:907–912. doi: 10.1056/NEJM198810063191404. [DOI] [PubMed] [Google Scholar]
- 23.Timio M., Monarca C., Pede S. The spectrum of cardiovascular abnormalities in autosomal dominant polycystic kidney disease: a 10-year follow-up in a five-generation kindred. Clin Nephrol. 1992;37:245–251. [PubMed] [Google Scholar]
- 24.Lumiaho A., Ikaheimo R., Miettinen R. Mitral valve prolapse and mitral regurgitation are common in patients with polycystic kidney disease type 1. Am J Kidney Dis. 2001;38:1208–1216. doi: 10.1053/ajkd.2001.29216. [DOI] [PubMed] [Google Scholar]
- 25.Ivy D.D., Shaffer E.M., Johnson A.M. Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2032–2036. doi: 10.1681/ASN.V5122032. [DOI] [PubMed] [Google Scholar]
- 26.Harrap S.B., Davies D.L., Macnicol A.M. Renal, cardiovascular and hormonal characteristics of young adults with autosomal dominant polycystic kidney disease. Kidney Int. 1991;40:501–508. doi: 10.1038/ki.1991.238. [DOI] [PubMed] [Google Scholar]
- 27.Pei Y. Molecular genetics of autosomal dominant polycystic kidney disease. Clin Invest Med. 2003;26:252–258. [PubMed] [Google Scholar]
- 28.Su Q., Hu F., Ge X. Structure of the human PKD1-PKD2 complex. Science. 2018:361. doi: 10.1126/science.aat9819. [DOI] [PubMed] [Google Scholar]
- 29.Shen P.S., Yang X., DeCaen P.G. The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell. 2016;167:763–773 e11. doi: 10.1016/j.cell.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grieben M., Pike A.C., Shintre C.A. Structure of the polycystic kidney disease TRP channel polycystin-2 (PC2) Nat Struct Mol Biol. 2017;24:114–122. doi: 10.1038/nsmb.3343. [DOI] [PubMed] [Google Scholar]
- 31.Wilkes M., Madej M.G., Kreuter L. Molecular insights into lipid-assisted Ca2+ regulation of the TRP channel polycystin-2. Nat Struct Mol Biol. 2017;24:123–130. doi: 10.1038/nsmb.3357. [DOI] [PubMed] [Google Scholar]
- 32.Liu X., Vien T., Duan J. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife. 2018;7 doi: 10.7554/eLife.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koulen P., Cai Y., Geng L. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 34.Cai Y., Anyatonwu G., Okuhara D. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem. 2004;279:19987–19995. doi: 10.1074/jbc.M312031200. [DOI] [PubMed] [Google Scholar]
- 35.Kleene S.J., Kleene N.K. The native TRPP2-dependent channel of murine renal primary cilia. Am J Phyiol Renal Physiol. 2017;312:F96–F108. doi: 10.1152/ajprenal.00272.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleene S.J., Siroky B.J., Landero-Figueroa J.A. The TRPP2-dependent channel of renal primary cilia also requires TRPM3. PloS One. 2019;14 doi: 10.1371/journal.pone.0214053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin S.C., Homsy J., Zaidi S. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. 2017;49:1593–1601. doi: 10.1038/ng.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshiba S., Shiratori H., Kuo I.Y. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science. 2012;338:226–231. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan S., Zhao L., Brueckner M., Sun Z. Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr Biol. 2015;25:556–567. doi: 10.1016/j.cub.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman A.B., Johnson A.M., Gabow P.A. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5:1178–1185. doi: 10.1681/ASN.V551178. [DOI] [PubMed] [Google Scholar]
- 41.Kaur S., McGlashan S.R., Ward M.L. Evidence of primary cilia in the developing rat heart. Cilia. 2018;7:4. doi: 10.1186/s13630-018-0058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villalobos E., Criollo A., Schiattarella G.G. Fibroblast primary cilia are required for cardiac fibrosis. Circulation. 2019;139:2342–2357. doi: 10.1161/CIRCULATIONAHA.117.028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paavola J., Schliffke S., Rossetti S. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J Mol Cell Cardiol. 2013;58:199–208. doi: 10.1016/j.yjmcc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delling M., DeCaen P.G., Doerner J.F. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delling M., Indzhykulian A.A., Liu X. Primary cilia are not calcium-responsive mechanosensors. Nature. 2016;531:656–660. doi: 10.1038/nature17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anyatonwu G.I., Estrada M., Tian X. Regulation of ryanodine receptor–dependent calcium signaling by polycystin-2. Proc Natl Acad Sci U S A. 2007;104:6454–6459. doi: 10.1073/pnas.0610324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo I.Y., Kwaczala A.T., Nguyen L. Decreased polycystin 2 expression alters calcium-contraction coupling and changes beta-adrenergic signaling pathways. Proc Natl Acad Sci U S A. 2014;111:16604–16609. doi: 10.1073/pnas.1415933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuo I.Y., Duong S.L., Nguyen L., Ehrlich B.E. Decreased polycystin 2 levels result in non-renal cardiac dysfunction with aging. PloS One. 2016;11 doi: 10.1371/journal.pone.0153632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giehl E., Lemos F.O., Huang Y. Polycystin 2-dependent cardio-protective mechanisms revealed by cardiac stress. Pflugers Arch. 2017;469:1507–1517. doi: 10.1007/s00424-017-2042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balbo B.E., Amaral A.G., Fonseca J.M. Cardiac dysfunction in Pkd1-deficient mice with phenotype rescue by galectin-3 knockout. Kidney Int. 2016;90:580–597. doi: 10.1016/j.kint.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedrozo Z., Criollo A., Battiprolu P.K. Polycystin-1 is a cardiomyocyte mechanosensor that governs L-type Ca2+ channel protein stability. Circulation. 2015;131:2131–2142. doi: 10.1161/CIRCULATIONAHA.114.013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altamirano F., Schiattarella G.G., French K.M. Polycystin-1 assembles with Kv channels to govern cardiomyocyte repolarization and contractility. Circulation. 2019;140:921–936. doi: 10.1161/CIRCULATIONAHA.118.034731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu T.M., Chuang Y.W., Yu M.C. New-onset atrial fibrillation is associated with polycystic kidney disease: a nationwide population-based cohort study. Medicine (Baltimore) 2016;95:e2623. doi: 10.1097/MD.0000000000002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huizar J.F., Ellenbogen K.A., Tan A.Y., Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2328–2344. doi: 10.1016/j.jacc.2019.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Criollo A., Altamirano F., Pedrozo Z. Polycystin-2-dependent control of cardiomyocyte autophagy. J Mol Cell Cardiol. 2018;118:110–121. doi: 10.1016/j.yjmcc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Sharif-Naeini R., Folgering J.H., Bichet D. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 57.Bulley S., Fernandez-Pena C., Hasan R. Arterial smooth muscle cell PKD2 (TRPP1) channels regulate systemic blood pressure. eLife. 2018;7 doi: 10.7554/eLife.42628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding A., Walton S.L., Moritz K.M., Phillips J.K. Impact of prenatal and postnatal maternal environment on nephron endowment, renal function and blood pressure in the Lewis polycystic kidney rat. J Dev Orig Health Dis. 2019;10:154–163. doi: 10.1017/S2040174418000673. [DOI] [PubMed] [Google Scholar]
- 59.Quek K.J., Boyd R., Ameer O.Z. Progressive vascular remodelling, endothelial dysfunction and stiffness in mesenteric resistance arteries in a rodent model of chronic kidney disease. Vascul Pharmacol. 2016;81:42–52. doi: 10.1016/j.vph.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Souders C.A., Bowers S.L., Baudino T.A. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian Q., Li M., Cai Y. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol. 2003;14:2280–2287. doi: 10.1097/01.asn.0000080185.38113.a3. [DOI] [PubMed] [Google Scholar]
- 62.Stypmann J., Engelen M.A., Orwat S. Cardiovascular characterization of Pkd2(+/LacZ) mice, an animal model for the autosomal dominant polycystic kidney disease type 2 (ADPKD2) Int J Cardiol. 2007;120:158–166. doi: 10.1016/j.ijcard.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Lorthioir A., Joannides R., Remy-Jouet I. Polycystin deficiency induces dopamine-reversible alterations in flow-mediated dilatation and vascular nitric oxide release in humans. Kidney Int. 2015;87:465–472. doi: 10.1038/ki.2014.241. [DOI] [PubMed] [Google Scholar]
- 64.Wang D., Strandgaard S., Borresen M.L. Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008;51:184–191. doi: 10.1053/j.ajkd.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 65.Kocyigit I., Taheri S., Sener E.F. Endothelial nitric oxide synthase gene expression is associated with hypertension in autosomal dominant polycystic kidney disease. Cardiorenal Med. 2014;4:269–279. doi: 10.1159/000369105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang D., Iversen J., Strandgaard S. Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11:1371–1376. doi: 10.1681/ASN.V1181371. [DOI] [PubMed] [Google Scholar]
- 67.Klawitter J., Reed-Gitomer B.Y., McFann K. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Phyiol Renal Physiol. 2014;307:F1198–F1206. doi: 10.1152/ajprenal.00327.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peterson K.M., Franchi F., Loeffler D.L. Endothelial dysfunction occurs prior to clinical evidence of polycystic kidney disease. Am J Nephrol. 2013;38:233–240. doi: 10.1159/000354236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCooke J.K., Appels R., Barrero R.A. A novel mutation causing nephronophthisis in the Lewis polycystic kidney rat localises to a conserved RCC1 domain in Nek8. BMC Genomics. 2012;13:393. doi: 10.1186/1471-2164-13-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salman I.M., Sarma Kandukuri D., Harrison J.L. Direct conscious telemetry recordings demonstrate increased renal sympathetic nerve activity in rats with chronic kidney disease. Front Physiol. 2015;6:218. doi: 10.3389/fphys.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]